Abstract
Purpose
Our purpose was to assess the diagnostic performance of positron emission tomography/computed tomography (PET/CT) and pelvic/abdominal magnetic resonance imaging (MRI) after concurrent chemoradiotherapy (CCRT) for posttherapy evaluation in patients with advanced cervical cancer.
Methods
Patients with cervical squamous cell carcinoma, either with advanced FIGO stage or with positive pelvic or para-aortic lymph node (PALN), received PET/CT using [18F]fluorodeoxyglucose and MRI including diffusion-weighted imaging between 2 and 3 months after CCRT completion. PET/CT were interpreted independently by two nuclear medicine physicians and MRI by two radiologists using the same scoring system. Active residual tumor was proven by pathological confirmation or disease progression on imaging studies within one year after CCRT and the disease regions were classified as local, regional, PALN, or distant. Patient-based and region-based comparison was performed using the receiver operating characteristic curve analysis.
Results
The study included 55 patients and 15 (27%) patients had active residual tumor. The diagnostic performance of PET/CT is significantly superior to that of MRI in patient-based analysis (P = 0.025) and in the detection of local (P = 0.045) and regional (P = 0.014) disease. The patient-based sensitivity, specificity, and accuracy of PET/CT are 60%, 100%, and 89% while those of MRI are 27%, 100%, and 80%.
Conclusions
PET/CT is superior to MRI for posttherapy evaluation in patients with advanced cervical cancer 2–3 months after definitive CCRT, mainly for the detection of residual local and regional disease. Patients with negative or equivocal results should be followed up regularly due to suboptimal sensitivities of imaging.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cervical cancer is the fourth most common cause of cancer death among women worldwide [1]. Advanced cervical cancer is not curable by surgery alone, and concurrent chemoradiotherapy (CCRT) has replaced radiotherapy alone as the treatment of choice based on the encouraging results of five randomized trials published in 1999 [2,3,4,5,6]. A meta-analysis reported a 6% improvement in 5-year OS with CCRT versus radiotherapy alone [7]. A decreasing beneficial effect with increasing FIGO stage has been noticed, with 10% survival improvement for stage Ia to IIa, 7% for stage IIb, and 3% for stage III to IVa. Approximately one third of patients receiving definitive therapy will experience persistent or recurrent disease and the majority of these can be detected within the first two years [8, 9]. It is thus desirable to assess the posttherapy disease status early after definitive treatment, in order to provide additional therapy for patients with persistent disease and to offer a surrogate marker for treatment efficacy assessment.
At the primary staging of cervical cancer, magnetic resonance imaging (MRI) has been considered to be the best imaging modality for local staging due to its soft tissue resolution [10]. Positron emission tomography/computed tomography (PET/CT) using fluorine-18-labeled fluorodeoxyglucose ([18F]FDG) has been shown to be better than MRI in detecting metastatic lymph nodes [11]. A prospective study by Schwarz et al. indicated the metabolic response assessed by PET/CT 2–4 months after completion of CCRT is predictive of survival outcome in 92 patients with cervical cancer [12]. Recent studies also suggested that MRI with diffusion-weighted imaging (DWI) may be useful in evaluating the therapeutic response to CCRT [13,14,15]. However, the diagnostic performance of PET/CT and MRI for detecting posttherapy disease early after definitive CCRT have not been addressed before. In this prospective observational study we evaluated PET/CT with [18F]FDG and pelvic/abdominal MRI with DWI scheduled 2–3 months after completion of CCRT in patients with advanced cervical cancer, with the hypothesis that MRI is superior in detecting local lesions and PET/CT is superior in detecting regional and distant metastases.
Materials and methods
Patients
A prospective, randomized Asian Gynecologic Oncology Group trial (AGOG 09–001, ClinicalTrials.gov identifier NCT00842660) with a parallel PET/CT imaging study had been conducted [8, 16]. The detailed inclusion and exclusion criteria were presented previously. Concisely, patients with cervical squamous cell carcinoma, either FIGO stage III-IVA or with positive pelvic or para-aortic lymph node (PALN) defined by pretreatment PET/CT, were enrolled. Eligibility also required a ECOG status of 0 or 1 and documentation of adequate bone marrow, liver, and renal function. Patients with prior pelvic radiation, prior systemic chemotherapy, or evidence of distant metastasis other than PALN were excluded. Fifty-five patients entering the parallel imaging study received posttherapy PET/CT and MRI 2–3 months after completion of CCRT. All participants gave their written informed consents.
Concurrent chemoradiotherapy
The patients were enrolled into a randomized trial comparing CCRT with single-agent cisplatin versus cisplatin plus gemcitabine. Chemotherapy consisted of weekly intravenous infusion of cisplatin (40 mg/m2) with or without gemcitabine (125 mg/m2) administered during the course of radiotherapy up to six cycles. The detailed protocol has been previously described [16].
Posttherapy PET/CT imaging and interpretation
Posttherapy PET/CT studies were arranged between 2 and 3 months after completion of CCRT. The patients were instructed to fast for 6 h before examination. Images were started at 50 min after the intravenous injection of [18F]FDG (370 ± 10% MBq). A non-enhanced CT scan from the head to the thigh followed by PET imaging was acquired on the PET/CT systems (Discovery ST16, GE Health Systems, Milwaukee, WI, USA, or Biograph mCT, Siemens Healthcare, Erlangen, Germany). PET scans were corrected for attenuation using the CT data with an ordered subset expectation maximization algorithm. PET/CT images were interpreted independently by two nuclear medicine physicians (Su TP and Liu FY, with 4 and 12 years of experience for PET/CT reporting in gynecologic oncology) who were aware of the study protocol but did not access the patients’ previous imaging studies, using a 4-point scoring system: 1 (normal or benign), 2 (equivocal), 3 (suspicious for malignancy) and 4 (highly confident for malignancy). The lesion localization was categorized as local, regional, PALN, or distant. A lesion or region is classified as negative, equivocal, or positive according the summed score of the two interpreters (2–3 as negative, 4–5 as equivocal, 6–8 as positive). For the calculation of sensitivity, specificity and accuracy, equivocal lesions were classified as negative. For the patient-based analysis, the lesion with the highest summed score was considered to be representative.
Posttherapy MRI and interpretation
Posttherapy MRI studies were also arranged between 2 and 3 months after completion of CCRT and the general imaging protocol had been described previously [17]. Pelvic and abdominal imaging was performed using a 1.5-Tesla scanner (SIGNA LX, GE Health Systems, Milwaukee, WI, USA) or a 3-Tesla scanner (Tim Trio, Siemens Healthcare, Erlangen, Germany) and included different sequences and axes of view, also with DWI and gadolinium enhancement. DWI was obtained using a single-shot spin-echo echo-planar technique with chemical-shift selective fat-suppression. The b-value was chosen to be 0 and 1000 s/mm2 to optimize the signal to noise ratio. MRI studies were interpreted by two radiologists (Huang YT and Lin G, with 7 and 12 years of experience for MRI reporting in gynecologic oncology) independently using the same scoring system described for the PET/CT studies.
Patient follow-up and determination of posttherapy disease status
The first patient follow-up had been scheduled at two months after CCRT completion, with posttherapy imaging studies performed within the following one month. Further posttherapy surveillance protocol including imaging studies was performed every 3–6 months for the first 2 years or whenever tumor recurrence was suspected. Posttherapy disease is defined to be positive if pathological confirmation or disease progression on the imaging studies have been documented within one year after CCRT completion. The sites of persistent or recurrent disease are classified into local, regional, PALN, and distant regions based on the pathological and imaging findings.
Statistical analysis
Statistical analysis was performed with the software MedCalc (version 16.8, MedCalc Software, Belgium), with a P value <0.05 considered statistically significant. Pearson’s chi-squared test was performed to compare the baseline characteristics between patients with and without posttherapy disease. Receiver operating characteristic (ROC) curve analysis and comparison were performed to evaluate the diagnostic performance of PET/CT and MRI in assessing posttherapy disease status, both for patient-based and region-based analyses. Weighted kappa values with linear weights were obtained to evaluate the inter-interpreter agreement of PET/CT and MRI, respectively. Weighted kappa values less or equal to 0.20, 0.21 to 0.40, 0.41 to 0.60, 0.61 to 0.80, and 0.81 to 1.00 indicated poor, fair, moderate, good, and excellent agreement.
Results
Baseline patient characteristics and posttherapy disease status
All 55 patients entering the parallel imaging study received posttherapy PET/CT and MRI during 2–3 months after completion of CCRT, with the interval between PET/CT and MRI being 1.3 ± 8.0 (mean ± standard deviation) days. The CONSORT diagram and patient characteristics of the study had been presented before [8]. The median age was 56 years at primary diagnosis. There were four patients with FIGO stage I, 28 patients with FIGO stage II, and 23 patients with FIGO stage III. Two patients with stage IVA enrolled in the AGOG 09–001 trial did not participate in the parallel imaging study.
A total of 15 (27%) patients were determined to have active residual tumor, with ten patients having pathological proof and five patients confirmed by follow-up imaging studies. The failure sites are presented in Table 1. Local, regional, PALN, and distant failures were present in five, seven, five, seven patients, respectively. Eight patients had failure in one region (two local, two regional, one PALN, and three distant lymph nodes). Two patients had combined local and regional failure. Combined regional and PALN failure, combined PALN failure and lung metastasis, and combined local failure and lung metastasis occurred in one patient, respectively. The remaining two patients had combined regional and PALN failure along with other distant metastases.
Baseline characteristics of patients and their association with active residual tumor are presented in Table 2. Among the baseline characteristics, only the presence of PALN metastasis is significantly associated with active residual tumor (P < 0.001). Nine (69%) out of 13 patients with initial PALN metastasis had residual disease, while six (14%) out of 42 patients without PALN metastasis had residual disease.
Patient-based comparison of posttherapy PET/CT and MRI
The ROC curves of PET/CT and MRI for detecting posththerapy disease on the patient-based analysis are illustrated in Fig. 1, with area under the curve (AUC) values of PET/CT and MRI being 0.828 (95% CI, 0.702 to 0.916) and 0.618 (95% CI, 0.477 to 0.746), respectively. The diagnostic performance of PET/CT is significantly superior to that of MRI (P = 0.025). The sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of PET/CT are 60%, 100%, 100%, 87%, and 89%, respectively, while those of MRI are 27%, 100%, 100%, 78%, and 80%. For the evaluation of inter-interpreter agreement, PET/CT interpreters achieved good agreement (ƙ = 0.654) while MRI interpreters achieved fair agreement (ƙ = 0.277).
Region-based comparison of posttherapy PET/CT and MRI
Region-based analytical results including the AUC value, sensitivity, specificity, positive predictive value, negative predictive value, and accuracy for PET/CT and MRI are listed in Table 3. The PET/CT is superior to MRI in detecting local (P = 0.045) and regional (P = 0.014) disease, manifested by higher sensitivities of PET/CT. A case with active residual local disease detected by PET/CT but not by MRI is illustrated in Fig. 2. The sensitivities for detecting regional disease are lower than those for detecting local disease, both for PET/CT and MRI. For detecting PALN, PET/CT and MRI have equal sensitivities and specificities. For distant metastases other than PALN, the sensitivity of PET/CT is higher than that of MRI but the difference is not significant by the ROC analysis (P = 0.105) (Fig. 3).
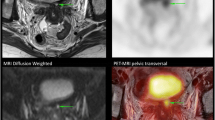
Posttherapy axial PET/CT and MRI images of a 56-year-old patient with FIGO stage II cervical cancer and right external iliac lymph node metastasis after definitive chemoradiotherapy. T2-weighted MRI (a) and fat-saturated contrast-enhanced T1-weighted MRI (b) showed a small-sized right external iliac node (arrows). Both radiologists considered this to be a benign reactive posttherapy lymph node (score 1) although the diffusion-weighted MRI (c) showed some diffusion restriction (arrow). On the PET/CT fusion image (d), obviously increased metabolic activity over the lymph node was noted (arrow) and both nuclear medicine physicians considered it to be with residual malignancy (score 3). Further right pelvic lymph node dissection proved the presence of residual nodal metastases
Posttherapy sagittal PET/CT and MRI images of a 58-year-old patient with FIGO stage III cervical cancer and nodal metastases. T2-weighted image (a) and contrast enhanced fat-saturated T1-weighted image (b) showed atrophy of uterine cervix without identifiable residual tumor. Diffusion weighted image (c) showed susceptibility artifacts in the cervical region due to the rectal gas without definite diffusion restriction. On the PET/CT fusion image (d), a hypermetabolic cervical lesion over the posterior lip of the uterine cervix (arrow) was found. Cervical biopsy proved the presence of residual local disease
For local assessment, PET/CT interpreters achieved good agreement (ƙ = 0.610) while MRI interpreters achieved moderate agreement (ƙ = 0.392). For regional assessment, PET/CT interpreters achieved good agreement (ƙ = 0.642) while MRI interpreters achieved only poor agreement (ƙ = 0.152). For PALN assessment, both PET/CT interpreters and MRI interpreters achieved moderate agreement (ƙ = 0.548 and 0.597, respectively). For assessing distant disease, both PET/CT interpreters and MRI interpreters achieved good agreement (ƙ = 0.700 and 0.796, respectively).
Discussion
PET/CT is found to have a higher sensitivity than MRI for posttherapy disease detection. In contrast to our hypothesis, PET/CT is superior to MRI in local disease detection. It seems that post-CCRT tissue reactions do interfere with the ability of MRI to detect residual local tumor. PET/CT is also superior in detecting residual regional nodes, as in the situation of primary staging. However, PET/CT is not superior to MRI for PALN detection. As for distant metastases, the AUC value of PET/CT is higher than that of MRI but the difference does not reach statistical significance. As a whole-body scan, PET/CT detected three more patients with metastatic lesions outside the scanning field of MRI. In another patient with supraclavicular lymph node and right lower lung metastases, PET/CT detected both lesions, while MRI detected the lower lung lesion, which was at the edge of its scanning field. This difference of performance may become significant if the sample size becomes larger. However, with the introduction of whole-body MRI, the advantage of PET/CT for detecting distant metastases may no longer persist.
Posttherapy PET/CT showed lower sensitivities for nodal and distant disease detection than for residual local tumor. This may be related to the lower sensitivities of PET/CT for detecting small volume tumors. A previous study by our group found the false-negative pelvic LN micrometastases on PET measured a median of 4 by 3 mm (range, 0.5 by 0.5 to 7 by 6 mm) [18]. Metastatic lymph nodes or distant foci with small volumes of viable tumor may not be detected. Ferrandina et al. assessed MRI and PET/CT in 96 cervical cancer patients receiving neoadjuvant CCRT or chemotherapy and showed that both imaging modalities performed 4–6 weeks from the end of neoadjuvant treatment and before surgery have low sensitivities and high specificities for residual lymph node detection [19]. However, they found MRI to have high sensitivity and low specificity for detecting residual local disease, in contrast to our results. This may be related to the shorter time between imaging and treatment in Ferrandina’s study. The volumetric and morphological regression of local tumors may not be complete at that time and this can result in a high false positive rate.
We had noticed some patients without active residual tumor to have mildly increased [18F]FDG activity at the cervix on the posttherapy PET/CT study. Since post-radiotherapy inflammation was thought be the cause, the interpreters usually gave a score of 2 (equivocal) for this local finding. The ability of PET/CT to differentiate between positive local disease and post-radiotherapy inflammation is thus based on the size and intensity of local abnormality and can be subjective and interpreter-dependent. Whether there are more objective and discriminative criteria for this differentiation deserves additional study.
Posttherapy disease is defined to be positive if pathological confirmation or disease progression on the imaging studies have been documented within one year after CCRT. Eight patients were confirmed to have residual or progressive disease during the first six months and seven during 7–12 months after CCRT. It is difficult to confirm the posttherapy disease within 6 months as small residual or metastatic tumors need more time to grow before identifiable. No patient was found to have recurrent disease during 12–24 months after CCRT. We thus consider our definition for posttherapy disease to be reasonable. However, late recurrence did occur in some patients and this may be associated with dormant tumor cells [20]. Since the sensitivities of early posttherapy imaging for detecting small volume disease are limited, we do not expect it to be able to detect or predict late recurrence.
Although DWI and ADC mapping have the potential to aid the traditional MRI in detecting posttherapy disease in patients with cervical cancer, their ability to detect small volume tumors is still uncertain. From the current study, the sensitivity of MRI for detecting posttherapy disease is suboptimal, especially for the detection of residual regional lymph node. Whether the PET/MR imaging introduced in recent years can outperform PET/CT and become the one-stop imaging modality for surveillance after CCRT will be an interesting topic for further study.
Due to the high specificities of posttherapy PET/CT and MRI, patients with positive imaging findings have pathological confirmation and evaluated for further management. Patients with negative or equivocal findings should be still followed up due to the low sensitivities of imaging. Our previous study showed a few clinically useful prognosticators for treatment failure from pre-treatment and during-treatment assessment [8]. For patients with high risk of failure, a more intensive follow-up may be considered even if the posttherapy imaging result is negative or equivocal.
There were limitations in our study. First, the sample size of this observational study was dependent on the enrolled patient number of the original trial. Although a larger patient number can lead to stronger results, we think the statistical results to be satisfactory. Second, we found the scoring of imaging findings to be interpreter-dependent as implied by the weighted kappa values. In general, the interpretation of PET/CT achieved better agreement than that of MRI. The inconsistency between interpreters may be related to either reader experience or the interpretability of images. Third, the definition of positive lymph node metastasis for the eligible patients was based on imaging rather than histopathological confirmation. Patients with positive or suspicious lymph node metastasis on the pretreatment MRI received PET/CT for clinical confirmation instead of invasive biopsy.
Conclusions
The diagnostic performance of PET/CT is superior to that of MRI for posttherapy evaluation in patients with advanced cervical cancer 2–3 months after definitive CCRT, mainly for the detection of residual local and regional disease. Patients with positive findings should be evaluated for further management due to the high specificities of PET/CT and MRI, while patients with negative or equivocal results should be still followed up regularly due to suboptimal sensitivities.
References
Stewart B, Wild CP, (editors). World Cancer Report 2014. International Agency for Research on Cancer, WHO; 2014. Available from: http://www.thehealthwell.info/node/725845. Accessed: 31 May 2017.
Keys HM, Bundy BN, Stehman FB, Muderspach LI, Chafe WE, Suggs CL, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med. 1999;340:1154–61.
Morris M, Eifel PJ, Lu J, Grigspy GW, Levenback C, Stevens RE, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340:1137–43.
Peters WA III, Liu PY, Barrett R, Gordon W Jr., Stock R, Berek JF, et al. Cisplatin, 5-fluorouracil plus radiation therapy are superior to radiation therapy as adjunctive therapy in high-risk, early-stage carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: report of a phase III intergroup study. Presented at Soc Gynecol Oncol 30th Annual Meeting, Gynecol Oncol. 1999;72:443.
Rose PG, Bundy BN, Watkins EB, Thigpen JT, Deppe G, Maiman MA, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340:1144–53.
Whitney CW, Sause W, Bundy BN, Malfetano JH, Hannigan EV, Fowler WC, et al. A randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stages IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: a gynecologic oncology group and southwest oncology group study. J Clin Oncol. 1999;17:1339–48.
Chemoradiotherapy for Cervical Cancer Meta-Analysis Collaboration. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: a systematic review and metaanalysis of individual patient data from 18 randomized trials. J Clin Oncol. 2008;26:5802–12.
Liu FY, Lai CH, Yang LY, Wang CC, Lin G, Chang CJ, et al. Utility of 18F-FDG PET/CT in patients with advanced squamous cell carcinoma of the uterine cervix receiving concurrent chemoradiotherapy: a parallel study of a prospective randomized trial. Eur J Nucl Med Mol Imaging. 2016;43:1812–23.
Hong JH, Tsai CS, Lai CH, Chang TC, Wang CC, Chou HH, et al. Recurrent squamous cell carcinoma of cervix after definitive radiotherapy. Int J Radiat Oncol Biol Phys. 2004;60:249–57.
Herrera FG, Prior JO. The role of PET/CT in cervical cancer. Front Oncol. 2013;3:34.
Choi HJ, Ju W, Myung SK, Kim Y. Diagnostic performance of computer tomography, magnetic resonance imaging, and positron emission tomography or positron emission tomography/computer tomography for detection of metastatic lymph nodes in patients with cervical cancer: meta-analysis. Cancer Sci. 2010;101:1471–9.
Schwarz JK, Siegel BA, Dehdashti F, Grigsby PW. Association of posttherapy positron emission tomography with tumor response and survival in cervical carcinoma. JAMA. 2007;298:2289–95.
Levy A, Caramella C, Chargari C, Medjhoul A, Rey A, Zareski E, et al. Accuracy of diffusion-weighted echo-planar MR imaging and ADC mapping in the evaluation of residual cervical carcinoma after radiation therapy. Gynecol Oncol. 2011;123:110–5.
Kim HS, Kim CK, Park BK, Huh SJ, Kim B. Evaluation of therapeutic response to concurrent chemoradiotherapy in patients with cervical cancer using diffusion-weighted MR imaging. J Magn Reson Imaging. 2013;37:187–93.
Onal C, Erbay G, Guler OC. Treatment response evaluation using the mean apparent diffusion coefficient in cervical cancer patients treated with definitive chemoradiotherapy. J Magn Reson Imaging. 2016;44:1010–9.
Wang CC, Chou HH, Yang LY, Lin H, Liou WS, Tseng CW, et al. A randomized trial comparing concurrent chemoradiotherapy with single-agent cisplatin versus cisplatin plus gemcitabine in patients with advanced cervical cancer: an Asian gynecologic oncology group study. Gynecol Oncol. 2015;137:462–7.
Ho KC, Lin G, Wang JJ, Lai CH, Chang CJ, Yen TC. Correlation of apparent diffusion coefficients measured by 3T diffusion-weighted MRI and SUV from FDG PET/CT in primary cervical cancer. Eur J Nucl Med Mol Imaging. 2009;36:200–8.
Chou HH, Chang TC, Yen TC, Ng KK, Hsueh S, Ma SY, et al. Low value of [18F]-fluoro-2-deoxy-d-glucose positron emission tomography in primary staging of early-stage cervical cancer before radical hysterectomy. J Clin Oncol. 2006;24:123–8.
Ferrandina G, Petrillo M, Restaino G, Rufini V, Macchia G, Carbone A, et al. Can radicality of surgery be safely modulated on the basis of MRI and PET/CT imaging in locally advanced cervical cancer patients administered preoperative treatment? Cancer. 2012;118:392–403.
Páez D, Labonte MJ, Bohanes P, Zhang W, Benhanim L, Ning Y, et al. Cancer dormancy: a model of early dissemination and late cancer recurrence. Clin Cancer Res. 2012;18:645–53.
Acknowledgements
This study was financially supported by grants from the Chang Gung Memorial Hospital (CMRPG381141 and CMRPG381142), Taiwan.
Funding
This study was funded by Chang Gung Memorial Hospital (CMRPG381141 and CMRPG381142).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Su, TP., Lin, G., Huang, YT. et al. Comparison of positron emission tomography/computed tomography and magnetic resonance imaging for posttherapy evaluation in patients with advanced cervical cancer receiving definitive concurrent chemoradiotherapy. Eur J Nucl Med Mol Imaging 45, 727–734 (2018). https://doi.org/10.1007/s00259-017-3884-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-017-3884-0