Abstract
-
I.
Introduction
-
A.
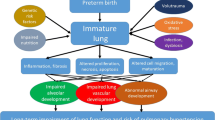
Bronchopulmonary dysplasia (BPD) remains the commonest complication of prematurity, and its incidence is rising most likely from improved survival of extremely low birthweight newborns. BPD is a complex disorder of the respiratory system affecting mostly preterm babies exposed to invasive mechanical ventilation and inspired oxygen. This complex disorder represents histologic distortion of normal lung architecture by factors that cause lung injury and disruption of lung development. Gestational age at birth and low birthweight are the strongest risk factors for BPD. Ninety-five percent of all infants with BPD are very low birthweight (VLBW). The reported incidence of BPD is around 80% at gestations of 22–24 weeks and around 20% at 28 weeks. Despite significant improvements in clinical practice, there has been no improvement in the prevalence of BPD.
-
A.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Bronchopulmonary dysplasia
- Growth
- Neurological outcome
- Respiratory function
- Pulmonary hypertension
- Nephrocalcinosis
-
I.
Introduction
-
A.
Bronchopulmonary dysplasia (BPD) remains the commonest complication of prematurity, and its incidence is rising most likely from improved survival of extremely low birthweight newborns. BPD is a complex disorder of the respiratory system affecting mostly preterm babies exposed to invasive mechanical ventilation and inspired oxygen. This complex disorder represents histologic distortion of normal lung architecture by factors that cause lung injury and disruption of lung development. Gestational age at birth and low birthweight are the strongest risk factors for BPD. Ninety-five percent of all infants with BPD are very low birthweight (VLBW). The reported incidence of BPD is around 80% at gestations of 22–24 weeks and around 20% at 28 weeks. Despite significant improvements in clinical practice, there has been no improvement in the prevalence of BPD.
-
B.
“Old BPD ” as described by Northway et al. in 1967 was characterized by extensive inflammatory and fibrotic changes in airways and lung parenchyma. With improved survival of more immature infants, in part from the use of antenatal steroids, gentler ventilatory strategies, and surfactant therapy, a different pattern of this disorder, “new BPD, ” has evolved. This condition represents a developmental disorder of immature lungs unable to reach full structural complexity. This is characterized by alveolar arrest and disordered pulmonary vasculature and a smaller effective surface area resulting in diffusion abnormalities.
-
A.
-
II.
Neonatal Morbidity
-
A.
Very preterm infants are more prone to complications of prematurity, such as nosocomial blood stream infections, ventilator-associated pneumonia, necrotizing enterocolitis (NEC), and growth failure. These comorbid factors trigger a systemic inflammatory response, adversely disrupting alveolarization and microvascular development in the immature lungs. These anatomical changes lead to abnormal gas exchange and abnormal lung mechanics. BPD infants require lengthy hospital stays and have delayed discharges, often requiring supplementary home oxygen for several months.
-
B.
Survivors with BPD are also more prone to other comorbidities such as IVH, PVL, and ROP; however, there is no direct causal relationship.
-
A.
-
III.
Long-Term Outcomes
-
A.
Growth and development
-
1.
Nutrition
-
(a)
Neonates and children with BPD have increased nutritional requirements compared to infants without BPD. Infants with BPD have increased needs from increased work of breathing secondary to poor lung compliance, chronic stress from hypoxic episodes and inflammation, along with growth suppression from the use of chronic steroid or diuretic therapy. Optimal nutritional support should meet expenditure as well as substrate for growth and repair of injured organ systems. Sicker neonates are less likely to be fed, and feeding difficulties associated with aversion, intolerance, and gastroesophageal reflux are well described in infants with BPD. Recent studies suggest that VLBW infants with poor enteral nutrition within the first 2 weeks of life were are more likely to develop BPD. Chronic and episodic hypoxia in infants with BPD during feeding and sleep can contribute to growth failure. VLBW infants with all forms of BPD have been shown to have lower weight, length, and head circumferences compared to VLBW infants without BPD. Infants with severe BPD appear to be more vulnerable to a negative growth outcome even when corrected for perinatal and demographic variables. Postnatal growth appears to be linked to improvement in respiratory function in childhood. Close monitoring of nutritional intake and supplemental oxygen therapy to avoid hypoxia post discharge is therefore recommended.
-
(b)
Recurrent illnesses, increased need for hospitalization in infancy, and increased metabolic demands associated with BPD also result in poor growth. However, after controlling for confounding factors, studies during childhood have not demonstrated significant differences in the growth of VLBW children with and without BPD.
-
(c)
Outcome in adult survivors with BPD is intricately linked to issues related to low gestational age and birthweight with higher rates of many adverse health outcomes in early adulthood; however, the majority lead productive and healthy lives. Longer-term studies are essential in evaluating the lifetime consequences of BPD and LBW on survivors.
-
(a)
-
2.
Neurocognitive development
-
(a)
Infants with BPD have multiple episodes of hypoxia, hypercapnia, and prolonged respiratory acidosis which could predispose their brains to injury. Additionally, a higher proportion of these infants receive postnatal corticosteroids. BPD independently predicts adverse developmental outcome in early infancy, roughly doubling the odds of an adverse neurologic outcome. Furthermore, BPD infants with associated pulmonary hypertension (PH) had significant lower motor, cognitive, and language scores at 18 to 24 months’ corrected age. The reason for this is thought to be an increased degree of hypoxemia and hemodynamic instability associated with PH. Volumetric magnetic resonance imaging study of preterm infants with BPD showed uniform reduction in cerebral volumes compared to a regional reduction in brain volume seen in preterm infants without BPD. The exact mechanism for this reduction in brain volume is unclear, but may correlate with functional deficits more frequently seen in survivors with BPD. Neuroimaging focusing on the brainstem has documented lower pontine and medullary volumes in BPD infants as a result of abnormal myelination, degeneration of descending white matter tracts, and focal necrotic changes in the brainstem. This is being attributed to an underdeveloped brainstem, which is frequent in babies requiring prolonged ventilation. There is increased awareness of the negative effects of episodic hypoxic episodes frequently seen in infants with BPD and adverse neurodevelopmental outcomes. Studies have also shown that patients with severe BPD have an increased incidence of neurodevelopmental disability at 6 and 12 months, which is less notable in mild to moderate BPD.
-
(b)
Motor development.
-
(1)
A recent meta-analysis exploring an association between BPD and cerebral palsy (CP) concluded that BPD was significantly associated with CP with an odds ratio of 2.1 (95% CI, 1.57–2.82). The risk of CP is related to severity of BPD. Infants with severe BPD requiring mechanical ventilation at 36 weeks’ postmenstrual age were 6 times more likely to have quadriplegic CP and 4 times more likely to have diplegic CP.
-
(2)
The most common types of cerebral palsy phenotypes associated with BPD are quadriparesis and diparesis. These forms reflect diffuse, bilateral cerebral hemispheric disease in these infants.
-
(3)
Poorer gross and fine motor skills are noted in BPD infants with scores approximately 1 SD lower in comparison to preterm controls at 10 years of age. Consequently, these infants need greater access to occupational and physiotherapy services.
-
(4)
Visual-spatial perceptual deficits are noted in about 30% of VLBW children with BPD on visual motor integration testing. This deficit persists into adolescence and correlates to duration of oxygen therapy.
-
(1)
-
(c)
Neurosensory impairments.
-
(1)
BPD independently predicts neurosensory impairment. PVL, severe ROP, and length of hospital stay are predictive of adverse neurosensory outcome.
-
(2)
BPD infants perform significantly below controls on Visual-Motor Integration (VMI) testing with roughly a third of BPD infants performing below age expectations. There is an association between difficulties in visual perception and BPD, which in turn is strongly related to the duration of oxygen requirement.
-
(1)
-
(d)
Cognitive and academic consequences.
-
(1)
Multiple studies have reported a correlation between cognitive deficits and BPD persisting to school age. BPD infants had lower scores when compared to matched VLBW controls without BPD, who in turn had lower scores than term controls. This pattern was seen in general intelligence, reading, mathematics, motor skills, memory, and attention. Severity of BPD correlated with worse scores.
-
(2)
Infants with BPD have significant language delay with lower scores in receptive language possibly as a consequence of auditory processing difficulties. Presence of a patent ductus arteriosus (PDA) along with BPD was found to predict poorer language development. The precise mechanism for this association is not known, but PDA may simply be a marker of severe BPD.
-
(3)
Attention-deficit hyperactivity disorders (ADHD) are reportedly as high as 15% in VLBW infants with BPD, twice as high compared to non-BPD VLBW children at school age.
-
(4)
A recent study demonstrated that there was persistence of impaired cognitive functions in adult survivors with BPD. This study showed that this population displayed deficits in executive functioning even at a mean age of 24.2 years, suggesting important implications for healthcare, social well-being, and cognitive rehabilitation.
-
(1)
-
(a)
-
1.
-
B.
Other systems
-
1.
Respiratory system
-
(a)
In early childhood (0–5 years), hospital readmission rates, mainly from reactive airway disease, pneumonia, and RSV infections, are higher in BPD infants in the first 2 years of life in comparison to term controls. For this reason, monoclonal antibodies for RSV prophylaxis are recommended in the first year for infants with BPD and for a further year while on supplemental oxygen, diuretics, or chronic steroid therapy. A recent meta-analysis concluded that FEV1 was diminished at the age of 5–23 years in preterm subjects with and without BPD. The nutritional status of children at 2 years of age influences respiratory outcomes in childhood. Respiratory function testing shows substantial expiratory flow impairment with modest reduction in Total Lung Capacity (TLC), increase in Functional Residual Capacity (FRC), and increased Residual Volume (RV) to TLC ratio consistent with gas trapping. Studies have shown improvement in expiratory flow abnormalities in the first 2 years; however, chronic coughing, wheezing, and other asthma-like symptoms requiring the use of inhaled bronchodilators are more common in comparison to term controls.
-
(b)
At school age (6–18 years), children with BPD had poorer lung function and reduced exercise tolerance in comparison to non-BPD survivors of similar weight. Spirometry shows persisting reduction in FEV1; however, TLC and FRC were normal or only modestly reduced. RV/TLC ratio remained elevated suggestive of air trapping. High-resolution computed tomography (CT) of the chest of children with BPD showed areas of multifocal emphysema, atelectasis extending to the pleura, bronchial wall thickening, bullae, and air trapping. These finding suggest that children with BPD are at risk of developing COPD later in life. Children with BPD have abnormal lung structure, lower lung function, and declining lung function over time with an increased risk of respiratory symptoms in later life. Healthy lifestyle choices and avoidance of smoking should be advocated.
-
(c)
Adulthood: Restriction in FEV1 persists into adulthood, and adult survivors with BPD are twice as likely to report wheezing and three times as likely to use asthma medications as term control subjects. Uncertainty remains about the respiratory consequences of BPD in later adult life (>40 years) with the potential for further decline in respiratory function, pulmonary hypertension, and development of COPD. Serial assessment of lung function by spirometry may identify patients at risk of COPD and related pulmonary vascular diseases in adulthood.
-
(a)
-
2.
Cardiovascular system
-
(a)
Pulmonary hypertension is reported in 6%, 12%, and 39% of infants with mild, moderate, and severe BPD, respectively. Pulmonary hypertension contributes to both increased morbidity, with a mortality rate of 16–40%. Altered lung development and postnatal lung injury can lead to compromised alveolar development and vascular pruning characteristic of BPD. Some infants with BPD develop increased pulmonary vascular resistance as a result of abnormal pulmonary vascularization, vascular remodeling, and increased vascular tone. Screening for pulmonary hypertension by echocardiography is recommended in infants with BPD prior to discharge.
-
(b)
BPD is an independent risk factor for systemic hypertension and the most common nonrenal cause of hypertension in preterm infants. Systemic hypertension is reported in about 7–43% in infants with BPD. Fluid retention, increased serum aldosterone levels, arterial wall thickening, and abnormal vasomotor tone have been implicated as mechanisms. Less than 50% of cases will require medical treatment for a short period. A retrospective study reported that most infants did not require treatment beyond 3–6 months of age.
-
(a)
-
3.
Renal
-
(a)
Nephrocalcinosis is reported in as many as 60% of VLBW infants with BPD. Nephrocalcinosis is more commonly seen in preterm infant who have had severe respiratory disease, acidosis, parenteral nutrition and treatment with diuretics, methylxanthines, and glucocorticoids in the neonatal period.
-
(b)
While up to 80% resolve spontaneously in the first 2 years, the long-term consequence of this condition, previously thought to be benign, is unknown with presumed risk of long-term systemic hypertension. Long-term follow-up and predischarge renal ultrasound surveillance should be considered.
-
(a)
-
1.
-
A.
-
IV.
Summary
-
A.
BPD is a multisystem disorder with consequences beyond the neonatal period. A multidisciplinary approach to the management and follow-up of preterm survivors with BPD is advocated.
-
B.
Further research and long-term follow studies are needed to understand the lifelong implications of this primarily respiratory disorder of the preterm.
-
A.
Suggested Reading
Bauer S, Vanderpool C, Huff K, Rose R, Cristea AI. Growth and nutrition in children with established bronchopulmonary dysplasia: a systematic review. Authorea Preprints. 2020; https://doi.org/10.22541/au.160430258.88068804/v1.
Berkelhamer SK, Mestan KK, Steinhorn R. An update on the diagnosis and management of bronchopulmonary dysplasia (BPD)-associated pulmonary hypertension. Semin Perinatol. 2018;42(7):432–43. WB Saunders.
Cheong JL, Doyle LW. An update on pulmonary and neurodevelopmental outcomes of bronchopulmonary dysplasia. Semin Perinatol. 2018;42(7):478–84. WB Saunders.
Choi EK, Shin SH, Kim EK, et al. Developmental outcomes of preterm infants with bronchopulmonary dysplasia-associated pulmonary hypertension at 18–24 months of corrected age. BMC Pediatr. 2019;19:26. https://doi.org/10.1186/s12887-019-1400-3.
Gou X, Yang L, Pan L, et al. Association between bronchopulmonary dysplasia and cerebral palsy in children: a meta-analysis. BMJ Open. 2018;8:e020735. https://doi.org/10.1136/bmjopen-2017-020735.
Guillot M, Guo T, Ufkes S, Schneider J, Synnes A, Chau V, Grunau RE, Miller SP. Mechanical ventilation duration, brainstem development, and neurodevelopment in children born preterm: a prospective cohort study. J Pediatr. 2020;226:87–95.e3. https://doi.org/10.1016/j.jpeds.2020.05.039. Epub ahead of print. PMID: 32454115.
Kim HS, Jeong K, Choi YY, Song ES. Risk factors and outcome of nephrocalcinosis in very low birth weight infants. Korean J Perinatol. 2015;26(1):35–45.
Northway WH Jr, Rosan RC, Porter DY. Pulmonary disease following respiratory therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med. 1967;276:357–68.
Rakow A, Laestadius Å, Liliemark U, Backheden M, Legnevall L, Kaiser S, Vanpée M. Kidney volume, kidney function, and ambulatory blood pressure in children born extremely preterm with and without nephrocalcinosis. Pediatr Nephrol. 2019;34(10):1765–76.
Sehgal A, Steenhorst JJ, Mclennan DI, Merkus D, Ivy D, McNamara PJ. The left heart, systemic circulation, and bronchopulmonary dysplasia: relevance to pathophysiology and therapeutics. J Pediatr. 2020;225:13–22.
Singer LT, Siegel AC, Lewis B, Hawkins S, Yamashita T, Baley JILL. Preschool language outcomes of children with history of bronchopulmonary dysplasia and very low birth weight. J Dev Behav Pediatr. 2001;22(1):19–26.
Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, Laptook AR, Sánchez PJ, Van Meurs KP, Wyckoff M, Das A. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA. 2015;314(10):1039–51.
Thébaud B, Goss KN, Laughon M, Whitsett JA, Abman SH, Steinhorn RH, Aschner JL, Davis PG, McGrath-Morrow SA, Soll RF, Jobe AH. Bronchopulmonary dysplasia. Nat Rev Dis Primers. 2019;5(1):78. https://doi.org/10.1038/s41572-019-0127-7. PMID: 31727986; PMCID: PMC6986462.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Thomas, S., Murthy, P. (2022). Long-Term Outcomes of Newborns with Bronchopulmonary Dysplasia. In: Donn, S.M., Mammel, M.C., van Kaam, A.H. (eds) Manual of Neonatal Respiratory Care. Springer, Cham. https://doi.org/10.1007/978-3-030-93997-7_80
Download citation
DOI: https://doi.org/10.1007/978-3-030-93997-7_80
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-93996-0
Online ISBN: 978-3-030-93997-7
eBook Packages: MedicineMedicine (R0)