Abstract
Purpose of review
The non-specific symptom profile and subclinical nature of disease along with variable region of cardiac involvement in systemic sarcoidosis make the diagnosis particularly challenging. The yield of endomyocardial biopsy, a gold standard for diagnosis, is not high unless coupled with additional imaging modalities to detect regional involvement. This review is focused on highlighting the major recent advances in imaging modalities and diagnosis of cardiac sarcoidosis.
Recent findings
There has been much interest and increasing research focused on developing newer and improved imaging modalities to establish diagnosis. CMR and 18F- FDG-PET are now considered imaging modalities of choice in most centers worldwide, but the data comparing both methodologies head-to-head is limited. Nevertheless, novel radiotracers (i.e. 68Ga-DOTANOC, 18F-Flurpiridaz, 13N-Ammonia) and hybrid combination PET/CMR imaging are coming to spotlight with improved sensitivity and specificity for earlier detection of myocardial sarcoid.
Summary
As CMR and PET are showing increased utilization in cardiac sarcoidosis, 201Th-SPECT, 99mTc MDP SPECT, 67Ga Scintigraphy, and 82Rb PET are falling out of favor. Newer imaging modalities, radionuclide tracers, and hybrid PET/CMR combinations have been promising in better detecting cardiac sarcoidosis and are currently being evaluated in larger trials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sarcoidosis has been present as a clinical entity over last 150 years. It was first described by Hutchinson in 1869 as a cutaneous manifestation [1]. The disease was named 30 years later, when Caesar Boeck, a Norwegian dermatologist in 1899, described nodular skin lesions of epithelioid cells. He named the cells “sarcoid” as he thought the appearance was similar to sarcoma cells [2]. Over time, knowledge about the disease process grew through contributions of numerous physicians and scientists. It was discovered that the disease’s hallmark was inflammation and granuloma formation in multiple organ systems. Cardiac sarcoidosis (CS) was first described by Bernstein in 1929, when he incidentally found epicardial granulomas in a post-mortem patient which were identical to their skin granulomas [3]. In 1937, G. Gentzen reported the first death directly attributed to myocardial sarcoidosis [4]. Subsequently, a case of myocardial sarcoidosis was diagnosed clinically and confirmed at autopsy by Adickes et al. [5]. There remains a paucity of data regarding different aspects of cardiac sarcoidosis and some may argue that the lack of information is due to the under-diagnosis of this condition. Additionally, the non-specific nature of symptoms and subclinical nature of disease often hinder prompt diagnosis.
The prevalence of CS amongst patients with systemic sarcoidosis has been reported to be 20 to 27% in the USA and as high as 58% in Japan [6,7,8]. Yet, autopsy studies indicate that subclinical cardiac involvement may be present in up to 80% of cases [9]. In the USA, 13 to 25% of deaths from sarcoidosis have been attributed to CS, while in Japan, 47 to 85% of deaths from sarcoidosis have been attributed to cardiac involvement [10].
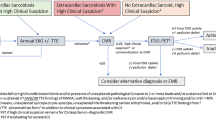
The diagnosis of CS has traditionally been confirmed by endomyocardial biopsy (EMB) from the affected region, with demonstration of non-caseating granulomas. However, the diagnosis becomes challenging due to variation in extent and distribution of affected region. Most of the data regarding distribution and extent of lesions are based on autopsy findings and establishing a diagnosis in the living remains difficult [11,12,13]. No single diagnostic imaging study has been identified to have sufficient sensitivity and specificity to reliably confirm or rule out CS. Although the imaging has been increasingly used, expert consensus still refers to EMB as the gold standard for diagnosis of CS.
The healthcare burden carried by sarcoidosis patients remains substantial. In a recent US-based population study of patients with sarcoidosis, the mean annual associated health care cost was $119,878 for patients in the 95th–99th percentile and $375,436 for patients in the top percentile, with higher-cost patients being associated with high odds-ratio (OR) of cardiac arrhythmia (OR 1.493; P < 0.001), having an inpatient admission (OR = 9.771; P < 0.001) and use of biologic therapies [14]. Therefore, an intelligent radio-pathologic algorithm is fundamental to an early and prompt diagnosis of this condition. There has been considerable progress in imaging studies over the recent years. These advances in imaging may not only allow for earlier diagnosis of CS but also for clinicians to follow therapeutic response. This review aims to highlight the most recent advances made in cardiac imaging for establishing a diagnosis and their utility in tracking therapeutic responses in patients with cardiac sarcoidosis.
Diagnostic imaging techniques
A. Two-dimensional (2D) echocardiography
Considering the advancements in modern medicine, cardiac imaging has now become integral to the diagnosis of CS [15]. Two-dimensional Doppler echocardiography is non-invasive, readily available at many institutions, and relatively inexpensive compared to other modalities. These characteristics make it an attractive initial screening test. Echocardiography offers detailed structural and functional properties of the myocardium and is the initial imaging study of choice for evaluation of patients with suspected or known CS [16, 17••]. Among patients with an established diagnosis, a majority have pathologic abnormalities [18, 19] on echocardiography including but not limited to hypokinesia, dyskinesia, [20, 21] chamber dilation, reduced ejection fraction, structural valvular regurgitation, and wall thinning or thickening [18, 20,21,22]. Specifically, localized thinning of the basal interventricular septum is present in approximately 90% of patients with CS [9]. Ventricular aneurysms, which commonly affect the infero-posterior wall in this patient population, can easily be distinguished from ischemic aneurysms, as the abnormalities do not follow typical coronary artery distributions.
Left ventricular systolic and diastolic dysfunction are also a common finding, seen in 32% [23] and 14% [19] of patients respectively. Diastolic dysfunction is more common amongst patients who also suffer from pulmonary sarcoid involvement; likewise, early mitral annular tissue velocity of the septal wall has been shown to be lower in this population [24]. Left ventricular systolic dysfunction, when present, may be global, segmental, or regional due to the variable granulomatous involvement of the myocardium. Regional wall motion abnormalities are also common, typically affecting the anterior and apical segments [21]. Longitudinal strain of the left ventricle may be a valuable predictor of therapeutic response as well as adverse outcomes as demonstrated in one small retrospective study of 117 patients in Greece [25, 26]. Right ventricular dysfunction, which may be due to direct granulomatous inflammation of the myocardium but most commonly secondary to pulmonary hypertension from sarcoid lung involvement, is present in up to 5.7% of patients [27].
Pericardial manifestations are not uncommon, with effusions occurring in 19% of patients [28]. These effusions are typically hemodynamically insignificant with rare occurrences of cardiac tamponade. Constrictive pericarditis may develop as well [29].
Valvular dysfunction may be also present and is typically classified as primary or secondary. Primary dysfunction results from direct granulomatous involvement of the valve leaflets. Secondary valvular dysfunction may result from a change in left ventricular geometry, with mitral regurgitation being most prevalent lesion [30]. Valvular lesions, are helpful in diagnosing CS but are unfortunately non-specific and can be associated with a myriad of other cardiac etiologies.
More specific findings, such as granulomatous inflammation, can also be detected on echocardiography via macroscopic echogenic lesions referred to as having a “snowstorm-speckled” pattern [7, 31]. Tissue Doppler derived global and left ventricular longitudinal strain patterns can be detected earlier with greater sensitivity using speckle tracking strain analysis [32,33,34,35] along with techniques like pulmonary capillary contrast assessment and cycle-dependent variation of myocardial integrated backscatter analysis [36]. Backscatter analysis specifically measures acoustic properties within the myocardium, which allows for differentiation between affected and normal tissue and is able to detect these abnormalities before conventional echocardiography [36].
Overall echocardiography is a valuable tool in the management of suspected or diagnosed CS. It is far from perfect however. Even in combination with electrocardiogram (ECG), ambulatory rhythm monitoring and thorough history taking, this imaging modality still has a relatively low sensitivity (85%) and specificity (55%) for diagnosing CS [37].
B. Cardiac magnetic resonance
Both the Heart Rhythm Society (HRS) expert consensus statement and a recent positional statement from the Cardiovascular and Inflammation and Infection Committees of the European Association of Nuclear Medicine, the European Association of Cardiovascular Imaging, and the American Society of Nuclear Cardiology recommend either cardiac magnetic resonance (CMR) or 18-fluorodeoxyglucose positron emission tomography (18F-FDG PET), for patients with any significant abnormality on echocardiography and suspected CS, with CMR being a preferred initial imaging choice [38, 39••]. Diagnosis of CS with CMR has a 100% sensitivity and specificity of 78%, greatly surpassing echocardiography [39••, 40].
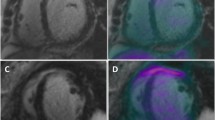
In a recent Danish study of 197 screened patients with systemic sarcoidosis, of patients with positive Japanese Ministry of Health and Welfare (JMHW) criteria, 53% were diagnosed by CMR alone compared to 29% with EMB [41]. Part of current diagnostic guidelines for CS, as published by the Heart Rhythm Society, includes late gadolinium enhancement (LGE) on CMR which is present in 97% of patients diagnosed. Similar to 2D-echocardiography, CMR allows for increased image clarity without added radiation risks, as well as the ability to differentiate between ischemic and non-ischemic lesions. Allergic reaction rates are also low, with only 0.005% of patients in a 37,788-patient cohort experiencing severe allergic reaction to gadolinium [42]. Additionally, in patients with a known glomerular filtration rate (GFR) greater than 30 ml/min, gadolinium contrast is considered to be low risk, and with recent advancements and development of newer contrast agents, reported complications (i.e., nephrogenic systemic fibrosis) are even lower [43]. Compared to echocardiography, CMR offers superior visualization of granulomatous infiltration, fibrosis, perfusion defects, and even microvasculitis [44]. As previously mentioned, the presence of LGE on CMR is a diagnostic criterion for CS in several established society guidelines. Late gadolinium enhancement is caused by delayed clearance of gadolinium in fibrotic myocardium [45]. The presence of myocardial scarring detected by LGE on CMR in a non-vascular distribution or in two orthogonal views without the presence of another known LGE process, like myocarditis or hypertrophic obstructive cardiomyopathy, is indicative of CS. The most prevalent myocardial fibrosis patterns detected as LGE on CMR in patients with diagnosed CS include sub-epicardial and mid-wall enhancements of the basal septum or inferolateral wall [46]. This modality of imaging also offers great detail of the right ventricle, often difficult to visualize using conventional 2D-echocardiography. It has been previously documented that right ventricular structural and functional changes may correlate directly with myocardial infiltration or be a result of pulmonary sarcoid involvement [47]. Isolated right ventricular involvement is rare, with large majority of patients rather having concomitant extensive left ventricular involvement. Right-sided involvement may often be patchy/multifocal [48], septal, or present with insertion point enhancement [49•]. Ventricular insertion point enhancement has been documented [50•] and directly correlates with pulmonary arterial pressures, right ventricular mass, volume, and ejection fraction. In cases of predominant right ventricular involvement, CS can be differentiated from arrhythmogenic right ventricular cardiomyopathy by several features: an older age of onset, a non-familiar pattern, wider QRS complexes, septal involvement with atrioventricular conduction disease, multiple arrhythmogenic foci, concomitant left ventricular disease, and the presence of mediastinal lymphadenopathy, all favoring sarcoid [51].
In diagnosis of CS, T2-weighted CMR sequences have been able to identify areas of definitive myocardial fibrosis for endomyocardial biopsy, increasing the successful detection of pathologic tissue [52]. Although LGE is not a diagnostic criterion by the modified JMHW guidelines, it has been shown that LGE is up to twice as sensitive than JHMW criteria [40, 49]. Additionally, presence of LGE on CMR was directly correlated with significantly higher rates of sudden cardiac death, ventricular arrhythmias, and all-cause mortality (11.9 vs 1.1%) in a meta-analysis of 10 studies (760 patients) [53]. Myocardial scarring identified on CMR via LGE has also been proven to be a potent independent risk factor for death, ventricular arrhythmias, and defibrillator discharges [54•]. Right ventricular involvement was also found to be predictive of ventricular tachyarrhythmias, probably because of higher coexisting left ventricular disease and resulting systolic dysfunction [48, 55, 56]. Newer techniques including circumferential strain (Ecc) and strain change per second (Ecc rate) to better detect focal myocardial damage have shown promising results in small patient samples, particularly in LGE-positive segments [57]. Given these mentioned findings, cardiac magnetic resonance imaging will remain a valuable tool in the evaluation process for suspected CS.
C. 18-fluorodeoxyglucose positron emission tomography
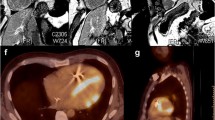
18-Fluorodeoxyglucose positron emission tomography or 18F-FDG PET takes advantage of the high glycolytic activity of the immunologic cells within cardiac sarcoid granulomas. 18-Fluorodeoxyglucose, a glucose analog, is retained within these metabolically active cells enabling high sensitivity imaging [58]. Unfortunately, the myocardium is also a very metabolically active tissue, requiring patient pre-imaging preparation to improve pathologic cellular detection. Many techniques have been developed to suppress normal myocardial tissue uptake of the 18F-FDG. Some strategies require high-fat and low-carbohydrate pre-imaging diets, intravenous unfractionated heparin administration, and long-term fasting. Additionally, 18F-FDG requires comparison-resting perfusion scans to determine active inflammation from scar tissue (SPECT or PET) and a low-dose CT scan for attenuation correction making this imaging modality a high radiation exposure option [59].
Despite several disadvantages and radiation risks associated with 18F-FDG PET, several studies have shown increased sensitivity and specificity when compared to other imaging modalities, including Gallium-67 uptake studies, Thallium-201 perfusion studies, and 99mTc imaging [60,61,62]. Some of its main advantages when compared with CMR are the ability to perform the tests in patients with implantable cardioverter-defibrillators and the ability to distinguish active inflammation from scar [63]. A recent small study out of France on patients with biopsy-proven sarcoidosis and suspected cardiac involvement reported 100% sensitivity and 91% specificity of FDG-PET/CT findings according to JMHW criteria [64].
There are limited studies comparing CMR to 18F-FDG PET, but one small study suggested that 18F-FDG PET has higher sensitivity (88%) when compared to CMR (75%). This study was quite small however, with only eight patients, and the results were not statistically significant [58, 65•]. A list of studies utilizing CMR and PET in patients with systemic sarcoidosis, known or suspected CS, and their respective sensitivities/specificities for cardiac involvement identification can be seen in Table 1. Initial reports also assume benefit of PET to reliably monitor disease progression and therapeutic monitoring of anti-inflammatory or immunosuppressive therapy [90] for CS, response to ablation therapy in ventricular arrhythmia patients using lesion metabolic activity (LMA) which correlated with a 20-fold higher risk of MACE in non-responders (p = 0.007) and may even parallel systolic function (p = 0.003) [91•], and association with higher adverse event rates in those with high-degree AVB [92]. Novel ECG parameters of ventricular remodeling (septal and inferolateral) and diffuse QRS fragmentation have also been found to have a strong association with myocardial FDG uptake on PET scans in sarcoidosis patients in a recent Finnish study of 133 patients [93]. FDG PET has also been shown to diagnose CS in asymptomatic patients [94] as this modality allows for detection of inflammation and infiltration via metabolic activity at the cellular level [95•]. In addition, in cases where myocardial LGE usually persists and T2-weighted edema CMR imaging may be unreliable, PET can reveal reduced FDG uptake that can signify a successful response to therapy in myocardial CS [96, 97].
Imaging classification for CS includes normal results (normal perfusion and 18F-FDG uptake), progressive disease (a moderate perfusion defect and increased 18F-FDG uptake), and fibrous disease (severe perfusion defect and minimal or no 18F-FDG uptake) [66]. Additionally, other quantitative techniques with 18F-FDG PET have been developed, like FDG-volume intensity detection, coined cardiac metabolic activity (CMA), which has been independently associated with adverse cardiac events in patients with CS [98•]. In summary, both imaging modalities, 18F-FDG PET and CMR, are very useful in CS and can be used in conjunction to diagnose and evaluate CS. Fibrosis can be identified as LGE on CMR, and inflammatory infiltration can be detected via increased 18F-FDG uptake on PET. Each modality can highlight different pathophysiologic processes in CS, and for this reason, both studies are valuable tools to diagnose and monitor the progression of the disease process.
D. Other imaging modalities: Thallium-201 myocardial perfusion single photon emission computed tomography (201Th-SPECT), technetium 99m-methylene diphosphonate single-photon emission computed tomography (99mTc MDP SPECT), 67-gallium citrate (67Ga) scintigraphy, and rubidium-82 positron emission computed tomography (82-Rb PET)
Like 18F-FDG PET, Thallium-201 myocardial perfusion single photon emission-computed tomography or 201Th-SPECT is a form of radionuclide imaging used in the evaluation of CS. 201Th-PET has a documented left ventricular perfusion defect detection rate of approximately 30% [22, 99] and has been also valuable in detection of abnormal right ventricular perfusion [100]. Similar to CMR and 18F-FDG PET, perfusion defects on 201Th-SPECT that do not follow coronary artery anatomy and exist in the presence of normal coronary studies are consistent with granulomatous disease [101]. Although this diagnostic process is similar to other myocardial perfusion imaging modalities, its reliability is questionable. There is evidence suggesting that microvascular vasoconstriction may be responsible for the perfusion defects seen on 201Th-SPECT, as reversibility of perfusion defects has been documented between post-injection rest and delayed imaging studies [101]. Evidence which depicts a relationship between abnormal 201Th-SPECT studies and clinical cardiac dysfunction is also lacking. Given this information, in the absence of clinical cardiac signs or symptoms, 201Th-SPECT is not recommended as a routine screening tool for CS.
There are other radionuclide imaging modalities available, although they have fallen out of favor in light of more sensitive and specific imaging techniques. Technetium 99m-methylene diphosphonate single photon emission-computed tomography (99mTc MDP SPECT) Scintigraphy and 67-Gallium Citrate (67Ga) Scintigraphy are amongst the sparsely used and now somewhat antiquated imaging techniques. Although 67Ga scintigraphy has been incorporated into the modified diagnostic criteria in the JMHW guidelines, most centers do not pursue such imaging, as studies have demonstrated its inferiority, in terms of sensitivity and diagnostic accuracy to 18F-FDG PET [102]. Similarly, perfusion abnormalities visualized on 99mTc MDP SPECT are not diagnostic of CS and their clinical significance has yet to be established [103].
Rubidium-82 positron emission computed tomography (82-Rb PET) is another less often used radionuclide imaging modality. It does offer some advantages when compared to when compared to 99mTc MDP SPECT. It has greater sensitivity (91%) and specificity (90%) with lower radiation exposure [104, 105]. Rest and stress 82-Rb PET imaging is roughly equivalent to the average person’s annual natural radiation exposure in the USA [105, 106]. Additionally, 82-Rb PET was able to outperform 99mTc MDP SPECT in terms of diagnostic accuracy in patients with body mass index greater than 30 kg/m2 and in women with large breast size, 85 and 67%, respectively [105, 107], and offer more information on myocardial blood flow (MBF) [108, 109], which may aid in the detection of inflammatory and fibrotic myocardial disease.
E. Novel PET tracers: 18F-flurpiridaz positron emission computed tomography (18F-Flurpiridaz PET), 13N-ammonia positron emission computed tomography (3N-Ammonia PET), and 68Ga-DOTANOC
Along with the advent of newer imaging modalities, novel radionuclide tracers have been developed which may greatly surpass their antiquated counterparts in terms of diagnostic accuracy for cardiac pathophysiology. 18F-Flurpiridaz positron emission computed tomography (18F-Flurpiridaz PET) and 13N-Ammonia positron emission computed tomography (13N-Ammonia PET) are two novel radionuclide tracers that are in development which may greatly aid in early detection of CS. 18F-Flurpiridaz binds to mitochondrial complex 1 which allows for high-resolution myocardial perfusion imaging (MPI) [110]. Similar to 82-Rb PET, 18F-Flurpiridaz PET offers enhanced clarity in obese patients or in women with large breasts, but with reduced radiation levels. Based on the clinical studies available, when compared to SPECT MPI which is the current standard of care, it appears that 18F-Flurpiridaz PET may provide higher resolution imaging, increased diagnostic certainty, reduced patient radiation exposure, and more accurate risk stratification [110,111,112]. 13N-Ammonia PET adds diagnostic value as it allows for quantification of myocardial flow reserve (MFR), particularly in patients with normal MPI which can potentially unmask clinically significant cardiac pathophysiology. In a Phase 2 trial focused on detection of coronary artery disease (> 50% stenosis by coronary angiography), 18F-Flurpiridaz had a higher sensitivity (78.8 vs 61.5%) and a stronger negative predictive value when compared to SPECT [112]. Considering their proposed increased detection rates of perfusion defects, it can be implied that these imaging modalities may offer potentially superior diagnostic accuracy for CS as perfusion defects (non-coronary artery distribution) are common. Currently, 18F-Flurpiridaz, 13N-Ammonia, in addition to other tracers (i.e., 11C-PBR28, F18-FSPG and DOTATAE) are undergoing further clinical trials investigating their potential use in CS patients and are demonstrated in Table 2.
Lastly, 68Ga-DOTANOC is an alternative radionucleotide tracer that binds to somatostatin receptors on inflammatory cells, specifically in sarcoid granulomas, which requires a less rigorous fasting protocol compared to 18F-FDG PET and has shown promising results in a small Danish study where 68Ga-DOTANOC tracer use yielded 100% diagnostic accuracy, proving it to be a viable alternative to 18F-FDG PET [113]. It has also shown greater sensitivity in disease activity monitoring when compared to conventional CMR; the reasoning being that LGE on CMR primarily depicts myocardial scarring, while 68Ga-DOTANOC is actively bound to activated macrophages, lymphocytes, and epithelioid cells. Unfortunately, with 68Ga-DOTANOC PET, immunosuppressive therapy at the time of imaging may alter results, increasing the rate of false-negative reports [114].
F. Simultaneous hybrid cardiac imaging: 18F-FDG PET/CMR
Recently, some centers have taken on a hybrid imaging approach by combining 18F-FDG PET with CMR [115]. A small single-center study (N = 51) compared simultaneous 18F-FDG PET and LGE on CMR to current accepted diagnostic imaging modalities (18F-FDG PET alone and CMR alone). This study found that simultaneous hybrid imaging was superior to conventional imaging in terms of sensitivity (94%), specificity (44%), positive predictive value (76%), and negative predictive value (80%). The sensitivity for 18F-FDG PET is 85% and LGE on CMR is 82%. The prevalence of CS in this patient population was 65% (N = 33) [88]. Simultaneous hybrid cardiac imaging allows for high-resolution morphologic and functional data collection via LGE-CMR [95•] while retaining the ability to assess for myocardial viability, perfusion, inflammatory metabolism processes, and abnormal cardiac deposition via 18F-FDG PET. Compared to PET-CT, 18F-FDG PET CMR offers many advantages, including attenuation correction without additional radiation, detection of edematous tissue, and improved image quality in regard to myocardial viability, perfusion, cardiac morphology, and function [95•]. Recent studies also predict that hybrid cardiac imaging may allow clinicians to differentiate between active and inactive disease, as both positive PET and LGE on MRI suggest active CS, while PET-negative and LGE MRI-positive imaging may correlate with inactive disease with residual fibrosis [95•]. This mentioned benefit may aid in the monitoring of CS and response to therapy [116]. As both 18F-FDG PET and CMR are indicated imaging studies for the evaluation of CS [83], and there is no clear consensus on which modality is superior [104, 117], simultaneous hybrid cardiac imaging should be considered.
Framework of combined PET/CMR studies establishes a new monumental mark in our role as imagers and caregivers for these patients. The combination of CMR and FDG-PET imaging may add significant incremental benefit for many patients. Vita et al. demonstrated this in a recent paper with utilization of CMR, FDG-PET, and combination of both in assessment of the likelihood of CS and guidance of patient management. The combination of FDG-PET and CMR image data reclassified 45% (high probable and higher likelihood groups) of patients compared with single-modality imaging [118•, 119]. On the contrary, Dweck et al. postulated in a recent prospective combined PET/MRI study only 32% of their patients with both PET and MRI (LGE)-positive tests to suffer from active CS. As mentioned previously, it is important to keep in mind that PET-negative findings with positive LGE in MRI can be rather considered as inactive CS with remaining scar [87, 95•].
Conclusion
Awareness about CS is growing with increased understanding of the disease process and case detection in autopsy studies. Due to the nature of cardiac involvement, diagnosis of CS remains a challenging task for the clinician. Even with detailed history and clinical exam with basic cardiac workup including ECG and transthoracic echocardiography, the diagnosis is frequently missed. This has prompted extensive research in cardiac imaging for prompt detection of cardiac involvement. There has been promising development in cardiac imaging studies in recent years, resulting in increasing pre-mortem identification of CS cases. CMR and 18F-FDG-PET have shown high sensitivity and specificity, making them the imaging modalities of choice. Newer imaging modalities with novel agents like 18F-Flurpiridaz PET, 13N-Ammonia PET, and 68Ga-DOTANOC are currently undergoing testing and have showed promise in initial small studies. However, further research is needed to establish a unified approach and increase detection rates of CS. Recent advancements in imaging techniques continue to shape the narrative on how best to diagnose this disease. At this point in time, there does not seem to be a gold standard non-invasive test that accomplishes the goals of being readily available, inexpensive, sensitive, and specific for diagnosis of CS.
Like many other diseases in medicine, CS requires awareness and clinical suspicion of the disease coupled with knowledge of the strengths and weaknesses of each imaging modality. This knowledge can help guide the clinician to order the best test or tests to make the diagnosis and monitor treatment of CS. As knowledge about the disease increases and diagnostic techniques are refined, one can hope for earlier diagnosis of the condition which hopefully will lead to better patient outcomes.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Doughan AR, Williams BR. Cardiac Sarcoidosis. Heart. 2006;92(2):282–8.
Boeck C. Multiple benign sarcoid of the skin. The Journal of Cutaneous and Genito-Urinary Diseases 1899;17:543–50.
Bernstein M, Konzlemann FW, Sidlick DM. Boeck’s sarcoid: report of a case with visceral involvement. Arch Intern Med. 1929;44:721–34.
Gentzen G. Über die Riesenzellen granulome bei zwei Fällen von Endokard-fibrose. Beitr. Path. Anat.. 1937;98:375–98.
Adickes GC, Zimmerman SL, Cardwell ES. Sarcoidosis with fatal cardiac involvement. Ann Intern Med. 1951;35:898–909.
Silverman KJ, Hutchins GM, Bulkley BH. Cardiac sarcoid: a clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation. 1978;58:1204–11.
Sharma OP, Maheshwari A, Thaker K. Myocardial sarcoidosis. Chest. 1993;103:253–8.
Matsui Y, Iwai K, Tachibana T, Fruie T, Shigematsu N, Izumi T, et al. Clinicopathological study of fatal myocardial sarcoidosis. Ann N Y Acad Sci. 1976;278:455–69.
Roberts WC, McAllister HA Jr, Ferrans VJ. Sarcoidosis of the heart: a clinicopathologic study of 35 necropsy patients (group 1) and review of 78 previously described necropsy patients (group 11). Am J Med. 1977;63:86–108.
Yigla M, Badarna-Abu-Ria N, Tov N, et al. Sarcoidosis in northern Israel; clinical characteristics of 120 patients. Sarcoidosis Vasc Diffuse Lung Dis. 2002;19:220.
Perry A, Vuitch F. Causes of death in patients with sarcoidosis: a morphologic study of 38 autopsies with clinicopathologic correlations. Arch Pathol Lab Med. 1995;119(2):167–72.
Bagwan IN, Hooper LV, Sheppard MN. Cardiac sarcoidosis and sudden death: the heart may look normal or mimic other cardiomyopathies. Virchows Arch. 2011;458(6):671–8.
Tavora F, Cresswell N, Li L, Ripple M, Solomon C, Burke A. Comparison of necropsy findings in patients with sarcoidosis dying suddenly from cardiac sarcoidosis versus dying suddenly from other causes. Am J Cardiol. 2009;104(4):571–7.
Bradford JR, White A, Lopez A, Nelson WW. High-cost sarcoidosis patients in the United States: patient characteristics and patterns of health care resource utilization. J Manag Care Spec Pharm. 2017;23(12):1261–9.
Lynch JP. III, Baughman RP, Deng JC. Cardiac involvement in sarcoidosis: evolving concepts in diagnosis and treatment. Semin Respir Crit Care Med. 2002;23(6).
Judson M, Costabel U, Drent M, et al. The WASOG sarcoidosis organ assessment instrument: an update of a previous clinical tool. Sarcoidosis Vasc Diffuse Lung Dis. 2014;31:19–27.
•• Birnie DH, Sauer WH, Bogun F, Cooper JM, Culver DA, Duvernoy CS, et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014;11(7):1304–23. https://doi.org/10.1016/j.hrthm.2014.03.043. The HRS Expert Consensus Statement recommends 2D-Echocardiography as the initial imaging study of choice for cardiac sarcoidosis as it provides structural and functional data.
Burstow DJ, Tajik A, Bailey KR, Deremee RA, Taliercio CP. Two-dimensional echocardiographic findings in systemic sarcoidosis. Am J Cardiol. 1989;63(7):478–82. https://doi.org/10.1016/0002-9149(89)90323-8.
Fahy GJ, Marwick T, Mccreery CJ, Quigley PJ, Maurer BJ. Doppler echocardiographic detection of left ventricular diastolic dysfunction in patients with pulmonary sarcoidosis. Chest. 1996;109(1):62–6. https://doi.org/10.1378/chest.109.1.62.
Gregor P, Widimsky P, Sladkova T, Petrikova J, Cervenka V, Visek V. Echocardiography in sarcoidosis. Jpn Heart J. 1984;25(4):499–508. https://doi.org/10.1536/ihj.25.499.
Yazaki Y, Isobe M, Hiramitsu S, Morimoto SI, Hiroe M, Omichi C, et al. Comparison of clinical features and prognosis of cardiac sarcoidosis and idiopathic dilated cardiomyopathy. Am J Cardiol. 1998;82(4):537–40. https://doi.org/10.1016/s0002-9149(98)00377-4.
Kinney EL, Jackson GL, Reeves WC, Zelis R. Thallium-scan myocardial defects and echocardiographic abnormalities in patients with sarcoidosis without clinical cardiac dysfunction. Am J Med. 1980;68(4):497–503. https://doi.org/10.1016/0002-9343(80)90292-2.
Chapelon-Abric C, Zuttere DD, Duhaut P, et al. Cardiac sarcoidosis. Medicine. 2004;83(6):315–34. https://doi.org/10.1097/01.md.0000145367.17934.75.
Kaderli AA, Gullulu S, Coskun F, Yilmaz D, Uzaslan E. Impaired left ventricular systolic and diastolic functions in patients with early grade pulmonary sarcoidosis. Eur J Echocardiogr. 2010;11(10):809–13. https://doi.org/10.1093/ejechocard/jeq070.
Elikowski W, Małek-Elikowska M, Świerkocki K, Wróblewski D, Bolewski A, Janus M. Utility of left ventricular longitudinal strain in the diagnosis and treatment monitoring of cardiac sarcoidosis. Pol Arch Intern Med. 2018;128(2):126–8. https://doi.org/10.20452/pamw.4212.
Felekos I, Aggeli C, Gialafos E, et al. Global longitudinal strain and long-term outcomes in asymptomatic extracardiac sarcoid patients with no apparent cardiovascular disease. Echocardiography. 2018. https://doi.org/10.1111/echo.13846.
Handa T, Nagai S, Miki S, Fushimi Y, Ohta K, Mishima M, et al. Incidence of pulmonary hypertension and its clinical relevance in patients with sarcoidosis. Chest. 2006;129(5):1246–52. https://doi.org/10.1378/chest.129.5.1246.
Kinney E, Murthy R, Ascunce G, Donohoe R, Zelts R. Pericardial effusions in sarcoidosis. Chest. 1979;76(4):476–8. https://doi.org/10.1378/chest.76.4.476.
Garrett J, Oneill H, Blake S. Constrictive pericarditis associated with sarcoidosis. Am Heart J. 1984;107(2):394. https://doi.org/10.1016/0002-8703(84)90394-6.
Fleming HA, Bailey SM. Sarcoid heart disease. J R Coll Physicians Lond. 1981;15(4):245–6. 9-53
Angomachalelis I, Kyriazis G, Angomachalelis N. Pericardial involvement frequently complicating pulmonary and/or systemic sarcoid granulomatosis: a lifelong, multistage disease, periodical study in 278 biopsy-proven patients. Chest. 2012;142(4):449A. https://doi.org/10.1378/chest.1388984.
Joyce E, Ninaber MK, Katsanos S, Debonnaire P, Kamperidis V, Bax JJ, et al. Subclinical left ventricular dysfunction by echocardiographic speckle-tracking strain analysis relates to outcome in sarcoidosis. Eur J Heart Fail. 2014;17(1):51–62. https://doi.org/10.1002/ejhf.205.
Pizarro C, Kluenker F, Hammerstingl C, Skowasch D. Diagnostic value of speckle-tracking echocardiography in confirmed cardiac sarcoidosis. Clin Res Cardiol. 2016;105(10):884–6. https://doi.org/10.1007/s00392-016-1004-y.
Schouver E-D, Moceri P, Doyen D, Tieulie N, Queyrel V, Baudouy D, et al. Early detection of cardiac involvement in sarcoidosis with 2-dimensional speckle-tracking echocardiography. Int J Cardiol. 2017;227:711–6. https://doi.org/10.1016/j.ijcard.2016.10.073.
Smedema J. Tissue Doppler imaging in cardiac sarcoidosis. Eur J Echocardiogr. 2008;9(4):579–80. https://doi.org/10.1093/ejechocard/jen073.
Hyodo E. Early detection of cardiac involvement in patients with sarcoidosis by a non-invasive method with ultrasonic tissue characterization. Heart. 2004;90(11):1275–80. https://doi.org/10.1136/hrt.2003.027763.
Perez IE, Garcia MJ, Taub CC. Multimodality imaging in cardiac sarcoidosis: is there a winner? Curr Cardiol Rev. 2016;12(1):3–11. https://doi.org/10.2174/1573403x11666150318110406.
Yufu K, Kondo H, Shinohara T, et al. Outcome of patients with cardiac sarcoidosis who received cardiac resynchronization therapy: comparison with dilated cardiomyopathy patients. J Cardiovasc Electrophysiol. 2016;28(2):177–81. https://doi.org/10.1111/jce.13119.
•• Slart RHJA, Glaudemans AWJM, Lancellotti P, Hyafil F, Blankstein R, Schwartz RG, et al. The joint procedural position statement from the cardiovascular, inflammation and infection committees of the European association of nuclear medicine, as well as the European association of cardiovascular imaging and the American society of nuclear cardiology, recommend imaging with FDG-PET or CMR after initial evaluation with echocardiography. J Nucl Cardiol. 2018;25(1):298–319 22 p. CMR is a very sensitive (100%) modality with relatively high specificity (78%) for cardiac involvement in sarcoidosis. Echocardiography in contrast has a sensitivity of 10% to 47% and a specificity of 82%-99% CMR can be used for both diagnosis and risk assessment of CS. The high-sensitivity of CMR makes it especially useful to exclude CS.
Smedema J, Snoep G, Kroonenburgh MPV, et al. Evaluation of the accuracy of gadolinium-enhanced cardiovascular magnetic resonance in the diagnosis of cardiac sarcoidosis. J Am Coll Cardiol. 2005;45(10):1683–90. https://doi.org/10.1016/j.jacc.2005.01.047.
Ghanizada M, Rossing K, Bundgaard H, Gustafsson F. Clinical presentation, management and prognosis of patients with cardiac sarcoidosis. Dan Med J. 2018;65(4).
Bruder O, Schneider S, Pilz G, van Rossum AC, Schwitter J, Nothnagel D, et al. 2015 update on acute adverse reactions to gadolinium based contrast agents in cardiovascular MR. large multi-national and multi-ethnical population experience with 37788 patients from the EuroCMR registry. J Cardiovasc Magn Reson. 2015;17(1):58. https://doi.org/10.1186/s12968-015-0168-3.
Reiter T, Ritter O, Prince MR, Nordbeck P, Wanner C, Nagel E, et al. Minimizing risk of nephrogenic systemic fibrosis in cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2012;14(1):31. https://doi.org/10.1186/1532-429X-14-31.
Ward EV, Nazari J, Edelman RR. Coronary artery vasculitis as a presentation of cardiac sarcoidosis. Circulation. 2012;125(6):e344–6. https://doi.org/10.1161/circulationaha.110.990747.
Blankstein R, Waller AH. Evaluation of known or suspected cardiac sarcoidosis. Circ Cardiovasc Imaging. 2016;9(3):e000867. https://doi.org/10.1161/circimaging.113.000867.
Okabe T, Yakushiji T, Hiroe M, Oyama Y, Igawa W, Ono M, et al. Steroid pulse therapy was effective for cardiac sarcoidosis with ventricular tachycardia and systolic dysfunction. ESC Heart Fail. 2016;3(4):288–92. https://doi.org/10.1002/ehf2.12095.
Hulten E, Aslam S, Osborne M, Abbasi S, Bittencourt MS, Blankstein R. Cardiac sarcoidosis—state of the art review. Cardiovasc Diagn Ther. 2016;6(1):50–63. https://doi.org/10.3978/j.issn.2223-3652.2015.12.13.
Crawford T, Mueller G, Sarsam S, Prasitdumrong H, Chaiyen N, Gu X, et al. Magnetic resonance imaging for identifying patients with cardiac sarcoidosis and preserved or mildly reduced left ventricular function at risk of ventricular arrhythmias. Circ Arrhythm Electrophysiol. 2014;7:1109–15.
• Patel MR, Cawley PJ, Heitner JF, Klem I, Parker MA, Jaroudi WA, et al. Detection of myocardial damage in patients with sarcoidosis. Circulation. 2009;120:1969–77. DE-CMR is more than twice as sensitive for cardiac sarcoidosis when compared to current consensus criteria and DE-CMR myocardial damage is associated with cardiac death.
• Smedema J, van Geuns R, Ainslie G, Ector J, Heidbuchel H, Crijns HJGM. Right ventricular involvement in cardiac sarcoidosis demonstrated with cardiac magnetic resonance. Esc Heart Fail. 2017;4(4):535–44. https://doi.org/10.1002/ehf2.12166. Right ventricular enhancement was detected in 16% of patients diagnosed pulmonary sarcoidosis and in 48% of patients with left ventricular enhancement and was directly linked with pulmonary arterial hypertension, right ventricular systolic dysfunction, hypertrophy and dilation.
Philips B, Madhavan S, James CA, te Riele ASJM, Murray B, Tichnell C, et al. Arrhythmogenic right ventricular dysplasia/cardiomyopathy and cardiac sarcoidosis. Distinguishing features when the diagnosis is unclear. Circ Arrhythm Electrophysiol. 2014;7:230–6.
Crouser ED, Ono C, Tran T, He X, Raman SV. Improved detection of cardiac sarcoidosis using magnetic resonance with myocardial T2 mapping. Am J Respir Crit Care Med. 2014;189(1):109–12. https://doi.org/10.1164/rccm.201309-1668LE.
Coleman GC, Shaw PW, Balfour PC, et al. Prognostic value of myocardial scarring on CMR in patients with cardiac sarcoidosis: a systematic review and meta-analysis. JACC Cardiovasc Imaging. 2017;10(4):411–20. https://doi.org/10.1016/j.jcmg.2016.05.009.
• Greulich S, Deluigi CC, Gloekler S, Wahl A, Zürn C, Kramer U, et al. CMR imaging predicts death and other adverse events in suspected cardiac sarcoidosis. J Am Coll Cardiol Img. 2013;6(4):501–11. https://doi.org/10.1016/j.jcmg.2012.10.021. The presence of myocardial scarring identified via LGE on CMR is the best independent risk factor for lethal events and adverse events, suggesting the need for future studies to establish LGE as an independent predictor of cardiac death in sarcoidosis patients.
Schuller JL, Zipse M, Crawford T, Bogun F, Beshai J, Patel AR, et al. Implantable cardioverter defibrillator therapy in patients with cardiac sarcoidosis. J Cardiovasc Electrophysiol. 2012;23:925–9.
Muser D, Santangeli P, Patahk RK, et al. Long-term outcomes of catheter ablation of ventricular tachycardia in patients with cardiac sarcoidosis. Circ Arrhythm Electrophysiol. 2016;9:e004333.
Watanabe Y, Nishii T, Shimoyama S, et al. Focal myocardial damage in cardiac sarcoidosis characterized by strain analysis on magnetic resonance tagged imaging in comparison with fluorodeoxyglucose positron emission tomography accumulation and magnetic resonance late gadolinium enhancement. J Comput Assist Tomogr. 2018. https://doi.org/10.1097/RCT.0000000000000733.
Rao DA, Dellaripa PF. Extrapulmonary manifestations of sarcoidosis. Rheum Dis Clin N Am. 2013;39(2):277–97. https://doi.org/10.1016/j.rdc.2013.02.007.
Skali H, Schulman AR, Dorbala S. 18F-FDG PET/CT for the assessment of myocardial sarcoidosis. Curr Cardiol Rep. 2013;15(4):352. https://doi.org/10.1007/s11886-013-0352-8.
Skali H, Schulman A, Dorbala S. 18F-FDG PET/CT for the assessment of myocardial sarcoidosis. Eur Heart J. 2005;26:1538–43.
Skali H, Schulman A, Dorbala S. 18F-FDG PET/CT for the assessment of myocardial sarcoidosis. Eur Heart J. 2005;26:1538–43.
Manabe O, Yoshinaga K, Ohira H, Masuda A, Sato T, Tsujino I, et al. The effects of 18-h fasting with low-carbohydrate diet preparation on suppressed physiological myocardial 18F-fluorodeoxyglucose (FDG) uptake and possible minimal effects of unfractionated heparin use in patients with suspected cardiac involvement sarcoidosis. J Nucl Cardiol. 2015;23(2):244–52. https://doi.org/10.1007/s12350-015-0226-0.
Pereira NL, Grogan M, Dec GW. Spectrum of restrictive and infiltrative cardiomyopathies: part 2 of a 2-part series. J Am Coll Cardiol. 2018;71(10):1149–66. https://doi.org/10.1016/j.jacc.2018.01.017. Review
Lebasnier A, Legallois D, Bienvenu B, Bergot E, Desmonts C, Zalcman G, et al. Diagnostic value of quantitative assessment of cardiac 18F-fluoro-2-deoxyglucose uptake in suspected cardiac sarcoidosis. Ann Nucl Med. 2018;32:319–27. https://doi.org/10.1007/s12149-018-1250-3.
• Ohira H, Tsujino I, Ishimaru S, Oyama N, Takei T, Tsukamoto E, et al. Myocardial imaging with 18F-fluoro-2-deoxyglucose positron emission tomography and magnetic resonance imaging in sarcoidosis. Eur J Nucl Med Mol Imaging. 2007;35(5):933–41. https://doi.org/10.1007/s00259-007-0650-8. This 21-patient study found that FDG-PET was superior to CMR for ruling out cardiac sarcoidosis with a sensitivity of 87.5% and specificity of 38.5%, compared to CMR with sensitivity of 75% and specificity of 76.9%. Specificity of FDG-PET was lower in this study compared to previous data.
Okumura W, Iwasaki T, Toyama T, et al. Usefulness of fasting 18F-FDG PET in identification of cardiac sarcoidosis. J Nucl Med. 2004;45:1986–98.
Ishimaru S, Tsujino I, Takei T, Tsukamoto E, Sakaue S, Kamigaki M, et al. Focal uptake on 18F-fluoro-2-deoxyglucose positron emission tomography images indicates cardiac involvement of sarcoidosis. Eur Heart J. 2005;26(15):1538–43. https://doi.org/10.1093/eurheartj/ehi180.
• Mehta D, Lubitz SA, Frankel Z, Wisnivesky JP, Einstein AJ, Goldman M, et al. Cardiac involvement in patients with sarcoidosis: diagnostic and prognostic value of outpatient testing. Chest. 2008;133(6):1426–35. https://doi.org/10.1378/chest.07-2784. This was a review of 62 patients with sarcoidosis; prevalence of cardiac involvement of 39%. The review concluded that sarcoid lesions in myocardial tissue as seen on CMR or PET did not predict arrhythmia in ambulatory patients who had preserved cardiac function. Additionally, as CS is common among patients with sarcoidosis, incorporating advanced cardiac imaging with FDG PET or CMR is recommended as it is more sensitive than established clinical criteria.
Matoh F, Satoh H, Shiraki K, Odagiri K, Saitoh T, Urushida T, et al. The usefulness of delayed enhancement magnetic resonance imaging for diagnosis and evaluation of cardiac function in patients with cardiac sarcoidosis. J Cardiol. 2008;51(3):179–88. https://doi.org/10.1016/j.jjcc.2008.03.002.
Langah R, Spicer K, Gebregziabher M, Gordon L. Effectiveness of prolonged fasting 18f-FDG PET-CT in the detection of cardiac sarcoidosis. J Nucl Cardiol. 2009;16(5):801–10. https://doi.org/10.1007/s12350-009-9110-0.
Tahara N, Tahara A, Nitta Y, Kodama N, Mizoguchi M, Kaida H, et al. Heterogeneous myocardial FDG uptake and the disease activity in cardiac sarcoidosis. JACC Cardiovasc Imaging. 2010;3(12):1219–28. https://doi.org/10.1016/j.jcmg.2010.09.015.
Youssef G, Leung E, Mylonas I, Nery P, Williams K, Wisenberg G, et al. The use of 18F-FDG PET in the diagnosis of cardiac sarcoidosis: a systematic review and meta-analysis including the Ontario experience. J Nucl Med. 2012;53(2):241–8. https://doi.org/10.2967/jnumed.111.090662.
Manabe O, Ohira H, Yoshinaga K, Sato T, Klaipetch A, Oyama-Manabe N, et al. Elevated 18F-fluorodeoxyglucose uptake in the interventricular septum is associated with atrioventricular block in patients with suspected cardiac involvement sarcoidosis. European Journal of Nuclear Medicine and Molecular Imaging. 2013;40(10):1558–66. https://doi.org/10.1007/s00259-013-2460-5.
McArdle BA, Birnie DH, Klein R, et al. Is there an association between clinical presentation and the location and extent of myocardial involvement of cardiac sarcoidosis as assessed by 18F-fluorodoexyglucose positron emission tomography? Circ Cardiovasc Imaging. 2013;6(5):617–26. https://doi.org/10.1161/CIRCIMAGING.112.000289.
Soussan M, Brillet PY, Nunes H, Pop G, Ouvrier MJ, Naggara N, et al. Clinical value of a high-fat and low-carbohydrate diet before FDG-PET/CT for evaluation of patients with suspected cardiac sarcoidosis. J Nucl Cardiol. 2013;20(1):120–7. https://doi.org/10.1007/s12350-012-9653-3.
Ahmadian A, Brogan A, Berman J, Sverdlov AL, Mercier G, Mazzini M, et al. Quantitative interpretation of FDG PET/CT with myocardial perfusion imaging increases diagnostic information in the evaluation of cardiac sarcoidosis. J Nucl Cardiol. 2014;21(5):925–39. https://doi.org/10.1007/s12350-014-9901-9.
Blankstein R, Osborne M, Naya M, Waller A, Kim CK, Murthy VL, et al. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol. 2014;63(4):329–36. https://doi.org/10.1016/j.jacc.2013.09.022.
Manabe O, Yoshinaga K, Ohira H, Sato T, Tsujino I, Yamada A, et al. Right ventricular 18F-FDG uptake is an important indicator for cardiac involvement in patients with suspected cardiac sarcoidosis. Ann Nucl Med. 2014;28(7):656–63. https://doi.org/10.1007/s12149-014-0860-7.
Wicks E, Menezes L, Pantazis A, Mohiddin S, Porter J, Booth H, et al. 135 novel hybrid positron emission tomography - magnetic resonance (PET-MR) multi-modality inflammatory imaging has improved diagnostic accuracy for detecting cardiac sarcoidosis. Heart. 2014;100(Suppl 3):A80–LP-A80. http://heart.bmj.com/content/100/Suppl_3/A80.1.abstract
Yokoyama R, Miyagawa M, Okayama H, Inoue T, Miki H, Ogimoto A, et al. Quantitative analysis of myocardial 18F-fluorodeoxyglucose uptake by PET/CT for detection of cardiac sarcoidosis. Int J Cardiol. 2015;195(2015):180–7. https://doi.org/10.1016/j.ijcard.2015.05.075.
Gormsen LC, Haraldsen A, Kramer S, Dias AH, Kim WY, Borghammer P. A dual tracer 68Ga-DOTANOC PET/CT and 18F-FDG PET/CT pilot study for detection of cardiac sarcoidosis. EJNMMI Res. 2016;6(1):52. https://doi.org/10.1186/s13550-016-0207-6.
Lapa C, Reiter T, Kircher M, Schirbel A, Werner RA, Pelzer T, et al. Somatostatin receptor based PET/CT in patients with the suspicion of cardiac sarcoidosis: an initial comparison to cardiac MRI. Oncotarget. 2016;7(47):77807–14. https://doi.org/10.18632/oncotarget.12799.
Ohira H, Birnie DH, Pena E, Bernick J, Mc Ardle B, Leung E, et al. Comparison of 18F-fluorodeoxyglucose positron emission tomography (FDG PET) and cardiac magnetic resonance (CMR) in corticosteroid-naive patients with conduction system disease due to cardiac sarcoidosis. Eur J Nucl Med Mol Imaging. 2016;43(2):259–69. https://doi.org/10.1007/s00259-015-3181-8.
Aikawa T, Oyama-Manabe N, Naya M, Ohira H, Sugimoto A, Tsujino I, et al. Delayed contrast-enhanced computed tomography in patients with known or suspected cardiac sarcoidosis: a feasibility study. Eur Radiol. 2017;27(10):4054–63. https://doi.org/10.1007/s00330-017-4824-x.
Norikane T, Yamamoto Y, Maeda Y, Noma T, Dobashi H, Nishiyama Y. Comparative evaluation of 18F-FLT and 18F-FDG for detecting cardiac and extra-cardiac thoracic involvement in patients with newly diagnosed sarcoidosis. EJNMMI Res. 2017;7:1–7. https://doi.org/10.1186/s13550-017-0321-0.
• Kouranos V, Tzelepis GE, Rapti A, et al. Complementary role of CMR to conventional screening in the diagnosis and prognosis of cardiac sarcoidosis. JACC Cardiovasc Imaging. 2017;10(12):1437–47. https://doi.org/10.1016/j.jcmg.2016.11.019. Although 2D-Electrocardiography holds a high positive predictive value (83.9%) and relative low risk to benefit margins, CMR is the most valuable diagnostic and prognostic imaging study for evaluation of myocardial tissue in patients with diagnosed sarcoidosis 2D-Electrocardiography has low sensitivity (27.1%). This study has the largest CMR cohort size (N=321) and was able to identify silent CS at a rate of nearly 10%, sensitivity 96.9% and specificity 100%.
Dweck MR, Abgral R, Trivieri MG, Robson PM, Karakatsanis N, Mani V, et al. Hybrid magnetic resonance imaging and positron emission tomography with fluorodeoxyglucose to diagnose active cardiac sarcoidosis.
• Wicks EC, Menezes LJ, Barnes A, et al. Diagnostic accuracy and prognostic value of simultaneous hybrid 18F-fluorodeoxyglucose positron emission tomography/magnetic resonance imaging in cardiac sarcoidosis. Eur Heart J Cardiovasc Imaging. 2018. doi:https://doi.org/10.1093/ehjci/jex340. This review of literature compares combined 18F-FDG PET and LGE on CMR against 18F-FDG PET alone and CMR alone. The study found hybrid imaging to be superior with sensitivity 94%, specificity 44%, positive predictive value 76%, negative predictive value 80%. Sensitivity for 18F-FDG PET alone is 85% and LGE on CMR alone is 82%. This review suggests combined imaging techniques for increased diagnostic accuracy of cardiac sarcoidosis.
Smedema JP, van Geus RJ, Truter R, Mayosi BM, Crijns H. Contrast-enhanced cardiac magnetic resonance: distinction between cardiac sarcoidosis and infarction scar. Sarcoidosis Vasculitis and Diffuse Lung Disease. 2018;34(4):307–14.
Bremer W, Sweiss NJ, Lu Y. Serial FDG-PET/CT imaging in the management of cardiac sarcoidosis. Clin Nucl Med. 2018;43(2):e50–2.
• Muser D, Santangeli P, Castro SA, et al. Prognostic role of serial quantitative evaluation of 18F-fluorodeoxyglucose uptake by PET/CT in patients with cardiac sarcoidosis presenting with ventricular tachycardia. Eur J Nucl Med Mol Imaging. 2018. https://doi.org/10.1007/s00259-018-4001-8. Ventricular arrhythmia response to ablation using lesion metabolic activity (LMA) correlated with a 20-fold higher risk of MACE in non-responders (p=0.007) as well as parallels systolic function (p=0.003). This study suggests that a reduction in FDG uptake is strongly associated with decreased MACE at long term follow up.
Danwade TA, Devidutta S, Shelke AB, Saggu DK, Yalagudri SD, Sridevi C, et al. Prognostic value of Fluorine-18 fluoro-2-deoxyglucose positron emission computed tomography in patients with unexplained atrioventricular block. Heart Rhythm. 2017;15:234–9. https://doi.org/10.1016/j.hrthm.2017.10.025.
Sipilä K, Tuominen H, Haarala A, Tikkakoski A, Kähönen M, Nikus K. Novel ECG parameters are strongly associated with inflammatory 18F-FDG PET findings in patients with suspected cardiac sarcoidosis. Int J Cardiol. 2017 Dec 15;249:454–60. https://doi.org/10.1016/j.ijcard.2017.07.027.
Travin MI, Bergmann SR. Assessment of myocardial viability. Semin Nucl Med. 2005;35:2–16.
• Krumm P, Mangold S, Gatidis S, et al. Clinical use of cardiac PET/MRI: current state-of-the-art and potential future applications. Jpn J Radiol. 2018. https://doi.org/10.1007/s11604-018-0727-2. This study suggests that FDG-PET negative imaging in the setting of positive LGE in CMR may be suggestive of inactive cardiac sarcoidosis and scarring while FDG-PET positive imaging may be suggestive of an active inflammatory process and active cardiac sarcoidosis.
Wada K, Niitsuma T, Yamaki T, Masuda A, Ito H, Kubo H, et al. Simultaneous cardiac imaging to detect inflammation and scar tissue with (18)F-fluorodeoxyglucose PET/MRI in cardiac sarcoidosis. J Nucl Cardiol. 2016;23:1180–2.
White JA, Rajchl M, Butler J, Thompson RT, Prato FS, Wisen- berg G. Active cardiac sarcoidosis: first clinical experience of simultaneous positron emission tomography–magnetic resonance imaging for the diagnosis of cardiac disease. Circulation. 2013;127:e639–41.
• Ohira H, Yoshinaga K, Manabe O, Oyama-Manabe N, Tsujino I, Nishimura M, et al. Clinical application of 18F-fluorodeoxyglucose PET and LGE CMR in cardiac sarcoidosis. Ann of Nuclear Cardiology. 2017;3(1):125–30. https://doi.org/10.17996/anc.17-00027. Volume intensity detection (cardiac metabolic activity) has been independently associated with adverse cardiac events; FDG-PET and CMR may be used independently or in combination to better improve detection and to reduce adverse cardiac events.
Haywood J, Sharma O, Siegel M, Siegal R, Gottlieb S, Caldwell J, et al. Detection of myocardial sarcoidosis by thallium 201 imaging. J Natl Med Assoc. 1982;74:959–64.
Kandolin R, Lehtonen J, Graner M, Schildt J, Salmenkivi K, Kivistö SM, et al. Diagnosing isolated cardiac sarcoidosis. J Intern Med. 2011;270:461–8.
Tellier P, Paycha F, Antony I, Nitenberg A, Valeyre FD, Foult JM, et al. Reversibility by dipyridamole of thallium-201 myocardial scan defects in patients with sarcoidosis. Am J Med. 1988;85(2):189–93. https://doi.org/10.1016/s0002-9343(88)80340-1.
Lee PI, Cheng G, Alavi A. The role of serial FDG PET for assessing therapeutic response in patients with cardiac sarcoidosis. J Nucl Cardiol. 2016;24(1):19–28. https://doi.org/10.1007/s12350-016-0682-1.
Le Guludec D, Menad F, Faraggi M, Weinmann P, Battesti J, Myocardial Sarcoidosis VD. Clinical value of technetium-99m Sestamibi tomoscintigraphy. Chest. 1994;106:1675–82.
Schatka I, Bengel FM. Advanced imaging of cardiac sarcoidosis. J Nucl Med. 2013;55(1):99–106. https://doi.org/10.2967/jnumed.112.115121.
Chatal JF, Rouzet F, Haddad F, Bourdeau C, Mathieu C, Guludec DL. Story of rubidium-82 and advantages for myocardial perfusion PET imaging. Front Med. 2015;2. https://doi.org/10.3389/fmed.2015.00065.
Senthamizhchelvan S, Bravo PE, Esaias C, Lodge MA, Merrill J, Hobbs RF, et al. Human biodistribution and radiation dosimetry of 82Rb. J Nucl Med. 2010;51(10):1592–9. https://doi.org/10.2967/jnumed.110.077669.
Bateman T, Heller G, Mcghie A, et al. Diagnostic accuracy of rest/stress ECG-gated Rb-82 myocardial perfusion PET: comparison with ECG-gated Tc-99m sestamibi SPECT. J Nucl Cardiol. 2006;13(1):24–33. https://doi.org/10.1016/j.nuclcard.2005.12.004.
Fakhri GE, Kardan A, Sitek A, et al. Reproducibility and accuracy of quantitative myocardial blood flow assessment with 82Rb PET: comparison with 13N-ammonia PET. J Nucl Med. 2009;50(7):1062–71. https://doi.org/10.2967/jnumed.104.007831.
Ben-Haim S, Murthy VL, Breault C, Allie R, Sitek A, Roth N, et al. Quantification of myocardial perfusion reserve using dynamic SPECT imaging in humans: a feasibility study. J Nucl Med. 2013;54(6):873–9. https://doi.org/10.2967/jnumed.112.109652.
Yalamanchili P, Wexler E, Hayes M, Yu M, Bozek J, Kagan M, et al. Mechanism of uptake and retention of F-18 BMS-747158-02 in cardiomyocytes: a novel PET myocardial imaging agent. J Nucl Cardiol. 2007;14(6):782–8. https://doi.org/10.1016/j.nuclcard.2007.07.009.
Berman DS, Maddahi J, Tamarappoo B, et al. Phase II safety and clinical comparison with single-photon emission computed tomography myocardial perfusion imaging for detection of coronary artery disease. J Am Coll Cardiol. 2013;61(4):469–77. https://doi.org/10.1016/j.jacc.2012.11.022.
Maddahi J, Huang S, Truong D, Lazewatsky JL, Ehlgen A, Schelbert H, et al. Preliminary results of absolute quantification of rest and stress myocardial blood flow with flurpiridaz F-18 PET in normal and coronary artery disease patients in a single-center study. J Nucl Cardiol. 2010;17:743.
Gormsen LC, Haraldsen A, Kramer S, Dias AH, Kim WY, Borghammer P. A dual tracer 68 Ga-DOTANOC PET/CT and 18F-FDG PET/CT pilot study for detection of cardiac sarcoidosis. EJNMMI Res. 2016;6(1):52.
Pizarro C, Kluenker F, Dabir D, Thomas D, Gaertner FC, Essler M, et al. Cardiovascular magnetic resonance imaging and clinical performance of somatostatin receptor positron emission tomography in cardiac sarcoidosis. ESC Heart Fail. 2018;5(2):249–61. https://doi.org/10.1002/ehf2.12243.
Bailey DL, Pichler BJ, Gückel B, Barthel H, Beer AJ, Botnar R, et al. Combined PET/MRI: from status quo to status go. Summary report of the fifth international workshop on PET/MR imaging; February 15–19, 2016; Tübingen, Germany. Mol Imaging Biol. 2016;18(5):637–50. https://doi.org/10.1007/s11307-016-0993-2.
Sobic-Saranovic D, Grozdic I, Videnovic-Ivanov J, Vucinic-Mihailovic V, Artiko V, Saranovic D, et al. The utility of 18F-FDG PET/CT for diagnosis and adjustment of therapy in patients with active chronic sarcoidosis. J Nucl Med. 2012;53(10):1543–9. https://doi.org/10.2967/jnumed.112.104380.
Kim JS, Judson MA, Donnino R, Gold M, Cooper LT Jr, Prystowsky EN, et al. Cardiac sarcoidosis. Am Heart J. 2009;157(1):9–21. https://doi.org/10.1016/j.ahj.2008.09.009.
•• Vita T, Okada DR, Veillet-Chowdhury M, et al. Complementary value of cardiac magnetic resonance imaging and positron emission tomography/computed tomography in the assessment of cardiac sarcoidosis. Circ Cardiovasc Imaging. 2018;11:e007030. This was a retrospective study on 107 patients referred for evaluation of cardiac sarcoidosis. The study found 85% had LGE on CMR and 76% had positive FDG PET scans, among the 85% of patients with LGE findings, 66% had abnormal FDG PET scans as well. When analyzing probability of cardiac sarcoidosis with combined imaging, 45% of patients would be re-classified. Amongst the 45% re-classified, 80% were correctly classified when compared to final diagnosis. This study suggest combined imaging is critical in increasing the accuracy of diagnosis and disease classification.
Miller EJ, Culver DA. Establishing an evidence-based method to diagnose cardiac sarcoidosis: the complementary use of cardiac magnetic resonance imaging and FDG-PET. Circulation: Cardiovascular Imaging. 2018;11(1):e007408. Editorial
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Authorship Declaration
All authors listed meet the authorship criteria according to the latest guidelines the International Committee of Medical Journal Editors. All the authors have contributed equally and are in agreement with the manuscript.
Additional information
This article is part of the Topical Collection on Imaging
Rights and permissions
About this article
Cite this article
Yatsynovich, Y., Valencia, D., Petrov, M. et al. Updates on the Role of Imaging in Cardiac Sarcoidosis. Curr Treat Options Cardio Med 20, 74 (2018). https://doi.org/10.1007/s11936-018-0670-7
Published:
DOI: https://doi.org/10.1007/s11936-018-0670-7