Abstract
Portal hypertension is the central driver of complications in patients with chronic liver diseases and cirrhosis. The diagnosis of portal hypertension has important prognostic and clinical implications. In particular, screening for varices in patients with portal hypertension can effectively reduce the morbidity and mortality of variceal bleeding. In this article, we review the invasive and non-invasive methods to assess portal hypertension. Hepatic venous pressure gradient remains the gold standard to measure portal pressure but is invasive and seldom performed outside expert centers and research settings. In recent years, a number of non-invasive tests of fibrosis have shown good correlation with liver histology. They also show promise in identifying patients with portal hypertension and large varices. As a result, the latest Baveno VI consensus guidelines endorse the use of liver stiffness measurement by transient elastography and platelet count as initial assessment to select patients for varices screening. On the other hand, the performance of non-invasive tests in assessing the response to non-selective beta-blockers or transjugular intrahepatic portosystemic shunting is either suboptimal or unclear.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
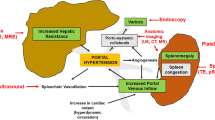
Portal hypertension is the central driver of complications in patients with chronic liver diseases and cirrhosis. While some manifestations of portal hypertension (e.g., ascites) are clinically apparent, others are more silent. For example, patients with varices remain asymptomatic until variceal bleeding develops. It is therefore important to identify patients with portal hypertension and offer endoscopic screening before bleeding occurs. Apart from cirrhotic complications, portal hypertension is strongly associated with mortality in patients with different liver conditions [1, 2].
While cirrhosis is the main cause of portal hypertension, the latter can arise in non-cirrhotic patients, a condition referred to as non-cirrhotic portal hypertension [3]. In addition, not every cirrhotic patient would have portal hypertension. Among cirrhotic patients, the thickness of fibrous septa and the degree of hepatic architectural distortion affect the portal pressure and clinical outcomes. In this article, we focus on portal hypertension as a result of cirrhosis. We describe the gold standard of assessing portal hypertension and review the role of non-invasive tests of fibrosis in the setting of portal hypertension.
Clinical and radiological features of portal hypertension
Splenomegaly and the associated hypersplenism are features of portal hypertension. A splenic craniocaudal length of >13 cm indicates splenomegaly. However, splenomegaly is a non-specific sign of portal hypertension. Using color Doppler ultrasound, the presence of small reflective channels within the splenic parenchyma may suggest that splenomegaly is caused by portal hypertension rather than from other causes [4]. Another specific radiological finding is Gamna-Gandy bodies, which can be detected on MRI as tiny hypointense foci in the spleen, but are present in only 6–12% of portal hypertensive patients [5].
A recent study showed that the splenic arterial resistive index (SARI), which measures a change in splenic hemodynamics, is highly correlated with the HVPG, especially in patients without splenomegaly (r = 0.830) [6]. This test can possibly serve as a non-invasive method for diagnosing clinically significant portal hypertension in patients without an enlarged spleen.
The umbilical vein is often reopened in patients with portal hypertension, acting as a portosystemic shunt between the left portal vein and superficial epigastric veins. In fact, it is the most specific ultrasonographic sign of portal hypertension. Recanalization of the umbilical vein is detected in 26% of portal hypertensive patients by percutaneous transhepatic portography [7], but this invasive procedure has been largely replaced by ultrasonography. The presence of a recanalized umbilical vein can be recognized as a central sonolucent region of the falciform ligament on ultrasound, known as a ‘bull’s-eye’ appearance [8]. It is present in 34% of patients detected by ultrasonography [9]. It has been suggested that the lumen of the umbilical vein should be more than 3 mm in diameter and extend for a considerable length away from the left portal vein for the diagnosis of portal hypertension [10]. Nevertheless, one study showed that the recanalised umbilical vein seen on ultrasound is in fact a paraumbilical vein by histological analysis on liver specimens [11]. While the actual recanalized structure is still a matter of debate, the presence of an enlarged vein in the falciform ligament on ultrasound should serve as a highly reliable sign of portal hypertension.
A dynamic CT scan performed with a bolus of contrast material can accurately detect most portosystemic collaterals in patients with portal hypertension [12]. It is superior to other modalities such as angiography, ultrasound and endoscopy in detecting varices, e.g., paraumbilical and abdominal wall varices, but its sensitivity is relatively low in detecting esophageal varices [12]. To this end, the PillCam ESO capsule endoscopy can detect esophageal varices and portal hypertensive gastropathy with a sensitivity and specificity of 80–90% [13, 14]. The high cost of capsule endoscopy makes it a less attractive option than the transient elastography-based assessment as recommended by the Baveno VI consensus guidelines.
Recently, the multiparametric MRI has been developed as a one-stop examination for hepatic steatosis, inflammation, fibrosis and iron content based on T1 and T2* imaging [15]. In a small prospective series, the liver inflammation fibrosis (LIF) score was associated with adverse clinical outcomes [16]. The same technique, when applied to the spleen, also correlated with the hepatic venous pressure gradient (HVPG).
Another recent multi-center study evaluated the use of shear-wave elastography of the liver and spleen in diagnosing patients with clinically significant portal hypertension [17]. Using a rule-in algorithm with both liver and spleen shear-wave elastography, the sensitivity and specificity in diagnosing clinically significant portal hypertension were 89.2 and 91.4%, respectively. This algorithm could save a proportion of patients from undergoing invasive HVPG measurements.
Hepatic venous pressure gradient
Measuring the HVPG is the gold standard for determining the portal pressure [18]. It is an invasive procedure that can diagnose portal hypertension with other clinical applications. Although the HVPG measures the portal pressure indirectly, it is highly consistent with direct measurements obtained through less invasive means [19–21]. The interpretation of HVPG readings is also highly reproducible [22].
The procedure
Under sedation and local anesthesia, a balloon-tipped catheter is passed through the right internal jugular vein (or femoral vein or antecubital vein) and into the hepatic vein under fluoroscopic guidance [18]. The HVPG is the free hepatic vein pressure (FHVP) subtracted from the wedged hepatic vein pressure (WHVP). The FHVP is measured in the hepatic vein 2–4 cm from the opening into the inferior vena cava. The inferior vena cava pressure (IVCP) is also measured below the diaphragm. Although FHVP and IVCP are almost identical, the FHVP can be falsely elevated by 1–3 mmHg by inadequate placement of the catheter, hence underestimating the HVPG; a difference of less than 2 mmHg is considered acceptable. Some surgeons always prefer using IVCP to FHVP.
The WHVP is measured in the hepatic vein after balloon occlusion. Compared to a straight-end catheter, a balloon occludes a greater area of hepatic veins, thereby improving the reliability and reproducibility of measurements [23]. Contrast dye injected into the hepatic vein confirms the occlusion by the absence of reflux.
The pulse rate, blood pressure and oxygen saturation are monitored during the procedure. Complications are minimal: major complications include bleeding, hematoma or arterial-venous fistula formation at the puncture site, which can be reduced by ultrasound guidance. Passing the catheter through the right atrium might cause self-limiting arrhythmias. Patients allergic to contrast dyes can use carbon dioxide as a contrast agent. Those with severe thrombocytopenia or prolonged prothombin time should consider platelet or fresh frozen plasma transfusion before the procedure.
Interpretation of results
The WHVP is equivalent to the sinusoidal pressure, which is roughly equivalent to portal pressure. The FHVP serves as a reference zero; when subtracted from the WHVP, the HVPG gives an accurate depiction of the portal pressure. HVPG of 6–9 mmHg indicates preclinical portal hypertension, and a HVPG ≥10 mmHg is diagnostic of significant portal hypertension. However, in prehepatic or intrahepatic presinusoidal hypertension, the WHVP cannot reflect the raised portal pressure as any blood that flows to the hepatic veins travels via unaffected sinusoids, which have large capacity and low resistance. Therefore, these patients have normal WHVP and HVPG (Fig. 1).
Applications of HVPG
The HVPG independently predicts mortality among patients with cirrhosis [24, 25]. The 1-year mortality rate is 1.9% among those with HVPG ≤17 mmHg versus 16.2% in patients with HVPG >17 mmHg [25]. However, when combined with the Model for End-Stage Liver Disease (MELD) score, it does not significantly improve the stratification [24].
A reduction of baseline HVPG to ≤12 mmHg or by ≥20% by beta-blockers significantly reduces the risk of variceal bleeding and mortality [26, 27]. Furthermore, patients with an acute response to intravenous propranolol, i.e. reduction of HVPG to ≤12 mmHg or by ≥10% within 20 min, are less likely to have a first episode of variceal bleeding in the next 2 years (4 vs. 46%, p < 0.001). These patients are also likely to be chronic responders to the beta-blocker treatment [27].
The HVPG can also be used to monitor the response to transjugular intrahepatic porto-systemic shunt (TIPSS). After TIPSS, the HVPG should be maintained at 5–12 mmHg to reduce the risk of ascites and post-TIPSS encephalopathy [28]. Compared to traditional parameters, the HVPG better predicts the post-operative course after hepatectomy in patients with hepatocellular carcinoma. Those with a preoperative HVPG ≥10 mmHg are at greater risk of hepatic decompensation and mortality after hepatectomy [29].
Despite these applications, HVPG measurement is invasive and has to be carried out in facilities with fluoroscopy and expertise. It is therefore seldom performed outside a research setting except in some expert centers.
Serum markers for portal hypertension
Serum tests may also serve as surrogate non-invasive markers of portal hypertension. For instance, osteopontin, von Willebrand factor and the VITRO score have been evaluated in the context of portal hypertension.
Osteopontin is associated with pathological conditions including inflammation, angiogenesis and fibrosis [30]. In one study comprising 157 liver cirrhosis patients [31], osteopontin was showed to distinguish clinically significant portal hypertension (CSPH) (HVPG >10 mmHg) at 75% sensitivity and 63% specificity, and has an AUROC of 0.763 using a cut-off value of 80 ng/mL. The study also described the prognostic value of osteopontin as similar to HVPG.
Von Willebrand factor was also proposed to be a clinically significant non-invasive predictor of CSPH [32]. Using a cut-off value of ≥241%, the AUROC for detection of CSPH in compensated patients was 0.85, with the mortality prediction similar to the MELD score.
Recently, researchers have proposed using a new score, the VITRO score (Von Willebrand Factor Antigen/Thrombocyte Ratio), as a possible marker for detecting CSPH [33]. The AUROC was found to be 0.86, which was higher than that of the von Willebrand factor, and the APRI and ELF score, but lower than that of transient elastography.
Non-invasive tests of liver fibrosis
Serum tests
Despite histological examination being the gold standard of liver fibrosis assessment, the liver biopsy procedure carries notable drawbacks. Variable sampling error [34] and potential complications, including severe hemorrhage or even death [35], are known limitations of liver biopsy. The demand for non-invasive assessments of liver fibrosis has become increasingly relevant. The lookout for feasible methods that allow frequent testing and regular monitoring of liver fibrosis is also becoming the new trend. Among different non-invasive techniques, serum-based tests offer one of the alternative approaches which is cost–effective and widely available [36]. They are also extensively discussed in most recent guidelines of the European Association for the Study of the Liver [37].
An ideal fibrosis biomarker should be liver-specific, not influenced by alterations in liver, renal, or reticulo-endothelial function, measure one or more of the processes related to fibrosis, and easy to perform [38]. Serum biomarkers for liver fibrosis can be divided into Class I and Class II. Class I markers aim to directly measure the activity of fibrogenesis or fibrinolysis, while class II markers aim to measure surrogate parameters that correlate with fibrosis [39]. There has been no consensus in giving preference to markers from one of the classes. Class I or II markers may be used individually, but are very commonly used in combination, especially in commercialized tests.
Generic and proprietary serum tests of liver fibrosis
While most of the serum tests are widely available, some have been patented and commercialized. These became ‘proprietary’ tests as compared to the unpatented ‘generic’ tests. When using generic tests, results can often instantly be calculated from its required biomarker(s), and can be carried out by any laboratory. On the other hand, proprietary tests often require blood samples to be sent to respective corporations, and payments are required. The formulae of proprietary tests are also protected or undisclosed.
This article reviews 13 popular serum tests of liver fibrosis, including 8 generic tests and 5 proprietary tests (Table 1). Selected generic tests include platelet count, aspartate aminotransferase (AST)-to-platetlet ratio index (APRI), FIB-4, AST/alanine aminotransferase (ALT) ratio, hyaluronic acid, Lok index, Forns’ index and Fibroindex. Selected proprietary tests include FibroMeter (Echosens, Paris, France), FibroTest/FibroSure (BioPredictive, Paris, France/LabCorp, Burlington, NC, USA), Hepascore (PathWest, University of Western Australia, Australia), FibroSpect (Prometheus, San Diego, CA, USA) and Enhanced Liver Fibrosis (Siemens Healthineers, Erlangen, Germany). Different studies often evaluated the same serum test using different cutoff thresholds (Table 2). Some studies aimed to detect F2-4 disease, and some aimed to detect F3-4 disease. While positive and negative predictive values are dependent on the disease prevalence; sensitivity and specificity are affected by the chosen cutoff value; the area under the receiver-operating characteristics curve (AUROC) provides better information on the accuracy of a dichotomous diagnostic test [40].
Some of the commonly used generic serum tests, such as APRI, have been more extensively studied. A meta-analysis evaluating APRI has included over 8000 patients from 40 studies [41]. The mean AUROC of APRI in detecting F2-4 disease was 0.77. Hyaluronic acid was suggested as the most validated single marker that most accurately predicts advanced fibrosis compared to other individual biomarkers [42]. It is a biomarker commonly included in many of the proprietary composite tests. It is a component of the extracellular matrix. It increases during collagen synthesis as a result of inflammation and decreased sinusoidal endothelial function, which reduces endothelial absorption and destruction of hyaluronic acid [43]. A Japanese study has reported the AUROC of hyaluronic acid to be 0.87 in detecting F2-4 disease and 0.89 in detecting F3-4 disease [43]. It was also suggested to use the high negative predictive value (98–100%) of hyaluronic acid at a high cut-off value to exclude advance fibrosis [44]. Another popular biomarker used in proprietary formulae is α2-macroglobulin. It is a wide-spectrum proteinase inhibitor which inhibits the catabolism of matrix proteins during fibrosis [45].
FibroTest (Biopredictive), also known as Fibrosure in the USA (LabCorp), was the first proprietary test calculated from combining several generic parameters [46]. It is one of the most extensively investigated proprietary serum tests of liver fibrosis [47]. Its AUROC has been reported by three studies [48–50], ranging from 0.69 to 0.81 in detecting F2-4 disease, and from 0.72 to 0.84 in detecting F3-4 disease. FibroMeter is another popular proprietary serum test of liver fibrosis, which has been claimed to surpass the accuracy of its peers [48, 51, 52]. In two studies, its AUROC was reported to range from 0.84 to 0.85 in detecting F2-4 disease and from 0.85 to 0.91 in detecting F3-4 disease [48, 49].
Assessment of varices
Many studies have been carried out in search of predictive markers related to varices [53–55]. A number of serum tests of liver fibrosis have been correlated with esophageal varices, variceal bleeding or mortality. There is considerable interest in developing accurate screening tests to detect or predict variceal complications [56], as more may benefit from primary prophylaxis and may reduce complications or healthcare burden from frequent endoscopy. A large retrospective cohort study has reported that platelet count, APRI, AST/ALT ratio and Lok index were useful in predicting the presence of varices prior to endoscopy, in differentiating patients with and without varices, and in predicting the likelihood of variceal bleeding [57].
Another study also provided cutoff values in seven generic serum tests to potentially predict the presence of large varices [58]. It also evaluated their AUROCs in the prediction, which range from 0.55 to 0.71. FibroTest has also been evaluated as a possible non-invasive aid in the detection of large esophageal varices [59], and the study suggested that using a high threshold may rule out the presence of large esophageal varices. Some serum tests have been correlated as prognostic markers of mortality, including FibroTest and APRI [60, 61].
However, there are also studies pointing out the low predictive values of using individual serum markers, such as APRI, to sufficiently predict varices itself. Another study evaluating four class I serum markers, including hyaluronic acid, has suggested their correlation in predicting the presence of varices, but they are not reliable enough for assessing the risk of variceal bleeding [62].
In portal hypertensive patients, non-selective beta-blockers and TIPSS are common treatments [63]. The discovery of non-invasive tests which can monitor changes induced by these treatments of portal hypertension can provide useful prognostic information. While the serum markers discussed here were initially for detecting liver fibrosis and not portal pressure, FibroTest was reported as having significant correlation with HVPG values [60]. Another study quoted the AUROC of FibroTest in detecting severe portal hypertension as 0.79 [64].
Non-invasive measurements of liver stiffness
The non-invasive measurements of liver stiffness or elasticity include transient elastography, shear-wave elastography, acoustic radiation force impulse and magnetic resonance elastography [65]. The first three are ultrasound-based, while magnetic resonance elastography (MRE) utilizes magnetic resonance imaging (MRI). It is possible to examine the liver parenchyma and perform HCC surveillance in the same session with the latter three techniques; transient elastography is only equipped with M mode ultrasound and cannot be used for structural examination. MRE has the advantage of examining the entire liver and not being affected by obesity. It is also more accurate in delineating milder degrees of liver fibrosis. However, existing data suggest that the ultrasound-based measurements are probably as good as MRE in detecting cirrhosis, with all techniques typically having AUROCs of over 0.90 [66–69]. The availability and cost are the major hurdles preventing broader application of MRE.
Liver stiffness or elasticity measurement is affected by high alanine aminotransferase level [70], congestive heart failure [71], biliary obstruction [72], food intake [73], amyloidosis [74], extreme body size [75], and, to a lesser extent, hepatic steatosis [76, 77]. Although most studies on the confounders of liver stiffness measurement were performed using transient elastography, the physical property likely applies to the other techniques as well. Caution should be exercised when interpreting liver stiffness values in such patients.
Liver stiffness has been correlated with HVPG. In a cross-sectional study of 150 patients undergoing liver biopsy, HVPG and transient elastography, liver stiffness had an AUROC of 0.95 in detecting significant portal hypertension (HVPG ≥10 mmHg) with an optimal cutoff of 21 kPa [78]. Likewise, patients with portal hypertension have increased splenic vein pressure, splenomegaly and high spleen stiffness [79, 80]. Nevertheless, transient elastography measures a core of tissue that is 4 cm in length and is not designed for measuring spleen stiffness, particularly in patients without splenomegaly. To this end, acoustic radiation force impulse has also been used to measure spleen stiffness, which correlated with the presence of esophageal varices [81]. This technique captures small regions of interest and can handle spleens of different sizes.
Other than correlation with HVPG, multiple studies have confirmed that liver and/or spleen stiffness can be used to predict the presence of all varices or large varices [82–86]. Importantly, liver stiffness is correlated with subsequent variceal bleeding and other complications of portal hypertension [87]. Based on these observations, the latest Baveno VI consensus guidelines recommend the use of liver stiffness and platelet count to select cirrhotic patients for varices screening [88]. For patients with liver stiffness <20 kPa and normal platelet count, the risk of having large varices or variceal bleeding is minimal, and the non-invasive tests may be repeated annually (Fig. 2) [89]. Otherwise, upper gastrointestinal endoscopy should be performed for formal screening.
Two related questions deserve further discussion. First, large varices and/or variceal bleeding may be treated with non-selective beta-blockers and TIPSS. While the HVPG response to these treatments can be used to predict who will develop variceal bleeding, few centers can provide HVPG monitoring. It is thus of interest to see if elastography may serve this purpose. Theoretically, a reduction in portal blood flow should result in decreased liver and spleen stiffness. However, liver stiffness is not expected to normalize because neither non-selective beta-blockers nor TIPSS affect liver fibrosis. In a small study of 10 patients, the spleen stiffness by acoustic radiation force impulse decreased from 3.65 to 3.27 m/s after TIPSS, but there was no significant change in liver stiffness [90].
Another question is whether annual examination according to the Baveno VI consensus is needed in patients whose liver disease is quiescent [88]. This question has become highly relevant now that we can suppress hepatitis B virus with antiviral drugs and cure chronic hepatitis C virus infection with direct-acting antivirals in almost all patients. Successful treatment of chronic viral hepatitis can prevent disease progression and reverse cirrhosis in the majority of patients [91–93]. Incident varices and variceal bleeding are also rare in patients on long-term antiviral therapy for chronic hepatitis B [94]. Therefore, it is likely that patients with quiescent liver diseases may not require further liver stiffness measurement once the value drops below a certain threshold. The notion should be explored in prospective studies.
Investigations for the cause of portal hypertension
Cirrhosis is the end result of all causes of chronic liver disease and is the most common cause of portal hypertension. It is important to identify the underlying cause of liver injury, not only to direct management decisions but also because it may have other implications such as family screening in cases of hereditary hemochromatosis and Wilson’s Disease. The etiology of cirrhosis can usually be made from patient history and serological testing.
Radiological assessment
Cirrhosis can lead to hemodynamic changes such as blood flow velocity in the portal and systemic circulation. Altered portal blood flow direction, velocity, stigmata of portal hypertension (e.g., splenomegaly,) portal vein thrombosis and evidence of portosystemic shunting can be detected using ultrasonography. Hemodynamic changes can directly influence the severity of portal hypertension. It may be detected by Doppler ultrasound even in those with normal B-mode findings and before the appearance of varices and splenomegaly [95]. A portal blood flow velocity of <15 cm/s had a sensitivity and specificity of 88 and 96%, respectively, for the detection of portal hypertension [96]. In addition, computed tomography (CT) and MRI can provide a detailed mapping of the portal venous system prior to TIPSS procedure and to detect radiological signs of portal hypertension.
Portal venography has been the standard method of mapping collateral vessels for many years and involved puncture of a branch artery which feeds the gastrointestinal system, such as the celiac, mesenteric, or splenic artery [97]. Because of the invasive nature of the procedure, venography is now generally performed using CT or MRI (with contrast enhancement), which has a high resolution and allows 3D-reconstruction of the portal venous system.
Carbon dioxide has been found in some studies to be better than iodinated-contrast for wedged hepatic venography and for visualizing the portal vein before TIPSS [98]. In cirrhotic patients, visualization of the portal vein and branches by carbon dioxide-occluded venography was achieved in 85% of patients versus 35% with iodinated-contrast venography.
Non-cirrhotic portal hypertension
Non-cirrhotic portal hypertension is usually caused by diseases that lead to changes in the hepatic vasculature, with preserved hepatic synthetic function and near-normal HVPG [99]. The etiology of portal hypertension is generally classified by the site of resistance to blood flow: pre-hepatic, hepatic, and post-hepatic. Hepatic causes can be further divided into pre-sinusoidal, sinusoidal and post-sinusoidal (Table 3).
Portal vein thrombosis can be classified as acute or chronic, and partially or completely occlusive. The most common causes of portal vein thrombosis are secondary including cirrhosis, hypercoagulable states (e.g., antiphospholipid syndrome, malignancy), abdominal trauma, surgery or infection.
Parasitic infestations such as schistosomiasis can cause extensive periportal fibrosis but retained hepatic function [100]. It is one of the leading causes of non-cirrhotic portal hypertension, particularly in low-income countries in southeast Asia and central Africa. Diagnosis can be made by demonstration of eggs in urine, feces, or tissue biopsies.
Acquired forms of prothrombotic states, e.g., hematological malignancies and myeloproliferative disorders, can be the underlying cause of venous occlusion. In a recent meta-analysis, the prevalence of V617F mutation of Janus kinase 2, which is associated with various myeloproliferative disorders, can be found in 27.7% of patients with portal vein thrombosis [101].
Idiopathic non-cirrhotic portal hypertension is rare in western countries and occurs mainly in India and Japan [102]. Proposed etiologies include childhood infections and prothrombotic states.
Conclusions
The diagnosis of portal hypertension in patients with chronic liver disease has important prognostic and management implications. HVPG, while invasive, will remain an important research tool for the pathophysiology of portal hypertension and may be used in specific situations at expert centers. The Baveno VI criteria based on liver stiffness measurement and platelet count are robust and may be applied clinically to select patients for varices screening. Further studies are required to determine the best method to monitor response to treatment in patients with portal hypertension.
Abbreviations
- ALT:
-
Alanine aminotransferase
- APRI:
-
AST-to-platelet ratio index
- AST:
-
Aspartate aminotransferase
- AUROC:
-
Area under the receiver-operating characteristics curve
- CT:
-
Computed tomography
- FHVP:
-
Free hepatic vein pressure
- HCC:
-
Hepatocellular carcinoma
- HVPG:
-
Hepatic vein pressure gradient
- IVCP:
-
Inferior vena cava pressures
- MRE:
-
Magnetic resonance elastography
- MRI:
-
Magnetic resonance imaging
- TIPSS:
-
Transjugular intrahepatic porto-systemic shunt
- WHVP:
-
Wedged hepatic vein pressure
References
Rincon D, Lo Iacono O, Ripoll C, Gomez-Camarero J, Salcedo M, Catalina MV, Hernando A, et al. Prognostic value of hepatic venous pressure gradient for in-hospital mortality of patients with severe acute alcoholic hepatitis. Aliment Pharmacol Ther. 2007;25:841–8.
Garg H, Kumar A, Garg V, Kumar M, Kumar R, Sharma BC, Sarin SK. Hepatic and systemic hemodynamic derangements predict early mortality and recovery in patients with acute-on-chronic liver failure. J Gastroenterol Hepatol. 2013;28:1361–7.
Sarin SK, Khanna R. Non-cirrhotic portal hypertension. Clin Liver Dis. 2014;18:451–76.
Kedar RP, Merchant SA, Malde HH, Patel VH. Multiple reflective channels in the spleen: a sonographic sign of portal hypertension. Abdom Imaging. 1994;19:453–8.
Elsayes KM, Narra VR, Mukundan G, Lewis JS Jr, Menias CO, Heiken JP. MR imaging of the spleen: spectrum of abnormalities. Radiographics. 2005;25:967–82.
Lee CM, Jeong WK, Lim S, Kim Y, Kim J, Kim TY, Sohn JH. Diagnosis of clinically significant portal hypertension in patients with cirrhosis: splenic arterial resistive index versus liver stiffness measurement. Ultrasound Med Biol. 2016;42:1312–20.
Aagaard J, Jensen LI, Sorensen TI, Christensen U, Burcharth F. Recanalized umbilical vein in portal hypertension. Am J Roentgenol. 1982;139:1107–10.
Schabel SI, Rittenberg GM, Javid LH, Cunningham J, Ross P. The, “bull’s-eye” falciform ligament: a sonographic finding of portal hypertension. Radiology. 1980;136:157–9.
Subramanyam BR, Balthazar EJ, Madamba MR, Raghavendra BN, Horii SC, Lefleur RS. Sonography of portosystemic venous collaterals in portal hypertension. Radiology. 1983;146:161–6.
Saddekni S, Hutchinson DE, Cooperberg PL. The sonographically patent umbilical vein in portal hypertension. Radiology. 1982;145:441–3.
Lafortune M, Constantin A, Breton G, Legare AG, Lavoie P. The recanalized umbilical vein in portal hypertension: a myth. Am J Roentgenol. 1985;144:549–53.
Cho KC, Patel YD, Wachsberg RH, Seeff J. Varices in portal hypertension: evaluation with CT. Radiographics. 1995;15:609–22.
Eisen GM, Eliakim R, Zaman A, Schwartz J, Faigel D, Rondonotti E, Villa F, et al. The accuracy of PillCam ESO capsule endoscopy versus conventional upper endoscopy for the diagnosis of esophageal varices: a prospective three-center pilot study. Endoscopy. 2006;38:31–5.
de Franchis R, Eisen GM, Laine L, Fernandez-Urien I, Herrerias JM, Brown RD, Fisher L, et al. Esophageal capsule endoscopy for screening and surveillance of esophageal varices in patients with portal hypertension. Hepatology. 2008;47:1595–603.
Banerjee R, Pavlides M, Tunnicliffe EM, Piechnik SK, Sarania N, Philips R, Collier JD, et al. Multiparametric magnetic resonance for the non-invasive diagnosis of liver disease. J Hepatol. 2014;60:69–77.
Pavlides M, Banerjee R, Sellwood J, Kelly CJ, Robson MD, Booth JC, Collier J, et al. Multiparametric magnetic resonance imaging predicts clinical outcomes in patients with chronic liver disease. J Hepatol. 2016;64:308–15.
Jansen C, Bogs C, Verlinden W, Thiele M, Moller P, Gortzen J, Lehmann J, et al. Shear-wave elastography of the liver and spleen identifies clinically significant portal hypertension: a prospective multicentre study. Liver Int. 2017;37:396–405.
Bosch J, Abraldes JG, Berzigotti A, Garcia-Pagan JC. The clinical use of HVPG measurements in chronic liver disease. Nat Rev Gastroenterol Hepatol. 2009;6:573–82.
Lin HC, Tsai YT, Lee FY, Chang TT, Wang SS, Lay CS, Lee SD, et al. Comparison between portal vein pressure and wedged hepatic vein pressure in hepatitis B-related cirrhosis. J Hepatol. 1989;9:326–30.
Perello A, Escorsell A, Bru C, Gilabert R, Moitinho E, Garcia-Pagan JC, Bosch J. Wedged hepatic venous pressure adequately reflects portal pressure in hepatitis C virus-related cirrhosis. Hepatology. 1999;30:1393–7.
Thalheimer U, Leandro G, Samonakis DN, Triantos CK, Patch D, Burroughs AK. Assessment of the agreement between wedge hepatic vein pressure and portal vein pressure in cirrhotic patients. Dig Liver Dis. 2005;37:601–8.
Tandon P, Ripoll C, Assis D, Wongcharatrawee S, Groszmann RJ, Garcia-Tsao G. The interpretation of hepatic venous pressure gradient tracings—excellent interobserver agreement unrelated to experience. Liver Int. 2016;36:1160–6.
Zipprich A, Winkler M, Seufferlein T, Dollinger MM. Comparison of balloon vs. straight catheter for the measurement of portal hypertension. Aliment Pharmacol Ther. 2010;32:1351–6.
Ripoll C, Banares R, Rincon D, Catalina MV, Lo Iacono O, Salcedo M, Clemente G, et al. Influence of hepatic venous pressure gradient on the prediction of survival of patients with cirrhosis in the MELD Era. Hepatology. 2005;42:793–801.
Kim TY, Lee JG, Sohn JH, Kim JY, Kim SM, Kim J, Jeong WK. Hepatic venous pressure gradient predicts long-term mortality in patients with decompensated cirrhosis. Yonsei Med J. 2016;57:138–45.
D’Amico G, Garcia-Pagan JC, Luca A, Bosch J. Hepatic vein pressure gradient reduction and prevention of variceal bleeding in cirrhosis: a systematic review. Gastroenterology. 2006;131:1611–24.
Villanueva C, Aracil C, Colomo A, Hernandez-Gea V, Lopez-Balaguer JM, Alvarez-Urturi C, Torras X, et al. Acute hemodynamic response to beta-blockers and prediction of long-term outcome in primary prophylaxis of variceal bleeding. Gastroenterology. 2009;137:119–28.
Garcia-Tsao G. Transjugular intrahepatic portosystemic shunt in the management of refractory ascites. Semin Intervent Radiol. 2005;22:278–86.
Bruix J, Castells A, Bosch J, Feu F, Fuster J, Garcia-Pagan JC, Visa J, et al. Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology. 1996;111:1018–22.
Nagoshi S. Osteopontin: versatile modulator of liver diseases. Hepatol Res. 2014;44:22–30.
Bruha R, Jachymova M, Petrtyl J, Dvorak K, Lenicek M, Urbanek P, Svestka T, et al. Osteopontin: a non-invasive parameter of portal hypertension and prognostic marker of cirrhosis. World J Gastroenterol. 2016;22:3441–50.
Ferlitsch M, Reiberger T, Hoke M, Salzl P, Schwengerer B, Ulbrich G, Payer BA, et al. von Willebrand factor as new noninvasive predictor of portal hypertension, decompensation and mortality in patients with liver cirrhosis. Hepatology. 2012;56:1439–47.
Hametner S, Ferlitsch A, Ferlitsch M, Etschmaier A, Schofl R, Ziachehabi A, Maieron A. The VITRO score (Von Willebrand Factor Antigen/Thrombocyte Ratio) as a new marker for clinically significant portal hypertension in comparison to other non-invasive parameters of fibrosis including ELF test. PLoS ONE. 2016;11:e0149230.
Abdi W, Millan JC, Mezey E. Sampling variability on percutaneous liver biopsy. Arch Intern Med. 1979;139:667–9.
Piccinino F, Sagnelli E, Pasquale G, Giusti G. Complications following percutaneous liver biopsy. A multicentre retrospective study on 68,276 biopsies. J Hepatol. 1986;2:165–73.
Zhou K, Lu LG. Assessment of fibrosis in chronic liver diseases. J Dig Dis. 2009;10:7–14.
European Association for Study of the Liver. Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH Clinical Practice Guidelines: non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63:237–64.
Afdhal NH, Nunes D. Evaluation of liver fibrosis: a concise review. Am J Gastroenterol. 2004;99:1160–74.
Wong GL, Espinosa WZ, Wong VW. Personalized management of cirrhosis by non-invasive tests of liver fibrosis. Clin Mol Hepatol. 2015;21:200–11.
Hajian-Tilaki K. Receiver operating characteristic (ROC) curve analysis for medical diagnostic test evaluation. Caspian J Intern Med. 2013;4:627–35.
Lin ZH, Xin YN, Dong QJ, Wang Q, Jiang XJ, Zhan SH, Sun Y, et al. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology. 2011;53:726–36.
Papastergiou V, Tsochatzis E, Burroughs AK. Non-invasive assessment of liver fibrosis. Ann Gastroenterol. 2012;25:218–31.
Suzuki A, Angulo P, Lymp J, Li D, Satomura S, Lindor K. Hyaluronic acid, an accurate serum marker for severe hepatic fibrosis in patients with non-alcoholic fatty liver disease. Liver Int. 2005;25:779–86.
Gressner OA, Weiskirchen R, Gressner AM. Biomarkers of liver fibrosis: clinical translation of molecular pathogenesis or based on liver-dependent malfunction tests. Clin Chim Acta. 2007;381:107–13.
Naveau S, Poynard T, Benattar C, Bedossa P, Chaput JC. Alpha-2-macroglobulin and hepatic fibrosis. Diagnostic interest. Dig Dis Sci. 1994;39:2426–32.
Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T, Group M. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet. 2001;357:1069–75.
Shaheen AA, Wan AF, Myers RP. FibroTest and FibroScan for the prediction of hepatitis C-related fibrosis: a systematic review of diagnostic test accuracy. Am J Gastroenterol. 2007;102:2589–600.
Cales P, Boursier J, Oberti F, Hubert I, Gallois Y, Rousselet MC, Dib N, et al. FibroMeters: a family of blood tests for liver fibrosis. Gastroenterol Clin Biol. 2008;32:40–51.
Leroy V, Sturm N, Faure P, Trocme C, Marlu A, Hilleret MN, Morel F, et al. Prospective evaluation of FibroTest(R), FibroMeter(R), and HepaScore(R) for staging liver fibrosis in chronic hepatitis B: comparison with hepatitis C. J Hepatol. 2014;61:28–34.
Friedrich-Rust M, Rosenberg W, Parkes J, Herrmann E, Zeuzem S, Sarrazin C. Comparison of ELF, FibroTest and FibroScan for the non-invasive assessment of liver fibrosis. BMC Gastroenterol. 2010;10:103.
Cales P, Oberti F, Michalak S, Hubert-Fouchard I, Rousselet MC, Konate A, Gallois Y, et al. A novel panel of blood markers to assess the degree of liver fibrosis. Hepatology. 2005;42:1373–81.
Rossi E, Adams LA, Bulsara M, Jeffrey GP. Assessing liver fibrosis with serum marker models. Clin Biochem Rev. 2007;28:3–10.
Madhotra R, Mulcahy HE, Willner I, Reuben A. Prediction of esophageal varices in patients with cirrhosis. J Clin Gastroenterol. 2002;34:81–5.
Pilette C, Oberti F, Aube C, Rousselet MC, Bedossa P, Gallois Y, Rifflet H, et al. Non-invasive diagnosis of esophageal varices in chronic liver diseases. J Hepatol. 1999;31:867–73.
Ng FH, Wong SY, Loo CK, Lam KM, Lai CW, Cheng CS. Prediction of oesophagogastric varices in patients with liver cirrhosis. J Gastroenterol Hepatol. 1999;14:785–90.
Silva G. New serum markers for predicting esophageal varices: is it a reality? J Gastroenterol Hepatol. 2013;28:4–5.
Rockey DC, Elliott A, Lyles T. Prediction of esophageal varices and variceal hemorrhage in patients with acute upper gastrointestinal bleeding. J Investig Med. 2016;64:745–51.
Sebastiani G, Tempesta D, Fattovich G, Castera L, Halfon P, Bourliere M, Noventa F, et al. Prediction of oesophageal varices in hepatic cirrhosis by simple serum non-invasive markers: results of a multicenter, large-scale study. J Hepatol. 2010;53:630–8.
Thabut D, Trabut JB, Massard J, Rudler M, Muntenau M, Messous D, Poynard T. Non-invasive diagnosis of large oesophageal varices with FibroTest in patients with cirrhosis: a preliminary retrospective study. Liver Int. 2006;26:271–8.
Poca M, Puente A, Graupera I, Villanueva C. Prognostic markers in patients with cirrhosis and portal hypertension who have not bled. Dis Markers. 2011;31:147–54.
Mao W, Sun Q, Fan J, Lin S, Ye B. AST to platelet ratio index predicts mortality in hospitalized patients with hepatitis b-related decompensated cirrhosis. Med (Baltimore). 2016;95:e2946.
Qi X, Li H, Chen J, Xia C, Peng Y, Dai J, Hou Y, et al. Serum liver fibrosis markers for predicting the presence of gastroesophageal varices in liver cirrhosis: a retrospective cross-sectional study. Gastroenterol Res Pract. 2015;2015:274534.
Bari K, Garcia-Tsao G. Treatment of portal hypertension. World J Gastroenterol. 2012;18:1166–75.
Thabut D, Imbert-Bismut F, Cazals-Hatem D, Messous D, Muntenau M, Valla DC, Moreau R, et al. Relationship between the Fibrotest and portal hypertension in patients with liver disease. Aliment Pharmacol Ther. 2007;26:359–68.
Chan TT, Wong VW. In search of new biomarkers for nonalcoholic fatty liver disease. Clin Liver Dis. 2016;8:19–23.
Wong VW, Vergniol J, Wong GL, Foucher J, Chan HL, Le Bail B, Choi PC, et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010;51:454–62.
Leung VY, Shen J, Wong VW, Abrigo J, Wong GL, Chim AM, Chu SH, et al. Quantitative elastography of liver fibrosis and spleen stiffness in chronic hepatitis B carriers: comparison of shear-wave elastography and transient elastography with liver biopsy correlation. Radiology. 2013;269:910–8.
Cui J, Heba E, Hernandez C, Haufe W, Hooker J, Andre MP, Valasek MA, et al. Magnetic resonance elastography is superior to acoustic radiation force impulse for the Diagnosis of fibrosis in patients with biopsy-proven nonalcoholic fatty liver disease: a prospective study. Hepatology. 2016;63:453–61.
Imajo K, Kessoku T, Honda Y, Tomeno W, Ogawa Y, Mawatari H, Fujita K, et al. Magnetic resonance imaging more accurately classifies steatosis and fibrosis in patients with nonalcoholic fatty liver disease than transient elastography. Gastroenterology. 2016;150(626–637):e627.
Wong GL, Wong VW, Choi PC, Chan AW, Chim AM, Yiu KK, Chan FK, et al. Increased liver stiffness measurement by transient elastography in severe acute exacerbation of chronic hepatitis B. J Gastroenterol Hepatol. 2009;24:1002–7.
Millonig G, Friedrich S, Adolf S, Fonouni H, Golriz M, Mehrabi A, Stiefel P, et al. Liver stiffness is directly influenced by central venous pressure. J Hepatol. 2010;52:206–10.
Millonig G, Reimann FM, Friedrich S, Fonouni H, Mehrabi A, Buchler MW, Seitz HK, et al. Extrahepatic cholestasis increases liver stiffness (FibroScan) irrespective of fibrosis. Hepatology. 2008;48:1718–23.
Mederacke I, Wursthorn K, Kirschner J, Rifai K, Manns MP, Wedemeyer H, Bahr MJ. Food intake increases liver stiffness in patients with chronic or resolved hepatitis C virus infection. Liver Int. 2009;29:1500–6.
Loustaud-Ratti VR, Cypierre A, Rousseau A, Yagoubi F, Abraham J, Fauchais AL, Carrier P, et al. Non-invasive detection of hepatic amyloidosis: fibroScan, a new tool. Amyloid. 2011;18:19–24.
Wong GL, Chan HL, Choi PC, Chan AW, Lo AO, Chim AM, Wong VW. Association between anthropometric parameters and measurements of liver stiffness by transient elastography. Clin Gastroenterol Hepatol. 2013;11(295–302):e291–3.
Petta S, Maida M, Macaluso FS, Di Marco V, Camma C, Cabibi D, Craxi A. The severity of steatosis influences liver stiffness measurement in patients with nonalcoholic fatty liver disease. Hepatology. 2015;62:1101–10.
Petta S, Wong VW, Camma C, Hiriart JB, Wong GL, Marra F, Vergniol J, et al. Improved noninvasive prediction of liver fibrosis by liver stiffness measurement in patients with nonalcoholic fatty liver disease accounting for controlled attenuation parameter values. Hepatology. 2017;65:1145–55.
Bureau C, Metivier S, Peron JM, Selves J, Robic MA, Gourraud PA, Rouquet O, et al. Transient elastography accurately predicts presence of significant portal hypertension in patients with chronic liver disease. Aliment Pharmacol Ther. 2008;27:1261–8.
Colecchia A, Montrone L, Scaioli E, Bacchi-Reggiani ML, Colli A, Casazza G, Schiumerini R, et al. Measurement of spleen stiffness to evaluate portal hypertension and the presence of esophageal varices in patients with HCV-related cirrhosis. Gastroenterology. 2012;143:646–54.
Zykus R, Jonaitis L, Petrenkiene V, Pranculis A, Kupcinskas L. Liver and spleen transient elastography predicts portal hypertension in patients with chronic liver disease: a prospective cohort study. BMC Gastroenterol. 2015;15:183.
Takuma Y, Nouso K, Morimoto Y, Tomokuni J, Sahara A, Toshikuni N, Takabatake H, et al. Measurement of spleen stiffness by acoustic radiation force impulse imaging identifies cirrhotic patients with esophageal varices. Gastroenterology. 2013;144(92–101):e102.
Kazemi F, Kettaneh A, N’Kontchou G, Pinto E, Ganne-Carrie N, Trinchet JC, Beaugrand M. Liver stiffness measurement selects patients with cirrhosis at risk of bearing large oesophageal varices. J Hepatol. 2006;45:230–5.
Castera L, Le Bail B, Roudot-Thoraval F, Bernard PH, Foucher J, Merrouche W, Couzigou P, et al. Early detection in routine clinical practice of cirrhosis and oesophageal varices in chronic hepatitis C: comparison of transient elastography (FibroScan) with standard laboratory tests and non-invasive scores. J Hepatol. 2009;50:59–68.
Vermehren J, Polta A, Zimmermann O, Herrmann E, Poynard T, Hofmann WP, Bojunga J, et al. Comparison of acoustic radiation force impulse imaging with transient elastography for the detection of complications in patients with cirrhosis. Liver Int. 2012;32:852–8.
Stefanescu H, Radu C, Procopet B, Lupsor-Platon M, Habic A, Tantau M, Grigorescu M. Non-invasive menage a trois for the prediction of high-risk varices: stepwise algorithm using lok score, liver and spleen stiffness. Liver Int. 2015;35:317–25.
Wong GL, Kwok R, Chan HL, Tang SP, Lee E, Lam TC, Lau TW, et al. Measuring spleen stiffness to predict varices in chronic hepatitis B cirrhotic patients with or without receiving non-selective beta-blockers. J Dig Dis. 2016;17:538–46.
Robic MA, Procopet B, Metivier S, Peron JM, Selves J, Vinel JP, Bureau C. Liver stiffness accurately predicts portal hypertension related complications in patients with chronic liver disease: a prospective study. J Hepatol. 2011;55:1017–24.
de Franchis R, Baveno VIF. Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743–52.
Maurice JB, Brodkin E, Arnold F, Navaratnam A, Paine H, Khawar S, Dhar A, et al. Validation of the Baveno VI criteria to identify low risk cirrhotic patients not requiring endoscopic surveillance for varices. J Hepatol. 2016;65:899–905.
Gao J, Ran HT, Ye XP, Zheng YY, Zhang DZ, Wang ZG. The stiffness of the liver and spleen on ARFI Imaging pre and post TIPS placement: a preliminary observation. Clin Imaging. 2012;36:135–41.
Chang TT, Liaw YF, Wu SS, Schiff E, Han KH, Lai CL, Safadi R, et al. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology. 2010;52:886–93.
Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, Washington MK, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468–75.
Mallet V, Gilgenkrantz H, Serpaggi J, Verkarre V, Vallet-Pichard A, Fontaine H, Pol S. Brief communication: the relationship of regression of cirrhosis to outcome in chronic hepatitis C. Ann Intern Med. 2008;149:399–403.
Lampertico P, Invernizzi F, Vigano M, Loglio A, Mangia G, Facchetti F, Primignani M, et al. The long-term benefits of nucleos(t)ide analogs in compensated HBV cirrhotic patients with no or small esophageal varices: a 12-year prospective cohort study. J Hepatol. 2015;63:1118–25.
Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749–61.
Zironi G, Gaiani S, Fenyves D, Rigamonti A, Bolondi L, Barbara L. Value of measurement of mean portal flow velocity by Doppler flowmetry in the diagnosis of portal hypertension. J Hepatol. 1992;16:298–303.
Pollard JJ, Nebesar RA. Catheterization of the splenic artery for portal venography. N Engl J Med. 1964;271:234–7.
Sheppard DG, Moss J, Miller M. Imaging of the portal vein during transjugular intrahepatic portosystemic shunt procedures: a comparison of carbon dioxide and iodinated contrast. Clin Radiol. 1998;53:448–50.
Khanna R, Sarin SK. Non-cirrhotic portal hypertension—diagnosis and management. J Hepatol. 2014;60:421–41.
Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. Lancet. 2014;383:2253–64.
Smalberg JH, Arends LR, Valla DC, Kiladjian JJ, Janssen HL, Leebeek FW. Myeloproliferative neoplasms in Budd-Chiari syndrome and portal vein thrombosis: a meta-analysis. Blood. 2012;120:4921–8.
Sarin SK, Kumar A, Chawla YK, Baijal SS, Dhiman RK, Jafri W, Lesmana LA, et al. Noncirrhotic portal fibrosis/idiopathic portal hypertension: APASL recommendations for diagnosis and treatment. Hepatol Int. 2007;1:398–413.
Pohl A, Behling C, Oliver D, Kilani M, Monson P, Hassanein T. Serum aminotransferase levels and platelet counts as predictors of degree of fibrosis in chronic hepatitis C virus infection. Am J Gastroenterol. 2001;96:3142–6.
Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, Fontaine H, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32–6.
Sirli R, Sporea I, Bota S, Popescu A, Cornianu M. A comparative study of non-invasive methods for fibrosis assessment in chronic HCV infection. Hepat Mon. 2010;10:88–94.
Koda M, Matunaga Y, Kawakami M, Kishimoto Y, Suou T, Murawaki Y. FibroIndex, a practical index for predicting significant fibrosis in patients with chronic hepatitis C. Hepatology. 2007;45:297–306.
Zaman A, Rosen HR, Ingram K, Corless CL, Oh E, Smith K. Assessment of FIBROSpect II to detect hepatic fibrosis in chronic hepatitis C patients. Am J Med. 2007;120:e280–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
None.
Conflict of interest
Vincent Wong has served as an advisory board member for Perspectum Diagnostics and a speaker for Echosens. The other authors report no conflict of interest.
Ethical approval
Not applicable because this is a review article.
Informed consent
Not applicable because this is a review article.
Rights and permissions
About this article
Cite this article
Leung, J.CF., Loong, T.CW., Pang, J. et al. Invasive and non-invasive assessment of portal hypertension. Hepatol Int 12 (Suppl 1), 44–55 (2018). https://doi.org/10.1007/s12072-017-9795-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-017-9795-0