Abstract
Pesticides are chemical agents used to destroy or control pests, both in agriculture and in public health. Despite the beneficial effects associated with the usage of them, these chemicals may cause adverse effects to humans and to the nature. In addition, many pesticides are persistent and may therefore bioaccumulate in the environment; also some of them are important carcinogens and mutagens. In the world, alarming levels of pesticides have been detected in air, water, soil, as well as in foods and biological materials. Because of the special character as sink and source of contaminants soil is a critical medium, and as an environmental contaminant that comes into contact with soil intensively, pesticides are one of the important issues of environmental soil forensics. The different classes and wide range of pesticides and environmental mediums containing them have made essential the development of sensitive and current methods for the analysis of pesticide residues for environmental monitoring and forensic investigations. This chapter describes pesticides, historical background of pesticide usage, pesticides classification, environmental impacts and fate of pesticides, misuse and overuse of them, and provides a general brief overview on the soil sampling and pre-treatment, the basic principles of the conventional and also modern extraction approaches (including their advantages and disadvantages), and the chromatographic-based determination techniques used for pesticide residue analysis in soil.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Pesticide Residue
- Supercritical Fluid Extraction
- Accelerate Solvent Extraction
- Pesticide Residue Analysis
- Carbamic Acid
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
The preservation of the environment and human health from exposure to persistent organic pollutant is nowadays a priority objective in the whole world. In this sense, pesticides constitute a very important group of target compounds owing to their persistence, bioaccumulation, toxicity, long-range environmental transport ability, and unavoidable usage. Because of that pesticides are of concern to both scientists and environmental quality managers or policy makers (Richter et al. 2003; Li et al. 2011).
As the presence of trace amounts of pesticide residues could be potential health hazards, UN organization has formed specialized groups: World Health Organization (WHO) and Food Agriculture Organization (FAO), with the aim to establish restrictive measures to protect the environment against pollution. These organizations and their experts groups on annual meetings summarize international achievements in pesticides domain, establish legislation and make recommendations obligating member states to act in accordance with international standards (Durovic and Dordevic 2011).
Since World War II, it has been impossible to imagine agriculture without the use of pesticides. Pesticides and their metabolites can be found everywhere: in fresh water, groundwater, soil, bottom sediments, food and even faraway oceans (den Hond et al. 2003). Among them soil has a different significance. Because, soil acts as a sink/receptor of the effects of human activities or environmental phenomena and it is an interface between earth, air and water, and additionally hosts most of the biosphere. Therefore, any contamination also affects other environmental media and ecosystems. Also it should not be forgotten that soil is considered as a non-renewable resource because of extremely low formation process (Commission of the European Communities 2006). Based on these reasons, the 6th Environmental Action Programme, published by the European Commission (EC) in 2001, established the basis for further actions to protect soil against adverse impacts on a European level. For this purpose, in 2002 a communication from the EC to the Council and the European Parliament, entitled: “Towards a Thematic Strategy for Soil Protection”, was developed and ratified by the 15 ministers of environment of the European Union in 2002. The Soil Thematic Strategy brings soil to a higher level of importance for water managers, policy makers and researchers (Blum et al. 2004).
Development of environmental regulations over the past few decades led to the need for analytical methods that determine qualitatively and quantitatively pesticides in the environment. This need is critical for forensic sciences that require sensitive and selective analytical methods to be useful in litigation (Wait 2000). Originally, the purpose of pesticide laws and regulations was to protect consumers. But, the focus now has shifted to the protection of health and the environment. The determination of pesticide residues is a requirement to support the enforcement of legislation, ensure trading compliance, conduct monitoring residue programs in environmental samples, and study their mode of action and movement within the environment (US EPA 2012a; Pico et al. 2004).
The use of pesticides is still increasing and since soil monitoring plays an important part in the assessment of impacts on environmental quality as well as forensic sciences, pesticide residues in soil continue to be studied more than any other environmental contaminant. This chapter aims to provide a general brief overview of pesticides, their environmental impacts and the main features of pesticide residue analysis in soil.
2 Pesticides
According to the Food and Agriculture Organization (FAO) pesticide means any substance or mixture of substances intended for preventing, destroying or controlling any pest, including vectors of human or animal disease, unwanted species of plants or animals causing harm during or otherwise interfering with the production, processing, storage, transport or marketing of food, agricultural commodities, wood and wood products or animal feedstuffs, or substances which may be administered to animals for the control of insects, arachnids or other pests in or on their bodies (FAO 2002).
There are 920 active ingredients used as pesticides worldwide, mostly in agriculture, and they are currently formulated in thousands of different commercial products (MacBean 2012).
2.1 Historical Background of Pesticides Usage
Since before 2000 BC, humans have used pesticides to protect their crops. However, the use of modern pesticides in agriculture and public health is dated back to the after World War II. The first generation pesticides were highly toxic compounds, such as arsenic, mercury, lead, and hydrogen cyanide. The second-generation pesticides included synthetic organic compounds. The first important synthetic organic pesticide was an organochlorine: dichlorodiphenyltrichloroethane (DDT). DDT was discovered in 1939 by a Swiss chemist Paul Muller. In its early days, it was hailed as a miracle because of its effectiveness against a wide range of insects, persistence, low cost and easiness of production. During the 1940s manufacturers began to produce large amounts of synthetic pesticides and their use became widespread. Consequently, in 1948, Dr. Paul Muller won the Nobel Prize in Medicine for discovering its insecticidal properties (Muir 2012).
However, even though the governments, universities and the public were hailing DDT as a miracle, by the mid-1940s some toxicological problems associated with it were being reported. In 1962, Rachel Carson published her best selling book “Silent Spring”. In this book, she alerted the public to the potential problems of pesticide misuse, and predicted massive destruction of the planet’s fragile ecosystems unless more was done to halt what she called the “rain of chemicals”. Afterwards, public confidence in pesticide use was shaken and the modern environmental movement started (Jarman and Ballschmiter 2012).
These concerns, and the resulting public outcry prompted the US Environmental Protection Agency (EPA) to cancel the registration of DDT in the US in 1972. Research activities concentrated on finding new pesticides, which have greater selectivity and better environmental and toxicological profiles. Organochlorines were replaced by organophosphates and carbamates by 1975. Then, pyrethroids have become the dominant insecticides (Unsworth 2010).
In 2004, the Stockholm Convention on Persistent Organic Pollutants (POPs), an international environmental treaty that aims to eliminate or restrict the production and use of POPs entered into force. At that time, the restricted compounds included nine POC pesticides: aldrin, chlordane, dieldrin, endrin, heptachlor, hexachlorobenzen, mirex, toxaphene and DDT. In 2009, α-hexachlorocyclohexane (HCH), β-HCH and γ-HCH were added to the restricted list (http://chm.pops.int).
The early 1990s a new kinds of pesticide entered the European market. The group of these pesticides are called neonicotinoids and presented as “modern”. Initially neonicotinoids were praised for their low-toxicity to many beneficial insects, including bees; however this claim has come into question. Since about 2006 there has been a world-wide dramatic rise in the number of hive losses and a reduction of wild bees. Recent research has suggested a potential toxicity to bees and other beneficial insects through low levels of contamination of nectar and pollen with neonicotinoid insecticides used in agriculture (Goulson 2013). Eventually, in 2013 three of them (clothianidin, imidacloprid and thiametoxam) have been temporarily banned by the European Commission, based on the growing scientific evidence regarding the negative effects they have on bees (Di Prisco et al. 2013).
Today, due to the adverse impact of chemical pesticides, there was resurgence in academic and industrial research related to biopesticide development (Fountain and Wratten 2013). And with the rapid expansion of organic agriculture during the past decade, adoption rates have rapidly increased. Biopesticides offer more sustainable solution to pest control than synthetic alternatives but still only make up a small percentage of pest control products (Glare et al. 2012). Also, limited scientific literature is available on the use and environmental impact of them and serious questions remain about the safety of biopesticide products from both a human and ecosystem health standpoint. Current regulations do not go nearly far enough in evaluating systemic broader impacts of biopesticides (Romero-Gonzalez et al. 2011; Chandler et al. 2008).
2.2 Classification of Pesticides
Pesticides can be classified or grouped in many different ways; according to the pests they control, their mode of action or their chemical structure.
According to the type of pest they control, pesticides are named after the name of target pest group as shown in Table 11.1.
Under the classification that according to the mode of action, pesticides are classified based on how they work. Contact pesticides generally control a pest as a result of direct contact. They do not penetrate plant tissues. On the other side, systemic pesticides are pesticides, which are absorbed by plants or animals and transported to untreated tissues. Systemic pesticides penetrate the plant tissues and move through the leaves, stems or roots. Stomach poisons kill animal pests after ingestion and so they have to be eaten. Fumigants are chemicals that are applied as toxic gas or as a solid or liquid which forms a toxic gas. The gas penetrates cracks and crevices of structures or soil or the spaces between products stored in containers and kill pests (Zacharia 2011).
Another way of classification is using their active ingredient. The chemical classification at the same time gives information about physical and chemical properties of pesticides so more useful for researchers. According to this, major chemical groups are organoclorines, organophosphates, carbamates and pyrethriods.
Organochlorine pesticides were commonly used in the past in agriculture and public health as insecticides, but many have been removed from the market due to their health and environmental effects, and their persistence (e.g. DDT and chlordane). Organoclorines act as central nervous system disruptors. Furthermore, due to their tendency to accumulate in fatty tissues of organisms they can stay in the body for a long time (US EPA 2012b).
Organophosphate pesticides affect the nervous system by disrupting the acetylcholinesterase enzyme (AChE) that regulates acetylcholine (a neurotransmitter) and stops nerve transmission. Most organophosphates are insecticides. They were developed during the early nineteenth century, but their effects on insects, which are similar to their effects on humans, were discovered in 1932. Organophosphates are efficiently absorbed by inhalation, ingestion, and skin penetration. They are highly toxic to bees, wildlife, and humans. Commonly used organophosphates have included malathion, parathion, chlorpyrifos and diaznon (Gupta et al. 2011; US EPA 2012b).
Carbamate pesticides are derivatives of carbamic acid. The mode of action of carbamates is very similar to that of the organophosphates as they suppress AChE. However, they differ in action from the organophosphate compounds in that the inhibitory effect on cholinesterase is brief. Thus, even though organophosphates inhibit AChE irreversibly, whereas carbamates inhibit AChE reversibly. They are relatively unstable compounds that break down in the environment within weeks or months. Some of the common used carbamates include aldicarb, carbofuran and carbaryl (Gupta et al. 2011).
The last major chemical group, pyrethroid pesticides, are synthetic derivatives of naturally occurring pyrethrins which are obtain from pyrethrum produced by chrysanthemum flowers. They have been modified to increase their stability in the environment. They act as contact poisons, affecting the insect’s nervous system but they are not cholinesterase inhibitors like organophosphates or carbamates. Their primary mode of action is inhibition of voltage-sensitive sodium channels. Pyrethroids have relatively low toxicity in humans but they are highly toxic to fish and aquatic invertebrates. They have an extremely low pesticide movement rating because of their tendency to bind the soil particles. The most widely used synthetic pyrethroids include cypermethrin, permethrin and deltamethrin (Acikkol et al. 2012; Zacharia 2011).
2.3 Environmental Impact of Pesticides
Despite the beneficial effects of pesticides, which include crop protection, preservation of food and materials and prevention of vector born diseases, their extensive applications have raised serious concerns about entire environment in general and the health of humans and over the years, more and more problems associated with the use of pesticides have shown up (Muir 2012).
In fact, it has been estimated that less than 0.1 % of the pesticide that applied to crops actually reaches the target pest; the rest enters environment gratuitously and contaminating soil, sediment, water, and air, where it can affect non-target organisms (Arias-Esteves et al. 2008). Besides being toxic to the pests they are intended to control, pesticides are also toxic to non-target species including different birds, fish species, animals, and humans (Henny et al. 1985; Stroud 1998; Jett 2011). Also, most of the pesticide residues were found to accumulate in human and biological food chain (Dewailly et al. 2000). Moreover, many studies presented that the low-level long-term exposure to pesticides can result in chronic effects like cancer and other genetic disorders, liver and kidney damage, disorders of the nervous system, damage to the immune system, endocrine disruption, and birth defects (Fortes and Aprea 2011; Mostafalou and Abdollahi 2013; Landau-Ossondo et al. 2009).
Furthermore, pesticides kill not only the pests but also the natural enemies of these pests. That means natural control mechanisms are disrupted and it allows the pest populations to rapidly build up again to levels that can cause serious crop damage (Hardin et al. 1995).
Also eventually, after repeated and more intensive use of the same pesticide to the same pest population, the pesticide becomes ineffective. Accordingly, an increasing number of fungi, weeds and insects have become resistant to the action of individual as well as groups of chemically related active ingredients. Indeed, some observers have noted a preserve effect of the general use of pesticides, namely that crop losses due to insect invasion have actually increased with increasing pesticide use (den Hond et al. 2003).
2.4 Environmental Fate and Persistence of Pesticides in Soil
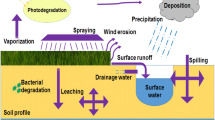
When a pesticide is used in the environment, it becomes distributed among four major compartments: soil, water, air, and biota. In addition, part of it goes to the air or to surface waters, due to emission or drift. Once on the target site, the pesticide may drain into surface waters or volatilize into the air. From the air it may deposit on humans, wildlife or plants or on the soil. From the animals or plants where it was applied the pesticide may leak into groundwater. Pesticides in surface water may go into aquatic organisms, and by sedimentation into other organisms that remain in the sediment (Linde 1994).
Careful consideration of these fate processes and their interactions is necessary to evaluate the risk to groundwater and surface water. All pesticides in groundwater, and most residues present in surface water enter by way of the soil, through surface runoff and leaching. In the case of pesticides presence in soil is mainly product of crop protection, reaching it in many ways: direct treatments, by aerial spraying, and vegetable waste after harvest (Arias-Estevez et al. 2008; Agrawal et al. 2010).
Soil is generally defined as the top layer of the earth’s crust, formed by mineral particles, organic matter, water, air, and living organisms. Over 320 major soil types have been identified in Europe and within each there are enormous variations in physical, chemical and biological properties (Commission of the European Communities 2006). The persistence and mobility of pesticides in soil depends on: soil factors (soil composition, soil chemistry, and microbial activity); pesticide properties (water solubility, vapor pressure, and the molecule’s susceptibility to chemical or microbial alteration or degradation); climatic factors (moisture, temperature, and sunlight); site conditions (elevation, slope, aspect, geographical conditions, presence of pollutants, tillage, irrigation, etc.); and application features (method, time, frequency, and amount) (Curran 1998; Kerle et al. 1994; Hao et al. 2008).
2.5 Misuse and Overuse of Pesticides
In the regulation of pesticides application, government bodies have an important and major role because both producers and users are not likely to limit themselves in the sales and use of pesticides. The weak enforcement of laws and regulations governing pesticide use results in misuse and overuse of pesticides, and consequently, increased environmental contamination and human exposure (Abhilash and Singh 2009; Grovermann et al. 2013).
Surveys show that farmers have overused pesticides in many developing countries including Turkey, Thailand, Bangladesh etc. as many farmers believe that the level of protection derived from pesticides is proportional to the amount applied. Further, they tend to mix more than two types of pesticides that should not be mixed (Abhilash and Singh 2009; Ali et al. 2012; Demircan and Yılmaz 2005). The use of unprescribed pesticides in inappropriate doses is not only disturbing the soil conditions but also destroying the healthy pool of biocontrol agents that normally coexist with the vegetation and affecting whole ecosystem. Therefore, after application of agrochemicals should be monitor closely by government authority and experts to minimize the health hazard towards human and environment (Ali et al. 2012).
Another problem beside the misuses and overuses is illegal use of pesticides. Despite the prohibition process and public announcements regarding the bans, numerous reports reveal continued widespread use of banned pesticides even today. Because of the effectiveness in controlling pests and low cost, they are still in high demand from farmers (Panuwet et al. 2012; Abhilash and Singh 2009; Rahman 2013).
Governments should emphasize on the issue of misuses and improper sale of pesticides among suppliers and farmers. These criminal activities must be observed in order to preserve the safety of consumer, human body, animal, growing crops and the conservation of ecosystem (Ali et al. 2012).
A vital component of investigating pesticide misuse is the collection of environmental forensic samples such as soil, air, water or any other medium that come into contact with a pesticide. The analysis for pesticide residue is an important aspect of many investigations. Monitoring of pesticide residues and enforcing the MRLs (maximum residue limits) are challenging for the responsible regulatory agencies particularly when relying on the use of non-quantitative and sensitive techniques. So, it is important to establish proper protocols for sampling and use sensitive and selective techniques for monitoring to ensure enforcement actions against companies and other individuals (Saxton and Engel 2005; Panuwet et al. 2012).
3 Analytical Procedures
Since soil is an extremely complex and variable medium, the analysis of pesticides in soil is a complicated procedure involving many steps; field sampling, soil pre-treatment, extraction, clean up (if necessary), and determination.
3.1 Soil Sampling
The first step in the process is to determine how soil samples would be taken in the field, packed, and transported to laboratory. Collection of environmental forensic sample such as soil is a vital component of investigating pesticide misuse (Saxton and Engel 2005).
The main objective in any soil sampling strategy is to obtain a representative portion of the sample. Because of its heterogenic structure soil sampling is very difficult and an effective sampling strategy must be include sample location, sample volume, sample number, sampling depth, sampling approach (random, systematic, judgmental or a combination of these), sample handling, transport and storage. Besides, it is necessary to collect proper blank samples from the same site as the samples. Blank samples are matrices that have no measurable amount of the analyte of interest so they must be free of the pesticide and all conditions will be carried out as the actual samples (Dean 1998).
After sampling, since probably soil samples are analyzed after some delay, care should be taken to preserve them from contamination and degradation, both during transportation and storage.
3.2 Soil Pre-treatment
Collected soil samples are commonly dried, ground and sieved through a mesh. Soil drying is necessary to limit microbial growth and other soil processes to provide protection of samples, and also to enable better homogenisation. The most used method in drying is a thin layer of soil air-dried at room temperature and protected from direct sunlight. Afterwards dry soil samples are grounded and passed through a sieve (the conventional 2 mm sieve has generally been accepted) (Theocharopoulos et al. 2001). Grounding allows the homogenisation and analyses of the soil sample to be carried out under standard conditions with the most physico-chemically active fine particles. The sieving will mostly reduce the fraction of the soil that is largely chemically inert such as coarse-grained, feldspar and carbonate minerals, and will increase the components active in pollutant enrichment. After sieving, obtained fully homogenized sample mechanically or manually mixing performed and homogenized powder is stored in brown glass bottles until chemical analysis (Andreu and Pico 2004; Theocharopoulos et al. 2004).
3.3 Extraction
Extraction aims to remove as much as possible of the analyte from the matrix, so it is important to select the appropriate extraction method and optimize the extraction parameters. The sample extraction step, which takes most of the total analysis time, is still the weakest link and the time-determining step in the whole analytical step and also the main reason of errors and differences between laboratories. Ideally, a sample extraction should be rapid, simple, low cost, environmentally friendly and provide clean extracts. For the isolation of pesticides from soil samples various extraction methods have been proposed.
3.3.1 Liquid-Solid Extraction
Conventional methodology frequently involves liquid-solid extraction (LSE). LSE can be sub-divided into approaches that utilize heat and those do not. The use of heat is typified by Soxhlet extraction and methods which no heat is added, but utilise some form of agitation i.e. shaking or sonication are shake-flask and ultrasonic solvent extraction (USE) (Dean 1998).
Baron von Soxhlet introduced Soxhlet extraction in the mid-nineteenth century. Soxhlet extraction normally requires large volumes (up to 150 ml per sample) of solvent and takes time 6–24 h. Also, only one sample can be extracted per set of apparatus. On the other hand, shake-flask and ultrasonic extraction require smaller volumes of organic solvent (20–100 ml) and are relatively fast (10–60 min). Besides, they allow multiple extractions to be carried out by the use of the simple laboratory mechanical shakers and ultrasonic bath or prob (Dean and Xiong 2000; Pozo et al. 2001; Babic et al. 1998).
These methods are inexpensive and easy to handle but they are laborious, time-consuming, requires large volumes of organic solvents and subject to problems arising from evaporation of large volumes of solvent and loss of some analyte quantity. As a result, modern sample extraction procedures based on instrumental techniques have been developed and applied to overcome the disadvantages of the traditional approaches (Fuentes et al. 2007).
3.3.2 Instrumental Techniques
The first of these new types of extraction techniques appeared almost 20 years ago in the form of supercritical fluid extraction (SFE). This technique makes use of the gas-like and liquid-like properties of a supercritical fluid (a fluid is any substance above its critical temperature and pressure), typically carbon dioxide, to extract organic analytes from solid environmental matrices at temperatures >31.1 °C and 74.8 atm. Initial limitations of the technique centered around its inability to extract polar molecules. By using combinations of CO2 mixed with an organic modifier, e.g. methanol and acetone, it is possible to extract a range of molecules of different polarity (Forero-Mendieta et al. 2012). All SFE systems contain six basic components, namely the supply of high purity CO2, a supply of high purity organic modifier, the pumps, the oven for the extraction cell, the pressure outlet or restrictor, and the collection vessel. In general, SFE lasts less than 2 h and requires low solvent volumes (10–40 ml). Besides these, it does not allow multiple extractions and has high cost of the equipment (Dean 1998).
The second of the instrumental techniques is microwave-assisted extraction (MAE). The first use of MAE for the extraction of analytes with organic solvents appeared in 1986. MAE utilizes electromagnetic radiation to desorb desired components from the matrix. In MAE, organic solvent and the sample are subjected to radiation from a magnetron in sealed vessels. In order to heat a solvent, part of it must be polar with high dielectric constant to absorb microwave energy efficiently, if it is not certain amount of water or a polar solvent must be added. MAE is a promising technique for soil samples, so in last years, applications of MAE for extracting pesticides from soil have increased rapidly (Paiga et al. 2008). For the optimization of the MAE procedures, several parameters such as volume and solvent composition, extraction temperature and time are usually studied. The high sample throughput (up to 14 vessels can be extracted simultaneously), need for minimum sample amount (2–5 g), low solvent consumption (10–40 ml), fast extraction, high level of automation and efficiency make this technique attractive (Dean and Xiong 2000; Durovic and Dordevic 2011). But due to its limited selectivity and simultaneous co-extraction of soil components together with the target analytes, it often requires a further clean-up step (Lesueur et al. 2008). Also, an alternative application of MAE using micellar media as extractans (MAME) to completely avoid the use of organic solvents has been reported and offers advantages, such as low toxicity and compatibility with aqueous-organic mobile phase in liquid chromatography (Padron-Sanz et al. 2005).
The final instrumental technique is pressurised liquid extraction (PLE), available commercially in the form of accelerated solvent extraction (ASE). This technique, which first appeared commercially in 1995, uses small amounts of water and organic solvents to sequentially extract analytes from the sample matrix under elevated temperature (up to 200 °C) and pressure (up to 20 MPa). ASE is an automated instrument capable of sequentially extracting up to 24 samples. A typical extraction time per sample is 12 min (Dean and Xiong 2000; Luo et al. 2010). The combination of high temperature and pressure results in better extraction efficiency, thus minimizing solvent use. For all that, high temperatures may lead to degradation of thermo labile analytes and also to the co-extraction of interfering species. Obvious ASE advantage is that it requires much less solvent and shorter extraction times than conventional techniques. Additionally, ASE is reduced both, waste levels and analysts exposure to harmful solvents. However, limited by high cost, its application is still not widespread (Durovic and Dordevic 2011).
Despite mentioned disadvantages related to conventional solvent extraction methods, they are still the most popular methods for routine analysis. To overcome the disadvantages of these methods, new approaches in pesticide residues analysis have appeared. In 2003, Anastassiades et al. developed a method for the multi-class, multi-residue extraction of pesticides in fruits and vegetables. This method was called QuEChERS, which stands for Quick, Easy, Cheap, Rugged and Safe, and it is based on dispersive solid phase extraction (dSPE). In dSPE analytes are extracted with an aqueous miscible solvent with a high amount of salt (MgSO4) and/or buffering agents, in order to induce liquid phase separation and stabilize acid and base pesticides (Pinto et al. 2010). In the recent studies, the QuEChERS method applied for the determination of pesticides from soil successfully (Lesueur et al. 2008; Drozzdzynski and Kowalska 2009). The QuEChERS advantages are the high recovery, accurate results, and low solvent and glassware usage. Besides, the main QuEChERS disadvantage is requirement of concentration of the final extract to provide the necessary sensitivity (Rouviere et al. 2012).
3.3.3 Miniaturized Techniques
Modern trends in analytical chemistry are towards the simplification and miniaturization of sample preparation, as well as the minimization of organic solvent used. In view of this aspect, several newer miniaturized procedures are being developed in order to reduce the analysis step, increase the sample throughput and to improve the quality and the sensitivity of analytical methods (Lambropoulou and Albanis 2007). Liquid-phase micro-extraction (LPME), solid-phase microextraction (SPME), and matrix solid-phase dispersion (MSPD) are some of the most representative procedures for pesticide analysis from soil.
One of the emerging techniques in this area is liquid-phase micro-extraction (LPME). LPME involves the use of a small amount (3 μl) of organic solvent impregnated in a hallow fiber membrane, which is attached to the needle of a conventional gas chromatography (GC) syringe. It is quick, inexpensive and can be automated but only a limited number of studies have performed on soil samples (Hou and Lee 2004; Lambropoulou and Albanis 2007).
Solid-phase microextraction (SPME) is a newly developed solvent-free analytical technology, which allows the simultaneous extraction and pre-concentration of analytes from a sample. It involves the use of a fiber coated with an extracting phase, that can be a liquid (polymer) or a solid (sorbent), which extracts different kinds of analytes (including both volatile and non-volatile) from different kinds of media (Möder et al. 1999). Several disadvantages related to fiber stability and sensitivity has been pointed out. Yet, only a few references on the application of SPME for the determination of pesticides in soil samples are available (Hernandez et al. 2000; Bouaid et al. 2001; Moreno et al. 2006). Recently, headspace SPME (HS-SPME) has also been used to determine pesticide compounds in soil. Sampling in the headspace presents a significant advantage in terms of selectivity because only volatile and semivolatile organic compounds can be released into the headspace (Doong and Liao 2001).
Matrix solid-phase dispersion (MSPD) is a relatively developed extraction–clean-up technique characterized by simplicity and sensitivity. In MSPD, extraction and clean-up are carried out in the same step, which can avoid the general disadvantages of other traditional methods, such as the use of a large amount of solvent and glassware, the laborious extraction procedure and the occurrence of troublesome emulsions (Li et al. 2002; Salemi et al. 2012).
These new techniques seem to provide good results but there are still few reports to establish their usefulness and to compare them with other techniques. Therefore, further study is required.
3.4 Clean-Up
Because of the complexity of the matrix, during the extraction step many interfering components (lipids, pigments or cholesterol and its derivatives) are co-extracted from soil samples together with target analytes. Clean-up stage requires removing these substances that could disturb determination and quantitation of target analytes (Shen et al. 2006).
There are several approaches for extract clean-up: liquid-liquid partitioning; solid liquid adsorption chromatography using long open columns packed with alumina, Florisil, ion-exchange resins, silica gel, and many silica-based sorbents; solid-phase extraction (SPE) on disposable cartridges packed with C18, NH2, or CN modified silica or graphitized carbon; thin layer chromatography; and gel permeation (GPC) chromatography (Yasin et al. 1996; Dabrowska et al. 2003; Andreu and Pico 2004).
Some other matrix components that falsify the results are non-organic components of the extract, such as elemental sulphur. They also should be eliminated in order to protect the column. To remove sulphur generally copper is used in different grain-size forms (Esteve-Turillas et al. 2004).
3.5 Determination
Most of the analytical methods for the single or multiresidue determination of pesticides in soil are based on chromatographic techniques; gas chromatography (GC), liquid chromatography (LC), and thin layer chromatography (TLC). Chromatography is based on separation and then identification and quantification of components in extracts. Separation is achieved by using differences in equilibrium constants of components between mobile phase (a liquid or gaseous) that tends to transport them and stationary phase (column or plate) that tends to retain them (Theocharopoulos et al. 2004; Chen and Wang 1996).
Pesticide residue analyses in soil are conducted often by GC with different detectors. Electron-capture detector (ECD) is specific for halogen containing compounds and is used to determine some of the organoclorine, organophosphorus, and pyrethroid pesticides (Sun et al. 2009; Ozcan et al. 2009; Wang et al. 2008). Nitrogen-phosphorus detection (NPD) is also used for many pesticides (Forero-Mendieta et al. 2012; EL-Saeid and AL-Dosari 2010). In addition, for the analysis of non-halogen containing pesticides flame ionization detection (FID) can be applied; however, detection limits are not sensitive enough for residue analysis (Naeeni et al. 2011).
Moreover, GC has been coupled to mass spectrometry (MS) to provide a highly sensitive detector, which also gives information on the molecular structure of the analytes and selective detectors have progressively been replaced by GC-MS, mainly using electron impact (EI) and chemical ionization (CI). Also in recent years, the use of ion-trap tandem MS (MS/MS) has allowed improvement in the selectivity and the sensitivity of GC-MS methods for analysis of pesticides in soil (Santos and Galceran 2002). Besides, GC is not suitable for thermo-labile, low volatility, and strongly polar compounds.
Vice versa LC is ideally suited for the analysis of polar compounds. In comparison with GC, LC has relatively low sensitivity. However, the development in the LC including the introduction of high-performance (HP) columns and the improvement of new detectors (UV, Fluorescence, and MS) have broadened the application of it in the pesticide residue analysis. HPLC does not have the same limitations of GC with regard to compounds of low volatility and low thermal stability (Theocharopoulos et al. 2004; Chen and Wang 1996). In the last years, there has been an increase of the scientific publications dealing with LC–MS and LC-MS/MS for the determination of pesticides in soil (Dagnac et al. 2005; Li et al. 2013). Reversed-phase LC is the technique most widely used, especially for acidic pesticides (Hogendoorn et al. 2001).
Furthermore, recent years, new active ingredients have been developed through more specific reactions. These new pesticides can be produced by the synthesis of more complex molecules, which normally cannot be analyzed by GC, but better respond to analysis by LC. The same holds true for their, usually even more polar, transformation /degradation products or metabolites (Pizzutti et al. 2007).
TLC is less widely used compared to GC and LC in recent years, due to the low detection limits. The development of modern, instrumantalized HPTLC, that perform the final determination by measuring the UV absorbance with TLC scanner, makes the TLC application more promising (Acikkol et al. 2012; Babic et al. 1998).
4 Conclusions
Due to intensive and widespread usage, pesticides residues have become an unavoidable part of the environment and soil is an important medium that is closely associated with humans and their health. As a result, the development of new analytical methods for the determination of pesticides in soil is currently a high interest research area.
Pesticides are extremely diverse with nearly a thousand active ingredients currently in use and comprise a great variety of compounds, mainly insecticides, herbicides and fungicides, as well as their metabolites, with extremely diverse physico-chemical characteristics and large differences in polarity, volatility and persistence. Moreover, newly developed pesticide products are being introduced in the market consistently. The monitoring of conventional priority pesticides, such as DDT, which have long been recognized as posing risks to human health and persistent in the environment, follows long established standards and certified methods. But sensitive and current analytical methods for environmental monitoring and forensic investigations are not available for all pesticides. The need for the detection of low levels of a wide variety of pesticide residues in soil samples both in individual and simultaneously, and on the other hand the complexity of the soil matrix (because of the strong diversity and heterogeneity) makes the development of efficient and reliable analytical methods quite a challenge.
References
Acikkol M, Semen S, Turkmen Z et al (2012) Determination of α-cypermethrin from soil by using HPTLC. JPC-J Planar Chromatogr-Mod TLC 25:48–53
Abhilash PC, Singh N (2009) Pesticide use and application: an Indian scenario. J Hazard Mater 165:1–12
Agrawal A, Pandey RS, Sharma B (2010) Water pollution with special reference to pesticide contamination in India. J Water Resour Prot 2:432–448
Ali A, Noah RM, Malik SA (2012) Legal implications on sales and purchase, uses and misuses of agro chemicals in smallholders’ agro production in Malaysia. Procedia Soc Behav Sci 68:156–163
Anastassiades M, Lehotay SJ, Stajnbaher D et al (2003) Fast and easy multiresidue method employing acetonitrile extraction/partitioning and ‘’Dispersive solid-phase extraction” for the determination of pesticide residues in produce. J AOAC Int 86:412–431
Andreu V, Pico Y (2004) Determination of pesticides and their degradation products in soil: critical review and comparison of methods. TrAC-Trend Anal Chem 23:772–789
Arias-Estevez M, Lopez-Periago E, Martinez-Carballo E et al (2008) The mobility and degradation of pesticides in soils and the pollution of groundwater resources. Agric Ecosyst Environ 123:247–260
Babic S, Petrovic M, Kastelan-Macan M (1998) Ultrasonic solvent extraction of pesticides from soil. J Chromatogr A 823:3–9
Blum WEH, Büsing J, Montanarella L (2004) Research needs in support of the European thematic strategy for soil protection. TrAC-Trends Anal Chem 23:680–685
Bouaid A, Ramos L, Gonzalez MJ et al (2001) Solid-phase microextraction method for the determination of atrazine and four organophosphorus pesticides in soil samples by gas chromatography. J Chromatogr A 939:13–21
Chandler D, Davidson G, Grant WP et al (2008) Microbial biopesticides for integrated crop management: an assessment of environmental and regulatory sustainability. Trends Food Sci Tech 19:275–283
Chen ZM, Wang YH (1996) Chromatographic methods for the determination of pyrethrin and pyrethroid pesticide residues in crops, foods and environmental samples. J Chromatogr A 754:367–395
Commission of the European Communities (2006) Thematic strategy for soil protection. Brussels, 22.9.2006 COM(2006)231 final
Curran WS (1998) Persistence of herbicides in soil, Agronomy facts 36. Penn State College of Agricultural Science, The Pennsylvania State University, Pennsylvania
Dabrowska H, Dabrowski L, Biziuk M et al (2003) Solid-phase extraction clean-up of soil and sediment extracts for the determination of various types of pollutants in a single run. J Chromatogr A 1003:29–42
Dagnac T, Bristeau S, Jeannot R et al (2005) Determination of chloroacetanilides, triazines and phenylureas and some of their metabolites in soils by pressurised liquid extraction, GC–MS/MS, LC–MS and LC–MS/MS. J Chromatogr A 1067:225–233
Dean JR (1998) Extraction methods for environmental analysis. Wiley, Chichester
Dean JR, Xiong G (2000) Extraction of organic pollutants from environmental matrices: selection of extraction technique. Trends Anal Chem 19:553–564
Demircan V, Yılmaz H (2005) The analysis of pesticide use in apple production in Isparta province in terms of economy and environmental sensitivity perspective (in Turkish). Ekoloji 14:15–25
den Hond F, Groenewegen P, van Straalen N (eds) (2003) Pesticides: problems, improvements, alternatives. Blackwell, London
Dewailly E, Ayotte P, Bruneau S et al (2000) Susceptibility to infections and immune status in inuit infants exposed to organochlorines. Environ Health Perspect 108:205–211
Di Prisco G, Cavaliere V, Annoscia D et al (2013) Neonicotinoid clothianidin adversely affects insect immunity and promotes replication of a viral pathogen in honey bees. PNAS 110(46):18466–18471
Doong RA, Liao PL (2001) Determination of organochlorine pesticides and their metabolites in soil samples using headspace solid-phase microextraction. J Chromatogr A 918:177–188
Drozzdzynski D, Kowalska J (2009) Rapid analysis of organic farming insecticides in soil and produce using ultra-performance liquid chromatography/tandem mass spectrometry. Anal Bioanal Chem 394:2241–2247
Durovic R, Dordevic T (2011) Modern extraction techniques for pesticide residues determination in plant and soil samples. In: Stoytcheva M (ed) Pesticides in the modern world – trends in pesticides analysis. InTech, Rijeka, Croatia, pp 221–246
EL-Saeid MH, AL-Dosari SA (2010) Monitoring of pesticide residues in Riyadh dates by SFE, MSE, SFC, and GC techniques. Arab J Chem 3(3):179–186
Esteve-Turrillas FA, Aman CS, Pastor A et al (2004) Microwave-assisted extraction of pyrethroid insecticides from soil. Anal Chim Acta 522:73–78
FAO (2002) International code of conduct on the distribution and use of pesticides. Food and Agriculture Organization of the United Nations, Rome
Forero-Mendieta JR, Castro-Vargas HI, Parada-Alfonso F et al (2012) Extraction of pesticides from soil using supercritical carbon dioxide added with methanol as co-solvent. J Supercrit Fluid 68:64–70
Fortes C, Aprea C (2011) Cancer risks from residential exposure to pesticides. In: Nriagu JO (ed) Encyclopedia of environmental health, vol 1. Elsevier, Burlington, pp 489–497
Fountain ED, Wratten SD (2013) Conservation biological control and biopesticides in agricultural? Reference module in earth systems and environmental sciences. doi:10.1016/B978-0-12-409548-9.00539-X
Fuentes E, Báez ME, Labra R (2007) Parameters affecting microwave-assisted extraction of organophosphorus pesticides from agricultural soil. J Chromatogr A 1169(1–2):40–46
Glare T, Caradus J, Gelernter W et al (2012) Have biopesticides come of age? Trends Biotechnol 30(5):250–258
Goulson D (2013) An overview of the environmental risks posed by neonicotinoid insecticides. J Appl Ecol 50:977–987
Grovermann C, Schreinemachers P, Berger T (2013) Quantifying pesticide overuse from farmer and societal points of view: an application to Thailand. Crop Prot 53:161–168
Gupta RC, Malik JK, Milatovic D (2011) Organophosphate and carbamate pesticides. In: Gupta RC (ed) Reproductive and developmental toxicology. Elsevier, London, pp 471–486
Hao H, Sun B, Zhao Z (2008) Effect of land use change from paddy to vegetable field on the residues of organochlorine pesticides in soils. Environ Pollut 156:1046–1052
Hardin MR, Benrey B, Coll M et al (1995) Arthropod pest resurgence: an overview of potential mechanisms. Crop Prot 14:3–18
Henny CJ, Blus LJ, Kolbe EJ et al (1985) Organophosphate insecticide (famphur) topically applied to cattle kills magpies and hawks. J Wildl Manage 49:648–658
Hernandez F, Beltran J, Lopez FJ et al (2000) Use of solid-phase microextraction for the quantitative determination of herbicides in soil and water samples. Anal Chem 15(72):2313–2322
Hogendoorn EA, Huls R, Dijkman E et al (2001) Microwave assisted solvent extraction and coupled-column reversed-phase liquid chromatography with UV detection: use of an analytical restricted-access-medium column for the efficient multi-residue analysis of acidic pesticides in soils. J Chromatogr A 938:23–33
Hou L, Lee HK (2004) Determination of pesticides in soil by liquid-phase micro-extraction, and gas chromatography–mass spectrometry. J Chromatogr A 1038:37–42
Jarman WM, Ballschmiter K (2012) From coal to DDT: the history of the development of the pesticide DDT from synthetic dyes till silent spring. Endeavour 36:131–142
Jett DA (2011) Neurotoxic pesticides and neurologic effects. Neurol Clin 29:667–677
Kerle EA, Jenkins JJ, Vogue PA (1994) Understanding pesticide persistence and mobility for groundwater and surface water protection. Oregon State University, Corvallis
Lambropoulou DA, Albanis TA (2007) Liquid-phase micro-extraction techniques in pesticide residue analysis. J Biochem Biophys Methdos 70:195–228
Landau-Ossondo M, Rabia N, Jos-Pelage J et al (2009) Why pesticides could be a common cause of prostate and breast cancers in the French Caribbean Island, Martinique. An overview on key mechanisms of pesticide-induced cancer. Biomed Pharmacother 63:383–395
Lesueur C, Gartner M, Mentler A et al (2008) Comparison of four extraction methods for the analysis of 24 pesticides in soil samples with gas chromatography–mass spectrometry and liquid chromatography–ion trap–mass spectrometry. Talanta 75:284–293
Li J, Lu Y, Shi Y et al (2011) Environmental pollution by persistent toxic substances and health risk in an industrial area of China. J Environ Sci 23:1359–1367
Li Y, Dong F, Liu X et al (2013) Development of a multi-residue enantiomeric analysis method for 9 pesticides in soil and water by chiral liquid chromatography/tandem mass spectrometry. J Hazard Mater 250–251:9–18
Li ZY, Zhang ZC, Zhou QL et al (2002) Fast and precise determination of phenthoate and its enantiomeric ratio in soil by the matrix solid-phase dispersion method and liquid chromatography. J Chromatogr A 977:17–25
Linde CD (1994) Physico-chemical properties and environmental fate of pesticides. Environmental Protection Agency, Sacramento
Luo L, Shao B, Zhang J (2010) Pressurized liquid extraction and cleanup procedure for the determination of pyrethroids in soils using gas chromatography/tandem mass spectrometry. Anal Sci 26:461–465
MacBean C (ed) (2012) The pesticide manual: a world compendium, 16th revised edn. British Crop Protection Council, Alton
Moreno DV, Ferrera ZS, Rodriguez JJS (2006) Microwave assisted micellar extraction coupled with solid phase microextraction for the determination of organochlorine pesticides in soil samples. Anal Chim Acta 571:51–57
Mostafalou S, Abdollahi M (2013) Pesticides and human chronic diseases: evidences, mechanisms, and perspectives. Toxicol Appl Pharm 268:157–177
Möder M, Popp P, Eisert R et al (1999) Determination of polar pesticides in soil by solid phase microextraction coupled to high-performance liquid chromatography electrospray/mass spectrometry. Fresenius J Anal Chem 363:680–685
Muir P (2012) A history of pesticide use. Oregon State University. http://people.oregonstate.edu/~muirp/pesthist.htm. Accessed 21 June 2012
Naeeni MH, Yamini Y, Rezaee M (2011) Combination of supercritical fluid extraction with dispersive liquid–liquid microextraction for extraction of organophosphoruspesticides from soil and marine sediment samples. J Supercrit Fluids 57:219–226
Ozcan S, Tor A, Aydin ME (2009) Application of miniaturised ultrasonic extraction to the analysis of organochlorine pesticides in soil. Anal Chim Acta 640:52–57
Padron-Sanz C, Halko R, Sosa-Ferrera Z et al (2005) Combination of microwave assisted micellar extraction and liquid chromatography for the determination of organophosphorous pesticides in soil samples. J Chromatogr A 1078:13–21
Paíga P, Morais S, Correia M et al (2008) A multiresidue method for the analysis of carbamate and urea pesticides from soils by microwave-assisted extraction and liquid chromatography with photodiode array detection. Anal Lett 41:1751–1772
Panuwet P, Siriwong W, Prapamontol T et al (2012) Agricultural pesticide management in Thailand: status and population health risk. Environ Sci Policy 17:72–81
Pico Y, Blasco C, Font G (2004) Environmental and food applications of LC–tandem mass spectrometry in pesticide-residue analysis: an overview. Mass Spectrom Rev 23:45–85
Pinto CG, Laespada MEF, Martín SH et al (2010) Simplified QuEChERS approach for the extraction of chlorinated compounds from soil samples. Talanta 81:385–391
Pizzuttia IR, de Kok A, Zanella R et al (2007) Method validation for the analysis of 169 pesticides in soya grain, without clean up, by liquid chromatography–tandem mass spectrometry using positive and negative electrospray ionization. J Chromatogr A 1142:123–136
Pozo O, Pitarch E, Sancho JV et al (2001) Determination of the herbicide 4-chloro-2-methylphenoxyacetic acid and its main metabolite, 4-chloro-2-methylphenol in water and soil by liquid chromatography-electrospray tandem mass spectrometry. J Chromatogr A 923:75–85
Rahman S (2013) Pesticide consumption and productivity and the potential of IPM in Bangladesh. Sci Total Environ 445–446:48–56
Richter P, Sepulveda B, Oliva R et al (2003) Screening and determination of pesticides in soil using continuous subcritical water extraction and gas chromatography–mass spectrometry. J Chromatogr A 994:169–177
Romero-Gonzalez R, Garrido Frenich A, Martínez Vidala JL et al (2011) Simultaneous determination of pesticides, biopesticides and mycotoxins in organic products applying a quick, easy, cheap, effective, rugged and safe extraction procedure and ultra-high performance liquid chromatography–tandem mass spectrometry. J Chromatogr A 1218:1477–1485
Rouvière F, Buleté A, Cren-Olivé C et al (2012) Multiresidue analysis of aromatic organochlorines in soil by gas chromatography–mass spectrometry and QuEChERS extraction based on water/dichloromethane partitioning. Comparison with accelerated solvent extraction. Talanta 93:336–344
Salemi A, Shafiei E, Vosough M (2012) Optimization of matrix solid phase dispersion coupled with gas chromatography electron capture detection for determination of chlorinated pesticides in soil. Talanta 101:504–509
Santos FJ, Galceran MT (2002) The application of gas chromatography to environmental analysis. TrAC-Trend Anal Chem 21:672–685
Saxton GN, Engel B (2005) A survey of soil sample handling procedures of state pesticide regulatory agencies. Environ Forensic 6:105–108
Shen X, Cai J, Gao Y et al (2006) Determination of organophosphorus pesticides in soil by MMSPD-GC-NPD and confirmation by GC-MS. Chromatographia 64:71–77
Stroud RK (1998) Wildlife forensics and the veterinary practitioner. Semin Avian Exot Pet 7:182–192
Sun K, Zhao Y, Gao B et al (2009) Organochlorine pesticides and polybrominated diphenyl ethers in irrigated soils of Beijing, China: levels, inventory and fate. Chemosphere 77:1199–1205
Theocharopoulos SP, Wagner G, Sprengart J et al (2001) European soil sampling guidelines for soil pollution studies. Sci Total Environ 264:51–62
Theocharopoulos SP, Mitsios IK, Arvanitoyannis I (2004) Traceabilty of environmental soil measurements. TrAC-Trends Anal Chem 23:237–251
Unsworth J (2010) History of pesticide use. http://agrochemicals.iupac.org/index.php? option=com_sobi2&sobi2Task=sobi2Details&catid=3&sobi2Id=31. Accessed 21 June 2012
US EPA (2012a) Agricultural pesticides. US Environmental Protection Agency. http://www.epa.gov/oecaagct/ag101/croppesticideuse.html. Accessed 10 Dec 2012
US EPA (2012b) Types of pesticides. US Environmental Protection Agency. http://www.epa.gov/pesticides/about/types.htm#chemical. Accessed 21 June 2012
Yasin M, Baugh PJ, Bonwick GA et al (1996) Analytical method development fort he determination of synthetic pyrethroid insecticides in soil by gas chromatography–mass spectrometry operated in negative-ion chemical-ionization mode. J Chromatogr A 754:235–243
Wait AD (2000) Evolution of organic analytical methods in environmental forensic chemistry. Environ Forensic 1:37–46
Wang X, Zhao X, Liu X et al (2008) Homogeneous liquid-liquid extraction combined with gas chromatography-electron capture detector for the determination of three pesticide residues in soils. Anal Chim Acta 620:162–169
Zacharia TJ (2011) Identity, physical and chemical properties of pesticides. In: Stoytcheva M (ed) Pesticides in the modern world – trends in pesticides analysis. InTech, Rijeka, pp 1–18
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this paper
Cite this paper
Semen, S., Mercan, S., Acikkol, M. (2016). A General Overview of Pesticides in Soil: Requirement of Sensitive and Current Residue Analysis Methods. In: Kars, H., van den Eijkel, L. (eds) Soil in Criminal and Environmental Forensics. Soil Forensics. Springer, Cham. https://doi.org/10.1007/978-3-319-33115-7_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-33115-7_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-33113-3
Online ISBN: 978-3-319-33115-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)