Abstract
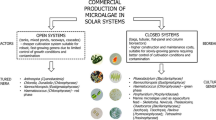
Two basic approaches to microalgae biomass production are used: one applies to cultivation in closed or semi-closed vessels – photobioreactors, while the other involves open reservoirs with direct contact of the microalgal culture with the environment. The most crucial variable for phototrophic growth is light availability. The amount of photon energy received by each cell is a combination of several factors: irradiance intensity, cell density, length of optical path (thickness of culture layer), rate of mixing as well as cultivation unit design. In practice, this should form a part of the considerations when designing cultivation systems.
The highest growth rate and productivity have been achieved in cultivation systems with microalgae layer thickness lower than 50 mm. The advantage of these thin-layer systems is that high biomass density is reached, which is advantageous for harvesting and processing. Basically, two thin-layer cultivation systems are being used that guarantee high areal or volumetric productivity due to high surface-to-volume ratio: vertical or inclined flat panels, and near-horizontal sloping cascades or raceways. The first type, flat-panel photobioreactors represent closed or semi-closed systems. In the other system, thin-layer sloping cascades – microalgae culture is grown on open, inclined-surface platforms that – by some means – combine the advantages of open systems (direct sun irradiance, easy heat dissipation, simple cleaning and maintenance, lower construction and biomass costs and efficient degassing) with positive features of closed systems (operation at high biomass densities achieving high volumetric productivity). Among the limitations of these systems, there can be a possibility of contamination by other microalgae strains which allows growing preferentially fast-growing strains or those cultivated in selective environments.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Biomass
- Cascades
- Chlorella
- Cyanobacteria
- Flat-panel
- Growth rate
- Mass culture
- Microalgae
- Nutrition
- Open pond
- Photobioreactor
- Productivity
- Thin-layer
- Light-dark cycle
1 Introduction
In microalgal biotechnology, suitable species can be grown as productive strains in extensive aquacultures (algacultures) facilitating the efficient manipulation of cultivation processes. Although many microalgal strains are cultivated worldwide for different purposes, the bulk of annual biomass production is represented by only a few species. Algacultures are an ideal platform for the large-scale production of biomass because they are fast-growing, solar-powered ‘biofactories’ with low nutrient requirements. Their substantial benefits over plants are based on their short life cycles and metabolic plasticity that offers the possibility of modifying their biochemical pathways and cellular composition by varying culture conditions.
Over the last 60 years, microalgal biotechnology has shown a range of applications: from the traditional extensive biomass production in human and animal nutrition, health food products, soil conditioning in agriculture, aquaculture colorants, technologies for waste-water treatment, products for cosmetics, pharmaceuticals, and most recently the possible production of a ‘third’ generation biofuels. Dense, well-mixed mass culture of microalgae Footnote 1 (>0.5 g biomass per litre) with sufficient nutrition and gas exchange represents an artificial system that is a suitable model for a biorefinery. Footnote 2
1.1 Cultivation Systems
Various cultivation systems and technologies have been developed to grow microalgal mass cultures. The choice of a suitable cultivation system and the adjustment of the cultivation regime must be worked out for each individual microalgal strain and production purpose. The key problem to solve in a cultivation unit design is how to use the photon flux at a maximum rate, i.e. how to allow each single microalgae cell to get access to an optimum number of photons every time. Two basic approaches to mass production are used: one applies to cultivation in open reservoirs (with direct contact of the microalgal culture with the environment), while the other involves closed or semi-closed vessels – photobioreactors (PBRs) with no direct contact between the culture and the atmosphere (for a recent review, see Zittelli et al. 2013). Large-scale outdoor PBRs for commercial production are usually designed as modules. There are major operational differences between open reservoirs and PBRs and, consequently, the growth physiology of the microalgae is different between the two systems (Grobbelaar 2009, 2012).
The systems are used for specific purposes and this will determine which cultivation system is the most suitable since there is no universal all-purpose unit. Crucial variables are the irradiance intensity, temperature, optical depth, turbulence, light acclimated state of the organism, nutrient availability and gas exchange (supply of CO2 and O2 degassing). From a commercial point of view, the price of the final product is often an important consideration. At present open reservoirs are the only feasible culture systems for the production of thousands of tons of biomass as production is cheaper than a culture from a closed PBR. Unfortunately, the use of open ponds is restricted to a relatively small number of microalgal species due to the limited control of cultivation conditions and contamination. Hence, open systems are suitable for “robust” microalgal strains (e.g., Chlorella or Scenedesmus) that grow rapidly, or under very selective conditions (e.g., Spirulina or Dunaliella).
Compared to open systems, photobioreactors have certain advantages: reproducible cultivation conditions with regard to environmental influences; reduced risk of contamination; low CO2 losses; lower cost of biomass down-stream processing; and smaller area requirements. On the downside, closed systems are: more difficult to clean; the construction material might partially decrease sunlight penetration; and the system must be cooled and degassed effectively since excessive oxygen produced by the growing cultures can reduce growth. Furthermore, the cost of construction is about one order of magnitude higher than that of open ponds.
The total ground area (i.e. including the ground area between panels) for the vertical flat plate PBR is significantly lower than that occupied by an open reservoir (e.g. raceway or cascade). Finally, the harvested cell density is close to one order of magnitude higher in the flat plate PBR than that in open ponds or raceways, which carries economic significance.
From a practical point of view, flat-panel PBRs have one serious disadvantage: biofouling at higher biomass density, especially of the channels, due to reduced turbulence in their narrow, rectangularly shaped channels.
1.1.1 Open Outdoor Systems
Open cultivation systems are usually artificial ponds, tanks, raceways (shallow race-tracks mixed by paddle wheels) and sloping cascades (i.e. inclined-surface platforms). An overview of open culture systems used for the mass cultivation of microalgae outdoors has been presented recently (Tredici 2004; Zittelli et al. 2013). Productivity in these open systems is usually low (∼1 g DM m−2 day −1) due to the lack of mixing and CO2 supply. To improve productivity, open systems are mixed by impellers, rotating arms, paddle wheels, or by a stream of CO2-enriched air supplied into the culture. The culture depth may vary between 10 and 30 cm. The cultures are usually grown at a biomass concentration ranging between 0.5 and 1 g L−1 depending on the culture depth. Outdoor cultures are considered a photo-limited system as they are operated at an optimum concentration rather than at a maximum growth rate.
1.1.2 Closed and Semi-closed Photobioreactors
Compared to open systems closed PBRs are more flexible and can be better optimized according to the biological and physiological characteristics of a selected microalgal strain. A variety of PBRs (using either natural or artificial illumination) has been designed consisting of glass or transparent plastic tubes, columns or panels, positioned horizontally or vertically, arranged as serpentine loops, fences, flexible coils, a series of panels or column ‘gardens’; these act as a photostage in which the microalgal suspension is continuously mixed. Necessary cooling is maintained by submerging the tubes in a pool of water, by heat exchangers, or by spraying water onto the PBR surface. In PBRs, a much greater biomass density can be maintained than in open systems. At present, panel or tubular PBRs are often mounted in greenhouses to maintain culture conditions for all-year functioning. Slowly-growing strains sensitive to contamination are grown in PBRs, e.g. Nannochloropsis, Haematococcus, Tetraselmis, Phaeodactylum, Skeletonema, Pavlova, Thalassiosira, Nostoc, Navicula, Isochrysis, Chaetoceros, etc.
Despite the higher biomass yields attainable with PBRs (as compared to open systems), their high construction and maintenance costs still make them uncompetitive for the industrial production of microalgal biomass. Their use can be foreseen for the production of high-value bioactive substances, which require the adoption of sterile conditions.
2 Culture Monitoring and Maintenance
Microalgal mass cultures grown in a cultivation unit should also have its physiological status checked operatively in order to optimize photosynthetic activity and growth. Successful cultivation requires a continuous monitoring of a culture’s physicochemical parameters, namely its pH, temperature, dissolved oxygen concentration, and nutrient status. One method of observation is to use basic biological examination under the microscope: in order to detect morphological changes of cells and contamination by other microorganisms (microalgae, bacteria, fungi and protozoa). The nutrient status can be followed by monitoring the concentration of nitrogen, and then using this as a measure for adding proportional amounts of other nutrients. Sufficient carbon (CO2 or bicarbonate) supply is a crucial point as the ambient CO2 concentration is very low (about 0.04 %; v/v) and is the limiting factor in extensive microalgal mass cultures exposed to high irradiances.
Photobiochemical monitoring methods reflect the general status of the cells’ photosynthetic apparatus and are thus often used to adjust the appropriate cultivation conditions. Oxygen production and chlorophyll (Chl) fluorescence have been used as reliable and sensitive techniques to monitor the photosynthetic activity of various photosynthetic organisms including microalgae (Bradbury and Baker 1984; Krause and Weis 1984, 1991; Walker 2009; Flameling and Kromkamp 1998; Gilbert et al. 2000; Figueroa et al. 2003; Wilhelm et al. 2004; Figueroa et al. 2013). Although providing analogous information, Chl fluorescence techniques are, as compared with measurements of O2 production, considerably faster, more sensitive and moreover, can give information on absorbed energy distribution between photochemical and dissipative (protective) processes and the balance between photosynthetic electron transport and the Calvin-Benson cycle (Schreiber et al. 1986, 1995; Baker and Oxborough 2004; Suggett et al. 2011).
Since the 1990s Chl fluorescence measurement has become one of the most common and useful approaches used for monitoring the physiological status of microalgal mass cultures due to its sensitivity, ease of use, as well as its prompt provision of results (Ting and Owens 1992; Büchel and Wilhelm 1993; Vonshak et al. 1994, 1996; Torzillo et al. 1996, 1998; Baker 2008; Enriquez and Borowitzka 2011; Masojídek et al. 2011b). One, direct approach is to measure photosynthesis on-line/in-situ during the diel cycle to monitor the actual situation in a culture. The other possibility is to measure Chl fluorescence off-line using dark-adapted microalgal samples taken from a cultivation unit at selected times (Masojídek et al. 2011a).
Chl fluorescence measurements in our experiments showed that changes of some fluorescence variables can be well correlated with changes of cultivation conditions, physiological status and growth of a given microalgal culture and/or the suitability of a selected cultivation system (Torzillo et al. 1996, 1998; Masojídek et al. 2000, 2003, 2009, 2011a; Malapascua et al. 2014). Using pulse-amplitude-modulation (PAM) technique to carry out saturation pulse analysis of fluorescence quenching some of the fluorescence variables can be calculated (for recent reviews see Maxwell and Johnson 2000; Schreiber 2004; Baker 2008; Masojídek et al. 2011b; Malapascua et al. 2014). For example the maximum photochemical yield of PSII (FV/FM), actual photochemical yield of PSII, ΔF′/FM′ (= [FM′ − F′]/FM′) and the relative electron transport rate rETR through PSII (the product of multiplication ΔF′/FM′ by the photosynthetically active radiation EPAR in the culture) reflect photosynthetic activity and can be correlated with analogous changes in the daily productivities of cultures grown under different conditions (Torzillo et al. 1996, 1998; Masojídek et al. 2000, 2011a). Namely, rETR proved to be a simple and reliable parameter to estimate growth and productivity in both indoor and outdoor mass cultures of microalgae (Malapascua et al. 2014). The so-called Stern-Volmer non-photochemical quenching NPQ (= [FM − FM′]/FM′) is, in principle, inversely related to photochemistry (ΔF′/FM′). It indicates an increased futile heat dissipation of absorbed energy and is considered a safety valve protecting PSII reaction centres from damage by excess irradiance (Bilger and Björkman 1990). It has been experimentally proven that a midday-depression of actual PSII photochemical yield (Fv/Fm) of between 20 and 30 % compared to morning values at high-cell density is compatible with well-performing cultures. A lower or higher depression of Fv/Fm indicated low-light acclimated or photoinhibited cultures, respectively (Masojídek et al. 2003, 2011a).
Although the theory is well described at present (Maxwell and Johnson 2000; Schreiber 2004, Strasser et al. 2004, Baker 2008), the interpretation of Chl fluorescence signals may not be straightforward, particularly when dealing with microalgae (Schreiber et al. 1995; Strasser et al. 1995, Campbell et al. 1998). Care must be taken when measuring fluorescence and evaluating data in cyanobacteria. This is because the fluorescence emission of phycobilisomes, as well as state transition effects and PSI fluorescence, can contribute significantly to the total signal, and this affects the correct determination of certain variables (Ting and Owens 1992; Büchel and Wilhelm 1993; Schreiber et al. 1995).
Culture growth might be estimated as changes in the optical density (OD) at 750 nm, the dry mass (biomass), or the number of cells. Pigment content is determined in solvent extracts using spectroscopy or liquid chromatography. Biomass productivity can be expressed as the areal or volumetric yield per unit time, for example in [g m−2 day−1] or in [g l−1 day−1].
Basically, two cultivation regimes are used for the growth of microalgal cultures. In the batch regime, the culture is inoculated and at a certain point of growth it is harvested. In the continuous regime, the culture is harvested continuously according to its growth rate and fresh medium is added to replace nutrients. In biotechnological practice, semi-continuous or semi-batch regimes are usually adopted, that is, where a part of the culture is harvested at regular intervals.
3 Biological Principles: Light-Regime, Biomass Density, Optical Path and the Importance of Time-Scales
The energy needed for the photosynthetic conversion of carbon dioxide into organic substances is delivered by photons, which come from the sun under natural conditions. Except light, the growth of microalgae biomass is further influenced by physico-chemical conditions for growth: a suitable temperature and pH, and a sufficient supply of carbon and nutrients in the growth medium. Since microalgal mass cultures grow in dense suspensions (as compared with natural populations of phytoplankton), turbulent mixing is critical to expose cells to light and to allow for an efficient mass transfer. In outdoor cultures, solar photosynthetically-active radiation (PAR) represents a major growth-limiting factor in well-maintained mass microalgal cultures (where temperature and nutrients are not limiting). The kinetic reply of microalgal cells to irradiance intensity is shown in a model of photosynthetic light-response curve (Fig. 1), provided that the irradiance intensity is the sole growth-limiting factor. At low, light-limited levels the photosynthetic rate is approximately linearly proportional to irradiance. At light saturation, the maximum rate of photosynthesis Pmax is reached. At irradiance values beyond the plateau region in Fig. 1, the rate of photon absorption exceeds the rate of electron turnover in the photosynthetic apparatus and it eventually leads to a decrease of photosynthesis, commonly referred to as photoinhibition. The ultimate rate limiting processes are the photosynthetic dark reactions (Gordon and Polle 2007; Richmond 2013; Masojídek et al. 2013).
A model of the light-response curve of photosynthesis. Three regions are present in the curve: light-limited, light-saturated and light inhibited. The intercept in the x-axis EC designate the compensation irradiance intensity between dark respiration and photosynthesis; and ES is an approximate irradiance level between photosynthesis limitation and saturation. At low, light-limited, levels the photosynthetic rate is approximately linearly proportional to irradiance. At light saturation, the maximum rate of photosynthesis Pmax is reached. By reaching ES the rate of photon absorption exceeds the rate of electron turnover in the PSII complex and excess energy is dissipated. Further irradiance increase eventually leads to a light-induced drop of photosynthesis, commonly referred as photoinhibition
From a practical point of view, flux requirement in commercial microalgal photobioreactors is typically ∼200–400 μmol photons m−2 s−1 corresponding to the irradiance saturating photosynthesis (ES in Fig. 1) that is only about 10–20 % of the maximum photosynthetic photon flux density (PPFD) (Gordon and Polle 2007; Richmond 2013; Masojídek et al. 2013). The requirements for efficient utilization of high light fluxes in microalgal cultures have been elucidated: the most important of these are a short light-path combined with a highly turbulent flow at high cell densities (i.e. >5 g DM L−1) (Hu and Richmond 1996; Hu et al. 1998; Richmond 2003; Grobbelaar 2012).
Since early reports in the 1930s, it has been clear that intermittent (pulsed) light is an important issue for microalgae growth (Emerson and Arnold 1932). Microalgal cells may utilize strong light only if it is delivered intermittently, in ‘pulses’. The so called ‘flashing light’ effect on photosynthesis in Chlorella was studied by Kok (1953). Later, the effect of L/D cycles was investigated by several research groups (e.g. Laws et al. 1983; Tennessen et al. 1995; Gordon and Polle 2007; Grobbelaar 2009; Zarmi et al. 2013). In the 1990s, the introduction of high-intensity LEDs to scientific use made it possible to measure the effect of intermittent illumination more preciously in the microsecond range (Matthijs et al. 1996; Nedbal et al. 1996).
In mass microalgal culture it is possible to achieve high photosynthetic yields in full sunlight when the turbulence and density of cells are adjusted to produce the proper pattern of light intermittence, i.e. the L/D cycles are sufficiently short in the order of tens to hundreds of microseconds (10–100 Hz), close to the time scale of the rate-limiting dark reactions of photosynthesis. The influence of L/D cycles of several seconds to tens of seconds does not appear to result in an improvement of the photosynthetic efficiency (Janssen et al. 2000, 2001, 2003). It was concluded that the averaged amount of photon energy received by each cell is a combination of several variables: irradiance intensity, cell population density, length of optical path (thickness of culture layer), spectral quality, light absorption, and the rate of mixing (Richmond 2003, 2004, 2013). As shown in Fig. 1, the maximum photochemical efficiency is achieved in the light-limited region, but maximum rates of photosynthesis are reached in microalgal cultures in which averaged cell irradiances are close to saturation ES (and energy losses are still low).
4 Thin-Layer Systems – Layer Thickness, High Surface-to-Volume Ratio and Biomass Productivity
The averaged irradiance intensity of a microalgal cell is modulated not only by ambient irradiance, but also by culture density, mixing, culture depth (light path), light-dark cycle frequency as well as cultivation unit design and spatial setting with respect to exposure to the sun. In practical terms, this should form part of the considerations when designing cultivation systems.
As discussed above, principally two thin-layer cultivation systems are being used that guarantee high areal or volumetric productivity due to their high exposed surface-to-volume ratio (S/V ratio): vertical or inclined flat panels, and sloping raceways or cascades. The higher the surface for light incidence and the smaller the volume for the microalgae culture (S/V ratio), the better the light supply. A crucial point is the sufficient mixing of microalgal culture to induce fast L/D cycling of cells in ‘short’ light-path (<50 mm) cultivation systems. For a given system the culture exhibits the highest photosynthetic efficiency at optimal cell density. The other aspect concerns the overall photic volume that should comprise ~5–10 % of optical path (Tredici 2010; Richmond 2013) as the depth the light penetrates into the culture is a function of cell density. The operation regime – suitable biomass density, culture layer (optical path), cell movement patterns (averaged light/dark cycles for cells) and mass exchange – has to be developed to maximise/photo-optimise the use of high photon flux densities reaching the surface of cultivation systems. In general, the shorter the length of the light-path, the smaller the areal volume and the higher the volumetric productivity (Richmond and Cheng-Wu 2001).
L/D cycle considerations have indicated that the cell travel time begins to represent a relevant parameter for enhancement in photosynthetic productivity when the optical path is reduced to about 10 mm. In such a system, e.g. flat-panel PBR, the time range of L/D cycles is hundreds of milliseconds, assuming a photic volume of 5 % and a fluid velocity of 30 cm s−1 (Richmond 2003). In another experiment, light penetration was measured in a sloping cascade using a culture with a biomass density of about 11 g L−1. As the culture depth was about 6 mm, a hydrodynamic model demonstrated highly turbulent flow allowing rapid L/D cycles (with a frequency of 0.47 s−1) in a culture layer (Masojídek et al. 2011a).
Higher cell density cultures require a higher Reynolds number (an indication of the extent of turbulence) in order to induce short L/D cycles. Efficient use of strong light requires high frequency of L/D cycles which in turn facilitates higher biomass productivity. Applied intermittently to the individual cells in the turbulent culture, high irradiance is diluted by being available in smaller doses to more cells within a given time span. Thus, the light is used more effectively, compared with light use of cells illuminated continuously at low-density, or in poorly stirred cultures. Therefore the increased L/D cycle frequency can be considered a form of light dilution (Richmond 2013).
4.1 Closed Systems – Flat-Panel Photobioreactors
The first type of thin-layer systems used for microalgae cultivation, flat-panel PBRs represent closed or semi-closed systems. They usually consist of vertical or inclined transparent rectangular vessels with a relatively short light-path of 1–5 cm, made of firm material, i.e. glass, Plexiglas or polycarbonate in which baffles can be mounted to create a labyrinth of channels (Fig. 2). The other possibility is to use flat-panels, 3–5 cm thick, made of flexible, polyethylene bags enclosed in a rigid framework. The flat-panel systems are placed either outdoors or in a greenhouse exploiting sun light.
Thin-layer photobioreactors for cultivation of microalgae. (a) Outdoor inclined flat-panel photobioreactors were arranged in series (Sde Boqer campus, J. Blaustein Institutes for Desert Research, Ben Gurion University, Israel,; courtesy of Prof. Amos Richmond); (b) Vertical-panel photobioreactors arranged in parallel and mounted in a greenhouse (Institut für Getreideverarbeitung, Potsdam-Rehbrücke, Germany)
The height and width of individual panels can be varied. In practice, panels can be connected to modules of several metres in length with a total volume of hundreds to thousands litres. The panels are in series or parallel, arranged vertically some distance apart to avoid self-shading. Flat-panel PBRs are mixed with air (+ CO2) introduced via a perforated tube at the bottom of panels to create a high degree of turbulence (air-bubble or air-lift), or the culture is circulated by a pump (for recent review see Zittelli et al. 2013; Pulz et al. 2013).
Flat-panel PBRs with high biomass density have been one of the devices to establish the potential for massive improvements in bioproductivity as biomass yield and optimum biomass density (OBD) in closed systems is generally much higher than in open systems. The other advantage is the possibility of growing some strains sensitive to contamination by other fast-growing microalgae (Nannochloropsis, Haematococcus, Tetraselmis, Isochrysis, Phaeodactylum, Skeletonema, Pavlova, Thalassiosira, Nostoc, Chaetoceros, etc.). Experiments in inclined flat-panel PBRs (30°–60° tilt angle to sun) connected in series (Fig. 2a) for mass cultivation of fast-growing cyanobacterium Spirulina (Arthrospira) platensis showed the interrelationship between light path and OBD: the stepwise decreasing thickness of flat-panel from 104, 52, 26 to 13 mm corresponded to an increase of OBD from 1.7, 3.1, 8.4 to 15.8 g L−1 resulting in the biomass productivity of 33.6, 38.9, 49.4 to 51.1 g m−2 day−1, respectively (Hu et al. 1996a, b). Naturally, biomass productivity depends on strain physiology and conditions. As compared to Arthrospira, productivities were much lower for slowly-growing marine microalga Nannochloropsis sp. which is the eminent producer of PUFA. In the same flat-panel PBRs with the culture layer thickness stepwise decreasing from 104, 52, 26 to 13 mm, the corresponding biomass productivities were 5.5, 7.3, 9.3 and 12.1 g m−2 day−1, respectively (Zou and Richmond 1999) which were almost an order of magnitude lower than those in Arthrospira.
In laboratory experiments, Arthrospira was grown in flat panels of only 7.5 mm (!) thick illuminated continuously by 900 μmol photons m−2 s−1 (provided by 1500 W halogen lamps) which were placed either on one or on both sides). In this case, it was possible to work with ultra-high biomass densities of about 27 g L−1 reaching biomass productivity of about 100 g m−2 day−1 (Hu et al. 1998). From a technical point of view the scaling up of this system is hardly feasible as the use of very thin flat-plate panels would be rather difficult for maintenance.
Most of industrial flat-panel PBRs have light-path between 20 and 40 mm which determines a high surface-to-volume ratio. Several commercial large-scale systems for microalgae production have been developed working on the principle of flat-plate panels. In one example, a large-scale flat plate photobioreactor was constructed employing vertically oriented plastic plates in a greenhouse, in which microalgae were flowed horizontally in narrow rectangular channels created by baffles (Fig. 2b). This system was a pioneering project of microalgae-based industrial CO2 fixation from flue gas produced by a lime kiln in Elbigerode (Germany). The productivity of this 6,000-L system was rather high, in the range of 30–50 g DM biomass m−2 day−1 using microalgae strains Chlorella and Scenedesmus (Pulz et al. 2013).
The concept of ‘disposable panels’ for large-scale applications was developed in the early 2000s by two groups working in Italy (University of Florence) and Israel (Ben Gurion University). The vertical photobioreactor called ‘Green Wall Panel (GWP I)’ consists of 100-litre bags (~4.5 × 100 × 250 cm; 800 L) made of a polyethylene foil enclosed in a rigid framework (Fig. 3) (Rodolfi et al. 2009; Zittelli et al. 2013). The GWP I modules (4.5 cm × 1 m × 20 m) can be connected, placing them in a single row or in parallel, 1-m apart to avoid mutual shading. For the outdoor experiments, air-flow is maintained for culture mixing and CO2 is supplied during daylight hours keeping pH in the range 7.5–8.0. The cooling is generally provided by water (even seawater) spraying, or by insertion of cooling loops inside the panels to prevent the culture overheating. This low-cost, easy-to-operate and low-contamination system with good scalability has been used to produce various microalgae (Tetraselmis, Nannochloropsis, Isochrysis, Cylindrotheca) although biomass densities are in the scale of grams. The GWP PBRs (developed at the University of Florence and commercialised by Fotosintetica & Microbiologica Srl) have been successfully used in several large-scale demonstration projects worldwide to grow microalgae for various purposes. The GPW design has been continuously modified in order to improve functioning and to reduce costs. The most recent model of these PBRs, GWP III PBRs have east-west orientation, shorter light-path and adjustable tilt from vertical to inclined position to use sun light effectively and decrease energy input by integration with photovoltaics.
Flat-plate photobioreactors for cultivation of microalgae. (a) Outdoor vertical flat-panel photobioreactor ‘Green Wall Panel’ arranged in a single row or in parallel (developed at the University of Florence and commercialised by Fotosintetica & Microbiologica Srl., Italy). (b) Vertical alveolar flat-panel photobioreactors produced by Subitec GmbH were arranged in series and mounted in a greenhouse at Vattenfall power plant in Senftenberg (Germany)
The technology provided by Subitec GmbH (Germany) enables the cultivation of microalgae at an industrial scale with an enclosed system based on flat-panel airlift photobioreactors to produce microalgae biomass as a source of feed, bulk chemicals and energy – clean biofuels from microalgae (Fig. 3).
A large-scale flat-panel photobioreactor ‘Hanging Gardens’ was developed and demonstrated by Ecoduna GmbH (Bruck a/L, Austria). One module unit (4.3 m3) consist of 12 parallel flat panels (3 cm × 2 m × 6 m) which are placed 15 cm apart in a movable frame that allows tracking the sun movements (Fig. 4). The panels are internally partitioned by baffles to allow culture circulation as air and CO2 are injected from the bottom to generate a gas-lift effect.
Large-scale flat-panel photobioreactor ‘Hanging Gardens’ (developed and demonstrated by Ecoduna GmbH in Bruck a/L, Austria). One module unit consisting (see insert) of 12 closely spaced parallel panels (3 cm × 2 m × 6 m) which are placed 15 cm apart in a movable frame that allows tracking of the sun movements. The panels are internally partitioned by vertical baffles to allow culture circulation using a gas-lift effect. The insert shows details of the arrangements of the panels in the module
4.2 Open System – Sloping Cascades
The other type of thin-layer systems used for microalgae cultivation represent sloping cascades which are known worldwide as the Třeboň’s or Šetlík’s type (Šetlík et al. 1967; 1970). In these cultivation units microalgae flow in thin-layer over open, inclined-surface platforms which – by some means – combine the advantages of open systems (direct sun irradiance, easy heat derivation, simple cleaning and maintenance, lower construction and biomass costs, efficient degassing) with positive features of closed systems (operation at high biomass densities achieving high volumetric productivity). The unique cultivation plant of 900 m2 was constructed in 1962–1963 which was one of the first large-scale research facilities for mass microalgae production (Fig. 5). Later, in the 1970s as a part of collaborative projects some thin-layer cascades (TLC) of the Třeboň’s type were also constructed in Bulgaria, Italy, Poland and Cuba, in order to compare microalgae cultivation under various climatic conditions (Bartoš 1967; Zahradník 1967; Vendlová 1969). A large-scale facility of 900 m2 was operated in Rupite, Bulgaria until the 2000s. In mass cultivations, green microalgae Scenedesmus and Chlorella were mostly used. Recently, outdoor TLC were used in pilot trials to study the growth of the cyanobacterium Arthrospira platensis (Torzillo et al., unpublished results) and the freshwater microalga Trachydiscus (Eustigmatophyceae) (Malapascua et al. 2014).
Outdoor large-scale cascades for cultivation of microalgae (50 and 900 m2) built in the 1960s. One of the first large-scale research facilities for mass microalgae production was located on the campus of the Opatovický mlýn, Institute of Microbiology, Třeboň (mid 1960s). The units had a plain glass surface, with a slope of 3 %, framed by a steel structure. The transverse baffles 3.5 cm high and 15 cm apart were fitted on the surface to create intensive turbulence in the microalgae layer of about 50 mm
Since the 1960s, the concept of thin-layer has been developed at the Laboratory of Algal Biotechnology of the Institute of Microbiology at Třeboň (for review see Masojídek and Prášil 2010). Originally, the design of microalgae cultivation in a relatively thin layer (<50 mm) has been based on turbulent flow using corrugated surfaces, or a plane fitted with transversal baffles (Fig. 5). As compared to open reservoirs (ponds, raceways) with the depth of suspension in the range of 100–300 mm where diluted cultures of microalgae (0.5–1 g DM L−1) are grown under limited light, poor mixing and gas exchange, the main advantage of TLCs was to grow well-mixed, thick microalgae culture with a much higher biomass density (>10 g DM L−1). Thus, a much lower volume of dense microalgae suspension can be handled during biomass processing.
In the 1990s, when the scientific atmosphere in the Czech Republic became more favourable, microalgae were put back on stage, and another generation of large-scale (650 m2) outdoor TLCs for microalgae cultivation was built and tested at the Institute of Microbiology in Třeboň (Fig. 6) (Doucha et al. 1993; Lívanský et al. 1995; Doucha and Lívanský 1995; Grobbelaar et al. 1995). Pilot units of 25–50 m2 of the same principle are also used in several institutions in the country (Institute of Botany at Třeboň, an agricultural farm at Dublovice, etc.). As compared to the TLCs used in the 1960–1970s, the second generation of TLCs employs a much thinner layer of microalgae – less than 10 mm. Instead of densely spaces baffles, plastic rods with a diameter of 13 mm diameter were placed 1.5 m apart and thus the flow velocity could be increased to 0.4–0.5 m s−1. It was just a small step to realize that the inclined-surface system could work best if operated as a smooth inclined surface (glass plates framed by an angle steel structure) without any baffles where the layer of microalgae is only 6–8 mm. First and foremost, the cleaning and maintenance of smooth surface units has been much simpler, as compared with the baffled system. Another advantage of TLCs was easy heating-up by solar irradiance, but on the other hand microalgae suspension was also spontaneously cooled by water evaporation avoiding overheating.
The cell layer thickness below 10 mm in combination with high flow speed (0.4–0.5 m s−1) generates the turbulent flow (Reynolds number of about 4,500) which prevents cell self-shading. Due to the short optical path, light utilisation is more efficient and high optimum biomass densities (15–35 g DM L−1) can be operated in semi-continuous regime, enabling cheaper harvesting (Masojídek et al. 2011a, Masojídek and Torzillo 2014, Doucha and Lívanský 2014). These units are characterized by their high ratio of exposed surface to total culture volume (S/V of ~100 m−1), which enables high volumetric and areal productivity as compared with that of open ponds (S/V ~10 m−1). The short light path in combination with the high cell density and intensive turbulence enables cells to be exposed to intermittent light with short light/dark cycles (10–100 Hz), thus avoiding over-reduction of photosynthetic electron carriers (Hu and Richmond 1996; Richmond 2004; Masojídek et al. 2004). This set-up has allowed achievement of high growth rates, up to biomass concentration of 40–50 g DM L−1. A 100 m2 pilot system was also tested in the Mediterranean climate where summer productivities were as high as 32 g DM m−2 day−1 as compared to Central Europe with productivity maxima of about 23 g DM m−2 day−1 (Doucha and Lívanský 2006). Improved construction of the retention tank caused a significant reduction of the dark phase to about 20 % of total volume; such high productivities as 50 g DM m−2 day−1 could be achieved in cascade cultivation units in summer days, even in temperate climate zones (Masojídek et al. 2011a). These TLCs have been used for research and biomass production until now.
Recently, thin-layer cascades have been used for the pilot cultivation of various microalgae strains in several countries: Italy (Torzillo et al. 2010), Spain (Jerez et al. 2014; Ihnken et al. 2014), Switzerland (University of Applied Sciences, Zürich) and Greece (Doucha and Lívanský 2006). A large-scale plant consisting of 2 cascade raceway modules of 1,500 m2 each (total volume of 180,000 L) was installed in Pataias, Portugal for the BIOFAT project (designed and built by the company A4F EU). The unit consists of two sloped platforms (declining of 0.5 %), 10 m wide and 75 m long which form a cascade-like system running in opposite directions. This facility is a hybrid technology between raceway pond and sloping cascade since the layer thickness is 40 mm, resulting in the S/V ratio of about 15 m−1. In this case, the operating biomass density is about 4 g DM L−1.
4.2.1 Latest Innovations of TLC Set-Up
TLCs are constructed in a way that the microalgae culture flows from the top to the bottom over sloping platforms and ends in a retention tank, from where it is pumped back to the top. The units are made up of five parts: cultivation surface – photostage, retention tank, pump, CO2 supply and aeration, and measurement and control sensors. The module consists of two sloped platforms (divided into lanes separated by bent edges) where the lower end of the upper platform is connected by a trough to the beginning of the lower platform, which is declined in the opposite direction (Fig. 7). The operation cycle starts in a retention tank (degasser) from where the microalgal suspension is circulated by a pump via a return pipe (riser) to the upper part of the cultivation area. Then, the suspension flows back into a retention tank which helps in degassing of excess oxygen produced by the microalgae. Pure CO2 is supplied directly into the microalgal suspension in the riser. Fast flow in a thin layer suspension shows great importance for growth of microalgae since the aquaculture is well mixed with sufficient light and nutrient availability and produced oxygen is released into the surrounding atmosphere. The culture is circulated over the surface only during the day; it is kept in a retention tank at night to reduce heat loss, or during rainfall to avoid dilution by rainwater. After collection in the retention tank, the culture is mixed by aeration to preserve biological activity. Special software has been designed to enable automatic control and data acquisition of the culture parameters in the experimental unit. The culture’s behaviour is monitored by temperature, pH, dissolved oxygen and Chl fluorescence sensors.
(Panel a) Outdoor thin-layer cascade, model Dahlia for cultivation of microalgae built in 2013. It has an area of 90 m2 and can contain a total volume of 500–1,500 l. The surface-to-total-volume ratio can be operated in the range between 60 and 180 m−1 corresponding to the layer thickness of suspension between 5 and 15 mm. (Panel b) Schematic diagram of the 90 m2 cascade. The module consists of two identical platforms (1) divided into lanes separated by bent edges made of stainless steel. They are supported by scaffolding and exposed to sunlight in a north-south orientation. The slope of each platform is adjustable (between 0.5 and 3°; see two-way arrows) which in combination with a variable-flow maintained by an open-impeller pump make it possible toFig. 7 (continued) set-up suspension layer thickness between 5 and 15 mm. The lower end of the upper platform is connected by a trough (8) to the beginning of the lower platform, which is declined in the opposite direction. The operation cycle starts in a retention tank (2; degasser) from where the microalgal suspension is circulated by a pump (3) via a riser (6; a return pipe) to the upper part of the cultivation area where it is distributed by a perforated tube (7; flow direction is indicated by an arrow). The lower end of the upper platform is connected by a trough (8) to the beginning of the lower platform, which is inclined in the opposite direction. Then, the suspension flows back into a retention tank via a screen (10) which helps in degassing of excess oxygen produced by the microalgae. Pure CO2 is supplied directly into the microalgal suspension in the riser (4). A three-way valve (11) is used for harvesting. Measurement and control sensors (pH, dissolved oxygen, temperature and liquid-level) are mounted in the degasser and in the connecting trough (5). The circulation cycle takes about 60–80 s which can be varied by the pump velocity. The suspension can be harvested via a three-way valve (11). The whole system is controlled by computer software which enables regulation of the cultivation process and data acquisition
As compared with the TLC units used in the 1990–2000, the latest generation of TLCs, model Dahlia designed and constructed in 2012–2013 as a modular system in the Institute of Microbiology in Třeboň. It has several innovations (see below) to improve cultivation process and ease of maintenance (Fig. 7). The module has an area of 90 m2 and is made up of two identical platforms where microalgal culture is exposed to sunlight in a north-south orientation. Compared to previous units made of fragile glass plates glued to metal frames, the cultivation surface is made of stainless steel which is easily cleaned and maintained, avoiding any problems with winter damage and corrosion. The slope of each platform is (independently) vertically adjustable between 0.5° and 3° which in combination with a variable-flow (20–60 cm s−1) using an open-impeller pump make it possible to set-up suspension layer thickness between 5 and 15 mm. Vertically-adjustable platforms and pumping speed make it possible to change layer thickness in order to study the optimal conditions for microalgal strains at varying biomass density. This is advantageous to regulate light supply to the microalgae culture according to its physiological demand to maintain optimum irradiance regime. The shape of the retention tank was designed to minimise the dark volume of the microalgal suspension which can be as low as 10 %. The S/V ratio can be operated in the range of 60–180 m−1. Hydraulic properties, suspension distribution and flow were improved to reduce energy demand and CO2 losses.
The cultivation unit is controlled and regulated via sensors to measure photosynthetic activity, temperature, dissolved oxygen concentration and pH. Thermoregulation of the culture can be partially controlled by a heat exchanger in the trough and heating cable in the retention tank. The CO2 supply is regulated as a pH-stat according to the demand of the microalgal culture. Easy access to all parts of the cultivation device is necessary for cleaning and maintenance purposes.
In order to lower the cultivation unit height (maximum height of 1.7 m above ground) the retention tank is buried of about 0.5 m below ground. The unit is supported by a lightweight scaffolding structure made of rectangular profiles with ground anchors which enhances axial and torsional stiffness. All materials used for construction are biocompatible (PVC, PE, zinc-galvanised parts); the cultivation area is made of stainless steel. This construction is durable to climate conditions and corrosive environmental factors for tens of years. The unit was made using standardized parts as construction modules connected by joints which ease disassembly of the system. These features make it transportable with a long working life and easy to repair.
This unit for cultivation of microalgae was registered at the Industrial Property Office of the Czech Republic (patent pending PV 2013-803; utility design CZ 27021U1). The use of this demonstration unit is intended for microalgae production as food and feed additives, especially enriched in certain bioactive compounds (e.g. carotenoids, polyunsaturated fatty acids, etc.) or chemical elements (Se, Cr, Fe, Zn), for biodegradation and waste water treatment or CO2 sequestration.
In model trials a culture of Chlorella sorokiniana was grown in an outdoor TLC of 90 m2 (model Dahlia; Fig. 7) during late summer (September). The S/V ratio was operated between 120–160 m−1 and the culture layer thickness was between 5 and 6 mm. The maximum daily irradiance was about 1800 μmol photons m−2 s−1 and usually cultivation temperature rose from about 15–19 °C in the morning to the midday maximum of 24–29 °C. The growth optimum of this fast growing microalga is rather broad, between 20 and 40 °C. The dissolved oxygen concentrations were between 9 and 10 g L−1 at the start of the cultivation area rising to 18–32 g L−1 (the variability is caused by ambient irradiance and temperature) before flowing to the retention tank. The measurement of irradiance intensity close to the surface in the photic zone by a spherical microsensor (US-SQS/B; H. Walz, Germany) showed the mean light intensity of about 400 μmol photons m−2 s−1 which is within the usual upper-limit of saturating irradiance for most microalgae and it guarantees high productivity. In one trial the growth of the thinner culture was rather fast since the culture biomass density increased from the starting point of 2 g L−1 to 18 g L−1 in 8 days. The starting biomass concentration was about 2 g L−1 which was relatively diluted culture in this thin-layer set-up; it resulted in an initial lag-phase for 2 days (Fig. 8, curve with open circles). It corresponded to a chlorophyll concentration of 50–550 mg L−1. In the exponential phase (5–15 g L−1) the specific growth rate was about 0.27 day−1. The diel course of photosynthetic activity of the Chlorella culture was monitored from 08:00 to 18:00 h as in samples taken from the culture. Maxima of the relative electron transport rate rETR and the maximum photochemical yield of PSII, Fv/Fm were calculated from rapid light-response curves (using saturation-pulse analysis of fluorescence quenching) (Fig. 9) as described in Malapascua et al. (2014). The so-called rapid light-response curve shows the dependency of photosynthetic electron transport (rETR) on the irradiance intensity E (Fig. 1; see also Kromkamp et al. 1998; White and Critchley 1999; Ralph and Gademann 2005) and provides detailed information on the saturation characteristics of electron transport, as well as the actual performance of a microalgal culture. The value of rETRmax at midday was about 350, 2.3-times higher than that in the morning which indicated that the culture was rather active as it responded well to high irradiance. The values of the FV/FM ratio usually range between 0.7 and 0.8 in normal non-stressed green microalgae (Masojídek et al. 2013). In this case, the morning value of Fv/Fm was 0.75 which indicated a ‘healthy’ culture; this variable decreased to 0.6 at 13:00 h, i.e. by about 20 %. The experiments in closed photobioreactors as well as TLCs showed that a midday-depression of PSII photochemical yields between 20 and 30 % as compared with maximal morning values is essential for well-performing cultures (Masojídek et al. 2003, 2011a). A lower or higher depression of photochemical yields indicated low-light acclimated or photoinhibited cultures, respectively. The night temperatures below 20 °C minimized the respiration losses of biomass to less than 10 %.
Growth curves of the Chlorella sorokiniana culture. Two trials – 8 and 15-day long – were carried out to measure biomass concentration changes (g L−1) using the 90-m2 cascade (S/V ratio = 120–160 m−1) in late summer (September). The starting biomass density was about 2 g L−1 (curve with open circles) and about 5.8 g L−1 (curve with closed circles), respectively
Diel changes in the maximum relative electron transport rate rETRmax and the maximum photochemical yield of PSII, Fv/Fm in Chlorella sorokiniana mass culture. The diel course of photosynthetic activity of the Chlorella culture was monitored from 08:00 to 18:00 h in samples taken from the culture at exponential phase of growth (see Fig. 7; Day 5). Maxima of the relative electron transport rate rETRmax and the maximum photochemical yield of PSII, Fv/Fm were estimated from rapid light-response curves (using saturation-pulse analysis of fluorescence quenching) as described in Malapascua et al. (2014). One typical experiment was taken for presentation of data in this graph
In the other trial the starting biomass concentration was about 5.8 g L−1 which was about 3-times denser than the previous one. In this case the lag phase was seen only during Day 1, but the growth of this culture was slower during the first week as compared with the lower starting biomass density (Fig. 8, curve with closed circles).
TLCs have, however, some advantages, among which the much higher operating cell concentration, very high daylight productivities, and the possibility to quickly store the culture at night or in case of unfavourable weather conditions. These results are important from a biotechnological point of view in order to optimize the growth of outdoor microalgae mass cultures under varying climatic conditions.
5 Future Prospects of Thin-Layer Systems
Two principles have to be considered, namely reducing layer thickness and using vertical extensions in the configuration of cultivation modules. In thin-layer systems the culture layer usually varies between 10 and 40 mm. Empirically, a further reduction of layer thickness seems to be possible and opens a way to higher cell densities and productivities. An ultrathin-layer system where the microalgae suspension flows by gravity in a thin vertical coating of 0.5–2 mm between two plastic foils to assure a uniform and optimal photon supply was patented in 1994 (DE 4411486 CI, 1994) (Pulz et al. 2013). This system uses the adhesion forces between hydrophilic materials such as foils, plastics, or glass to grow microalgae culture in a thin flowing layer. Subject to research, various configurations of ultrathin layers in the space of a cultivation system are accessible, both for the formation of static (immobilized) and dynamic (flowing) biofilms. The next improvement is the use of light-penetrable and gas-permeable materials such as transparent textile tissues or meshes. These materials allow controlled light and gas supply (O2, CO2) within the culture suspension. The ultra-thin layer units with their uniform distribution of microalgae in the photostage allow high culture densities and high productivities.
Recently, a similar principle was used to design the so called ‘accordion’ PBR which consists of two adjacent transparent sheets, sealed together along to form thin-layer vessels holding microalgae culture arranged like a ‘pleated sheet’ (US 8709808 B2, 2014 by J.L Cuello & J.W Ley). The PBR includes a support structure and a base reservoir from which the culture is pumped to the upper edge of the photobioreactor between the two sheets.
The most recent development patented by IGV GmdH (Nuthetal, Bergholz-Rehbrücke, Germany) is the so called ‘rain PBR’ where microalgae are grown in ultrathin-layer (DE 10 2009 027, WO 2010/14154) (Pulz et al. 2013). The key issue of this approach is that a dynamic biofilm in the form of droplets and films is created using meshes or grids, distributing the high-cell-density microalgae culture into tiny fog-to-rain-like droplets in PBR space. While in the present PBR technology, biomass concentrations of 1–5 g DM L−1 are usually achievable, this system allows biomass concentrations of 20–40 g DM L−1. Biomass productivity (footprint based) 80 g DM m−2 day−1 can be estimated for this system. Another advantage of the new system is the predictable reduction of investment costs.
Notes
- 1.
The term – microalgae – is used by phycologists pragmatically for oxygenic phototrophic microorganisms, which include prokaryotic cyanobacteria and various eukaryotic algae and diatoms; it has no taxonomic significance.
- 2.
A biorefinery is a facility that integrates equipment and biomass conversion processes to produce fuels, power, heat, and value-added chemicals from biomass.
References
Baker NR (2008) Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol 59:89–113
Baker NR, Oxborough K (2004) Chlorophyll fluorescence as a probe of photosynthetic productivity. In: Papageorgiou GC, Govindjee (eds) Chlorophyll-a fluorescence: a signature of photosynthesis. Springer, Dordrecht, pp 65–82
Bartoš J (1967) Outdoor algae culture and selection of strains in Cuba. In: Nečas J, Lhotský O (eds) Ann Rep Algolog Lab Třeboň for 1966, pp 123–125
Bilger W, Björkman O (1990) Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth Res 25:173–185
Bradbury M, Baker NR (1984) A quantitative determination of photochemical and nonphotochemical quenching during the slow phase of the chlorophyll fluorescence induction curve of bean leaves. Biochim Biophys Acta 765:275–281
Büchel C, Wilhelm C (1993) In vivo analysis of slow chlorophyll fluorescence induction kinetics in algae: progress problems and perspectives. Photochem Photobiol 58:137–148
Campbell D, Hurry V, Clarke A, Gustafsson P, Öquist G (1998) Chlorophyll fluorescence analysis of cyanobacterial photosynthesis and acclimation. Microbiol Mol Biol Rev 62:667–683
Doucha J, Lívanský K (1995) Novel outdoor thin-layer high density microalgal culture system: productivity and operation parameters. Arch Hydrobiol Algol Stud 76:129–147
Doucha J, Lívanský K (2006) Productivity, CO2/O2 exchange and hydraulics in outdoor open high density microalgal (Chlorella sp.) photobioreactors operated in a Middle and Southern European climate. J Appl Phycol 18:811–826
Doucha J, Lívanský K (2014) High density outdoor microalgal culture. In: Bajpai R, Prokop A, Zappi M (eds) Algal biorefinery, vol 1. Springer, Dordrecht, pp 147–173
Doucha J, Lívanský K, Bínová J, Kubičko P, Novotný P (1993) Thin-layer high density microalgal culture systém: productivity and operational energy costs. In: Masojidek J, Šetlík, I (eds) Progress in biotechnology of phototrophic microorganisms. Proceedings of 6th international conference on apple algol, Institute of Microbiology, Třeboň, p 40
Emerson R, Arnold W (1932) The photochemical reactions in photosynthesis. J Gen Physiol 16:191–205
Enriquez S, Borowitzka MA (2011) The use of the fluorescence signal in studies of seagrasses and macroalgae. In: Suggett DJ, Prášil O, Borowitzka MA (eds) Chlorophyll a fluorescence in aquatic sciences: methods and applications. Springer, Dordrecht, pp 187–208
Figueroa FL, Conde-Álvarez R, Gómez I (2003) Relations between electron transport rates determined by pulse amplitude modulated chlorophyll fluorescence and oxygen evolution in macroalgae under different light conditions. Photosynth Res 75:259–275
Figueroa FL, Jerez CG, Korbee N (2013) Use of in vivo chlorophyll fluorescence to estimate photosynthetic activity and biomass productivity in microalgae grown in different culture systems. Lat Am J Aquat Res 41:801–819
Flameling IA, Kromkamp J (1998) Light dependence of quantum yields for PSII charge separation and oxygen evolution in eukaryotic algae. Limnol Oceanogr 43:284–297
Gilbert M, Wilhelm C, Richter M (2000) Bio-optical modelling of oxygen evolution using in vivo fluorescence: comparison of measured and calculated photosynthesis/irradiance (P-E) curves in four representative phytoplankton species. J Plant Physiol 157:307–314
Gordon JM, Polle JEW (2007) Ultrahigh bioproductivity from algae. Appl Microbiol Biotechnol 76:969–975
Grobbelaar JU (2009) Factors governing algal growth in photobioreactors: the “open” versus “closed” debate. J Appl Phycol 21:489–492
Grobbelaar JU (2012) Microalgae mass culture: the constrains of scaling-up. J Appl Phycol 24:315–318
Grobbelaar JU, Nedbal L, Tichy L, Šetlík I (1995) Variation in some photosynthetic characteristics of microalgae cultured in outdoor thin-layered sloping reactors. J Appl Phycol 7:175–184
http://en.wikipedia.org/w/index.php?search=biorefinary&title=Special%3ASearch&fulltext=1
Hu Q, Richmond A (1996) Productivity and photosynthetic efficiency of Spirulina platensis as affected by light intensity, algal density and rate of mixing in a flat plate photobioreactor. J Appl Phycol 8:139–145
Hu Q, Guterman H, Richmond A (1996a) A flat inclined modular photobioreactor for outdoor mass cultivation of photoautotrophs. Biotechnol Bioeng 51:51–60
Hu Q, Guterman H, Richmond A (1996b) Physiological characteristics of Spirulina platensis (cyanobacteria) cultured at ultrahigh cell densities. J Phycol 32:1066–1073
Hu Q, Zarmi Y, Richmond A (1998) Combined effects of light intensity, light-path and culture density on output rate of Spirulina platensis (Cyanobacteria). Eur J Phycol 33:165–171
Ihnken S, Beardall J, Kromkamp JC, Gómez Serrano C, Torres MA, Masojídek J, Malpartida I, Abdala R, Jerez CG, Malapascua JR, Navarro E, Rico RM, Peralta E, Ezequil JPF, Figueroa FL (2014) Light acclimation and pH perturbations affect photosynthetic performance in Chlorella mass culture. Aquat Biol 22:95–110
Janssen M, de Bresser L, Baijens T, Tramper J, Mur LR, Snel JFH, Wijffels RH (2000) Scale-up aspects of photobioreactors: effects of mixing-induced light/dark cycles. J Appl Phycol 12:225–237
Janssen M, Slenders P, Tramper J, Mur LR, Wijffels RH (2001) Photosynthetic efficiency of Dunaliella tertiolecta under short light/dark cycles. Enzyme Microb Techol 29:298–305
Janssen M, Tramper J, Mur LR, Wijfels RH (2003) Enclosed outdoor photobioreactors: light regime, photosynthetic efficiency, scale-up and future prospects. Biotechnol Bioeng 81:193–210
Jerez CG, Navarro E, Abdala R, Malpartida I, Rico RM, Masojídek J, Figueroa FL (2014) Hydrodynamics and photosynthesis performance of Chlorella fusca grown in a thin-layer cascade. Aquat Biol 22:111–122
Kok B (1953) Experiments on photosynthesis by Chlorella in flashing light. In: Burlew JS (ed) Algal culture: from laboratory to pilot plant, vol 600. Carnegie Institution of Washington Publication, Washington, pp 63–75
Krause GH, Weis E (1984) Chlorophyll fluorescence as a tool in plant physiology. 2. Interpretation of fluorescence signals. Photosynth Res 5:139–157
Krause GH, Weis E (1991) Chlorophyll fluorescence and photosynthesis – the basics. Annu Rev Plant Physiol 42:313–349
Kromkamp JC, Barranguet C, Peene J (1998) Determination of microphytobenthos PSII quantum efficiency and photosynthetic activity by means of variable chlorophyll fluorescence. Mar Ecol Prog Ser 162:45–55
Laws EA, Terry KL, Wickman J, Challup MS (1983) A simple algal production system designed to utilize the flashing light effect. Biotechnol Bioeng 25:2319–2335
Lívanský K, Kajan M, Pilarski PS (1995) Productivity, respiration and chemical composition of the green alga Scenedesmus incrassatulus grown in outdoor cultivation units with and without baffles. Arch Hydrobiol Algol Stud 76:111–128
Malapascua JRF, Jerez CG, Sergejevová M, Figueroa FL, Masojídek J (2014) Photosynthesis monitoring to optimize growth of microalgal mass cultures: application of chlorophyll fluorescence techniques. Aquat Biol 22:124–140
Masojídek J, Prášil O (2010) The development of microalgal biotechnology in the Czech Republic. J Ind Microbiol Biotechnol 37:1307–1317
Masojídek J, Torzillo G, Kopecký J, Koblížek M, Nidiaci L, Komenda J, Lukavská A, Sacchi A (2000) Changes in chlorophyll fluorescence quenching and pigment composition in the green alga Chlorococcum sp grown under nitrogen deficiency and salinity stress. J Appl Phycol 12:417–426
Masojídek J, Papáček Š, Sergejevová M, Jirka V, Červený J, Kunc J, Korečko J, Verbovikova O, Kopecký J, Štys D, Torzillo G (2003) A closed solar photobioreactor for cultivation of microalgae under supra-high irradiance: basic design and performance. J Appl Phycol 15:239–248
Masojídek J, Koblížek M, Torzillo G (2004) Photosynthesis in microalgae. In: Richmond A (ed) Handbook of microalgal mass cultures, 1st edn. Wiley Blackwell, Chichester, pp 20–39
Masojídek J, Sergejevová M, Rottnerová K, Jirka V, Korečko J, Kopecký J, Zaťková I, Torzillo G, Štys D (2009) A two-stage solar photobioreactor for cultivation of microalgae based on solar concentrators. J Appl Phycol 21:55–63
Masojídek J, Kopecký J, Giannelli L, Torzillo G (2011a) Productivity correlated to photobiochemical performance of Chlorella mass cultures grown outdoors in thin-layer cascades. J Ind Microbiol Biotechnol 38:307–317
Masojídek J, Vonshak A, Torzillo G (2011b) Chlorophyll fluorescence applications in microalgal mass cultures. In: Suggett DJ, Prášil O, Borowitzka MA (eds) Chlorophyll a fluorescence in aquatic sciences: methods and applications. Springer, Dordrecht, pp 277–292
Masojídek J, Koblížek M, Torzillo G (2013) Photosynthesis in microalgae. In: Richmond A, Hu Q (eds) Handbook of microalgal culture: applied phycology and biotechnology. Wiley Blackwell, Chichester, pp 20–39
Masojídek J, Torzillo G (2014) Mass cultivation of freshwater microalgae, 2nd edn. On-line database Earth Systems and Environmental Sciences, Elsevier, 13 p. http://dx.doi.org/10.1016/B978-0-12-409548-9.09373-8
Matthijs HCP, Balke H, VanHes UM et al (1996) Application of light-emitting diodes in bioreactors: flashing light effects and energy economy in algal culture (Chlorella pyrenoidosa). Biotechnol Bioeng 50:98–107
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence – a practical guide. J Exp Bot 51:659–668
Nedbal L, Tichy V, Xiong FH, Grobbelaar JU (1996) Microscopic green algae and cyanobacteria in high-frequency intermittent light. J Appl Phycol 8:325–333
Pulz O, Broneske J, Waldeck P (2013) IGV Gmb H experience report, industrial production of microalgae under controlled conditions: innovative prospects. In: Richmond A, Hu Q (eds) Handbook of microalgal culture: applied phycology and biotechnology. Wiley Blackwell, Chichester, pp 445–460
Ralph PJ, Gademann R (2005) Rapid light curves: a powerful tool to assess photosynthetic activity. Aquat Bot 82:222–237
Richmond A (2003) Growth characteristics of ultrahigh-density microalgal cultures. Biotechnol Bioprocess Eng 8:349–353
Richmond A (2004) Biological principles of mass cultivation. In: Richmond A (ed) Handbook of microalgal mass cultures. Wiley Blackwell, Chichester, pp 125–177
Richmond A (2013) Biological principles of mass cultivation of photoautotrophic microalgae. In: Richmond A, Hu Q (eds) Handbook of microalgal culture: applied phycology and biotechnology. Wiley Blackwell, Chichester, pp 171–204
Richmond A, Cheng-Wu Z (2001) Optimization of a flat plate glass reactor for mass production of Nannochloropsis sp. Outdoors. J Biotech 85:259–269
Rodolfi L, Chini Zittelli G, Bassi N, Padovani G, Biondi N, Bonini G, Tredici MR (2009) Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol Bioeng 102:100–112
Schreiber U (2004) Pulse-amplitude-modulation (PAM) fluorometry and saturation pulse method: an overview. In: Papageorgiou GC, Govindjee (eds) Chlorophyll a fluorescence: a signature of photosynthesis, Advances in photosynthesis and respiration, vol 19. Springer, Dordrecht, pp 279–319
Schreiber U, Schliwa U, Bilger W (1986) Continuous recording of photochemical and nonphotochemical fluorescence quenching with a new type of modulation fluorometer. Photosynth Res 10:51–62
Schreiber U, Endo T, Mi H, Asada K (1995) Quenching analysis of chlorophyll fluorescence by the saturation pulse method: particular aspects relating to the study of eukaryotic algae and cyanobacteria. Plant Cell Physiol 36:873–882
Šetlík I, Komárek J, Prokeš B (1967) Short account of the activities from 1960 to 1965 and some future prospects, Annu. Rep. Algolog. Lab for 1966. Institute of Microbiology, Třeboň
Šetlík I, Šust V, Málek I (1970) Dual purpose open circulation units for large scale culture of algae in temperate zones. I. Basic design considerations and scheme of a pilot plant. Algol Stud 1:111–164
Strasser RJ, Srivastava A, Govindjee (1995) Polyphasic chlorophyll a fluorescence transient in plants and cyanobacteria. Photochem Photobiol 61:33–42
Strasser RJ, Tsimili-Michael M, Srivastava A (2004) Analysis of the chlorophyll a fluorescence transient. In: Papageorgiou GC, Govindjee (eds) Chlorophyll a fluorescence: a signature of photosynthesis. Advances in photosynthesis and respiration, vol 19. Springer, pp 321–362
Suggett DJ, Moore CM, Geider RJ (2011) Estimating aquatic productivity from active fluorescence measurements. In: Suggett DJ, Prášil O, Borowitzka MA (eds) Chlorophyll a fluorescence in aquatic sciences: methods and Applications. Springer, Dordrecht, pp 103–128
Tennessen DJ, Bula RJ, Sharkey TD (1995) Efficiency of photosynthesis in continuous and pulsed light emitting diode irradiation. Photosynth Res 44:261–269
Ting CS, Owens TG (1992) Limitation of the pulse-modulated technique for measuring the fluorescence characteristics of algae. Plant Physiol 100:367–373
Torzillo G, Accolla P, Pinzani E, Masojídek J (1996) In situ monitoring of chlorophyll fluorescence to assess the synergistic effect of low temperature and high irradiance stresses in Spirulina cultures grown outdoors in photobioreactors. J Appl Phycol 8:283–291
Torzillo G, Bernardini P, Masojídek J (1998) On-line monitoring of chlorophyll fluorescence to assess the extent of photoinhibition of photosynthesis induced by high oxygen concentration and low temperature and its effect on the productivity of outdoor cultures of Spirulina platensis (Cyanobacteria). J Phycol 34:504–510
Torzillo G, Giannelli L, Martinez-Roldan AJ (2010) Microalgae culturing in thin-layer photobioreactors. In: Bardone E, Viglia A (eds) 2nd international conference on industrial biotechnology. Chemical engineering transactions 20, pp 265–270
Tredici M (2004) Mass production of microalgae: photobioreactors. In: Richmond A (ed) Handbook of microalgal mass cultures. Wiley Blackwell, Chichester, pp 178–214
Tredici M (2010) Photobiology of microalgae mass cultures: understanding the tools for the next green revolution. Biofuels 1:143–162
Vendlová J (1969) Outdoor cultivation in Bulgaria. Mass culture of Scenedesmus in outdoor units. In: Nečas J, Lhotský O (eds) Ann Rep Algolog Lab Třeboň for 1968, Institute of Microbiology, Třeboň, pp 143–152
Vonshak A, Torzillo G, Tomaselli L (1994) Use of chlorophyll fluorescence to estimate the effect of photoinhibition in outdoor cultures of Spirulina platensis. J Appl Phycol 6:31–34
Vonshak A, Torzillo G, Accolla P, Tomaselli L (1996) Light and oxygen stress in Spirulina platensis (Cyanobacteria) grown outdoors in tubular reactors. Physiol Plant 97:175–179
Walker DA (2009) Biofluels, facts, fantasy, and feasibility. J Appl Phycol 21:509–517
White AJ, Critchley C (1999) Rapid light curves: a new fluorescence method to assess the state of the photosynthetic apparatus. Photosynth Res 59:63–72
Wilhelm C, Becker A, Vieler A, Rautenberger R (2004) Photophysiology and primary production of phytoplankton in freshwater. Physiol Plant 120:347–357
Zahradník J (1967) Bioengineering. Mass culture of Scenedesmus in outdoor units. In: Nečas J, Lhotský O (eds) Ann Rep Algolog Lab Třeboň for 1966, Institute of Microbiology, Třeboň, pp 103–122
Zarmi Y, Bel G, Aflalo C (2013) Theoretical analysis of culture growth in flat-plate bioreactors: the essential role of timescales. In: Richmond A, Hu Q (eds) Handbook of microalgal mass culture: applied phycology and biotechnology. Wiley Blackwell, Chichester, pp 205–224
Zittelli GC, Biondi N, Rodolfi L, Tredici MR (2013) Photobioreactors for mass production of microalgae. In: Richmond A, Hu Q (eds) Handbook of microalgal mass culture: applied phycology and biotechnology. Wiley Blackwell, Chichester, pp 225–266
Zou N, Richmond A (1999) Effect of light-path length in outdoor flat plate reactors on output rate of cell mass and of EPA in Nannochloropsis sp. J Biotech 70:351–356
Acknowledgements
The authors thank Mr Pavel Souček, Mr Petr Novotný, Ms. Karolina Ranglová and Ms. Soňa Pekařová for technical assistance and Mr Jason Dean for language corrections. The Ministry of Education, Youth and Sports and the Technology Agency of the Czech Republic supported this work through the project Algatech CZ.1.05/2.1.00/03.0110, Algain CZ.1.07/2.3.00/30.0059 and Algaman CZ.1.07/2.3.00/20.0203 and project TA03011027.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Abbreviations
- Chl
-
chlorophyll
- DM
-
dry mass
- E
-
irradiance
- F0, Fv, Fm
-
minimum, variable and maximum fluorescence in dark-adapted state
- F′, Fv′, Fm′
-
minimum, steady-state, variable and maximum fluorescence in light-adapted state
- Fv/Fm, ΔF′/Fm′
-
maximum, resp. actual photochemical yield of PSII
- GWP
-
green-wall panel
- L/D
-
light-dark
- LED
-
light-emitting diode
- OBD
-
optimum biomass density
- OD
-
optical density
- PAM
-
pulse-amplitude-modulation
- PAR
-
photosynthetically active radiation
- PBR
-
photobioreactor
- Pmax
-
maximum rate of photosynthesis
- PPFD
-
photosynthetic photon flux density
- PSII
-
Photosystem II
- rETR
-
relative electron transport rate through PSII
- RLC
-
rapid light-response curve
- S/V
-
surface-to-volume ratio
- TLC
-
thin-layer cascade
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Masojídek, J., Sergejevová, M., Malapascua, J.R., Kopecký, J. (2015). Thin-Layer Systems for Mass Cultivation of Microalgae: Flat Panels and Sloping Cascades. In: Prokop, A., Bajpai, R., Zappi, M. (eds) Algal Biorefineries. Springer, Cham. https://doi.org/10.1007/978-3-319-20200-6_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-20200-6_7
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-20199-3
Online ISBN: 978-3-319-20200-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)