Abstract
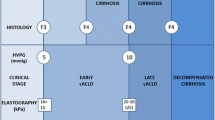
There are two clinically relevant questions when screening for portal hypertension: first, identification of patients at high risk for clinically significant portal hypertension (CSPH; defined by an hepatic venous pressure gradient (HVPG) ≥ 10 mmHg); second, identification of patients at high risk for esophageal varices (OV). Thanks to the improvements in the noninvasive methods, most patients are currently diagnosed in a very initial stage of cirrhosis, in which clinically CSPH and OV are often absent. In this new scenario, a large proportion of HVPG measurements and screening endoscopies may be unnecessary. Among available noninvasive methods, liver stiffness measurement using transient elastography (TE) has been the most extensively studied. The diagnostic performance of TE for predicting OV is not as good as for CSPH (AUROC 0.84 and 0.93, respectively). TE is better at ruling in than ruling out CSPH, whereas it is better at ruling out than ruling in OV. Strategies combining liver stiffness measurement with platelet count and spleen diameter could be useful to rule out OV. Finally, the evidence accumulated so far indicates that noninvasive methods cannot replace HVPG for a detailed portal hypertension evaluation and upper GI endoscopy for detecting OV. However, in settings where HVPG is not available, TE could be considered to stratify the risk of CSPH. Similarly, strategies combining TE with platelet count and spleen diameter could be useful to rule out OV in patients at low risk of having portal hypertension.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
The Baveno V consensus conference recommended 5 years ago that all patients with newly diagnosed cirrhosis should undergo screening endoscopy for assessing gastroesophageal varices in order to begin primary prophylaxis, if required, and hepatic vein pressure gradient (HVPG) measurement should be obtained for prognostic aims whenever available [1]. However, in the meantime noninvasive methods have been increasingly validated and used not only for staging liver fibrosis but also to predict complications of cirrhosis including those related to portal hypertension [2]. Among noninvasive methods, transient elastography (TE) (FibroScan™, Echosens, Paris, France) has reached an established role in clinical practice, particularly in viral hepatitis-induced chronic liver diseases and is now routinely used worldwide [3]. Several meta-analyses [4–8] have confirmed the excellent performance of liver stiffness (LS) measurement using TE for diagnosing cirrhosis in patients with chronic liver disease, with mean AUROC values of 0.94 and a suggested optimal cut-off of 13 kPa [6]. In clinical practice, TE is better at ruling out than ruling in cirrhosis with negative and positive predictive values of 96 % and 74 %, respectively [9]. Although different cut-offs have been proposed for cirrhosis according to etiologies (ranging, for instance, from 11 kPa in chronic hepatitis B [10] to 22.6 kPa in alcoholic liver disease [11]), it should be kept in mind that these cut-off values have been defined in a single population using ROC curves in order to maximize sensitivity and specificity – and not applied to a validation cohort. Difference between cut-offs may be simply related to difference in cirrhosis prevalence in the studied populations, known as the spectrum bias [12]. Finally, the cut-off choice should also consider the pretest probability of cirrhosis in the target population (varying from less than 1 % in the general population to 10–20 % in tertiary referral centers). For instance, it has been shown that in a population with a pretest probability of 13.8 %, at a cut-off <7 kPa, cirrhosis postestprobability ranged from 0 to 3 %, whereas at a cut-off >17 kPa cirrhosis probability was 72 % [13]. Interestingly, several recent studies have shown that in patients with chronic liver disease, LS could also predict clinical decompensation as well as survival [14–17]. For instance, Robic et al. [15] found that TE was as effective as HVPG measurement in predicting clinical decompensation in 100 patients with chronic liver disease with a 2 years follow-up. Both HVPG ≤ 10 mmHg and liver stiffness ≤21.1 kPa had 100 % negative predictive value for portal-hypertensive complications. However in clinical practice, TE results should always be interpreted being aware of the risk of overestimating liver stiffness values with confounding factors such as ALT flares, food intake, extrahepatic cholestasis, congestive heart failure, and excessive alcohol intake [18].
Thanks to the improvements in the noninvasive methods, most patients are currently diagnosed in a very initial stage of cirrhosis, in which CSPH and esophageal varices (EV) are often absent. In this new scenario, a large proportion of HVPG measurements and screening endoscopies may be unnecessary. Therefore, efforts should be directed at limiting these procedures to those patients at higher risk of CSPH and varices, so as to reducing healthcare cost and lessen patients’ discomfort [19]. There are two clinically relevant questions when screening for portal hypertension: first, identification of patients at high risk for clinically significant portal hypertension (CSPH) defined by an HVPG ≥ 10 mmHg [20]; second, identification of patients at high risk for EV.
Detection of Patients at High Risk for CSPH
Among available noninvasive tests, LS measurement using TE has been the most extensively studied. There is substantial evidence indicating that TE can be quite effective in detecting patients with a high risk of having (or not having) developed CSPH. Several studies have shown that there is a good correlation between liver stiffness values and HVPG in patients with advanced liver diseases [21–24]. According to a recent meta-analysis (based on 5 studies including 420 patients), the diagnostic performance of TE for predicting CSPH in the setting of patients with compensated chronic liver disease/cirrhosis is excellent, with an AUROC of 0.93 [25]. TE was very informative with 81 % probability of correctly detecting significant portal hypertension following a “positive” measurement (over the threshold value) and lowering the probability of disease to as low as 11 % when “negative” measurement (below the threshold value) was found when the pretest probability was 50 %. However, it should be noted that when the pretest probability of significant portal hypertension was as low as 25 %, the probability of correctly identifying significant portal hypertension decreased markedly. The studies addressing the diagnostic performances of TE for the detection of CSPH [22–24, 26–31] are summarized in Table 6.1. The results of these studies deserve several comments: most if not all of them have been conducted in European expert centers where HVPG is available with a likely referral bias. Indeed, studied populations are heterogeneous in terms of etiologies and Child-Pugh classes (ranging from 20 to 100 % for Child-Pugh class A) with small sample size (<100 patients) and high prevalence of CSPH (51–86 %). These are limitations that are inherent to the HVPG technique and thus will be difficult to overcome but that hamper the applicability of these results to the target population of patients with early cirrhosis eligible for screening. Finally, TE cut-offs vary from 13.6 to 34.9 kPa, making the optimal TE cut-offs for prediction of CSPH difficult to be defined. In the largest studied population (n = 502), Reiberger et al. [29] have shown that at a cut-off of 18 kPa, TE was better at ruling in than ruling out CSPH (positive and negative predictive values of 86 and 81 %) [29]. Other authors [27] have proposed a dual cut-off strategy (<13.6 kPa with a 90 % sensitivity for CSPH diagnosis and ≥21 kPa with a 90 % specificity), allowing a correct stratification of presence/absence of CSPH in patients with compensated cirrhosis and potentially resectable hepatocellular carcinoma, reducing the need for invasive hemodynamic assessment in around 50 % of patients. However, while the correlation is excellent for HVPG values between 5 and 10–12 mmHg (typical of cirrhosis without evident clinical manifestations related to portal hypertension), it hardly reaches statistical significance for values above 12 mmHg [22]. This is because, with the progression of cirrhosis, the mechanisms of portal hypertension (PH) become less and less dependent on the intrahepatic resistance to portal flow due to tissue fibrosis and progressively more dependent on extrahepatic factors (i.e., hyperdynamic circulation, splanchnic vasodilatation) [32]. This observation sets a key limitation to the use of liver stiffness measurements as a noninvasive surrogate of HVPG beyond the prediction of clinically significant (HVPG ≥ 10 mmHg) and severe (HVPG ≥ 12 mmHg) PH, and, accordingly, TE of the liver is unlikely to be useful in monitoring the hemodynamic response to the administration of beta-blockers or disease progression in the decompensated phase.
Several biological parameters have been proposed for the noninvasive detection of clinically significant portal hypertension including prothrombin time [23], a score combining platelet count and total bilirubin [33], and FibroTest® [34]. In particular, a score combining platelet count with total bilirubin had an AUROC of 0.91 for predicting clinically significant portal hypertension with 88 % sensitivity and 86 % specificity at a cut-off of −1.0.
Finally, in order to increase diagnostic accuracy, some authors have proposed scores combining LS with platelet count and spleen diameter by ultrasound, referred as LSPS for LSM-spleen diameter to Platelet ratio score [35] or PH risk score [30]. For instance, in a population of 117 patients with compensated cirrhosis, more than 80 % of patients were accurately classified for CSPH using LSPS and PH risk score. These promising results require further external validation but could represent an attractive strategy for screening patients for CSPH as proposed by some authors [36].
Detection of Patients at High Risk for GOV
More uncertain and controversial is the possibility of predicting the presence and the size of OV based on LS measurements (LSM). In a recent meta-analysis [25] (based on 18 studies and 3644 patients), the diagnostic performance of TE for predicting OV and large OV (LOV) was not as good as for CSPH with AUROCS of 0.84 and 0.78, respectively. The studies addressing the performance of TE for prediction of OV [22–24, 28, 37–52] are summarized in Table 6.2. AUROCs range from 0.62 to 0.90 and cut-offs from 13.9 to 48.0 kPa. Although the sensitivity for the prediction of the presence of OV was high (56–100 %), specificity was much lower (32–87 %) and less satisfactory. Regardless, the general features of these studies, i.e., single-center retrospective, heterogeneous etiology of cirrhosis and stages of disease progression, and subjective assessment of OV size, do not allow any sound conclusion on the utility of liver stiffness assessment in predicting the presence of OV and to screen cirrhotic patients without endoscopy [54].
Similarly, several biomarkers have been proposed for the detection of OV including routine biological parameters [55], FibroTest® [56], and combination of simple biological and ultrasound parameters [57]. In the largest study to date comparing retrospectively a panel of serum markers (platelet count, AST/ALT ratio, APRI, Forns index, Lok index, FIB-4, and Fibroindex) in more than 500 patients with chronic liver diseases, the combination of Lok index (cut-off = 1.5) and Forns index (cut-off = 8.8) had the best diagnostic performance (AUROC of 0.80 and negative predictive value of 90 %) for predicting clinically relevant OV [55]. Finally, as mentioned before for CSPH, scores combining LS with platelet count and spleen diameter by ultrasound such as LSPS or variceal risk score have been proposed [30, 35]. For instance, in 401 Korean patients with HVB cirrhosis (280 in the training set and 121 in the validation set), the LSPS had a significantly better AUROC than TE alone for prediction of high-risk OV (0.95 vs. 0.88 in the training set, respectively, p < 0.001) [35]. At a cut-off < 3.5, LSPS had a 94.0 % negative predictive value and a 94.2 % positive predictive value at a cut-off > 5.5. Overall, upper GI endoscopy could be saved in 90.3 % patients. Interestingly, LSPS appeared as a reliable predictor of OV bleeding risk [58]. The performance of LSPS has also been confirmed externally [28, 30]. Using a similar strategy in 173 patients, Berzigotti et al. [30] have shown that only 3 of 70 with varices (4 %; all with small varices) would have been missed if endoscopy was delayed using the varices risk score. These scores appear thus as an attractive strategy in clinical decision making for detecting patients with high-risk OV.
Spleen Stiffness: A New Surrogate of Portal Hypertension?
Recently, studies employing different technical approaches have highlighted the potential utility of spleen stiffness (SS) assessment for the prediction of the presence of OV and the degree of portal hypertension in cirrhotic patients [28, 51, 59]. Colecchia et al. measured SS and LS by TE in 100 consecutive patients with hepatitis C virus-induced cirrhosis patients who underwent measurement of HVPG and upper GI endoscopy [28]. The ability of both SS and LS to predict CSPH and the presence of OV was compared to that of the previously proposed methods, i.e., LSPS and platelet count to spleen diameter [35, 57, 60]. SS and LS were more accurate than other noninvasive parameters in identifying patients with OV and different degrees of portal hypertension. However, TE may not be the most appropriate tool for SS measurement, as ultrasound examination of the spleen was mandatory before performing TE to ensure that the ultrasound beam remained within the spleen parenchyma. Indeed, SS could not be measured in 13 % of patients particularly those with an anteroposterior spleen diameter measuring <4 cm. Alternative ultrasound-based elastography techniques such as acoustic radiation force impulse imaging (ARFI) (Virtual touch tissue quantification™, Siemens) or 2D-shear-wave elastography (2D-SWE) (Aixplorer™, Supersonic Imagine, France) have been proposed for measuring SS with much lower failure rates of 4.5 % [61] and 3 % [62], respectively. Another technical advantage of ARFI and 2D-SWE over TE is that they can be performed using a regular ultrasound machine, allowing during a single procedure to choose the region of interest where the shear-wave velocity is measured under direct visualization of the spleen [63]. Although not clearly demonstrated in the study by Colecchia et al. [28], the study by Takuma et al. [61] in 340 patients showed that SS was better than LS measurement, particularly for ruling out the presence of OV. Finally, there may be a ceiling effect with TE that showed significantly higher kPa values (up to 70 kPa) with SS values compared with LS at any given HVPG level, suggesting that even an upper detection limit of 75 kPa could be too restrictive for a satisfactory SS measurement and would need to be extended as proposed by some authors [48]. Thus, SS is not ready yet for “prime time,” and further validation is needed before its exact place in clinical practice can be defined.
Conclusions and Perspectives
In conclusion, the evidence accumulated so far indicates that noninvasive methods cannot replace HVPG for a detailed portal hypertension evaluation and upper GI endoscopy for detecting OV. However, in settings where HVPG is not available, TE could be considered to stratify the risk of CSPH. Similarly, strategies combining LS measurement with platelet count and spleen diameter could be useful to rule out OV in patients at low risk of having portal hypertension. One would foresee different levels of invasiveness, starting with simple laboratory tests, followed by measurements of LS and, only in a minority of patients, would we need to perform an invasive test.
References
de Franchis R (2010) Revising consensus in portal hypertension: report of the Baveno consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol 53(4):762–768
EASL-ALEH Clinical practice guidelines: non-invasive tests for evaluation of liver disease severity and prognosis (2015) J Hepatol 63:237–264
Castera L (2012) Noninvasive methods to assess liver disease in patients with hepatitis B or C. Gastroenterology 142(6):1293–1302
Shaheen AA, Wan AF, Myers RP (2007) FibroTest and FibroScan for the prediction of hepatitis C-related fibrosis: a systematic review of diagnostic test accuracy. Am J Gastroenterol 102(11):2589–2600
Talwalkar JA, Kurtz DM, Schoenleber SJ, West CP, Montori VM (2007) Ultrasound-based transient elastography for the detection of hepatic fibrosis: systematic review and meta-analysis. Clin Gastroenterol Hepatol 5(10):1214–1220
Friedrich-Rust M, Ong MF, Martens S, Sarrazin C, Bojunga J, Zeuzem S, Herrmann E (2008) Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology 134(4):960–974
Stebbing J, Farouk L, Panos G, Anderson M, Jiao LR, Mandalia S, Bower M, Gazzard B, Nelson M (2010) A meta-analysis of transient elastography for the detection of hepatic fibrosis. J Clin Gastroenterol 44(3):214–219
Tsochatzis EA, Gurusamy KS, Ntaoula S, Cholongitas E, Davidson BR, Burroughs AK (2011) Elastography for the diagnosis of severity of fibrosis in chronic liver disease: a meta-analysis of diagnostic accuracy. J Hepatol 54(4):650–659
Ganne-Carrie N, Ziol M, de Ledinghen V, Douvin C, Marcellin P, Castera L, Dhumeaux D, Trinchet JC, Beaugrand M (2006) Accuracy of liver stiffness measurement for the diagnosis of cirrhosis in patients with chronic liver diseases. Hepatology 44(6):1511–1517
Marcellin P, Ziol M, Bedossa P, Douvin C, Poupon R, de Ledinghen V, Beaugrand M (2009) Non-invasive assessment of liver fibrosis by stiffness measurement in patients with chronic hepatitis B. Liver Int 29(2):242–247
Nahon P, Kettaneh A, Tengher-Barna I, Ziol M, de Ledinghen V, Douvin C, Marcellin P, Ganne-Carrie N, Trinchet JC, Beaugrand M (2008) Assessment of liver fibrosis using transient elastography in patients with alcoholic liver disease. J Hepatol 49(6):1062–1068
Ransohoff DF, Feinstein AR (1978) Problems of spectrum and bias in evaluating the efficacy of diagnostic tests. N Engl J Med 299(17):926–930
Degos F, Perez P, Roche B, Mahmoudi A, Asselineau J, Voitot H, Bedossa P (2010) Diagnostic accuracy of FibroScan and comparison to liver fibrosis biomarkers in chronic viral hepatitis: a multicenter prospective study (the FIBROSTIC study). J Hepatol 53(6):1013–1021
Vergniol J, Foucher J, Terrebonne E, Bernard PH, le Bail B, Merrouche W, Couzigou P, de Ledinghen V (2011) Noninvasive tests for fibrosis and liver stiffness predict 5-year outcomes of patients with chronic hepatitis C. Gastroenterology 140(7):1970–1979
Robic MA, Procopet B, Metivier S, Peron JM, Selves J, Vinel JP, Bureau C (2011) Liver stiffness accurately predicts portal hypertension related complications in patients with chronic liver disease: a prospective study. J Hepatol 55(5):1017–1024
Kim SU, Lee JH, Kim Y, Ahn SH, Jung KS, Choi EH, Park YN, Han KH, Chon CY, Park JY (2012) Prediction of liver-related events using fibroscan in chronic hepatitis B patients showing advanced liver fibrosis. PLoS One 7(5):e36676. doi:10.1371/journal.pone.0036676
Corpechot C, Gaouar F, El Naggar A, Kemgang A, Wendum D, Poupon R, Carrat F, Chazouilleres O (2014) Baseline values and changes in liver stiffness measured by transient elastography are associated with severity of fibrosis and outcomes of patients with primary sclerosing cholangitis. Gastroenterology 146(4):970–979
Tapper EB, Castera L, Afdhal NH (2015) Contemporary assessment of hepatic fibrosis. Clin Gastroenterol Hepatol 13:60–67
Berzigotti A, Bosch J, Boyer TD (2014) Use of noninvasive markers of portal hypertension and timing of screening endoscopy for gastroesophageal varices in patients with chronic liver disease. Hepatology 59(2):729–731
Garcia-Tsao G, Bosch J (2010) Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med 362(9):823–832
Carrion JA, Navasa M, Bosch J, Bruguera M, Gilabert R, Forns X (2006) Transient elastography for diagnosis of advanced fibrosis and portal hypertension in patients with hepatitis C recurrence after liver transplantation. Liver Transpl 12(12):1791–1798
Vizzutti F, Arena U, Romanelli RG, Rega L, Foschi M, Colagrande S, Petrarca A et al (2007) Liver stiffness measurement predicts severe portal hypertension in patients with HCV-related cirrhosis. Hepatology 45(5):1290–1297
Bureau C, Metivier S, Peron JM, Selves J, Robic MA, Gourraud PA, Rouquet O, Dupuis E, Alric L, Vinel JP (2008) Transient elastography accurately predicts presence of significant portal hypertension in patients with chronic liver disease. Aliment Pharmacol Ther 27(12):1261–1268
Lemoine M, Katsahian S, Ziol M, Nahon P, Ganne-Carrie N, Kazemi F, Grando-Lemaire V, Trinchet JC, Beaugrand M (2008) Liver stiffness measurement as a predictive tool of clinically significant portal hypertension in patients with compensated hepatitis C virus or alcohol-related cirrhosis. Aliment Pharmacol Ther 28(9):1102–1110
Shi KQ, Fan YC, Pan ZZ, Lin XF, Liu WY, Chen YP, Zheng MH (2013) Transient elastography: a meta-analysis of diagnostic accuracy in evaluation of portal hypertension in chronic liver disease. Liver Int 33(1):62–71
Sanchez-Conde M, Montes-Ramirez ML, Miralles P, Alvarez JM, Bellon JM, Ramirez M, Arribas JR et al (2010) Comparison of transient elastography and liver biopsy for the assessment of liver fibrosis in HIV/hepatitis C virus-coinfected patients and correlation with noninvasive serum markers. J Viral Hepat 17(4):280–286
Llop E, Berzigotti A, Reig M, Erice E, Reverter E, Seijo S, Abraldes JG, Bruix J, Bosch J, Garcia-Pagan JC (2012) Assessment of portal hypertension by transient elastography in patients with compensated cirrhosis and potentially resectable liver tumors. J Hepatol 56(1):103–108
Colecchia A, Montrone L, Scaioli E, Bacchi-Reggiani ML, Colli A, Casazza G, Schiumerini R et al (2012) Measurement of Spleen Stiffness to Evaluate Portal Hypertension and the Presence of Esophageal Varices in Patients With HCV-Related Cirrhosis. Gastroenterology 143(3):646–654, doi:S0016-5085(12)00800-1 [pii]
Reiberger T, Ferlitsch A, Payer BA, Pinter M, Schwabl P, Stift J, Trauner M, Peck-Radosavljevic M (2012) Noninvasive screening for liver fibrosis and portal hypertension by transient elastography–a large single center experience. Wien Klin Wochenschr 124(11–12):395–402
Berzigotti A, Seijo S, Arena U, Abraldes JG, Vizzutti F, Garcia-Pagan JC, Pinzani M, Bosch J (2013) Elastography, spleen size, and platelet count identify portal hypertension in patients with compensated cirrhosis. Gastroenterology 44(1):102–111, e101
Kitson MT, Roberts SK, Colman JC, Paul E, Button P, Kemp W (2015) Liver stiffness and the prediction of clinically significant portal hypertension and portal hypertensive complications. Scand J Gastroenterol 50(4):462–469
Reiberger T, Ferlitsch A, Payer BA, Pinter M, Homoncik M, Peck-Radosavljevic M (2012) Non-selective beta-blockers improve the correlation of liver stiffness and portal pressure in advanced cirrhosis. J Gastroenterol 47(5):561–568
Park SH, Park TE, Kim YM, Kim SJ, Baik GH, Kim JB, Kim DJ (2009) Non-invasive model predicting clinically-significant portal hypertension in patients with advanced fibrosis. J Gastroenterol Hepatol 7:1289–1293
Thabut D, Imbert-Bismut F, Cazals-Hatem D, Messous D, Muntenau M, Valla DC, Moreau R, Poynard T, Lebrec D (2007) Relationship between the Fibrotest and portal hypertension in patients with liver disease. Aliment Pharmacol Ther 26(3):359–368
Kim BK, Han KH, Park JY, Ahn SH, Kim JK, Paik YH, Lee KS, Chon CY, Kim Y (2010) A liver stiffness measurement-based, noninvasive prediction model for high-risk esophageal varices in B-viral liver cirrhosis. Am J Gastroenterol 105(6):1382–1390
Augustin S, Millan L, Gonzalez A, Martell M, Gelabert A, Segarra A, Serres X, Esteban R, Genesca J (2014) Detection of early portal hypertension with routine data and liver stiffness in patients with asymptomatic liver disease: a prospective study. J Hepatol 60(3):561–569
Kazemi F, Kettaneh A, N’Kontchou G, Pinto E, Ganne-Carrie N, Trinchet JC, Beaugrand M (2006) Liver stiffness measurement selects patients with cirrhosis at risk of bearing large oesophageal varices. J Hepatol 45(2):230–235
Foucher J, Castéra L, Bernard PH, Adhoute X, Bertet J, Couzigou P, de Lédinghen V (2006) Assessment of cirrhosis and its severity by FibroScan® and biochemical markers in alcoholic patients (abstract). J Hepatol 44(Suppl 2):S39
Castera L, Le Bail B, Roudot-Thoraval F, Bernard PH, Foucher J, Merrouche W, Couzigou P, de Ledinghen V (2009) Early detection in routine clinical practice of cirrhosis and oesophageal varices in chronic hepatitis C: comparison of transient elastography (FibroScan) with standard laboratory tests and non-invasive scores. J Hepatol 50(1):59–68
Pineda JA, Recio E, Camacho A, Macias J, Almodovar C, Gonzalez-Serrano M, Merino D, Tellez F, Rios MJ, Rivero A (2009) Liver stiffness as a predictor of esophageal varices requiring therapy in HIV/hepatitis C virus-coinfected patients with cirrhosis. J Acquir Immune Defic Syndr 51(4):445–449
Nguyen-Khac E, Saint-Leger P, Tramier B, Coevoet H, Capron D, Dupas JL (2010) Noninvasive diagnosis of large esophageal varices by fibroscan: strong influence of the cirrhosis etiology. Alcohol Clin Exp Res 34(7):1146–1153
Malik R, Lai M, Sadiq A, Farnan R, Mehta S, Nasser I, Challies T, Schuppan D, Afdhal N (2010) Comparison of transient elastography, serum markers and clinical signs for the diagnosis of compensated cirrhosis. J Gastroenterol Hepatol 25(9):1562–1568
Pritchett S, Cardenas A, Manning D, Curry M, Afdhal NH (2011) The optimal cut-off for predicting large oesophageal varices using transient elastography is disease specific. J Viral Hepat 18(4):e75–e80. doi:10.1111/j.1365-2893.2010.01375.x
Sporea I, Ratiu I, Sirli R, Popescu A, Bota S (2011) Value of transient elastography for the prediction of variceal bleeding. World J Gastroenterol 17(17):2206–2210
Stefanescu H, Grigorescu M, Lupsor M, Maniu A, Crisan D, Procopet B, Feier D, Badea R (2011) A new and simple algorithm for the noninvasive assessment of esophageal varices in cirrhotic patients using serum fibrosis markers and transient elastography. J Gastrointestin Liver Dis 20(1):57–64
Chen YP, Zhang Q, Dai L, Liang XE, Peng J, Hou JL (2012) Is transient elastography valuable for high-risk esophageal varices prediction in patients with hepatitis-B-related cirrhosis? J Gastroenterol Hepatol 27(3):533–539
Wang HM, Lo GH, Chen WC, Hsu PI, Yu HC, Lin CK, Chan HH et al (2012) Efficacy of transient elastography in screening for large esophageal varices in patients with suspicious or proven liver cirrhosis. J Dig Dis 13(8):430–438
Calvaruso V, Bronte F, Conte E, Simone F, Craxi A, Di Marco V (2013) Modified spleen stiffness measurement by transient elastography is associated with presence of large oesophageal varices in patients with compensated hepatitis C virus cirrhosis. J Viral Hepat 20(12):867–874
Fraquelli M, Giunta M, Pozzi R, Rigamonti C, Della Valle S, Massironi S, Conti CB et al (2014) Feasibility and reproducibility of spleen transient elastography and its role in combination with liver transient elastography for predicting the severity of chronic viral hepatitis. J Viral Hepat 21(2):90–98. doi:10.1111/jvh.12119
Hu Z, Li Y, Li C, Huang C, Ou Z, Guo J, Luo H, Tang X (2015) Using Ultrasonic Transient Elastometry (FibroScan) to Predict Esophageal Varices in Patients with Viral Liver Cirrhosis. Ultrasound Med Biol. doi:10.1016/ j.ultrasmedbio.2015.02.005
Sharma P, Kirnake V, Tyagi P, Bansal N, Singla V, Kumar A, Arora A (2013) Spleen stiffness in patients with cirrhosis in predicting esophageal varices. Am J Gastroenterol 108(7):1101–1107. doi:10.1038/ajg.2013.119
Stefanescu H, Radu C, Procopet B, Lupsor-Platon M, Habic A, Tantau M, Grigorescu M (2015) Non-invasive menage a trois for the prediction of high-risk varices: stepwise algorithm using lok score, liver and spleen stiffness. Liver Int 35(2):317–325
Wang JH, Chuah SK, Lu SN, Hung CH, Chen CH, Kee KM, Chang KC, Tai WC, Hu TH (2012) Transient elastography and simple blood markers in the diagnosis of esophageal varices for compensated patients with hepatitis B virus-related cirrhosis. J Gastroenterol Hepatol 27(7):1213–1218. doi:10.1111/j.1440-
Castera L, Pinzani M, Bosch J (2012) Non invasive evaluation of portal hypertension using transient elastography. J Hepatol 56:696–703
Sebastiani G, Tempesta D, Fattovich G, Castera L, Halfon P, Bourliere M, Noventa F, Angeli P, Saggioro A, Alberti A (2010) Prediction of oesophageal varices in hepatic cirrhosis by simple serum non-invasive markers: results of a multicenter, large-scale study. J Hepatol 53(4):630–638
Thabut D, Trabut JB, Massard J, Rudler M, Muntenau M, Messous D, Poynard T (2006) Non-invasive diagnosis of large oesophageal varices with FibroTest in patients with cirrhosis: a preliminary retrospective study. Liver Int 26(3):271–278
Giannini E, Botta F, Borro P, Risso D, Romagnoli P, Fasoli A, Mele MR et al (2003) Platelet count/spleen diameter ratio: proposal and validation of a non-invasive parameter to predict the presence of oesophageal varices in patients with liver cirrhosis. Gut 52(8):1200–1205
Kim BK, Kim do Y, Han KH, Park JY, Kim JK, Paik YH, Lee KS, Chon CY, Ahn SH (2011) Risk assessment of esophageal variceal bleeding in B-viral liver cirrhosis by a liver stiffness measurement-based model. Am J Gastroenterol 106(9):1654–1662
Stefanescu H, Grigorescu M, Lupsor M, Procopet B, Maniu A, Badea R (2011) Spleen stiffness measurement using Fibroscan for the noninvasive assessment of esophageal varices in liver cirrhosis patients. J Gastroenterol Hepatol 26(1):164–170
Giannini EG, Zaman A, Kreil A, Floreani A, Dulbecco P, Testa E, Sohaey R et al (2006) Platelet count/spleen diameter ratio for the noninvasive diagnosis of esophageal varices: results of a multicenter, prospective, validation study. Am J Gastroenterol 101(11):2511–2519
Takuma Y, Nouso K, Morimoto Y, Tomokuni J, Sahara A, Toshikuni N, Takabatake H et al (2012) Measurement of spleen stiffness by acoustic radiation force impulse imaging identifies cirrhotic patients with esophageal varices. Gastroenterology 144(1):92–101, e102
Elkrief L, Rautou PE, Ronot M, Lambert S, Dioguardi Burgio M, Francoz C, Plessier A et al (2015) Prospective comparison of spleen and liver stiffness by using shear-wave and transient elastography for detection of portal hypertension in cirrhosis. Radiology 275:589–598
Castera L, Garcia-Tsao G (2013) When the spleen gets tough, the varices get going. Gastroenterology 144(1):19–22
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this paper
Cite this paper
Castera, L. (2016). How to Screen?. In: de Franchis, R. (eds) Portal Hypertension VI. Springer, Cham. https://doi.org/10.1007/978-3-319-23018-4_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-23018-4_6
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-23017-7
Online ISBN: 978-3-319-23018-4
eBook Packages: MedicineMedicine (R0)