Abstract
Transplant osteoporosis has become a prevalent problem, as the numbers of patients receiving organ transplants and posttransplant survival have increased. Initially, transplant-related bone disease was considered to be due to glucocorticoid therapy. However, even after calcineurin inhibitors were introduced and glucocorticoid-sparing regimens adopted, patients continued to sustain rapid bone loss and fractures. In this chapter, we review both the evolution of and our current understanding of the effects of transplantation-immunosuppressive therapy on bone and mineral metabolism, as well as the potential effects of vitamin D in the immune system. We also review the epidemiology, natural history, and pathogenesis of bone loss and fracture in organ transplant candidates or recipients and summarize the results of therapeutic trials and make recommendations for prevention of bone loss during the initial acute phase following organ transplantation and treatment of established osteoporosis in organ transplant recipients.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Transplant osteoporosis
- Bone and mineral metabolism
- Vitamin D
- Immunity
- Graft rejection
- Kidney transplant
- Heart transplant
- Liver transplant
- Lung transplant
- Bone marrow transplant
- Glucocorticoids
- Calcineurin inhibitors
- Tacrolimus
- Alendronate
- Teriparatide
11.1 Skeletal Effects of Immunosuppressive Drugs
11.1.1 The Bone-Remodeling System
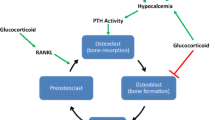
Bone remodeling is an orderly series of events in which old, damaged bone is replaced by new, mechanically stronger bone. Osteoclast first excavates small (0.5 mm3) resorption pits or Howship’s lacunae on cancellous and cortical bone surfaces, a process that takes 2–3 weeks (Ross 2006). After a brief rest period (the reversal phase), local mesenchymal marrow stem cells differentiate into osteoblasts and accumulate in the resorption pits (Aubin et al. 2006). Clusters of plump cuboidal osteoblasts first produce new bone matrix or osteoid and then mineralize it. Osteoclasts express receptors for NFkB ligand (RANKL), calcitonin, prostaglandins, calcium, and vitronectin (integrin α1β3). Osteoblasts express receptors for several hormones (parathyroid hormone, estrogens, vitamin D3), cell adhesion molecules (integrins), and cytokines. Bone remodeling is regulated by the tumor necrosis factor (TNF) ligand and receptor-signaling family: RANKL, RANK, and osteoprotegerin (OPG) (Gori et al. 2000; Hofbauer et al. 2000). RANKL is expressed by osteoblasts and by bone marrow stromal cells. In the presence of sufficient macrophage colony-stimulating factor (mCSF), RANKL binds to RANK receptors on surfaces of osteoclast lineage cells, resulting in rapid differentiation of osteoclast precursors to mature osteoclasts, increased osteoclast activity, and reduced apoptosis of mature osteoclasts. RANKL also binds to another osteoblast product, OPG. Competitive binding of RANKL to either RANK or OPG regulates bone remodeling by increasing (RANK) or decreasing (OPG) osteoclastogenesis. Immunosuppressants exert their effects on remodeling by interacting with the RANK/RANKL/OPG system (Hofbauer et al. 2001). When bone remodeling becomes “uncoupled,” such that the rate of resorption exceeds the rate of formation, bone loss occurs. Transplantation-related bone loss results from both an increase in the rate of bone resorption and a decrease in the rate of bone formation (Cohen et al. 2006a; Epstein 1996).
11.1.2 Glucocorticoids
Glucocorticoids (GCs), which are well recognized to cause osteoporosis, are included in the majority of posttransplant immunosuppression regimens. Prednisone or methylprednisolone is often prescribed in high doses (50–100 mg of prednisone or its equivalent daily) immediately after transplantation and during episodes of severe rejection, with gradual reduction over weeks to months. Total exposure varies with the organ transplanted, the number and management of rejection episodes, and the practice of individual transplantation programs.
The mechanisms by which GCs cause bone loss and fractures have been summarized in several reviews (Mazziotti et al. 2006; Sambrook 2006; van Staa 2006; Kulak et al. 2012; Amiche et al. 2016). Their predominant effect is an immediate and profound inhibition of bone formation: decreased osteoblast recruitment, differentiation and synthesis of type I collagen, induction of osteoblast and osteocyte apoptosis, and stimulation of osteocyte autophagy (Weinstein et al. 1998; Piemontese et al. 2015). Mechanistically, increased microRNA expression in osteoblasts exposed to GCs suppresses Wnt signaling, which is important for osteoblast expansion and function (Shi et al. 2015a; Westendorf et al. 2004). These effects are reflected biochemically by low serum levels of osteocalcin, a major non-collagenous bone matrix protein secreted by osteoblasts. GCs also inhibit growth hormone secretion and decrease production or bioactivity of certain skeletal growth factors (IGF-1, PGE2, and TGF-β), actions that also reduce bone formation.
GCs also increase bone resorption by decreasing osteoblast expression of OPG and increasing osteoblast expression of RANKL. The effects of GCs on osteoclast formation and function are dose dependent and mediated via increases in reactive oxygen species, which facilitate osteoclastogenesis (Shi et al. 2015b). GC stimulatory effects on resorption are not as profound as their inhibitory effects on formation and are generally limited to the first 6–12 months. GCs may be associated with secondary hyperparathyroidism due to inhibition of intestinal calcium absorption and stimulation of urinary calcium excretion. Multiple studies have shown direct relationships between serum PTH levels and rates of bone loss after organ transplantation (Gupta et al. 2012; Torregrosa et al. 1995; Savaj and Ghods 2012; Obi et al. 2014; Mazzaferro and Pasquali 2016). GCs also cause hypogonadotrophic hypogonadism and reduced secretion of adrenal androgens and estrogens, which may also increase bone resorption. These contrasting effects of GCs upon bone formation (decreased) and resorption (increased) prevent osteoblasts from replacing the increased amount of bone resorbed at each remodeling site, and rapid bone loss ensues.
New data suggest that GCs decrease bone strength as well as bone mass (Mellibovsky et al. 2015). Reference point indentation of the anterior tibia allows tissue-level assessment of bone mechanical characteristics and detects subtle treatment-induced changes in bone material properties (Mellibovsky et al. 2015). In 52 patients tested within 4 weeks of GC initiation, strength declined in those on GCs for 7 and 20 weeks (Mellibovsky et al. 2015). In contrast, bone densitometry (BMD) by DXA did not decline, suggesting that subtle changes in bone material strength occur soon after GC initiation, before bone loss is detected by DXA (Mellibovsky et al. 2015).
GCs cause bone loss in individuals of all races, ages, and of both genders. However, postmenopausal Caucasian women, in whom GC effects are superimposed upon bone loss due to aging and estrogen deficiency, are at greatest risk for fracture (Sambrook 2006; van Staa 2006). In general, bone loss is most rapid during the first 12 months and is directly related to dose and duration of therapy. Areas of the skeleton rich in cancellous bone (ribs, vertebrae, and distal ends of long bones) and the cortical rim of the vertebral body are most severely affected and are the most common sites for fracture. This bone loss can be tempered with the use of anti-osteoporosis medications (Overman et al. 2015). One study found a 48 % decrease in fractures by 1 year and a 32 % decrease by 3 years if patients on GCs are treated with agents such as bisphosphonates, denosumab, or teriparatide within 90 days of GC initiation (Overman et al. 2015).
11.1.3 Calcineurin Inhibitors
Cyclosporine A (CsA) was introduced to posttransplant immunosuppression regimens in the early 1980s. CsA is a small fungal cyclic peptide that forms a heterodimer with its cytoplasmic receptor, cyclophilin, and inhibits phosphatase activity of calcineurin, which also regulates osteoblasts and osteoclasts (Kahan 1989; Sun et al. 2005; Sun et al. 2007). The calcineurin gene has been identified in osteoclasts and extracted whole rat bone, but does not appear to be affected by CsA administration (Awumey et al. 1999). When administered to rodents, albeit in doses higher than those currently used to prevent allograft rejection, CsA was associated with a marked increase in osteoclast-mediated bone resorption and rapid and severe cancellous bone loss (Epstein 1996; Tamler and Epstein 2006; Movsowitz et al. 1988, 1989), likely mediated by T lymphocytes (Buchinsky et al. 1996; Rucinski et al. 1994; Zahner et al. 1997). CsA also increases gene expression of the bone-resorbing cytokines, IL-1 and IL-6(Marshall et al. 1995). In contrast to GCs, bone formation is increased in CsA-treated animals, although insufficiently to compensate for the increased resorption. Rodent studies suggest that hepatic clearance of CsA was six times lower in male than female rats, leading to increased rates of osteopenia (Jager et al. 2012), although this has not been shown in humans. CsA-induced bone loss may be promoted by PTH (Epstein et al. 2001). Drugs that inhibit bone resorption (estrogen, raloxifene, calcitonin, alendronate) prevent or attenuate CsA-induced bone loss in the rat (Bowman et al. 1995; Joffe et al. 1992; Sass et al. 1997; Stein et al. 1991). Similarly, 1,25(OH)2D and prostaglandin E2 prevent bone loss in CsA-treated rats (Epstein et al. 1990; Katz et al. 1992). Regimens that combine CsA with GCs found an additive rather than synergistic effect on bone loss (Shimizu et al. 2013).
Human studies have yielded conflicting results. Although kidney transplant patients receiving CsA in a GC-free regimen did not lose bone (Ponticelli and Aroldi 2001; Grotz et al. 1994; McIntyre et al. 1995; Cueto-Manzano et al. 2003), a prospective study found that cumulative CsA dose was associated with bone loss in the 2 years following transplant, independent of GCs (Josephson et al. 2004). In a recent study of patients on GC-sparing regimens, 27 % sustained a fracture within 6 months after transplant, similar to rates in patients on conventional GC-containing regimens (Edwards et al. 2011).
Tacrolimus (FK506), a macrolide that binds to an immunophilin FK-binding protein, blocks T-cell activation in a similar manner to CsA. FK506 causes bone loss in rodents similar in degree and mechanism to that observed with CsA (Cvetkovic et al. 1994). Rapid bone loss has been documented in patients after both cardiac (Stempfle et al. 1998) and liver (Park et al. 1996) transplantation. Rates of bone loss are directly associated with serum levels of FK506 (Luo et al. 2012). High serum FK506 levels were associated with high serum tartrate-resistant acid phosphatase (TRAP-5b) levels in male kidney transplant recipients, suggesting accelerated bone resorption as the underlying mechanism for FK506-mediated bone loss (Luo et al. 2012). FK506 may cause less bone loss than CsA (Goffin et al. 2002; Monegal et al. 2001a), likely because lower doses of GCs are required for immunosuppression. Whether FK506 confers any benefit over CsA with regard to fracture incidence is not known.
11.1.4 Other Immunosuppressive Agents
Sirolimus (rapamycin), a macrocyclic lactone, is structurally similar to FK506 and binds to the same binding protein, but induces immunosuppression via distinct mechanisms. In rat studies, low-dose CsA combined with rapamycin was bone sparing (Goodman et al. 2001). In an open-label study, markers of bone turnover (N-telopeptide and osteocalcin) were lower in kidney transplant recipients who received sirolimus than CsA; as BMD was not measured, no data on bone loss are available (Campistol et al. 2005). Mycophenolate mofetil does not have deleterious effects on bone in the rat (Dissanayake et al. 1998). The skeletal effects of other immunosuppressant agents such as mizoribine, deoxyspergualin, brequinar sodium, leflunomide, and azaspirane are unclear.
11.2 Vitamin D, Immunity, and Graft Rejection
Effects of vitamin D on immune function may be relevant after organ transplantation (Mazzaferro and Pasquali 2016; Lemire 1995; Stein and Shane 2011). Vitamin D potentiates the innate immune system and may protect against bacterial infections and tuberculosis (Bikle 2008). When 1,25(OH)2D is produced by monocytes and macrophages in sufficient quantities, it has intracellular antimicrobial effects. 1,25(OH)2D can also interact with and govern the cytokine profiles of activated T and B lymphocytes (Adams and Hewison 2010a). The ability of monocytes and macrophages to synthesize 1,25(OH)2D is dependent on the availability of adequate serum concentrations of 25-OHD and increases in response to vitamin D supplementation (Adams and Hewison 2010a). When sufficient 25-OHD is not available, there are declines in both local production of 1,25(OH)2D and activity of 1,25(OH)2D against ingested microbes (Adams and Hewison 2010b). The antimicrobial actions of 1,25(OH)2D also occur in barrier epithelial cells of the skin (Peric et al. 2008; Schauber et al. 2008), gut (Liu et al. 2008), and lungs (Hansdottir et al. 2008). Animals treated with the 1,25(OH)2D analogue, calcitriol, were resistant to infection with Candida albicans and Herpes simplex virus-1 (Cantorna et al. 1998), two common opportunistic infections in transplant patients.
There is evidence supporting a role for vitamin D in the regulation of immune cell proliferation, differentiation, and responsiveness (Lemire 1995; Bikle 2011; Haroon and Fitzgerald 2012; Dimitrov et al. 2014; Adams et al. 2014; Wei and Christakos 2015). Animal studies suggest that 1,25(OH)2D prevents acute allograft rejection following liver (Zhang et al. 2003; Redaelli et al. 2001), kidney (Becker et al. 2002), and heart (Hullett et al. 1998) transplantation. While there are limited data from human studies, kidney transplant recipients supplemented with calcitriol had fewer episodes of acute cellular rejection (Tanaci et al. 2003), required lower GC doses (Uyar et al. 2006), and had decreased expression of co-stimulatory and HLA-DR molecules (Ahmadpoor et al. 2009). In one study, patients treated with calcitriol after heart transplantation required less CsA (Briffa et al. 2003). In contrast, our study of heart transplant recipients found no differences between those randomized to calcitriol and alendronate with respect to CsA or prednisone dose (Shane et al. 2004). In a retrospective study of cardiac transplant patients, those with lower preoperative serum 25-OHD levels had more moderate-to-severe rejection episodes in the first 2 months after transplant (Bitetto et al. 2010). In 293 kidney transplant recipients, low serum 25-OHD levels predicted both decline in GFR and requirement for GCs to treat allograft rejection (Obi et al. 2014). In an observational study of liver transplant patients, those supplemented with cholecalciferol had fewer rejection episodes (Bitetto et al. 2010). Several studies have found increased BMD at the lumbar spine and femoral neck in renal transplant patients receiving activated vitamin D (calcitriol and alfacalcidol) (Sarno et al. 2015; Courbebaisse et al. 2014). The optimal amount of cholecalciferol that is both beneficial and safe in transplant recipients remains to be determined. The vitamin D supplementation in renAL transplant recipients (VITALE) trial is a prospective, multicenter, randomized trial of the efficacy and safety of cholecalciferol 100,000 IU monthly to maintain serum levels of 25-hydroxyvitamin D between 30 and 80 ng/mL (Courbebaisse et al. 2014). The results of this study will be interesting especially in light of studies that have found higher rates of falls and fractures in people treated with high doses of cholecalciferol (Sanders et al. 2010; Bischoff-Ferrari et al. 2016).
11.3 Effect of Transplantation on Bone and Mineral Metabolism
11.3.1 Bone Loss Before Transplantation
Many patients awaiting organ transplantation have multiple established risk factors for osteoporosis (older age, hypogonadism, physical inactivity, excessive tobacco and alcohol use, and exposure to drugs known to cause bone loss, such as GCs, heparin, loop diuretics). Most have already sustained considerable bone loss (Shane et al. 1997a; Ball et al. 2002; Hay 2003).
11.3.2 Kidney and Kidney-Pancreas Transplantation
Chronic kidney disease-mineral and bone disorder (CKD-MBD) refers to complex abnormalities in bone and mineral metabolism that affect most patients with chronic kidney disease (CKD) and end-stage renal disease (ESRD). Factors that contribute to development of CKD-MBD include disturbances in calcium and phosphate metabolism, low serum 25-OHD levels and decreased calcitriol synthesis, increased synthesis and secretion of PTH, metabolic acidosis, and defective bone mineralization (Martin et al. 2006; Beique et al. 2013). Patients with CKD-MBD may manifest extraskeletal calcifications, abnormal immune and cardiovascular function, and variable abnormalities in bone histology termed renal osteodystrophy (ROD). Almost 70–90 % of CKD stage 2–4 patients and all with stage 5 CKD have some form of ROD: high bone turnover with or without osteitis fibrosa (due to hyperparathyroidism), low bone turnover or adynamic bone disease, osteomalacia, or “mixed” renal osteodystrophy, a combination of one or more of the aforementioned lesions. The specific histological type depends on many factors that regulate bone remodeling and mineralization, such as PTH, acidosis, hypogonadism, and type I diabetes, as well as various therapeutic interventions used to manage patients with CKD and ESRD. Measurement of BMD by Dual-energy X-ray absorptiometry (DXA) does not distinguish among the various types of ROD. Fracture risk is estimated to be 4.4–14 times greater in patients with ESRD than the general population (Coco and Rush 2000; Mittalhenkle et al. 2004). In one study, 34 % of hemodialysis (HD) patients had a history of previous fracture (Kohlmeier et al. 1998). In another, fracture incidence was 0.1 per dialysis year in patients with osteitis fibrosa and 0.2 per dialysis year in patients with adynamic bone disease (Piraino et al. 1988). In people with moderate-to-severe CKD not on HD, hip fractures are twice as common as in those with normal kidney function (Nickolas et al. 2006).
As patients with ESRD are highly likely to have compromised bone health before kidney transplantation, it is not surprising that low spine and/or hip BMD has been reported in many cross-sectional studies of long-term kidney transplant recipients (Gupta et al. 2012; Grotz et al. 1994; Shane 2003; Cohen and Shane 2003; Epstein and Shane 2001; Shane and Epstein 2001; Braga Junior et al. 2006; Dolgos et al. 2008; Marcen et al. 2007; Dounousi et al. 2015; Boot et al. 1995; Cueto-Manzano et al. 1999), although the prognostic significance of low BMD in this group is unclear. Depending on the study and site of measurement, prevalence of low BMD long-term kidney transplant recipients ranges between 11 and 41 %. Factors associated with low BMD include increasing time since transplantation, high PTH concentrations, advanced age, female gender, and diabetic nephropathy.
A large number of longitudinal studies have documented high rates of bone loss at the spine and hip after kidney transplantation, ranging from 3 to 10 % during the first 6–12 months (Torregrosa et al. 1995; Savaj and Ghods 2012; Almond et al. 1994; Gallego et al. 2006; Horber et al. 1994; Kwan et al. 1992; Mikuls et al. 2003; Techawathanawanna et al. 2005; Yun et al. 1996; Bayat et al. 2007). Several studies have reported that GC dose is directly associated with bone loss (Rizzari et al. 2012; Woodle et al. 2008). However, by early 2000s, most kidney transplant programs were using lower GC doses and an increasing number of programs discontinue GCs altogether a few days after surgery. This shift in management seems to be associated with both reduced rates of bone loss and recovery of bone loss to pretransplant levels by 2 years after kidney transplantation in some (Aroldi et al. 1997; van den Ham et al. 2003; Nishioka et al. 2014), although not all studies (Meulen et al. 2004). For example, in 326 patients who had received a kidney transplant between 1996 and 2011, BMD Z scores were only slightly below average for age and sex at the baseline exam (approximately 6 months after transplant) and were not different from normal at 2.7 years (Naylor et al. 2014). In 47 patients managed without GCs and followed for 1 year after kidney transplant, DXA did not detect any bone loss at the spine or hip (Iyer et al. 2014). On a cautionary note, however, DXA did detect significant bone loss at the ultradistal and 1/3 radius sites, which are seldom measured (Iyer et al. 2014). Thus, despite the lower doses of GCs and alternative antirejection drugs used today (Gaston 2006; Paterson et al. 2005; Pescovitz 2006; Regazzi et al. 2005), significant bone loss occurs, although it may be less rapid than documented in the 1980s and 1990s (Van Staa et al. 2000). Moreover, high-resolution peripheral quantitative computed tomography (HRpQCT), which separately measures cortical and trabecular volumetric BMD and microarchitecture at the distal radius and tibia, documented cortical and trabecular bone loss and significant decreases in whole bone strength (Iyer et al. 2014). Cortical bone loss was directly related to an increase in cortical porosity at the endocortical surface and to higher PTH levels (Nishiyama et al. 2015). In contrast, seven patients, in whom GCs were stopped shortly after kidney transplantation and who underwent transiliac bone biopsy within 2 months and again 2–5 years after surgery, showed significant deterioration of trabecular bone microstructure, no significant changes in cortical bone, and no relationship between cortical bone parameters and PTH (Carvalho et al. 2015).
Fractures after renal transplantation typically affect appendicular sites (feet, ankles, long bones, hips) more than axial sites (spine, ribs) (Roe et al. 2005). In one study, non-vertebral fractures were far more prevalent in renal transplant recipients than in the normal population (Ramsey-Goldman et al. 1999), although another study found that renal transplant recipients had a lower risk of non-vertebral fracture compared to members of the general population who had previously sustained a non-vertebral fracture (Naylor et al. 2015). More recent data from a large cohort of first-time kidney transplant recipients suggests that hip fracture incidence may be lower in recent years with a hazard ratio for new hip fracture in the year 2010 of 0.56 (0.41–0.77) when compared to 1997 (Sukumaran Nair et al. 2014); this is noteworthy as patients transplanted in 2010 were older and had more medical comorbidities than the 1997 cohort (Sukumaran Nair et al. 2014). In a 3-year retrospective study of 35 kidney-pancreas recipients, approximately half had sustained from one to three symptomatic, non-vertebral fractures (Chiu et al. 1998). Men with type 1 diabetes who undergo kidney-pancreas transplants were recently reported to have approximately 30 % lower fracture rates than those who undergo kidney transplant alone; this relationship was not seen among women (Nikkel et al. 2011, 2013). Older age, white race, prior dialysis, and pretransplantation fracture were associated with increased fracture risk (Nikkel et al. 2013). Fracture incidence may be lower in patients managed with early GC withdrawal (Nikkel et al. 2012). A recent systematic review of fracture incidence in kidney transplant recipients analyzed 10 studies totaling 262,678 recipients and found a highly variable incidence rate ranging from 3.3 to 99.6 fractures per 1000 person-years (Naylor et al. 2013). Similarly, the 5-year cumulative incidence for fracture varied ranging from 0.85 to 27 %. Common factors associated with increased fracture risk were older age, female sex, the presence of diabetes, and receipt of dialysis before transplantation (Naylor et al. 2013). Other less common but statistically significant risk factors were a previous history of fracture and receipt of a kidney from a deceased versus living donor (Naylor et al. 2013). The authors concluded that there is poor consensus on the incidence and risk factors for fractures in kidney transplant recipients.
After kidney transplantation, there are several fairly consistent changes in biochemical indices of mineral metabolism and bone turnover (Julian et al. 1991; Kulak and Shane 2006; Sprague et al. 2008; Molnar et al. 2014; Alshayeb et al. 2013). PTH levels are often very high before transplantation, and while levels typically decline markedly immediately after transplantation (Akaberi et al. 2006; Rathi et al. 2015), they may remain above normal throughout the first year (Iyer et al. 2014) and may never completely normalize. Mild hypercalcemia and hypophosphatemia are common during the first few months after transplant (Rathi et al. 2015), but usually resolve within a few months. In long-term transplant recipients, persistent elevations in PTH may be associated with reduced hip BMD (Akaberi et al. 2006). Low calcitriol levels may persist after transplantation (Uyar et al. 2006; Sprague et al. 2008; Fleseriu and Licata 2007; Tripathi et al. 2008; Querings et al. 2006). Low serum 25-OHD levels were recently reported in nearly half of renal transplant recipients; this may represent improvement from older studies that reported vitamin D deficiency in over 75 % of renal transplant candidates, likely because of greater awareness of vitamin D (Beique et al. 2013). Sclerostin, secreted by osteocytes, is an inhibitor of the Wnt-signaling pathway which leads to decreased bone formation (Bonani et al. 2014). Sclerostin levels tend to be high in patients before renal transplantation. Although sclerostin levels decline within 15 days after transplant, it is unclear whether this is due to increased sclerostin catabolism or decreased sclerostin secretion, as phosphorus levels normalize (Bonani et al. 2014; Pelletier et al. 2013).
11.3.3 Cardiac Transplantation
The prevalence of osteoporosis in patients awaiting cardiac transplantation ranges from 4 to 23 %(Shane et al. 1997a, 2004; Dolgos et al. 2010; Anijar et al. 1999; Cohen and Shane 2005; Lee et al. 1994; Iqbal et al. 2008). Observational studies, primarily conducted during the 1990s, documented rates of bone loss ranging from 4 to 11 %, with the most rapid losses during the first 3–12 months, with greater losses at the hip than the spine (Stempfle et al. 1998; Shane et al. 2004; Berguer et al. 1994; Cremer et al. 1999; Henderson et al. 1995; Sambrook et al. 1994a; Shane et al. 1997b; Thiebaud et al. 1996; Valimaki et al. 1999a; Van Cleemput et al. 1995). The few longitudinal data after the first year suggest that the rate of bone loss slows or stops in the majority of patients, with partial recovery of bone mass at the spine though not at the hip (Shane et al. 2004; Shane et al. 1997b). More recent studies suggest lower rates of spine and hip bone loss (2–3 %) than previously reported, likely related to lower GC exposure than patients transplanted in the 1990s (Shane et al. 2004, 2012). However, while some GC-sparing protocols are associated with less bone loss, these results are not consistent across studies suggesting factors other than GCs are responsible for posttransplant bone loss (Baraldo et al. 2014).
Observational studies conducted during the 1990s found that approximately one-third of cardiac transplant recipients sustained a fragility fracture during the first 1–3 years after grafting (Shane et al. 1996a; Leidig-Bruckner et al. 2001). Fractures were associated with female gender, lower pretransplant BMD, and higher rates of bone loss after transplantation (Shane et al. 1996a; Leidig-Bruckner et al. 2001). However, many patients who fractured had normal BMD prior to transplant (Shane et al. 1996a; Leidig-Bruckner et al. 2001). In contrast, more recent studies report fracture incidence to be considerably lower than in the past, in the range of 12–14 %(Shane et al. 2004; Kerschan-Schindl et al. 2008). One study compared fracture risk between GC-free protocols, low-dose GC protocols (prednisone ≤ 5 mg daily by the end of the first posttransplant year), and high-dose GC protocols (prednisone > 5 mg daily by the end of the first posttransplant year). At 2 years, there was no difference in fracture incidence among the three groups (1.1 %, 1.5 %, and 2.5 %, respectively) (Crespo Leiro et al. 2012). A recent retrospective study of 105 patients who received a heart transplant between 2005 and 2010 reported that 8.6 % sustained a fragility fracture, with a median time to first fracture of 12 months. A slightly larger proportion of women than men fractured (11.8 % versus 8.0 %). There was a high rate of bisphosphonate use in both fracture and non-fracture groups, 56 % versus 39 %, which may have reduced fracture rates (Hariman et al. 2014).
Early studies reported biochemical evidence of uncoupled bone remodeling (increased resorption and decreased formation) during the first 3–6 months after heart transplant, with restitution of coupling when glucocorticoid doses are lowered (Shane et al. 1997b; Valimaki et al. 1999a; Sambrook et al. 1994b). Later in the posttransplant period, many studies suggest that bone remodeling is increased (Lee et al. 1994; Thiebaud et al. 1996; Valimaki et al. 1999a; Sambrook et al. 1994b; Glendenning et al. 1999; Guo et al. 1998; Rich et al. 1992; Shane et al. 1993). Vitamin D deficiency is extremely common among heart and liver transplant recipients at the time of transplantation. In one study of patients assessed within the first 1–2 weeks after transplant, 55 % had deficiency with serum 25-OHD levels between 10 and 20 ng/ml, and 16 % had levels < 10 ng/ml (Stein et al. 2009).
11.3.4 Liver Transplantation
Osteoporosis is present in 20–43 % of patients with end-stage liver disease awaiting transplantation (Dolgos et al. 2010; Crosbie et al. 1999; Monegal et al. 1997; Bai et al. 2007; Guichelaar et al. 2006). A recent Spanish study that compared two groups of liver transplant candidates, one evaluated between 1992 and 1993 and the second between 2010 and 2011, found that the prevalence of osteoporosis (22 % versus 30 %, respectively) and vertebral fractures (36 % versus 33 %, respectively) was high and approximately the same in both (Monegal et al. 2013). Vitamin D deficiency is also very common, affecting >80 % of liver transplant candidates (Chaney et al. 2015; Venu et al. 2013). Chronic liver disease, of various etiologies, is associated with low levels of serum RANKL and high levels of OPG, possibly leading to an increased OPG/RANKL ratio, osteoclast inhibition, and a low bone-turnover state (Gatta et al. 2014). In fibrotic liver diseases, the synthesis of type I collagen is markedly increased and thus collagen-related bone-turnover markers do not reflect skeletal turnover in patients with liver disease (Guanabens et al. 1998). Serum osteocalcin and tartrate-resistant acid phosphatase (TRAP) may be more valid markers of bone remodeling activity.
In studies published before 2000 (Leidig-Bruckner et al. 2001; Eastell et al. 1991; Haagsma et al. 1988; Hawkins et al. 1994; Hussaini et al. 1999; Lopez et al. 1992; McDonald et al. 1991; Meys et al. 1994; Navasa et al. 1994; Porayko et al. 1991; Keogh et al. 1999), spine BMD fell by 2–24 %, primarily in the initial few months after liver transplantation, with recovery to levels equal to or even higher than before transplant by 1 year. More recent studies have reported lower rates of bone loss at the femoral neck (Keogh et al. 1999) and no significant bone loss at the spine (Ninkovic et al. 2002),(Floreani et al. 2001). In a recent cohort study of 201 liver transplant recipients from the Netherlands, the majority treated with prednisone for at least 6 months and either tacrolimus or cyclosporine, lumbar spine BMD declined by 2.5 % but returned to pretransplant levels by 2 years. In contrast, femoral neck BMD had declined by 6.5 % at 6 months and remained stable thereafter, with no recovery over 5 years of follow-up (Krol et al. 2014a). Lower bone mass and/or higher rates of bone loss after liver transplant have been associated with older age, female gender, cholestatic liver disease, and higher prednisone dose (Smallwood et al. 2005).
In older studies, fracture incidence was extremely high, ranging from 24 to 65 %. Some recent studies suggest that fracture rates are lower (Ninkovic et al. 2002; Kaemmerer et al. 2012). However, in the Netherland cohort study, radiographic vertebral fractures were present in 56 % at screening, 68 % at 6 months, and 71 % at 12 months (Krol et al. 2014a), suggesting that vertebral fractures remain a significant problem (Krol et al. 2014a). Factors that have been associated with increased risk of fracture include male gender (Krol et al. 2014a), primary sclerosing cholangitis (Guichelaar et al. 2006) and alcoholic cirrhosis (Millonig et al. 2005), older age and lower pretransplant BMD (Ninkovic et al. 2002; Monegal et al. 2001b), prevalent pretransplant vertebral fractures, and longer survival (Leidig-Bruckner et al. 2001; Ninkovic et al. 2000).
PTH increases significantly during the first 3–6 months after liver transplant (Floreani et al. 2001; Monegal et al. 2001b; Compston et al. 1996). Serum 25-OHD concentrations are low in recent liver transplant recipients, likely because of disease-related factors such as malabsorption, impaired hepatic 25-hydroxylation of vitamin D, and reduced production of vitamin D-binding protein (Stein et al. 2009). Bone turnover, measured by markers of bone formation (osteocalcin and carboxyterminal peptide of type I collagen) and resorption are higher in liver transplant recipients than in normal controls in most (Haagsma et al. 1988; Abdelhadi et al. 1995; Watson et al. 1990; Valero et al. 1995), though not all studies (Rabinowitz et al. 1992). OPG and RANKL levels are significantly elevated in the first 2 weeks following liver transplant (Fabrega et al. 2006). In 21 patients who underwent tetracycline-labeled bone biopsies before and 3 months after transplantation, low turnover transitioned to a high turnover state (Compston et al. 1996; Vedi et al. 1999).
11.3.5 Lung Transplantation
Osteoporosis is extremely common among patients with end-stage lung disease. In cross-sectional studies, low bone mass and osteoporosis have been documented in 31–86 % of candidates for lung transplantation (Aris et al. 1996, 1998, 2000; Donovan et al. 1998; Ott and Aitken 1998; Shane et al. 1996b; Tschopp et al. 2006; Caplan-Shaw et al. 2006; Jastrzebski et al. 2010). All etiologies appear to be vulnerable. Markedly increased rates of all fractures and severe kyphosis due to vertebral fractures have been reported in adults with cystic fibrosis (Aris et al. 1998; Donovan et al. 1998; Ott and Aitken 1998). Low BMD and osteoporosis are also highly prevalent in patients with emphysema (Aris et al. 1996; Shane et al. 1996b), diffuse parenchymal lung disease (Caplan-Shaw et al. 2006), and primary pulmonary hypertension (Tschopp et al. 2006). The etiology of osteoporosis in patients with end-stage lung disease is multifactorial. In addition to prior GC exposure (Park et al. 1996; Aris et al. 1996; Shane et al. 1996b; Jastrzebski et al. 2010; Wang et al. 2013), low body weight, chronic hypoxemia, tobacco exposure, and chronic infection/inflammation have been implicated. In some studies, BMD correlated with pulmonary vascular resistance, functional measures, and walking distance (Tschopp et al. 2006; Caplan-Shaw et al. 2006). In patients with cystic fibrosis, additional etiologic factors include calcium malabsorption, hypogonadism, small body size, and vitamin D deficiency (Donovan et al. 1998).
Not surprisingly, therefore, osteoporosis affects a very high proportion of lung transplant recipients. In fact, a population-based cohort study from Taiwan found that those at highest risk for osteoporosis and various types of fracture after solid organ transplant were lung transplant recipients (Yu et al. 2014). Prospective studies have shown that spine and hip BMD declines by 4–5 % during the first year (Ferrari et al. 1996; Spira et al. 2000). An HRpQCT study comparing 58 lung transplant recipients and 60 controls found lung transplant recipients had greater cortical porosity, lower trabecular number and thickness, bone stiffness, and failure load (Fischer et al. 2015). In studies from the 1990s, fracture incidence varied from 11 to 42 %(Spira et al. 2000; Aringer et al. 1998; Rutherford et al. 2005; Shane et al. 1999). Risk factors for fracture and bone loss after lung transplant include female gender; low pretransplant BMD; pretransplant GC therapy; GC dose in some (Spira et al. 2000), but not all studies (Shane et al. 1999); pretransplant fractures; and high bone turnover after transplantation (Wang et al. 2013). In contrast, a retrospective study of patients transplanted between 2005 and 2010 reported a somewhat lower fracture rate of 8.0 %, perhaps because approximately 50 % were receiving bisphosphonates (Hariman et al. 2014).
11.3.6 Bone Marrow Transplantation (BMT)
As in the case of solid organ transplantation, low BMD is common in patients who require BMT. Bone loss may be related both to the underlying disease and to pretransplant myeloablative therapy with alkylating agents and/or total body irradiation, which may precipitate profound and frequently permanent hypogonadism, particularly in women, and may also have toxic effects on bone marrow osteoprogenitor cells (Banfi et al. 2001). Pretransplant exposure to GCs may also contribute. In patients studied after myeloablative chemotherapy but before BMT, osteopenia was present in 24 % and osteoporosis in 4 %(Schulte et al. 2000).
Adverse skeletal effects are more common after allogeneic BMT, in which donor and recipient are not genetically identical, than after autologous BMT, which involves removal and reinfusion of the patient’s own stem cells after high-dose myeloablative therapy (Ebeling et al. 1999). After allogeneic BMT, there is a substantial risk of acute or chronic graft-versus-host disease (GVHD), in which donor immune cells react against the recipient. GVHD is typically treated with combinations of high-dose GCs, methotrexate, cyclosporine A, or tacrolimus. Several studies have documented low total body BMD and spine BMD measured by DXA and CT (Kelly et al. 1990; Bhatia et al. 1998; Yao et al. 2008; Nysom et al. 2000; Kauppila et al. 1999; Kerschan-Schindl et al. 2004). Those younger than 18 at transplantation may be more profoundly affected, perhaps because of failure to achieve optimal peak bone mass and smaller bone size (Bhatia et al. 1998, Frisk et al. 2012). Factors associated with low BMD 6 months after BMT include GVHD, GC use, low vitamin D levels, and family history of osteoporosis (Campos et al. 2014). In adult BMT recipients, hematopoietic stem cell transplantation before age 10 was associated with lower total body and femoral neck BMD than after age 18 (Petryk et al. 2014). Bone mass is low in hypogonadal women after BMT and hormone replacement therapy is associated with significant increases in BMD (Branco et al. 1996; Castaneda et al. 1997).
After BMT, spine bone loss ranges between 2 and 6 %, while FN BMD declines by 6–12 % (Serio et al. 2013; Ebeling 2005; Valimaki et al. 1999b; Kashyap et al. 2000). Duration of GC exposure may be more prognostic than cumulative dose (Serio et al. 2013). While there appears to be little bone loss after the first year (Kauppila et al. 1999), FN bone loss may not be regained. A recent study from the MD Anderson Cancer Center found that 8 % of patients fractured after BMT (Pundole et al. 2015). Risk factors included age over 50, multiple myeloma, solid organ tumors, and autologous BMT (Pundole et al. 2015). Vertebral fractures were slightly more common (53 %) than non-vertebral fractures (47 %) (Pundole et al. 2015), which mostly affected the ribs, upper limbs, and femur (Pundole et al. 2015).
The pathogenesis of post-BMT bone loss is complex. Cellular or cytokine-mediated bone marrow dysfunction may affect bone remodeling and loss after BMT (Lee et al. 2002a). High-dose chemotherapy, total body irradiation, and treatment with GCs and/or CsA may reduce osteoblast differentiation. Colony-forming unit fibroblasts (CFU-f) are reduced for up to 12 years following BMT (Banfi et al. 2001; Tauchmanova et al. 2002). During the first 3 months after BMT, bone formation markers decrease and resorption markers increase (Valimaki et al. 1999b), a pattern consistent with uncoupling of formation from resorption. After 3 months, bone formation markers rebound and elevated turnover persists during the latter half of the year (Kauppila et al. 1999; Valimaki et al. 1999b; Carlson et al. 1994; Withold et al. 1996; Lee et al. 2002b; Kang et al. 2000). Long-term survivors after BMT may have persistent abnormalities in bone turnover and vitamin D (Kananen et al. 2002).
11.4 Evaluation and Management of Osteoporosis in Patients Awaiting Transplantation
Candidates for all types of transplantation should be evaluated so that potentially treatable abnormalities of bone mineral metabolism may be addressed and bone health optimized preoperatively (Table 11.1). BMD of the spine and hip should be measured by DXA and spine x-rays or vertebral fracture analysis (VFA) performed to detect prevalent fractures, which are associated with an increased risk of incident fractures. If pretransplant BMD is low, a thorough evaluation should be performed to detect potentially treatable causes of low bone mass and guide therapy. On a cautionary note, however, one study found no association between BMD and vertebral fractures in OLT recipients suggesting that BMD may not provide a complete radiologic assessment of pretransplant bone health (Krol et al. 2014b). Patients found to have osteoporosis before transplantation should receive treatment, typically antiresorptive therapy with a bisphosphonate. Teriparatide can be considered for patients with very low BMD or fractures if they do not have hyperparathyroidism. The pretransplant waiting period is often long enough (1–2 years) for significant improvement in BMD before transplantation. Patients with renal osteodystrophy should be managed in accordance with accepted clinical guidelines (Kidney Disease: Improving Global Outcomes 2009).
11.5 Prevention and Treatment of Transplantation Osteoporosis
As bone loss is most rapid during the first 6–12 months after transplantation and fractures may also occur early, strategies to prevent bone loss should be instituted immediately after transplantation, particularly in patients with low BMD and those who will be on GC therapy (Table 11.2).
The most commonly tested therapies for transplantation osteoporosis include bisphosphonates and active vitamin D analogues. While teriparatide is approved for the treatment of GC-induced osteoporosis with increases in BMD and decreases in non-vertebral fractures (Saag et al. 2009), the only study of teriparatide after organ transplantation found that FN BMD did not decline in kidney transplant patients randomized to teriparatide but decreased significantly in those on placebo (Cejka et al. 2008).
11.5.1 Vitamin D Analogues
Active vitamin D analogues prevent GC-induced decreases in intestinal calcium absorption, reduce secondary hyperparathyroidism, promote differentiation of osteoblast precursors into mature cells, and may potentiate immunosuppressive effects of CsA (Briffa et al. 2003; Lemire 1992; Lemire et al. 1994). Alfacalcidol (1-α-OHD) prevented or attenuated bone loss at the LS and FN (El-Agroudy et al. 2003; De Sevaux et al. 2002; El-Agroudy et al. 2005). Calcitriol (0.5–0.75 ug/day) prevented bone loss at the spine and hip during the first 6 months after heart or lung transplantation (Henderson et al. 2001); those who received it for 24 months sustained significantly less FN bone loss, while those who stopped at 12 months had no evidence of benefit at the FN at 24 months (Sambrook et al. 2000). Calcitriol was as effective as daily alendronate in preventing bone loss after heart transplantation (Cohen et al. 2006b). In one study, daily calcitriol was associated with an increase in BMD at the LS, FN, and forearm during the first year after kidney transplantation (Josephson et al. 2004), while in another, intermittent calcitriol prevented bone loss at the hip but not the spine after kidney transplant (Torres et al. 2004). As hypercalcemia and hypercalciuria may develop rapidly and at any time during therapy with active vitamin D analogues, frequent monitoring of serum and urine calcium is necessary.
11.5.2 Bisphosphonates
Two meta-analyses of bisphosphonate trials in kidney transplant recipients found that bisphosphonates prevented bone loss at the spine and hip (Mitterbauer et al. 2006; Palmer et al. 2005). Similarly, a meta-analysis of randomized controlled trials in diverse transplant types showed that treatment with bisphosphonates improved spine and hip BMD by approximately 3 % on average (Cohen et al. 2011).
Intravenous bisphosphonates consistently prevent bone loss after transplantation (Valero et al. 1995; Aris et al. 2000; Fan et al. 2000, 2003; Garcia-Delgado et al. 1997; Shane et al. 1998; Bianda et al. 2000; Hommann et al. 2002; Grotz et al. 2001; Arlen et al. 2001; Trombetti et al. 2000; Krieg et al. 2001; Haas et al. 2003; Coco et al. 2003; Walsh et al. 2009; Jeon et al. 2013; Okamoto et al. 2014). Repeated doses of intravenous pamidronate prevented spine and hip bone loss in the kidney (Fan et al. 2000, 2003; Coco et al. 2003; Torregrosa et al. 2011), heart (Bianda et al. 2000; Krieg et al. 2001), liver (Pennisi et al. 2007; Monegal et al. 2009), lung (Aris et al. 2000; Trombetti et al. 2000), and bone marrow transplant recipients (Kananen et al. 2005; Grigg et al. 2004). A meta-analysis of six studies (144 kidney transplant patients) found that pamidronate, given 3–7 times at 30–90 mg per dose, was associated with significant reduction of bone loss in the spine without adverse effects on graft function (Wang et al. 2014). Similarly, both zoledronic acid and ibandronate prevent bone loss at 6 and 12 months in recipients of heart (Fahrleitner-Pammer et al. 2009), liver (Hommann et al. 2002; Crawford et al. 2006; Bodingbauer et al. 2007), and kidney (Grotz et al. 2001; Haas et al. 2003; Walsh et al. 2009; Smerud et al. 2012) transplants. Zoledronic acid administered monthly for the first 6 months and at 9 and 12 months after liver transplantation was associated with stable BMD at the spine and reduced losses at the FN compared to controls and was associated with a reduction in vertebral fractures (Bodingbauer et al. 2007). Ibandronate, 2 mg IV every 3 months for 1 year, maintained spine BMD and attenuated hip bone loss after liver transplantation, with a significant reduction in number of fractures (Kaemmerer et al. 2010). In long-term liver transplant recipients, quarterly IV ibandronate and calcitriol were associated with improvements in BMD at the hip and a reduction in vertebral fractures over 3 years compared to an untreated reference group (Wagner et al. 2012).
In two large prospective studies of patients after allogeneic BMT, IV pamidronate prevented bone loss at the spine and ameliorated losses at the hip (Kananen et al. 2005; Grigg et al. 2004). Similarly, IV zoledronic acid (4 mg) given 12 months after grafting and in another study, given every 3 months for 2 years, prevented spine and hip bone loss (Tauchmanova et al. 2005; Hausmann et al. 2012). In a small uncontrolled study, BMD was stable over 3 years after BMT in patients who received zoledronic acid once at transplant and 6 months later (Ganguly et al. 2012). When BMT patients were treated with zoledronic acid, FN BMD increased at 12 months (Lee et al. 2002c; Hari et al. 2013). Similarly, monthly zoledronic acid infusions for the first 3 months after BMT restored BMD at the LS and FN (Serio et al. 2013).
Results with oral bisphosphonates are less compelling. There was no change in BMD in 42 renal transplant patients randomized to risedronate or placebo, although risedronate appeared to preserve BMD in women (Coco et al. 2012). Monthly oral ibandronate (150 mg) or weekly oral risedronate (35 mg) initiated 12 months after kidney transplant provided comparable increases in spine and hip BMD after 1 year (Sanchez-Escuredo et al. 2015). A study of 76 kidney transplant recipients treated with weekly oral alendronate (70 mg) found improvements in lumbar spine BMD both in patients with and without preexisting osteoporosis after 14 months of therapy (Huang et al. 2012). Alendronate reduced bone-turnover markers in 24 kidney transplant recipients after 36 months of treatment (Yamamoto et al. 2013). Kidney transplant patients treated with alendronate (10 mg daily), calcitriol (0.25 μg daily), and calcium carbonate (2 g daily) had marked increases in spine BMD compared to decreases in those who received only calcium and calcitriol (Kovac et al. 2001). There were similar increases in spine BMD in patients treated with alendronate or risedronate after kidney transplant (Nowacka-Cieciura et al. 2006; Torregrosa et al. 2007; Giannini et al. 2001). Weekly alendronate (70 mg) improved BMD in kidney (Toro et al. 2005) and liver transplant recipients (Atamaz et al. 2006). Risedronate prevented bone loss at the spine but not the hip 12 months after liver transplant (Guadalix et al. 2011). In contrast, monthly oral ibandronate increased spine and hip BMD in 74 liver transplant patients, and fracture rates were relatively low (5.4 %) (Kaemmerer et al. 2012). Both alendronate (10 mg daily) and calcitriol (0.25 μg twice daily) initiated immediately after heart transplant prevented bone loss at the spine and hip compared with a reference group receiving only calcium and vitamin D (Shane et al. 2004); BMD remained stable during the second year after alendronate and calcitriol were discontinued (Cohen et al. 2006a). A single infusion of zoledronic acid (5 mg) or weekly alendronate (70 mg) initiated immediately after heart and liver transplantation prevented bone loss at the hip and, in liver transplant patients, at the spine compared to a reference group that received only calcium and vitamin D (Cohen et al. 2011). In heart transplant patients, spine bone mineral density increased with IV zoledronic acid and decreased on alendronate (Cohen et al. 2011). In BMT patients, risedronate started 12 months after BMT improved LS BMD and prevented loss at the FN (Tauchmanova et al. 2003).
Although fracture is a very important clinical outcome, few studies have had adequate power to detect differences in fracture. A meta-analysis of 11 randomized controlled clinical trials showed that treatment with either bisphosphonates or vitamin D analogues significantly reduced the number of subjects with fracture by 50 % and number of vertebral fractures by 76 %. When vitamin D analogue trials were excluded and bisphosphonate trials examined separately, there was a significant 47 % reduction in number of subjects with fractures but the 66 % reduction in vertebral fractures was not significant (Cohen et al. 2011). In our opinion, bisphosphonates represent the most promising approach to the prevention of transplantation osteoporosis, but data on fracture prevention is needed.
11.6 Summary and Conclusions
The prevalence of osteoporosis among candidates for solid organ and bone marrow transplantation is high. During the 1990s, prospective longitudinal studies demonstrated rapid bone loss and high fracture rates, particularly during the first posttransplant year. More recent studies suggest that rates of bone loss and fracture are considerably lower than in the past, although rates remain unacceptably high. All patients should be evaluated before transplantation and receive treatment for prevalent osteoporosis, if present. Primary prevention therapy should be initiated immediately after transplantation in patients with low BMD, advanced age, or other significant risk factors, as the most rapid bone loss occurs in the first few months after grafting. Long-term transplant recipients should be monitored and treated for bone disease as well (Table 11.3). At present, bisphosphonates are the most consistently effective agents for prevention and treatment of bone loss in organ transplant recipients. In the future, the use of denosumab or the introduction of newer, bone-sparing immunosuppressive agents may further reduce rates of bone loss after transplant. It is hoped that these advances will further reduce morbidity from transplantation osteoporosis.
References
Abdelhadi M, Eriksson SA, Ljusk Eriksson S, Ericzon BG, Nordenstrom J (1995) Bone mineral status in end-stage liver disease and the effect of liver transplantation. Scand J Gastroenterol 30:1210–1215
Adams JS, Hewison M (2010) Update in vitamin D. J Clin Endocrinol Metab 95:471–478
Adams JS, Rafison B, Witzel S et al (2014) Regulation of the extrarenal CYP27B1-hydroxylase. J Steroid Biochem Mol Biol 144(Pt A):22–27
Ahmadpoor P, Ilkhanizadeh B, Ghasemmahdi L, Makhdoomi K, Ghafari A (2009) Effect of active vitamin D on expression of co-stimulatory molecules and HLA-DR in renal transplant recipients. Exp Clin Transplant 7:99–103
Akaberi S, Lindergard B, Simonsen O, Nyberg G (2006) Impact of parathyroid hormone on bone density in long-term renal transplant patients with good graft function. Transplantation 82:749–752
Almond MK, Kwan JTC, Evans K, Cunningham J (1994) Loss of regional bone mineral density in the first 12 months following renal transplantation. Nephron 66:52–57
Alshayeb HM, Josephson MA, Sprague SM (2013) CKD-mineral and bone disorder management in kidney transplant recipients. Am J Kidney Dis 61:310–325
Amiche MA, Albaum JM, Tadrous M et al (2016) Efficacy of osteoporosis pharmacotherapies in preventing fracture among oral glucocorticoid users: a network meta-analysis. Osteoporos Int 6:1989–98
Anijar JR, Szejnfeld VL, Almeida DR, Fernandes AR, Ferraz MB (1999) Reduced bone mineral density in men after heart transplantation. Braz J Med Biol Res 32:413–420
Aringer M, Kiener H, Koeller M et al (1998) High turnover bone disease following lung transplantation. Bone 23:485–488
Aris R, Neuringer I, Weiner M, Egan T, Ontjes D (1996) Severe osteoporosis before and after lung transplantation. Chest 109:1176–1183
Aris RM, Renner JB, Winders AD et al (1998) Increased rate of fractures and severe kyphosis: sequelae of living into adulthood with cystic fibrosis. Ann Intern Med 128:186–193
Aris RM, Lester GE, Renner JB et al (2000) Efficacy of pamidronate for osteoporosis in patients with cystic fibrosis following lung transplantation. Am J Respir Crit Care Med 162:941–946
Arlen DJ, Lambert K, Ioannidis G, Adachi JD (2001) Treatment of established bone loss after renal transplantation with etidronate. Transplantation 71:669–673
Aroldi A, Tarantino A, Montagnino G, Cesana B, Cocucci C, Ponticelli C (1997) Effects of three immunosuppressive regimens on vertebral bone density in renal transplant recipients: a prospective study. Transplantation 63:380–386
Atamaz F, Hepguler S, Karasu Z, Kilic M, Tokat Y (2006) The prevention of bone fractures after liver transplantation: experience with alendronate treatment. Transplant Proc 38:1448–1452
Aubin J, Lian J, Stein G (2006) Bone formation: maturation and functional activities of osteoblast lineage cells. In: Favus M (ed) Primer on the metabolic bone diseases and other disorders of bone and mineral metabolism. Wiley-Blackwell. Iowa,USA pp 20–29
Awumey E, Moonga B, Sodam B et al (1999) Molecular and functional evidence for calcineurin alpha and beta isoforms in the osteoclasts. Novel insights into the mode of action of cyclosporine A. Biochem Biophys Res Commun 254:148–252
Bai XL, Liang TB, Wu LH et al (2007) Elevation of intact parathyroid hormone level is a risk factor for low bone mineral density in pretransplant patients with liver diseases. Transplant Proc 39:3182–3185
Ball AM, Gillen DL, Sherrard D et al (2002) Risk of hip fracture among dialysis and renal transplant recipients. JAMA 288:3014–3018
Banfi A, Podesta M, Fazzuoli L et al (2001) High-dose chemotherapy shows a dose-dependent toxicity to bone marrow osteoprogenitors: a mechanism for post-bone marrow transplantation osteopenia. Cancer 92:2419–2428
Baraldo M, Gregoraci G, Livi U (2014) Steroid-free and steroid withdrawal protocols in heart transplantation: the review of literature. Transpl Int 27:515–529
Bayat N, Einollahi B, Pourfarzian V et al (2007) Bone mineral density changes within 11 months of renal transplantation in Iranian patients. Transplant Proc 39:1039–1043
Becker BN, Hullett DA, O’Herrin JK, Malin G, Sollinger HW, DeLuca H (2002) Vitamin D as immunomodulatory therapy for kidney transplantation. Transplantation 74:1204–1206
Beique LC, Kline GA, Dalton B, Duggan K, Yilmaz S (2013) Predicting deficiency of vitamin D in renal transplant recipients in northern climates. Transplantation 95:1479–1484
Berguer DG, Krieg MA, Thiebaud D et al (1994) Osteoporosis in heart transplant recipients: a longitudinal study. Transplant Proc 26:2649–2651
Bhatia S, Ramsay NK, Weisdorf D, Griffiths H, Robison LL (1998) Bone mineral density in patients undergoing bone marrow transplantation for myeloid malignancies. Bone Marrow Transplant 22:87–90
Bianda T, Linka A, Junga G et al (2000) Prevention of osteoporosis in heart transplant recipients: a comparison of calcitriol with calcitonin and pamidronate. Calcif Tissue Int 67:116–121
Bikle DD (2008) Vitamin D, and the immune system: role in protection against bacterial infection. Curr Opin Nephrol Hypertens 17:348–352
Bikle DD (2011) Vitamin D, regulation of immune function. Vitam Horm 86:1–21
Bischoff-Ferrari HA, Dawson-Hughes B, Orav EJ et al (2016) Monthly high-dose vitamin D treatment for the prevention of functional decline: a randomized clinical trial. JAMA Intern Med 176:175–183
Bitetto D, Fabris C, Falleti E et al (2010) Vitamin D and the risk of acute allograft rejection following human liver transplantation. Liver Int 30:417–444
Bodingbauer M, Wekerle T, Pakrah B et al (2007) Prophylactic bisphosphonate treatment prevents bone fractures after liver transplantation. Am J Transplant 7:1763–1769
Bonani M, Rodriguez D, Fehr T et al (2014) Sclerostin blood levels before and after kidney transplantation. Kidney Blood Press Res 39:230–239
Boot AM, Nauta J, Hokken-Koelega ACS, Pols HAP, Ridder MAJ, Keizer-Schrama SMPF (1995) Renal transplantation and osteoporosis. Arch Dis Child 72:502–506
Bowman A, Sass D, Marshall I et al (1995) Raloxifene analog (Ly 117018-HCL) ameliorates cyclosporin A induced osteopenia. J Bone Miner Res 10(Suppl 1):350
Braga Junior JW, Neves RM, Pinheiro MM et al (2006) Prevalence of low trauma fractures in long-term kidney transplant patients with preserved renal function. Braz J Med Biol Res 39:137–147
Branco CC, Rovira M, Pons F et al (1996) The effect of hormone replacement therapy on bone mass in patients with ovarian failure due to bone marrow transplantation. Maturitas 23:307–312
Briffa NK, Keogh AM, Sambrook PN, Eisman JA (2003) Reduction of immunosuppressant therapy requirement in heart transplantation by calcitriol. Transplantation 75:2133–2134
Buchinsky FJ, Ma Y, Mann GN et al (1996) T lymphocytes play a critical role in the development of cyclosporin A-induced osteopenia. Endocrinology 137:2278–2285
Campistol JM, Holt DW, Epstein S, Gioud-Paquet M, Rutault K, Burke JT (2005) Bone metabolism in renal transplant patients treated with cyclosporine or sirolimus. Transpl Int 18:1028–1035
Campos DJ, Boguszewski CL, Funke VA et al (2014) Bone mineral density, vitamin D, and nutritional status of children submitted to hematopoietic stem cell transplantation. Nutrition 30:654–659
Cantorna MT, Hullett DA, Redaelli C et al (1998) 1,25-Dihydroxyvitamin D3 prolongs graft survival without compromising host resistance to infection or bone mineral density. Transplantation 66:828–831
Caplan-Shaw CE, Arcasoy SM, Shane E et al (2006) Osteoporosis in diffuse parenchymal lung disease. Chest 129:140–146
Carlson K, Simonsson B, Ljunghall S (1994) Acute effects of high dose chemotherapy followed by bone marrow transplantation on serum markers of bone metabolism. Calcif Tissue Int 55:408–411
Carvalho C, Magalhaes J, Pereira L, Simoes-Silva L, Castro-Ferreira I, Frazao JM (2015) Evolution of bone disease after kidney transplantation: a prospective histomorphometric analysis of trabecular and cortical bone. Nephrology (Carlton) 21:55–61
Castaneda S, Carmona L, Carjaval I, Arranz B, Diaz A, Garcia-Vadillo A (1997) Reduction of bone mass in women after bone marrow transplantation. Calcif Tissue Int 60:343–347
Cejka D, Benesch T, Krestan C et al (2008) Effect of teriparatide on early bone loss after kidney transplantation. Am J Transplant 8:1864–1870
Chaney A, Heckman MG, Diehl NN, Meek S, Keaveny AP (2015) Effectiveness and outcomes of current practice in treating vitamin D deficiency in patients listed for liver transplantation. Endocr Pract 21:761–769
Chiu MY, Sprague SM, Bruce DS, Woodle ES, Thistlethwaite JR Jr, Josephson MA (1998) Analysis of fracture prevalence in kidney-pancreas allograft recipients. J Am Soc Nephrol 9:677–683
Coco M, Rush H (2000) Increased incidence of hip fractures in dialysis patients with low serum parathyroid hormone. Am J Kidney Dis 36:1115–1121
Coco M, Glicklich D, Faugere MC et al (2003) Prevention of bone loss in renal transplant recipients: a prospective, randomized trial of intravenous pamidronate. J Am Soc Nephrol 14:2669–2676
Coco M, Pullman J, Cohen HW et al (2012) Effect of risedronate on bone in renal transplant recipients. J Am Soc Nephrol 23:1426–1437
Cohen A, Shane E (2003) Osteoporosis after solid organ and bone marrow transplantation. Osteoporos Int 14:617–630
Cohen A, Shane E (2005) Bone disease in patients before and after cardiac transplantation. In: Compston JE, Shane E (eds) Bone disease of organ transplantation. Elsevier Academic Press, Burlington, pp 287–301
Cohen A, Ebeling P, Sprague S, Shane E (2006a) Transplantation osteoporosis. In: Favus M (ed) Primer on the metabolic bone diseases and disorders of bone and mineral metabolism. Wiley-Blackwell. Iowa, USA pp 302–309
Cohen A, Addesso V, McMahon DJ et al (2006b) Discontinuing antiresorptive therapy one year after cardiac transplantation: effect on bone density and bone turnover. Transplantation. Wiley-Blackwell. Iowa, USA 81:686–691
Cohen A, Stein EM, Boutroy S et al (2011) Weekly alendronate versus a single infusion of zoledronic acid: effects on bone turnover markers, areal and volumetric BMD and bone microarchitecture during the first year after heart or liver transplantation. J Bone Miner Res 26
Compston J, Greer S, Skingle S et al (1996) Early increase in plasma parathyroid hormone level following liver transplantation. J Hepatol 25:715–718
Courbebaisse M, Alberti C, Colas S et al (2014) VITamin D supplementation in renAL transplant recipients (VITALE): a prospective, multicentre, double-blind, randomized trial of vitamin D estimating the benefit and safety of vitamin D3 treatment at a dose of 100,000 UI compared with a dose of 12,000 UI in renal transplant recipients: study protocol for a double-blind, randomized, controlled trial. Trials 15:430
Crawford BA, Kam C, Pavlovic J et al (2006) Zoledronic acid prevents bone loss after liver transplantation: a randomized, double-blind, placebo-controlled trial. Ann Intern Med 144:239–248
Cremer J, Struber M, Wagenbreth I et al (1999) Progression of steroid-associated osteoporosis after heart transplantation. Ann Thorac Surg 67:130–133
Crespo Leiro MG, Bonet LA, Paniagua Martin MJ et al (2012) Steroid withdrawal during 5 years following heart transplantation, and the relationship between steroid dosage at 1-year follow-up and complications during the next 2 years: results from the RESTCO study. Transplant Proc 44:2631–2634
Crosbie OM, Freaney R, McKenna MJ, Hegarty JE (1999) Bone density, vitamin D status, and disordered bone remodeling in end- stage chronic liver disease. Calcif Tissue Int 64:295–300
Cueto-Manzano A, Konel S, Hutchinson AJ et al (1999) Bone loss in long term renal transplantation. Histopathology and densitometry analysis. Kidney Int 55:2021–2029
Cueto-Manzano AM, Konel S, Crowley V et al (2003) Bone histopathology and densitometry comparison between cyclosporine a monotherapy and prednisolone plus azathioprine dual immunosuppression in renal transplant patients. Transplantation 75:2053–2058
Cvetkovic M, Mann GN, Romero DF et al (1994) The deleterious effects of long term cyclosporin A, cyclosporin G and FK506 on bone mineral metabolism in vivo. Transplantation 57:1231–1237
De Sevaux RG, Hoitsma AJ, Corstens FH, Wetzels JF (2002) Treatment with vitamin D and calcium reduces bone loss after renal transplantation: a randomized study. J Am Soc Nephrol 13:1608–1614
Dimitrov V, Salehi-Tabar R, An BS, White JH (2014) Non-classical mechanisms of transcriptional regulation by the vitamin D receptor: insights into calcium homeostasis, immune system regulation and cancer chemoprevention. J Steroid Biochem Mol Biol 144(Pt A):74–80
Dissanayake IR, Goodman GR, Bowman AR et al (1998) Mycophenolate mofetil; a promising new immunosuppressant that does not cause bone loss in the rat. Transplantation 65:275–278
Dolgos S, Hartmann A, Bonsnes S et al (2008) Determinants of bone mass in end-stage renal failure patients at the time of kidney transplantation. Clin Transplant 22:462–468
Dolgos S, Hartmann A, Isaksen GA et al (2010) Osteoporosis is a prevalent finding in patients with solid organ failure awaiting transplantation – a population based study. Clin Transplant 24:E145–E152
Donovan DS Jr, Papadopoulos A, Staron RB et al (1998) Bone mass and vitamin D deficiency in adults with advanced cystic fibrosis lung disease. Am J Respir Crit Care Med 157:1892–1899
Dounousi E, Leivaditis K, Eleftheriadis T, Liakopoulos V (2015) Osteoporosis after renal transplantation. Int Urol Nephrol 47:503–511
Eastell R, Dickson RE, Hodgson SF et al (1991) Rates of vertebral bone loss before and after liver transplantation in women with primary biliary cirrhosis. Hepatology 14:296–300
Ebeling PR (2005) Defective osteoblast function may be responsible for bone loss from the proximal femur despite pamidronate therapy. J Clin Endocrinol Metab 90:4414–4416
Ebeling P, Thomas D, Erbas B, Hopper L, Szer J, Grigg A (1999) Mechanism of bone loss following allogeneic and autologous hematopoeitic stem cell transplantation. J Bone Miner Res 14:342–350
Edwards BJ, Desai A, Tsai J et al (2011) Elevated incidence of fractures in solid-organ transplant recipients on glucocorticoid-sparing immunosuppressive regimens. J Osteoporos 2011:591793
El-Agroudy AE, El-Husseini AA, El-Sayed M, Ghoneim MA (2003) Preventing bone loss in renal transplant recipients with vitamin D. J Am Soc Nephrol 14:2975–2979
El-Agroudy AE, El-Husseini AA, El-Sayed M, Mohsen T, Ghoneim MA (2005) A prospective randomized study for prevention of postrenal transplantation bone loss. Kidney Int 67:2039–2045
Epstein S (1996) Post-transplantation bone disease: the role of immunosuppressive agents on the skeleton. J Bone Miner Res 11:1–7
Epstein S, Shane E (2001) Transplantation osteoporosis. In: Marcus R, Feldman D, Kelsey J (eds) Osteoporosis. Academic Press, San Diego, pp 327–340
Epstein S, Schlosberg M, Fallon M, Thomas S, Movsowitz C, Ismail F (1990) 1,25 dihydroxyvitamin D3 modifies cyclosporine induced bone loss. Calcif Tissue Int 47:152–157
Epstein S, Dissanayake A, Goodman GR et al (2001) Effect of the interaction of parathyroid hormone and cyclosporine A on bone mineral metabolism in the rat. Calcif Tissue Int 68:240–247
Fabrega E, Orive A, Garcia-Unzueta M, Amado JA, Casafont F, Pons-Romero F (2006) Osteoprotegerin and receptor activator of nuclear factor-kappaB ligand system in the early post-operative period of liver transplantation. Clin Transplant 20:383–388
Fahrleitner-Pammer A, Piswanger-Soelkner JC, Pieber TR et al (2009) Ibandronate prevents bone loss and reduces vertebral fracture risk in male cardiac transplant patients: a randomized double-blind, placebo-controlled trial. J Bone Miner Res 24:1335–1344
Fan S, Almond MK, Ball E, Evans K, Cunningham J (2000) Pamidronate therapy as prevention of bone loss following renal transplantation. Kidney Int 57:684–690
Fan SL, Kumar S, Cunningham J (2003) Long-term effects on bone mineral density of pamidronate given at the time of renal transplantation. Kidney Int 63:2275–2279
Ferrari SL, Nicod LP, Hamacher J et al (1996) Osteoporosis in patients undergoing lung transplantation. Eur Respir J 9:2378–2382
Fischer L, Valentinitsch A, DiFranco MD et al (2015) High-resolution peripheral quantitative CT imaging: cortical porosity, poor trabecular bone microarchitecture, and low bone strength in lung transplant recipients. Radiology 274:473–481
Fleseriu M, Licata AA (2007) Failure of successful renal transplant to produce appropriate levels of 1,25-dihydroxyvitamin D. Osteoporos Int 18:363–368
Floreani A, Mega A, Tizian L et al (2001) Bone metabolism and gonad function in male patients undergoing liver transplantation: a two-year longitudinal study. Osteoporos Int 12:749–754
Frisk P, Arvidson J, Ljunggren O, Gustafsson J (2012) Decreased bone mineral density in young adults treated with SCT in childhood: the role of 25-hydroxyvitamin D. Bone Marrow Transplant 47:657–662
Gallego R, Oliva E, Vega N et al (2006) Steroids and bone density in patients with functioning kidney allografts. Transplant Proc 38:2434–2437
Ganguly S, Divine CL, Aljitawi OS, Abhyankar S, McGuirk JP, Graves L (2012) Prophylactic use of zoledronic acid to prevent early bone loss is safe and feasible in patients with acute myeloid leukemia undergoing allogeneic stem cell transplantation. Clin Transplant 26:447–453
Garcia-Delgado I, Prieto S, Fragnas LG, Robles E, Rufilanchas T, Hawkins F (1997) Calcitonin, etidronate and calcidiol treatment in bone loss after cardiac transplantation. Calcif Tissue Int 60:155–159
Gaston RS (2006) Current and evolving immunosuppressive regimens in kidney transplantation. Am J Kidney Dis 47:S3–S21
Gatta A, Verardo A, Di Pascoli M, Giannini S, Bolognesi M (2014) Hepatic osteodystrophy. Clin Cases Miner Bone Metab 11:185–191
Giannini S, Dangel A, Carraro G et al (2001) Alendronate prevents further bone loss in renal transplant recipients. J Bone Miner Res 16:2111–2117
Glendenning P, Kent GN, Adler BD et al (1999) High prevalence of osteoporosis in cardiac transplant recipients and discordance between biochemical turnover markers and bone histomorphometry. Clin Endocrinol (Oxf) 50:347–355
Goffin E, Devogelaer JP, Lalaoui A et al (2002) Tacrolimus and low-dose steroid immunosuppression preserves bone mass after renal transplantation. Transpl Int 15:73–80
Goodman GR et al (2001) Immunosuppressant use without bone loss implications for bone loss after transplantation. J Bone Miner Res. 16:72–78
Gori F, Hofbauer LC, Dunstan CR, Spelsberg TC, Khosla S, Riggs BL (2000) The expression of osteoprotegerin and RANK ligand and the support of osteoclast formation by stromal-osteoblast lineage cells is developmentally regulated. Endocrinology 141:4768–4776
Grigg AC, Shuttleworth P, Reynolds J et al (2004) Pamidronate therapy for one year after allogeneic bone marrow transplantation (AlloBMT) reduces bone loss from the lumbar spine, femoral neck and total hip. Blood 104:A2253
Grotz WH, Mundinger A, Gugel B, Exner V, Kirste G, Schollmeyer PJ (1994) Bone fracture and osteodensitometry with dual energy x-ray absorptiometry in kidney transplant recipients. Transplantation 58:912–915
Grotz W, Nagel C, Poeschel D et al (2001) Effect of ibandronate on bone loss and renal function after kidney transplantation. J Am Soc Nephrol 12:1530–1537
Guadalix S, Martinez-Diaz-Guerra G, Lora D et al (2011) Effect of early risedronate treatment on bone mineral density and bone turnover markers after liver transplantation: a prospective single-center study. Transpl Int 24:657–665
Guanabens N, Pares A, Alvarez L et al (1998) Collagen-related markers of bone turnover reflect the severity of liver fibrosis in patients with primary biliary cirrhosis. J Bone Miner Res 13:731–738
Guichelaar MM, Kendall R, Malinchoc M, Hay JE (2006) Bone mineral density before and after OLT: long-term follow-up and predictive factors. Liver Transpl 12:1390–1402
Guo C, Johnson A, Locke T, Eastell R (1998) Mechanism of bone loss after cardiac transplantation. Bone 22:267–271
Gupta AK, Huang M, Prasad GV (2012) Determinants of bone mineral density in stable kidney transplant recipients. J Nephrol 25:373–383
Haagsma EB, Thijn CJP, Post JG, Slooff MJH, Gisp CH (1988) Bone disease after liver transplantation. J Hepatol 6:94–100
Haas M, Leko-Mohr Z, Roschger P et al (2003) Zoledronic acid to prevent bone loss in the first 6 months after renal transplantation. Kidney Int 63:1130–1136
Hansdottir S, Monick MM, Hinde SL, Lovan N, Look DC, Hunninghake GW (2008) Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. J Immunol 181:7090–7099
Hari P, DeFor TE, Vesole DH, Bredeson CN, Burns LJ (2013) Intermittent zoledronic acid prevents bone loss in adults after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 19:1361–1367
Hariman A, Alex C, Heroux A, Camacho P (2014) Incidence of fractures after cardiac and lung transplantation: a single center experience. J Osteoporos 2014:573041
Haroon M, Fitzgerald O (2012) Vitamin D and its emerging role in immunopathology. Clin Rheumatol 31:199–202
Hausmann A, Hill W, Stemmler HJ et al (2012) Bone loss after allogeneic haematopoietic stem cell transplantation: a pilot study on the use of zoledronic acid. Chemother Res Pract 2012:858590
Hawkins FG, Leon M, Lopez MB et al (1994) Bone loss and turnover in patients with liver transplantation. Hepatogastroenterology 41:158–161
Hay JE (2003) Osteoporosis in liver diseases and after liver transplantation. J Hepatol 38:856–865
Henderson NK, Sambrook PN, Kelly PJ et al (1995) Bone mineral loss and recovery after cardiac transplantation [letter]. Lancet 346:905
Henderson K, Eisman J, Keogh A et al (2001) Protective effect of short-tem calcitriol or cyclical etidronate on bone loss after cardiac or lung transplantation. J Bone Miner Res 16:565–571
Hofbauer LC, Khosla S, Dunstan CR, Lacey DL, Boyle WJ, Riggs BL (2000) The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J Bone Miner Res 15:2–12
Hofbauer LC, Shui C, Riggs BL et al (2001) Effects of immunosuppressants on receptor activator of NF-kappaB ligand and osteoprotegerin production by human osteoblastic and coronary artery smooth muscle cells. Biochem Biophys Res Commun 280:334–339
Hommann M, Abendroth K, Lehmann G et al (2002) Effect of transplantation on bone: osteoporosis after liver and multivisceral transplantation. Transplant Proc 34:2296–2298
Horber FF, Casez JP, Steiger U, Czerniack A, Montandon A, Jaeger PH (1994) Changes in bone mass early after kidney transplantation. J Bone Miner Res 9:1–9
Huang WH, Lee SY, Weng CH, Lai PC (2012) Use of alendronate sodium (Fosamax) to ameliorate osteoporosis in renal transplant patients: a case–control study. PLos One 7:e48481
Hullett DA, Cantorna MT, Redaelli C et al (1998) Prolongation of allograft survival by 1,25-dihydroxyvitamin D3. Transplantation 66:824–828
Hussaini SH, Oldroyd B, Stewart SP et al (1999) Regional bone mineral density after orthotopic liver transplantation. Eur J Gastroenterol Hepatol 11:157–163
Iqbal N, Ducharme J, Desai S et al (2008) Status of bone mineral density in patients selected for cardiac transplantation. Endocr Pract 14:704–712
Iyer SP, Nikkel LE, Nishiyama KK et al (2014) Kidney transplantation with early corticosteroid withdrawal: paradoxical effects at the central and peripheral skeleton. J Am Soc Nephrol 25:1331–1341
Jager W, Xu H, Wlcek K, Schuler C, Rubel F, Erben RG (2012) Gender- and dose-related effects of cyclosporin A on hepatic and bone metabolism. Bone 50:140–148
Jastrzebski D, Lutogniewska W, Ochman M et al (2010) Osteoporosis in patients referred for lung transplantation. Eur J Med Res 15(Suppl 2):68–71
Jeon HJ, Han M, Jeong JC et al (2013) Impact of vitamin D, bisphosphonate, and combination therapy on bone mineral density in kidney transplant patients. Transplant Proc 45:2963–2967
Joffe I, Katz I, Jacobs T et al (1992) 17 beta estradiol prevents osteopenia in the oophorectomized rat treated with cyclosporin A. Endocrinology 130:1578–1586
Josephson MA, Schumm LP, Chiu MY, Marshall C, Thistlethwaite JR, Sprague SM (2004) Calcium and calcitriol prophylaxis attenuates posttransplant bone loss. Transplantation 78:1233–1236
Julian BA, Laskow DA, Dubovsky J, Dubovsky EV, Curtis JJ, Quarrles LD (1991) Rapid loss of vertebral bone density after renal transplantation. N Engl J Med 325:544–550
Kaemmerer D, Lehmann G, Wolf G, Settmacher U, Hommann M (2010) Treatment of osteoporosis after liver transplantation with ibandronate. Transpl Int 23:753–759
Kaemmerer D, Schmidt B, Lehmann G, Wolf G, Hommann M, Settmacher U (2012) Monthly ibandronate for the prevention of bone loss in patients after liver transplantation. Transplant Proc 44:1362–1367
Kahan BD (1989) Cyclosporine. N Engl J Med 321:1725–1738
Kananen K, Volin L, Tahtela R, Laitinen K, Ruutu T, Valimaki MJ (2002) Recovery of bone mass and normalization of bone turnover in long-term survivors of allogeneic bone marrow transplantation. Bone Marrow Transplant 29:33–39
Kananen K, Volin L, Laitinen K, Alfthan H, Ruutu T, Valimaki MJ (2005) Prevention of bone loss after allogeneic stem cell transplantation by calcium, vitamin D, and sex hormone replacement with or without pamidronate. J Clin Endocrinol Metab 90:3877–3885
Kang MI, Lee WY, Oh KW et al (2000) The short-term changes of bone mineral metabolism following bone marrow transplantation. Bone 26:275–279
Kashyap A, Kandeel F, Yamauchi D et al (2000) Effects of allogeneic bone marrow transplantation on recipient bone mineral density: a prospective study. Biol Blood Marrow Transplant 6:344–351
Katz IA, Jee WSS, Joffe I et al (1992) Prostaglandin E2 alleviates cyclosporin A-induced bone loss in the rat. J Bone Miner Res 4:1191–1200
Kauppila M, Irjala K, Koskinen P et al (1999) Bone mineral density after allogeneic bone marrow transplantation. Bone Marrow Transplant 24:885–889
Kelly PJ, Atkinson K, Ward RL, Sambrook PN, Biggs JC, Eisman JA (1990) Reduced bone mineral density in men and women with allogeneic bone marrow. Transplantation 50:881–883
Keogh JB, Tsalamandris C, Sewell RB et al (1999) Bone loss at the proximal femur and reduced lean mass following liver transplantation: a longitudinal study. Nutrition 15:661–664
Kerschan-Schindl K, Mitterbauer M, Fureder W et al (2004) Bone metabolism in patients more than five years after bone marrow transplantation. Bone Marrow Transplant 34:491–496
Kerschan-Schindl K, Ruzicka M, Mahr S et al (2008) Unexpected low incidence of vertebral fractures in heart transplant recipients: analysis of bone turnover. Transpl Int 21:255–262
Kidney Disease: Improving Global Outcomes CKDMBDWG (2009) KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 113:S1–S130
Kohlmeier M, Saupe J, Schaefer K, Asmus G (1998) Bone fracture history and prospective bone fracture risk of hemodialysis patients are related to apolipoprotein E genotype. Calcif Tissue Int 62:278–281
Kovac D, Lindic J, Kandus A, Bren AF (2001) Prevention of bone loss in kidney graft recipients. Transplant Proc 33:1144–1145
Krieg M, Seydoux C, Sandini L et al (2001) Intravenous pamidronate as a treatment for osteoporosis after heart transplantation: a prospective study. Osteoporos Int 12:112–116
Krol CG, Dekkers OM, Kroon HM, Rabelink TJ, van Hoek B, Hamdy NA (2014a) Longitudinal changes in BMD and fracture risk in orthotopic liver transplant recipients not using bone-modifying treatment. J Bone Miner Res 29:1763–1769
Krol CG, Dekkers OM, Kroon HM, Rabelink TJ, van Hoek B, Hamdy NAT (2014b) No association between BMD and prevalent vertebral fractures in liver transplant recipients at time of screening before transplantation. J Clin Endocrinol Metab 99:3677–3685
Kulak C, Shane E (2006) Transplantation osteoporosis: biochemical correlates of pathogenesis and treatment. In: Seibel M, Robins S, Bilezikian J (eds) Dynamics of bone and cartilage metabolism: principles and clinical applications, 2nd edn. Elsevier, San Diego, pp 701–716
Kulak CA, Borba VZ, Kulak J Jr, Custodio MR (2012) Osteoporosis after transplantation. Curr Osteoporos Rep 10:48–55
Kwan JTC, Almond MK, Evans K, Cunningham J (1992) Changes in total body bone mineral content and regional bone mineral density in renal patients following renal transplantation. Miner Electrolyte Metab 18:166–168
Lee AH, Mull RL, Keenan GF et al (1994) Osteoporosis and bone morbidity in cardiac transplant recipients. Am J Med 96:35–41
Lee WY, Kang MI, Oh ES et al (2002a) The role of cytokines in the changes in bone turnover following bone marrow transplantation. Osteoporos Int 13:62–68
Lee WY, Cho SW, Oh ES et al (2002b) The effect of bone marrow transplantation on the osteoblastic differentiation of human bone marrow stromal cells. J Clin Endocrinol Metab 87:329–335
Lee WY, Kang MI, Baek KH et al (2002c) The skeletal site-differential changes in bone mineral density following bone marrow transplantation: 3-year prospective study. J Korean Med Sci 17:749–754
Leidig-Bruckner G, Hosch S, Dodidou P et al (2001) Frequency and predictors of osteoporotic fractures after cardiac or liver transplantation: a follow-up study. Lancet 357:342–347
Lemire JM (1992) Immunomodulatory role of 1,25 Dihydroxyvitamin D3. J Cell Biochem 49:26–31
Lemire JM (1995) Immunomodulatory actions of 1,25-dihydroxyvitamin D3. J Steroid Biochem Mol Biol 53:599–602
Lemire JM, Archer DC, Reddy GS (1994) Dihydroxy-24-oxo-16-ene-vitamin D3, a renal metabolite of the vitamin D analog 1,25-dihydroxy-16ene-vitamin D3, exerts immunosuppressive activity equal to its parent without causing hypercalcemia in vivo. Endocrinology 135:2818–2821
Liu N, Nguyen L, Chun RF et al (2008) Altered endocrine and autocrine metabolism of vitamin D in a mouse model of gastrointestinal inflammation. Endocrinology 149:4799–4808
Lopez MB, Pinto IG, Hawkins F et al (1992) Effect of liver transplantation and immunosuppressive treatment on bone mineral density. Transplant Proc 24:3044–3046
Luo L, Shi Y, Bai Y et al (2012) Impact of tacrolimus on bone metabolism after kidney transplantation. Int Immunopharmacol 13:69–72
Marcen R, Caballero C, Uriol O et al (2007) Prevalence of osteoporosis, osteopenia, and vertebral fractures in long-term renal transplant recipients. Transplant Proc 39:2256–2258
Marshall I, Isserow JA, Buchinsky FJ, Paynton BV, Epstein S (1995) Expression of interleukin 1 and interleukin 6 in bone from normal and cyclosporine treated rats. XIIth International Conference on calcium regulating hormones, Melbourne
Martin K, Al-Aly Z, Gonzalez E (2006) Renal osteodystrophy. In: Favus M (ed) Primer on the metabolic bone diseases and other disorders of bone and mineral metabolism, Wiley-Blackwell. Iowa, USA pp 359–366
Mazzaferro S, Pasquali M (2016) Vitamin D: a dynamic molecule. How relevant might the dynamism for a vitamin be? Nephrol Dial Transplant 31:23–30
Mazziotti G, Angeli A, Bilezikian JP, Canalis E, Giustina A (2006) Glucocorticoid-induced osteoporosis: an update. Trends Endocrinol Metab 17:144–149
McDonald JA, Dunstan CR, Dilworth P et al (1991) Bone loss after liver transplantation. Hepatology 14:613–619
McIntyre HD, Menzies B, Rigby R, Perry-Keene DA, Hawley CM, Hardie IR (1995) Long-term bone loss after renal transplantation: comparison of immunosuppressive regimens. Clin Transplant 9:20–24
Mellibovsky L, Prieto-Alhambra D, Mellibovsky F et al (2015) Bone tissue properties measurement by reference point indentation in glucocorticoid-induced osteoporosis. J Bone Miner Res 30:1651–1656
Meys E, Fontanges E, Fourcade N, Thomasson A, Pouyet M, Delmas P (1994) Bone loss after orthotopic liver transplantation. Am J Med 97:445–450
Mikuls TR, Julian BA, Bartolucci A, Saag KG (2003) Bone mineral density changes within six months of renal transplantation. Transplantation 75:49–54
Millonig G, Graziadei IW, Eichler D et al (2005) Alendronate in combination with calcium and vitamin D prevents bone loss after orthotopic liver transplantation: a prospective single-center study. Liver Transpl 11:960–966
Mittalhenkle A, Gillen DL, Stehman-Breen CO (2004) Increased risk of mortality associated with hip fracture in the dialysis population. Am J Kidney Dis 44:672–679
Mitterbauer C, Schwarz C, Haas M, Oberbauer R (2006) Effects of bisphosphonates on bone loss in the first year after renal transplantation – a meta-analysis of randomized controlled trials. Nephrol Dial Transplant 21:2275–2281
Molnar MZ, Naser MS, Rhee CM, Kalantar-Zadeh K, Bunnapradist S (2014) Bone and mineral disorders after kidney transplantation: therapeutic strategies. Transplant Rev (Orlando) 28:56–62
Monegal A, Navasa M, Guanabens N et al (1997) Osteoporosis and bone mineral metabolism in cirrhotic patients referred for liver transplantation. Calcif Tissue Int 60:148–154
Monegal A, Navasa M, Guanabens N et al (2001a) Bone mass and mineral metabolism in liver transplant patients treated with FK506 or cyclosporine A. Calcif Tissue Int 68:83–86
Monegal A, Navasa M, Guanabens N et al (2001b) Bone disease after liver transplantation: a long-term prospective study of bone mass changes, hormonal status and histomorphometric characteristics. Osteoporos Int 12:484–492
Monegal A, Guanabens N, Suarez MJ et al (2009) Pamidronate in the prevention of bone loss after liver transplantation: a randomized controlled trial. Transpl Int 22:198–206
Monegal A, Navasa M, Peris P et al (2013) Bone disease in patients awaiting liver transplantation. Has the situation improved in the last two decades? Calcif Tissue Int 93:571–576
Movsowitz C, Epstein S, Fallon M, Ismail F, Thomas S (1988) Cyclosporin a in vivo produces severe osteopenia in the rat: effect of dose and duration of administration. Endocrinology 123:2571–2577
Movsowitz C, Epstein S, Ismail F, Fallon M, Thomas S (1989) Cyclosporin A in the oophorectomized rat: unexpected severe bone resorption. J Bone Miner Res 4:393–398
Navasa M, Monegal A, Guanabens N et al (1994) Bone fractures in liver transplant patients. Br J Rheumatol 33:52–55
Naylor KL, Li AH, Lam NN, Hodsman AB, Jamal SA, Garg AX (2013) Fracture risk in kidney transplant recipients: a systematic review. Transplantation 95:1461–1470
Naylor KL, Garg AX, Hodsman AB, Rush DN, Leslie WD (2014) Long-term changes in bone mineral density in kidney transplant recipients. Transplantation 98:1279–1285
Naylor KL, Jamal SA, Zou G et al (2015) Fracture incidence in adult kidney transplant recipients. Transplantation 100:167–175
Nickolas TL, McMahon DJ, Shane E (2006) Relationship between moderate to severe kidney disease and hip fracture in the United States. J Am Soc Nephrol 17:3223–3232
Nikkel L, Mohan S, Tanriover B et al (2011) Does simultaneous pancreas-kidney transplantation reduce fracture risk? An analysis of the USRDS. Am Soc Nephrol
Nikkel LE, Mohan S, Zhang A et al (2012) Reduced fracture risk with early corticosteroid withdrawal after kidney transplant. Am J Transplant 12:649–659
Nikkel LE, Iyer SP, Mohan S et al (2013) Pancreas-kidney transplantation is associated with reduced fracture risk compared with kidney-alone transplantation in men with type 1 diabetes. Kidney Int 83:471–478
Ninkovic M, Skingle SJ, Bearcroft PW, Bishop N, Alexander GJ, Compston JE (2000) Incidence of vertebral fractures in the first three months after orthotopic liver transplantation. Eur J Gastroenterol Hepatol 12:931–935
Ninkovic M, Love S, Tom BD, Bearcroft PW, Alexander GJ, Compston JE (2002) Lack of effect of intravenous pamidronate on fracture incidence and bone mineral density after orthotopic liver transplantation. J Hepatol 37:93–100
Nishioka S, Sofue T, Inui M et al (2014) Mineral and bone disorder is temporary in patients treated with early rapid corticosteroid reduction after kidney transplantation: a single-center experience. Transplant Proc 46:514–520
Nishiyama KK, Pauchard Y, Nikkel LE et al (2015) Longitudinal HR-pQCT and image registration detects endocortical bone loss in kidney transplantation patients. J Bone Miner Res 30:554–561
Nowacka-Cieciura E, Cieciura T, Baczkowska T et al (2006) Bisphosphonates are effective prophylactic of early bone loss after renal transplantation. Transplant Proc 38:165–167
Nysom K, Holm K, Michaelsen KF et al (2000) Bone mass after allogeneic BMT for childhood leukaemia or lymphoma. Bone Marrow Transplant 25:191–196
Obi Y, Hamano T, Ichimaru N et al (2014) Vitamin D deficiency predicts decline in kidney allograft function: a prospective cohort study. J Clin Endocrinol Metab 99:527–535
Okamoto M, Yamanaka S, Yoshimoto W, Shigematsu T (2014) Alendronate as an effective treatment for bone loss and vascular calcification in kidney transplant recipients. J Transplant 2014:269613
Ott SM, Aitken ML (1998) Osteoporosis in patients with cystic fibrosis. Clin Chest Med 19:555–567
Overman RA, Gourlay ML, Deal CL, Farley JF, Brookhart MA, Layton JB (2015) Fracture rate associated with quality metric-based anti-osteoporosis treatment in glucocorticoid-induced osteoporosis. Osteoporos Int 26:1515–1524
Palmer SC, Strippoli GF, McGregor DO (2005) Interventions for preventing bone disease in kidney transplant recipients: a systematic review of randomized controlled trials. Am J Kidney Dis 45:638–649
Park KM, Hay JE, Lee SG et al (1996) Bone loss after orthotopic liver transplantation: FK 506 versus cyclosporine. Transplant Proc 28:1738–1740
Paterson AM, Yates SF, Nolan KF, Waldmann H (2005) The new immunosuppression: intervention at the dendritic cell-T-cell interface. Curr Drug Targets Immune Endocr Metabol Disord 5:397–411
Pelletier S, Dubourg L, Carlier MC, Hadj-Aissa A, Fouque D (2013) The relation between renal function and serum sclerostin in adult patients with CKD. Clin J Am Soc Nephrol 8:819–823
Pennisi P, Trombetti A, Giostra E, Mentha G, Rizzoli R, Fiore CE (2007) Pamidronate and osteoporosis prevention in liver transplant recipients. Rheumatol Int 27:251–256
Peric M, Koglin S, Kim SM et al (2008) IL-17A enhances vitamin D3-induced expression of cathelicidin antimicrobial peptide in human keratinocytes. J Immunol 181:8504–8512
Pescovitz MD (2006) B cells: a rational target in alloantibody-mediated solid organ transplantation rejection. Clin Transplant 20:48–54
Petryk A, Polgreen LE, Zhang L et al (2014) Bone mineral deficits in recipients of hematopoietic cell transplantation: the impact of young age at transplant. Bone Marrow Transplant 49:258–263
Piemontese M, Onal M, Xiong J et al (2015) Suppression of autophagy in osteocytes does not modify the adverse effects of glucocorticoids on cortical bone. Bone 75:18–26
Piraino B, Chen T, Cooperstein L, Segre G, Puschett J (1988) Fractures and vertebral bone mineral density in patients with renal osteodystrophy. Clin Nephrol 30:57–62
Ponticelli C, Aroldi A (2001) Osteoporosis after organ transplantation. Lancet 357:1623
Porayko MK, Wiesner RH, Hay JE et al (1991) Bone disease in liver transplant recipients: incidence, timing, and risk factors. Transplant Proc 23:1462–1465
Pundole XN, Barbo AG, Lin H, Champlin RE, Lu H (2015) Increased incidence of fractures in recipients of hematopoietic stem-cell transplantation. J Clin Oncol 33:1364–1370
Querings K, Girndt M, Geisel J, Georg T, Tilgen W, Reichrath J (2006) 25-hydroxyvitamin D deficiency in renal transplant recipients. J Clin Endocrinol Metab 91:526–529
Rabinowitz M, Shapiro J, Lian J, Bloch GD, Merkel IS, Thiel DHV (1992) Vitamin D and osteocalcin levels in liver transplant recipients. Is osteocalcin a reliable marker of bone turnover in such cases? J Hepatol 16:50–55
Ramsey-Goldman R, Dunn JE, Dunlop DD et al (1999) Increased risk of fracture in patients receiving solid organ transplants. J Bone Miner Res 14:456–463
Rathi M, Kumar D, Bhadada SK et al (2015) Sequential changes in bone biochemical parameters and bone mineral density after renal transplant. Saudi J Kidney Dis Transpl 26:671–677
Redaelli CA, Wagner M, Tien YH et al (2001) 1 alpha,25-Dihydroxycholecalciferol reduces rejection and improves survival in rat liver allografts. Hepatology 34:926–934
Regazzi MB, Alessiani M, Rinaldi M (2005) New strategies in immunosuppression. Transplant Proc 37:2675–2678
Rich GM, Mudge GH, Laffel GL, LeBoff MS (1992) Cyclosporine A and prednisone-associated osteoporosis in heart transplant recipients. J Heart Lung Transplant 11:950–958
Rizzari MD, Suszynski TM, Gillingham KJ et al (2012) Ten-year outcome after rapid discontinuation of prednisone in adult primary kidney transplantation. Clin J Am Soc Nephrol 7:494–503
Roe SD, Porter CJ, Godber IM, Hosking DJ, Cassidy MJ (2005) Reduced bone mineral density in male renal transplant recipients: evidence for persisting hyperparathyroidism. Osteoporos Int 16:142–148
Ross F (2006) Osteoclast biology and bone resorption. In: Favus M (ed) Primer on the metabolic bone diseases and other disorders of bone and mineral metabolism, Wiley-Blackwell. Iowa, USA pp 30–35
Rucinski B, Liu CC, Epstein S (1994) Utilization of cyclosporine H to elucidate the possible mechanisms of cyclosporine A induced osteopenia in the rat. Metabolism 43:1114–1118
Rutherford RM, Fisher AJ, Hilton C et al (2005) Functional status and quality of life in patients surviving 10 years after lung transplantation. Am J Transplant 5:1099–1104
Saag KG, Zanchetta JR, Devogelaer JP et al (2009) Effects of teriparatide versus alendronate for treating glucocorticoid-induced osteoporosis: thirty-six-month results of a randomized, double-blind, controlled trial. Arthritis Rheum 60:3346–3355
Sambrook P (2006) Glucocorticoid-induced osteoporosis. In: Favus M (ed) Primer on the metabolic bone diseases and other disorders of bone and mineral metabolism, Wiley-Blackwell. Iowa, USA pp 296–302
Sambrook PN, Kelly PJ, Keogh A et al (1994a) Bone loss after cardiac transplantation: a prospective study. J Heart Lung Transplant 13:116–121
Sambrook PN, Kelly PJ, Fontana D et al (1994b) Mechanisms of rapid bone loss following cardiac transplantation. Osteoporos Int 4:273–276
Sambrook P, Henderson NK, Keogh A et al (2000) Effect of calcitriol on bone loss after cardiac or lung transplantation. J Bone Miner Res 15:1818–1824
Sanchez-Escuredo A, Fuster D, Rubello D et al (2015) Monthly ibandronate versus weekly risedronate treatment for low bone mineral density in stable renal transplant patients. Nucl Med Commun 36:815–818
Sanders KM, Stuart AL, Williamson EJ et al (2010) Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA 303:1815–1822
Sarno G, Daniele G, Tirabassi G et al (2015) The impact of vitamin D deficiency on patients undergoing kidney transplantation: focus on cardiovascular, metabolic, and endocrine outcomes. Endocrine 50:568–574
Sass DA, Bowman AR, Marshall I et al (1997) Alendronate prevents cyclosporin-induced osteopenia in the rat. Bone 21:65–70
Savaj S, Ghods FJ (2012) Vitamin D, parathyroid hormone, and bone mineral density status in kidney transplant recipients. Iran J Kidney Dis 6:295–299
Schauber J, Oda Y, Buchau AS et al (2008) Histone acetylation in keratinocytes enables control of the expression of cathelicidin and CD14 by 1,25-dihydroxyvitamin D3. J Invest Dermatol 128:816–824
Schulte C, Beelen DW, Schaefer UW, Mann K (2000) Bone loss in long-term survivors after transplantation of hematopoietic stem cells: a prospective study. Osteoporos Int 11:344–353
Serio B, Pezzullo L, Fontana R et al (2013) Accelerated bone mass senescence after hematopoietic stem cell transplantation. Transl Med UniSa 5:7–13
Shane E (2003) Transplantation osteoporosis. In: Orwoll E, Bliziotes M (eds) Osteoporosis: pathophysiology and clinical management. Humana Press, Totowa, pp 537–567
Shane E, Epstein S (2001) Transplantation osteoporosis. Transplant Rev 15:11–32
Shane E, Rivas MDC, Silverberg SJ, Kim TS, Staron RB, Bilezikian JP (1993) Osteoporosis after cardiac transplantation. Am J Med 94:257–264
Shane E, Rivas M, Staron RB et al (1996a) Fracture after cardiac transplantation: a prospective longitudinal study. J Clin Endocrinol Metab 81:1740–1746
Shane E, Silverberg SJ, Donovan D et al (1996b) Osteoporosis in lung transplantation candidates with end stage pulmonary disease. Am J Med 101:262–269
Shane E, Mancini D, Aaronson K et al (1997a) Bone mass, vitamin D deficiency and hyperparathyroidism in congestive heart failure. Am J Med 103:197–207
Shane E, Rivas M, McMahon DJ et al (1997b) Bone loss and turnover after cardiac transplantation. J Clin Endocrinol Metab 82:1497–1506
Shane E, Rodino MA, McMahon DJ et al (1998) Prevention of bone loss after heart transplantation with antiresorptive therapy: a pilot study. J Heart Lung Transplant 17:1089–1096
Shane E, Papadopoulos A, Staron RB et al (1999) Bone loss and fracture after lung transplantation. Transplantation 68:220–227
Shane E, Addesso V, Namerow PB et al (2004) Alendronate versus calcitriol for the prevention of bone loss after cardiac transplantation. N Engl J Med 350:767–776
Shane E, Cohen A, Stein EM et al (2012) Zoledronic acid versus alendronate for the prevention of bone loss after heart or liver transplantation. J Clin Endocrinol Metab 97:4481–4490
Shi C, Huang P, Kang H et al (2015a) Glucocorticoid inhibits cell proliferation in differentiating osteoblasts by microRNA-199a targeting of WNT signaling. J Mol Endocrinol 54:325–337
Shi J, Wang L, Zhang H et al (2015b) Glucocorticoids: dose-related effects on osteoclast formation and function via reactive oxygen species and autophagy. Bone 79:222–232
Shimizu C, Fujita T, Fuke Y et al (2013) Effects of cyclosporine on bone mineral density in patients with glucocorticoid-dependent nephrotic syndrome in remission. Int Urol Nephrol 45:803–808
Smallwood GA, Burns D, Fasola CG, Steiber AC, Heffron TG (2005) Relationship between immunosuppression and osteoporosis in an outpatient liver transplant clinic. Transplant Proc 37:1910–1911
Smerud KT, Dolgos S, Olsen IC et al (2012) A 1-year randomized, double-blind, placebo-controlled study of intravenous ibandronate on bone loss following renal transplantation. Am J Transplant 12:3316–3325
Spira A, Gutierrez C, Chaparro C, Hutcheon MA, Chan CK (2000) Osteoporosis and lung transplantation: a prospective study. Chest 117:476–481
Sprague SM, Belozeroff V, Danese MD, Martin LP, Olgaard K (2008) Abnormal bone and mineral metabolism in kidney transplant patients--a review. Am J Nephrol 28:246–253
Stein EM, Shane E (2011) Vitamin D in organ transplantation. Osteoporos Int 22:2107–2118
Stein B, Halloran P, Reinhardt T et al (1991) Cyclosporin A increases synthesis of 1,25 dihydroxyvitamin D3 in the rat and mouse. Endocrinology 128:1369–1373
Stein EM, Cohen A, Freeby M et al (2009) Severe vitamin D deficiency among heart and liver transplant recipients. Clin Transplant 23:861–865
Stempfle HU, Werner C, Echtler S et al (1998) Rapid trabecular bone loss after cardiac transplantation using FK506 (tacrolimus)-based immunosuppression. Transplant Proc 30:1132–1133
Sukumaran Nair S, Lenihan CR, Montez-Rath ME, Lowenberg DW, Chertow GM, Winkelmayer WC (2014) Temporal trends in the incidence, treatment and outcomes of hip fracture after first kidney transplantation in the United States. Am J Transplant 14:943–951
Sun L, Blair HC, Peng Y et al (2005) Calcineurin regulates bone formation by the osteoblast. Proc Natl Acad Sci U S A 102:17130–17135
Sun L, Peng Y, Zaidi N et al (2007) Evidence that calcineurin is required for the genesis of bone-resorbing osteoclasts. Am J Physiol Renal Physiol 292:F285–F291
Tamler R, Epstein S (2006) Nonsteroid immune modulators and bone disease. Ann N Y Acad Sci 1068:284–296
Tanaci N, Karakose H, Guvener N, Tutuncu NB, Colak T, Haberal M (2003) Influence of 1,25-dihydroxyvitamin D3 as an immunomodulator in renal transplant recipients: a retrospective cohort study. Transplant Proc 35:2885–2887
Tauchmanova L, Serio B, Del Puente A et al (2002) Long-lasting bone damage detected by dual-energy x-ray absorptiometry, phalangeal osteosonogrammetry, and in vitro growth of marrow stromal cells after allogeneic stem cell transplantation. J Clin Endocrinol Metab 87:5058–5065
Tauchmanova L, Selleri C, Esposito M et al (2003) Beneficial treatment with risedronate in long-term survivors after allogeneic stem cell transplantation for hematological malignancies. Osteoporos Int 14:1013–1019
Tauchmanova L, Ricci P, Serio B et al (2005) Short-term zoledronic acid treatment increases bone mineral density and marrow clonogenic fibroblast progenitors after allogeneic stem cell transplantation. J Clin Endocrinol Metab 90:627–634
Techawathanawanna N, Avihingsanon Y, Praditpornsilpa K et al (2005) The prevalence and risk factors of osteoporosis in Thai renal-transplant patients. J Med Assoc Thai 88(Suppl 4):S103–S109
ter Meulen CG, van Riemsdijk I, Hene RJ et al (2004) No important influence of limited steroid exposure on bone mass during the first year after renal transplantation: a prospective, randomized, multicenter study. Transplantation 78:101–106
Thiebaud D, Krieg M, Gillard-Berguer D, Jaquet A, Goy JJ, Burckhardt P (1996) Cyclosporine induces high turnover and may contribute to bone loss after heart transplantation. Eur J Clin Invest 26:549–555
Toro J, Gentil MA, Garcia R et al (2005) Alendronate in kidney transplant patients: a single-center experience. Transplant Proc 37:1471–1472
Torregrosa JV, Campistol JM, Montesinos M et al (1995) Factors involved in the loss of bone mineral density after renal transplantation. Transplant Proc 27:2224–2225
Torregrosa JV, Fuster D, Pedroso S et al (2007) Weekly risedronate in kidney transplant patients with osteopenia. Transpl Int 20:708–711
Torregrosa JV, Fuster D, Monegal A et al (2011) Efficacy of low doses of pamidronate in osteopenic patients administered in the early post-renal transplant. Osteoporos Int 22:281–287
Torres A, Garcia S, Gomez A et al (2004) Treatment with intermittent calcitriol and calcium reduces bone loss after renal transplantation. Kidney Int 65:705–712
Tripathi SS, Gibney EM, Gehr TW, King AL, Beckman MJ (2008) High prevalence of vitamin D deficiency in African American kidney transplant recipients. Transplantation 85:767–770
Trombetti A, Gerbase MW, Spiliopoulos A, Slosman DO, Nicod LP, Rizzoli R (2000) Bone mineral density in lung-transplant recipients before and after graft: prevention of lumbar spine post-transplantation-accelerated bone loss by pamidronate. J Heart Lung Transplant 19:736–743
Tschopp O, Schmid C, Speich R, Seifert B, Russi EW, Boehler A (2006) Pretransplantation bone disease in patients with primary pulmonary hypertension. Chest 129:1002–1008
Uyar M, Sezer S, Arat Z, Elsurer R, Ozdemir FN, Haberal M (2006) 1,25-dihydroxyvitamin D(3) therapy is protective for renal function and prevents hyperparathyroidism in renal allograft recipients. Transplant Proc 38:2069–2073
Valero M, Loinaz C, Larrodera L, Leon M, Morena E, Hawkins F (1995) Calcitonin and bisphosphonate treatment in bone loss after liver transplantation. Calcif Tissue Int 57:15–19
Valimaki MJ, Kinnunen K, Tahtela R et al (1999a) A prospective study of bone loss and turnover after cardiac transplantation: effect of calcium supplementation with or without calcitonin. Osteoporos Int 10:128–136
Valimaki M, Kinnunen K, Volin L et al (1999b) A prospective study of bone loss and turnover after allogeneic bone marrow transplantation: effect of calcium supplementation with or without calcitonin. Bone Marrow Transplant 23:355–361
Van Cleemput J, Daenen W, Nijs J, Geusens P, Dequeker J, Vanhaecke J (1995) Timing and quantification of bone loss in cardiac transplant recipients. Transpl Int 8:196–200
van den Ham EC, Kooman JP, Christiaans ML, van Hooff JP (2003) The influence of early steroid withdrawal on body composition and bone mineral density in renal transplantation patients. Transpl Int 16:82–87
van Staa TP (2006) The pathogenesis, epidemiology and management of glucocorticoid-induced osteoporosis. Calcif Tissue Int 79:129–137
Van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C (2000) Use of oral corticosteroids and risk of fractures. J Bone Miner Res 15:993–1000
Vedi J, Greer S, Skingle S et al (1999) Mechanism of bone loss after liver transplantation: a histomorphometric analysis. J Bone Miner Res 14:281–287
Venu M, Martin E, Saeian K, Gawrieh S (2013) High prevalence of vitamin A deficiency and vitamin D deficiency in patients evaluated for liver transplantation. Liver Transpl 19:627–633
Wagner D, Amrein K, Dimai HP et al (2012) Ibandronate and calcitriol reduces fracture risk, reverses bone loss, and normalizes bone turnover after LTX. Transplantation 93:331–336
Walsh SB, Altmann P, Pattison J et al (2009) Effect of pamidronate on bone loss after kidney transplantation: a randomized trial. Am J Kidney Dis 53:856–865
Wang TK, O’Sullivan S, Gamble GD, Ruygrok PN (2013) Bone density in heart or lung transplant recipients – a longitudinal study. Transplant Proc 45:2357–2365
Wang Z, Han Z, Tao J et al (2014) Clinical efficacy and safety of pamidronate therapy on bone mass density in early post-renal transplant period: a meta-analysis of randomized controlled trials. PLoS One 9:e108106
Watson RG, Coulton L, Kanis JA et al (1990) Circulating osteocalcin in primary biliary cirrhosis following liver transplantation and during treatment with cyclosporine. J Hepatol 11:354–358
Wei R, Christakos S (2015) Mechanisms underlying the regulation of innate and adaptive immunity by vitamin D. Nutrients 7:8251–8260
Weinstein R, Jilka R, Parfitt M, Manolagas S (1998) Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids: potential mechanisms of their deleterious effects on bone. J Clin Invest 102:274–282
Westendorf JJ, Kahler RA, Schroeder TM (2004) Wnt signaling in osteoblasts and bone diseases. Gene 341:19–39
Withold W, Wolf H, Kollbach S, Heyll A, Schneider W, Reinauer H (1996) Monitoring of bone metabolism after bone marrow transplantation by measuring two different markers of bone turnover. Eur J Clin Chem Clin Biochem 34:193–197
Woodle ES, First MR, Pirsch J, Shihab F, Gaber AO, Van Veldhuisen P (2008) A prospective, randomized, double-blind, placebo-controlled multicenter trial comparing early (7 day) corticosteroid cessation versus long-term, low-dose corticosteroid therapy. Ann Surg 248:564–577
Yamamoto S, Suzuki A, Sasaki H et al (2013) Oral alendronate can suppress bone turnover but not fracture in kidney transplantation recipients with hyperparathyroidism and chronic kidney disease. J Bone Miner Metab 31:116–122
Yao S, McCarthy PL, Dunford LM et al (2008) High prevalence of early-onset osteopenia/osteoporosis after allogeneic stem cell transplantation and improvement after bisphosphonate therapy. Bone Marrow Transplant 41:393–398
Yu TM, Lin CL, Chang SN, Sung FC, Huang ST, Kao CH (2014) Osteoporosis and fractures after solid organ transplantation: a nationwide population-based cohort study. Mayo Clin Proc 89:888–895
Yun YS, Kim BJ, Hong TW, Lee CG, Kim MJ (1996) Changes of bone metabolism indices in patients receiving immunosuppressive therapy including low doses of steroids after transplantation. Transplant Proc 28:1561–1564
Zahner M, Teraz F, Pacifici R (1997) T cells mediate a stimulatory effect of cyclosporine A on human osteoclastogenesis while immature osteoclast precursors are directly regulated by glucocorticoids. J Bone Miner Res 12(Suppl 1):198
Zhang AB, Zheng SS, Jia CK, Wang Y (2003) Effect of 1,25-dihydroxyvitamin D3 on preventing allograft from acute rejection following rat orthotopic liver transplantation. World J Gastroenterol 9:1067–1071
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Furst, J., Shane, E. (2016). Osteoporosis in Organ Transplant Patients. In: Pietschmann, P. (eds) Principles of Osteoimmunology. Springer, Cham. https://doi.org/10.1007/978-3-319-34238-2_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-34238-2_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-34236-8
Online ISBN: 978-3-319-34238-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)