Abstract
Sarcoidosis is a multisystem disease of unknown origin, predominantly affecting young adults, and characterized by compact noncaseating epithelioid cell granulomas. The term sarkoid was first introduced in 1899 by Caesar Boeck, a Norwegian dermatologist, to describe benign cutaneous lesions that resembled sarcoma on histopathological examination. With the development and progress of new imaging modalities including positron emission tomography (PET) and magnetic resonance imaging (MRI), there has been a recent increase in interest from clinicians and researchers regarding the diagnosis and treatment of cardiac sarcoidosis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Positron Emission Tomography
- Cardiac Sarcoidosis
- Pulmonary Sarcoidosis
- Heart Rhythm Society
- Ottawa Heart Institute
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
4.1 Introduction
Sarcoidosis is a multisystem disease of unknown origin, predominantly affecting young adults, and characterized by compact noncaseating epithelioid cell granulomas. The term sarkoid was first introduced in 1899 by Caesar Boeck, a Norwegian dermatologist, to describe benign cutaneous lesions that resembled sarcoma on histopathological examination [1]. With the development and progress of new imaging modalities including positron emission tomography (PET) and magnetic resonance imaging (MRI), there has been a recent increase in interest from clinicians and researchers regarding the diagnosis and treatment of cardiac sarcoidosis.
4.2 Sarcoidosis
4.2.1 Epidemiology and Pathophysiology
The incidence of sarcoidosis is estimated to be 5 per 100,000 person-years in whites, with slight female predominance, and up to 39 per 100,000 person-years in African-Americans [2–4]. However, recent data suggest even higher prevalence, reaching 3–10 and 35–80 per 100,000 person-years in whites and African-Americans, respectively [5]. Underestimation of sarcoidosis incidence is likely due in part to lack of accurate diagnostic tests, leading to under recognition and misdiagnosis of the disease [6]. Even though the exact causes of sarcoidosis are unknown, current evidence suggests that the disease is the result of inflammation mediated by T lymphocytes and activated macrophages following exposure to specific agents in subjects with genetic predispositions [2, 6–13]. Some agents that have been linked to increased incidence of sarcoidosis are listed in Table 4.1.
4.2.2 Clinical Presentation and Treatment
Presenting symptoms of sarcoidosis typically include dyspnea and cough due to lung involvement as well as systemic symptoms including fatigue, weight loss, and fever. However, sarcoidosis can affect virtually any organ of the human body and can present with a wide variety of symptoms depending on organ involvement (Table 4.2).
Most patients with sarcoidosis present with mild symptoms and will recover spontaneously. Decision to initiate treatment should be based on risk-benefit analysis [14]. The mainstay of sarcoidosis treatment is corticosteroids. Current guidelines recommend the use of 20–40 mg of oral prednisone per day [2], and response to therapy should be assessed within 1–3 months. In case of improvement, prednisone treatment can be tapered down to dosage of 5–15 mg per day for an additional 9–12 months, whereas lack of response suggests irreversible fibrotic disease [14]. Given the nonspecific nature of sarcoidosis symptoms, it can be difficult to accurately monitor response to therapy. The use of cytotoxic medication such as methotrexate and immunomodulatory agents such as tumor necrosis factor alpha has been shown to be a viable therapeutic option in patient not responding to corticosteroids or unable to pursue corticosteroid treatment due to adverse effects [15]. Other therapeutic options include hydroxychloroquine to treat patients with skin involvement and hypercalcemia [14].
4.2.3 Diagnosis of Sarcoidosis
The diagnosis of systemic sarcoidosis is established when there is radiographic evidence of sarcoidosis with compatible clinical features and noncaseating granulomas on biopsy with other causes of granulomas ruled out. Response to corticosteroids is not enough to establish diagnosis of sarcoidosis [14]. There are no specific serum markers for sarcoidosis. C reactive protein (CRP) and erythrocyte sedimentation rate (ESR) are sensitive inflammation markers but are not specific for sarcoidosis. Serum angiotensin-converting enzyme (ACE) levels are elevated in approximately 60 % of patients with sarcoidosis due to production of ACE by the granulomas [14]. However, serum ACE is neither sensitive nor specific enough to screen patients with suspected sarcoidosis with its positive predictive value of 84 % and negative predictive value of 74 % [16]. Furthermore, serum ACE should not be used to diagnose sarcoidosis or guide therapy [14].
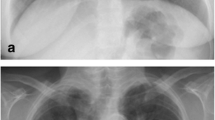
Due to lack of useful serum markers, diagnosis of sarcoidosis relies greatly on imaging. Classical features of sarcoidosis on chest radiographs include mediastinal and hilar lymphadenopathy, parenchymal opacities, and parenchymal fibrosis in more advanced cases [17]. A staging based on chest radiography has been described more than 40 years ago to monitor chest disease in patient with sarcoidosis (Fig. 4.1) [18]. Although this scale shows poor interobserver agreement, especially for advanced disease, it remains widely used due to its prognostic value [19].
Computed tomography (CT) imaging is only indicated when chest radiograph is atypical for sarcoidosis or when patients present with hemoptysis. Lymphadenopathy and parenchymal disease are more readily seen on CT. Classically, parenchymal lesions present as small pulmonary nodules in a peribronchovascular distribution or following the fissures [17]. Lymphadenopathy with coarse calcification or “icing sugar” lymphadenopathies are frequently described in sarcoidosis. In more advanced disease, lung fibrosis is seen.
Gallium-67 citrate has been used for the diagnosis, staging, and treatment follow-up of patients with sarcoidosis. Classical findings include the so-called lambda sign, arising from uptake in the mediastinal and hilar lymphadenopathy, and the panda sign, from abnormal parotid and lacrimal gland uptake (Fig. 4.2). However, accuracy of gallium scintigraphy varies greatly from one study to another with reported sensitivity ranging from 60 to 90 % and suboptimal interobserver variability [20–25].
4.2.4 Role of PET/CT in Sarcoidosis
18F-fluorodeoxyglucose (FDG) is a diagnostic PET radiopharmaceutical with half-life of 109.7 min. FDG is a glucose analog that is transported from the blood into the cells by glucose transporters GLUT 1 and GLUT 4. Once intracellular, FDG is then phosphorylated by hexokinase to form FDG-6-phosphate [26]. Unlike glucose, FDG-6-phosphate is not further metabolized, which leads to trapping of the radiopharmaceutical into the cells (Fig. 4.3). Uptake of FDG is proportional to glucose metabolism, and therefore, increased FDG uptake is seen in several conditions including infection, inflammation, and malignancies.
FDG-PET has several practical and technical advantages over 67Ga, including favorable tracer kinetics, lower radiation exposure, and better quality images [27]. FDG-PET is more accurate and allows better evaluation of extrapulmonary involvement compared to 67Ga [25, 28].
4.2.4.1 Evaluation of Disease Extent
FDG-PET imaging permits whole-body assessment in a single-step examination. A large proportion of patients with suspected pulmonary sarcoidosis have extrapulmonary involvement visualized on FDG-PET [29]. Frequent extrapulmonary sites include the bone, spleen, liver, and abdominal lymph nodes [30]. Accurate determination of the spread of active disease with FDG-PET provides an explanation for persistent disabling symptoms and has been shown to influence patient management [29, 31]. Moreover, identification of sites of extrapulmonary sarcoidosis can identify potential biopsy sites (Fig. 4.4) [29].
4.2.4.2 FDG-PET as a Marker of Disease Activity and in Assessment of Therapy Response
The goal of treatment in sarcoidosis is to treat reversible granulomatous inflammation. Still, there is currently no gold standard method for monitoring inflammatory activity in patients with sarcoidosis. Because of the serious side effects associated with sarcoidosis therapy, it is important to differentiate active and treatable inflammation from quiescent fibrosis. Sarcoidosis often presents with nonspecific systemic symptoms such as fatigue. Monitoring of these nonspecific symptoms does not allow to assess disease progression or response to therapy [32]. Besides, some patients are completely asymptomatic. Serum ACE is also used to monitor response to treatment but does not accurately reflect inflammatory activity [33]. Changes on conventional anatomical imaging, including x-ray and high-resolution CT, are not reliable markers of active inflammation, as they can remain positive even without active disease [34]. On the contrary, PET was shown to provide additional value to assess inflammatory activity in patients with pulmonary fibrosis [35]. Bronchoalveolar lavage (BAL) is an important tool in diagnosis of pulmonary sarcoidosis, but is not recommended for assessment of treatment response [36]. The prognostic value of BAL depends on the number of T lymphocytes, neutrophils, and mast cells [37–39]. Although gallium-67 has been used to detect active granulomas, it lacks correlation with BAL lymphocyte counts [23, 40]. Because FDG accumulates in inflammatory cells and allows imaging of active inflammation, FDG-PET could be used to monitor response to therapy (Figs. 4.4 and 4.5) [41]. FDG-PET has been shown to be a useful adjunct to other diagnostic methods for detecting active inflammation, especially in patients with persistent symptoms and normal ACE levels [31]. FDG-PET is also used to monitor response to therapy. So far, only few studies, most retrospective and with a small sample size, have looked at FDG-PET before and after corticosteroid therapy [20, 42–45] and TNF-α inhibitor therapy [46, 47]. FDG-PET post therapy was shown to correlate with symptoms [44, 46] as well as corticosteroid dosage [42]. It also correlates with endoscopic findings in patients with sinonasal sarcoidosis [45]. On the other hand, FDG-PET does not correlate with serum ACE, serum IL-2R, or pulmonary function tests (PFT) [20, 44, 46, 47]. These findings suggest that FDG-PET could provide useful information to assess response to treatment and adjust steroid dosage.
4.3 Cardiac Sarcoidosis
4.3.1 Epidemiology
In the United States, myocardial involvement is recognized in approximately 25 % of patients with sarcoidosis [48]. In the Japanese population, the prevalence of cardiac involvement in patients with sarcoidosis is significantly greater, reaching 58 % [49–51]. Cardiac sarcoidosis is characterized by noncaseating granulomas in the myocardium surrounded by lymphocytes [52]. The left ventricular free wall and septum are the most frequently affected myocardial regions [49, 52].
4.3.2 Clinical Presentation and Treatment
Patients with cardiac sarcoidosis may have different presentations depending on the extension and location of the disease [14]. They can present with conduction abnormalities due to direct infiltration of inflammatory cell and/or scar formation. These can also manifest as atrioventricular block or bundle branch block [49]. Atrioventricular block affects approximately 25 % of patients with cardiac sarcoidosis and is caused by inflammation or scar in the basal septum [53, 54]. Diagnosis of cardiac sarcoidosis should be considered in young patients with atrioventricular heart block of unknown etiology [54, 55]. Patients with cardiac sarcoidosis can also present with congestive heart failure due to extensive infiltration of the myocardium by granulomas [49, 56].
As with pulmonary sarcoidosis, cardiac inflammation from sarcoidosis is typically treated with corticosteroids. Several uncontrolled series and small sample retrospective studies suggest that steroids may be valuable to treat cardiac sarcoidosis [53, 54, 57–59]. Thus far, there are no randomized controlled trials available to confirm these results and treatment of cardiac sarcoidosis with corticosteroids remains controversial [60]. Initiation of corticosteroid therapy, even with antiarrhythmic drugs, does not prevent arrhythmic event [61]. Pacemakers and ICD are often required when there is extensive involvement of the conduction system or when there is significant heart failure [60]. A systematic review of corticosteroid therapy in cardiac sarcoidosis revealed a wide range of steroid regimens, with prednisone dosage of 20–60 mg daily or 50–60 mg on alternate day [62]. In small single-center retrospective studies, corticosteroid therapy was associated with maintenance of LV function in patients with normal function at diagnosis and improvement of ejection fraction in patients with mild to moderate LV dysfunction [58, 59, 63]. However, no improvement was observed in patients with severe LV dysfunction. As regards to ventricular arrhythmias, some studies showed benefits of immunosuppression therapy [59, 64, 65], whereas others showed no benefit or worsening [61, 66]. As for LV dysfunction, immunosuppressive therapy appears more beneficial in early phase of disease [58, 59]. There is, however, currently not enough data to establish mortality benefit from steroid therapy [62].
4.3.3 PET Imaging
4.3.3.1 Patient Preparation
The myocardium can metabolize different substrates, including free fatty acids, glucose, pyruvate, and ketone bodies. Under physiological conditions, the heart metabolizes mainly free fatty acids and glucose, with free fatty acids accounting for 60–90 % of the energy substrates [67–69]. The source of energy used by the myocardium depends mainly on free fatty acids and glucose plasma concentration and insulin levels [70]. In order to obtain diagnostic FDG-PET images for assessing active cardiac sarcoidosis, pretest preparation is required to suppress physiological uptake in the normal myocardium. Adequate suppression of physiological myocardial FDG uptake is crucial to maximize diagnostic accuracy (Fig. 4.6).
4.3.3.1.1 Fasting
When fasting, the myocardial metabolism is shifted and 90 % of its energy comes from metabolism of free fatty acids [71]. For this reason, prolonged fasting is routinely used to suppress physiological myocardial glucose uptake. Several protocols have been described using different fasting time to suppress physiological myocardial FDG uptake, typically between 5 and 18 h [72–75]. To maximize specificity of myocardial FDG uptake, current guidelines recommend fasting for at least 12 h [76].
4.3.3.1.2 Diet
Twelve-hour fasting alone can lead to variable myocardial FDG uptake and additional measures are important to consider to minimize physiological uptake [76]. Changing diet prior to imaging has been shown to be at least as effective as fasting to suppress physiological myocardial uptake [72]. Indeed, the fatty acid-glucose cycle, known as the Randle cycle, indicates that glucose loading suppresses fatty acid utilization and that fatty acid loading suppresses glucose utilization by the myocardium [77]. Different low-carbohydrate diets and low-carbohydrate high-fat (LCHF) diets have been used to minimize myocardial FDG uptake (Table 4.3). In a small randomized study, Cheng et al. showed that a simple low-carbohydrate diet in combination with prolonged fasting of 6 h provided adequate myocardial suppression of FDG uptake, while addition of fatty acid loading did not, suggesting that low carbohydrate might be preferable [78]. Others suggested that high-fat meal 3–6 h or 1 h prior to imaging provided adequate myocardial suppression [79]. Harisankar et al. demonstrated that LCHF diet is superior to prolonged fasting of 12 h to suppress myocardial FDG uptake [80]. More recently, Soussan et al. demonstrated that a LCHF diet for the dinner and breakfast preceding imaging followed by 4 h fasting provided sufficient myocardial suppression of uptake for the diagnosis of cardiac sarcoidosis [81]. There is currently no evidence that clearly established usefulness of LCHF diet over low carbohydrate alone. Furthermore, there is no data on improvement of diagnosis of cardiac sarcoidosis using such diets. Nevertheless, because diet modification is an easily applicable measure, it is routine to follow a diet with less than 5 g of carbohydrate, with or without high-fat meal prior to imaging. Table 4.4 shows an example of the LCHF recommendations provided to the patients prior to FDG imaging.
4.3.3.1.3 Heparin
Nuutila et al. showed that increased free fatty acid serum levels induced by heparin infusion were associated with decreased myocardial FDG uptake in healthy volunteers [86]. The protocol used in their study consisted of two 200 IU boluses of unfractionated heparin, injected intravenously, 90 min before FDG injection and at time of injection, with continuous infusion of 15 IU/min starting 90 min prior to FDG injection. The increased in FFA levels is mediated via activation of the lipoprotein and hepatic lipase [87]. Heparin injection is now routinely done to suppress myocardial FDG uptake, and typical dosage is 50 IU/kg divided in one or two intravenous bolus injections [25, 74, 88, 89], without reports of increased bleeding risk [25, 89]. However, using heparin infusion alone to suppress myocardial uptake is not sufficient since approximately half of the patients still present diffuse myocardial FDG uptake following heparin injection and 6 h fasting [89, 90]. Administration of unfractionated heparin prior to injection of FDG has not been shown to improve detection of cardiac sarcoidosis yet. Nevertheless, many centers have implemented heparin injection in their imaging protocol to maximize diagnostic accuracy.
4.3.3.1.4 Diabetic Patients
While many studies on patient preparation prior for FDG imaging exclude patients with diabetes [80, 82, 83, 85], others include only very small number of patients with diabetes [78]. In the small number of studies that do include patients with diabetes, there are discrepancies between the protocols with some suggesting to continue diabetic medication [79], while others are prescribing to hold diabetic medication on the day of the scan [81]. Currently, there are no data addressing specifically the optimal pretest preparation of patients with diabetes prior to FDG-PET in the context of cardiac sarcoidosis.
Different centers have different protocols to image diabetic patients. At the University of Ottawa Heart Institute, diabetic patients are scheduled as the first case of the day, following 12 h fasting and low-carbohydrate high-fat diet, to minimize patient discomfort and glycemic issues. It is recommended that diabetic patients monitor their blood sugar levels following their usual routine with one measurement obtained the morning of the PET scan. If the glycemia falls to less than 4 mmol/L, patients are instructed to drink juice or take glucose to raise their sugar level to higher than 4 mmol/L. Some oral medications should be held on the morning of the scan, while others should be continued (Table 4.5).
In many cases, usual doses of long-acting and intermediate-acting insulin can be continued the night before the PET scan. Short-acting insulin dosage and premixed insulin may need to be reduced the evening before the scan given the low-carbohydrate diet. Since the patients will be fasting on the day of the test, the usual breakfast dose of short-acting insulin should not be taken. Finally, for patients with type 1 diabetes using an insulin pump, basal insulin should be continued on the day of the test.
4.3.4 Imaging Protocol
Imaging protocol includes three components: (1) cardiac rest perfusion scan, (2) whole-body FDG scan, and (3) dedicated cardiac FDG scan. The imaging protocol used at the University of Ottawa Heart Institute (UOHI) is illustrated in Fig. 4.7.
Cardiac rest perfusion scan is typically performed using PET perfusion tracers such as rubidium-82 or ammonia-N13 and follows regular procedure guidelines for rest myocardial perfusion [91]. With Rb-82, dosages of 10–20 MBq/kg up to 2,200 MBq are used and ECG-gated images are acquired over 4–8 min after injection. Ammonia-N13 rest images are acquired over 15–30 min approximately 5 min after intravenous injection of 5–10 MBq/kg (up to 750 MBq). Images are reconstructed using CT-based attenuation correction and displayed in the usual short and long axes.
Approximately 1 h after intravenous injection of 5 MBq/kg (up to 550 MBq) of 18F-FDG, whole-body PET scan is acquired. The patient is positioned supine with his or her arms up above his head. A low-dose CT for attenuation correction and localization is first acquired. Then, whole-body FDG-PET images are acquired from the base of the skull to mid thighs, 3 min per bed position. Following whole-body imaging, dedicated cardiac PET scan is performed. This acquisition includes a static and, if available, ECG-gated acquisitions. Images are reconstructed by OSEM with attenuation correction and displayed along the perfusion images in the usual short and long axes (Fig. 4.6).
4.3.5 Image Interpretation
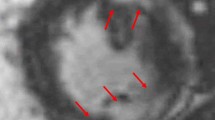
Cardiac FDG interpretation must ensure adequate patient preparation. Indeed, poor patient preparation can result in equivocal study due to physiological myocardial FDG uptake. For interpretation, cardiac FDG-PET images are displayed along with rest perfusion images with conventional cardiac display software in the usual short and long axes. Ishimaru et al. described four patterns of FDG uptake: none, focal, focal on diffuse, and diffuse (Fig. 4.8) [89]. This classification has been adopted by other studies and is now frequently used [88, 92]. Focal and focal on diffuse uptake patterns are considered suspicious for cardiac sarcoidosis, whereas diffuse or absence of uptake are considered negative. In order to increase specificity of the diagnostic test, correlation with clinical history is crucial in the interpretation of FDG-PET scan. The interventricular septum is known to be frequently affected in patients with CS and is associated with second- and third-degree atrioventricular block [53, 54]. Therefore, uptake in that region in a patient presenting with atrioventricular block is highly suspicious for sarcoidosis (Fig. 4.9) [93].
(a) ECG of a patient without known history of sarcoidosis presenting with complete atrioventricular block and junctional escape beat with right bundle branch block. (b) Subsequent FDG-PET/CT with ammonia-N13 rest imaging shows focal increased uptake in the basal septal wall, right ventricular insertion, as well as right ventricular free wall
Similarly, if the onset site of VT demonstrates focal FDG uptake on FDG-PET, the likelihood that the uptake represents active cardiac sarcoidosis is increased [76]. Although not routinely used, quantification of heterogeneity of uptake can be used to increased specificity. Quantification of heterogeneity of uptake using SUV coefficient of variation has been shown to be significantly higher in patients with CS compared to normal healthy subjects and in patients with DCM [92].
Image interpretation should include comparison with perfusion images. This allows detection of myocardial scar and assessment of active sarcoidosis in these areas [94]. Interpretation criteria including perfusion images are summarized in Table 4.6 [95]. Finally, whole-body FDG-PET scan should be reviewed to assess for extracardiac sarcoidosis and other incidental findings. Detection of extrathoracic sarcoidosis can facilitate and accelerate the diagnosis of sarcoidosis by identifying potential biopsy sites [29]. It must be noted that imaging represents one of the criteria used for the diagnosis of cardiac sarcoidosis and diagnosis cannot be established solely on the base of a positive FDG-PET scan.
4.3.6 Role of FDG-PET in Cardiac Sarcoidosis
4.3.6.1 Diagnosis of Cardiac Sarcoidosis
Revision of the original Japanese Ministry of Health and Welfare (JMHW) guidelines for the diagnosis of cardiac sarcoidosis was presented by the joint committee of the Japan Society of Sarcoidosis and Other Granulomatous Disorders and the Japanese College of Cardiology in 2006 (Table 4.7) [96]. These new guidelines, simply referred as the JMHW guidelines, are currently used in many studies as a gold standard for cardiac sarcoidosis. Although FDG-PET is not explicitly included in these criteria, gallium-67 scintigraphy is often substituted by FDG-PET. According to these criteria, diagnosis of CS requires histopathological or clinical diagnosis of extracardiac sarcoidosis. Endomyocardial biopsy has limited sensitivity due to sampling method and heterogeneous myocardial involvement [97]. Unlike amyloidosis, cardiac involvement with sarcoidosis is typically patchy; therefore, even in the cases of negative endomyocardial biopsy, the diagnostic of cardiac sarcoidosis can still be established in the appropriate clinical setting [76]. Because of poor correlation between imaging findings and JMHW diagnosis of CS, some authors have suggested that JMHW has limited sensitivity for detection of CS [88, 95, 98].
More recently, the Heart Rhythm Society (HRS), in collaboration with many organisms including the World Association for Sarcoidosis and Other Granulomatous Disorders (WASOG), published an expert consensus recommendation on criteria for the diagnosis of cardiac sarcoidosis (Table 4.8) [99]. Imaging modalities recommended in these guidelines include FDG-PET, Ga-67 scintigraphy, and cardiac MRI. Furthermore, these criteria are more stringent, requiring histological diagnosis of cardiac or extracardiac sarcoidosis.
4.3.6.2 Diagnostic Accuracy
Establishing diagnostic accuracy for cardiac sarcoidosis (CS) of any imaging modality is challenging because of lack of a proper gold standard. Ideally, diagnostic accuracy studies would use myocardial biopsy on all subjects. However, this gold standard is invasive and has a poor sensitivity below 25 % due to the heterogeneity of myocardial involvement [100, 101]. Because of this, studies tend to have small sample size and/or rely on JMWH criteria as their gold standard. A meta-analysis reviewing seven studies with a total of 164 patients reported sensitivity of FDG-PET scan for cardiac sarcoidosis ranging from 79 to 100 % with pooled sensitivity of 89 % [74]. They reported a wide range of specificity values from 38 to 100 % with pooled specificity of 78 %. It was hypothesized that this wide range of specificity is due to physiological myocardial uptake leading to false-positive studies and low sensitivity of the use of JMHW criteria as gold standard [74].
4.3.6.2.1 FDG-PET vs Gallium 67
As for other sites of extrapulmonary sarcoidosis, sensitivity of Ga-67 to detect cardiac sarcoidosis is very low, ranging from 15 to 40 % [25, 75, 102]. In a retrospective study including 76 patients with suspected cardiac sarcoidosis using the 1993 JMHW criteria as their gold standard, fasting FDG-PET sensitivity and specificity were 85 % and 90 %, respectively, compared to 15 % and 80 % for Ga-67 scintigraphy [75]. These results reinforce the importance of revising JMHW diagnostic criteria to incorporate FDG-PET instead of Ga-67.
4.3.6.2.2 Pitfalls
The wide range of reported specificity of FDG-PET to detect cardiac sarcoidosis has been attributed in part to a suboptimal gold standard [74]. Additionally, other conditions can render false-positive results. One of the major causes of false-positive study is related to poor patient preparation. Without preparation, uptake in the myocardium is usually diffuse but can be focal or focal on diffuse [103]. Respect of protocol can be optimized by good communication with patients and an interview to ensure adequate preparation prior to imaging. Nevertheless, even after proper fasting, normal myocardium can demonstrate uptake with regional disparities, with the lateral and inferior wall containing more activity compared to the septum and anterior wall [104]. Indeed, lateral wall uptake in a normal heart following adequate preparation was reported and is thought to represent a normal variation [74, 88, 89], possibly related to regional substrate availability (Fig. 4.10) [92].
(a) Rest ammonia-N13 and FDG-PET scan of a patient with suspected cardiac sarcoidosis. After following fasting and HCLF diet to suppress physiological myocardial uptake, there is FDG uptake in the lateral wall of the left ventricle, representing a normal variant. (b) Anterior projection of maximal intensity projection showing no evidence of extracardiac sarcoidosis. Note the diffuse uptake in muscle of the upper extremities, in keeping with recent strenuous activity
Dilated cardiomyopathy (DCM) can also present as focal on diffuse FDG uptake, possibly due to myocardial cell loss or regional fibrosis. Consequently, in the appropriate clinical context, DCM should be included in the differential diagnosis of a positive FDG-PET study [92]. Other causes of myocarditis, cardiac amyloidosis, infection, and myocardial metastases can also demonstrate abnormal myocardial uptake. Finally, patients with pulmonary hypertension can demonstrate increased right ventricular FDG [105, 106], mimicking RV involvement.
FDG-PET is a sensitive examination to detect CS, and therefore, there are few circumstances under which FDG-PET will be falsely negative [74]. Patient receiving corticosteroids or with “burned-out” sarcoidosis can have a negative FDG-PET scan [107]. In the case of burned-out sarcoidosis, rest perfusion scan will demonstrate a perfusion defect in the area of granulomatous scar [95]. Finally, another potential cause of false negative is related to the size of the area of inflammation. Although spatial resolution of FDG-PET is significantly greater than [67] Ga scintigraphy, it remains lower than MRI and very small foci of inflammation could be missed.
4.3.6.3 Prognostic Value of PET/CT
Cardiac involvement in sarcoidosis can lead to congestive heart failure due to progressive infiltration of the myocardium by granulomas [49, 56]. In a survey conducted in the United Kingdom, the 5-year survival rate of patients with sarcoidosis and suspected cardiac involvement was 40 %, with most of these patients receiving steroid treatments [108]. In a more recent study by Yazaki et al., the 5-year survival rate of patients treated for cardiac sarcoidosis and systolic impairment was 75 %, while the 5-year survival rate reached 90 % in patients treated for cardiac sarcoidosis with normal systolic function [56]. These results suggest that identification and treatment of patients with active cardiac sarcoidosis before the occurrence of systolic dysfunction is fundamental in the prevention of adverse outcomes. Unfortunately, approximately half of the patients with cardiac sarcoidosis are asymptomatic [109]. Furthermore, the severity of pulmonary involvement assessed either by imaging or PFTs does not correlate with the presence of CS [109, 110]. Therefore, identification of patients with CS before occurrence of systolic dysfunction can be challenging. For this reason, it is thought that imaging could play a central role in the identification of patients with CS and could lead to prevention of adverse outcome.
Other complications of patients with cardiac sarcoidosis are ventricular arrhythmias and sudden death [49]. Patients with cardiac sarcoidosis diagnosed based on the JMHW presenting with unsustained VT display significantly higher FDG uptake when compared with those with AV blocks and asymptomatic controls [111]. Conversely, patients demonstrating both perfusion abnormality and inflammation on FDG-PET have threefold increased rate of event rate (VT or sudden death) [95]. Blankstein et al. demonstrated, using a multivariable model including left ventricular ejection fraction (LVEF), JMHW criteria, and pattern of abnormality on PET scan, that the presence of both abnormal FDG uptake and perfusion abnormalities had the strongest link to death or VT (Fig. 4.11) [95]. Furthermore, they showed that risk was greater when there was RV involved [95]. There is likely a relationship between arrhythmias and FDG findings, but there is currently only sparse data on prognostic value of FDG-PET in CS, and therefore, current guidelines on ICD implantation in patients with CS do not rely specifically on FDG-PET [99].
Survival free of death or ventricular tachycardia stratified by perfusion and FDG-PET results, demonstrating that combination of perfusion abnormality and abnormal FDG-PET uptake is associated with increased risk of death and ventricular tachycardia (Reprinted from Blankstein et al. [95] with permission from the author and Elsevier)
FDG-PEt allows detection of CS before functional and structural changes are detectable, allowing early diagnosis and appropriate management. Furthermore, close monitoring of patients with myocardial inflammation demonstrated on FDG-PET is warranted and may prevent progressive cardiac disease [109].
4.3.6.4 Assessment of Response to Therapy
Another potential role of FDG-PET in CS is the assessment of response to therapy. Because FDG accumulates in active inflammation and not fibrosis [41], it could be used to evaluate response to therapy on serial studies. Several studies showed that following steroid therapy, there is a decrease in FDG uptake in both extracardiac lesions [20, 42–45] and cardiac lesions (Fig. 4.12) [73]. Resolution of FDG uptake in the basal septum has been associated with resolution of third-degree atrioventricular block and progression of FDG uptake after steroid tapering was associated with VT in case reports [112, 113].
(a) Baseline rest ammonia-N13 perfusion images with (b) corresponding FDG-PET images of a patient with diagnosis of cardiac sarcoidosis. Following a treatment of 9 months of corticosteroid, a FDG-PET study was repeated 13 months following baseline evaluation, showing no evidence of active inflammation in the myocardium. The LVEF, as measured by radionuclide ventriculography prior to each FDG-PET scan, remained unchanged, from 53 to 51 %
In a retrospective study of 23 patients with cardiac sarcoidosis treated with immunosuppressive therapies guided by serial PET scans, reduction in the intensity and extent of inflammation, as quantified by FDG uptake, was associated with an increase in LVEF [107]. These results reinforce the potential role of FDG-PET in guiding the duration and intensity of immunosuppressive treatment. Nonetheless, further validation of the role of FDG-PET in assessment of response to therapy by prospective trials is necessary.
4.3.7 When to Consider PET/CT in Patients with Sarcoid Disease
Metha et al. studied prevalence of cardiac symptoms, ECG findings, Holter monitoring, and transthoracic echocardiography findings of 62 patients with known sarcoidosis [109]. Description of abnormalities studied is presented in Table 4.9. They showed that patients with cardiac sarcoidosis had more cardiac symptoms and more findings on Holter monitoring and on transthoracic echocardiography compared to patients without cardiac sarcoidosis. All patients with cardiac sarcoidosis had symptoms or at least one abnormal test result [109]. Based on these results, the HRS expert consensus recommends screening of patients with established extracardiac sarcoidosis with clinical symptoms, ECG and echocardiography; [99] if one or more of these screening tests is abnormal, the patient is then referred for further cardiac imaging with FDG-PET or cardiac MRI.
4.3.8 When to Consider PET/CT in Patients Without Evidence of Sarcoid Disease
Multiple studies have reported cardiac manifestations as the initial presentation of patients with sarcoidosis [54, 114]. These patients were younger than 55 years old and initially presented with new onset of unexplained atrioventricular block and VT [115]. Nery et al. prospectively evaluated patients younger than 60 years old who presented with unexplained second- or third-degree atrioventricular block, with no previous history of sarcoidosis [55]. Of the 32 patients studied, CS was diagnosed in 11 (34 %) subjects and 11/11 were subsequently diagnosed with extracardiac sarcoidosis. Similar results were obtained in patients presenting with sustained VT of unknown etiology [116]. Nery et al. prospectively evaluated patients presenting with monomorphic VT of unknown etiology, excluding patients with idiopathic VT, ischemic VT, or known sarcoidosis. In their sample, 6 out of 14 (28 %) patients had CS as the underlying etiology. Based on these results, it is recommended to perform advanced cardiac imaging with FDG-PET or cardiac MRI in patients younger than 60 years old presenting with unexplained second- or third-degree atrioventricular block, and it could be useful in patients with VT of unknown etiology [99].
4.4 Future Directions
The rationale behind detection of patient with cardiac sarcoidosis is prevention of life-threatening arrhythmias and to preserve left ventricular function. Over the past decade, FDG-PET has been playing a central role in detecting cardiac sarcoidosis. Nevertheless, the current criteria used in the diagnosis of CS require further refinement. For example, an optimal pretest preparation protocol should be determined and image interpretation should be standardized. Additionally, understanding physiological uptake after preparation is central to improve accuracy. Some authors reported the possibility of FDG uptake in areas without inflammation due to regional substrate availability [92]. Furthermore, very little data is available on accuracy of FDG-PET in patients with insulin resistance. Given the high prevalence of diabetes and that corticosteroids can induce insulin resistance, many patients referred for FDG-PET imaging may present with insulin resistance. For this reason, understanding the effects of insulin resistance on FDG-PET accuracy and optimization of pretest preparation in these patients is necessary.
Although promising results have emerged so far, further prospective studies to establish the role of FDG uptake in therapy monitoring are needed. Using FDG-PET to differentiate between quiescent granulomas and active inflammation could be used to adjust therapy dosage, and decision to discontinue treatment could be based on assessment of inflammation rather than fixed time periods.
New hybrid imaging systems combining PET and MRI are now available, allowing combination of tissue characterization capabilities of MRI with PET. Two reports have highlighted the complementary role of PET and MRI in cardiac sarcoidosis with PET-MRI hybrid imaging [117, 118].
Although FDG-PET is an effective tool in the diagnosis of CS, FDG remains a nonspecific marker and interpretation of results should always be conducted in light of clinical information. Some authors have described combination of FDG with other PET tracers including 11C-methionine [119, 120], fluorine-18-alpha-methyltyrosine (FMT) [121], and 18F-fluorothymidine (FLT) [122] to improve specificity of FDG imaging in extracardiac sarcoidosis. Clinical trials are under way to assess the potential role of 68Ga-DOTANOC, a neuroendocrine tumor marker, in cardiac sarcoidosis. To this day, only anecdotal reports are available on imaging sarcoidosis with other tracers than FDG. In order to replace FDG in clinical practice, a new PET tracer would need to be more specific and less dependent on patient preparation and not require on-site cyclotron. Until then, FDG-PET remains an accurate and efficient way of imaging patients for sarcoidosis.
References
Boeck C. Multiple benign sarkoid of the skin. J Cutan Genitourinary Dis. 1899;17:543–50.
Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160:736–55.
Henke CE, Henke G, Elveback LR, et al. The epidemiology of sarcoidosis in Rochester, Minnesota: a population-based study of incidence and survival. Am J Epidemiol. 1986;123:840–5.
Bresnitz EA, Strom BL. Epidemiology of sarcoidosis. Epidemiol Rev. 1983;5:124–56.
Erdal BS, Clymer BD, Yildiz VO, et al. Unexpectedly high prevalence of sarcoidosis in a representative U.S. Metropolitan population. Respir Med. 2012;106:893–9.
Hennessy TW, Ballard DJ, DeRemee RA, et al. The influence of diagnostic access bias on the epidemiology of sarcoidosis: a population-based study in Rochester, Minnesota, 1935–1984. J Clin Epidemiol. 1988;41:565–70.
Buck AA, Sartwell PE. Epidemiologic investigations of sarcoidosis. II. Skin sensitivity and environmental factors. Am J Hyg. 1961;74:152–73.
Buck AA, McKusick VA. Epidemiologic investigations of sarcoidosis. III. Serum proteins; syphilis; association with tuberculosis: familial aggregation. Am J Hyg. 1961;74:174–88.
Buck AA. Epidemiologic investigations of sarcoidosis. IV. Discussion and summary. Am J Hyg. 1961;74:189–202.
Hills SE, Parkes SA, Baker SB. Epidemiology of sarcoidosis in the Isle of Man–2: Evidence for space-time clustering. Thorax. 1987;42:427–30.
Prezant DJ, Dhala A, Goldstein A, et al. The incidence, prevalence, and severity of sarcoidosis in New York City firefighters. Chest. 1999;116:1183–93.
Kajdasz DK, Lackland DT, Mohr LC, Judson MA. A current assessment of rurally linked exposures as potential risk factors for sarcoidosis. Ann Epidemiol. 2001;11:111–7.
Kucera GP, Rybicki BA, Kirkey KL, et al. Occupational risk factors for sarcoidosis in African-American siblings. Chest. 2003;123:1527–35.
Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007;357:2153–65.
Baughman RP, Lower EE. Novel therapies for sarcoidosis. Semin Respir Crit Care Med. 2007;28:128–33.
Studdy PR, Bird R. Serum angiotensin converting enzyme in sarcoidosis–its value in present clinical practice. Ann Clin Biochem. 1989;26(Pt 1):13–8.
Prabhakar HB, Rabinowitz CB, Gibbons FK, et al. Imaging features of sarcoidosis on MDCT, FDG PET, and PET/CT. AJR Am J Roentgenol. 2008;190:S1–6.
Scadding JG. Prognosis of intrathoracic sarcoidosis in England. A review of 136 cases after five years’ observation. Br Med J. 1961;2:1165–72.
Baughman RP, Shipley R, Desai S, et al. Changes in chest roentgenogram of sarcoidosis patients during a clinical trial of infliximab therapy: comparison of different methods of evaluation. Chest. 2009;136:526–35.
Braun JJ, Kessler R, Constantinesco A, Imperiale A. 18 F-FDG PET/CT in sarcoidosis management: review and report of 20 cases. Eur J Nucl Med Mol Imaging. 2008;35:1537–43.
Abrar ML, Agrawal K, Bhattacharya A, Mittal BR. Diffuse lung uptake in stress myocardial perfusion scintigraphy with Thallium-201 in a patient with sarcoidosis. Indian J Nucl Med. 2013;28:57.
Lebtahi R, Crestani B, Belmatoug N, et al. Somatostatin receptor scintigraphy and gallium scintigraphy in patients with sarcoidosis. J Nucl Med. 2001;42:21–6.
Beaumont D, Herry JY, Sapene M, et al. Gallium-67 in the evaluation of sarcoidosis: correlations with serum angiotensin-converting enzyme and bronchoalveolar lavage. Thorax. 1982;37:11–8.
Klech H, Kohn H, Kummer F, Mostbeck A. Assessment of activity in sarcoidosis. Sensitivity and specificity of 67Gallium scintigraphy, serum ACE levels, chest roentgenography, and blood lymphocyte subpopulations. Chest. 1982;82:732–8.
Nishiyama Y, Yamamoto Y, Fukunaga K, et al. Comparative evaluation of 18 F-FDG PET and 67Ga scintigraphy in patients with sarcoidosis. J Nucl Med. 2006;47:1571–6.
Phelps ME, Hoffman EJ, Selin C, et al. Investigation of [18 F]2-fluoro-2-deoxyglucose for the measure of myocardial glucose metabolism. J Nucl Med. 1978;19:1311–9.
Basu S, Zhuang H, Torigian DA, et al. Functional imaging of inflammatory diseases using nuclear medicine techniques. Semin Nucl Med. 2009;39:124–45.
Mañá J. Nuclear imaging. 67Gallium, 201thallium, 18 F-labeled fluoro-2-deoxy-D-glucose positron emission tomography. Clin Chest Med. 1997;18:799–811.
Cremers JP, Van Kroonenburgh MJ, Mostard RL, et al. Extent of disease activity assessed by 18 F-FDG PET/CT in a Dutch sarcoidosis population. Sarcoidosis Vasc Diffuse Lung Dis. 2014;31:37–45.
Ambrosini V, Zompatori M, Fasano L, et al. 18 F-FDG PET/CT for the assessment of disease extension and activity in patients with sarcoidosis: results of a preliminary prospective study. Clin Nucl Med. 2013;38:e171–7.
Sobic-Saranovic D, Grozdic I, Videnovic-Ivanov J, et al. The utility of 18 F-FDG PET/CT for diagnosis and adjustment of therapy in patients with active chronic sarcoidosis. J Nucl Med. 2012;53:1543–9.
De Vries J, Rothkrantz-Kos S, van Dieijen-Visser MP, Drent M. The relationship between fatigue and clinical parameters in pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2004;21:127–36.
Schoenberger CI, Line BR, Keogh BA, et al. Lung inflammation in sarcoidosis: comparison of serum angiotensin-converting enzyme levels with bronchoalveolar lavage and gallium-67 scanning assessment of the T lymphocyte alveolitis. Thorax. 1982;37:19–25.
Nunes H, Brillet P-Y, Valeyre D, et al. Imaging in sarcoidosis. Semin Respir Crit Care Med. 2007;28:102–20.
Mostard RLM, Vöö S, van Kroonenburgh MJPG, et al. Inflammatory activity assessment by F18 FDG-PET/CT in persistent symptomatic sarcoidosis. Respir Med. 2011;105:1917–24.
Drent M, Mansour K, Linssen C. Bronchoalveolar lavage in sarcoidosis. Semin Respir Crit Care Med. 2007;28:486–95.
Bjermer L, Rosenhall L, Angström T, Hällgren R. Predictive value of bronchoalveolar lavage cell analysis in sarcoidosis. Thorax. 1988;43:284–8.
Keogh BA, Hunninghake GW, Line BR, Crystal RG. The alveolitis of pulmonary sarcoidosis. Evaluation of natural history and alveolitis-dependent changes in lung function. Am Rev Respir Dis. 1983;128:256–65.
Lin YH, Haslam PL, Turner-Warwick M. Chronic pulmonary sarcoidosis: relationship between lung lavage cell counts, chest radiograph, and results of standard lung function tests. Thorax. 1985;40:501–7.
Turner-Warwick M, McAllister W, Lawrence R, et al. Corticosteroid treatment in pulmonary sarcoidosis: do serial lavage lymphocyte counts, serum angiotensin converting enzyme measurements, and gallium-67 scans help management? Thorax. 1986;41:903–13.
Treglia G, Taralli S, Giordano A. Emerging role of whole-body 18 F-fluorodeoxyglucose positron emission tomography as a marker of disease activity in patients with sarcoidosis: a systematic review. Sarcoidosis Vasc Diffuse Lung Dis. 2011;28:87–94.
Brudin LH, Valind SO, Rhodes CG, et al. Fluorine-18 deoxyglucose uptake in sarcoidosis measured with positron emission tomography. Eur J Nucl Med. 1994;21:297–305.
Teirstein AS, Machac J, Almeida O, et al. Results of 188 whole-body fluorodeoxyglucose positron emission tomography scans in 137 patients with sarcoidosis. Chest. 2007;132:1949–53.
Sobic-Saranovic DP, Grozdic IT, Videnovic-Ivanov J, et al. Responsiveness of FDG PET/CT to treatment of patients with active chronic sarcoidosis. Clin Nucl Med. 2013;38:516–21.
Imperiale A, Riehm S, Braun JJ. Interest of [18 F]FDG PET/CT for treatment efficacy assessment in aggressive phenotype of sarcoidosis with special emphasis on sinonasal involvement. Q J Nucl Med Mol Imaging. 2013;57:177–86.
Keijsers RGM, Verzijlbergen JF, van Diepen DM, et al. 18 F-FDG PET in sarcoidosis: an observational study in 12 patients treated with infliximab. Sarcoidosis Vasc Diffuse Lung Dis. 2008;25:143–9.
Milman N, Graudal N, Loft A, et al. Effect of the TNF-α inhibitor adalimumab in patients with recalcitrant sarcoidosis: a prospective observational study using FDG-PET. Clin Respir J. 2012;6:238–47.
Silverman KJ, Hutchins GM, Bulkley BH. Cardiac sarcoid: a clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation. 1978;58:1204–11.
Matsui Y, Iwai K, Tachibana T, et al. Clinicopathological study of fatal myocardial sarcoidosis. Ann N Y Acad Sci. 1976;278:455–69.
Tadamura E, Yamamuro M, Kubo S, et al. Effectiveness of delayed enhanced MRI for identification of cardiac sarcoidosis: comparison with radionuclide imaging. AJR Am J Roentgenol. 2005;185:110–5.
Sekhri V, Sanal S, Delorenzo LJ, et al. Cardiac sarcoidosis: a comprehensive review. Arch Med Sci. 2011;7:546–54.
Roberts WC, McAllister HA, Ferrans VJ. Sarcoidosis of the heart. A clinicopathologic study of 35 necropsy patients (group 1) and review of 78 previously described necropsy patients (group 11). Am J Med. 1977;63:86–108.
Banba K, Kusano KF, Nakamura K, et al. Relationship between arrhythmogenesis and disease activity in cardiac sarcoidosis. Heart Rhythm. 2007;4:1292–9.
Kandolin R, Lehtonen J, Kupari M. Cardiac sarcoidosis and giant cell myocarditis as causes of atrioventricular block in young and middle-aged adults. Circ Arrhythm Electrophysiol. 2011;4:303–9.
Nery PB, Beanlands RS, Nair GM, et al. Atrioventricular block as the initial manifestation of cardiac sarcoidosis in middle-aged adults. J Cardiovasc Electrophysiol. 2014;25:875–81.
Yazaki Y, Isobe M, Hiroe M, et al. Prognostic determinants of long-term survival in Japanese patients with cardiac sarcoidosis treated with prednisone. Am J Cardiol. 2001;88:1006–10.
Chapelon-Abric C, de Zuttere D, Duhaut P, et al. Cardiac sarcoidosis: a retrospective study of 41 cases. Medicine (Baltimore). 2004;83:315–34.
Kato Y, Morimoto S, Uemura A, et al. Efficacy of corticosteroids in sarcoidosis presenting with atrioventricular block. Sarcoidosis Vasc Diffuse Lung Dis. 2003;20:133–7.
Yodogawa K, Seino Y, Ohara T, et al. Effect of corticosteroid therapy on ventricular arrhythmias in patients with cardiac sarcoidosis. Ann Noninvasive Electrocardiol. 2011;16:140–7.
Doughan AR, Williams BR. Cardiac sarcoidosis. Heart. 2006;92:282–8.
Winters SL, Cohen M, Greenberg S, et al. Sustained ventricular tachycardia associated with sarcoidosis: assessment of the underlying cardiac anatomy and the prospective utility of programmed ventricular stimulation, drug therapy and an implantable antitachycardia device. J Am Coll Cardiol. 1991;18:937–43.
Sadek MM, Yung D, Birnie DH, et al. Corticosteroid therapy for cardiac sarcoidosis: a systematic review. Can J Cardiol. 2013;29:1034–41.
Chiu C-Z, Nakatani S, Zhang G, et al. Prevention of left ventricular remodeling by long-term corticosteroid therapy in patients with cardiac sarcoidosis. Am J Cardiol. 2005;95:143–6.
Jefic D, Joel B, Good E, et al. Role of radiofrequency catheter ablation of ventricular tachycardia in cardiac sarcoidosis: report from a multicenter registry. Heart Rhythm. 2009;6:189–95.
Futamatsu H, Suzuki J, Adachi S, et al. Utility of gallium-67 scintigraphy for evaluation of cardiac sarcoidosis with ventricular tachycardia. Int J Cardiovasc Imaging. 2006;22:443–8.
Hiramitsu S, Morimoto S, Uemura A, et al. National survey on status of steroid therapy for cardiac sarcoidosis in Japan. Sarcoidosis Vasc Diffuse Lung Dis. 2005;22:210–3.
Liedtke AJ. Alterations of carbohydrate and lipid metabolism in the acutely ischemic heart. Prog Cardiovasc Dis. 1981;23:321–36.
Camici P, Ferrannini E, Opie LH. Myocardial metabolism in ischemic heart disease: basic principles and application to imaging by positron emission tomography. Prog Cardiovasc Dis. 1989;32:217–38.
Stanley WC, Lopaschuk GD, Hall JL, McCormack JG. Regulation of myocardial carbohydrate metabolism under normal and ischaemic conditions. Potential for pharmacological interventions. Cardiovasc Res. 1997;33:243–57.
Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85:1093–129.
Wisneski JA, Gertz EW, Neese RA, Mayr M. Myocardial metabolism of free fatty acids. Studies with 14C-labeled substrates in humans. J Clin Invest. 1987;79:359–66.
Ohira H, Tsujino I, Yoshinaga K. 18 F-Fluoro-2-deoxyglucose positron emission tomography in cardiac sarcoidosis. Eur J Nucl Med Mol Imaging. 2011;38:1773–83.
Yamagishi H, Shirai N, Takagi M, et al. Identification of cardiac sarcoidosis with (13)N-NH(3)/(18)F-FDG PET. J Nucl Med. 2003;44:1030–6.
Youssef G, Leung E, Mylonas I, et al. The use of 18 F-FDG PET in the diagnosis of cardiac sarcoidosis: a systematic review and metaanalysis including the Ontario experience. J Nucl Med. 2012;53:241–8.
Langah R, Spicer K, Gebregziabher M, Gordon L. Effectiveness of prolonged fasting 18f-FDG PET-CT in the detection of cardiac sarcoidosis. J Nucl Cardiol. 2009;16:801–10.
Ishida Y, Yoshinaga K, Miyagawa M, et al. Recommendations for (18)F-fluorodeoxyglucose positron emission tomography imaging for cardiac sarcoidosis: Japanese Society of Nuclear Cardiology recommendations. Ann Nucl Med. 2014;28:393–403.
Frayn KN. The glucose-fatty acid cycle: a physiological perspective. Biochem Soc Trans. 2003;31:1115–9.
Cheng VY, Slomka PJ, Ahlen M, et al. Impact of carbohydrate restriction with and without fatty acid loading on myocardial 18 F-FDG uptake during PET: a randomized controlled trial. J Nucl Cardiol. 2010;17:286–91.
Williams G, Kolodny GM. Suppression of myocardial 18 F-FDG uptake by preparing patients with a high-fat, low-carbohydrate diet. AJR Am J Roentgenol. 2008;190:W151–6.
Harisankar CNB, Mittal BR, Agrawal KL, et al. Utility of high fat and low carbohydrate diet in suppressing myocardial FDG uptake. J Nucl Cardiol. 2011;18:926–36.
Soussan M, Brillet P-Y, Nunes H, et al. Clinical value of a high-fat and low-carbohydrate diet before FDG-PET/CT for evaluation of patients with suspected cardiac sarcoidosis. J Nucl Cardiol. 2013;20:120–7.
Lum DP, Wandell S, Ko J, Coel MN. Reduction of myocardial 2-deoxy-2-[18 F]fluoro-D-glucose uptake artifacts in positron emission tomography using dietary carbohydrate restriction. Mol Imaging Biol. 2002;4:232–7.
Wykrzykowska J, Lehman S, Williams G, et al. Imaging of inflamed and vulnerable plaque in coronary arteries with 18 F-FDG PET/CT in patients with suppression of myocardial uptake using a low-carbohydrate, high-fat preparation. J Nucl Med. 2009;50:563–8.
Coulden R, Chung P, Sonnex E, et al. Suppression of myocardial 18 F-FDG uptake with a preparatory “Atkins-style” low-carbohydrate diet. Eur Radiol. 2012;22:2221–8.
Kobayashi Y, Kumita S, Fukushima Y, et al. Significant suppression of myocardial 18 F-fluorodeoxyglucose uptake using 24-h carbohydrate restriction and a low-carbohydrate, high-fat diet. J Cardiol. 2013;62:314–9.
Nuutila P, Koivisto VA, Knuuti J, et al. Glucose-free fatty acid cycle operates in human heart and skeletal muscle in vivo. J Clin Invest. 1992;89:1767–74.
Persson E. Lipoprotein lipase, hepatic lipase and plasma lipolytic activity. Effects of heparin and a low molecular weight heparin fragment (Fragmin). Acta Med Scand. 1988;724(Suppl):1–56.
Ohira H, Tsujino I, Ishimaru S, et al. Myocardial imaging with 18 F-fluoro-2-deoxyglucose positron emission tomography and magnetic resonance imaging in sarcoidosis. Eur J Nucl Med Mol Imaging. 2008;35:933–41.
Ishimaru S, Tsujino I, Takei T, et al. Focal uptake on 18 F-fluoro-2-deoxyglucose positron emission tomography images indicates cardiac involvement of sarcoidosis. Eur Heart J. 2005;26:1538–43.
Morooka M, Moroi M, Uno K, et al. Long fasting is effective in inhibiting physiological myocardial 18 F-FDG uptake and for evaluating active lesions of cardiac sarcoidosis. EJNMMI Res. 2014;4:1.
Strauss HW, Miller DD, Wittry MD, et al. Procedure guideline for myocardial perfusion imaging 3.3. J Nucl Med Technol. 2008;36:155–61.
Tahara N, Tahara A, Nitta Y, et al. Heterogeneous myocardial FDG uptake and the disease activity in cardiac sarcoidosis. JACC Cardiovasc Imaging. 2010;3:1219–28.
Manabe O, Ohira H, Yoshinaga K, et al. Elevated (18)F-fluorodeoxyglucose uptake in the interventricular septum is associated with atrioventricular block in patients with suspected cardiac involvement sarcoidosis. Eur J Nucl Med Mol Imaging. 2013;40:1558–66.
Skali H, Schulman AR, Dorbala S. 18 F-FDG PET/CT for the assessment of myocardial sarcoidosis. Curr Cardiol Rep. 2013;15:1–11.
Blankstein R, Osborne M, Naya M, et al. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol. 2014;63:329–36.
Diagnostic standard and guidelines for sarcoidosis. Jpn J Sarcoidosis Granulomatous Disorders [in Japanese]. 2007;27:89–102.
Deng JC, Baughman RP, Lynch JP. Cardiac involvement in sarcoidosis. Semin Respir Crit Care Med. 2002;23:513–27.
Patel MR, Cawley PJ, Heitner JF, et al. Detection of myocardial damage in patients with sarcoidosis. Circulation. 2009;120:1969–77.
Birnie DH, Sauer WH, Bogun F, et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014;11:1305–23.
Cooper LT, Baughman KL, Feldman AM, et al. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Circulation. 2007;116:2216–33.
Ardehali H, Howard DL, Hariri A, et al. A positive endomyocardial biopsy result for sarcoid is associated with poor prognosis in patients with initially unexplained cardiomyopathy. Am Heart J. 2005;150:459–63.
Okumura W, Iwasaki T, Toyama T, et al. Usefulness of fasting 18 F-FDG PET in identification of cardiac sarcoidosis. J Nucl Med. 2004;45:1989–98.
Khandani AH, Isasi CR, Donald Blaufox M. Intra-individual variability of cardiac uptake on serial whole-body 18 F-FDG PET. Nucl Med Commun. 2005;26:787–91.
Gropler RJ, Siegel BA, Lee KJ, et al. Nonuniformity in myocardial accumulation of fluorine-18-fluorodeoxyglucose in normal fasted humans. J Nucl Med. 1990;31:1749–56.
De Keizer B, Scholtens AM, van Kimmenade RRJ, de Jong PA. High FDG uptake in the right ventricular myocardium of a pulmonary hypertension patient. J Am Coll Cardiol. 2013;62:1724.
Can MM, Kaymaz C, Tanboga IH, et al. Increased right ventricular glucose metabolism in patients with pulmonary arterial hypertension. Clin Nucl Med. 2011;36:743–8.
Osborne MT, Hulten EA, Singh A, et al. Reduction in 18 F-fluorodeoxyglucose uptake on serial cardiac positron emission tomography is associated with improved left ventricular ejection fraction in patients with cardiac sarcoidosis. J Nucl Cardiol. 2014;21:166–74.
Fleming HA, Bailey SM. The prognosis of sarcoid heart disease in the United Kingdom. Ann N Y Acad Sci. 1986;465:543–50.
Mehta D, Lubitz SA, Frankel Z, et al. Cardiac involvement in patients with sarcoidosis: diagnostic and prognostic value of outpatient testing. Chest. 2008;133:1426–35.
Judson MA, Baughman RP, Teirstein AS, et al. Defining organ involvement in sarcoidosis: the ACCESS proposed instrument. ACCESS Research Group. A Case Control Etiologic Study of Sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16:75–86.
Mc Ardle BA, Birnie DH, Klein R, et al. Is there an association between clinical presentation and the location and extent of myocardial involvement of cardiac sarcoidosis as assessed by 18 F- fluorodoexyglucose positron emission tomography? Circ Cardiovasc Imaging. 2013;6:617–26.
Takeda N, Yokoyama I, Hiroi Y, et al. Positron emission tomography predicted recovery of complete A-V nodal dysfunction in a patient with cardiac sarcoidosis. Circulation. 2002;105:1144–5.
Pandya C, Brunken RC, Tchou P, et al. Detecting cardiac involvement in sarcoidosis: a call for prospective studies of newer imaging techniques. Eur Respir J. 2007;29:418–22.
Uusimaa P, Ylitalo K, Anttonen O, et al. Ventricular tachyarrhythmia as a primary presentation of sarcoidosis. Eurospace. 2008;10:760–6.
Nery PB, Leung E, Birnie DH. Arrhythmias in cardiac sarcoidosis: diagnosis and treatment. Curr Opin Cardiol. 2012;27:181–9.
Nery PB, Mc Ardle BA, Redpath CJ, et al. Prevalence of cardiac sarcoidosis in patients presenting with monomorphic ventricular tachycardia. Pacing Clin Electrophysiol. 2014;37:364–74.
White JA, Rajchl M, Butler J, et al. Active cardiac sarcoidosis: first clinical experience of simultaneous positron emission tomography–magnetic resonance imaging for the diagnosis of cardiac disease. Circulation. 2013;127:e639–41.
Schneider S, Batrice A, Rischpler C, et al. Utility of multimodal cardiac imaging with PET/MRI in cardiac sarcoidosis: implications for diagnosis, monitoring and treatment. Eur Heart J. 2014;35:312.
Yamada Y, Uchida Y, Tatsumi K, et al. Fluorine-18-fluorodeoxyglucose and carbon-11-methionine evaluation of lymphadenopathy in sarcoidosis. J Nucl Med. 1998;39:1160–6.
Hain SF, Beggs AD. C-11 methionine uptake in granulomatous disease. Clin Nucl Med. 2004;29:585–6.
Kaira K, Oriuchi N, Otani Y, et al. Diagnostic usefulness of fluorine-18-alpha-methyltyrosine positron emission tomography in combination with 18 F-fluorodeoxyglucose in sarcoidosis patients. Chest. 2007;131:1019–27.
Kim S-K, Im HJ, Kim W, et al. F-18 fluorodeoxyglucose and F-18 fluorothymidine positron emission tomography/computed tomography imaging in a case of neurosarcoidosis. Clin Nucl Med. 2010;35:67–70.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Pelletier-Galarneau, M., Mc Ardle, B., Ohira, H., Leung, E., Ruddy, T.D. (2015). Role of PET/CT in Assessing Cardiac Sarcoidosis. In: Schindler, T., George, R., Lima, J. (eds) Molecular and Multimodality Imaging in Cardiovascular Disease. Springer, Cham. https://doi.org/10.1007/978-3-319-19611-4_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-19611-4_4
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-19610-7
Online ISBN: 978-3-319-19611-4
eBook Packages: MedicineMedicine (R0)