Abstract
Respiratory disease is one of the most common complications of preterm birth. Survivors of prematurity have increased risks of morbidities and mortalities independent of prematurity, and frequently require multiple medications, home respiratory support, and subspecialty care to maintain health. Although advances in neonatal and pulmonary care have improved overall survival, earlier gestational age, lower birth weight, chorioamnionitis and late onset sepsis continue to be major factors in the development of bronchopulmonary dysplasia. These early life events associated with prematurity can have respiratory consequences that persist into adulthood. Furthermore, after initial hospital discharge, air pollution, respiratory tract infections and socioeconomic status may modify lung growth trajectories and influence respiratory outcomes in later life. Given that the incidence of respiratory disease associated with prematurity remains stable or increased, there is a need for pediatric and adult providers to be familiar with the natural history, manifestations, and common complications of disease.
Similar content being viewed by others
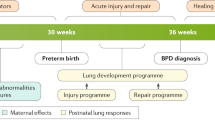
Introduction
Over 13 million infants were born prematurely worldwide in 2020. Of those, 15% were born less than 32 weeks gestation [1]. Preterm infants are subject to a number of complications or sequelae as a result of their prematurity including post-prematurity respiratory disease [2]. There are number of respiratory phenotypes associated with preterm birth, the archetype being bronchopulmonary dysplasia (BPD) [3]. BPD includes elements of alveolar, airway, and pulmonary vascular disease, and is associated with significant morbidity and mortality [4]. Increased morbidity and mortality risks associated with BPD may persist into early adolescence [5] and adulthood [6]. Infants and young children with BPD can require supplemental oxygen or ventilatory support in the home setting, and often require multiple daily medications, and/or frequent medical appointments [7]. Despite advances in neonatal care, the incidence of BPD has remained stable or increased, thus respiratory disease associated with prematurity will remain a condition that pediatric and adult providers will need to be familiar with [8].
Longstanding and emerging evidence suggests that in utero and early life events have life-long respiratory consequences [9]. In a study of men born during 1911–1930, men with low birth weights were more likely to die from Chronic Obstructive Lung Disease (COPD) [10]. Moreover, a study of young adults found lower measures of lung function by spirometry in individuals with a prior history of radiological confirmed pneumonia prior to 3 years of age compared to peers without such a history [11]. In offspring born preterm, maternal tobacco smoke exposure during pregnancy was a risk factor for BPD development and later onset respiratory disease during childhood [12]. Studies of individuals born prematurely also demonstrate lower lung function in those with a history of BPD compared to those born prematurely without a history of BPD [13]. Congenitally and/or remodeled small airways may encompass a pediatric origin of COPD in adults [14]. In a study of adults aged 30–45 years, gestational age was inversely proportional with mortality, with the authors defining preterm birth as a chronic condition that requires long term care and treatment of health sequelae such as cardiovascular and respiratory diseases [15].
However, the extent of respiratory disease associated with prematurity that persists into adulthood is poorly understood, owing to a paucity of longitudinal studies, no consensus definitions for preterm respiratory disease after 36 weeks corrected age, and continual advances in lung-protective neonatological care [16].
Despite recognition of the fact that BPD carries a risk for life-long respiratory consequences, there is limited knowledge about the natural history of the disease, particularly in adults, an absence of biomarkers or clinical definitions to predict which individuals may be at risk for worse outcomes as older children or adults, and an absence of proven interventions to optimize long-term outcomes. This review will summarize existing data on long-term pulmonary sequelae and management in patients with BPD.
Natural history of BPD
Gestational age is the major driver of BPD development in preterm infants, with earlier gestational age associated with a higher likelihood of BPD and long-term expiratory airflow abnormalities [17, 18]. Nevertheless, any degree of prematurity has been associated with short and long-term pulmonary issues. BPD is a condition that occurs when normal lung development is disrupted during the canalicular/saccular stage of development. Premature disruption of normal lung development contributes to short-term and long-term cardiopulmonary abnormalities that can be seen throughout the lifespan. These abnormalities can manifest as dysanaptic airway growth, alveolar simplification, airway inflammation and pulmonary vascular dysfunction. Preterm infants and young children are also more likely to have a higher burden of RSV-associated disease compared to term children [19]. Minimizing the long-term effects of disrupted lung growth caused by prematurity continues to be an ongoing challenge.
Although gestational age is the primary driver of BPD, there are a number of antenatal and postnatal determinants that contribute to the development and severity of BPD and its long-term respiratory outcomes (Table 1) [20]. Antenatal determinants of BPD may include maternal exposures such as infection and smoking, intrauterine growth restriction, genetic factors, sex, and degree of prematurity [21]. A meta-analysis of over 240,000 preterm infants reported that maternal chorioamnionitis was associated with a 2.32-fold increased risk of bronchopulmonary dysplasia as defined by a supplemental oxygen requirement at 28 days of life and a 1.29-fold risk of moderate or severe BPD at 36 weeks postmenstrual age [22]. Similarly, a meta-analysis of over 340,000 infants reported that intrauterine growth restriction or small for gestational age status was associated with a 1.56-fold increased risk of moderate or severe BPD at 36 weeks postmenstrual age [23]. Influences within the first few months of life play a substantial role as well, including ventilator-related trauma, lung inflammation from infectious and non-infectious sources, oxidative stress, and aberrant lung repair [21, 24]. Genetic factors have also been implicated in the development of BPD and more recently, an association between gut dysbiosis and BPD has been found [25,26,27].
The formal diagnosis of BPD is most commonly made at 36 weeks corrected gestational age based on respiratory support needs in the NICU, however there are varied definitions that are commonly used for clinical or research purposes [28,29,30]. While several of these definitions are associated with respiratory outcomes in early childhood [31,32,33], as well as spirometry at 4–8 years of age [34, 35], their utility in predicting longer-term outcomes is unknown. Additionally, it should be recognized that BPD is a highly heterogenous disease with varying components of several phenotypes present, which include the components in the original published description for BPD (i.e., alveoli, small airways, pulmonary vascular), as well as other phenotypic elements associated with prematurity (e.g., large airway malacia) [36, 37]. There are also numerous patients who may not meet one of the formal definitions of BPD [28,29,30] who have chronic respiratory disease associated with their prematurity, that may be more accurately described as post-prematurity respiratory disease.
Alveolar disease in BPD
Alveolar disease is frequently manifested as diminished pulmonary reserve with limitations in gas exchange. Infants with hypoplastic alveoli commonly have abnormal lung compliance with tachypnea and poor growth. Although national statistics are not readily available, it is estimated that as many as 12,000 infants with BPD are discharged to home on supplemental oxygen annually in the U.S., with a smaller subset on home ventilation [36]. Although home supplemental oxygen is frequently discontinued by 1 year of age [38, 39], and home ventilation by 2 years of age [40], reductions in lung function can persist into school-age, adolescence, and adulthood [13]. Infants and children with severe BPD may have cystic disease readily visible on thoracic imaging that may resemble features of COPD [41]. However, it is unknown whether this cystic disease translates into early onset COPD or a similar disease in later life, given the absence of longitudinal data over decades. Given the different pathophysiologies contributing to the cystic disease in BPD versus COPD, the natural history of disease and response to therapies is likely to vary.
Airway disease in BPD
Multiple studies have documented increased incidences of obstructive lung disease in individuals born prematurely [42]. Small airways disease in BPD often resembles asthma with a component of airway hyperreactivity [43], but its pathophysiology may also be linked to developmental dysanapsis as observed on infant pulmonary function testing (Fig. 1) [44]. Dysanapsis refers to a mismatch between airway tree caliber and lung size, which may affect airway resistance and ventilation of dead space [45]. In a child with a history of prematurity and airway obstruction it is often difficult to differentiate the contributions of airway inflammation, airway hyperactivity and developmental lung abnormalities caused by premature birth and early lung injury. It is not unusual in BPD for typical pharmaceutical therapies for asthma management to be not as efficacious for treating airflow obstruction, given the higher likelihood of dysanapsis, which has been theorized to persist into adulthood [46]. Furthermore, dysanapsis, has been associated with a greater risk for COPD in adults [47]. Nevertheless, children with BPD are also commonly diagnosed with asthma during early childhood. Diagnosis of asthma is more common in children with a family history, likely due to genetic or shared environmental factors [48]. The causes of an asthma-like phenotype in children with BPD is likely multifactorial, but may include exposure to respiratory viruses, gastroesophageal reflux and early life sensitization to environmental factors [49]. Those with asthma-like symptoms often are prescribed bronchodilators and inhaled corticosteroids and are burden with greater acute care usage, particularly during the first few years of life. In a single center study, children with BPD who had a family history of asthma were more likely to have respiratory symptoms and greater acute care usage in the first three years of life, versus those without a family history of asthma [48].
A Normal lung. B Compared with healthy bronchioles (top right), those from individuals with asthma (middle right) and BPD (lower right) are significantly narrowed. While asthma is driven primarily by inflammatory airflow obstruction, the origins of airway obstruction in BPD likely include a combination of developmental differences (dysanapsis) and lung injury-related factors (dysregulated repair/remodeling).
Although, clinical symptoms of airway obstruction in BPD may resolve or lessen during childhood [50], spirometry may detect obstructive disease into adulthood (Fig. 2) [51]. Additionally, formerly preterm children and adults with and without BPD have reported an increase in exercise induced intolerance and shortness of breath with activities associated with lower peak oxygen consumption measurements [52,53,54,55]. A recent meta-analysis of 11 studies (935 patients) reported expiratory flow outcomes for individuals born premature in the pre-surfactant era and aged beyond 16 years. Very preterm or very low birthweight infants did not reach their airway growth potential in adolescence or early adulthood, with lowest lung function in patients with a diagnosis of BPD [56]. Moreover, longitudinal data from 297 individuals born in the modern surfactant era who survived to age 25 demonstrated reduced airway function for those born very premature or very low birth weight compared with 260 controls [57]. One study examined three population-based cohorts from 10 to 35 years of age and found that extremely preterm individuals had parallel lung function trajectories compared to term individuals but trajectories in the preterm individuals were significantly lower than those born at term. Other studies however reported an accelerated loss in lung function in preterm and preterm children with BPD compared to term controls [58, 59]. A meta-analysis of cohorts including both preterm survivors and term individuals found that with increasing age, preterm survivors had lower FEV1/FVC values; with BPD survivors having the greatest decline in FEV1/FVC compared to term controls [58, 60]. Differences in lung function between preterm and term individuals, however was less in cohorts born more recently, in the post surfactant era [61]. Other prospective longitudinal studies of lung function have reported associations between lower lung function trajectories in adult life and risk factors, including secondhand smoke and pneumonia during early life [9, 11, 62]. Additional studies are needed to identify individuals at risk for early onset COPD and to introduce interventions and treatments that can minimize this risk and optimize lung growth potential in children and adolescents with BPD [63].
A Infants born very premature or with very low birthweight often display impaired alveolar growth and small airway abnormalities. B In older children and adults alveolar catch-up growth often occurs but obstructive airway disease can persist, reducing airway function potential, and increasing risk for COPD.
Pulmonary vascular disease in BPD
Pulmonary vascular disease manifesting as pulmonary hypertension (PH) is common in BPD (Fig. 3), affecting 17–43% of those with BPD [64] and is one of the most common causes (21.7%) of pulmonary hypertension in children [65]. It is associated with substantial morbidity and mortality, with up to 14–38% of infants with BPD and pulmonary hypertension dying even with care in tertiary care centers [64]. For most patients, however echocardiographic resolution of PH occurs during infancy [66]. Multidisciplinary BPD care, including respiratory support optimization, has been shown to be associated with pulmonary hypertension resolution in infants with BPD [67]. However, longer term case series have reported the presence of residual cardiac findings at 8–14 years of age, including right-sided cardiac strain and elevated pulmonary pressures [68, 69]. Also, a case series of former preterm individuals in their 20s found the presence of early pulmonary vascular disease, including elevated pulmonary pressures and right ventricular dysfunction [70]. In one study, overt pulmonary vascular disease was found in 18% of preterm adults who had an average gestational age of 28 weeks [70]. These studies suggest that preterm adults are at higher risk of pulmonary vascular disease compared to term adults, likely due to developmental disruption of lung and pulmonary vascular growth during infancy. How significant this risk is for developing pulmonary hypertension and other premature cardiovascular conditions is unknown, necessitating the need for longitudinal studies and guidelines for followup and treatment in this population.
Tracheobronchomalacia in BPD
Large airway malacia involving the trachea and/or bronchi is perhaps less common than the classic triad of manifestations of BPD, but may be underdiagnosed, as only some patients undergo procedures involving direct visualization of the lower airways [71]. Large airway malacia is estimated to affect only up to 2% of infants born less than 28 weeks gestation, but up to 30% of these may require tracheostomy placement [72]. It is presumed to improve for most patients by 2 years of age as the airway cartilage matures and with airway growth [73], but it is unknown whether early life airway malacia predisposes to the development of airway malacia in adult life, which can be seen with COPD [74]. Longitudinal airway evaluations are needed to answer this question.
Follow-up care
The initial transition for most patients with BPD is discharge or transfer from the neonatal intensive care unit; however, the trajectories may vary widely depending on local and institutional resources and may include locations such as the pediatric intensive care unit, general inpatient floor or step-down unit, subacute facility, community hospital, or to home. These transitions can create significant stress for caregivers. A thematic review of familial experiences with transitions for ill infants revealed that not being informed or involved in decisions to transition between settings, poor communication, and perceptions about care between settings, resulted in parental stress [75]. Similarly, the initial discharge to home may be associated with parental feelings of both hope and inadequacy [76]. However, coordination between inpatient and outpatient interdisciplinary teams may improve outcomes for infants with BPD during critical transitions [7].
Care models for the follow-up of respiratory disease vary widely depending on local institutional practices. Some infants and young children may receive care through NICU follow-up clinics staffed with neonatologists for the first 24–36 months of life prior to being transitioned to other providers. In other areas, pediatric pulmonologists may assume care after discharge from the NICU or later at the time of transition from NICU follow-up clinics. In areas where subspecialty resources are scarce, pediatricians or other primary care providers may take responsibility for management after NICU discharge. These providers may also assume all aspects of respiratory care when patients are discharged from subspecialty care as their disease improves. There are no current guidelines to inform healthcare providers when to refer to subspecialty care or when to transition patients to primary care providers, although a recent expert consensus suggested that “infants with moderate-severe BPD and those discharged on oxygen should be targeted for in-person pulmonary follow-up within 1 month of hospital discharge” [77].
Once discharged from the NICU, many infants and children with BPD will need multiple medications, require frequent primary care and subspecialty care appointments, may be technology dependent and will likely have more acute care use compared to the general population. This creates a large burden of responsibility for families and caregivers [7]. This burden of care may have negative effects on quality of life for both caregivers and the patients themselves. While quality of life is at its lowest in the immediate post-discharge period, it does improve over time [78].
There is emerging evidence that respiratory outcomes in the outpatient setting do not always correlate with BPD severity scores in the NICU [39, 79]. This means that some children with mild, moderate or severe BPD severity scores from the NICU will have significant respiratory morbidities as outpatients, while others will not, making it difficult to predict outpatient outcomes in this population. As such, better outpatient phenotyping could help with better assignment of appropriate therapies, identification of children at higher risk for adverse outcomes and improved prediction of long-term outcomes. Phenotypic characterization could include respiratory medication use, chronic and acute respiratory symptoms, spirometry measurements and exercise tolerance, radiographic imaging, and family history. As of yet, no biomarkers exist that predict respiratory outcomes in BPD children following initial hospital discharge. Outpatient phenotyping may also help guide the need for subspecialty management, follow-up care and timing of longitudinal testing of respiratory function in this population.
Given the expected course of disease, there is also a need to establish guidelines for an ongoing care plan throughout the lifespan. For example, patients with cystic fibrosis are recommended to have quarterly spirometry after 6 years of age [80], but for BPD, only a conditional recommendation exists to monitor lung function with no guidance on specific tests or intervals [81]. Care models for long-term followup of cancer survivors also exist [82].
Lifelong BPD care models could help with timely identification of children and adults with clinically significant cardiopulmonary abnormalities, allowing more personalize therapy to help mitigate cardiopulmonary morbidities and other complications associated with preterm birth. One potential model could include ongoing respiratory subspecialty follow-up every 1–12 months as long as the patient requires supplemental oxygen, invasive or non-invasive ventilatory support, or daily respiratory medications. Respiratory subspecialty follow-up could be transitioned to every 2–5 years if the patient did not meet those criteria or had minimal or no respiratory symptoms. Screening for pulmonary hypertension should also be done, especially in extremely premature, low birth and IUGR infants. The optimal intervals for screening are unknown, however frequency of screening should be personalized based on clinical history but should also include asymptomatic children during periods of growth spurts and during early adult life. Additional, screening for systemic hypertension should be performed since preterm birth is associated with a higher likelihood of high blood pressure during adolescence [83]. There are also no specific models for transitioning patients from pediatric to adult care as young adults. A lifelong BPD care model should also involve a carefully orchestrated hand-off between pediatric and adult providers similar to that in cystic fibrosis care centers, acknowledging health, developmental and psychosocial challenges in patients with complex chronic disease [84, 85]. It is important to emphasize that respiratory care should not occur in a vacuum; given the complexity of preterm respiratory disease, ideally patients should have access to comprehensive care, including therapists, nutritionists, cardiologists, otolaryngologists, gastroenterologists, etc., depending on individual needs [7]. In terms of testing, basic spirometry, pulse-oximetry, and 6-minute walk test should be considered every 1–5 years depending on follow-up intervals, with consideration for imaging if any of these tests are abnormal. In terms of imaging, computed tomography of the chest could be considered to identify heterogenous lung disease, cystic disease, etc. Novel techniques in magnetic resonance imaging can identify airway malacia, pulmonary hypertension, and regional hypoventilation and may represent a future way to monitor lung structure without radiation [86]. Polysomnography is indicated if signs or symptoms of sleep disordered breathing are present, or supplemental oxygen needs persist past 2 years of age [87]. Sleep disordered breathing has been shown to be higher in children born premature [88]. Surveillance airway endoscopy is indicated in infants with tracheostomy to reduce outpatient mortality in this population [89].
Altering trajectories
As individuals born prematurely frequently have lower lung function, it is critical to undertake interventions that have the potential to improve overall lung function trajectories. There have been advances in understanding altered lung development in BPD, including the roles of growth factors and other molecular mediators of lung growth, inflammation, oxidative stress, injury from proteases, aberrant repair and extracellular matrix deposition, and epithelial cell plasticity [90], and these features will guide future therapy development. Interventions can take the form of enhancing the potential for catch-up lung growth, preventative guidance against potentially detrimental environmental exposures, prophylaxis against infectious agents and associated pulmonary inflammation, avoidance of other inflammatory processes such as chronic aspiration, or therapies to improve lung function and respiratory health. Interventions undertaken earlier in life may have greater benefits than in later life when lung function trajectories are established [91].
In contrast to many other lung diseases, the development of BPD during infancy implies that there is a long period of somatic growth for the individual whereby potentially lung repair and catch-up growth may occur. Ensuring adequate nutrition and growth is essential for premature infants and children; many of them continue to have growth failure at 3 years of age [92]. Unfortunately, metrics for lung growth during infancy and early childhood are frequently not readily available at many centers (i.e., infant pulmonary function testing or forced oscillometry), leaving spirometry at 6 years of age as often the first measure of lung growth. Growth parameters, such as height and weight, obtained at 2 and 6 years of age do not necessarily correlate with spirometry [34], thus somatic growth may not be a perfect proxy for lung growth in young children. However, improved linear growth is associated with the ability to wean respiratory support in patients with BPD [93] and may represent an imperfect measure of lung growth/ improved lung function.
As the lungs are exposed to air constantly, any pollutant or particulate matter can have significant consequences. As infants and children spend a significant proportion of their time indoors, indoor air pollution may have an outsized effect on the developing lung. Indoor air pollutants that have been associated with acute care use or chronic respiratory symptoms for infants and young children with BPD include secondhand tobacco smoke [94, 95], and other sources of indoor air pollution such as gas stoves [96]. There are no published data on the effects of emissions from electronic cigarettes and other vaping devices on infants and children with BPD, but household use of these devices is often associated with conventional cigarette use [97]. Outdoor air pollution in general or that specifically associated with traffic may also affect infants and young children with BPD [98, 99]. It should be noted that while preventative counseling leading to decreased exposure may have benefits for short-term respiratory morbidities, long-term benefits for avoidance to air pollutants have not been established for BPD.
Socioeconomic status may also influence outpatient respiratory outcomes in children with BPD.
One study found that after initial hospital discharge, children with BPD who resided in areas of high deprivation were more likely to be re-hospitalized and have emergency department visits for respiratory complaints compared to children from low deprivation areas [100]. Another study reported that preterm infants with BPD from disadvantaged areas were more likely to be hospitalized, during the first year following initial hospital discharge [101]. Early interventions are needed to minimize outcome disparities due to socioeconomic status in children with BPD. Longitudinal studies are also needed to track the relationship between socioeconomic status and lung growth trajectories in later life.
As previously mentioned, severe respiratory infections in early life can have life-long sequela [11], thus prophylaxis against respiratory infections is highly recommended. Benefits are well established for RSV and influenza prophylaxis in BPD [102]. RSV prophylaxis may take place prior to birth with RSV vaccines given in the third trimester, or postnatally with palivizumab given monthly through the RSV season or nirsevimab given once at the beginning of the season. Influenza vaccines are recommended to be given annually at the beginning of the influenza season. The specific benefits for COVID-19 vaccines for BPD are not established; however, COVID-19 vaccines have been shown to be efficacious in the general pediatric population [103]. The odds of contracting viral respiratory infections may be increased with exposure to additional children either in a daycare setting or living in the home. Specifically, the odds of emergency department visits or hospital readmissions may be three-fold for children aged 0–3 years who attend daycare versus those who do not [79, 104]. Similarly, albeit with lower odds ratios, the presence of every additional child in the household, increases the risk of hospital readmission, other acute care usage, and chronic respiratory symptoms by 10–18% depending on the outcome [105].
The evidence for the chronic daily use for therapies to improve outpatient outcomes in BPD is weak. Routine use of diuretics is discouraged, and trials of bronchodilators or inhaled corticosteroids are recommended to be limited to only those with chronic cough or wheezing in the recent American Thoracic Society guidelines for outpatient BPD [87]; the European Respiratory Society guidelines offer similar guidance [81]. Nevertheless, novel approaches to therapeutics, such as SMART for asthma [106], may have some benefits for BPD management. In a similar vein, use of inhaled therapies in COPD such as salmeterol/fluticasone and/or tiotropium may slow lung function decline [107]. However, limited outpatient data exists for commonly used outpatient medications in BPD such as diuretics or inhaled steroids, let alone novel therapies such as these.
Lastly, experimental cell-based therapies are poised to alter BPD development and downstream pulmonary trajectories in premature infants [108]. Mesenchymal Stem Cells (MSCs) have anti-inflammatory, antifibrotic, antioxidative, and proangiogenic properties. Preclinical data and early clinical trials involving MSCs release bioactive compounds packaged in extracellular vesicles (EVs) or exosomes are ongoing [109].
Conclusions
Despite advances in neonatal care, bronchopulmonary dysplasia remains a common sequela of preterm birth with altered respiratory trajectories into adulthood. Current interventions to improve outcomes are largely limited to preventative and prophylactic measures owing to the paucity of data on outpatient therapies though benefits of new research in these areas are on the horizon. Rectifying gaps in therapy and knowledge will require better understanding of the long-term course of respiratory disease in people born preterm, including the important developing of BPD phenotypes, longitudinal studies to define disease trajectories, care models designed to provide care and support throughout the lifespan, and clinical trials to assess the efficacy of novel therapies.
References
Ohuma EO, Moller AB, Bradley E, Chakwera S, Hussain-Alkhateeb L, Lewin A, et al. National, regional, and global estimates of preterm birth in 2020, with trends from 2010: a systematic analysis. Lancet. 2023;402:1261–71.
Pryhuber GS, Maitre NL, Ballard RA, Cifelli D, Davis SD, Ellenberg JH, et al. Prematurity and respiratory outcomes program (PROP): study protocol of a prospective multicenter study of respiratory outcomes of preterm infants in the United States. BMC Pediatrics. 2015;15:37.
Northway WH Jr., Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med. 1967;276:357–68.
Jensen EA, Edwards EM, Greenberg LT, Soll RF, Ehret DEY, Horbar JD. Severity of Bronchopulmonary Dysplasia Among Very Preterm Infants in the United States. Pediatrics. 2021;148:e2020030007.
Aoyama BC, Rice JL, McGrath-Morrow SA, Collaco JM. Mortality in Outpatients with Bronchopulmonary Dysplasia. J Pediatrics. 2022;241:48–53.e1.
Sillers L, Alexiou S, Jensen EA. Lifelong pulmonary sequelae of bronchopulmonary dysplasia. Curr Opin Pediatr. 2020;32:252–60.
Abman SH, Collaco JM, Shepherd EG, Keszler M, Cuevas-Guaman M, Welty SE, et al. Interdisciplinary Care of Children with Severe Bronchopulmonary Dysplasia. J Pediatrics. 2017;181:12–28.e1.
Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993-2012. JAMA J Am Med Assoc. 2015;314:1039–51.
Martinez FD. Early-Life Origins of Chronic Obstructive Pulmonary Disease. N Engl J Med. 2016;375:871–8.
Barker DJ, Godfrey KM, Fall C, Osmond C, Winter PD, Shaheen SO. Relation of birth weight and childhood respiratory infection to adult lung function and death from chronic obstructive airways disease. BMJ. 1991;303:671–5.
Chan JY, Stern DA, Guerra S, Wright AL, Morgan WJ, Martinez FD. Pneumonia in childhood and impaired lung function in adults: a longitudinal study. Pediatrics. 2015;135:607–16.
Morrow LA, Wagner BD, Ingram DA, Poindexter BB, Schibler K, Cotten CM, et al. Antenatal Determinants of Bronchopulmonary Dysplasia and Late Respiratory Disease in Preterm Infants. Am J Respir Crit Care Med. 2017;196:364–74.
Caskey S, Gough A, Rowan S, Gillespie S, Clarke J, Riley M, et al. Structural and Functional Lung Impairment in Adult Survivors of Bronchopulmonary Dysplasia. Ann Am Thorac Soc. 2016;13:1262–70.
Allen JL. Airway function throughout the lifespan: Pediatric origins of adult respiratory disease. Pediatr Investig. 2019;3:236–44.
Crump C, Sundquist J, Winkleby MA, Sundquist K. Gestational age at birth and mortality from infancy into mid-adulthood: a national cohort study. Lancet Child Adolesc Health. 2019;3:408–17.
Collaco JM, McGrath-Morrow SA. Bronchopulmonary dysplasia as a determinant of respiratory outcomes in adult life. Pediatr Pulmonol. 2021;56;3464–71.
Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–56.
Doyle LW, Ranganathan S, Cheong J. Bronchopulmonary dysplasia and expiratory airflow at 8 years in children born extremely preterm in the post-surfactant era. Thorax. 2023;78:484–8.
Wang X, Li Y, Shi T, Bont LJ, Chu HY, Zar HJ, et al. Global disease burden of and risk factors for acute lower respiratory infections caused by respiratory syncytial virus in preterm infants and young children in 2019: a systematic review and meta-analysis of aggregated and individual participant data. Lancet. 2024;403:1241–53.
Thébaud B, Goss KN, Laughon M, Whitsett JA, Abman SH, Steinhorn RH, et al. Bronchopulmonary dysplasia. Nat Rev Dis Prim. 2019;5:78.
Kalikkot Thekkeveedu R, Guaman MC, Shivanna B. Bronchopulmonary dysplasia: A review of pathogenesis and pathophysiology. Respir Med. 2017;132:170–7.
Villamor-Martinez E, Alvarez-Fuente M, Ghazi AMT, Degraeuwe P, Zimmermann LJI, Kramer BW, et al. Association of Chorioamnionitis With Bronchopulmonary Dysplasia Among Preterm Infants: A Systematic Review, Meta-analysis, and Metaregression. JAMA Netw Open. 2019;2:e1914611.
Pierro M, Villamor-Martinez E, van Westering-Kroon E, Alvarez-Fuente M, Abman SH, Villamor E. Association of the dysfunctional placentation endotype of prematurity with bronchopulmonary dysplasia: a systematic review, meta-analysis and meta-regression. Thorax. 2022;77:268–75.
Bonadies L, Zaramella P, Porzionato A, Perilongo G, Muraca M, Baraldi E. Present and Future of Bronchopulmonary Dysplasia. J Clin Med. 2020;9:1539.
Lavoie PM, Pham C, Jang KL. Heritability of bronchopulmonary dysplasia, defined according to the consensus statement of the national institutes of health. Pediatrics. 2008;122:479–85.
Lavoie PM, Rayment JH. Genetics of bronchopulmonary dysplasia: An update. Semin Perinatol. 2023;47:151811.
Chen SM, Lin CP, Jan MS. Early Gut Microbiota Changes in Preterm Infants with Bronchopulmonary Dysplasia: A Pilot Case-Control Study. Am J Perinatol. 2021;38:1142–9.
Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–9.
Jensen EA, Dysart K, Gantz MG, McDonald S, Bamat NA, Keszler M, et al. The Diagnosis of Bronchopulmonary Dysplasia in Very Preterm Infants. An Evidence-based Approach. Am J Respir Crit Care Med. 2019;200:751–9.
Higgins RD, Jobe AH, Koso-Thomas M, Bancalari E, Viscardi RM, Hartert TV, et al. Bronchopulmonary Dysplasia: Executive Summary of a Workshop. J Pediatrics. 2018;197:300–8.
Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, et al. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116:1353–60.
Katz TA, van Kaam AH, Schuit E, Mugie SM, Aarnoudse-Moens CSH, Weber EH, et al. Comparison of New Bronchopulmonary Dysplasia Definitions on Long-Term Outcomes in Preterm Infants. J Pediatrics. 2023;253:86–93.e4.
Perez-Tarazona S, Marset G, Part M, Lopez C, Perez-Lara L. Definitions of Bronchopulmonary Dysplasia: Which One Should We Use?. J Pediatrics. 2022;251:67–73.e2.
Aoyama BC, Collaco JM, McGrath-Morrow SA. Predictors of pulmonary function at 6 years of age in infants with bronchopulmonary dysplasia. Pediatric Pulmonol. 2021;56:974–81.
Rite S, Martin de Vicente C, Garcia-Iniguez JP, Couce ML, Samper MP, Montaner A, et al. The Consensus Definition of Bronchopulmonary Dysplasia Is an Adequate Predictor of Lung Function at Preschool Age. Front Pediatr. 2022;10:830035.
Collaco JM, McGrath-Morrow SA. Respiratory Phenotypes for Preterm Infants, Children, and Adults: Bronchopulmonary Dysplasia and More. Ann Am Thorac Soc. 2018;15:530–8.
Wu KY, Jensen EA, White AM, Wang Y, Biko DM, Nilan K, et al. Characterization of Disease Phenotype in Very Preterm Infants with Severe Bronchopulmonary Dysplasia. Am J Respir Crit Care Med. 2020;201:1398–406.
Yeh J, McGrath-Morrow SA, Collaco JM. Oxygen weaning after hospital discharge in children with bronchopulmonary dysplasia. Pediatr Pulmonol. 2016;51:1206–11.
McGlynn JR, Aoyama BC, Martin A, Collaco JM, McGrath-Morrow SA. Outpatient respiratory outcomes in children with BPD on supplemental oxygen. Pediatr Pulmonol. 2023;58:1535–41.
Manimtim WM, Agarwal A, Alexiou S, Levin JC, Aoyama B, Austin ED, et al. Respiratory Outcomes for Ventilator-Dependent Children With Bronchopulmonary Dysplasia. Pediatrics. 2023;151:e2022060651.
Tonson la Tour A, Spadola L, Sayegh Y, Combescure C, Pfister R, Argiroffo CB, et al. Chest CT in bronchopulmonary dysplasia: clinical and radiological correlations. Pediatr Pulmonol. 2013;48:693–8.
Kotecha SJ, Edwards MO, Watkins WJ, Henderson AJ, Paranjothy S, Dunstan FD, et al. Effect of preterm birth on later FEV1: a systematic review and meta-analysis. Thorax. 2013;68:760–6.
Royce SG, Nold MF, Bui C, Donovan C, Lam M, Lamanna E, et al. Airway Remodeling and Hyperreactivity in a Model of Bronchopulmonary Dysplasia and Their Modulation by IL-1 Receptor Antagonist. Am J Respir Cell Mol Biol. 2016;55:858–68.
Nelin LD, Kielt MJ, Jebbia M, Jadcherla S, Shepherd EG. Bronchodilator responsiveness and dysanapsis in bronchopulmonary dysplasia. ERJ Open Res. 2022;8:00682-2021.
Mortola JP. Dysanaptic lung growth: an experimental and allometric approach. J Appl Physiol Respir Environ Exerc Physiol. 1983;54:1236–41.
Duke JW, Lewandowski AJ, Abman SH, Lovering AT. Physiological aspects of cardiopulmonary dysanapsis on exercise in adults born preterm. J Physiol. 2022;600:463–82.
Smith BM, Kirby M, Hoffman EA, Kronmal RA, Aaron SD, Allen NB, et al. Association of Dysanapsis With Chronic Obstructive Pulmonary Disease Among Older Adults. JAMA. 2020;323:2268–80.
McGlynn JR, Aoyama BC, Collaco JM, McGrath-Morrow SA. Family history of asthma influences outpatient respiratory outcomes in children with BPD. Pediatr Pulmonol. 2021;56:3265–72.
Caffarelli C, Gracci S, Giannì G, Bernardini R. Are Babies Born Preterm High-Risk Asthma Candidates? J Clin Med. 2023;12:5400.
Jaakkola JJ, Ahmed P, Ieromnimon A, Goepfert P, Laiou E, Quansah R, et al. Preterm delivery and asthma: a systematic review and meta-analysis. J Allergy Clin Immunol. 2006;118:823–30.
Been JV, Lugtenberg MJ, Smets E, van Schayck CP, Kramer BW, Mommers M, et al. Preterm birth and childhood wheezing disorders: a systematic review and meta-analysis. PLoS Med. 2014;11:e1001596.
Lovering AT, Elliott JE, Laurie SS, Beasley KM, Gust CE, Mangum TS, et al. Ventilatory and sensory responses in adult survivors of preterm birth and bronchopulmonary dysplasia with reduced exercise capacity. Ann Am Thorac Soc. 2014;11:1528–37.
MacLean JE, DeHaan K, Fuhr D, Hariharan S, Kamstra B, Hendson L, et al. Altered breathing mechanics and ventilatory response during exercise in children born extremely preterm. Thorax. 2016;71:1012–9.
Peralta GP, Piatti R, Haile SR, Adams M, Bassler D, Moeller A, et al. Respiratory morbidity in preschool and school-age children born very preterm and its association with parents’ health-related quality of life and family functioning. Eur J Pediatrics. 2023;182:1201–10.
Delfrate J, Girard-Bock C, Curnier D, Perie D, Cloutier A, Gascon G, et al. Cardiopulmonary response to exercise in adults born very preterm. Eur Respir J. 2023:2300503.
Doyle LW, Andersson S, Bush A, Cheong JLY, Clemm H, Evensen KAI, et al. Expiratory airflow in late adolescence and early adulthood in individuals born very preterm or with very low birthweight compared with controls born at term or with normal birthweight: a meta-analysis of individual participant data. Lancet Respir Med. 2019;7:677–86.
Doyle LW, Adams AM, Robertson C, Ranganathan S, Davis NM, Lee KJ, et al. Increasing airway obstruction from 8 to 18 years in extremely preterm/low-birthweight survivors born in the surfactant era. Thorax. 2017;72:712–9.
Simpson SJ, Turkovic L, Wilson AC, Verheggen M, Logie KM, Pillow JJ, et al. Lung function trajectories throughout childhood in survivors of very preterm birth: a longitudinal cohort study. Lancet Child Adolesc health. 2018;2:350–9.
Levin JC, Sheils CA, Gaffin JM, Hersh CP, Rhein LM, Hayden LP. Lung function trajectories in children with post-prematurity respiratory disease: identifying risk factors for abnormal growth. Respir Res. 2021;22:143.
Gibbons JTD, Course CW, Evans EE, Kotecha S, Kotecha SJ, Simpson SJ. Increasing airway obstruction through life following bronchopulmonary dysplasia: a meta-analysis. ERJ Open Res. 2023;9:00046-2023.
Bårdsen T, Røksund OD, Benestad MR, Hufthammer KO, Clemm HH, Mikalsen IB, et al. Tracking of lung function from 10 to 35 years after being born extremely preterm or with extremely low birth weight. Thorax. 2022;77:790–8.
Bui DS, Walters HE, Burgess JA, Perret JL, Bui MQ, Bowatte G, et al. Childhood Respiratory Risk Factor Profiles and Middle-Age Lung Function: A Prospective Cohort Study from the First to Sixth Decade. Ann Am Thorac Soc. 2018;15:1057–66.
Moschino L, Bonadies L, Baraldi E. Lung growth and pulmonary function after prematurity and bronchopulmonary dysplasia. Pediatr Pulmonol. 2021;56:3499–508.
Collaco JM, Romer LH, Stuart BD, Coulson JD, Everett AD, Lawson EE, et al. Frontiers in pulmonary hypertension in infants and children with bronchopulmonary dysplasia. Pediatr Pulmonol. 2012;47:1042–53.
Abman SH, Mullen MP, Sleeper LA, Austin ED, Rosenzweig EB, Kinsella JP, et al. Characterisation of paediatric pulmonary hypertensive vascular disease from the PPHNet Registry. Eur Respir J. 2021;59:2003337.
Aoyama BC, McGrath MSA, Collaco JM. Characteristics of Children with Bronchopulmonary Dysplasia with Prolonged and/or Later-Onset Pulmonary Hypertension. Ann Am Thorac Soc. 2021;18:1746–8.
Yung D, Jackson EO, Blumenfeld A, Redding G, DiGeronimo R, McGuire JK, et al. A multidisciplinary approach to severe bronchopulmonary dysplasia is associated with resolution of pulmonary hypertension. Front Pediatr. 2023;11:1077422.
Kwon HW, Kim HS, An HS, Kwon BS, Kim GB, Shin SH, et al. Long-Term Outcomes of Pulmonary Hypertension in Preterm Infants with Bronchopulmonary Dysplasia. Neonatology. 2016;110:181–9.
Zivanovic S, Pushparajah K, Calvert S, Marlow N, Razavi R, Peacock JL, et al. Pulmonary Artery Pressures in School-Age Children Born Prematurely. J Pediatrics. 2017;191:42–9.e3.
Goss KN, Beshish AG, Barton GP, Haraldsdottir K, Levin TS, Tetri LH, et al. Early Pulmonary Vascular Disease in Young Adults Born Preterm. Am J Respir Crit Care Med. 2018;198:1549–58.
Hysinger EB, Friedman NL, Padula MA, Shinohara RT, Zhang H, Panitch HB, et al. Tracheobronchomalacia Is Associated with Increased Morbidity in Bronchopulmonary Dysplasia. Ann Am Thoracic Soc. 2017;14:1428–35.
Wai KC, Keller RL, Lusk LA, Ballard RA, Chan DK, Trial of Late Surfactant Study G. Characteristics of Extremely Low Gestational Age Newborns Undergoing Tracheotomy: A Secondary Analysis of the Trial of Late Surfactant Randomized Clinical Trial. JAMA Otolaryngol Head Neck Surg. 2017;143:13–9.
Carden KA, Boiselle PM, Waltz DA, Ernst A. Tracheomalacia and tracheobronchomalacia in children and adults: an in-depth review. Chest. 2005;127:984–1005.
Sverzellati N, Rastelli A, Chetta A, Schembri V, Fasano L, Pacilli AM, et al. Airway malacia in chronic obstructive pulmonary disease: prevalence, morphology and relationship with emphysema, bronchiectasis and bronchial wall thickening. Eur Radiol. 2009;19:1669–78.
Ballantyne M, Orava T, Bernardo S, McPherson AC, Church P, Fehlings D. Parents’ early healthcare transition experiences with preterm and acutely ill infants: a scoping review. Child Care Health Dev. 2017;43:783–96.
Hua W, Zhou J, Wang L, Li C, Zheng Q, Yuwen W, et al. It turned my life upside down’: Parents’ emotional experience of the transition with their preterm infant from birth to discharge Home-A qualitative study. Aust Crit Care. 2023;36:679–86.
Levin JC, Annesi CA, Williams DN, Abman SH, McGrath-Morrow SA, Nelin LD, et al. Discharge Practices for Infants with Bronchopulmonary Dysplasia: A Survey of National Experts. J Pediatrics. 2023;253:72–8.e3.
Lee DMX, Tan AKS, Ng YPM, Amin Z. Quality of life of patients and caregivers affected by bronchopulmonary dysplasia: a systematic review. Qual Life Res. 2023;32:1859–69.
McGrath-Morrow SA, Agarwal A, Alexiou S, Austin ED, Fierro JL, Hayden LP, et al. Daycare Attendance is Linked to Increased Risk of Respiratory Morbidities in Children Born Preterm with Bronchopulmonary Dysplasia. J Pediatr. 2022;249:22–8.
Castellani C, Duff AJA, Bell SC, Heijerman HGM, Munck A, Ratjen F, et al. ECFS best practice guidelines: the 2018 revision. J Cyst Fibros. 2018;17:153–78.
Duijts L, van Meel ER, Moschino L, Baraldi E, Barnhoorn M, Bramer WM, et al. European Respiratory Society guideline on long-term management of children with bronchopulmonary dysplasia. Eur Respir J. 2020;55:1900788.
Cai J, Cheung YT, Hudson MM. Care Models and Barriers to Long-Term Follow-Up Care Among Childhood Cancer Survivors and Health Care Providers in Asia: A Literature Review. JCO Glob Oncol. 2024;10:e2300331.
Hochmayr C, Ndayisaba JP, Gande N, Staudt A, Bernar B, Stock K, et al. Cardiovascular health profiles in adolescents being born term or preterm-results from the EVA-Tyrol study. BMC Cardiovasc Disord. 2023;23:371.
American Academy of P, American Academy of Family P, American College of Physicians-American Society of Internal M. A consensus statement on health care transitions for young adults with special health care needs. Pediatrics. 2002;110:1304–6.
Singh J, Towns S, Jayasuriya G, Hunt S, Simonds S, Boyton C, et al. Transition to adult care in cystic fibrosis: The challenges and the structure. Paediatr Respir Rev. 2022;41:23–9.
Higano NS, Bates AJ, Gunatilaka CC, Hysinger EB, Critser PJ, Hirsch R, et al. Bronchopulmonary dysplasia from chest radiographs to magnetic resonance imaging and computed tomography: adding value. Pediatr Radio. 2022;52:643–60.
Cristea AI, Ren CL, Amin R, Eldredge LC, Levin JC, Majmudar PP, et al. Outpatient Respiratory Management of Infants, Children, and Adolescents with Post-Prematurity Respiratory Disease: An Official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2021;204:e115–e33.
Ortiz LE, McGrath-Morrow SA, Sterni LM, Collaco JM. Sleep disordered breathing in bronchopulmonary dysplasia. Pediatr Pulmonol. 2017;52:1583–91.
Ong T, Liu CC, Elder L, Hill L, Abts M, Dahl JP, et al. The Trach Safe Initiative: A Quality Improvement Initiative to Reduce Mortality among Pediatric Tracheostomy Patients. Otolaryngol Head Neck Surg. 2020;163:221–31.
Surate Solaligue DE, Rodriguez-Castillo JA, Ahlbrecht K, Morty RE. Recent advances in our understanding of the mechanisms of late lung development and bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2017;313:L1101–L53.
Berry CE, Billheimer D, Jenkins IC, Lu ZJ, Stern DA, Gerald LB, et al. A Distinct Low Lung Function Trajectory from Childhood to the Fourth Decade of Life. Am J Respir Crit Care Med. 2016;194:607–12.
Aoyama BC, McGrath-Morrow SA, Psoter KJ, Collaco JM. Patterns of early life somatic growth in infants and children with a history of chronic lung disease of prematurity. Pediatr Pulmonol. 2023;58:2592–9.
Miller AN, Moise AA, Cottrell L, Loomis K, Polak M, Gest A. Linear growth is associated with successful respiratory support weaning in infants with bronchopulmonary dysplasia. J Perinatol. 2022;42:544–5.
Collaco JM, Aherrera AD, Ryan T, McGrath-Morrow SA. Secondhand smoke exposure in preterm infants with bronchopulmonary dysplasia. Pediatr Pulmonol. 2014;49:173–8.
Collaco JM, Aherrera AD, Breysse PN, Winickoff JP, Klein JD, McGrath-Morrow SA. Hair nicotine levels in children with bronchopulmonary dysplasia. Pediatrics. 2015;135:e678–86.
Rice JL, McGrath-Morrow SA, Collaco JM. Indoor Air Pollution Sources and Respiratory Symptoms in Bronchopulmonary Dysplasia. J Pediatrics. 2020;222:85–90.e2.
Mazza D, McGrath-Morrow SA, Collaco JM. Use and Perceptions of Electronic Cigarettes Among Caregivers of Infants and Children with Bronchopulmonary Dysplasia. Pedia Aller Imm Pul. 2017;30:141–7.
Collaco JM, Morrow M, Rice JL, McGrath-Morrow SA. Impact of road proximity on infants and children with bronchopulmonary dysplasia. Pediatr Pulmonol. 2020;55:369–75.
Kelchtermans J, Aoyama BC, Rice JL, Martin A, Collaco JM, McGrath-Morrow SA. Ambient Air Pollution and Outpatient Morbidities in Bronchopulmonary Dysplasia. Ann Am Thorac Soc. 2024;21:88–93.
Banwell E, Collaco JM, Oates GR, Rice JL, Juarez LD, Young LR, et al. Area Deprivation and Respiratory Morbidities in Children with Bronchopulmonary Dysplasia. Pediatr Pulmonol. 2022;57:2053–9.
Deschamps J, Boucekine M, Fayol L, Dubus JC, Nauleau S, Garcia P, et al. Neighborhood Disadvantage and Early Respiratory Outcomes in Very Preterm Infants with Bronchopulmonary Dysplasia. J Pediatrics. 2021;237:177–82.e1.
O’Leary ST, Yonts AB, Gaviria-Agudelo C, Kimberlin DW, Paulsen GC. Summer 2023 ACIP Update: RSV Prevention and Updated Recommendations on Other Vaccines. Pediatrics. 2023;152:e2023063955.
Klein NP, Stockwell MS, Demarco M, Gaglani M, Kharbanda AB, Irving SA, et al. Effectiveness of COVID-19 Pfizer-BioNTech BNT162b2 mRNA Vaccination in Preventing COVID-19-Associated Emergency Department and Urgent Care Encounters and Hospitalizations Among Nonimmunocompromised Children and Adolescents Aged 5-17 Years - VISION Network, 10 States, April 2021-January 2022. MMWR Morbidity Mortal Wkly Rep. 2022;71:352–8.
McGrath-Morrow SA, Lee G, Stewart BH, McGinley BM, Lefton-Greif MA, Okelo SO, et al. Day care increases the risk of respiratory morbidity in chronic lung disease of prematurity. Pediatrics. 2010;126:632–7.
Collaco JM, Tsukahara KR, Tracy MC, Sheils CA, Rice JL, Rhein LM, et al. Number of children in the household influences respiratory morbidities in children with bronchopulmonary dysplasia in the outpatient setting. Pediatr Pulmonol. 2024;59:314–22.
Beasley R, Harrison T, Peterson S, Gustafson P, Hamblin A, Bengtsson T, et al. Evaluation of Budesonide-Formoterol for Maintenance and Reliever Therapy Among Patients With Poorly Controlled Asthma: A Systematic Review and Meta-analysis. JAMA Netw Open. 2022;5:e220615.
Gladysheva ES, Malhotra A, Owens RL. Influencing the decline of lung function in COPD: use of pharmacotherapy. Int J Chron Obstruct Pulmon Dis. 2010;5:153–64.
Thebaud B, Kourembanas S. Can We Cure Bronchopulmonary Dysplasia? J Pediatr. 2017;191:12–4.
Lesage F, Thébaud B. Mesenchymal Stromal Cell-Derived Extracellular Vesicles for Neonatal Lung Disease: Tiny Particles, Major Promise, Rigorous Requirements for Clinical Translation. Cells. 2022;11:1176.
Funding
This work was supported by a R03HD109442 (JMC, SAM), The funding sources had no involvement in the writing of the manuscript or the decision to submit.
Author information
Authors and Affiliations
Contributions
Joseph M. Collaco: contributed to the conception and design of the work, drafting and writing and editing of the final manuscript. Laurie C. Eldredge: contributed to the conception and design of the work, drafting and writing and editing of the final manuscript. Sharon A. McGrath-Morrow: to the conception and design of the work, drafting writing and editing of the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Collaco, J.M., Eldredge, L.C. & McGrath-Morrow, S.A. Long-term pulmonary outcomes in BPD throughout the life-course. J Perinatol (2024). https://doi.org/10.1038/s41372-024-01957-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41372-024-01957-9
- Springer Nature America, Inc.