Abstract
The cryopreservation of exfoliated deciduous teeth and harvesting of stem cells from them as required would reduce the costs and efforts associated with banking stem cells from primary teeth. The aim of this study was determine whether the viability of pulp stromal cells from deciduous teeth was influenced by the cryopreservation process itself or the period of cryopreservation. In total, 126 deciduous teeth were divided into three groups: (1) fresh, (2) cryopreserved for <3 months (cryo<3), and (3) cryopreserved for 3–9 months (cryo3–9). The viability of the pulp tissues was compared among the three groups by evaluating the outgrowth from pulp tissues and cell activity within those pulp tissues. In addition, the terminal deoxynucleotidyl transferase-mediated dUTP–biotin nick end labeling (TUNEL) assay was performed to compare cell apoptosis within fresh pulp tissue and pulp tissue that had been cryopreserved for 4 months. The outgrowth from and cell activity within the pulp tissues did not differ significantly between the fresh and cryo<3 pulp tissues. However, these parameters were significantly reduced in the cryo3–9 pulp tissue. In TUNEL assay, 4-month cryopreserved pulp tissues has more apoptotic cells than fresh group. In conclusion, it is possible to acquire pulp stromal cells from cryopreserved deciduous teeth. However, as the period of cryopreservation becomes longer, it is difficult to get pulp cells due to reduced cell viability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Interest in the ability of stem cells to differentiate into a variety of cells has been increasing, and there has been a concomitant increase in the number of studies regarding the treatment of damaged tissues with stem cells. In particular, many studies have investigated mesenchymal stem cells derived from bone marrow (Bruder et al. 1997; Fernandez-Aviles et al. 2004; Yang et al. 2011). Studies on stem cells from tooth-related tissues such as the pulp (Iohara et al. 2006; Zhang et al. 2006), periodontal ligament (Huang et al. 2009), and dental follicles (Yao et al. 2008) are currently in progress. The tissues of deciduous teeth can be acquired noninvasively, since almost everyone experiences shedding of such teeth without requiring a painful iatrogenic procedure. The cells of deciduous teeth are of particular interest because they have a high proliferation rate (Miura et al. 2003; Nakamura et al. 2009), which has prompted many studies using deciduous teeth as a source of stem cells (Arora et al. 2009; Karaoz et al. 2010; Miura et al. 2003; Nakamura et al. 2009).
Studies into the cryopreservation of teeth have traditionally focused on the transplantation of permanent teeth (Izumi et al. 2007; Laureys et al. 2001; Schwartz 1986; Schwartz et al. 1985; Schwartz and Rank 1986), and the question of survival of the replanted teeth has led to studies of the viability of periodontal ligament and pulpal tissues of the cryopreserved teeth (Oh et al. 2005; Price and Cserepfalvi 1972; Schwartz and Rank 1986; Temmerman et al. 2010). Recent interest in stem cells has also resulted in many studies on cells obtained from cryopreserved permanent teeth (Lee et al. 2010; Min et al. 2010; Oh et al. 2005; Perry et al. 2008; Woods et al. 2009).
Since deciduous teeth cannot be used to replant or transplant, it has not been considered necessary to cryopreserve them. However, the ability to cryopreserve deciduous teeth themselves and obtain stem cells from such teeth whenever necessary would decrease the costs and efforts associated with banking stem cells from deciduous teeth (Arora et al. 2009). However, no study has investigated obtaining stem cells from cryopreserved deciduous teeth or even the effects of cryopreservation itself and the cryopreservation period on cell viability within pulpal tissues.
The aim of this study was thus to determine the effect of the cryopreservation process and the cryopreservation period on the viability of pulp stromal cells in deciduous teeth.
Materials and methods
Subjects and cryopreservation
The experimental protocol was approved by the Institutional Review Board of the Dental Hospital, Yonsei University, and informed consent to participate was obtained from all of the subjects and their parents (approval no. #2-2011-0034). In total, 122 deciduous teeth were obtained from 105 healthy persons (69 males and 36 females, aged 3–16 years). The experimental subjects were deciduous incisors and deciduous molars close to natural exfoliation with less than one-third of the root remaining. Deciduous teeth with deep caries, any type of restoration, periapical lesions or internal resorption were excluded.
After extracting the deciduous teeth they were divided randomly in a single-blind manner into three groups: (1) fresh, (2) cryopreserved for <3 months (cryo<3), and (3) cryopreserved for 3–9 months (cryo3–9). The teeth of the cryopreservation groups were kept in fetal bovine serum (FBS; Invitrogen, Carlsbad, CA, USA) supplemented with 10 % dimethyl sulfoxide (DMSO; Sigma, St. Louis, MO, USA) for 1 h at 4 °C, and then placed into a deep freezer (Cryo 1 °C freezing container, Nalge Nunc International, Rochester, NY, USA) in which they were slowly frozen from 4 to −80 °C at a rate of 1 °C/min. The teeth were then plunged into liquid nitrogen at −196 °C. After storage in liquid nitrogen for 1–9 months (depending upon the experimental group) the teeth were rapidly thawed in a 37 °C water bath in preparation for the subsequent experiments. Immediately after extraction, teeth in the fresh group were kept in a cell culture medium comprising α-minimum essential medium (α-MEM; Invitrogen) with 10 % FBS, 100 U/ml penicillin, 100 μg/ml streptomycin (Invitrogen), 2 mM l-glutamine (Invitrogen), and 10 mM l-ascorbic acid (Sigma) for 1 h at 4 °C, in preparation for the subsequent experiments.
Primary culture
Pulp stromal cells were obtained using the outgrowth method from fresh teeth (n = 30), cryo<3 teeth (n = 13), and cryo3–9 teeth (n = 17). Pulpal tissue was removed using a barbed broach (Mani, Utsunomiya Toshi-ken, Japan) and then cut into several 1-mm3 fragments, placed on a 60-mm culture dish (BD Falcon, Lincoln Park, NJ, USA), covered with cover glass (Superior, Lauda-Königshofen, Germany), and incubated with the aforementioned culture medium at 37 °C in a humid atmosphere containing 5 % CO2. The culture was classified as successful if fibroblast-like cells were observed around the attached tissues.
Cell activity in pulp tissues
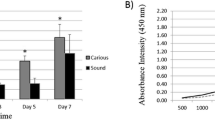
Cell activity in the pulp tissue was measured using a cell-counting assay kit (CCK-8, Dojindo Laboratories, Kumamoto, Japan). The number of living cells was measured indirectly by colorimetric determination of the amount of formazan salt that was formed as a result of the reaction of the WST-8 reagent in the kit with dehydrogenase in the living cells. Tissues from the fresh (n = 30), cryo<3 (n = 17), and cryo3–9 (n = 13) groups were used in these experiments. In brief, 10 μl of CCK-8 solution and 100 μl of cell culture medium were placed into the each well of a 96-well plate (BD Falcon), followed by the pulp tissues. After incubation in the wells for 3 h at 37 °C in a 5 % CO2 atmosphere, the pulp tissues were removed and then the optical density of each well was measured using a spectrophotometer (Benchmark Plus Microplate Reader, Bio-Rad, Hercules, CA, USA) at a wavelength of 450 nm.
Terminal deoxynucleotidyl transferase-mediated dUTP–biotin nick end labeling assay
The degree of apoptotic cell death in the pulp tissues was evaluated by the terminal deoxynucleotidyl transferase-mediated dUTP–biotin nick end labeling (TUNEL; Travigen, Gaithersburg, MD, USA) assay. A fresh deciduous tooth and a 4-month-cryopreserved deciduous tooth were fixed with 10 % buffered formalin (Sigma) at 4 °C overnight, decalcified with 10 % EDTA (pH 7.4; Fisher Scientific, Houston, TX, USA) for 4 weeks, embedded in paraffin, and then sectioned at a thickness of 4 μm. The sections were attached to slide glasses, deparaffinized, and then dehydrated. Staining procedures were performed according to the manufacturer’s instructions. In brief, the sections were preincubated with proteinase K (Sigma), treated with 3 % hydrogen peroxide, and then immersed in 1 × transferase-mediated deoxyuridine triphosphate (TdT; Sigma) labeling buffer for 5 min. After treatment with the Labeling Reaction Mix (Sigma), the sections were incubated at 37 °C for 30 min in a humidity chamber and immersed in 1 × TdT Stop Buffer (Sigma) for 5 min at room temperature to stop the labeling reaction. The samples were washed twice in deionized water for 5 min at room temperature and then incubated in streptavidin–horseradish-peroxidase solution (Sigma). The sections were then immersed in 3,3′-diaminobenzidine (Vector Laboratories, Burlingame, CA, USA) solution for 2 min. They were examined under an optical microscope (BX40, Olympus, Tokyo, Japan).
Statistical analysis
Statistical analysis was performed with SPSS (version 19.0, IBM, Chicago, IL, USA). Cell activity in pulp tissues was analyzed using the Kruskal–Wallis test followed by the Mann–Whitney U test with Bonferroni correction (p < 0.017).
Results
Primary culture
The cell outgrown from the pulp tissues exhibited a typical spindle-shaped fibroblastic morphology (Fig. 1a, b). The morphologies evident in optical microscopy were same for fresh and cryopreserved teeth (Fig. 1c, d). The success and failure cases of primary culture in each group are detailed in Table 1. The success rate was lowest in the cryo3–9 samples.
Cell activity in pulp tissue
Cell activity in the pulp tissues was significantly lower in the cryo3–9 group than in the other two groups, as illustrated in Figure 2 (p > 0.017).
TUNEL assays
The number of apoptotic cells was far higher in the pulp of the apical portion in contact with the outside environment than in the coronal portion in both the fresh and 4-month-cryopreserved teeth, and was higher in all portions of the cryopreserved tooth than in the fresh tooth (Fig. 3).
Apoptotic cell staining by the TUNEL assay in fresh and cryopreserved pulp tissues. a, c, e A fresh deciduous tooth. b, d, f A deciduous tooth that was cryopreserved for 4 months. Arrows indicate apoptotic cells stained positively with a dark-brown color. Scale bars 1 mm in (a, b), 100 μm in (c, d), and 20 μm in (e, f)
Discussion
It is necessary to use an adequate freezing rate and cryoprotective agents in order to protect against cell injury during the cryopreservation procedure. Freezing too rapidly can allow ice crystals to form inside the cells because it becomes impossible to transport sufficient water out of the cell, and this may cause cell necrosis during thawing (Day and Stacey 2007; Han and Bischof 2004), while a freezing process that is too slow may cause cell shrinkage due to the loss of intracellular fluid. Therefore, rate-controlled freezing is recommended to reduce cell damage (Kawasaki et al. 2004). Cryoprotectants such as DMSO and glycerol are also commonly used to increase the probability of cell survival. Several studies have demonstrated that the use of 10 % DMSO solution and a slow freezing protocol produces the highest cell viability (Miyamoto et al. 2001; Schwartz et al. 1985; Woods et al. 2009), and hence the teeth in our study were slowly frozen in 10 % DMSO.
The survival of the pulpal cells within a tooth depends on the degree of DMSO penetration. Several studies (Jomha et al. 2002; Muldrew et al. 2001) have shown that cryopreservation results in more nonviable cells at deeper portions than in the superficial layer of the tissue where cryoprotectants can penetrate easily. Another study found that the survival rate was higher for immature permanent teeth than for mature teeth because DMSO was more easily absorbed through the root apices (Temmerman et al. 2010). Moreover, drilling holes in teeth can improve the post-cryopreservation outcome (Gioventu et al. 2012). Therefore, in our study, to allow sufficient cryoprotective agent to diffuse through the entire pulp tissue via the apical portion, we stored the extracted teeth at 4 °C for 1 h before freezing them. Since the roots of deciduous teeth are already almost resorbed, the area of contact with the outside environment is relatively large, which allowed DMSO to be readily absorbed into the deep pulp tissues through the root apices.
Several methods can be used to culture cells from tissues, including enzyme digestion and outgrowth (Freshney 2005); in our study we estimated the cell viability by evaluating the cell outgrowth from pulp tissues. The outgrowth method takes longer but it can result in homogeneous cells being harvested and is particularly advantageous in cases involving small amounts of tissue (Huang et al. 2006). Although enzyme digestion has been the most common method used to acquire adult stem cells (Arora et al. 2009; Gay et al. 2007; Nishino et al. 2011), it has been reported that the outgrowth method can be used to acquire multipotent stem cells (Bakopoulou et al. 2011; Spath et al. 2010). We used the outgrowth method to isolate the cells from pulp tissue because only a very small amount of pulp tissues is available from deciduous teeth that are close to natural exfoliation.
The use of the CCK-8 assay to measure the cell activity in tissues represents a limitation of this study since we did not know how much of the CCK-8 solution had penetrated into the deeper portion of the tissues, and hence the results may have reflected more the effects on the outer portion than the deeper portion. In addition, we performed the viability assessment immediately post-thaw, which could provide elevated results since it does not take into account the delayed onset of cell death due to apoptosis. Nevertheless, the assay is useful for evaluating the cell survival in tissues indirectly, and is easy to apply, requires no organic solvents or isotopes, and is more sensitive than other viability assay tests.
The cell activity and cell-outgrowth ability did not differ between the fresh and cryo<3 groups in this study. This is consistent with (Temmerman et al. 2010) finding that the primary culture success rates did not differ significantly between pulpal cells from immature third molars cryopreserved for 1 month and those cultured immediately after extraction. (Oh et al. 2005) also found that the activities of periodontal ligament cells from human premolars cryopreserved for 1 week did not differ significantly from those obtained from fresh teeth. In contrast, other studies found that the pulp viability was lower for cryopreserved permanent teeth than for fresh teeth (Chen et al. 2011; Woods et al. 2009). However, those findings might have been due to the use of permanent teeth with a mature apex, which cannot absorb DMSO as easily as can immature pulp or periodontal ligament. Our study used deciduous teeth with large apices through which DMSO could be absorbed, and thus the activity of the cryopreserved tooth pulp was similar to that of fresh tooth pulp.
Cells can generally be stored safely for many years or even decades in liquid nitrogen. Previous studies have found that stem cells could be isolated from even 6-month-cryopreserved teeth and that they exhibit similar functionality (Perry et al. 2008; Woods et al. 2009). However, the survival rate of cells is lower when they are frozen for much longer periods (Kobylka et al. 1998; Liseth et al. 2009; Mugishima et al. 1999) because photophysical events such as the formation of free radicals and the production of breaks in macromolecules as direct results of impacts by background ionizing radiation or cosmic rays (Mazur 1984) can occur even in frozen aqueous systems at −196 °C. Unlike previous studies, our study revealed that cryopreservation for more than 3 months (cryo3–9) reduced the cell activity and outgrowth ability, which represents a rather rapid decrease in cell viability despite the use of liquid nitrogen. We do not know the exact reason for this phenomenon. During the cryopreservation periods of up to 9 months the cryovials with teeth could have been moved from liquid nitrogen to the outside environment in order to cryopreserve other teeth or cells and thereby be repeatedly and rapidly thawed and frozen. Our TUNEL assays revealed that exfoliated deciduous teeth already have many cells undergoing apoptosis—especially in the apical portion of the pulp and the periodontal ligament—induced by inflammatory reactions associated with root resorption of deciduous teeth (Domon et al. 2008; Rodrigues et al. 2009). Even a small amount of the natural radiation might enhance or accelerate the apoptotic process in cryopreserved tissue cells.
Our TUNEL assays also revealed that the extent and amount of apoptosis were greater in teeth that had been cryopreserved for 4 months than in fresh teeth. This is inconsistent with a previous study finding that the number of apoptotic periodontal ligament cells per unit area was similar in 1-week-cryopreserved mandibular premolars and fresh premolars (Oh et al. 2005). In our study the tissues were cryopreserved for 4 months before performing TUNEL assays, so it can be assumed that the longer cryopreservation period had negatively affected the cell viability.
The TUNEL assays also showed that the degree of apoptosis was greater in the apical than the coronal portion of the pulp. This finding contrasts with a previous study finding that the viability of cryopreserved rat tooth pulp was greater in the apical than the coronal portion of the pulp, which would be difficult for DMSO to reach (Lee et al. 2012). However, our study used nearly exfoliated teeth that exhibited advanced root resorption. It may be that the apical portion of the pulp exhibited more apoptosis than the coronal portion because of apical inflammatory reactions induced by the root resorption process.
We found that the cell viability did not differ significantly between pulp tissues acquired from cryopreserved deciduous teeth and fresh teeth, and hence it appears to be possible to acquire viable stromal cells from cryopreserved deciduous teeth. However, the viability of the cells decreases as the period of cryopreservation lengthens. Future studies should explore methods for increasing cell survival rates after long-term cryopreservation and determine whether the cells from the pulp of cryopreserved deciduous teeth indeed represent useful stem cells.
References
Arora V, Arora P, Munshi AK (2009) Banking stem cells from human exfoliated deciduous teeth (SHED): saving for the future. J Clin Pediatr Dent 33:289–294
Bakopoulou A, Leyhausen G, Volk J, Tsiftsoglou A, Garefis P, Koidis P et al (2011) Assessment of the impact of two different isolation methods on the osteo/odontogenic differentiation potential of human dental stem cells derived from deciduous teeth. Calcif Tissue Int 88:130–141
Bruder SP, Jaiswal N, Haynesworth SE (1997) Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biochem 64:278–294
Chen YK, Huang AH, Chan AW, Shieh TY, Lin LM (2011) Human dental pulp stem cells derived from different cryopreservation methods of human dental pulp tissues of diseased teeth. J Oral Pathol Med 40:793–800
Day JG, Stacey G (2007) Cryopreservation and freeze-drying protocols, 2nd edn. Humana Press, Totowa, NJ
Domon T, Taniguchi Y, Inoue K, Ushijima N, Taishi Y, Hiramatsu A et al (2008) Apoptosis of odontoclasts under physiological root resorption of human deciduous teeth. Cell Tissue Res 331:423–433
Fernandez-Aviles F, San Roman JA, Garcia-Frade J, Fernandez ME, Penarrubia MJ, de la Fuente L et al (2004) Experimental and clinical regenerative capability of human bone marrow cells after myocardial infarction. Circ Res 95:742–748
Freshney RI (2005) Culture of animal cells: a manual of basic technique, 5th edn. Wiley-Liss, Hoboken, NJ
Gay IC, Chen S, MacDougall M (2007) Isolation and characterization of multipotent human periodontal ligament stem cells. Orthod Craniofac Res 10:149–160
Gioventu S, Andriolo G, Bonino F, Frasca S, Lazzari L, Montelatici E et al (2012) A novel method for banking dental pulp stem cells. Transfus Apher Sci 47:199–206
Han B, Bischof JC (2004) Direct cell injury associated with eutectic crystallization during freezing. Cryobiology 48:8–21
Huang GT, Sonoyama W, Chen J, Park SH (2006) In vitro characterization of human dental pulp cells: various isolation methods and culturing environments. Cell Tissue Res 324:225–236
Huang CY, Pelaez D, Dominguez-Bendala J, Garcia-Godoy F, Cheung HS (2009) Plasticity of stem cells derived from adult periodontal ligament. Regen Med 4:809–821
Iohara K, Zheng L, Ito M, Tomokiyo A, Matsushita K, Nakashima M (2006) Side population cells isolated from porcine dental pulp tissue with self-renewal and multipotency for dentinogenesis, chondrogenesis, adipogenesis, and neurogenesis. Stem Cells 24:2493–2503
Izumi N, Yoshizawa M, Ono Y, Kobayashi T, Hamamoto Y, Saito C (2007) Periodontal regeneration of transplanted rat teeth subcutaneously after cryopreservation. Int J Oral Maxillofac Surg 36:838–844
Jomha NM, Lavoie G, Muldrew K, Schachar NS, McGann LE (2002) Cryopreservation of intact human articular cartilage. J Orthop Res 20:1253–1255
Karaoz E, Dogan BN, Aksoy A, Gacar G, Akyuz S, Ayhan S et al (2010) Isolation and in vitro characterisation of dental pulp stem cells from natal teeth. Histochem Cell Biol 133:95–112
Kawasaki N, Hamamoto Y, Nakajima T, Irie K, Ozawa H (2004) Periodontal regeneration of transplanted rat molars after cryopreservation. Arch Oral Biol 49:59–69
Kobylka P, Ivanyi P, Breur-Vriesendorp BS (1998) Preservation of immunological and colony-forming capacities of long-term (15 years) cryopreserved cord blood cells. Transplantation 65:1275–1278
Laureys W, Beele H, Cornelissen R, Dermaut L (2001) Revascularization after cryopreservation and autotransplantation of immature and mature apicoectomized teeth. Am J Orthod Dentofacial Orthop 119:346–352
Lee SY, Chiang PC, Tsai YH, Tsai SY, Jeng JH, Kawata T et al (2010) Effects of cryopreservation of intact teeth on the isolated dental pulp stem cells. J Endod 36:1336–1340
Lee SY, Sun CH, Kuo TF, Huang YH, Jeng JH, Yang JC et al (2012) Determination of cryoprotectant for magnetic cryopreservation of dental pulp tissue. Tissue Eng Part C Methods 18:397–407
Liseth K, Ersvaer E, Abrahamsen JF, Nesthus I, Ryningen A, Bruserud O (2009) Long-term cryopreservation of autologous stem cell grafts: a clinical and experimental study of hematopoietic and immunocompetent cells. Transfusion 49:1709–1719
Mazur P (1984) Freezing of living cells: mechanisms and implications. Am J Physiol 247:C125–C142
Min KS, Lee HW, Lee HS, Lee JH, Park SH (2010) Comparison of gene expression in human periodontal ligament cells cultured from teeth immediately after extraction and from teeth cryopreserved for 1 week. Cryobiology 60:326–330
Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG et al (2003) SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA 100:5807–5812
Miyamoto M, Balamurugan AN, Nozawa Y, Sakurai T, Xu B, Yoshimura S et al (2001) Development of a cryopreservation procedure employing a freezer bag for pancreatic islets using a newly developed cryoprotectant. Cell Transplant 10:363–371
Mugishima H, Harada K, Chin M, Suzuki T, Takagi K, Hayakawa S et al (1999) Effects of long-term cryopreservation on hematopoietic progenitor cells in umbilical cord blood. Bone Marrow Transplant 23:395–396
Muldrew K, Novak K, Studholme C, Wohl G, Zernicke R, Schachar NS et al (2001) Transplantation of articular cartilage following a step-cooling cryopreservation protocol. Cryobiology 43:260–267
Nakamura S, Yamada Y, Katagiri W, Sugito T, Ito K, Ueda M (2009) Stem cell proliferation pathways comparison between human exfoliated deciduous teeth and dental pulp stem cells by gene expression profile from promising dental pulp. J Endod 35:1536–1542
Nishino Y, Yamada Y, Ebisawa K, Nakamura S, Okabe K, Umemura E et al (2011) Stem cells from human exfoliated deciduous teeth (SHED) enhance wound healing and the possibility of novel cell therapy. Cytotherapy 13:598–605
Oh YH, Che ZM, Hong JC, Lee EJ, Lee SJ, Kim J (2005) Cryopreservation of human teeth for future organization of a tooth bank—a preliminary study. Cryobiology 51:322–329
Perry BC, Zhou D, Wu X, Yang FC, Byers MA, Chu TM et al (2008) Collection, cryopreservation, and characterization of human dental pulp-derived mesenchymal stem cells for banking and clinical use. Tissue Eng Part C Methods 14:149–156
Price PJ, Cserepfalvi M (1972) Pulp viability and the homotransplantation of frozen teeth. J Dent Res 51:39–43
Rodrigues LV, Vasconcelos AC, Campos PA, Brant JM (2009) Apoptosis in pulp elimination during physiological root resorption in human primary teeth. Braz Dent J 20:179–185
Schwartz O (1986) Cryopreservation as long-term storage of teeth for transplantation or replantation. Int J Oral Maxillofac Surg 15:30–32
Schwartz O, Rank CP (1986) Autotransplantation of cryopreserved tooth in connection with orthodontic treatment. Am J Orthod Dentofacial Orthop 90:67–72
Schwartz O, Andreasen JO, Greve T (1985) Cryopreservation before replantation of mature teeth in monkeys. (II). Effect of preincubation, different freezing and equilibration rates and endodontic treatment upon periodontal healing. Int J Oral Surg 14:350–361
Spath L, Rotilio V, Alessandrini M, Gambara G, De Angelis L, Mancini M et al (2010) Explant-derived human dental pulp stem cells enhance differentiation and proliferation potentials. J Cell Mol Med 14:1635–1644
Temmerman L, Beele H, Dermaut LR, Van Maele G, De Pauw GA (2010) Influence of cryopreservation on the pulpal tissue of immature third molars in vitro. Cell Tissue Bank 11:281–289
Woods EJ, Perry BC, Hockema JJ, Larson L, Zhou D, Goebel WS (2009) Optimized cryopreservation method for human dental pulp-derived stem cells and their tissues of origin for banking and clinical use. Cryobiology 59:150–157
Yang Y, Schumacher A, Liu J, Shi X, Hill WD, Hu TC (2011) Monitoring bone marrow-originated mesenchymal stem cell traffic to myocardial infarction sites using magnetic resonance imaging. Magn Reson Med 65:1430–1436
Yao S, Pan F, Prpic V, Wise GE (2008) Differentiation of stem cells in the dental follicle. J Dent Res 87:767–771
Zhang W, Walboomers XF, Shi S, Fan M, Jansen JA (2006) Multilineage differentiation potential of stem cells derived from human dental pulp after cryopreservation. Tissue Eng 12:2813–2823
Acknowledgments
This study was supported by a faculty research grant of Yonsei University College of Dentistry for 2011 (No. 6-2011-0050). There were no competing financial interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Eun Hye Ji and Je Seon Song equally contributed to this work.
Rights and permissions
About this article
Cite this article
Ji, E.H., Song, J.S., Kim, SO. et al. Viability of pulp stromal cells in cryopreserved deciduous teeth. Cell Tissue Bank 15, 67–74 (2014). https://doi.org/10.1007/s10561-013-9375-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-013-9375-z