Abstract
Cervical cancer (CC) is diagnosis based on histopathological analysis. Although cancer staging is performed clinically, using the International Federation of Gynecology and Obstetrics (FIGO) criteria, cancer staging systems have been shown to be deficient for the examination of prognostic factors of CC, including tumor size and infiltration of the parametria or pelvic wall, as well as for the evaluation of nodal metastases. In this regard, imaging methods are considered crucial in the assessment of CC, as imaging data complement the information obtained from a clinical examination.
Ultrasound has a limited role in CC staging; it is inadequate for assessing nodal status or pelvic wall involvement. Tomography is used to evaluate adenopathies, define the extent of disease progression, assess metastasis, plan radiotherapy, and guide percutaneous biopsy. Magnetic resonance imaging (MRI) has been established to be superior for the characterization of lesions and local extension of disease. The main indications for 18F-FDG positron emission tomography-computed tomography (PET-CT) are initial staging (determination of locoregional nodal involvement and extrapelvic extension), evaluation of the response to therapy and detection of recurrence.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
9.1 Introduction
Given its epidemiological features, cervix cancer is still diagnosis based on a clinical examination, despite an error rate of up to 32% in stage IB patients and up to 65% in stage III patients according to the International Federation of Gynecology and Obstetrics (FIGO). Clinical staging has been shown to be unsuitable for the evaluation of prognostic factors, such as invasion of the parametria and pelvic wall, tumor size, and locoregional and distant nodal metastases [1].
Cervical Cancer (CC) staging is fundamental for treatment planning, and this process implies a reliable, practical and reproducible staging method that facilitates the assessment of the treatment response, prediction of prognosis, and exchange of information among different oncological treatment centers. Magnetic resonance imaging (MRI) is an imaging modality that plays a predominant role in the evaluation of disease extension and spread [2].
Recent studies have reported discrepancies between clinical evaluation results and MRI data; for instance, the characteristics endocervical lesions are often conflicting, with an underestimation of the tumor size based on the clinical examination compared to the tumor size based on MRI. In general, the accuracy of MRI in estimating the tumor size is 93%, while the accuracy of clinical staging is less than 60% [3].
The revised FIGO staging guidelines recommend performing computed tomography (CT) or MRI whenever such an imaging method is available. The use of CT is unsuited for local staging but is highly useful for detecting extrauterine affection, including nodal affection, fistula, or distant disease. Conversely, MRI provides high-contrast resolution of soft tissue and enables the clear definition of the local extension of the primary tumor and metastatic lesions [4].
Currently, MRI has no defined indication in stage IA patients, as stage IA cancer is by definition a microscopic disease that is undetectable on MRI. Thorax radiography (TR) and PET are considered for patients with distant disease. Early stage (IIA1 and IB1) CC patient treatment includes surgery; thus, it is crucial to identify tumor extension beyond cervix in order to plan the course chemotherapy and radiotherapy for these patients.
9.2 Technical Recommendations Concerning Cervical Cancer Assessment Using Different Diagnostic Tools
9.2.1 Ultrasound
This method is affordable, non-invasive, and widely used in different studies and treatment centers. Hence, ultrasound has gained popularity for CC analysis during the last two decades. Reports by Fisherova and Testa have described its high sensitivity and specificity in the detection of primary lesions as well as locoregional extension [5, 6].
In the study of this type of neoplasia, tone must combine abdominal and pelvic suprapubic ultrasound to search for adenopathies. Additionally, transvaginal ultrasound and transrectal ultrasound are needed to evaluate local extension and Doppler color ultrasound is applied to assess, tumor angiogenesis in vivo in a non-invasive manner [7].
To achieve high accuracy using ultrasound, it is recommended to consider three variables: the operator, who needs some degree of experience; a high-resolution ultrasound system equipped with multifrequency convex and endocavitary transducers that is capable of color, spectral and power Doppler imaging. Additionally, the ultrasound data must be stored as static images or video for post-procedural analysis. Finally, during each evaluation, the patient’s habitus and general conditions must be considered to perform a complete assessment [8].
Transvaginal exploration is the optimal approach to the study of the uterus, as this approach adequately locates the anatomy, the annexes, and the pelvic peritoneum [8]. Cervix-dependent lesions are identified as non-compressible lesions that can vary in morphology in relation to their growth pattern (exophytic or endophytic). A color Doppler system enables the identification of cervix-dependent lesions based on a notable increase in their vascularity [9].
After detection and measurement of the orthogonal diameters of a tumor, stromal infiltration is evaluated (classified as one-third, two-thirds, or complete invasion), and tumor extension to anterior, lateral, or posterior parametria is explored. Parametrial invasion is detecting using color Doppler imaging, which facilitates the discrimination of vascular structures from tumoral tisssue [9].
The transrectal approach is beneficial to patients with an intact hymen, with stenotic vagina, with previous brachytherapy; the main indication of this approach in patients with CC is the evaluation of advanced disease extending to the parametrium, rectum, and vagina (Fig. 9.1) [8].
9.2.2 Computed Tomography
Among its different methods, CT is widely available and provides images with high spatial resolution within a short period. However, even after image reconstruction and contrast medium administration, the resolution of soft tissue remains unsatisfactory.
Small primary CC lesions usually appear isodense compared to the cervix, while larger lesions tend to be hypodense, necrotic and heterogeneous [10]. However, even further extended neoplasias may appear only as an unspecific increase in cervical volume (Fig. 9.2).
It is currently necessary for a patient to ingest 750–1000 ml of hydrosoluble contrast solution; oral contrast medium is useful for discriminating intestinal loops from lesions, particularly in patients with recurrence or distant disease. Subsequently, 120 ml of non-ionic contrast material is injected intravenously through an injector at a rate of 3 ml/s. After a 50 s delay, the images are acquired in a caudo-cephalic direction to observe the uterus and the cervix in their greatest relief. Additionally, a collimation of 5 mm, a table speed of 12.5 mm per rotation, and 3–5-mm-thick reconstructions are suggested [10].
The adequate application of contrast medium and table direction during study acquisition allows for discrimination of the uterine body from the cervix, which shows characteristic secondary zonal relief against the different components of the central glandular mucosa, internal fibromuscular stroma, and external fibroglandular stroma, conferring the uterine body with a “target-like” appearance in axial plane images [11].
9.2.3 Magnetic Resonance Imaging
In the authors’ experience in CC patient diagnosis and follow-up at their institute, imaging studies are performed using a 3 T Discovery 750 W imager (General Electric) equipped with a 16-channel superficial antenna, although diagnostic images can also be obtained using a 1.5 T magnet.
Patient preparation includes a 4- to 6-h fast, with the aim of diminishing peristalsis during imaging. The creatinine concentration in serous fluid must be known before deciding whether to administer endovenous contrast. To this end, veins in the forearm fold are preferentially chosen; it is also preferable to administer 20 mg (1 ampule) of hyoscine N-butylbromide 30–40 min before the initiation of imaging [3].
The key to adequate staging with MRI images is on the utilization of sequences that use a field of view (FOV) of 16–24 cm, a slice thickness of 3–5 mm, a base sequence for the evaluation of parametrial invasion of T2, and an imaging plane perpendicular to the longitudinal axis of the cervical canal, especially for imaging of the supravaginal cervix; the protocol duration is 30–40 min [3].
The T2 imaging sequences obtained using the below protocol are produced in the sagittal and oblique planes (acquired perpendicularly to the long cervical canal). The slice thickness is 5 mm, with a 1 mm inter-slice interval. The images are acquired with a 24 cm FOV and a 288 × 288 matrix. Optionally, sequences with fat saturation can be obtained to evaluate lesions showing signal that is distinct from that of the stroma and cervical canal and to evaluate tumor extension to the uterus, parametrium, and adjacent organs [3].
T1 sequences in the oblique plane are obtained using a 5 mm slice thickness, a 1 mm inter-slice interval, and a 320 × 320 matrix. Additionally, Diffusion-weighted imaging (DWI) sequences with a B value of 700 s/mm2 in the sagittal and oblique planes can be employed. Whenever needed, the apparent diffusion coefficient (ADC) is processed in the work module to evaluate the behavior of the lesions that have greater signal than the remainder of the stroma and to perform quantitative measurements of the area of interest; ADCs less than 1.2 × 10−3 mm2/s are considered positive as hypointense [3]. In some centers, gel is applied to the vagina for adequate evaluation of the vaginal and cervical fornices; this method is useful in patients with exophytic tumor growth. In our institution, this is not routinely performed because most of the patients do not agree to use it.
9.3 Main Diagnostic Aspects to Evaluate in Each Image Method
9.3.1 Imaging Features Considered in the Detection of Cervix Cancer
The imaging modalities used to evaluate CC extension include excretory urography (EU), barium enema, ultrasound, MRI, and PET. An important goal in CC staging is to discriminate early disease (IA and IB stages) that can be treated with surgical resection from more advanced disease requiring radiotherapy and probably chemotherapy.
Recent studies showed a decrease in the use of EU, barium enema, and lymphangiography and an increase in the use of CT. In spite of its proven superiority over other techniques, MRI remains underused in the detection of recurrent disease [5]. Iodine-based and barium-based contrasts are useful in the evaluation of treatment complications, such as fistulae, stenotic zones, vesicoureteral reflux, and ureteral lesion-related hydronephrosis (Fig. 9.3).
9.3.1.1 Ultrasonography
Transvaginal ultrasonography (TVU) has limited utility for cervix cancer tumor evaluation.
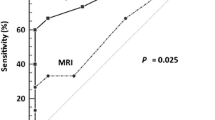
Transrectal ultrasonography (TRU) has shown higher accuracy than clinical examination (83% vs. 78%) and, in some studies, even MRI (90% vs. 81%) for the detection of tumors and 99% vs. 95% for the detection of parametrial invasion [5].
TRU has higher accuracy than CT or IRM for the detection of bladder invasion, based upon intact mobility over the uterine neck. However, the main disadvantages of TUR are the inability to evaluate the lateral walls of the pelvis and locoregional nodes [7].
As for the role of echography in the detection of lymphatic nodes in CC, Madsen et al. showed that the sensitivity of this technique is low (23%) but that it has an acceptable positive predictive value (71%) in a series of 109 women.
Fisherova et al. described the ultrasound evaluation technique in women with CC. Detailed evaluation allows for measurement of tumor size to determine the depth of stromal infiltration; the tumor location; parametrial, bladder and rectal involvement; and even the presence of pelvic lymphatic nodes [8].
9.3.1.1.1 Doppler Ultrasound in Cervical Cancer
Angiogenesis, the production of new vessels in a specific area, has been demonstrated to be an essential event in tumor growth and progression. In the specific case of CC, angiogenesis has been shown to constitute an independent prognostic factor and to predict recurrence. The first studies that evaluated blood flow hemodynamics in CC were published in the 90s and were focused on the main cervical supply vessels: the uterine artery and its cervical branches; these studies found that the pulsation index (PI) was lower in healthy women [7].
The first report on an analysis of intratumoral vessels in CC was published by Hsieh et al. in 1995. These authors found that 46.2% of CC patients showed an increase in blood flow signals on color Doppler ultrasound. It was reported that patients with detectable color signals more frequently showed lymphatic node involvement than those who showed no changes on color Doppler imaging (33% vs 5.7%, p = 0.005), and detection of color Doppler imaging signals correlated with a higher cell proliferation index. No differences in tumor stage, patient age, clinical staging, histologic type, or the DNA ploidy of the tumor cells were observed (Fig. 9.4) [7].
26 years-old women with cervical lesion, sagittal (a) and axial (b) transrectal ultrasound images show an hypoechoic, solid cervical tumor (arrow) with increase in blood flow signals on color Doppler ultrasound, (c) sagittal transvaginal ultrasound image, shows a cervical tumor obliterates the endocervix and is present an intrauterine fluid collection, hematometra (*)
9.3.1.2 Computed Tomography
CT has an accuracy in CC ranging from 32% to 80%. Its sensitivity and specificity in the detection of parametrial invasion vary from 17% to 100%, with a mean of 64%, and 50% to 100%, with a mean of 81%, respectively. The consensus from the literature is that the value of CT is increased in the most advanced stages of the disease but that CT has limited value (a positive predictive value of 58%) in the evaluation of early parametrial invasion. CT has high accuracy in the detection of advanced disease. However, a recent study by ACRIN® reported that CT has a sensitivity of only 42% for the detection of advanced disease, with ranges of sensitivity and specificity for detecting parametrial invasion of 14–38% and 84–100%, respectively (Fig. 9.5) [10].
Axial enhanced CT image of the tumors of the 4 cm or more size, in (a) women (52 years old), (b) and other patient with 46 years old, in both of them: enhancement and hypodense areas and isodensity (*) which represent necrosis and parametrial extension (arrows), in the left the enhancement the ligaments uterosacral. In (c) shows infiltration to the vesical wall (*) by epidermoid carcinoma in a patient 46 years old and cervical cancer clinical stage IIIB
The main limitation of CT to local staging is inadequate discrimination between the tumor and normal cervical stromal tissue or parametrial structures (because of tissue isodensity). Thus, CT is chiefly used to evaluate advanced disease and metastasis to lymph nodes. The positive predictive value for nodal affection is between 51% and 65%, with a negative predictive value of 86–96% and a sensitivity range of 31–65%. A finding of tumor size (>1 cm) alone on CT can be a low-sensitivity parameter for malignant adenopathies and can miss microscopic metastases. Other uses of CT are detection of distant metastases, planning of radiotherapy and guidance of intervention procedures (Fig. 9.6) [10, 11].
Others have stated that CT has an accuracy of 76–80% for the detection of parametrial invasion, which is identified as eccentric parametrial masses, obliteration of periureteral fat planes and shrinkage of vascular structures. Due to its poor resolution of soft tissue, CT may overestimate parametrial invasion [11].
9.3.1.3 Magnetic Resonance Imaging
MRI offers high-resolution contrast and high-quality contrast in soft tissue, enabling the generation of multiplanar slices and three-dimensional reconstructions with maximum intensity projections. The use of functional MRI parameters, such as flux, temperature, tissue oxygenation, dynamic perfusion, and diffusion, further assist in treatment planning and treatment response evaluation [3].
MRI allows for the determination of the size, location, local extension, and stromal invasion depth of the tumor. Thus, MRI is superior to clinical evaluation, considering that lesions smaller than 5 mm have been found in surgical specimens in 70–94% of the cases.
The accuracy of MRI varies from 75% to 96%; its sensitivity and specificity for parametrial invasion range from 40% to 57% and from 77% to 80%, respectively. In studies comparing MRI with CT in the evaluation of parametrial invasion, MRI was superior; it was also demonstrated that the accuracy of MRI is not improved by the use of a 3.0-T magnet. The ADC of MRI for CC is inferior to that for normal cervical stroma, providing higher resolution between normal cervical stromal tissue (1.33–2.0 × 10−3 mm2/s) and cervical tumoral tissue (0.757–1.11 × 10−3 mm2/s). Applying DWI sequences, which do not require intravenous contrast, adds approximately 2 min to the established protocol (Fig. 9.7) [3].
The addition of DWI examination improves interobserver agreement and is greatly helpful, particularly when T2 sequences are not conclusive or the image acquisition is deficient. Metastatic lymph nodes also show significantly lower ADCs than benign lymph nodes, as observed in abnormal nodes as small as 5 mm. MR spectroscopy with C-choline measurement does not provide any additional benefit. In the evaluation of nodal disease, the sensitivity and specificity ranges of MRI are 30–73% and 93–95%, respectively (similar to those of CT) [3].
9.3.1.4 PET-CT
The prognosis of invasive CC is based on the stage, size, and histologic grade of the primary tumor, as well as on the nodal status; therefore, staging of the disease is essential in treatment determination. PET-CT shows higher sensitivity and specificity than conventional imaging methods for the evaluation of extrapelvic extension and tumor recurrence [12].
The principal indications for 18F-FGD PET-CT are initial staging (the determination of locoregional nodal involvement and extrapelvic extension), evaluation of the response to therapy, and detection of recurrence.
18FGD PET-CT is also useful for tumor margin determination and ureteral obstruction identification, even in the absence of significant dilation. Furthermore, 18FGD PET-CT is useful in the discrimination of residual or recurrent disease from post-radiation fibrosis when tomography findings are inconclusive [13, 14].
In recent studies, PET-CT showed the highest sensitivity (79–84%) and specificity (95–99%) for nodal staging, compared to 47–50%, and 92–97%, respectively, for tomography, and 56–72% and 90–97%, respectively, for MRI.
Due to its high sensitivity and positive predictive value, PET-CT should be the imaging technique of choice for the evaluation of extrapelvic disease prior to surgical treatment (Fig. 9.8).
Woman 52 years old. (a) CT with contrast, and coronal reconstruction. They identify themselves lymphadenopathies: supraclaviculares, mediastinales and retroperitoneal. (b) The hypermetabolism by MIP, shows hypermetabolism in the morphologic study, and additionally is evident the pulmonary alteration. (c) Study with fusion of PET/CT with 18FDG
PET-CT has been used in some cases to assist in radiotherapy planning because the standard external radiotherapy dose is presumably insufficient to provide locoregional control of advanced disease. Because 18-fluoromisonidazole (F-MISO) labels hypoxic areas, this agent is useful in the planning of intensity-modulated radiotherapy [15, 16].
9.3.2 Imaging Characteristics of Cervix Cancer that Are Useful for Staging
9.3.2.1 Radiological Approach and FIGO Staging
In June 2009, the FIGO staging committee presented a review on CC staging, updating the previous 1998 version. Although the revised FIGO staging system does not include imaging studies in the evaluation of CC, for the first time, the committee recommended imaging techniques, when available, for the assessment of important prognostic factors, such as tumor size, parametrial and/or pelvic lateral wall invasion, adjacent organ invasion, and distant lymph node metastasis. Imaging studies are complementary to clinical evaluation. According to the FIGO, MRI is the optimal option for the assessment of CC of IB1 or higher stage.
On the other hand, young patients bearing small tumors and desiring to preserve fertility may choose a more conservative surgical procedure (trachelectomy). In such cases, MRI is mandatory to determine the tumor size (<2 cm), the cervical length (>2.5 cm), and the distance from the tumor to the internal cervical orifice (>1 cm) [17].
9.3.2.1.1 Stage I
The normal cervix has a round shape typically observed in axial oblique images. In enhanced T2 images, three distinct uterine neck images are observed: the mucosa is thin and hyperintense, the stoma is thick and hypointense, and the inner smooth muscle layer shows an intermediate signal. Generally, MRI is not indicated for the evaluation of stage IA CC, as such tumors do not show signal abnormalities.
Stage IB tumors have intermediate to high signal intensity relative to that of the cervical stroma in T2 enhanced images and have variable hyperintensity compared to the stroma on dynamic contrast-enhanced MRI (DCE-MRI). The tumor growth pattern may vary between exophytic, infiltrative, and endocervical [3, 12, 18].
Stage IB tumors are subclassified in IB1 and IB2 based on their size (<4 cm or ≥4 cm, respectively). The tumor size must be measured in two orthogonal directions and in three dimensions. MRI has 90% accuracy in the assessment of tumor size. Accuracy of MRI in tumor size is very important because the clinical examination is not reliable in this regard, especially fir endocervical lesions. The tumor size can also determine the type of treatment; fertility-preserving surgery is possible for tumors smaller than 2 cm, and chemo/radiotherapy may be chosen for tumors larger than 4 cm, even in the absence of parametrial invasion. MRI is helpful in the evaluation of cervical length and of the distance of the tumor from the internal orifice in patients who are candidates for fertility-preserving surgery (Fig. 9.9) [3, 18].
9.3.2.1.2 Stage II
Stage II is defined as the involvement of two thirds of the superior vagina, without parametrial invasion. Vaginal extension is detected as a focal loss of signal towards the vaginal wall in T2-weighted contrast-enhanced sequences. Similarly to stage IB, stage IIA is subclassified into IIA1 and IIA2 based on tumor size (<4 cm and ≥4 cm, respectively). Cervical exophytic tumors can occasionally imitate apparent vaginal extension; however, the hypointense vaginal ring remains intact in such cases.
Invasion to the parametrium may occur in stage IIB CC, and such invasion alters the hypointensity of the cervical stroma without extending to the pelvic lateral wall. The presence of an intact cervical stroma (with a thickness greater than 3 mm) has a very high negative predictive value of 96% for parametrial invasion. Conversely, the positive predictive value of this factor is low (82–86%). Other potential indicators of parametrial invasion include distortion of the uterine neck, lateral displacement of the invaded parametrium, loss of the interphase between the tumor and the parametrium, increased soft tissue signal after gadolinium administration and spread along the uterosacral ligaments (Fig. 9.10) [3, 18].
Mistakes in the interpretation of parametrial invasion can occur in the presence of a previous biopsy with hemorrhage or of peripheral stromal edema, with consequent loss of detectability of the actual tumor margins; other misdiagnosed cases are endometriosis and voluptuous intravaginal tumors. Interruption of the stromal ring in tumors that involve the vaginal portion of the uterine neck together with an intact fornix excludes parametrial invasion.
When the entire thickness of the cervical stroma is interrupted, even in the absence of parametrial invasion, microscopic invasion cannot be excluded. The sensitivity and specificity of IRM for the evaluation of parametrial invasion have been shown to vary from 44% to 100% and from 87% to 93%, respectively [3, 18].
9.3.2.1.3 Stage III
Invasion directly to the inferior third of the vagina without extension to the lateral wall of the pelvis is present in stage IIIA CC.
Stage IIIB CC extends through the lateral wall of the pelvis and is defined as the presence of tumor tissue within 3 mm of the lateral pelvic wall muscles. Hydronephrosis caused by obstruction of the ureter and the iliac vessel is also present in stage IIIB disease (Fig. 9.11).
Direct invasion of the bladder or the rectal mucosa is defined as stage IVA CC: interruption of the muscularis propa showing hypointensity and mucosal invasion by a polypoid mass result in high signal intensity along the anterior face of the posterior wall of the bladder. Vesicovaginal or rectovaginal fistula is observed in some cases. Invasion of the bladder is more common than rectal invasion because the area at the back wall of the bladder is exposed and because rectovaginal septum separates the posterior rectal fornix. Bullous edema can mimic bladder invasion but can be distinguished based on the presence of an intact bladder mucosa on DCE-MRI (Fig. 9.13). The sensitivity and specificity of MRI for predicting bladder and/or rectal invasion vary between 83% and 100% and between 88% and 100%, respectively. Stage IVB refers to distant metastases beyond the pelvic lymph nodes to para-aortic and inguinal lymph node, lung, liver and bone tissue (Fig. 9.12) [3, 18, 19].
Another important aspect to evaluate is nodal status. The sensitivity and specificity of MRI for evaluating nodal disease are from 50% to 70% and from 90% to 96%, respectively. MRI using ultra-small paramagnetic iron oxide particles (lymphography) increases the sensitivity and specificity for detecting such metastatic nodes to 90–100% and to 95%, respectively [19].
Corine et al. stated that the DWI sequence is an appropriate tool in MRI for assessing not only primary lesions but also suspicious lymph nodes after chemotherapy. DWI alone has comparable value to PET. Therefore, Corine et al. recommended the use of DWI for evaluation of the pelvis and abdomen.
The criteria used to diagnose suspicion of lymph node metastasis are a maximum tumor size of the short axis greater than 1 cm (pelvis and abdomen), round shape, irregular margins, signal similar to the primary lesion, and the presence of necrosis [20, 21]. Pannu et al. reported that the maximum dimensions of normal lymph nodes at specific sites are 7 mm in the internal iliac chain, 9 mm in the common iliac chain and 10 mm in the external iliac chain (Fig. 9.6) [22].
Nodal spread is characteristic of CC and commonly occurs in a defined order. The paracervical lymph nodes are the first affected, followed by the parametrial, paraureteral, obturator, hypogastric, external iliac, and common iliac lymph nodes. When the common iliac lymph nodes are affected, further extension to the inguinal, presacral and para-aortic lymph nodes is common. Finally, CC metastasizes to the mediastinal and supraclavicular nodes. When suspicious extrapelvic retroperitoneal lymph nodes are identified, performance of guided biopsy is recommended for confirmation of the histological status and the treatment plan [20, 21].
9.4 Recurrence Evaluation
In 2003, 24,094 new cases of CC were diagnosed in Mexico, of which 38.2% were invasive lesions and 61.7% were in situ carcinomas. In the worldwide literature, 50% of CC patients are diagnosed with stage I disease; the 5-year survival rate for this group is higher than 90%. However, 35% of patients have persistent or recurrent disease after ending their therapy, and 75% of recurrences occur within the 2nd or 3rd year [1, 4, 17].
According to the FIGO, the risk of recurrence depends on the histologic type (e.g., clear cell carcinoma has a worse prognosis than other subtypes), tumor volume, depth of stromal invasion, lympho-vascular invasion, parametrial extension, lymph node involvement at the time of surgery, margin status, and histology.
The vagina and the central pelvis are the most frequent sites of recurrence. Recurrences are asymptomatic in 46–95% of patients. Symptoms include abdominal and pelvic pain, symptoms affecting the legs (pain or lymphedema), vaginal discharge or bleeding, urinary symptoms, cough and weight loss.
The clinical evaluation for CC recurrence includes a complete bimanual and rectovaginal examination of areas that are susceptible to HPV using a mirror (recurrent asymptomatic disease detection rate of 29–75%). Physical examination can detect many cases of recurrent disease in high-risk patients (based on positive pelvic lymph nodes, surgical margins and/or positive parametrium). Exfoliative cytology and physical examination have limitations that, in combination with factors such as obesity and radiotherapy-induced changes, contribute to its low accuracy. Therefore, imaging and laboratory studies are indicated in cases of strong clinical suspicion of recurrence [17].
The imaging modality selected for the evaluation of recurrence should include the entire body to exclude the presence of distant disease. Chest radiography has a detection rate of 20–47%, and its use is recommended in patients who have received radiotherapy.
In pelvic ultrasound studies, the appearance of vaginal recurrence has been described as vascularized solid nodules on color Doppler imaging. The spectra of recurrent tumors have low resistance, high systolic peaks compared with benign lesions. The infiltrated nodes are round and display loss of hilum and a hypoechoic structure. Changes in the appearance of infiltrated nodes may occur due to necrosis and calcification. Partial infiltration of a node produces a heterogeneous echo structure. When nodes show extracapsular invasion, the margins are poorly defined and irregular, and infiltrative growth is observed.
The nodal disease detection rates using ultrasound and CT are low; their main limitation is differentiation between fibrotic and tumor tissue. The use of these imaging methods is based upon the patient symptoms and the physical examination findings and should be individualized.
To evaluate local disease, MRI serves as an optimal method for analyzing disease recurrence. In a site that has been altered by surgery and radiation therapy, MRI is used to differentiate tumor recurrence from fibrosis (Fig. 9.13).
18F-FDG PET-CT has a sensitivity, specificity, and accuracy of 92%, 92.6% and 92.3%, respectively, for the detection of local recurrence as well as lung metastasis, peritoneal spread, paraaortic lymph node spread, and pelvic lymph node metastasis. A complete metabolic response is associated with 3-year disease-free survival. Therefore, women with lesions smaller than 2 cm or with negative lymph nodes do not require follow-up imaging unless they are symptomatic; patients with tumors greater than 2 cm in size with positive lymph nodes are indicated for follow-up via PET-CT13-15.
Approximately 30% of women treated for invasive cervical carcinoma have residual or recurrent disease. Pelvic recurrence is often central, extending from the uterus or cervix after radiotherapy or from the uterine or vaginal bed after surgery. Recurrence can be asymptomatic and can be detected on clinical examination or imaging. Tumor extension may involve the pelvic wall or rectum and can become further complicated by the appearance of a fistula. Recurrence in the pelvic wall can occur via direct extension or metastatic nodal disease, which can manifest as hydronephrosis, edema or pain in the extremities. Patients who received pelvic radiotherapy can have recurrence in extrapelvic sites such as the liver and lungs, despite adequate local control.
CT and MRI play an important role in the detection of recurrence. The accuracy of these modalities in showing recurrence after surgery and/or radiotherapy is 85%. The main limitation of CT and MRI is the differentiation of post-radiation fibrosis from disease recurrence after surgery; the presence of fistulae or rectovesical pouches also limits their usefulness.
MRI has been shown to be more useful than CT after radiotherapy. On MRI, tumor recurrence is detected as a high signal intensity mass within a low signal intensity cervix in the uterine bed or on the wall of the pelvis. Fibrosis usually has low signal intensity on T2 sequences, but high signal intensity areas can also represent inflammation. Thus, inflammation may produce false positives, especially during the first 6 months after radiation therapy. The use of contrast media has not improved the ability of MRI to detect tumor recurrence after radiotherapy, but dynamic imaging sequences have produced better results. Generally, MRI is also preferred for the evaluation of fistulae (Fig. 9.13) [3, 9, 10].
In patients with clinically suspected recurrence, PET-CT has been reported to have a sensitivity of 90.3–92.7% and a specificity of 81–100% for confirming the presence of disease recurrence. The performance of PET-CT on asymptomatic patients has not been shown to improve survival [12, 13].
9.5 The Future of Imaging Methods in the Evaluation of Cervical Cancer
Currently, imaging methods, including tomography, MRI and PET-CT, play a leading role in restaging, response to therapy and treatment failure prognosis in CC.
MRI is especially useful in delineating the extent of the primary tumor to support the planning of radiotherapy. Compared with hybrid PET-CT studies, MRI improves the search for lymphadenopathies with infiltration, enabling a requisite increase in the therapeutic dose [22].
Diffusion sequences have been shown to be useful in assessing the extent of these injuries, responses to therapy and prognosis. Currently, it is considered that the lesion size is ideally evaluated with T2-weighted contrast-enhanced MRI; however, this sequence may be suboptimal in cases of invasive adenocarcinoma, isointense lesions or early stage cancer in which fertility could be preserved. In these cases, considering that the lesions restrict diffusion, these sequences may be useful for disease characterization.
Diffusion MRI sequences can improve the initial staging, facilitating assessment of tumor size, and extrauterine spread of disease. In addition, they can be useful in histologic analysis of CC: the ADC has been suggested to be useful in determining the pathological grade of carcinoma because the ADC represents tumor cell density. The ADC has been reported to be significantly smaller in squamous cell carcinoma than in adenocarcinoma [23].
Considering the recent progress in oncologic PET-CT imaging, it is natural that the search for new applications that combine anatomical and functional imaging, such as PET/MRI fusion, which merges metabolic activity assessment with the spatial resolution of MRI, is promising. Moreover, the efficiency of MRI in local CC staging and the effectiveness of PET in the detection of suspicious lymph nodes and distant disease support the application of PET/MRI fusion. Currently, this hybrid method is limited by its scarce availability and its inherently high cost [24].
Only few studies have demonstrated the effectiveness of PET/MRI in the assessment of gynecological tumors. However, small studies have shown similar effectiveness between PET/MRI and PET-CT in detecting recurrence [24].
References
Odicino F, Pecorelli S, Zigliani L, et al. History of the FIGO cancer staging system. Int J Gynaecol Obstet. 2008;101(2):205–10.
Okamoto Y, Tanaka YO, Nishida M, et al. MR imaging of the uterine cervix: imaging-pathologic correlation. RadioGraphics. 2003;23(2):425–45.
Rauch GM, Kaur H, Choi H, Ernst RD, et al. Optimization of MR imaging for pretreatment evaluation of patients with endometrial and cervical cancer. RadioGraphics. 2014;34:1082–98.
Freeman S, Kataoka M, Addley H, et al. The revised FIGO staging system for uterine malignancies: implications for MR imaging. RadioGraphics. 2012;32:1805–27.
Fischerova D, Cibula D, Stenhova H, et al. Transrectal ultrasound and magnetic resonance imaging in staging of early cervical cáncer. Int J Gynecol Cancer. 2008;18:766–72.
Testa AC, Ludovisi M, Manfredi R, et al. Transvaginal ultrasonography and magnetic resonance imaging for assesment of presence, size and extent of invasive cervical cáncer. Ultrasound Obstet Gynecol. 2009;34:335–44.
Alcázar JL, Arribas S, Mínguez JA, Jurado M, et al. The role of ultrasound in the assesment of uterine cervical cáncer. J Obstet Gynaecol India. 2014;64(5):311–6.
Fischerova D. Ultrasound scanning of the pelvis and abdomen for staging of gynecologic tumors: a review. Ultrasound Obstet Gynecol. 2011;38(3):246–66.
Testa A, Di Legge A, et al. Imaging techniches for the evaluation of cervical cáncer. Best Pract Res Clin Obstet Gynaecol. 2014;28:741–68.
Pannu HK, Corl FM, Fishman EK. CT evaluation of cervical cáncer: spectrum of disease. Radiographics. 2001;21(5):1155–68.
Yitta S, Hecht EM, Mausner EV, Bennett GL. Normal or abnormal? Demystifying uterine and cervical contrast enhancement at multidetector CT. RadioGraphics. 2011;31(3):647–61.
Hildebrandt G, Kodhal R, et al. (18F) fluordeoxyglucose PET/computed tomography in breast cancer and gynecologic cancers, a literature review. PET Clin. 2015;10:89–104.
Grant P, Sakellis C. Gynecologic imaging with PET/CT. Semin Nucl Med. 2014;44(6):461–78.
Son H, Kositwattanarerk A, Hayes MP, et al. PET/CT evaluation of cervical cancer: spectrum of disease. RadioGraphics. 2010;30(5):1251–68.
Tachibana I, Nishimura Y, Shibata T, et al. A prospective clinical trial of tumor hypoxia imaging with 18F-fluoromisonidazole positron emission tomography and computed tomography (F-MISO PET/CT) before and during radiation therapy. J Radiat Res. 2013;54(6):1078–84.
Toma-Dasu I, Uhrdin J, Antonovic L, et al. Dose prescription and treatment planning based on FMISO-PET hypoxia. Acta Oncol. 2012;51(2):222–30.
Sree H, Alampady S, Srinivasa P. Current concepts in the diagnosis and management of endometrial and cervical carcinomas. Radiol Clin N Am. 2013;51:1087–110.
Shaaban. Blodgett. Diagnóstico por Imagen Oncología. Marbán. 2012;Sección 6:726–51.
Balleyguier C et al. Staging of uterine cervical cáncer with MRI: guidelines of the European Society of Urogenital Radiology. Eur Radiol. 2011;21:1102–10.
Harpreet K, Frank M, Elliot K. CT evaluation of cervical cancer: spectrum of disease. RadioGraphics. 2001;21:1155–68.
Welliver M-X, Yuh William TC, et al. Imaging across life span: innovations in imaging and therapy for gynecologic cáncer. Radiographics. 2014;34(4):1062–81.
Sunita D, Meenkski T, et al. Diffusion weighted imaging in gynecologic tumors: diagnostic imaging and potential pitfalls. Radiographics. 2014;34(5):1393–416.
Sasan P, Andres K, et al. clinical oncologic aplications of PET/MRI: a new horizon. Am J Med Mol Imaging. 2014;4(2):202–12.
Bashir U, Mallia A, et al. PET/MRI in oncologic imaging: state of the art. Diagnostics. 2015; doi:10.3390/diagnostics5030333.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Villaseñor-Navarro, Y. et al. (2017). Imaging in Cervical Cancer. In: de la Garza-Salazar, J., Morales-Vásquez, F., Meneses-Garcia, A. (eds) Cervical Cancer. Springer, Cham. https://doi.org/10.1007/978-3-319-45231-9_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-45231-9_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-45230-2
Online ISBN: 978-3-319-45231-9
eBook Packages: MedicineMedicine (R0)