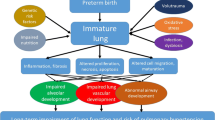
Abstract
With advances in neonatal care over the last three decades, a greater proportion of preterm infants are surviving the initial complications of prematurity and are now reaching adulthood in ever increasing numbers. The impact of preterm birth and its respiratory complications have lasting consequences on respiratory health. Bronchopulmonary dysplasia (BPD) and the long-term respiratory consequences of prematurity are unfamiliar and under-recognized entities to adult clinicians. Well described by the pediatric scientific community, these young adults who were born prematurely and suffered respiratory complications are joining the rank of a growing population of adults with chronic lung disease.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Bronchopulmonary dysplasia
- Airflow obstruction
- Preterm birth
- Bronchial hyperresponsiveness
- Respiratory health
- Long-term consequences of preterm birth
Introduction
Bronchopulmonary dysplasia (BPD) and the long-term respiratory consequences of prematurity are unfamiliar and under-recognized entities to adult clinicians. Well described by the pediatric scientific community , these young adults who were born prematurely and suffered respiratory complications are joining the ranks of a growing population of adults with chronic lung disease.
BPD is a chronic respiratory disease that develops as a consequence of neonatal lung injury and is one of the most important sequelae of preterm birth [1]. It occurs most commonly in preterm infants who have needed mechanical ventilation and oxygen therapy for respiratory distress syndrome of the newborn (RDS) [2]. BPD was first described four decades ago in children born slightly preterm with severe RDS who were exposed to aggressive mechanical ventilation and high concentrations of inspired oxygen [3]. Over time, this has been largely replaced by a new form of the condition occurring in more extreme preterm infants, often with less severe RDS following pulmonary surfactant administration [4].
Despite notable advances in prenatal and neonatal care, BPD remains an important complication of preterm births, frequently resulting in mortality as well as short-term and long-term morbidities, including airflow limitations and bronchial hyperresponsiveness [5]. With high rates of preterm birth worldwide, and more notably in North America [6], and with the improved survival associated with preterm birth, numerous young adults who were born prematurely and suffered respiratory complications are now manifesting chronic obstructive lung disease at a much younger age than their contemporaries with smoking-related chronic obstructive pulmonary disease (COPD). Their relative contribution to the growing adult populations with chronic pulmonary diseases has clinical and healthcare resources implications.
After the initial description of BPD in 1967 by Northway [7], many advances have been made in our understanding of the pathophysiology of this disease. With advances in neonatal care over the last three decades, a greater proportion of preterm infants are surviving the initial complications of prematurity and are now reaching adulthood in ever increasing numbers. Furthermore, the improved survival of very preterm infants has led to increasing incidence of pulmonary complications among these infants [2] and increased numbers of adolescents and young adults with sequelae of BPD [8]. This has clinical implications because their course, prognosis, and treatment are largely unknown and health system implications because of increased resource utilization.
Old BPD Versus New BPD
The form originally described in 1967 by Northway is now commonly referred to as “old BPD.” Lung histology of “old BPD” showed prominent signs of barotraumas with fibroproliferative reaction. These findings were reminiscent of the histopathology observed in adults suffering from the acute respiratory distress syndrome (ARDS) when high ventilatory pressures were used during mechanical ventilation [9, 10] for the management of acute lung injury.
However, the form of BPD seen nowadays (“new BPD”) often develops in more extreme preterm or very low birth weight (VLBW) newborns who received surfactant and needed less initial ventilator support or supplemental oxygen in their early days for the treatment of respiratory distress or apnea of prematurity [6]. It is increasingly recognized that the new BPD has a different underlying pathology and clinical course than was first described three decades ago. The lung histology of “new BPD” shows signs of truncated lung growth with abnormal alveolarization and dysmorphic vascular growth with a dysregulated pulmonary microvasculature. There is more uniform inflation, less fibrosis, little or no metaplasia of small and large airways, and only minimal smooth muscle hypertrophy [11]. These changes in histology may reflect the routine use of exogenous surfactants and the advances in mechanical ventilation techniques that were introduced in order to prevent barotrauma. It is to be expected that both forms of BPD will result in different phenotypes in the long-term and that they cannot be expected to behave the same way and, thus, should be considered as different entities .
Definition of BPD
In 2000, the National Institute of Child Health and Human Development (NICHD) sponsored a workshop to arrive at a consensus definition of BPD [12]. The final definition was that an infant requiring at least 28 days of supplemental oxygen should be considered to have BPD and that infants should be reassessed at 36 weeks postmenstrual date (or 56 days of life if born after 32 weeks) to establish the disease severity.
Long-Term Respiratory Outcomes of BPD Survivors
In the last 10 years, several studies have described the long-term sequelae of survivors of preterm births as these subjects reach adolescence and young adulthood [1, 13–20]. Most of these studies focused on pulmonary and neurological outcomes, the two systems most affected by prematurity and low birth weight [21, 22].
Early Childhood
Respiratory morbidity is common in infants and young children who were born prematurely, especially if they had developed BPD. Up to 50 % of children with BPD are readmitted in their first year of life for respiratory illnesses [18]. Lower respiratory tract infections contribute to this high rate of readmission. Rates of readmission and episodes of respiratory distress fall after the first year of life, as lung growth and remodeling of the airway take place, resulting in progressive improvement of pulmonary function [23]. Few children remain dependent on oxygen after 2 years of age [24].
BPD is associated with persistent pulmonary function abnormality in preschool children. In a cohort of 28 children diagnosed with BPD during their infancy, lung function testing before the age of 3 in a subset of seven asymptomatic children showed mild to moderate obstruction and air trapping [25]. In this same study, the 21 symptomatic children (with wheezing) had moderate to severe expiratory flow limitation, hyperinflation, and airway hyperresponsiveness .
Late Childhood and Adolescence
In most children who survive BPD, pulmonary function will improve, permitting normal activities, although increased airway resistance and hyperreactivity can remain present until adolescence [26]. Compared to controls matched for age and size, children with BPD have reduced absolute and size-corrected flow rates, a finding consistent with poor airway growth and persistent airflow limitation [27]. Among school age children, those who were born prematurely, particularly if they had BPD, were more likely to be symptomatic than their classroom colleagues who had been born at term. In a cohort of 125 children aged 7–8 years old, wheezing was present in 30 % of those who had had preterm birth and BPD, 24 % of those with preterm birth but did not develop BPD, and only 7 % of children born at term. This study ascertained symptoms, but did not measure lung function [23]. In a second study composed of 300 children, those who had had VLBW and who were aged 8–9 years old were significantly more likely to use inhalers, have school absences, or have a history of hospital admission for respiratory illness than 590 classroom controls with a normal birth weight. This study did not comment on the presence or absence of BPD among those born with VLBW [28].
One other study has reported 10 % lower forced expiratory volume in 1 s (FEV1 % predicted) in 12 survivors of moderate to severe BPD (mean age: 17.7 years) when compared to preterm infants with either mild or no BPD [29].
Adulthood
There are few published data from comprehensive longitudinal studies of patients followed into adulthood and few data on pulmonary function of adults with a history of preterm birth and BPD. One case–control study included 26 subjects with a history of preterm birth and BPD born between 1964 and 1973 and two control groups [15]. The first group was composed of 26 controls matched for gestational age and weight at birth that did not undergo mechanical ventilation, and the second group was composed of 53 age-matched subjects who were born at term. In this study, 76 % of 26 young adults with prior BPD had measurable pulmonary dysfunction (increased bronchial resistance, bronchial hyperreactivity, and hyperinflation) compared to none of the 53 controls that had been born at term. The cases also had significantly more frequent wheezing and need for long-term medication than subjects born prematurely (p-value: 0.047) and normal controls (p-value: 0.0001) [15].
A second study compared 690 19-year-old subjects, all of whom were born prematurely (prior to 32 weeks of gestation) and with VLBW (less than 1 500 g), to a control group of Dutch participants in the European Community Respiratory Health Survey . In this study, females with a history of BPD were more likely to report shortness of breath on exertion (43 vs. 16 %) and wheezing without a cold (35 vs. 13 %) and have doctor-diagnosed asthma (24 vs. 5 %) The prevalence of symptoms in the males with prior BPD was comparable to the control s [30].
Pulmonary Lung Function and Bronchial Hyperresponsiveness
In a more recent prospective cohort study, young adults (mean age of 22 years) born between 1987 and 1993 and who were living in the province of Quebec at the time of the study were included in one of four groups based on their hospital discharge diagnosis at birth: (1) preterm subjects (born at less than 37 weeks of gestation) with no respiratory complications (preterm), (2) preterm subjects with RDS but without BPD (RDS), (3) preterm subjects with BPD (with or without preceding RDS) (BPD), and (4) subjects born at term without respiratory complications following birth (term).
In the majority of subjects, the hospital discharge diagnosis data at birth were obtained from the Régie de l’assurance maladie du Québec (RAMQ) using the ICD-9 diagnostic codes for preterm birth (765.**) & RDS (769.*), and BPD (770.7). These data were obtained as part of a previously published cross-sectional study that surveyed the population of BPD and RDS and a portion of the preterm subjects born in the province of Quebec during the same period of time [31, 32]. The cohort study took place between 2011 and 2014 and consisted in two distinct visits at a research center (Montreal, Canada).
A study questionnaire was used to assess medical and smoking history, Medical Research Council (MRC) dyspnea score , and education level. The SF-36v2 and Saint George’s Respiratory (SGRQ) questionnaires were completed by participants to respectively evaluate their quality of life and their respiratory health .
Full pulmonary function tests were performed following the American Thoracic Society (ATS) guidelines [33–35]. Reversibility to bronchodilator was tested independently of the lung function test results, and a methacholine challenge test was also performed using the 2-min tidal breathing dosing protocol [36].
The BPD group was composed of 31 subjects, whereas the RDS group had 31, the preterm group had 26, and the term group had 35 subjects. Table 15.1 summarized the demographic and clinical information as well as the medical history across the four groups.
The mean age of the cohort participants was between 21 and 22 years and male subjects composed 35 % of the BPD and the term groups. The degree of dyspnea and the highest level of education achieved did not differ across the four groups. There were significantly more diagnoses of asthma, attention deficit hyperactivity disorder (ADHD) , and learning disabilities in the preterm subjects who suffered respiratory complications at birth (RDS and BPD) compared to preterm and term controls.
Lung Functions, Bronchodilator Response, and Bronchial Hyperresponsiveness to Methacholine
BPD participants had mild but significant airflow obstruction compared to the others, defined using a FEV1/forced vital capacity (FVC) ratio of 70 or less. The group with BPD also had significantly more gas trapping with a mean residual volume of 158 % of predicted. The diffusion capacity was also diminished compared to the other groups but was still within the lower limit of normal (Table 15.2). These findings are similar to those reported in another study looking at young adults, aged between 18 and 25 years, following a preterm birth complicated by BPD, which revealed a significant reduction in FEV1 when compared to control subjects born at term [37].
Forty-six percent of the BPD subjects had a significant bronchodilator response compared to RDS (18 %), preterm (12 %), and term (6 %) participants (p-value < 0.0001).
Results of methacholine challenge can be seen in Table 15.3. Seventy-one percent of the BPD subjects had evidence of bronchial hyperresponsiveness, but it was not found to be significantly different than the RDS group and the preterm group with 62 % and 69 %, respectively, although 25 % of the BPD subjects could not undergo the challenge because of a FEV1 that was less than 60 % of predicted.
With regard to their respiratory health and quality of life, the symptoms, activity, and impact scores of the SGRQ were not found to be significantly different across the four groups or different when compared to the general population. The total score on the SGRQ was 11 [standard deviation (SD): 12], 8 (SD: 8), 10 (SD: 12), and 5 (SD: 5) for the BPD, RDS, preterm, and term subjects, respectively (p-value: 0.06).
Similar findings were found with the SF-36v2 health survey questionnaire where the physical component summary and mental component summary scores were not different across the four groups.
In this study, the presence of mild airflow obstruction and a mean FEV1 value at the lower limit of normal in the BPD subjects at the age of 21–22 is somewhat of a worrisome finding, especially since 25 % of subjects in the BPD group were found to have an FEV1 less than 60 % of predicted, placing them in the category of moderate airflow obstruction. It is well known that the FEV1 value peaks at age 25 and then assumes a steady decline over time, ranging between 30 ml per year in nonsmokers to 60 ml per year in susceptible smokers [38, 39].
The annual rate of decline of the FEV1 in BPD subjects over time has still not yet been clearly defined, and there is a theoretical risk that the BPD subjects will have an accelerated loss of lung function with time, similar to what is seen in the COPD population.
The high prevalence of bronchial hyperresponsiveness in all preterm subjects that was found in this study is well supported by population studies that describe a fourfold increase in the incidence of asthma in preterm populations [40–42]. This raises an interesting issue of delineating the phenotypic features of a preterm lung from true asthma and a possible overlap between the two conditions.
Despite the abnormalities found in the pulmonary function, it is encouraging to see that the respiratory health and health-related quality of life do not differ significantly among the four groups, despite higher incidence of ADHD and learning difficulties in the BPD and RDS groups .
The Initial Severity of BPD and Its Impact on Long-Term Lung Function
BPD has undergone a radical change in its pathogenesis and its presentation over the past three decades since it was initially described. This prompted a consensus conference in order to better define the disease in 2000, as mentioned earlier. It combined two existing BPD definitions—oxygen dependency at 28 days of life and at 36 weeks of postmenstrual age. The new definition divided BPD into three forms: mild, moderate, and severe and clearly delineated criteria for judging severity. However, these criteria to differentiate mild from moderate disease or moderate from severe disease were chosen arbitrarily and were not based on analysis of factors associated with differences in outcome [12]. Knowing that the severity of BPD is associated with long-term outcomes would greatly add to the validity of the definition.
In a study looking at postnatal risk factors according to the new BPD definition, a chart review of 244 cases of VLBW preterm infants born between 1999 and 2004 showed that the frequency of BPD in VLBW neonates was high (76 %) but in the majority of cases the disease was mild (67 %). The authors concluded that severe BPD was more common in neonates with late onset sepsis and intraventricular hemorrhage grade III or IV and that the BPD risk factors were low gestational age, low birth weight, as well as late onset sepsis, late pneumonia, and patent ductus arteriosus [43].
In a retrospective study conducted to provide a detailed description of the long-term clinical characteristics of a cohort of preterm subjects who developed BPD of varying degrees of severity, as defined using the National Institutes of Health (NIH) consensus criteria, the initial severity of BPD was found to have an impact on the long-term lung function [41].
This cohort consisted of preterm infants admitted to the Montreal Children’s Hospital between the year 1980 and 1992 that were identified as suffering from BPD using the NIH consensus definition. The objective of this study was to provide a detailed description of the long-term consequences of BPD and to examine the association of initial BPD disease severity with these outcomes.
The inclusion criteria for the study population was a diagnosis of BPD, as defined by the NIH consensus [2] in subjects born before 37 weeks gestational age and admitted between January 1, 1980 and December 31, 1992 to a tertiary pediatric hospital with specialized neonatal care that serves as a referral center for the province of Quebec (Canada).
The presence of BPD was defined as the need for supplemental oxygen for at least 28 days [44]. Disease severity was graded based on an assessment done at 36 weeks postmenstrual age (or 56 days of life if born after 32 weeks). Mild disease was defined as breathing room air at that time (FiO2 of 0.21), moderate disease as requiring a FiO2 less than 0.30, and severe disease was defined as needing FiO2 of 0.30 or more or requiring positive pressure ventilation. Infants with BPD, who died of respiratory causes before the assessment date, were considered to have severe disease. These definitions are based on the NIH consensus definition of BPD established in 2000 [45].
Infants were grouped into three categories of disease severity (mild, moderate, and severe as defined above). Baseline and follow-up characteristics were compared between severity categories and/or age groups and differences between these groups tested for statistical significance. The statistical significance for trend across levels of disease severity was assessed using regression analysis.
Three hundred and twenty-two preterm infants with BPD identified during their hospital course were admitted over the 12-year study period. Of the 322 infants, 60 had mild, 123 had moderate, and 107 had severe disease. For 32 subjects (9.9 %), their disease severity was not assessed, mostly because they were transferred to other medical institutions before the severity assessment date but after having met the criterion for diagnosis of BPD of 28 days of oxygen treatment. Fifty-three infants died in the hospital. Among all infants discharged alive, 62 (26 %) were discharged on oxygen. The mean (± standard deviation) gestational age at discharge was 334.4 ± 117.7 days. The severity of BPD was associated with 1-min APGAR score, gestational age, presence of VLBW, and the occurrence of neonatal pneumonia/sepsis [41]. The duration of invasive and noninvasive mechanical ventilation as well as the total duration of oxygen therapy was significantly greater in more severe BPD cases, as seen in Table 15.4.
FEV1, FVC, and their ratio were significantly associated with the initial BPD severity as shown in Table 15.5. Of all lung function indices, only the FEV1 was associated with another parameter—the total duration of oxygen therapy (p-value: 0.02). No other parameters of lung function were associated with mechanical ventilation parameters (maximum pressure, positive end-expiratory pressure (PEEP), use of traditional mechanical ventilation versus nasal continuous positive airway pressure (CPAP), or level of FiO2 used.
This study described the post-hospitalization course of a retrospective cohort of infants with preterm birth who suffered the complication of BPD. Its major findings were the association of the initial BPD severity with numerous morbidities such as hospital readmissions in the first 2 years of life, the presence of developmental delay, and lung function abnormalities later in childhood. These findings have implications for the care of preterm infants and for planning of healthcare services.
In this cohort, 83 % of infants who were discharged alive had at least one admission in the first 2 years of their life. This is substantially higher than the 50 % readmission rate reported previously [24]. Oxygen therapy was discontinued after a mean duration of 20 weeks, but severe cases were maintained on oxygen for an average of 40 weeks, which, when considering their corrected gestational age at discharge, meant that subjects with severe BPD were weaned off oxygen after an average of 1 year and 2 months. More than half of the children (54 %) received inhaled short-acting beta-agonist in their first few years of life, presumed to represent the occurrence of wheezing episodes. Inhaled corticosteroids were used in 20.4 % of subjects, but their use was not associated with disease severity. Although not associated with disease severity, 68 % of BPD survivors had persistent radiological abnormalities compatible with chronic changes of BPD. Others have reported that radiological abnormalities improve with time [18], which was not observed in our dataset, a phenomenon possibly attributable to a selection bias, since chest X-rays were available for only a small proportion of subjects.
In previous studies, lung function abnormalities were not related to severity of neonatal respiratory illness [18, 46, 47], in contrast to our finding of a clear association between greater severity of BPD and greater abnormalities of FEV1, the FEV1/ FVC ratio, forced expiratory flow from 25 to 75 % of vital capacity (FEF25–75), and FRC. This finding is supported by another study showing a trend toward lower FEV (% predicted) in moderate to severe BPD subjects among former preterm infants born between 1982 and 1985 [29]. These findings are in agreement with the principle of “disynaptic” or unequal lung growth previously described, which means normal growth of lung volume but not of airway size [18]. When comparing to Northway’s earlier report of “old” BPD subjects born between 1964 and 1973, the overall airway obstruction and hyperinflation found in our study were more severe, likely to represent an evolving phase of the pathophysiology of BPD between “old” and “new,” accounting for the effects of greater prematurity and lower birth weight [15].
In one earlier study, infants with BPD had higher risk of cerebral palsy and delays in cognitive and motor function compared with controls matched for gestational age at birth [24]. A NICHD review on neurological developmental outcome of extreme low birth weight infants reported abnormal outcomes in 50–60 % of infants with BPD. Findings in these reports are comparable to our BPD cohort, of whom 52 % were found to have development delay and 20 % had neurological impairment during childhood [24]. Poor developmental outcomes were previously associated with prolonged hospital admissions and prolonged mechanical ventilation [24]. In our study, we found an association between the presence of a developmental delay and the following variables: length of the initial hospital admission, duration of mechanical ventilation, and birth weight when correcting for BPD severity. Other associations were found between neurological impairment and the presence of neonatal seizures or a history of anoxic encephalopathy. In another study, the risks of impaired neurological development, cerebral palsy, and/or low intellectual quotient were more than doubled in infants with severe BPD compared with infants with mild BPD [48]; however, this trend of neurological impairment with BPD of increased severity was not found in our study. The prevalence of ADHD in our BPD population was also significantly different than the general population (6.5 vs. 5.3 %) [49, 50], an observation previously reported in preterm infants [51] but never linked to chronic lung disease or its treatment. In our study, ADHD was associated with smaller gestational age and the use of steroids postnatally, but not with the degree of BPD severity.
T his study described an association between the initial BPD severity and hospital readmission rate, long-term lung function, and developmental delay. Severity of BPD was not associated with greater long-term use of inhalers or with persistent radiological abnormalities. In addition, the duration of mechanical ventilation and oxygen therapy was associated with developmental delay during childhood, and the use of postnatal corticosteroids was associated with the development of ADHDs. Initial BPD severity was an important predictor of pulmonary function abnormality and healthcare utilization during childhood. These findings contribute to a better description of the impact of BPD and its severity on long-term outcomes and will help sensitize the adult caregivers to the long-term consequences of preterm birth.
Conclusion
The impact of preterm birth and its respiratory complications have lasting consequences on respiratory health. Clinicians have to be better informed of its manifestation and the long-term consequences in order to better assist their young patients in maintaining their health and lung function. Prevention and education focusing on smoking avoidance or smoking cessation, health lifestyle, and physical activity will be key factors in ensuring that our young patients born prematurely will maintain an optimal respiratory health and quality of life.
The long-term effect of BPD on the evolution of FEV1 is unclear, particularly whether adults who had BPD as children will have normal, early, or accelerated decline in respiratory function [21]. Some investigators have expressed concern that survivors of preterm birth and BPD may be susceptible to COPD in later life, underlying the need for longer follow-up data [15, 18, 20, 21, 29, 52].
References
Wohl ME. Bronchopulmonary dysplasia in adulthood. N Engl J Med. 1990;323(26):1834–6.
Bancalari E, Claure N, Sosenko IR. Bronchopulmonary dysplasia: changes in pathogenesis, epidemiology and definition. Semin Neonatol. 2003;8(1):63–71.
Bancalari E, Abdenour GE, Feller R, Gannon J. Bronchopulmonary dysplasia: clinical presentation. J Pediatr. 1979;95(5 Pt 2):819–23.
Russell RB, Green NS, Steiner CA, Meikle S, Howse JL, Poschman K, et al. Cost of hospitalization for preterm and low birth weight infants in the United States. Pediatrics. 2007;120(1):e1–9.
Jobe AH. The new bronchopulmonary dysplasia. Curr Opin Pediatr. 2011;23(2):167–72.
Bhandari A, McGrath-Morrow S. Long-term pulmonary outcomes of patients with bronchopulmonary dysplasia. Semin Perinatol. 2013;37(2):132–7.
Northway Jr WH, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med. 1967;276(7):357–68.
Hulsmann AR, van den Anker JN. Evolution and natural history of chronic lung disease of prematurity. Monaldi Arch Chest Dis. 1997;52(3):272–7.
Lessard MR. New concepts in mechanical ventilation for ARDS. Can J Anaesth. 1996;43(5 Pt 2):R42–54.
Malhotra A. Low-tidal-volume ventilation in the acute respiratory distress syndrome. N Engl J Med. 2007;357(11):1113–20.
Bhandari A, Bhandari V. Pitfalls, problems, and progress in bronchopulmonary dysplasia. Pediatrics. 2009;123(6):1562–73.
Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163(7):1723–9.
Churg A, Golden J, Fligiel S, Hogg JC. Bronchopulmonary dysplasia in the adult. Am Rev Respir Dis. 1983;127(1):117–20.
Samuels MP, Warner JO. Bronchopulmonary dysplasia: the outcome. Arch Dis Child. 1987;62(11):1099–101.
Northway Jr WH, Moss RB, Carlisle KB, Parker BR, Popp RL, Pitlick PT, et al. Late pulmonary sequelae of bronchopulmonary dysplasia. N Engl J Med. 1990;323(26):1793–9.
Northway Jr WH. Bronchopulmonary dysplasia: twenty-five years later. Pediatrics. 1992;89(5 Pt 1):969–73.
Parat S, Moriette G, Delaperche MF, Escourrou P, Denjean A, Gaultier C. Long-term pulmonary functional outcome of bronchopulmonary dysplasia and premature birth. Pediatr Pulmonol. 1995;20(5):289–96.
Eber E, Zach MS. Long term sequelae of bronchopulmonary dysplasia (chronic lung disease of infancy). Thorax. 2001;56(4):317–23.
Greenough A. Bronchopulmonary dysplasia—long term follow up. Paediatr Respir Rev. 2006;7 Suppl 1:S189–91.
Vrijlandt EJ, Gerritsen J, Boezen HM, Grevink RG, Duiverman EJ. Lung function and exercise capacity in young adults born prematurely. Am J Respir Crit Care Med. 2006;173(8):890–6.
Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med. 2007;357(19):1946–55.
Fanaroff AA, Stoll BJ, Wright LL, Carlo WA, Ehrenkranz RA, Stark AR, et al. Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol. 2007;196(2):147.e1–8.
Greenough A. Long-term pulmonary outcome in the preterm infant. Neonatology. 2008;93(4):324–7.
Kinsella JP, Greenough A, Abman SH. Bronchopulmonary dysplasia. Lancet. 2006; 367(9520):1421–31.
Robin B, Kim YJ, Huth J, Klocksieben J, Torres M, Tepper RS, et al. Pulmonary function in bronchopulmonary dysplasia. Pediatr Pulmonol. 2004;37(3):236–42.
Koumbourlis AC, Motoyama EK, Mutich RL, Mallory GB, Walczak SA, Fertal K. Longitudinal follow-up of lung function from childhood to adolescence in prematurely born patients with neonatal chronic lung disease. Pediatr Pulmonol. 1996;21(1):28–34.
Filippone M, Sartor M, Zacchello F, Baraldi E. Flow limitation in infants with bronchopulmonary dysplasia and respiratory function at school age. Lancet. 2003;361(9359):753–4.
McLeod A, Ross P, Mitchell S, Tay D, Hunter L, Hall A, et al. Respiratory health in a total very low birthweight cohort and their classroom controls. Arch Dis Child. 1996;74(3):188–94.
Halvorsen T, Skadberg BT, Eide GE, Roksund OD, Carlsen KH, Bakke P. Pulmonary outcome in adolescents of extreme preterm birth: a regional cohort study. Acta Paediatr. 2004;93(10): 1294–300.
Vrijlandt EJ, Gerritsen J, Boezen HM, Duiverman EJ. Gender differences in respiratory symptoms in 19-year-old adults born preterm. Respir Res. 2005;6:117.
Landry JS, Croitoru D, Menzies D. Validation of ICD-9 diagnostic codes for bronchopulmonary dysplasia in Quebec’s provincial healthcare databases. Chronic Dis Inj Can. 2012;33(1):47–52.
Beaudoin S, Tremblay GM, Croitoru D, Benedetti A, Landry JS. Healthcare utilization and health-related quality of life of adult survivors of preterm birth complicated by bronchopulmonary dysplasia. Acta Paediatr. 2013;102(6):607–12.
Macintyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten CP, Brusasco V, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26(4):720–35.
Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–38.
Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26(3):511–22.
Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, et al. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 2000;161(1):309–29.
Vollsaeter M, Roksund OD, Eide GE, Markestad T, Halvorsen T. Lung function after preterm birth: development from mid-childhood to adulthood. Thorax. 2013;68(8):767–76.
Mangham LJ, Petrou S, Doyle LW, Draper ES, Marlow N. The cost of preterm birth throughout childhood in England and Wales. Pediatrics. 2009;123(2):e312–27.
Lam HS, Wong SP, Liu FY, Wong HL, Fok TF, Ng PC. Attitudes toward neonatal intensive care treatment of preterm infants with a high risk of developing long-term disabilities. Pediatrics. 2009;123(6):1501–8.
Kirkby S, Greenspan JS, Kornhauser M, Schneiderman R. Clinical outcomes and cost of the moderately preterm infant. Adv Neonatal Care. 2007;7(2):80–7.
Landry JS, Menzies D. Occurrence and severity of bronchopulmonary dysplasia and respiratory distress syndrome after a preterm birth. Paediatr Child Health. 2011;16(7):399–403.
Landry JS, Chan T, Lands L, Menzies D. Long-term impact of bronchopulmonary dysplasia on pulmonary function. Can Respir J. 2011;18(5):265–70.
Woynarowska M, Rutkowska M, Szamotulska K. Risk factors, frequency and severity of bronchopulmonary dysplasia (BPD) diagnosed according to the new disease definition in preterm neonates. Med Wieku Rozwoj. 2008;12(4 Pt 1):933–41.
Lavoie PM, Pham C, Jang KL. Heritability of bronchopulmonary dysplasia, defined according to the consensus statement of the national institutes of health. Pediatrics. 2008;122(3): 479–85.
Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, et al. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116(6):1353–60.
Chan KN, Noble-Jamieson CM, Elliman A, Bryan EM, Silverman M. Lung function in children of low birth weight. Arch Dis Child. 1989;64(9):1284–93.
Kitchen WH, Olinsky A, Doyle LW, Ford GW, Murton LJ, Slonim L, et al. Respiratory health and lung function in 8-year-old children of very low birth weight: a cohort study. Pediatrics. 1992;89(6 Pt 2):1151–8.
Vohr BR, Coll CG, Lobato D, Yunis KA, O’Dea C, Oh W. Neurodevelopmental and medical status of low-birthweight survivors of bronchopulmonary dysplasia at 10 to 12 years of age. Dev Med Child Neurol. 1991;33(8):690–7.
Greenbaum RL, Stevens SA, Nash K, Koren G, Rovet J. Social cognitive and emotion processing abilities of children with fetal alcohol spectrum disorders: a comparison with attention deficit hyperactivity disorder. Alcohol Clin Exp Res. 2009;33(10):1656–70.
Merrill RM, Lyon JL, Baker RK, Gren LH. Attention deficit hyperactivity disorder and increased risk of injury. Adv Med Sci. 2009;54:20–6.
Delobel-Ayoub M, Arnaud C, White-Koning M, Casper C, Pierrat V, Garel M, et al. Behavioral problems and cognitive performance at 5 years of age after very preterm birth: the EPIPAGE Study. Pediatrics. 2009;123(6):1485–92.
Doyle LW, Faber B, Callanan C, Freezer N, Ford GW, Davis NM. Bronchopulmonary dysplasia in very low birth weight subjects and lung function in late adolescence. Pediatrics. 2006;118(1):108–13.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Landry, J.S., Banbury, S.P. (2016). Pulmonary Function in Survivors of Bronchopulmonary Dysplasia. In: Bhandari, V. (eds) Bronchopulmonary Dysplasia. Respiratory Medicine. Humana Press, Cham. https://doi.org/10.1007/978-3-319-28486-6_15
Download citation
DOI: https://doi.org/10.1007/978-3-319-28486-6_15
Published:
Publisher Name: Humana Press, Cham
Print ISBN: 978-3-319-28484-2
Online ISBN: 978-3-319-28486-6
eBook Packages: MedicineMedicine (R0)