Abstract
Background
Spleen stiffness measurement (SSM) correlates with the severity of portal hypertension.
Aims
We investigated the utility of SSM in individuals with metabolic dysfunction-associated steatotic liver disease (MASLD) for detecting cirrhosis, esophageal varices (EV), and high-risk EV.
Methods
154 study participants with MASLD underwent simultaneous liver stiffness measurement (LSM) and SSM. 96 (62%) participants had an upper endoscopy (73 participants, i.e., 47% undergoing within a year). The diagnostic performance of SSM, as well as the BAVENO VII proposed SSM cutoffs (≥ 21 kPa, > 40 kPa, and > 50 kPa), was examined.
Results
The failure rate for SSM was 19% compared to 5% for LSM. An invalid SSM was statistically significantly associated with a higher body mass index, a larger waist circumference, and a lower fibrosis stage. The area under the receiver operating characteristics for SSM to diagnose cirrhosis, EV, and high-risk EV was 0.78 (95% CI 0.70–0.85), 0.74 (95% CI 0.61–0.84), and 0.82 (95% CI 0.75–0.98), respectively. SSM ≥ 21 kPa cutoff had a sensitivity > 96% for all three outcomes, with a positive predictive value (PPV) of 88% for cirrhosis. In contrast, SSM > 40 kPa and SSM > 50 kPa cutoffs had better diagnostic abilities for identifying EV, particularly high-risk EV (sensitivity of 100% and 93% with NPV of 100% and 96%, respectively).
Conclusion
SSM has a higher failure rate in individuals who are non-cirrhotic or have a higher BMI, or larger waist circumference. Although useful for diagnosing NASH cirrhosis, SSM is most reliable in excluding EV and high-risk EV.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Metabolic dysfunction-associated steatotic liver disease (MASLD), formerly known as nonalcoholic fatty liver disease (NAFLD), is one of the most prevalent forms of liver disease evaluated in gastroenterology and hepatology clinics in the United States [1,2,3]. The severity of MASLD is graded on a spectrum and ranges from steatotic liver with no steatohepatitis or fibrosis to metabolic dysfunction-associated steatohepatitis (MASH) with fibrosis that can ultimately lead to cirrhosis complicated by portal hypertension [4,5,6]. Since MASH is a histologic diagnosis requiring an invasive and time-consuming liver biopsy, the field has embraced non-invasive tests (NITs) to recognize the presence of fibrosis (clinically significant, advanced or cirrhosis) [7,8,9,10,11]. Several cross-sectional studies have demonstrated a correlation between liver stiffness measurement (LSM) and the severity of fibrosis in MASLD [8, 12,13,14,15,16,17,18]. More recently, a few investigators have reported a correlation between baseline LSM and change in LSM over time with liver-related clinical outcomes [18, 19]. The recent BAVENO VII established the “rule of five,” which proposes that there is no concern for compensated advanced chronic liver disease (cACLD) for those with LSM < 10 kilopascals (kPa), while an LSM ≥ 25 kPa suggests the presence of clinically significant portal hypertension (CSPH) [20]. In patients with MASLD, due to increased probe-to-capsule distance resulting in a false increase in LSM, the ANTICIPATE-NASH model is recommended, incorporating LSM with platelet count and body mass index (BMI) to predict the presence of CSPH[21].
There is a need for NITs that can estimate the presence of cACLD and CSPH with high diagnostic accuracy in patients with MASLD. Although the evaluation of a compensated cirrhotic by hepatic vein pressure gradient is ideal for risk stratification, the procedure is cumbersome, invasive, non-standardized, and often restricted to tertiary care centers due to limited expertise [22]. Spleen stiffness measurement (SSM) is an emerging NIT that has been studied to assess the severity of portal hypertension. In 2011, the utility of the standard liver 50-Hz vibration-controlled elastography to measure spleen stiffness as an alternative means to successfully predict the presence of esophageal varices (EV) was proposed [23]. Additional studies analyzing this standard 50-Hz elastography have demonstrated inconsistencies in detecting cutoff values for the presence of EV and the accuracy with which it can differentiate between variceal grades [23,24,25]. The 50-Hz elastography is limited by its narrow range of measurement [1.5–75 kPa]. As a result, the FibroScan 630 Expert was recently made available to include a broader range of SSM (5–100 kPa) and a B‐mode ultrasound to locate the spleen [26]. Prior studies have concluded that the FibroScan 630 Expert can more accurately detect EV than the original 50-Hz elastography probe [27].
Currently, the main diagnostic NITs utilized to assess the severity of liver disease rely on measuring LSM using elastography combined with blood tests [13, 28, 29]. However, using LSM to predict the severity of CSPH in individuals with liver disease has limited accuracy [30,31,32]. To address this issue, some studies have suggested that adding SSM to LSM may improve the ability to predict the presence of high-risk EV in those with liver disease and elevated LSM [33,34,35]. However, the usefulness of SSM by itself in routine clinical practice still needs to be clarified, as the data on its diagnostic accuracy are limited, particularly in MASLD [36,37,38].
Recently, the BAVENO VII guidelines recommended an SSM cutoff value of < 21 kPa to rule out CSPH and a cutoff of > 50 kPa to rule it in [20]. Additionally, an SSM cutoff of ≤ 40 kPa was proposed to identify individuals who are unlikely to have high-risk EV [20]. However, these recommendations were based on studies of patients with predominantly viral hepatitis, and it is unclear if they are applicable to individuals with MASLD [20]. Moreover, it is unknown whether SSM alone can detect cirrhosis and EV in those with MASLD. To address these gaps in knowledge, this study aims to assess SSM's clinical usefulness for detecting cirrhosis, EV, and high-risk EV in individuals with MASLD. Additionally, the study also evaluated the diagnostic performance of SSM cutoffs proposed by the BAVENO VII guidelines (≥ 21 kPa, > 40 kPa, and > 50 kPa) for identification of cirrhosis, EV (any size), and high-risk EV.
Methods
Study Design
In this prospective study (ClinicalTrials.gov Identifier: NCT03778411), subjects ≥ 18 years of age presenting to the hepatology clinics at Indiana University Hospital for the care of their liver disease were recruited to participate. All subjects provided Institutional Review Board or Ethics Committee approved informed consent before undergoing study-specific procedures. Patients were excluded if they had clinically apparent ascites or declined to provide informed consent. Simultaneous LSM, SSM, and controlled attenuation parameter (CAP) were obtained during the study using the FibroScan 630 Expert (Echosens, Paris) without SmartExam. All measurements were obtained by a single technician (AM). All scans were performed in the fasting state (at least 3 h) using a standard protocol per manufacturer recommendations.
Study Participants and Clinical Data
Between November 2020 and May 2022, a total of 419 patients presenting to the liver clinic were assessed for simultaneous LSM and SSM using the FibroScan 630 Expert. Pertinent demographics, clinical data, and variables related to blood tests, histology, imaging modalities, and endoscopy were extracted from the electronic health records. Of these, 154 patients with MASLD met the study inclusion criteria of a clinical diagnosis of MASLD by imaging or liver biopsy without a clinical history of excess alcohol use. Of these, 93 (60.1%) had MASH cirrhosis diagnosed through a combination of either imaging with thrombocytopenia (n = 33) and/or a liver biopsy (n = 60) [39].
Patients were excluded if they had primary biliary cholangitis (n = 23), primary sclerosing cholangitis (n = 16), autoimmune hepatitis (n = 25), hepatitis B (n = 11), hepatitis C (n = 48), alcohol-associated liver disease (n = 79), hereditary hemochromatosis (n = 3), Budd-Chiari syndrome (n = 3), alpha-1 antitrypsin deficiency (n = 8), hepatocellular carcinoma (n = 18), current alcohol use (n = 17), or an unknown liver etiology (n = 14). Fibrosis-4 (FIB4) was calculated for all enrolled study participants from data extracted through the electronic health record using the following formula ([age]x[AST]/[platelet count]x[√ALT]) [40].
Measurement of LSM and SSM
The FibroScan 630 Expert is a vibration-controlled transient elastography (VCTE) was used to measure the stiffness of the spleen and liver in kilopascals. Briefly, each patient was placed supine with right arm raised behind his or her head and remained still during the procedure. The probe was placed over the liver region, and measurements were obtained. After the completion of the liver elastography, the device was moved and positioned to obtain SSM as per the manufacturer's protocol. For LSM, the measurement was considered successful if at least 10 measurements were available and reliable when the interquartile range/median (IQR/M) was ≤ 30%, as per the previous recommendations [8, 41]. In contrast, SSM was considered successful if at least 10 measurements were available. Currently, there are no criteria to establish a reliable SSM.
Endoscopic Evaluation
In the current study cohort, 96 (62%) participants had undergone an upper endoscopy, and 73 (47%) had an upper endoscopy within a year of undergoing VCTE (Supplementary Fig. 1). The endoscopy records were reviewed, and the EV were classified according to Baveno VI consensus: no varix, small varices, moderately enlarged, beady varices, and large varices [42, 43]. EV were categorized as high-risk if they were large, required endoscopic band ligation, or had a red wale sign irrespective of the size of the varices as per BAVENO VI criteria [42, 43].
Statistical Analysis
Statistical analyses were performed using SPSS version 28 (IBM, New York, 10,504) and Stata MP version 17.0 (StataCorp, College Station, TX). Data were analyzed by the Mann–Whitney U test and chi-squared for continuous and categorical variables, respectively. P values less than 0.05 were deemed statistically significant. Area under the receiver operating characteristic curves (AUROC), specificity, sensitivity, positive predictive values (PPV), and negative predictive values (NPV) was established to determine the aptitude for the three non-invasive markers (SSM, LSM, and FIB4) to detect cirrhosis, EV (any size), and high-risk EV. The best Youden index was identified to determine the sensitivity and specificity of these NITs. The diagnostic performance of SSM cutoffs as proposed in the recent BAVENO VII consensus document, i.e., SSM cutoffs of ≥ 21 kPa, > 40 kPa, and > 50 kPa, was examined in the current cohort and reported in terms of sensitivity, specificity, PPV, and NPV [20].
Results
Study Participant Demographics
Table 1 illustrates the demographics and laboratory findings of the total cohort, valid SSM, and invalid SSM subgroups. The median age was 59 years (range 24–79 years), 46% were males, 88% were Caucasian, and the mean BMI was 34.6 ± 6.3 kg/m2 for the total cohort (Table 1). The mean platelet count, INR, and albumin were significantly different between the valid and invalid SSM subgroups (Table 1). The median duration between those who received an upper endoscopy and VCTE evaluation within one year was 67 days (IQR, 25th-75th: 4–168), with 18 (25%) undergoing both tests on the same day.
FibroScan Metrics
Of the 154 participants, 29 had an invalid SSM [inability to locate the spleen (n = 28) or < 10 valid spleen stiffness measurements (n = 1)] with a failure rate of 19% (Fig. 1). 8 participants had an invalid LSM [< 10 valid liver stiffness measurements (n = 8) or IQR/M > 30% (n = 0)] with a failure rate of 5% (Fig. 1).
Proportion of study participants with valid and invalid vibration-controlled transient elastography in the current study cohort of study participants with metabolic dysfunction-associated steatotic liver disease (N = 154). a Evaluation for spleen stiffness measurement (SSM) with failure rate of 18.8%. b Liver stiffness measurements (LSM) with failure rate of 5.2%
Patients with a valid SSM had a lower BMI [33.8 ± 5.9 vs. 37.9 ± 6.9 kg/m2, P = 0.002] and waist circumference [109.2 ± 14.4 vs. 123.2 ± 17.4) centimeters, P = 0.004] compared to those with an invalid SSM (Table 1). The valid SSM cohort had a greater degree of fibrosis than the invalid SSM group (P = 0.015) (Table 1). There were no significant differences between the two groups regarding the presence of EV, gastric varices, portal hypertensive gastropathy, splenomegaly, ascites, or hepatomegaly (Table 1).
Discriminating Ability of SSM
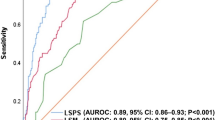
The mean SSM for MASH cirrhosis vs. non-cirrhotic MASLD was 56.2 ± 25.8 kPa vs. 33.0 ± 15.9 (P < 0.001), respectively (Table 2). The AUROC for SSM was 0.78 (95% CI 0.70–0.85) for detecting MASH cirrhosis (Fig. 2a). SSM cutoff of ≥ 21 kPa had a sensitivity of 96% and PPV of 88%, while SSM cutoff of > 50 kPa had a specificity of 78% and PPV of 94% for identifying NASH cirrhosis (Table 3). Of the patients with a valid SSM, 79 patients had an SSM < 50 kPa, 37 (47%) of whom had MASH cirrhosis and 42 (53%) who did not. Forty-six individuals had an SSM > 50 kPa, of which 43 (93%) had MASH cirrhosis and 3 (7%) did not. With this cutoff point, 68% of the cohort was correctly classified.
Diagnostic performance characteristics of spleen stiffness measurement in the current study cohort of subjects with metabolic dysfunction-associated steatotic liver disease: area under the curve for identifying cirrhosis (a) and associated manifestations of clinically significant portal hypertension (CSPH) such as esophageal varices (b) and high-risk esophageal varices (c)
73 participants had an upper endoscopy within one year of their VCTE and were included in this analysis. Of these 73 individuals, 63 had a valid SSM. 33 subjects in this valid SSM cohort had EV (19 small, 5 moderate, 9 large), of which 13 were banded, and 35 had portal hypertensive gastropathy. The valid LSM subgroup had 30 patients with EV (17 small, 4 moderate, and 9 large). The values of SSM in the groups with EV (any size) and without EV were 69.2 ± 23.4 kPa vs. 47.8 ± 25.1 kPa, respectively (P < 0.001) (Table 2). The mean SSM for small, medium, and large EV was 57.6 ± 19.7, 79.8 ± 27.8, and 85.0 ± 17.2 kPa, respectively (P = 0.007) (Table 2). SSM values were statistically different among small, medium, and large varices, with the largest difference between small vs. moderate and large groups. The LSM and FIB4 were not different among small, medium, and large varices (P = 0.48 and 0.65, respectively) (Table 2).
The AUROC for SSM was 0.74 (95% CI 0.61–0.84) for detecting the presence of EV (any size) (Fig. 2b). The AUROC for SSM for detecting high-risk EV was higher at 0.82 (95% CI: 0.75–0.98) (Fig. 2c). The optimal cutoff based on the Youden index for SSM associated with the presence of high-risk EV was > 67 kPa with sensitivity of 79%, specificity of 76%, PPV of 48%, and NPV of 93% (Table 4). 40 patients had SSM < 67 kPa, 3 had high-risk EV, and 37 did not. Among the 23 individuals that had SSM > 67 kPa, 11 had high-risk EV, and 12 did not. With this cutoff, 76% of the subjects were correctly classified.
Comparative Analysis
We next compared the discrimination ability of LSM and FIB4 in comparison with SSM in the overall cohort and in the cirrhosis subgroup for cirrhosis, EV (any size), and high-risk EV. For the overall cohort, the AUROC for LSM was 0.89 (95% CI 0.82–0.93, P < 0.001) and was higher than SSM in detecting MASH cirrhosis (Table 4). The LSM cutoff of > 18.0 kPa demonstrated a sensitivity of 74%, specificity of 88%, PPV of 90%, and NPV of 69% for NASH cirrhosis (Table 4). The valid LSM had 30 patients with EV (17 small, 4 moderate, 9 large), of which 11 were banded. The AUROC of LSM was low for detecting EV at 0.61 (95% CI 0.49–0.73, P = 0.1) (Table 4). For the detection of high-risk EV, LSM had an AUROC of 0.59 (95% CI 0.35–0.76, P = 0.4) (Table 4). The AUROC for FIB4 was excellent at 0.90 (95% CI 0.83–0.94, P < 0.0001) with a sensitivity of 84%, specificity of 79%, PPV of 89%, and NPV of 72% for detecting MASH cirrhosis with a FIB4 cutoff of > 0.375 (Table 4). The values of FIB4 in the group with EV were 1.236 ± 0.819 and were statistically significantly higher compared to those without varices (P = < 0.001) (Table 2). The FIB4 values were not statistically significantly different between the different sizes of EV (small, moderate, and large) (P = 0.65) (Table 2). The AUROC for FIB4 was 0.76 (95% CI 0.65–0.85) for detecting EV (Table 4). A FIB4 cutoff of > 0.481 had a sensitivity of 92%, specificity of 55%, PPV of 64%, and NPV of 89% for EV (Table 4). For detecting high-risk EV, FIB4 > 0.976, we had a sensitivity of 72%, specificity of 69%, PPV of 39%, and NPV of 90% (Table 4).
In the cirrhosis subgroup (n = 93), 64 subjects had undergone an upper endoscopy within a year of undergoing evaluation with FibroScan 630 Expert. The median age was 63 years, with 44% male and a body mass index of 34 ± 6 kg/m2. The mean platelet count was 120 ± 64 k/cumm with a FIB4 score of 0.963 ± 0.707. There were 55 cirrhosis patients with valid SSM and 57 cirrhosis patients with valid LSM who underwent an upper endoscopy within a year. The AUROC of SSM, LSM, and FIB4 for detecting the presence of EV was 0.70 (95% CI 0.56–0.84), 0.54 (95% CI 0.39–0.70), and 0.72 (95% CI 0.60–0.84), respectively. In those subjects who had all three NITs and upper endoscopy within a year (n = 48), the AUROC of SSM, LSM, and FIB4 were 0.74 (95% CI 0.60–0.89), 0.54 (95% CI 0.37–0.71), and 0.69 (95% CI 0.54–0.84), respectively, for the presence of EV. For the diagnosis of high-risk EV, the AUROC of SSM, LSM, and FIB4 was 0.85 (95% CI 0.73–0.98), 0.50 (95% CI 0.28–0.72), and 0.64 (95% CI 0.47–0.82), respectively.
Discussion
SSM is an additional NIT in the toolbox for clinicians caring for patients with chronic liver disease. In the current study, we obtained SSM measurements from all participants without any selection bias to examine its independent role as an NIT in evaluating MASLD. The failure rate for obtaining a valid SSM was 19% in the current study and higher than the 3–4% failure rates reported in prior studies that used the FibroScan 630 Expert [44, 45]. We believe this higher failure rate is related to patient factors and device limitations. Notably, higher BMI and larger waist circumference decreased the probability of acquiring a valid SSM. We suspect this is related to the limitation of measuring the SSM with the medium (M+) probe and the lack of the SmartExam option for SSM. In previous studies, failure rates were lower, likely because participants had lower BMIs [44, 45]. Another reason for the higher failure rate in the current study was the inability to locate the spleen with the probe despite the availability of an ultrasound probe for visualization in the FibroScan 630 Expert. We suspect that the lack of splenomegaly in non-cirrhotic MASLD adds an additional limitation along with the M + probe and lack of the SmartExam. Although one might argue that failure to measure SSM could be a good qualitative prognostic sign indicating the absence of CSPH, caution should be exercised with that assumption in a patient with MASLD with a higher waist circumference or higher BMI. We hope that newer versions of the device will incorporate both the SmartExam and extra-large (XL+) probe to measure SSM. The SmartExam option has overcome the limitation associated with increased probe-to-capsule distance regarding LSM, as there were no patients with unreliable criteria of > 30% IQR/M.
In this study, SSM is a useful tool for detecting MASH cirrhosis using the SSM ≥ 21 kPa cutoff proposed by Baveno VII. This cutoff had a sensitivity of 96% with a PPV of 88%. Alternatively, SSM > 50 kPa has both a high specificity (78%) and high PPV (94%). Both these cutoffs performed well in the current MASLD cohort and, therefore, would be very useful in the evaluation of MASLD patients, particularly in those with invalid LSM or where data to calculate FIB4 are not readily available or in the indeterminate range. In our opinion, it is not unreasonable to routinely evaluate SSM in those with LSM > 15 kPa to evaluate for the presence of cirrhosis and CSPH. Furthermore, SSM ≥ 21 kPa cutoff had high NPV for EV (any size) and high-risk EV, making it clinically useful to prognosticate patients with suspected cirrhosis. Lastly, SSM > 50 kPa had a high NPV. Clinically, this would be useful to prioritize these patients for early evaluation with an upper endoscopy. As the mortality rate for bleeding EV can be high, it is important to have an NIT such as SSM with a high NPV for detecting high-risk EV [46]. Finally, these findings suggest that SSM by itself may be used as an alternative diagnostic tool for risk prognostication in patients with MASLD.
Certain aspects of our study merit further discussion. The study enrolled patients from a hepatology clinic in a prospective manner, but there is still a possibility of referral bias, as the number of patients with advanced fibrosis or cirrhosis in the study is higher than what would be expected in a primary care clinic. To detect high-risk EV, the cutoff value for SSM, > 67 kPa, needs to be validated in a separate independent cohort, even though it is in line with the > 50 kPa proposed by the BAVENO VII. The current dataset could have been more reliable if all participants had same-day FibroScan and upper endoscopies. However, we think there may not be a significant increase in portal hypertension within a year. Additionally, several participants without cirrhosis did not have an upper endoscopy, as an endoscopy was not clinically warranted. The combination of LSM < 20 kPa and platelet count of > 150 k/cumm criteria did not show high diagnostic accuracy (data not shown) in the current cohort, presumably due to the high proportion of patients with cirrhosis. Interestingly, SSM was the only NIT demonstrating a positive correlation with variceal size. Lastly, FIB4 appears to perform with high diagnostic accuracy to identify CSPH but has limited potential to differentiate between cirrhosis, the presence of EV (any size), and high-risk EV.
In conclusion, our study supports using SSM as a NIT for detecting cirrhosis and portal hypertensive manifestations associated with CSPH in patients with MASLD. SSM by itself is most effective in identifying the presence of EV, particularly high-risk EV. The availability of SSM, in addition to other NITs, may provide additional insights for risk stratification of patients with MASLD.
References
Younossi Z, Golabi P, Paik J et al. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH): a systematic review. Hepatology 2023;77:1335.
Younossi ZM, Stepanova M, Younossi Y et al. Epidemiology of chronic liver diseases in the USA in the past three decades. Gut 2020;69:564–568.
Rinella ME, Lazarus JV, Ratziu V, et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. Ann Hepatol 2023:101133.
Sanyal AJ, Van Natta ML, Clark J et al. Prospective study of outcomes in adults with nonalcoholic fatty liver disease. N Engl J Med 2021;385:1559–1569.
Chalasani N, Younossi Z, Lavine JE et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328–357.
Vilar-Gomez E, Calzadilla-Bertot L, Wai-Sun Wong V et al. Fibrosis severity as a determinant of cause-specific mortality in patients with advanced nonalcoholic fatty liver disease: a multi-national cohort study. Gastroenterology 2018;155(443–457):e17.
Siddiqui MS, Vuppalanchi R, Van Natta ML et al. Vibration-controlled transient elastography to assess fibrosis and steatosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2019;17(156–163):e2.
Vuppalanchi R, Siddiqui MS, Van Natta ML et al. Performance characteristics of vibration-controlled transient elastography for evaluation of nonalcoholic fatty liver disease. Hepatology 2018;67:134–144.
Vuppalanchi R, Unalp A, Van Natta ML et al. Effects of liver biopsy sample length and number of readings on sampling variability in nonalcoholic Fatty liver disease. Clin Gastroenterol Hepatol 2009;7:481–486.
Vilar-Gomez E, Chalasani N. Non-invasive assessment of non-alcoholic fatty liver disease: Clinical prediction rules and blood-based biomarkers. J Hepatol 2018;68:305–315.
Vilar-Gomez E, Vuppalanchi R, Mladenovic A et al. Prevalence of High-risk Nonalcoholic Steatohepatitis (NASH) in the United States: Results From NHANES 2017–2018. Clin Gastroenterol Hepatol 2023;21(115–124):e7.
Eddowes PJ, Sasso M, Allison M et al. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology 2019;156:1717–1730.
Newsome PN, Sasso M, Deeks JJ et al. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol 2020;5:362–373.
Younossi Z, Alkhouri N, Cusi K et al. A practical use of noninvasive tests in clinical practice to identify high-risk patients with nonalcoholic steatohepatitis. Aliment Pharmacol Ther 2022;57:304–312.
Woreta TA, Van Natta ML, Lazo M et al. Validation of the accuracy of the FAST score for detecting patients with at-risk nonalcoholic steatohepatitis (NASH) in a North American cohort and comparison to other non-invasive algorithms. PLoS One 2022;17:e0266859.
Tamaki N, Kurosaki M, Huang DQ et al. Noninvasive assessment of liver fibrosis and its clinical significance in nonalcoholic fatty liver disease. Hepatol Res 2022;52:497–507.
Huang DQ, Sharpton SR, Amangurbanova M et al. Clinical Utility of Combined MRI-PDFF and ALT Response in Predicting Histologic Response in Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol 2022;21:2682–2685.
Ajmera V, Nguyen K, Tamaki N et al. Prognostic utility of magnetic resonance elastography and MEFIB index in predicting liver-related outcomes and mortality in individuals at risk of and with nonalcoholic fatty liver disease. Therap Adv Gastroenterol 2022;15:17562848221093868.
Gidener T, Dierkhising RA, Mara KC et al. Change in serial liver stiffness measurement by magnetic resonance elastography and outcomes in NAFLD. Hepatology 2023;77:268–274.
de Franchis R, Bosch J, Garcia-Tsao G et al. Baveno VII - Renewing consensus in portal hypertension. J Hepatol 2022;76:959–974.
Pons M, Augustin S, Scheiner B et al. Noninvasive diagnosis of portal hypertension in patients with compensated advanced chronic liver disease. Am J Gastroenterol 2021;116:723–732.
Silva-Junior G, Baiges A, Turon F et al. The prognostic value of hepatic venous pressure gradient in patients with cirrhosis is highly dependent on the accuracy of the technique. Hepatology 2015;62:1584–1592.
Stefanescu H, Grigorescu M, Lupsor M et al. Spleen stiffness measurement using Fibroscan for the noninvasive assessment of esophageal varices in liver cirrhosis patients. J Gastroenterol Hepatol 2011;26:164–170.
Song J, Huang J, Huang H et al. Performance of spleen stiffness measurement in prediction of clinical significant portal hypertension: a meta-analysis. Clin Res Hepatol Gastroenterol 2018;42:216–226.
Sharma P, Kirnake V, Tyagi P et al. Spleen stiffness in patients with cirrhosis in predicting esophageal varices. Am J Gastroenterol 2013;108:1101–1107.
Mladenovic A, Vuppalanchi R, Desai AP. A primer to the diagnostic and clinical utility of spleen stiffness measurement in patients with chronic liver disease. Clin Liver Dis (Hoboken) 2022;19:124–130.
Nagai K, Ogawa Y, Kobayashi T et al. Gastroesophageal varices evaluation using spleen-dedicated stiffness measurement by vibration-controlled transient elastography. JGH Open 2022;6:11–19.
Ajmera V, Kim BK, Yang K et al. Liver stiffness on magnetic resonance elastography and the MEFIB index and liver-related outcomes in nonalcoholic fatty liver disease: a systematic review and meta-analysis of individual participants. Gastroenterology 2022;163(1079–1089):e5.
Kim BK, Tamaki N, Imajo K et al. Head-to-head comparison between MEFIB, MAST, and FAST for detecting stage 2 fibrosis or higher among patients with NAFLD. J Hepatol 2022;77:1482–1490.
Munoz-Codoceo C, Amo M, Martin A et al. Diagnostic accuracy of liver and spleen stiffness measured by fibroscan(R) in the prediction of esophageal varices in HCV-related cirrhosis patients treated with oral antivirals. Gastroenterol Hepatol 2021;44:269–276.
Abraldes JG, Bureau C, Stefanescu H et al. Noninvasive tools and risk of clinically significant portal hypertension and varices in compensated cirrhosis: The “Anticipate” study. Hepatology 2016;64:2173–2184.
Ma X, Wang L, Wu H et al. Spleen stiffness is superior to liver stiffness for predicting esophageal varices in chronic liver disease: a meta-analysis. PLoS One 2016;11:e0165786.
Dajti E, Ravaioli F, Marasco G et al. A combined Baveno VII and Spleen stiffness algorithm to improve the noninvasive diagnosis of clinically significant portal hypertension in patients with compensated advanced chronic liver disease. Am J Gastroenterol 2022;117:1825–1833.
Fofiu R, Bende F, Popescu A et al. Spleen and liver stiffness for predicting high-risk varices in patients with compensated liver cirrhosis. Ultrasound Med Biol 2021;47:76–83.
Colecchia A, Ravaioli F, Marasco G et al. A combined model based on spleen stiffness measurement and Baveno VI criteria to rule out high-risk varices in advanced chronic liver disease. J Hepatol 2018;69:308–317.
Karagiannakis DS, Stefanaki K. Spleen stiffness: a predictive factor of dismal prognosis in liver cirrhosis. Clin J Gastroenterol 2023;16:121–129.
Karagiannakis DS, Voulgaris T, Markakis G et al. Spleen stiffness can predict liver decompensation and survival in patients with cirrhosis. J Gastroenterol Hepatol 2023;38:283–289.
Odriozola A, Puente A, Cuadrado A, et al. High accuracy of spleen stiffness measurement in diagnosing clinically significant portal hypertension in metabolic-associated fatty liver disease. Liver Int 2023.
Noureddin M, Chan JL, Barradas K et al. Attribution of nonalcoholic steatohepatitis as an etiology of cirrhosis for clinical trials eligibility: recommendations from the multi-stakeholder liver forum. Gastroenterology 2020;159(422–427):e1.
Shah AG, Lydecker A, Murray K et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009;7:1104–1112.
Boursier J, Zarski JP, de Ledinghen V et al. Determination of reliability criteria for liver stiffness evaluation by transient elastography. Hepatology 2013;57:1182–1191.
Cardenas A, Mendez-Bocanegra A. Report of the Baveno VI consensus workshop. Ann Hepatol 2016;15:289–290.
de Franchis R, Baveno VIF. Expanding consensus in portal hypertension: report of the Baveno VI consensus workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol 2015;63:743–752.
Moia R, Cittone MG, Boggione P et al. Stiffer spleen predicts higher bone marrow fibrosis and higher JAK2 Allele Burden in patients with myeloproliferative neoplasms. Front Oncol 2021;11:777730.
Rigamonti C, Cittone MG, Manfredi GF et al. High reproducibility of spleen stiffness measurement by vibration-controlled transient elastography with a spleen-dedicated module. Hepatol Commun 2022;6:3006–3014.
Lay CS, Tsai YT, Teg CY et al. Endoscopic variceal ligation in prophylaxis of first variceal bleeding in cirrhotic patients with high-risk esophageal varices. Hepatology 1997;25:1346–1350.
Funding
None.
Author information
Authors and Affiliations
Contributions
EW, AM, DR, RW, NS, SG, EV-G, NC, and RV all contributed to study concept, design, data analysis, writing, editing, and approving of the resulting manuscript. RV, EW, and EV-G contributed to statistical analysis. EW, RV, and NC contributed to writing, editing, and approving the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
RV declares consulting agreement with EchoSens. The rest of the authors have no conflict to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
An invited commentary on this article is available at https://doi.org/10.1007/s10620-024-08270-7.
Supplementary Information
Below is the link to the electronic supplementary material.
10620_2024_8272_MOESM1_ESM.tif
Flow chart to demonstrate study participant flow from the initial cohort and those that were included in the final analysis for examining the diagnostic accuracy for identification of esophageal varices (the duration between Fibroscan and upper endoscopy is 1 year or less). Supplementary file1 (TIF 954 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Williams, E.E., Mladenovic, A., Ranginani, D. et al. Role of Spleen Stiffness Measurement in the Evaluation of Metabolic Dysfunction-Associated Steatotic Liver Disease. Dig Dis Sci 69, 1444–1453 (2024). https://doi.org/10.1007/s10620-024-08272-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-024-08272-5