Abstract
Joint pain related to osteoarthritis (OA) is often linked to an initial cartilage injury. Immunogenic cartilage breakdown products cause inflammation of the synovium, leading to the release of inflammatory markers and cytokines. Various non-operative modalities exist to help treat patients, ranging from physical therapy, braces, and oral pharmacologic medications. Injectable treatments include intra-articular corticosteroids and viscosupplementation. These treatments are widely accepted, but the literature varies on their reported efficacy. Recently, platelet rich plasma and stem cell injections have emerged as alternative treatment options. The data on these treatment options is in its infantile stages but shows promise for the future.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Viscosupplementation
- Injection Treatment
- Intra-articular Corticosteroid Injections
- Platelet-rich Plasma (PRP)
- Bone Marrow-derived Stromal Cells (BMSCs)
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Pathophysiology of Osteoarthritis Pain
Joint pain related to osteoarthritis (OA) is often linked to an initial cartilage injury. Immunogenic cartilage breakdown products cause inflammation of the synovium, leading to the release of inflammatory markers and cytokines. Quiescent adult chondrocytes are subsequently activated, resulting in the further release of a host of inflammatory markers—most notably IL-1, IL-8, TNF-alpha, reactive oxygen species such as nitric oxide (NO), prostaglandins, matrix metalloproteinases, and leukotrienes [1,2,3,4,5,6]. This reaction leads to a breakdown of the cartilage matrix, chondrocyte apoptosis, and the activation of pain nociceptors within the cartilage, synovium, and subchondral bone [7]. This chronic inflammatory process can be viewed histologically, as synovial biopsies will demonstrate increased blood vessel proliferation, vascular endothelial growth factor (VEGF) expression, and increased mononuclear cell infiltration [8].
Pain within the osteoarthritic joint is unlikely to be immediately related to cartilage breakdown, as cartilage lacks nerve endings. However, the synovium, subchondral bone, and periosteum have dense concentrations of nociceptors [9]. Polymodal Aδ and C nociceptors include groups with low firing threshold for normal activity, while others maintain a high threshold for more injurious stimuli [10, 11]. A lesser-known third class is the sleeping nociceptor, which does not respond to typical pain stimuli, but instead to the endogenous stimuli from the injury itself. These in concert act to create the crescentic pain reaction typical in acute OA flares. An initial pain event may result in persistent hyperalgesia for years following the insult, leaving the patient prone to both central and peripheral sensitization [12, 13]. Resulting neuroplasticity may be a strong contributor to the compounding pain complaint central to chronic osteoarthritis [14].
Different inflammatory cascades are required for each region of the joint to evoke a pain response. As such, the degree of pain related to synovial inflammation is location-specific. This is especially true in the knee, where inflammation around the infrapatellar fat pad has been correlated with increased pain [15]. Further evidence of synovial inflammation due to osteoarthritis includes synovial hyperplasia [16], lymphocytic infiltrate, fibrosis, and thickening of the synovial capsule [17, 18]. Synovial fibrosis in particular may be a primary contributor to joint pain and stiffness in OA [19]. Subchondral bone edema is one of the earliest observed signs of osteoarthritis, and bone marrow edema-like lesions (BML) can be seen on advanced imaging prior to the onset of clinical symptoms [20]. Areas of the subchondral bone with BMLs have been correlated with increased pain and cartilage erosion [21]. Current theory suggests that these BMLs allow for the rapid ingrowth of sensory fibers and vascular channels, leading to increased pain and sensitivity to inflammatory cytokines [22].
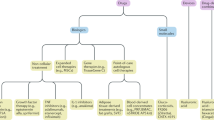
Nonsurgical Management of OA
While conservative management algorithms are often dictated by the individual physician, consensus guidelines for the management of osteoarthritis by the American College of Rheumatology (ACR) were released in 2012. These recommendations stratify patients by the severity and anatomic involvement of OA, specifically of the hand, hip, and knee.
Physical Therapy
The initial management of osteoarthritis includes targeted stretching and strengthening with physical therapy. Restrictive motion devices (i.e., splints, orthotics, braces, taping) for pain control and therapy guidance may provide relief to some patients although the literature demonstrates varying results on their efficacy [23, 24]. Similarly, aquatic exercise for the management of lower-extremity OA may help with pain relief and strengthening, although the global utility of these exercises has been inconsistently demonstrated among various studies [25,26,27]. For these reasons, the ACR does not recommend a specific exercise modality for hip or knee OA, but recommends tailored treatment based on the patient’s aerobic capacity [23].
Several alternative therapies not included in consensus recommendations have been studied extensively. High-level evidence has demonstrated acupuncture to provide long-term pain relief for OA, comparable to exercise and off-loading modalities [28,29,30]. However, other studies demonstrated no significant improvement when compared to physical therapy [31]. Given this, the American Academy of Orthopaedic Surgery (AAOS) clinical guidelines state there is inconclusive proof of efficacy to fully support acupuncture [24]. Other alternative therapies such as yoga [32], massage [33, 34], and tai chi [35,36,37] have demonstrated varying levels of pain improvement for OA. The ACR does not provide any specific therapeutic recommendations, although acknowledges potential efficacy in patients with end-stage arthritis who are poor surgical candidates or deny surgical treatment [23].
Mild to Moderate OA: Pharmacologic Management
The initial therapy for OA includes non-narcotic analgesia and nonsteroidal anti-inflammatory (NSAID) drugs. Acetaminophen (paracetamol) has been found to decrease subjective pain scores by more than 4 on a 100-point scale when used as monotherapy and is, therefore, recommended as baseline analgesia for lower-extremity OA [38,39,40]. A maximum daily dose of 4000 mg/day may be taken. Topical capsaicin is recommended as concurrent first-line therapy in cases of hand OA [23]. However, the ACR notes that this recommendation is not based on any validated therapeutic benefit. A meta-analysis of mostly retrospective work found a modest but significant improvement in pain following 4 weeks of continuous use [41, 42]. Unfortunately, this was not observed in randomized controlled trials.
NSAID therapy is considered a second-line treatment for lower-extremity OA and may be administered concurrently with acetaminophen to provide supplemental pain relief [43]. Given the risk of upper gastrointestinal (GI) complications related to long-term NSAID use [44], the ACR recommends that patients with a history of GI complications or concurrently on a prescribed full-dose aspirin use a COX-2 selective inhibitor only or begin a proton pump inhibitor (i.e., pantoprazole) concurrently [23, 45, 46]. The therapeutic benefit of selective COX-2 inhibitors has been found to be equivalent to nonselective NSAIDs, with no difference in complication rate observed [47]. The ACR contraindicates NSAIDs for patients over the age of 75, in line with previously published recommendations by the American Geriatrics Society [48].
The ACR has few recommendations for non-injectable monotherapies in the case of OA pain refractory to acetaminophen and NSAIDs . Pain improvement with tramadol monotherapy has been inconsistently demonstrated. However, tramadol has shown benefit as an adjunct therapy [49]. Randomized controlled trials show significant improvement in the pain ratings of patients with moderate to severe OA when tramadol is administered in conjunction with acetaminophen or NSAIDs [50]. Findings suggest that the analgesic effects of tramadol with NSAIDs are synergistic.
Chondroitin Sulfate + Glucosamine
Chondroitin sulfate and glucosamine, an amino sugar and carbohydrate naturally found within healthy cartilage, have been commonly utilized as an alternative or supplemental therapy for osteoarthritis. Supporters believe the reduction in chondroitin sulfate concentration and chain length seen in OA can be exogenously replenished [51, 52]. Several clinical trials have focused on the clinical benefits of these supplements.
While available in injectable form, chondroitin sulfate and glucosamine are predominately taken orally [53]. Randomized controlled trials using chondroitin sulfate as an adjunct to NSAID therapy showed both pain and structural improvements, with a reduced loss of cartilage volume at a 2-year endpoint [54]. Additional studies have demonstrated a reduction in NSAID use and improvement in mobility with long-term glucosamine as well [55, 56]. While the conclusions of these clinical trials are encouraging, several other trials refuted their conclusions [57, 58]. A large randomized controlled trial evaluating chondroitin sulfate and glucosamine as both monotherapy and adjunct failed to show any improvement in a global cohort compared to NSAID therapy alone [59].
While randomized trials and high-quality meta-analyses have been attempted to study the benefit of chondroitin sulfate and glucosamine, the inconsistent dosage, preparation, and administration has made it difficult to demonstrate an irrefutable clinical benefit. For this reason, the ACR only recommends the use of these supplements as adjuncts to traditional pharmacologic therapies, while the AAOS does not recommend their usage at all [23, 24]. Given low toxicity of these drugs and over-the-counter availability, they remain popular alternative therapies for OA [60].
Opioids in Osteoarthritis
Consensus recommendations regarding the role opioids should play in the management of OA are inconclusive [24]. A Cochrane review noted a modest pain improvement with opioid therapy, but also noted a high rate of opioid abuse and addiction [61]. While objective pain improvements have failed to demonstrate clinical significance, the high patient perception of opioid efficacy in the USA complicates therapeutic guidance [62]. As such prescription rates of opioids are significantly higher in the USA than in Europe and elsewhere in the world [63, 64].
Injectable Treatments
Intra-articular Corticosteroids
Intra-articular corticosteroids injections (CSI) remain the mainstay of injectable clinical therapy for moderate to severe osteoarthritis. There exists conflicting evidence supporting their efficacy, and as such, official recommendations have often been inconclusive. Recent revisions of the ACR recommendations recommend CSI for those patients with soft tissue inflammation and joint effusion [23], although the AAOS clinical guidelines remain inconclusive [24]. Advantages of CSI include a rapid onset of action, significant long-term local anti-inflammatory effects, and a limited, although significant, risk of side effects [65, 66].
Mechanism of action : The onset of corticosteroid activity begins upon activation by the surface receptors of the synovial membrane. The activated corticosteroid acts on the nuclear steroid receptors directly, reducing the rate of mRNA and protein synthesis. This in turn inhibits the function of T and B cells as well as phospholipase A2 and, consequently, arachidonic acid [67, 68]. Besides inhibiting the pro-inflammatory milieu produced as a downstream effect of osteoarthritis progression, corticosteroids may further exert a disease-modifying role. In vivo animal studies suggest that CSI decrease the severity of osteophyte formation and cartilage fibrillations related to OA. These effects were observed in both prophylactic and therapeutic trials [69, 70].
Composition : Corticosteroid preparations are largely derivatives of prednisolone (Table 7.1). The creation of large particle suspensions poorly soluble in water, such as with triamcinolone acetonide, allows the drug to remain in the joint for a longer period and requires hydrolysis by cellular esterases to release its active component [72, 73]. However, the rate of cellular uptake is slow, leading to a delayed onset of effect [73]. In contrast, drugs that are “clear,” or non-particulate, suspensions such as dexamethasone salt are rapidly taken up by cells and, therefore, are faster acting. However, the drug spends less time within the joint, theoretically reducing the duration of effect [73]. Recent clinical trials comparing ester and salt preparations have found that their efficacy and duration may be equivalent, suggesting that pharmacodynamics may not be of clinical significance [74]. Mixed preparations have not shown any benefit. A randomized trial assessing the benefit of a combination approach utilizing a mixture of the salt and ester forms of betamethasone has failed to show any improvement in duration or onset time with this approach compared to ester-only preparations [72, 75].
Efficacy : CSI have been shown to provide short- to mid-term pain relief in patients with OA [76]. Some evidence suggests that clinical improvement in pain and range of motion can be expected from 2 to 12 weeks post-injection, with an average reduction in pain score of over 20% [65, 77, 78]. A longer duration of efficacy has been demonstrated in patients with preexisting soft tissue involvement [79, 80], although soft tissue involvement in general had poorer therapeutic responses overall [81]. The long-term efficacy and safety of CSI remain a concern. Randomized trials in patients with knee OA suggest that regular injections every 3 months out to 2 years following initiation resulted in prolonged reductions in pain and stiffness [82].
Side effects: In addition to the side effect of transiently elevated blood glucose levels, particularly in diabetics, both local and systemic complications related to CSI have been described [83]. In vitro studies have demonstrated that direct exposure of corticosteroids to cartilage has a chondrotoxic effect [84, 85]. The clinical manifestation of this has yet to be determined. Skin atrophy, hypersensitivity, and hypopigmentation with a classic “linear ray” appearance have long been associated with intralesional or intra-articular steroid therapy. The mechanism behind this clinical manifestation is not fully understood, although current theory suggests that the lymphatic system may play an important role [86]. Tendon rupture and in vitro cellular degeneration following exposure to corticosteroids have been reported. While this mechanism is also poorly understood, recent findings suggest that increased apoptosis, transient increases in matrix metalloproteinases (e.g., MMP-3), and an active inhibition of repair mechanisms may be important contributors to tendon degeneration [87,88,89].
Viscosupplementation
In the pro-inflammatory arthritic cascade, the synovial fluid undergoes several compositional changes. Synovial fluid , traditionally responsible for the lubrication and smooth motion of joints, functions due to the presence of hyaluronate. Hyaluronate , a high-molecular-mass polysaccharide, gives synovial fluid its abilities to act as shock absorber and lubricant medium. In the osteoarthritic joint, the amount and quality of hyaluronate are both decreased, in part due to increased degradation rates. There is some evidence that the degradation of synovial fluid may be slowed or reversed with viscosupplementation.
Mechanism of action : Viscosupplementation exerts a anti-inflammatory effect on synovium, inhibiting the release of prostaglandins and the immunologic response typical in osteoarthritis [90]. Further theories suggesting that cartilage degeneration may be reversed with viscosupplementation have not borne out conclusively in the literature.
Composition : Current formulations of hyaluronic acid vary in molecular weight to modulate elastoviscosity. High-molecular-weight formulations, such as the well-tested hylan G-F-20, will have higher elastoviscosity compared to low-molecular-weight preparations. This property appears to be critical to the therapeutic effect of viscosupplementation, with an initial randomized trial showing that hylan G-F-20 improved pain and patient reported outcomes compared to low-molecular-weight preparations [91]. However, subsequent trials have been inconsistent in replicating this finding [92]. Moreover, studies in animal models suggest that high-molecular-weight hyaluronic acid may be more effective at binding to its cellular receptor and as a result more effective at reducing synovial inflammation and stabilizing synovial fluid [93].
Efficacy : Randomized controlled trials and meta-analysis exhibit significant variability and disagreement as to the efficacy of viscosupplementation. In general, many randomized controlled trials suggest that viscosupplementation is associated with some degree of pain relief in osteoarthritis patients. However, the degree and duration of pain relief is a source of disagreement. In general, trials agree that the longest expected efficacy of viscosupplementation is 5–6 months [94, 95], although Campbell et al. [96] found no improvement at any time point. Benefits beyond pain have also been proposed, with improvements demonstrated in gait kinematics following a course of viscosupplementation [97]. Viscosupplementation has traditionally been given in three separate weekly injections. However, this methodology has not been well validated in the literature, with randomized trials finding no significant clinical differences between three separate injections compared to one alone [98]. Given the disparate evidence, while some consensus opinions suggest that viscosupplementation may be of some clinical benefit, they do not establish guidelines for its use as differing trial metrics make comparison difficult [99]. The AAOS does not recommend viscosupplementation given the lack of conclusive evidence [24].
Side effects : Proponents of viscosupplementation have pointed to its low toxicity and paucity of side effects. Meta-analyses have demonstrated that viscosupplementation is safe, with an increased risk of minor adverse effect rate of less than 1% [100]. Reported complications include pseudosepsis secondary to an exaggerated immune reaction against a component of hyaluronic acid with higher rates found in avian-derived viscosupplementation products [101].
Platelet-Rich Plasma (PRP)
Mechanism of action : The beneficial mechanism behind a platelet concentrate compared with the injection of pure activated growth factor is not fully understood. However, recent in vitro studies suggest that there is a new class of cytokines present only in platelets, dedicated toward inflammatory regulation, protecting host tissues, and promoting angiogenesis [102]. Early studies on the impact of exogenous PRP on chemotaxis showed an increase in pro-inflammatory IL-1β, as well as phenotypic conversion of neutrophils and monocytes. Following this pro-inflammatory state, PRP may influence the expression of growth factors such as VEGF, TGF-β, and hepatocyte growth factor (HGF), which in turn inhibit the NF-κB inflammatory cascade. This may ultimately lead to an induction of immunologic quiescence, improving the inflammatory cascade seen in osteoarthritic joints. This immunologic quiescence has also been associated with the in vitro restoration of collagen-2 and aggrecan function around collagen scaffolds. Further functions of PRP include angiogenic proliferation via alpha-granules, although the balance between PRP-induced blood vessel growth and regression is not fully understood. PRP is also noted for other potentially chondroprotective functions, mediated via matrix metalloproteinases, alpha-2-macroglobulins, and overexpression of TGF-β.
Composition : Inconsistency in the preparation and delivery of PRP has made it difficult to study its efficacy. Currently, there are no standardized recommendations for the preparation of PRP. It is therefore often influenced by the experience and decision-making of the practitioner, the cost of the system in both time and laboratory expense, and the nature of the individual patient.
PRP is created when whole blood extracted from the patient is spun down in a centrifuge, removing red blood cells. There are multiple techniques currently employed to perform blood centrifugation, and a description of each is beyond the scope of this chapter. However, depending on the method of preparation, the platelet concentration and leukocyte levels will vary. Whether leukocytes should be removed from the platelet concentrate is not fully understood. Leukocyte-poor preparations may be beneficial in pro-inflammatory processes such as osteoarthritis; leukocyte-rich preparations may be better suited for chronic tendinopathy. Simplifying biology, platelets are anabolic sources, while leukocytes are catabolic. While one would expect leukocytes to counteract the benefit of a platelet concentrate, this has not been fully demonstrated [103]. Concentrates that contain leukocytes are labeled “L-PRP,” while pure platelet concentrate is “P-PRP.” The timing of PRP “activation” is not fully standardized and is also a product of individual technique. Pre-activation of platelets is stimulated with calcium chloride or thrombin introduced prior to injection. In contrast, postinjection activation is accomplished by endogenous tissue factors.
Efficacy: Studies evaluating the objective efficacy of PRP on osteoarthritic pain and function are varied. Multiple studies comparing a series of three weekly injections of exogenously activated P-PRP with viscosupplementation showed a significant improvement in pain, stiffness, and functional capacity at 5 weeks following the initiation of therapy [104, 105]. Gobbi et al. [106] noted improvement in symptoms beyond 1 year after administration. Other studies, however, have failed to corroborate these findings. Filardo et al. [107] found an improvement among younger patients with mild osteoarthritis, but their subsequent study failed to find any evidence of the superiority of PRP over viscosupplementation [108]. In those studies that reported clinical improvement, consistencies included PRP that underwent at least two centrifugations, at least two injections spaced by 1 week, and exogenous activation [109].
Collectively, the science surrounding PRP is in the infantile stages. Of the studies that exist, the methodologies are significantly varied, making generalizations difficult and meta-analyses limited [110]. Within the last few years, increasing evidence supports the formulation of PRP plays a substantial role as to its efficacy. Several factors exist within the PRP such as platelet-derived growth factor (PDGF), transforming growth factor (TGF), vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), interleukin-1 (IL-1), and matrix metalloproteinase-9 (MMP-9) depending on the preparation used [111]. These molecules in the proper concentrations have been shown to protect and heal cartilage [112]. Leukocyte-rich versus leukocyte-poor PRP has been suspected to matter as well. As research is suggesting the exact composition of PRP matters, it calls into question the results of early studies which did not take PRP creation, composition, or concentration into account. Further research is needed to identify the effects of the components of PRP and produce standardized formulations in order to study its clinical efficacy [113, 114]. As such, the current AAOS consensus recommendation on PRP remain inconclusive [24].
Side effects: Side effects related to PRP therapy may be related to both preparation and host factors. In a comparison of single and double spinning of PRP, a greater incidence of swelling and local pain reaction were noted in the double spun sample [104]. This was consistent between both L-PRP and P-PRP [103]. All side effects were transient and did not change long-term clinical outcomes. Additional basic science and clinical studies are needed to further define the mechanisms of action and side effect profiles of PRP therapies. Various compositions of PRP and a lack of well-known mechanisms of action pose important challenges to evaluating its efficacy, determining its adverse effects profile, and thus standardizing its use.
Stem Cell Therapy
While many cell types have been tested as a potential therapy for osteoarthritis and other musculoskeletal pathologies, bone marrow-derived stromal cells (BMSCs) appear prominently in the literature and are best understood.
Mechanism of action: The mechanism of action of BMSCs is thought to be through the induction of a chondroprotective cascade consisting of anti-inflammatory, antiapoptotic, and immunosuppressive functions, thereby permitting cartilage regeneration. Systemic mediators are key to chondrocyte differentiation and include parathyroid hormone-like peptide and basic fibroblast growth factor (FGF).
Composition : Given the lack of regulatory guidance on stem call preparation, delivery technique is variable. In the case of BMSCs , cells are typically isolated via bone marrow aspiration from the iliac crest. Cells are spun down to a concentrate in a manner similar to PRP, followed by resuspension in culture medium. These cells may be utilized immediately, or frozen in liquid nitrogen for later use. The stem cell quantity or concentration needed for therapeutic effect is variable, as is the way in which they are delivered into the osteoarthritic joint. Previously published mechanisms for stem cell delivery range from BMSCs loaded onto a scaffold [115] to the direct injection of incubated BMSCs [116]. As the cellular environment has been shown to be critical, most preparations are suspended within a growth factor-rich milieu. Human studies have utilized 1–12 million cell count preparations, and the exact cell concentration and count is inconsistently reported and varies widely. How the cells are cultured and how they are delivered remain an evolving research topic.
Efficacy : Studies utilizing autologous BMSC injection are generally encouraging, although irregularities related to stem cell composition and preparation complicate the interpretation of findings. A close examination of the cartilage defects post-injection in an in vivo rat model shows evidence of hypertrophic hyaline-like cartilage growth [117]. A synergy of BMSC therapy with PRP and physical therapy has been suggested, with synergistic improvement in patient reported knee and quality-of-life scores [118]. Allogeneic delivery of cultured BMSCs has also shown promising early findings. A randomized controlled trial delivering 40 million allogeneic BMSCs showed a significant increase in pain and function over the 1-year trial period [119]. While these early studies are encouraging, the lack of high-quality trials and the lack of standardized preparation protocols complicate a full assessment of the benefits of BMSC therapy [120]. As a result, the AAOS currently has no consensus opinion on stem cell therapy for osteoarthritis [24].
Side effects : There is a paucity of reported complications when utilizing stem cell therapy other than donor site morbidity. Long-term outcomes within knees and shoulders and with a matrix preparation have not been studied extensively owing to the recent development of stem cell technology. Well-powered randomized controlled trials examining stem cell therapy will be needed as the field develops.
References
Hardy MM, Seibert K, Manning PT, et al. Cyclooxygenase 2-dependent prostaglandin E2 modulates cartilage proteoglycan degradation in human osteoarthritis explants. Arthritis Rheum. 2002;46(7):1789–803.
Lee AS, Ellman MB, Yan D, et al. A current review of molecular mechanisms regarding osteoarthritis and pain. Gene. 2013;527(2):440–7.
Bian Q, Wang YJ, Liu SF, et al. Osteoarthritis: genetic factors, animal models, mechanisms, and therapies. Front Biosci (Elite Ed). 2012;4:74–100.
Fernandes JC, Martel-Pelletier J, Pelletier JP. The role of cytokines in osteoarthritis pathophysiology. Biorheology. 2002;39(1–2):237–46.
Goldring MB, Berenbaum F. The regulation of chondrocyte function by proinflammatory mediators: prostaglandins and nitric oxide. Clin Orthop Relat Res. 2004;(427 Suppl):S37–46.
Eyre DR, McDevitt CA, Billingham ME, et al. Biosynthesis of collagen and other matrix proteins by articular cartilage in experimental osteoarthrosis. Biochem J. 1980;188(3):823–37.
Im HJ, Li X, Muddasani P, et al. Basic fibroblast growth factor accelerates matrix degradation via a neuro-endocrine pathway in human adult articular chondrocytes. J Cell Physiol. 2008;215(2):452–63.
Goldring MB. Osteoarthritis and cartilage: the role of cytokines. Curr Rheumatol Rep. 2000;2(6):459–65.
Hunter DJ, McDougall JJ, Keefe FJ. The symptoms of OA and the genesis of pain. Rheum Dis Clin N Am. 2008;34(3):623–43.
Grigg P, Schaible HG, Schmidt RF. Mechanical sensitivity of group III and IV afferents from posterior articular nerve in normal and inflamed cat knee. J Neurophysiol. 1986;55(4):635–43.
Schaible HG, Schmidt RF. Effects of an experimental arthritis on the sensory properties of fine articular afferent units. J Neurophysiol. 1985;54(5):1109–22.
Coderre TJ, Katz J, Vaccarino AL, et al. Contribution of central neuroplasticity to pathological pain: review of clinical and experimental evidence. Pain. 1993;52(3):259–85.
Gwilym SE, Keltner JR, Warnaby CE, et al. Psychophysical and functional imaging evidence supporting the presence of central sensitization in a cohort of osteoarthritis patients. Arthritis Rheum. 2009;61(9):1226–34.
Melzack R, Coderre TJ, Katz J, et al. Central neuroplasticity and pathological pain. Ann N Y Acad Sci. 2001;933:157–74.
Ballegaard C, Riis RG, Bliddal H, et al. Knee pain and inflammation in the infrapatellar fat pad estimated by conventional and dynamic contrast-enhanced magnetic resonance imaging in obese patients with osteoarthritis: a cross-sectional study. Osteoarthr Cartil. 2014;22(7):933–40.
Hill CL, Gale DG, Chaisson CE, et al. Knee effusions, popliteal cysts, and synovial thickening: association with knee pain in osteoarthritis. J Rheumatol. 2001;28(6):1330–7.
Roach HI, Aigner T, Soder S, et al. Pathobiology of osteoarthritis: pathomechanisms and potential therapeutic targets. Curr Drug Targets. 2007;8(2):271–82.
Aigner T, Sachse A, Gebhard PM, et al. Osteoarthritis: pathobiology-targets and ways for therapeutic intervention. Adv Drug Deliv Rev. 2006;58(2):128–49.
Remst DF, Blaney Davidson EN, van der Kraan PM. Unravelling osteoarthritis-related synovial fibrosis: a step closer to solving joint stiffness. Rheumatology (Oxford). 2015;54:1954.
Felson DT, Niu J, Guermazi A, et al. Correlation of the development of knee pain with enlarging bone marrow lesions on magnetic resonance imaging. Arthritis Rheum. 2007;56(9):2986–92.
Felson DT, Chaisson CE, Hill CL, et al. The association of bone marrow lesions with pain in knee osteoarthritis. Ann Intern Med. 2001;134(7):541–9.
Wood JN. Nerve growth factor and pain. N Engl J Med. 2010;363(16):1572–3.
Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012;64(4):465–74.
Jevsevar DS, Brown GA, Jones DL, et al. The American Academy of Orthopaedic Surgeons evidence-based guideline on: treatment of osteoarthritis of the knee, 2nd edition. J Bone Joint Surg Am. 2013;95(20):1885–6.
Lu M, Su Y, Zhang Y, et al. Effectiveness of aquatic exercise for treatment of knee osteoarthritis: systematic review and meta-analysis. Z Rheumatol. 2015;74(6):543–52.
Bressel E, Wing JE, Miller AI, et al. High-intensity interval training on an aquatic treadmill in adults with osteoarthritis: effect on pain, balance, function, and mobility. J Strength Cond Res. 2014;28(8):2088–96.
Waller B, Ogonowska-Slodownik A, Vitor M, et al. Effect of therapeutic aquatic exercise on symptoms and function associated with lower limb osteoarthritis: systematic review with meta-analysis. Phys Ther. 2014;94(10):1383–95.
Ashraf A, Zarei F, Hadianfard MJ, et al. Comparison the effect of lateral wedge insole and acupuncture in medial compartment knee osteoarthritis: a randomized controlled trial. Knee. 2014;21(2):439–44.
Manheimer E, Linde K, Lao L, et al. Meta-analysis: acupuncture for osteoarthritis of the knee. Ann Intern Med. 2007;146(12):868–77.
Kwon YD, Pittler MH, Ernst E. Acupuncture for peripheral joint osteoarthritis: a systematic review and meta-analysis. Rheumatology (Oxford). 2006;45(11):1331–7.
Foster NE, Thomas E, Barlas P, et al. Acupuncture as an adjunct to exercise based physiotherapy for osteoarthritis of the knee: randomised controlled trial. BMJ. 2007;335(7617):436.
Garfinkel MS, Schumacher HR Jr, Husain A, et al. Evaluation of a yoga based regimen for treatment of osteoarthritis of the hands. J Rheumatol. 1994;21(12):2341–3.
Perlman AI, Sabina A, Williams AL, et al. Massage therapy for osteoarthritis of the knee: a randomized controlled trial. Arch Intern Med. 2006;166(22):2533–8.
Yip YB, Tam AC. An experimental study on the effectiveness of massage with aromatic ginger and orange essential oil for moderate-to-severe knee pain among the elderly in Hong Kong. Complement Ther Med. 2008;16(3):131–8.
Kang JW, Lee MS, Posadzki P, et al. T'ai chi for the treatment of osteoarthritis: a systematic review and meta-analysis. BMJ Open. 2011;1(1):e000035.
Wang C, Schmid CH, Hibberd PL, et al. Tai chi is effective in treating knee osteoarthritis: a randomized controlled trial. Arthritis Rheum. 2009;61(11):1545–53.
Ni GX, Song L, Yu B, et al. Tai chi improves physical function in older Chinese women with knee osteoarthritis. J Clin Rheumatol. 2010;16(2):64–7.
Chou R. Review: acetaminophen reduces pain in hip or knee osteoarthritis by a small amount, but not low back pain. Ann Intern Med. 2015;163(2):Jc10.
Verkleij SP, Luijsterburg PA, Bohnen AM, et al. NSAIDs vs acetaminophen in knee and hip osteoarthritis: a systematic review regarding heterogeneity influencing the outcomes. Osteoarthr Cartil. 2011;19(8):921–9.
Towheed TE, Maxwell L, Judd MG, et al. Acetaminophen for osteoarthritis. Cochrane Database Syst Rev. 2006;(1):CD004257.
Laslett LL, Jones G. Capsaicin for osteoarthritis pain. Prog Drug Res. 2014;68:277–91.
McCarthy GM, McCarty DJ. Effect of topical capsaicin in the therapy of painful osteoarthritis of the hands. J Rheumatol. 1992;19(4):604–7.
Makris UE, Abrams RC, Gurland B, et al. Management of persistent pain in the older patient: a clinical review. JAMA. 2014;312(8):825–36.
Lanas A, Boers M, Nuevo J. Gastrointestinal events in at-risk patients starting non-steroidal anti-inflammatory drugs (NSAIDs) for rheumatic diseases: the EVIDENCE study of European routine practice. Ann Rheum Dis. 2015;74(4):675–81.
Richette P, Latourte A, Frazier A. Safety and efficacy of paracetamol and NSAIDs in osteoarthritis: which drug to recommend? Expert Opin Drug Saf. 2015;14(8):1259–68.
Sostek MB, Fort JG, Estborn L, et al. Long-term safety of naproxen and esomeprazole magnesium fixed-dose combination: phase III study in patients at risk for NSAID-associated gastric ulcers. Curr Med Res Opin. 2011;27(4):847–54.
Angiolillo DJ, Datto C, Raines S, et al. Impact of concomitant low-dose aspirin on the safety and tolerability of naproxen and esomeprazole magnesium delayed-release tablets in patients requiring chronic nonsteroidal anti-inflammatory drug therapy: an analysis from 5 phase III studies. J Thromb Thrombolysis. 2014;38(1):11–23.
American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc. 2009;57(8):1331–46.
Dhillon S. Tramadol/paracetamol fixed-dose combination: a review of its use in the management of moderate to severe pain. Clin Drug Investig. 2010;30(10):711–38.
Silverfield JC, Kamin M, Wu SC, et al. Tramadol/acetaminophen combination tablets for the treatment of osteoarthritis flare pain: a multicenter, outpatient, randomized, double-blind, placebo-controlled, parallel-group, add-on study. Clin Ther. 2002;24(2):282–97.
Ishimaru D, Sugiura N, Akiyama H, et al. Alterations in the chondroitin sulfate chain in human osteoarthritic cartilage of the knee. Osteoarthr Cartil. 2014;22(2):250–8.
Bruyere O, Reginster JY. Glucosamine and chondroitin sulfate as therapeutic agents for knee and hip osteoarthritis. Drugs Aging. 2007;24(7):573–80.
Rivera F, Bertignone L, Grandi G, et al. Effectiveness of intra-articular injections of sodium hyaluronate-chondroitin sulfate in knee osteoarthritis: a multicenter prospective study. J Orthop Traumatol. 2016;17(1):27–33.
Martel-Pelletier J, Roubille C, Abram F, et al. First-line analysis of the effects of treatment on progression of structural changes in knee osteoarthritis over 24 months: data from the osteoarthritis initiative progression cohort. Ann Rheum Dis. 2015;74(3):547–56.
Rovati LC, Girolami F, D’Amato M, et al. Effects of glucosamine sulfate on the use of rescue non-steroidal anti-inflammatory drugs in knee osteoarthritis: results from the Pharmaco-Epidemiology of GonArthroSis (PEGASus) study. Semin Arthritis Rheum. 2016;45(4 Suppl):S34–41.
Kanzaki N, Ono Y, Shibata H, et al. Glucosamine-containing supplement improves locomotor functions in subjects with knee pain: a randomized, double-blind, placebo-controlled study. Clin Interv Aging. 2015;10:1743–53.
Sawitzke AD, Shi H, Finco MF, et al. The effect of glucosamine and/or chondroitin sulfate on the progression of knee osteoarthritis: a report from the glucosamine/chondroitin arthritis intervention trial. Arthritis Rheum. 2008;58(10):3183–91.
Fransen M, Agaliotis M, Nairn L, et al. Glucosamine and chondroitin for knee osteoarthritis: a double-blind randomised placebo-controlled clinical trial evaluating single and combination regimens. Ann Rheum Dis. 2015;74(5):851–8.
Clegg DO, Reda DJ, Harris CL, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med. 2006;354(8):795–808.
Sawitzke AD, Shi H, Finco MF, et al. Clinical efficacy and safety of glucosamine, chondroitin sulphate, their combination, celecoxib or placebo taken to treat osteoarthritis of the knee: 2-year results from GAIT. Ann Rheum Dis. 2010;69(8):1459–64.
Hitzeman N, Athale N. Opioids for osteoarthritis of the knee or hip. Am Fam Physician. 2010;81(9):1094.
Posnett J, Dixit S, Oppenheimer B, et al. Patient preference and willingness to pay for knee osteoarthritis treatments. Patient Prefer Adherence. 2015;9:733–44.
Wilson N, Sanchez-Riera L, Morros R, et al. Drug utilization in patients with OA: a population-based study. Rheumatology (Oxford). 2015;54(5):860–7.
Solomon DH, Avorn J, Wang PS, et al. Prescription opioid use among older adults with arthritis or low back pain. Arthritis Care Res. 2006;55(1):35–41.
Bellamy N, Campbell J, Robinson V, et al. Intraarticular corticosteroid for treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;(2):CD005328.
Hepper CT, Halvorson JJ, Duncan ST, et al. The efficacy and duration of intra-articular corticosteroid injection for knee osteoarthritis: a systematic review of level I studies. J Am Acad Orthop Surg. 2009;17(10):638–46.
Creamer P. Intra-articular corticosteroid injections in osteoarthritis: do they work and if so, how? Ann Rheum Dis. 1997;56(11):634–6.
D’Acquisto F, Paschalidis N, Raza K, et al. Glucocorticoid treatment inhibits annexin-1 expression in rheumatoid arthritis CD4+ T cells. Rheumatology (Oxford). 2008;47(5):636–9.
Pelletier JP, DiBattista JA, Raynauld JP, et al. The in vivo effects of intraarticular corticosteroid injections on cartilage lesions, stromelysin, interleukin-1, and oncogene protein synthesis in experimental osteoarthritis. Lab Investig. 1995;72(5):578–86.
Pelletier JP, Martel-Pelletier J. In vivo protective effects of prophylactic treatment with tiaprofenic acid or intraarticular corticosteroids on osteoarthritic lesions in the experimental dog model. J Rheumatol Suppl. 1991;27:127–30.
MacMahon PJ, Eustace SJ, Kavanagh EC. Injectable corticosteroid and local anesthetic preparations: a review for radiologists. Radiology. 2009;252(3):647–61.
Blankenbaker DG, De Smet AA, Stanczak JD, et al. Lumbar radiculopathy: treatment with selective lumbar nerve blocks--comparison of effectiveness of triamcinolone and betamethasone injectable suspensions. Radiology. 2005;237(2):738–41.
Wright JM, Cowper JJ, Page Thomas DP, et al. The hydrolysis of cortisol 21-esters by a homogenate of inflamed rabbit synovium and by rheumatoid synovial fluid. Clin Exp Rheumatol. 1983;1(2):137–41.
Lomonte AB, de Morais MG, de Carvalho LO, et al. Efficacy of triamcinolone hexacetonide versus methylprednisolone acetate intraarticular injections in knee osteoarthritis: a randomized, double-blinded, 24-week study. J Rheumatol. 2015;42:1677.
Stanczak J, Blankenbaker DG, De Smet AA, et al. Efficacy of epidural injections of Kenalog and Celestone in the treatment of lower back pain. AJR Am J Roentgenol. 2003;181(5):1255–8.
Hirsch G, Kitas G, Klocke R. Intra-articular corticosteroid injection in osteoarthritis of the knee and hip: factors predicting pain relief—a systematic review. Semin Arthritis Rheum. 2013;42(5):451–73.
Godwin M, Dawes M. Intra-articular steroid injections for painful knees. Systematic review with meta-analysis. Can Fam Physician. 2004;50:241–8.
Yavuz U, Sokucu S, Albayrak A, et al. Efficacy comparisons of the intraarticular steroidal agents in the patients with knee osteoarthritis. Rheumatol Int. 2012;32(11):3391–6.
Kruse DW. Intraarticular cortisone injection for osteoarthritis of the hip. Is it effective? Is it safe? Curr Rev Musculoskelet Med. 2008;1(3–4):227–33.
Kullenberg B, Runesson R, Tuvhag R, et al. Intraarticular corticosteroid injection: pain relief in osteoarthritis of the hip? J Rheumatol. 2004;31(11):2265–8.
Chao J, Wu C, Sun B, et al. Inflammatory characteristics on ultrasound predict poorer long-term response to intraarticular corticosteroid injections in knee osteoarthritis. J Rheumatol. 2010;37(3):650–5.
Raynauld JP, Buckland-Wright C, Ward R, et al. Safety and efficacy of long-term intraarticular steroid injections in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2003;48(2):370–7.
Habib G, Safia A. The effect of intra-articular injection of betamethasone acetate/betamethasone sodium phosphate on blood glucose levels in controlled diabetic patients with symptomatic osteoarthritis of the knee. Clin Rheumatol. 2009;28(1):85–7.
Syed HM, Green L, Bianski B, et al. Bupivacaine and triamcinolone may be toxic to human chondrocytes: a pilot study. Clin Orthop Relat Res. 2011;469(10):2941–7.
Dragoo JL, Danial CM, Braun HJ, et al. The chondrotoxicity of single-dose corticosteroids. Knee Surg Sports Traumatol Arthrosc. 2012;20(9):1809–14.
Venkatesan P, Fangman WL. Linear hypopigmentation and cutaneous atrophy following intra-articular steroid injections for de Quervain’s tendonitis. J Drugs Dermatol. 2009;8(5):492–3.
Tempfer H, Gehwolf R, Lehner C, et al. Effects of crystalline glucocorticoid triamcinolone acetonide on cultered human supraspinatus tendon cells. Acta Orthop. 2009;80(3):357–62.
Muto T, Kokubu T, Mifune Y, et al. Temporary inductions of matrix metalloprotease-3 (MMP-3) expression and cell apoptosis are associated with tendon degeneration or rupture after corticosteroid injection. J Orthop Res. 2014;32(10):1297–304.
Hossain MA, Park J, Choi SH, et al. Dexamethasone induces apoptosis in proliferative canine tendon cells and chondrocytes. Vet Comp Orthop Traumatol. 2008;21(4):337–42.
Hunter DJ. Viscosupplementation for osteoarthritis of the knee. N Engl J Med. 2015;372(26):2570.
Wobig M, Bach G, Beks P, et al. The role of elastoviscosity in the efficacy of viscosupplementation for osteoarthritis of the knee: a comparison of hylan G-F 20 and a lower-molecular-weight hyaluronan. Clin Ther. 1999;21(9):1549–62.
Kotevoglu N, Iyibozkurt PC, Hiz O, et al. A prospective randomised controlled clinical trial comparing the efficacy of different molecular weight hyaluronan solutions in the treatment of knee osteoarthritis. Rheumatol Int. 2006;26(4):325–30.
Ghosh P, Guidolin D. Potential mechanism of action of intra-articular hyaluronan therapy in osteoarthritis: are the effects molecular weight dependent? Semin Arthritis Rheum. 2002;32(1):10–37.
Modawal A, Ferrer M, Choi HK, et al. Hyaluronic acid injections relieve knee pain. J Fam Pract. 2005;54(9):758–67.
Medina JM, Thomas A, Denegar CR. Knee osteoarthritis: should your patient opt for hyaluronic acid injection? J Fam Pract. 2006;55(8):669–75.
Campbell J, Bellamy N, Gee T. Differences between systematic reviews/meta-analyses of hyaluronic acid/hyaluronan/hylan in osteoarthritis of the knee. Osteoarthr Cartil. 2007;15(12):1424–36.
Tang SF, Chen CP, Chen MJ, et al. Changes in sagittal ground reaction forces after intra-articular hyaluronate injections for knee osteoarthritis. Arch Phys Med Rehabil. 2004;85(6):951–5.
Zoboli AA, de Rezende MU, de Campos GC, et al. Prospective randomized clinical trial: single and weekly viscosupplementation. Acta Ortop Bras. 2013;21(5):271–5.
Altman RD, Schemitsch E, Bedi A. Assessment of clinical practice guideline methodology for the treatment of knee osteoarthritis with intra-articular hyaluronic acid. Semin Arthritis Rheum. 2015;45:132.
Strand V, McIntyre LF, Beach WR, et al. Safety and efficacy of US-approved viscosupplements for knee osteoarthritis: a systematic review and meta-analysis of randomized, saline-controlled trials. J Pain Res. 2015;8:217–28.
Goldberg VM, Coutts RD. Pseudoseptic reactions to hylan viscosupplementation: diagnosis and treatment. Clin Orthop Relat Res. 2004;419:130–7.
Zhu Y, Yuan M, Meng HY, et al. Basic science and clinical application of platelet-rich plasma for cartilage defects and osteoarthritis: a review. Osteoarthr Cartil. 2013;21(11):1627–37.
Riboh JC, Saltzman BM, Yanke AB, et al. Effect of leukocyte concentration on the efficacy of platelet-rich plasma in the treatment of knee osteoarthritis. Am J Sports Med. 2016;44(3):792–800.
Sanchez M, Anitua E, Azofra J, et al. Intra-articular injection of an autologous preparation rich in growth factors for the treatment of knee OA: a retrospective cohort study. Clin Exp Rheumatol. 2008;26(5):910–3.
Spakova T, Rosocha J, Lacko M, et al. Treatment of knee joint osteoarthritis with autologous platelet-rich plasma in comparison with hyaluronic acid. Am J Phys Med Rehabil. 2012;91(5):411–7.
Gobbi A, Lad D, Karnatzikos G. The effects of repeated intra-articular PRP injections on clinical outcomes of early osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc. 2015;23(8):2170–7.
Filardo G, Kon E, Di Martino A, et al. Platelet-rich plasma vs hyaluronic acid to treat knee degenerative pathology: study design and preliminary results of a randomized controlled trial. BMC Musculoskelet Disord. 2012;13:229.
Filardo G, Di Matteo B, Di Martino A, et al. Platelet-rich plasma intra-articular knee injections show no superiority versus viscosupplementation: a randomized controlled trial. Am J Sports Med. 2015;43(7):1575–82.
Chang KV, Hung CY, Aliwarga F, et al. Comparative effectiveness of platelet-rich plasma injections for treating knee joint cartilage degenerative pathology: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2014;95(3):562–75.
Campbell KA, Saltzman BM, Mascarenhas R, et al. Does intra-articular platelet-rich plasma injection provide clinically superior outcomes compared with other therapies in the treatment of knee osteoarthritis? A systematic review of overlapping meta-analyses. Arthroscopy. 2015;31(11):2213–21.
Oh JH, Kim W, Park KU, et al. Comparison of the cellular composition and cytokine-release kinetics of various platelet-rich plasma preparations. Am J Sports Med. 2015;43(12):3062–70.
Sakata R, McNary SM, Miyatake K, et al. Stimulation of the superficial zone protein and lubrication in the articular cartilage by human platelet-rich plasma. Am J Sports Med. 2015;43(6):1467–73.
Mishra AK, Skrepnik NV, Edwards SG, et al. Efficacy of platelet-rich plasma for chronic tennis elbow: a double-blind, prospective, multicenter, randomized controlled trial of 230 patients. Am J Sports Med. 2014;42(2):463–71.
Ornetti P, Nourissat G, Berenbaum F, et al. Does platelet-rich plasma have a role in the treatment of osteoarthritis? Joint Bone Spine. 2016;83(1):31–6.
Lee WD, Hurtig MB, Pilliar RM, et al. Engineering of hyaline cartilage with a calcified zone using bone marrow stromal cells. Osteoarthr Cartil. 2015;23(8):1307–15.
Qi Y, Feng G, Yan W. Mesenchymal stem cell-based treatment for cartilage defects in osteoarthritis. Mol Biol Rep. 2012;39(5):5683–9.
Matsumoto T, Cooper GM, Gharaibeh B, et al. Cartilage repair in a rat model of osteoarthritis through intraarticular transplantation of muscle-derived stem cells expressing bone morphogenetic protein 4 and soluble Flt-1. Arthritis Rheum. 2009;60(5):1390–405.
Gibbs N, Diamond R, Sekyere EO, et al. Management of knee osteoarthritis by combined stromal vascular fraction cell therapy, platelet-rich plasma, and musculoskeletal exercises: a case series. J Pain Res. 2015;8:799–806.
Vega A, Martin-Ferrero MA, Del Canto F, et al. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: a randomized controlled trial. Transplantation. 2015;99(8):1681–90.
Rodriguez-Merchan EC. Intra-articular injections of mesenchymal stem cells for knee osteoarthritis. Am J Orthop (Belle Mead NJ). 2014;43(12):E282–91.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Fourman, M.S., Kalawadia, J.V., Bradley, J. (2018). Injectable, Biologics, and Stem Cells. In: Wright, V., Middleton, K. (eds) Masterful Care of the Aging Athlete. Springer, Cham. https://doi.org/10.1007/978-3-319-16223-2_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-16223-2_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-16222-5
Online ISBN: 978-3-319-16223-2
eBook Packages: MedicineMedicine (R0)