Abstract
Oral or parenteral administered drugs used to treat knee osteoarthritis (OA) enter the joint through complicated pharmacokinetic processes. Intra-articular (IA) injection therapy has a number of advantages over systemic administration such as bypassing this process and avoiding systemic adverse events. For IA injection therapy to work effectively, drugs must be injected accurately into the joints. Image guided injection using ultrasound is more useful than blind method for accurate IA injection. IA therapeutic agents for the treatment of knee OA include corticosteroids (CS), hyaluronic acid (HA), biologics. CS has a short-term effect on improving symptoms of knee OA, but HA has a relatively longer term effect. Biologic agents either target specific catabolic proinflammatory mediators or affect anabolism because OA results from an imbalance between catabolic and anabolic factors. Biologics used for treatment of knee OA are categorized into non-cellular or cell therapy. Non-cellular therapy includes human serum albumin, growth factors, cytokine antagonists. In particular, the recombinant human fibroblast growth factor 18 and the wnt receptor inhibitor have an anabolic effect. Cell therapy includes cell concentrates, mesenchymal stromal cells, and gene therapy. Recently, cell concentrates are commonly used for knee OA treatment as autologous point-of-care cell therapy regardless of its efficacy. Cell concentrates include stromal vascular fraction (SVF), bone marrow aspirate concentrate (BMAC), plasma rich platelet (PRP), and autologous protein solution. The therapeutic effects of PRP remain for more than 6 months, but effect size has not reached minimal clinical important difference. Mesenchymal stromal cells (MSCs) are grown from cell concentrates in vitro and separated with only cells with MSC characteristics. MSCs used in the treatment of knee OA include bone marrow-derived MSCs and adipose-derived MSCs. Despite the clinical potential of MSCs, clinical efficacy in knee OA treatment is limited. According to guidelines from non-profit organizations, PRP and MSC injections are strongly recommended against in patients with knee OA.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Intra-articular injection
- Pharmacokinetics
- Ultrasound
- Corticosteroid
- Hyaluronic acid
- Polydeoxyribonucleotide
- Hypertonic dextrose
- Biologics
- Growth factors
- Cytokine antagonists
- Plasma rich platelet
- Mesenchymal stromal cells
10.1 Introduction
Osteoarthritis (OA) is defined as an irreversible and progressive damage to the articular cartilage of the knee. It was once known as a ‘wear and tear’ disease . However, complex interactions between aging, genetic, metabolic, biochemical, and biomechanical factors play an important role in the development of OA. Clinical manifestations of knee OA are pain, stiffness, joint swelling, and loss of motion. These symptoms can interfere with work and normal daily activities. The incidence of knee OA is increasing rapidly in the aging society. Knee OA produces a huge economic burden to society due to high prevalence and functional disabilities [1].
Current treatment for OA focuses on relieving symptoms and improving function. General guidelines for the management of knee OA mostly recommend a combination of non-pharmacological and pharmacological treatment [2]. Oral medications such as acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs) are commonly used as pharmacological treatments. However, long-term use of oral medications has raised concerns about their risk/benefit ratio issues, especially for patients with cardiovascular, gastrointestinal, and metabolic comorbidities [3]. Intra-articular (IA) injections , such as a corticosteroid (CS) and hyaluronic acid (HA), may be used to treat knee OA after other conservative treatments have failed [4]. IA injection became popular in the late twentieth century since the introduction of IA CS. IA drug delivery might be the ideal mode of drug delivery for these patients because it has many advantages such as increased local bioavailability, reduced systemic exposure, and lower total drug cost [5].
Despite a number of advantages of IA drug delivery, there are many controversial issues regarding the safety and efficacy of IA injection procedure and drug delivery. The incidence of adverse events (AEs) attributable to IA therapies in knee OA is very low, furthermore, these events are rarely serious. Clinician can distinguish self-limited AEs, such as post-injection pain and swelling that are the most frequently reported AEs, from not self-limited AEs, such as septic arthritis that is rarely reported [6]. For a safe and accurate IA drug delivery, direct IA injection with a needle is most preferred. There is a question about the efficacy of IA injection therapy because IA injections itself have a strong placebo effect. The effect size of placebo injection is significantly greater than did oral or topical placebos [7]. IA-normal saline placebo injection yields a significant improvement in symptoms up to 6 months after the injection in knee OA [8]. This placebo effect might be caused by dilution and reduction of inflammatory mediators in the joint effusion, providing relief of perceived pain and subjective stiffness. As to the efficacy of IA drug, there are problems that need to be solved such as the number of injections, bioavailability of injection vehicle, effect size of injections, long-term efficacy, or exact mechanism of action because of a lack of science based evidence of IA injections for OA [9]. Despite various controversies over efficacy, IA injection treatments are still commonly used in clinical practice.
CS and HA injections have been attempted for decades to treat the symptoms of knee OA and its pros and cons are already well known [10]. Recently, there is a growing interest in the delivery of autologous blood products, recombinant proteins, particles, cells, and gene therapy vectors to diseased joints. Local delivery in this way is potentially safer, less expensive, and more effective than parenteral delivery. However, better drug formulations with longer lasting efficacy are needed to reduce the need for burdensome repeated injections of soluble therapeutic agents [11]. Many biologic agents, including non-cellular or cellular components, were introduced and investigated extensively for OA treatment since a decade ago. The efficacy of biological agents has yet to be proven, but some of these drugs are undergoing clinical research, producing promising results [5].
In this chapter, we briefly describe the pharmacokinetics of synovial joint and explain the injection technique to increase the accuracy of needle placement by manual or using imaging modalities. We also describe the mechanism of action of the IA therapeutic agents including biologics and discuss clinical studies that have investigated small molecule drug therapies and provide a high-level overview of biologics including cell-based therapies. Finally, we deliver an update on a critical assessment of some of the most anticipated and promising IA injection therapies of knee OA currently in clinical development.
10.2 Influx and Efflux of the Molecules in the Synovial Joint
The drug delivery via systemic administration is not efficient because the cartilage is an avascular tissue. When a therapeutic substance is administered through oral or parenteral, the substance does not reach the cartilage directly, instead, reaches the joint fluid through the capillary network and interstitial tissue in the synovial membrane and then diffused to the extracellular matrix (ECM) of the cartilage. In order to treat knee OA using certain therapeutic substances, the influx and efflux of the therapeutic substances or molecules, residence time within the joint, and diffusion in the ECM of the cartilage must be well understood.
In order for the molecules to enter the joint cavity from the synovial capillaries, the molecule must first pass through the walls of the capillaries and then through the ECM of synovial intima [12]. About 50% of synovial capillaries has a fenestration in the endothelial lining that is faced to the joint cavity. Such orientation facilitates the transport of molecules from these capillaries to synovial interstitium, vice versa. The synovial membrane is characterized by non-epithelial cells, wide intercellular gaps, no cell junctions, and no basement membrane [13]. The synovial membrane acts as a semipermeable membrane controlling molecular traffic into and out of the joint space. Furthermore, no basement membrane in the synovial layer facilitates molecular transport. The molecules exiting the blood vessels through the fenestration diffuse to synovial interstitium and then pass through the synovial membrane into the joint cavity. The factors determining the movement of the molecules are the pore size of the fenestration in the endothelium of the capillaries and the tight space of the synovial interstitial matrix. Small molecular weight (MW) compounds (MW <10 kDa) freely transport through passive diffusion. Because the fenestration in the endothelial lining of the capillaries roles as a size-dependent sieving effect, transportation of molecules from blood into synovial fluid is quantitatively related to molecular size. Therefore, relatively large molecules have different synovial fluid:serum concentration ratios compared to smaller molecules. For example, the concentration ratio of normal synovial fluid:serum for albumin (MW 67 kDa) is ~0.40; for the much larger molecules α2–macroglobulin (MW 820 kDa) is 0.03 [14]. In inflamed joint, capillary permeability increases, improving the entry of macromolecules into the joint space. Evidence indicating this effect can be found in the protein content of synovial fluid from patients with rheumatoid arthritis, which increases compared to healthy controls and significantly increases the ratio of large and small molecular components in rheumatoid arthritis samples [5, 14] (Fig. 10.1).
Influx and efflux of the molecules in the synovial joint. Systematically administered molecules in the capillaries enter the joint cavity through capillary wall, extracellular matrix (ECM), and synovial membrane. Small molecules pass through capillary wall and synovium relatively easily but major resistance to their entry is the ECM of the synovial interstitium. Large molecules in the capillary are sieved by the fenestrated endothelium of the capillaries, which are obstacles to enter the joint cavity. Intra-articular injection is a way of circumventing this resistance. Molecules in synovial fluid are excreted through the vasculature and lymphatic system in synovium. Small molecules also leave via the vasculature whereas large molecules such as proteins exit via the lymphatic system. (Copyright permission: Nat. Rev. Rheumatol. Evans, 2013)
Molecules in synovial fluid are excreted through the vasculature and lymphatic system in synovium. Small molecules also leave via the vasculature whereas macromolecules such as proteins exit via the lymphatic system [15]. The residence time of molecules in the joint is affected by the rate at which they enter and exit the joint. Small molecules have short IA residence time because they easily enter and rapidly exit from the joint via the synovial capillaries. The entry of macromolecules into joints is constrained by a size-dependent sieving effect of the endothelial fenestration of capillaries. Although IA injection with macromolecules can bypass the capillary sieving effect, the IA residence time of macromolecules is typically a few hours or less because its removal from joints occurs via the lymphatic system regardless of its size. The half-lives of various substances reported are 1.23–13.1 h for albumin (MW 67 kDa), 0.35 h for lidocaine (MW 234 Da), and 26.3 h for hyaluronic acid (MW 3 × 106 Da). IA half-lives of NSAIDs were around 1.1–5.2 h and hydrocortisone at around 0.3–4.2 h [16].
Likewise, the residence time of molecules is short regardless of its size. Furthermore, in case of joint inflammation, the residence time of molecules becomes shorter because of increased vascularity and lymphatic flow [17]. This short residence time of the molecules is a major barrier to successful therapy. The IA injected therapeutic drug must be maintained within the joint for sufficient time to work. To do so, it is necessary to develop various methods to increase the drug residence time .
10.3 Intra-articular Injection Technique
The IA injection of therapeutic agents is an attractive method for the local treatment of joint diseases. To achieve the maximal potential treatment, various therapeutic agents should be correctly delivered into the joint. It is important to position the needle precisely inside the joint in order to achieve sufficient therapeutic effect and reduce the AEs of the injection. Incorrect placement of the needle also causes more pain and discomfort to the patient during and after the procedure, which can have a negative influence on the efficacy of the product being injected [4]. Injection technique is a very important factor for accuracy of IA knee injection, especially in symptomatic knee OA with no effusion [4]. However, it is difficult to place accurately a needle into the IA space of the knee without effusion. Jones et al. [18] evaluated the accuracy of needle placement into the IA space of the knee in the absence of a joint effusion and reported that 39 (66%) of 59 knee joint injections were IA and almost one-third were extra-articular. If therapeutic materials are injected into extra-synovial tissue, it may result in either painful blockage of the injected material outflow or the development of acute pseudoseptic arthritis. Therefore, an accurate needle placement is very important for IA drug delivery for knee OA treatment. There are two techniques, such as blind (palpation guided or landmark guided) or image guided injection, for IA knee injection. Accurate IA knee injection is not easy for patients who have dry joint, especially obese and/or severe arthritic knees by blind technique. Various methods such as injection of contrast or air with radiography [19], ultrasonography (US)[20] or fluoroscopy [21], magnetic resonance imaging (MRI) [22], surgical confirmation of intra- or extra-articular placement of drugs [23] have been used to evaluate the accuracy of IA knee injection. The backflow technique is also a helpful method for confirmation of the IA placement of needle during injection that has an accuracy of about 97% [24]. However, because all of these techniques are influenced by observer error in the evaluation of images, there is no gold standard for evaluating the accuracy of IA knee injections. Accuracy is improved by fluoroscopic and US guided techniques, and these tools are particularly useful for treating joints that are difficult to access, such as obese or dry joint. In this section, we included all studies independently attempted to confirm IA placement, including successful aspiration of synovial fluid. Furthermore, the results on accuracy of the sites of injection were described by injection site and whether image guidance was used or not, and this review did not look separately at accuracy by image guided method.
10.3.1 Injection Techniques: Sites, Approaches
A number of IA injection sites have been proposed to maximize therapeutic benefits and avoid incorrect knee injection when performing IA knee injections using blind or image guided techniques . Injection “sites” refer to specific areas in the knee for needle entry, and injection “approaches” refer to techniques that deliver the needle, including angle of the needle or position of the knee. There are various approaches to the same injection site, and there is a lot of controversy over which area is the best injection area for accurate injection. In a systematic review, eight different knee injection sites were identified regardless of injection technique that were (1) anteromedial, (2) medial mid-patellar, (3) superomedial patellar, (4) anterolateral, (5) lateral mid-patellar, (6) superolateral patellar, (7) lateral suprapatellar bursa, and (8) infrapatellar [25]. Infrapatellar site will not be described due to lack of literatures (Fig. 10.2).
10.3.1.1 Anteromedial (AM)
For AM joint site , the needle is inserted into the portal formed by inferomedial patellar border, patellar tendon, and medial tibial plateau, directing the needle toward intercondylar notch with the knee flexed 30° [21] or 90° with the patient’s leg hanging over the side of the examination table [23]. Accuracy rates of AM site in knee 30° and 90° flexion were 86% and 71%, respectively [26]. The use of the standard AM site may result in pain and potential harm with accidental injection into the cruciate ligaments, the ligamentum mucosum, or the fat pad.
10.3.1.2 Medial Mid-Patellar (MMP)
This technique is performed with the knee in extension . The patella is pulled medially or laterally, and a needle is advanced under the patella. Injection via the MMP approach is performed with the needle placed on the medial side of the knee joint under the middle of the patella (midpole) and directed towards the lateral patellar midpole [27]. The needles were inserted carefully to avoid injuring special structures such as the patella retinacula, periosteum, retropatella cartilage, and fat pad. MMP portal showed the least accuracy rate, which was 56% [23], 77.3% in blind injection [27], 75% in US guided injection [28].
10.3.1.3 Superomedial Patellar (SMP)
The SMP injection is performed by inserting a needle in a 45° cephalomedial to caudolateral direction between the femoral condyle and the lateral border of patella at the superior 1/3 margin of the patella with the knee extended. Gentle shaking of the patella will identify its border to facilitate the needle insertion. This portal provides 80% accuracy rate when using blind techniques [29].
10.3.1.4 Anterolateral (AL)
The standard AL injection was performed in the site formed by inferolateral patellar border, patellar tendon, and lateral tibial plateau with the patient sitting with the knees hanging freely over the side of the examination table and flexed to approximately 90°. However, there were several modified AL approaches regarding knee flexion degrees or needle direction. Different knee position had been proposed according to knee bending such as the knee flexed between 30° and 40° [21] or flexed to 90° [30] or full flexion ranging from 100° to 130° [31]. After palpation of the anatomic landmarks, the injection portal was selected one-fingerbreadth proximal to the tibial joint surface and one-fingerbreadth lateral to the patellar tendon. The needle was advanced obliquely toward the intercondylar notch or directing the needle toward medial femoral condyle. Accuracy rate of this approach was 71% [4], 85% [23], 97% [30], 97.1% [31]. These approaches are useful when the knee cannot be extended sufficiently, or when there is only a minimal amount of fluid in the knee joint. The AL site would provoke less pain as compared to the SL site [32]. The major problem with these approaches is that it is difficult to obtain fluid from the affected joint.
10.3.1.5 Lateral Mid-Patellar (LMP)
The LMP site is the most commonly utilized (64%) technique for knee arthrography among the North American radiologists [33]. For the LMP approach, the injection was made between patella and patellar groove at the mid lateral patellar junction with the knee extended. The needle was advanced transversely directing the needle at a 45° angle between the articular surfaces of the patellofemoral joint at the midpoint of the patella and pulled laterally [34]. Accuracy rate of the LMP approach was 55%–86%, that rate was directly proportional to severity of radiographic OA assessment [26], 76% [23], 91.5% [35], 93% [4]. The LMP has the advantage of allowing the needle to pass through the minimal soft tissue and reach the IA space. On the other hand, for those apprehensive individuals who involuntarily and forcefully contract the quadriceps muscles during a procedure, the elderly, individuals with knee contractures, the obese, large patellofemoral osteophytes, or wheelchair-bound individuals, the LMP approach can be difficult and/or inconvenient in these individuals. In addition, because less subcutaneous fat is transversed by the needle in the LMP portal, local AEs of injections may occur which can be easily observed, including visible ecchymosis, hematoma, and cutaneous atrophy or foreign body granuloma at the puncture site caused by reflux of CS or HA back through the needle tract [36].
10.3.1.6 Superolateral Patellar (SLP)
For the standard SLP approach, the patient is positioned supine on the examination table with the knee extended, and the patella and soft spot are palpated. The landmark is the intersection of the horizontal line from the upper border of the patella and another line crossing the lateral border of the patella. The needle was inserted in a 45° angle cephalolateral to caudomedial direction with parallel to the anterior femoral cortex. Accuracy rate of SLP site ranged from 55% to 100%, but the SLP approach resulted in the highest pooled accuracy rate of 91% (95% CI 84–99) among IA knee injection sites [37]. However, the SLP approach will not be suitable for several reasons: large osteophytes blocked the path for passage of the needle; the pain associated with patellar manipulation; determination of the configuration of bony landmarks is difficult, especially in obese patients.
10.3.1.7 Lateral Suprapatellar Bursa (LSB)
In the LSB approach , the needle is inserted from the superolateral aspect of patella, one-fingerbreadth above and one-fingerbreadth lateral to the patella with the knee extended [35, 38]. Accuracy rate of the LSB approach was 83.7% by blind and 96.0% by US guided injection [39], 82% by blind and 100% by US guided injection [40]. One of the advantages of an IA injection through the SB is that it reduces the risk of injuring other tissues in the knee joint [39]. When small effusions within the SB are detected, dynamic examination, such as isometric contraction of the quadriceps muscle or forced dorsiflexion of the foot with the knee extended, may be helpful. Quadriceps activation and hyperextension induce proximal shifting of fluid by displacing the Hoffa fat pad against the femoral condyles and tightening the posterior fascia.
10.3.2 Factors Related to Intra-articular Knee Accuracy
Accuracy of IA knee injections is affected by intrinsic factors such as obesity, severity of OA, presence or absence of joint effusion, etc. and external factors such as clinician’s experience, site of needle insertion, and use of image guide. Accurate IA knee injection is not easy for patients who have dry joint, especially obese and/or severe arthritic knees. These intrinsic factors cannot be modified when they first appear in the clinic, but extrinsic factors can be modified by clinician efforts.
In a systematic review with statistical pooling of accuracy rates, the SLP site resulted in high accuracy rates, with the highest pooled accuracy of 91% (95% CI 84–99%) and pooled accuracy rates for the LMP, AL, and AM site were 85% (95% CI 68–100%), 67% (95% CI 43–91%), and 72% (95% CI 65–78%), respectively [37]. This systematic review did not mention about guided injection data. The SLP site resulted in 100% accuracy rate, especially, in patient with effusion by blinded injection [19]. When attempting blind injection, SMP site (82%) had the highest injection accuracy among the three medial sites, followed by AM (74%) and MMP (64%), while SLP site (87%) had the greatest injection accuracy among the four lateral sites, followed by LMP (84%), LSB (83%), and AL (70%). When US guided injection was attempted, the accuracy was 100% on the SMP site and 86% on the MMP, which significantly increased the accuracy compared to the blind injection. Moreover, all four lateral sites (AL, LMP, SLP, and LSB) had 95–100% accuracy rate, when US guided injections were performed, and the best lateral site was still the SLP. The accuracy of medial sites was improved largely than the lateral sites by US guided injections. Therefore, US guided injections at MMP, SMP, LSB, and SLP sites were found to be significantly more accurate than their respective blinded injections. The experience of injector affected the accuracy rate of the blinded injections at the SLP site, which was 55% (95% CI 34–74) for the less experienced injector compared to 100% (95% CI 81–100) for the more experienced injector [38]. However, similar accuracy was found for less experienced junior clinicians and injectors when US guided injections were performed. When a research fellow performed the injections using US guided, accuracy rate of IA injection was 91% [41]. US guided has significantly improved the performance and effectiveness of the procedure, with a 43% decrease in pain associated with US guided procedures and a 26% increase in the proportion of treatment responses [40]. Accuracy is improved by US guided techniques that are particularly valuable for treating joints that are difficult to access, such as obese or dry joint.
10.3.3 Ultrasound Guided Injection Technique
Although the guideline of 2016 European League against Rheumatism (EULAR) recommends that IA injections using image guidance be used in specific situations but not routinely, IA injections using US have many advantages. US allows real-time monitoring during needle placement in a fast and less invasive manner without the risk of radiation exposure. In addition, a US machine is less expensive and widely available than a fluoroscopy or computed tomography/magnetic resonance scanner.
Choosing the right US device is important for accurate IA injection. In particular, appropriate transducers and ultrasonic frequency should be selected depending on the region of the musculoskeletal system. High frequency (7–15 MHz) line transducer is appropriate for the IA knee injection. This helps to obtain images of relatively superficial areas, such as knee joints, because of their low penetration and good resolution. Echogenicity refers to the ability to reflect a US wave. Each tissue type has a particular echogenicity in its normal state. Based on its echogenicity, a structure can be distinguished by hyperechoic (white on the screen), hypoechoic (gray on the screen), and anechoic (black on the screen). Bone appears anechoic or black on US and has a bright hyperechoic rim. Because the US beam cannot pass through the cortical bone, it casts an acoustic shadow underneath intensely hyperechoic bony structure. Articular cartilage appears grey or hypoechoic rim over a hyperechoic bony cortex. Blood vessels and joint fluid also appear anechoic. Muscles are hypoechoic with striate structure and fat is almost anechoic. Fascia/fascicles and other connective tissue appear as hyperechoic lines. Nerves appear hyperechoic with a stippled honeycomb pattern with hypo-anechoic fascia scattered between bright backgrounds. Tendons and ligaments appear hyperechoic, similar to the distal nerves. Tendons can be observed with characteristic striation in the long-axis view and are more anisotropic than nerves [42].
In order to inject needles accurately under US guided, meticulous manipulation of ultrasonic transducer is needed. To get a better image, clinician need to understand the angle of incidence. The angle of incidence is an angle that the US waves encounter a line perpendicular to the structure (Fig. 10.3). The closer the ultrasonic transducer and the surface of the object are to the perpendicular, the more US waves are reflected by the transducer to obtain a better image. Conversely, if the US waves become more parallel to the surface of the object, the image will have less definition. Better images can be obtained by adjusting the angle of incidence of the transducer with manipulation such as tilting or rotation. A close-to-perpendicular angle of incidence is also critical for better needle visualization during US guided needle insertion. To achieve better needle visualization, in addition to transducer manipulation, it is recommended to change the needle approach and advance more vertically to the US waves. Manipulating methods of ultrasonic transducers to obtain better images include pressure, alignment (sliding), rotation, and tilting [42].
Effects of the different angle of incidence. (a) Trajectory of US wave with probe 2 is more perpendicular to the surface of the artery and nerve. It shows more rounded and defined image of the artery and nerve than image obtained with probe1, of which US wave trajectory has a more parallel to the surface of object. (b) Oblique angle of incidence shows less defined image of anterior tibial artery (white arrow) and vein (black arrow). (c) Perpendicular angle of incidence shows a more round and defined image of the same vasculatures (Ultrasound photographs were provided in favor of Dr. BS Koo)
US wave produces many responses, such as scatter reflection, transmission, refraction, and specular reflection, when traveling through tissue or materials. Scatter reflection is caused by the deflection of the US wave in several directions toward or away from the probe. Scattering occurs on small or irregular objects. Transmission refers to the continuing US wave through tissue away from the probe. Refraction is caused by when the US waves come into contact the interface between two mediums with different propagation velocities, the US wave is refracted bent depending upon the difference in velocities. Specular reflection is caused by reflection from a large, smooth surface such as a bone and returns the US wave toward the probe when it is perpendicular to the US beam [43]. As result, various kinds of artifacts can be seen on the monitor in addition to normal anatomy. Operators performing IA needle injection should discriminate and understand US artifacts such as reverberation, scattering, and acoustic shadowing caused by air bubble in the needle tip. Reverberation artifact is caused when a US beam encounters two strong parallel reflectors. This represents a linear density at the same interval representing multiple visualized needles under the actual needle. When the US energy returns to the probe to process finally, a duplicate image of the needle is displayed on the screen. This duplicate image appears deeper than the actual needle because more time has elapsed for the US energy to return to the probe. Because air does not conduct US, a small amount of air serves as the perfect medium for generating dropout shadow. The presence of an air bubble at the needle tip generated an acoustic shadow [44] (Fig. 10.4).
US artifacts observed during needle insertion. (a) Reverberation artifact is that there are multiple needles visualized under the actual needle and equally spaced linear densities that represent ultrasound waves bouncing back and forth within the lumen of the needle. (b) A small amount of air (black arrow) serves as the perfect medium to generate an acoustic shadow (white arrow), as air does not conduct ultrasound. This image looks like the tip of needle is broken
There are in-plane and out-of-plane methods for inserting needles into the joints under US guide. In-plane needle placement is a method that the needle can be seen on the US monitor in the long-axis view because long axis of the needle is located within the US scanning plane. Out-of-plane needle placement is a method that the long axis of the needle is directed at right angle to the scanning plane so the needle can be seen as a white dot of echo in the short-axis view (Fig. 10.5). The in-plane mode is a commonly preferred approach because it can visualize the entire needle. When performing needle insertion, especially with out-of-plane method, dynamic tilting or sliding of the transducer may help track the tip of the needle because the US beam has a very thin width of about 1 mm, so the needle can enter and exit viewing field even with subtle movements. Visualizing the tip of the needle is essential for accurate needle insertion. However, inexperienced operators often miss the tip of the needle or the entire needle from viewing field. In these instances, it is necessary to look at the probe again and re-align the needle to the US plane. If only the tip of the needle is out of sight, the operator can pull the needle back a little and try again with a slight reorientation [42].
Methods of needle insertion into joints under ultrasonic guide. (a) In-plane needle placement. Long axis of the needle is located within the US scanning plane. (b) Needle can be seen on the US monitor in the long-axis view. (c) Out-of-plane needle placement is a method that the long axis of the needle is directed at right angle to the scanning plane. (d) Needle can be seen as a white dot of echo in the short-axis view
There are several technical tips for enhancing needle placement under US guide. Use larger needles than smaller ones as possible. Large needles are more easily visualized. Direct the US beam perpendicular to the needle rather than parallel to it. Use styletted needles if possible, which decreases reverberation artifact. Fill the needle with a clear solution rather than air. Insert the needle with its bevel either pointing towards the US probe or away from it. The relatively rougher bevel results in more US scatter, enhancing the tip. Try to needle movement, which the needle is inserted in a short “in-and-out, side-to-side” motion causes deflection of the adjacent soft tissues and makes the trajectory of the needle more discernible. Use hydrolocation technique that injecting a small amount of a clear solution to the targeting site can enhance the visibility of the needle tip.
10.3.4 Skin Preparation and Aseptic Technique, Choice of Needle, and Adverse Events
To reduce the risk of infection, IA injections should always be performed under sterile conditions using an aseptic technique . Povidone-iodine and/or alcohol was used to disinfect the skin around the injection portal. Operators should wear aseptic gloves and use sterilized gel for US probes. Only a sterilization wrap with pores to expose the applicable site might be needed during the procedure. Local anesthesia is usually not required before treatment, cooling spray or local anesthetic may be used for large and thick joints or pain-sensitive areas or patients. In general, drug injection usually uses 22–25 gauge needles and 18–21 gauge thick needles for joint aspiration. No.25 gauge needle was occasionally used to decrease pain from injection. The length of the needle mostly chosen is regular-length (1.25 in and 1.5 in). When performing injection using the standard AM and AL site, the distance from the skin edge to the articular surface of the femoral condyle ranged from 4.5 cm to 5.5 cm (1.8 in to 2.2 in). The needle length of 2 inches is needed to clear the IA fat pad and reach the IA space in these sites [4]. Therefore, the length of the needle can be determined by measuring the expected distance of the injection path on the US or MRI, and it is necessary to prepare enough needle length to fit the path.
AEs from IA injection therapy may occur either by injection itself or by drugs used. If therapeutic materials are injected into extra-synovial tissue, such as the anterior fat pad and extra-synovial tissue layers, it may result in injection site pain due to painful blockage of the injected material outflow. HA injection might develop acute pseudoseptic arthritis. CS injection into extra-articular tissue produces skin hypopigmentation, atrophy of subcutaneous fat and muscle. Although there are relatively few AEs associated with IA injection of CSs or HAs, IA infections are serious AEs. Incidences of infection 1 in 3000 to 1 in 50,000 have been reported in association with IA CS injection. Although these rates are low, AEs of CSs, such as the increased cumulative risk of infection with repeat administration and concern about cartilage damage, create reluctance to inject CS into the joints too frequently. No rigid guidelines on this matter exist, but most practitioners are reluctant to inject a joint more than once every 3–6 months, unless delivering agents such as HA, which require multiple injections. Minor AEs include injection site pain (1 to 33%), local swelling (<1 to 30%), and local skin problems (3 to 21%). Pseudoseptic reactions can occur in 1 to 3%, usually after repeated multiple HA injections. It is characterized by joint inflammation and swelling not associated with joint infection [29]. According to a recent retrospective Danish study (n = 22,370), actual joint infections (septic arthritis) had a very low incidence (0.08%, 95% CI 0.03–0.12), and only 11 patients were diagnosed with septic arthritis (~1 in 2000 injections). Risk factors for this serious condition include old age, male, and pre-existing articular disease [30]. As the IA injection is an invasive procedure, there are absolute and relative contraindications. Absolute contraindications include known hypersensitivity to the injection, significant skin breakdown or osteochondral fracture at the injection site, bacteremia, osteomyelitis, sepsis, septic arthritis, periarticular conditions such as cellulitis, joint prosthesis, or uncontrolled coagulopathy. Relative contraindications are not clear, so they should be decided on a case-by-case basis [31].
10.4 Intra-articular Therapeutic Agents
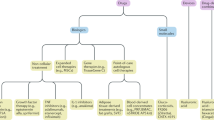
Various kinds of materials have been developed as IA therapeutic agents for knee OA. CS and HA are most commonly used drugs for management of pain with knee OA failed to respond to non-pharmacologic treatment, NSAIDs or analgesics despite questions have been raised about the effectiveness. Standard IA treatment includes CS and HA, and its efficacy and AEs have been extensively investigated. Besides standard IA treatment, polydeoxyribonucleotide (PDRN) and hypertonic dextrose are frequently used for knee OA in the clinics. However, there are a few, low level clinical research with them for evaluating its efficacy of treatment of knee OA. OA results from an imbalance between catabolic and anabolic factors, and biologic agents either target specific catabolic proinflammatory mediators or affect anabolism more generally. Biologic agents show excellent clinical results in other rheumatic inflammatory diseases. There has been a lot of clinical research on biologic agents, assuming that biologic agents will have similar effects in the treatment of OA. Results of clinical studies did not support the routine use of biologic agents for OA management. However, there is still hope for biologic agents in the future treatment of OA [45]. Biologics have four sub-categories such as non-cellular therapeutics, expanded cell therapies, gene therapies, and point-of care autologous cell therapies [9].
10.4.1 Standard Intra-articular Treatments
10.4.1.1 Corticosteroids
Steroids have variable structures, functions, and sites of effect. Steroid molecules vary mainly due to changes in functional groups attached to their carbon rings. Human CSs are produced in the adrenal gland and have a wide range of physiologic effects. CSs can be classified as mineralocorticoids (e.g., aldosterone) that control water and electrolyte physiology and glucocorticoids (e.g., cortisol) that control metabolism and inflammation. CS-like molecules have been synthesized for use in drug therapy because of its powerful anti-inflammatory effects. The synthetic CSs are derivatives of prednisolone (an analogue of human cortisol). Methylprednisolone is the methyl derivative of prednisolone, whereas dexamethasone, betamethasone, and triamcinolone are all fluorinated derivatives of prednisolone [46]. Pharmacologic properties with anti-inflammatory effect can be improved through fluorination of CSs.
Action Mechanism
CSs act directly on nuclear steroid receptors to regulate the rate of synthesis of mRNA and proteins. CSs have both anti-inflammatory and immunosuppressive effects, and CS’s mechanisms of action are highly complex including changes in T and B cell functions, changes in white blood cell traffic, changes in levels of cytokines and enzymes, inhibition of phospholipase A2 and arachidonic acid metabolism [47]. This mechanism is largely divided into altered movement of leukocytes, altered function of leukocytes, reduced microvascular dilation and permeability in inflamed areas, and reduced prostaglandin synthesis. Leukocyte migration alteration occurs 4–6 h after drug administration and includes lymphocyte reduction, T-lymphocyte selective depletion, and inhibition of neutrophil and monocyte-macrophage accumulation in the inflammatory site. Leukocyte function alteration is associated with an immune response. This includes processes such as inhibition of lymphocyte proliferation and inhibition of T-lymphocyte mediated cytotoxicity. In addition, these inhibitory effects inhibit the release of interleukin-1, leukotrienes, and prostaglandins. The reduction of these inflammatory mediators often improves pain symptoms and increases the relative viscosity as the concentration of HA in the joint increases [48].
Composition, Pharmacodynamics, and Pharmacokinetics
When a CS is injected into a joint, it is absorbed by synovial cells and then diffused into the blood and removed. The purpose of IA injection therapy is to achieve prolonged concentrations of CS in the synovial fluid and synovium. The duration and effectiveness of the drug depend on the anti-inflammatory potency, solubility, and dosage. Based on the chemical structure, the duration of effect should be inversely proportional to the solubility of the steroid. The less water soluble a CS is, the slower its onset and the longer its duration [49]. The CS formulations used for IA injection are microcrystalline suspensions of CS esters. When injected into the joint cavity, these esters are slowly hydrolyzed in synovial cells to form activated CSs. In this moment, if the solubility of esters is low, absorption in the synovial cell is delayed and the duration of the effect is increased [50]. The duration of the local effect of the drug is divided into short, intermediate, and long acting and is generally consistent with the anti-inflammatory effect. Several kinds of synthetic CS have been tried to improve the anti-inflammatory effect (Table 10.1). Triamcinolone acetate (TA) is completely absorbed from joint and can be detected in plasma for 2–6 weeks. Systemic TA absorption in plasma is relatively rapid after IA injection, the observed C max of 11.06 ng/mL in plasma was reached at a median t max of 6 h. The terminal t½ varies between 3.0 and 6.4 days and MRT (mean residence time) is 2.5–4.3 days depending on the products and the dose [51, 52]. Triamcinolone hexacetonide (TH) is the least soluble injectable CS, which is absorbed from the joint completely over a period of 2–3 weeks. The terminal t½ is 4.6 days and dose-independent, MRT is 6 and 6.1 days at a dose of 20 and 40 mg, respectively [51]. Betamethasone was investigated in plasma after the single IA injection, the terminal t½ in plasma is 6.3 days, and MRT is 2.8 days [51]. FX006 is an extended-release (ER) IA formulation of TA (TA-ER) in 75:25 poly (lactic-co-glycolic acid) microspheres designed to maintain prolonged drug concentration in the joint [11]. Synovial fluid (SF) TA-ER concentrations were quantifiable through 12 weeks. SF TA-ER reached Cmax 231.3 ng/mL at tmax 7 days. Plasma TA-ER reached C max 0.97 ng/mL at t max 7 h. The median t½ was 14.5 days and MRT was 19 days [52]. By delaying the absorption of drugs, a significant pain improvement at 10 weeks and lower peak plasma concentration were reported using an ER microsphere-based formulation of TA (FX006 or Zilretta®) instead of a crystalline suspension formulation [52] (Table 10.1).
Choosing a Corticosteroid Preparation and Dose
Several injectable CS preparations are commercially available. The choice is usually based on availability, cost, versatility, and pharmacokinetics. Methylprednisolone and triamcinolone are the two most common injectable CS used for knee OA. It is believed that more soluble preparations have a shorter duration of action than less soluble preparations. However, this may not always be the case. Research results on which CS preparation is effective in treating knee OA vary. Hepper et al. [53] reported that triamcinolone appeared to be more efficacious than either betamethasone or methylprednisolone. Pyne et al. [54] reported that triamcinolone was statistically more efficient in pain relief 3 weeks after injection than methylprednisolone, but its effect is lost by week 8. On the contrary, Yavuz et al. [55] stated that methylprednisolone was statistically more effective in relieving pain than triamcinolone until 6 weeks after injection. In another studies, comparing the efficacy of TH and methylprednisolone acetate (MA) injections in knee OA, both IA therapies have similar efficacy in relieving pain and improving function, and improvement in pain and function can be sustained for up to 24 weeks [56, 57]. From these clinical results, the effects of choice of CS preparation on the treatment of knee OA are not much different. Doses needed have not been systematically studied. One study showed that an 80 mg dose of TA had no additional benefit compared with 40 mg as treatment for knee arthritis [58]. Some general dosing guidelines and CSs preparations are provided in Table 10.2.
Procedural Precaution
There are no contraindications to use of IA CS therapy. However, if there is infection in or around the joint, IA CS injection should be postponed. Other potential complication risk factors, such as allergy, coagulopathy/anticoagulant use, very poorly controlled diabetes, possible fracture, or uncooperative patient should be considered [49]. IA CSs for knee OA are mostly administered with local anesthetics (lidocaine or bupivacaine). There are concerns that the preservatives in some local anesthetic preparations can cause aggregation when combined with other compounds. However, CS crystals do not aggregate or change particle size when mixed with local anesthetics [59]. Other concern is chondrotoxic effect of local anesthetics, which had been occurred after a single IA injection of 0.5% bupivacaine [60]. Rest and/or ice pack application for 24–48 h after IA CS injection are commonly advised because it helps delaying clearance of the agent from the joint space theoretically. One study reported that IA steroid injection into the knee joint followed by strict inpatient bed rest for 24 h results in a greater degree of clinical and serological improvement, compared to outpatient injections for up to 6 months [61]. But there is no strong evidence for non-weight bearing after IA CS injection of knee [62].
Efficacy and Clinical Guideline
IA CS injection for symptomatic treatment of knee OA has been successfully used for over 60 years. However, questions about the efficacy of this treatment have been raised. This treatment could improve symptoms in a short period of time, but it did not help with the treatment of fundamental arthritic lesions. Moreover, this method masks the patient’s pain, allowing them to resume activity, but it has the potential to cause further destruction to the joint. Several systematic reviews showed a short-term effect of IA CS injection for treatment for knee OA. There is evidence of pain reduction between 2 and 3 weeks, but a lack of evidence for efficacy in functional improvement. Longer term (from 4 weeks on) benefits have not been confirmed [63]. Up to 4 weeks after injection, IA CS appears to be relatively more effective for pain than IA HA. By week 4, both approaches have equal efficacy, but after week 8, HA has more effective. Understanding this tendency is useful to clinicians when treating knee OA [64]. IA CS injections were significantly and clinically efficacious at reducing knee OA pain for at least 1 week [53]. However, an updated meta-analysis study showed that, although IA CS injection seemed to offer small-to-moderate benefits over placebo for up to 6 weeks, it was unclear whether the difference was clinically important. The authors also concluded that there is no evidence that an effect remains 6 months after a CS injection [65]. Although the therapeutic effects from IA CSs are typically short-lived, a newly developed TA-ER is formulated in poly lactic-co-glycolic acid (PLGA) microspheres that slowly release TA in the synovium, enabling them to exist for a long time in the joint [66]. A single, 5 mL IA injection TA-ER 32 mg (Zilretta®) significantly improved pain, stiffness, and physical function in patients with knee OA compared to placebo over 24 weeks and reduced CS-related systemic AEs such as blood glucose elevation [67].
There are many factors affecting the response after IA CS injection. The presence of effusion, absence of synovitis, US guided injection, and greater symptoms at baseline may all improve the response to IA CS injection [68]. Clinical benefits of IA injections in patients with obesity and/or advanced arthritis are less predictable [69]. Compared with those who have mild joint damage, persons with more severe joint damage on either X-ray or MRI are less likely to respond to knee IA CS injection [70].
In 2013, American Academy Orthopaedic Surgeon (AAOS) guideline had published that there is inconclusive evidence (unable to recommend for or against) to support the use of IA CS injection for knee OA [71]. On the contrary, Osteoarthritis Research Society International (OARSI) guidelines showed that IA CS injections were appropriate and the quality of the evidence of its use was good [72]. Despite AAOS guideline for use of IA CS, many orthopedic surgeons have poor compliance to the guideline, resulting in lack of treatment consensus and continued use of modalities with no proven patient benefits [73]. Adherence to the recommendations contained within the AAOS guidelines was modest regardless of the Kellgren-Lawrence (KL) grade or history of treatment. IA injection with either CSs or HA was the most common intervention (32%) despite inconclusive to strong recommendation against their use [74]. A recently updated OARSI guidelines has published that IA CS injections are conditionally recommended for acute (1–2 weeks) and short-term (4–6 weeks) pain relief [75]. In 2019, European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) proposed a weak recommendation to the use of IA CSs, in the case of contraindications to NSAIDs, or if the patient is still symptomatic despite use of NSAIDs. IA CSs are more effective than IA HA in the first 2–4 weeks of treatment and its efficacy may be higher in patients with more severe pain [76]. IA CSs for knee OA are conditionally or weakly recommended for short-term effect for symptomatic treatment regarding recently updated guidelines , even though 2012 American College of Rheumatology (ACR) guidelines strongly recommended IA glucocorticoid injections for knee OA [2].
Adverse Events
Local AEs to IA CSs are postinjection flares, infectious arthritis, subcutaneous lipoatrophy, and chondrolysis. The incidence of postinjection flares is about 2–6% of patients, which has been known as a result from chemical irritation of crystals including steroids suspension. Infectious arthritis is an uncommon complication of which incidences range from 1 in 3000 to 1 in 50,000. Symptoms of septic arthritis occur 3–4 days after injection, so they are distinguished from postinjection flare, where symptoms occur within 24 h of postinjection. Subcutaneous lipoatrophy is sometimes observed on extra-articular injection and may be more common with less soluble agents, such as the triamcinolone compounds. Cartilage destruction might occur after excessive use of IA CS injection, which is caused by the catabolic effect of the agent. Among patients with symptomatic knee OA, comparing IA triamcinolone and IA saline over 2 years, the group using IA triamcinolone had significant loss of cartilage volume compared to IA saline group, but there was no significant difference in knee pain [77]. However, no significant deleterious effects of the steroids on the anatomical joint structure were observed in patients receiving TA injections every 3 months for up to 2 years for knee OA [78].
Physicians commonly administer IA CS injections to patients who are candidates for total knee arthroplasty (TKA) but may be unaware of the potential long-term complications. The incidence of infection within 3 months and 6 months after TKA within 3 months of knee injection was significantly higher. Ipsilateral knee injection within 3 months prior to TKA is associated with a significant increase in infection [79]. Preoperative CS or HA injection 3 months before TKA increased the risk of periprosthetic joint infection [80]. Therefore, pre- and peri-operative IA CS injections might be associated with a higher incidence of postoperative periprosthetic infection, so caution is advised [81].
Systemic effects after IA CSs are uncommon. However, attention should be paid to the AEs of systemic absorption of CSs after injection. Typically, it can reduce the inflammatory response of other joints and inhibit the hypothalamic–pituitary axis. In one study, plasma cortisol was low 2 weeks after an IA injection of TH (20 mg) and 4 weeks after an injection of methylprednisolone acetate (40 mg) [82]. Systemic effects, such as flushing, CS-induced osteoporosis, myopathy are not a major concern in patients receiving reasonable numbers of IA CS injections. Although CSs can occasionally affect blood glucose level, diabetic patients who received IA injections of methylprednisolone acetate did not detect a significant effect on blood glucose levels [49].
10.4.1.2 Hyaluronic Acid
HA is a high MW molecule that naturally occurs within the cartilage and the synovial fluid. It is a linear glycosaminoglycan composed of repeating disaccharides of β-D-glucuronic acid and β-D-N-acetyl-glucosamine. In normal human synovial fluid, the MW of HA range from 6500 to 10,900 kDa, and the concentration is 2.5 to 4.0 mg/ml [83]. High molecular weight (HMW) HA has viscoelastic properties . It behaves as a viscous liquid at low shear rates (lubricant) and as an elastic solid at high shear rates (shock absorber). In OA, the MW of synovial fluid HA is reduced to 2700 to 4500 kDa and cleared at higher rates than normal. The average half-life of HA is about 20 h in the normal synovial joint, while this half-life is reduced to 11–12 h in the inflamed joint [84]. As a result, viscoelastic properties of the fluid in OA joint are decreased [85]. Exogenous IA HA is available as a treatment for the symptoms of knee OA because it helps to restore the viscoelasticity of the synovial fluid, which called viscosupplementation [86]. In the past, US Food and Drug Administration(FDA) approved injectable HA as medical device because of its viscosupplement effect. In Dec 2018, US FDA reclassified IA HA as drug because current published scientific literature supports that HA achieves its primary intended purpose of treatment through chemical action within the body.
Action Mechanism
IA HA has not only mechanical role as viscosupplement, but also chemical role, which suppresses inflammation and promotes HA production. HA injected into the joint cavity restores the normal viscoelasticity of pathological SF, which called “viscosupplementation”. Viscosupplements also have disease-modifying effects, such as reducing synovial inflammation, preventing cartilage erosion, and promoting IA HA production [87]. In addition to these roles, IA HA therapy produces anti-inflammatory effects through a multifactorial mechanism of action mediated through receptor-binding relationships with cluster determinant 44 (CD44), toll-like receptor 2 (TLR2) and 4 (TLR-4), intercellular adhesion molecule-1 (ICAM-1), and layilin (LAYN) cell surface receptors. HMW HA promotes anti-inflammatory responses inhibiting the expression of proinflammatory cytokines, matrix metalloproteinases, prostaglandins, and nitric oxide, whereas short HA oligosaccharides produce inflammatory reactions [88]. Also, other action mechanism of HA has been suggested that exogenous HA decreased joint pain by directly suppressing of nociceptors and reducing the synthesis of bradykinin and substance P [89].
Hyaluronic Acid Formulations for Intra-articular Injection
Ideal injectable HA for knee OA is capable of recreating the full range of biological activities attributed to naturally occur HA. MW and concentration of HA should be considered before choosing HA formulation because it may be one of the most important differentiating characteristics between HA formulations. MW and concentration in the HA formulation is important for recreating the effects of endogenous HA for joint homeostasis. Exogenous HA of higher MW (>5 × 105 Da) may not only exert a greater protective effect but also encourage endogenous HA production . Also, higher HA concentration makes recreating the activities of endogenous HA and stimulating endogenous HA production [90]. There are several injectable HA formulations used for clinical use (Table 10.3). Each product differs in many characteristics, including source (rooster combs versus bacterial bio-fermentation using modified organisms), mean MW ranging from 500–6000 kDa, distribution of MW, structure of molecule (linear, cross-linked, or both), cross-linking method, concentration (0.8–30 mg/mL), injection dose (0.5–6.0 mL), number of injection [91]. Number of injection per treatment course varies from 1 to 5 injections per week according to the particular product being used. The number of injections is usually in accordance with the manufacturer’s instructions. HA can also be injected repeatedly. Meta-analysis study showed that repeated IA injections of HA are effective and safe treatment for knee OA [92]. The US FDA has approved repeat courses of IA HA injection; however, many insurance plans require at least a 6-month interval between treatments [93]. Although cross-linked HA or HMW HA has been known for its effectiveness regarding improvement of pain and function, series of systematic and meta-analysis study did not show a superior effectiveness comparing to non-crosslinked HA or LMW HA [94,95,96]. There is no reliable evidence that any one brand of viscosupplement is superior to other brands.
Indications, Contraindications, and Adverse Events
IA HA is FDA approved for the treatment of knee OA in patients who have failed conservative non-pharmaceutical therapy or simple pain medication. Patients with mild-to-moderate OA (grades 1–2) and those responding positively to the first injection were twice as likely to respond positively to the injection series. Patients who did not show improvement by injection therapy were more likely to undergo arthroplasty [97]. However, patients 65 years of age or older and those with terminal stage of OA (complete loss of joint space) were less likely to get better with IA HA injections [98]. In addition, IA HA injections are less favorable for patients with significant inflammation or suspected synovitis and those with advanced patellofemoral OA with anterior knee pain tend to be less effective [99]. IA HA injection is contraindicated in patients with known hypersensitivity to hyaluronate products, patients with targeted knee or around infections, bacteremia patients, children, pregnant or lactating women [100].
Serious AEs after IA HA injection are rare but minor one is not uncommon. Minor AEs include pain at the injection site, local joint pain and swelling, and local skin reactions, which are usually subsided with rest, cold compression, or analgesics . More serious AEs are infectious arthritis and pseudoseptic arthritis. Joint infection after IA HA injections is rare. Pseudoseptic arthritis occurred in 1–3% of patients, which are clinically characterized by severe joint inflammation with pain and swelling occurring 24–72 h after an IA injection. These reactions usually occur after sensitization with the second or third injection of a series or with a repeat treatment course. Infectious arthritis and crystalline arthropathy are ruled out with a negative synovial fluid examination. Pseudoseptic arthritis is not self-limited, requiring treatment with NSAIDs or an IA steroid injection or arthroscopic debridement. The exact cause of pseudosepsis after HA injection is currently not well understood [84]. It seems to be occurred secondary to increased immunogenicity associated with the cross-linking process used in certain HA formulations. A meta-analysis of AEs showed that the frequency of flares of pain and swelling was higher after IA injections of hylan (chemically cross-linked HA molecules with average MW up to 23 x 106 Da, and resulting half-lives of 1.5–9 days) than after injections of the standard form of IA HA [94].
Efficacy and Clinical Guideline
There have been several studies of the efficacy and safety of IA HA injection for knee OA over the past few decades. In a comparison of IA HA injection, oral NSAID treatment, and placebo, IA HA injection provided superior pain relief and functional improvement compared with placebo at 6-month follow-up [101]. Comparing to NSAIDs alone, patients treated with either HA supplementation alone or HA supplementation combined with NSAIDs had superior outcomes at 6-month follow-up [102]. In comparison with IA HA and CS injections, maximal benefit of steroids appeared more rapidly (within 2 weeks) but pain reduction and functional improvement were significantly better with HA supplementation during the 3- to 6-month follow-up period [103]. IA HA may delay the need for knee arthroplasty. The IA HA injection was associated with a longer time-to-knee arthroplasty of 8.7 months compared with the no IA HA injection [104]. In knee OA patients, the time-to-knee arthroplasty was increased by the dose of HA injections. Patients who did not receive HA injection underwent knee arthroplasty at an average of 0.7 years. In the patient group who received a single course of HA injection, the average time-to-knee arthroplasty was 1.4 years; patients who received 5 courses delayed knee arthroplasty by 3.6 years [105]. Furthermore, IA HA injection has beneficial effect on cartilage preservation. In patients with radiologically milder disease at baseline and receiving IA HA, the joint space narrowing was significantly reduced compared with placebo [106]. When IA HA was injected to patients with symptomatic OA of the knee for 6 months, the results of measuring cartilage volume and cartilage defect using MRI showed beneficial effects on knee cartilage preservation [107].
Despite numerous trials and meta-analyses, the effectiveness of IA HA injections in knee OA patients remains controversial and uncertain. Divine et al. [108] performed a systematic review of the five published meta-analyses and concluded that although they differ in several methods for determining individual trial quality, each of the five meta-analyses presented offers scientifically sound level 1 evidence to support the efficacy of HA use in select patients with OA. IA HA injections are effective at 4 weeks, peak effect at 8 weeks, and residues detected by 24 weeks. The maximum effect size is greater than the published effects of other OA pain relievers. Therefore, IA HA injections can be useful in certain clinical situations or combined with other therapies [109]. On the contrary, Rutjes et al. [94] concluded that the benefits of viscosupplement were small and clinically irrelevant and associated with an increased risk of serious AEs. Jevsevar et al. [96] concluded that the clinical significance of the results related to pain relief and functional improvement does not support the routine use of IA HA because patient benefit of IA HA was not clinically important when compared with IA saline solution injections used as a placebo. Meta-analyses assessing the efficacy of IA HA have had discordant findings because each review used different search strategies and selection criteria to identify trials for inclusion in the analysis [100].
Consistent with the contradictory meta-analyses, available guidelines also have conflicting recommendations, despite being based on the same research evidence. In the 2012 AAOS clinical practice guideline, it was determined that the evidence was inconclusive and a recommendation could not be made for or against the use of IA HA [71]. In 2019 ACR revised guideline, IA HA injections are conditionally recommended for knee OA patients when other alternatives have been depleted or have not provided satisfactory benefits [2]. The 2019 OARSI guidelines conditionally recommended IA HA for all patients at different stages of treatment depending on their comorbidity profiles. For example, in patients with knee OA who have no comorbidities, IA HA is recommended after failure to respond to core treatments, topical NSAIDs and oral NSAIDs (including COX2 inhibitors). IA HA may have beneficial effects after 12 weeks of treatment, and a long-term safety profile may be more favorable than repetitive IA CS [75]. The 2019 ESCEO working group gives a weak recommendation to the use of IA HA in patients who have contraindications to NSAIDs, or those who is still symptomatic despite the use of NSAIDs [76].
10.4.1.3 Hyaluronic Acid-Corticosteroid Combination
Both HA and CS IA injections have demonstrated therapeutic efficacy for knee OA. According to literature, CS injections relieve pain within 2–4 weeks after injection, but these effects decrease over time. On the other hand, HA injections take almost 2–3 months to induce pain relief, but these effects last longer [64]. Both of these treatments tend to be more popular, but show very different treatment trajectories [109]. Their combination in the management of OA symptoms may provide improved symptomatic relief for these patients, both early and late period. In a systematic review, the WOMAC pain score was further reduced at 2–4 weeks in the CS and HA combined group compared to the HA alone group. With a longer term follow-up, the WOMAC pain scores at 24–26 and 52 weeks also preferred the combined CS and HA groups over HA alone. There were no significant differences in treatment-related AEs [110]. Cingal® is an HA-TH combination drug. Comparing to HA and saline injection, the use of Cingal® IA injection provided better symptomatic relief than placebo, as measured by the WOMAC pain score at 26 weeks. At 1 and 3 weeks, Cingal® was significantly better than HA for most endpoints but Cingal® and HA were similar in the 6–26 weeks. The incidence of related AEs has been reported as low [110].
10.4.2 Other IA Treatments Including Small Molecules
10.4.2.1 Polydeoxyribonucleotide (PDRN)
Polydeoxyribonucleotide (PDRN) is a linear polymer consisting of a mixture of double stranded deoxyribonucleotides with a chain length of 80–2200 base pairs and a MW ranging between 50 and 1500 kDa [111]. PDRN is commonly extracted from salmon trout gonads. PDRN was originally introduced to enhance wound regeneration in difficult wound problems. A pharmacokinetic profile of PDRN is that PDRN in plasma reached its peak level at ~1 h, half-life is ~3.5 h, and bioavailability is 80–90%. It is not metabolized by the liver but is degraded by unspecific plasma or membrane-bound DNA nucleases and is finally excreted through urine and, to a lesser extent, feces [112]. PDRN may be considered a pro-drug providing active deoxyribonucleotides that interact with purinergic receptors, such as adenosine A2A receptors (A2ARs). In addition, PDRN has been shown to act by promoting DNA synthesis or repairing and restoring cell proliferation and growth through the so-called salvage pathway that PDRN supplies cells with nucleotides and bases deriving from its degradation [111]. Polynucleotides are polymers that can bind to a large amount of water molecules, and by adjusting water molecules to form a 3-dimensional gel, when injected into the joint cavity, they provide moisture to the joint surface and can reshape the cartilage structure [113]. When PDRN is enzymatically decomposed, it releases water molecules and smaller-sized oligonucleotides to maintain moisture and viscoelasticity in the joint. In addition, PDRN protects basic fibroblast growth factor (bFGF) from oxidation at the storage site, inhibits proinflammatory factors (TNF-a, IL-6, HMGB-1) by activating the adenosine A2A receptor, and increases anti-inflammatory cytokine (IL-10) [114]. Therefore, PDRNs have therapeutic effects on chondrocytes by protecting cartilage because it can inhibit the degradation of proteoglycan [115]. From these scientific bases , IA PDRN injections to treat knee OA have been tried. However, there has been little known about what is effective PDRN formulations for relieving pain and function of knee OA. According to the literature, the number of injections of PDRN used to treat knee OA varies from 3 to 5 times a week, injected volume ranged from 2 to 3 ml and a concentration of PDRN ranged from 5.6 to 30 mg/ml [116].
In a randomized double-blind clinical trial (DB RCT), 5 weekly IA PDRN (2 ml, 20 mg/ml) injection showed better pain relief and KOOS scores improvement than IA HA injection at 3 months after the end of treatment [113]. Zazgyva et al. [117] conducted a study to assess the efficacy of IA injections of PDRN versus HA in knee OA. IA PDRN (2 ml) was injected 3 weekly and followed till 16 weeks post-injection. The symptomatic and functional improvements in PDRN injection were superior to those obtained by HA injection. Giarratana et al. [118] conducted a study to investigate the equivalence of IA PDRN compared to standard HA injection. IA PDRN (2 ml, 20 mg/ml) was injected 3 weekly and followed until 26 weeks post-injection. There was statistically significant improvement of pain and KOOS scores from baseline in both treatments. PDRN injection showed significant KOOS symptoms subscore after 2 weeks while the results with HA injection became significant only after 18 weeks. In comparison with IA HA injection alone, IA injection of HA combined with PDRN showed better outcomes in VAS, WOMAC, and KSS scores at study periods. Study drugs were injected 3 times per week in both groups. There were no AEs and any other complications [119]. In another comparison study, KSS total score showed significantly better results in a combination of PDRN(10 mg/ml) and HA (10 mg/ml) injection, 3 times per week, compared with HA (20 mg/ml) alone at each follow-up time. However, no significant differences were observed for the WOMAC score between groups [120].
10.4.2.2 Hypertonic Dextrose (Prolotherapy )
Prolotherapy , also known as proliferative therapy, or regeneration injection therapy, is a complementary injection treatment for musculoskeletal pains. Hypertonic dextrose with concentrations ranging from 12.5 to 25% is the most commonly injected solution among prolotherapy agents. In this treatment, appropriate amount of hypertonic dextrose is injected into the painful ligament, the attachment of the tendon, or into the joint cavity. The mechanism of action behind hypertonic dextrose injection is not completely understood. Hypertonic dextrose solutions dehydrate the cells at the injection site and create a local inflammatory cascade. This induces growth factor release, collagen deposition, granulocytes, and macrophages activity and promotes healing [121]. In addition, it stimulates fibroblast and vascular proliferation, causing local recovery to damaged tissues inside and outside of the joint, and contributing to joint stability by strengthening ligament. According to animal study, it is reported that cartilage specific anabolic growth is possible with IA dextrose injection [122]. Furthermore, chondrogenic effects were observed after prolotherapy with hypertonic dextrose injection in symptomatic severe knee OA patients [123]. Dextrose proliferant has been approved for injection by US FDA but not for prolotherapy; thus, it is currently used as an off-label substance in prolotherapy . There are some procedural precautions in prolotherapy. Patients received prolotherapy suffered from post-injection pain. Use of prescribed pre- and/or post-procedure opioid drug dramatically reduced injection-related pain. Patients with prolotherapy should not take NSAIDs because it interferes with healing process (inflammation).
Reeves et al. [124] conducted a DB RCT to investigate the efficacy of dextrose in knee OA patients with or without ACL laxity. The tibiofemoral joint was injected with 9 ml of 10% glucose 3 times bimonthly, and an additional 10% glucose was injected 3 times bimonthly in open-label fashion. They concluded that prolotherapy with 10% dextrose significantly improves knee OA clinically and statistically. Dumais et al. [125] performed randomized crossover study to assess the effectiveness of dextrose injection to improve pain and function in knee OA. 1 cc of 15% dextrose and 0.6% lidocaine were injected into 8 administration sites in the collateral ligaments and 5 cc of 20% dextrose and 0.5% lidocaine was also administered inside the knee joint. They concluded that dextrose injection significantly reduced symptoms and lasted more than 24 weeks. Rubago et al. [126] conducted a 3-arm, DB RCT to assess the efficacy of 25% dextrose injection for knee OA. Injections were given at 1, 5, and 9 weeks with optional sessions at 13 and 17 weeks. Patients were given an optional 5 mg oxycodone tablet 30 min prior to prolotherapy to relieve pain during injection. Extra-articular injections were performed to painful attachment site of tendon and ligament with up to 15 cutaneous punctures and total amount of 22.5 mL hypertonic dextrose were used. 6 mL was injected into the knee joint. WOMAC scores exceeded minimal clinically significant difference. There were no AEs. Sit et al. [127] have performed a systematic review with meta-analysis to comprehensive clinical evidence of the effectiveness of prolotherapy for knee OA. Prolotherapy is superior to exercise alone by the WOMAC scale. Overall, prolotherapy has clearly conferred beneficial effects on knee OA treatment.
Prolotherapy has long been used to treat musculoskeletal pain, but use in knee OA is relatively rare. There is a lack of scientific evidence to use prolotherapy generally in the treatment of knee OA. Therefore, various clinical studies regarding dextrose concentration and dose, number and duration of injection, and specific utility of intra- compared with extra-articular injections are needed in the future [127]. In 2019 ACR guideline, prolotherapy is conditionally recommended for knee OA patients [2]. A limited number of trials with a small number of participants have shown small effect sizes of prolotherapy in knee. Moreover, injection schedules, injection sites, and comparators have varied substantially between trials.
10.4.2.3 SM04690
Wnt is an extracellular secreted glycoprotein whose signals act on 19 Wnt genes and various Wnt receptors, regulating canonical β-catenin-dependent and non-canonical β-catenin-independent signaling pathways. Both pathways are associated with the occurrence and development of OA [128]. Excessive activation of β-catenin-dependent signaling pathways inhibits cartilage formation, while inhibition results in chondrogenesis. SM04690 is a new small molecule Wnt-β-catenin signaling pathway inhibitor with potential as a disease-modifying OA drug (DMOAD) [128]. Yazici et al. [129] reported a phase IIb study to assess the safety and efficacy of SM04690. Inclusion criteria was KL grades 2–3, and NRS range 4 and 8. A single 2 mL IA injection of SM04690 (0.03, 0.07, 0.15, 0.23 mg, respectively), vehicle placebo, or sham (dry needle only) were given. This study showed statistically significant improvements in the 0.07 mg and 0.23 mg dose groups compared to vehicle placebo for NRS score, WOMAC pain and physical function score, and patient global assessment.
10.4.2.4 CNTX-4975
Capsaicin is an agonist for the transient receptor potential cation channel subfamily V member 1 (TRPV1). TRPV1 is a non-specific cationic channel which is opened by heat, acids, and certain fatty acids [130]. This channel is selectively expressed at the ends of the nociceptors (pain sensory fibers) in the peripheral nervous system [131]. CNTX-4975 is a high-purity injectable trans-capsaicin that targets the capsaicin receptor (TRPV1). The analgesic effects of capsaicin-based treatments have been attributed to several different mechanisms (collectively referred to as the “defunctionalization” of nociceptive fibers), including the transient retraction of nerve fiber terminals [131]. Stevens et al. [132] reported a phase 2 DB RCT results. Patients ages 45–80 years who had moderate-to-severe OA were randomized into a single IA injection of placebo, CNTX-4975 0.5 mg, or CNTX-4975 1.0 mg. At week 12, injections of CNTX-4975 in the 0.5 mg and 1.0 mg groups showed a greater reduction in AUC for pain scores compared to placebo. At week 24, significant improvements were maintained in the 1.0 mg group. AEs were similar in both groups. CNTX-4975 has shown a dose-dependent improvement in pain of knee OA patients. CNTX-4975 1.0 mg was well tolerated, with a safety profile similar to that of the placebo throughout the study. In conclusion, CNTX-4975 1.0 mg significantly reduced OA knee pain for 24 weeks; CNTX-4975 0.5 mg significantly reduced pain at 12 weeks, but the effect was not clear at 24 weeks.
10.4.3 Biologic Treatments
Biologics are defined as any pharmaceutical drug product manufactured, extracted or semi-synthetic from biological sources. Different from totally synthesized pharmaceuticals, they include vaccines, whole blood and its components, allergens, somatic cells and tissues, gene therapies, recombinant therapeutic protein, and live medicines used in cell therapy. Biologics may consist glucoses, proteins, nucleic acids, or complex combinations of these substances, or may be living cells or tissues. Biologics have some promising applications to pain relief and healing of damaged tissues in many different areas of health and medical research [133]. OA is caused by an imbalance between anabolic and catabolic factors, and biologics target certain catabolic proinflammatory mediators or affect anabolism more generally. Moreover, biologic agents have dramatic effects in other inflammatory arthritis such as rheumatoid arthritis. Taking into consideration of this point, biologic agents are thought to have similar effects in treating OA [45]. Biologics used for treatment of knee OA are categorized into non-cellular therapy or cell therapy. Non-cellular therapy includes human serum albumin, growth factors, cytokine antagonists. Cell therapy includes cell concentrates as autologous point-of-care cell therapy, expanded cell therapy as mesenchymal stromal (or stem) cells (MSCs), and gene therapy. Cell therapies can be classified by the method used to produce them or by their relative heterogeneity compared with the source tissue [9] (Fig. 10.6).
Biologic treatments for knee osteoarthritis. Biologics for knee OA are categorized into non-cellular therapy or cell therapy. Non-cellular therapy includes human serum albumin, growth factors, cytokine antagonists. Cellular therapy includes cell concentrates as autologous point-of-care cell therapy, expanded cell therapy as mesenchymal stromal (or stem) cells, and gene therapy. rhFGF recombinant human fibroblast growth factor, IL interleukin, TNF tumor necrotizing factor, APS autologous protein solution, BMAC bone marrow aspirate concentrate, MSC mesenchymal stem/stromal cell, PRP platelet-rich plasma, SVF stromal vascular fraction
10.4.3.1 Non-Cellular Therapy
10.4.3.1.1 Human Serum Albumin (LMWF-5A)
LMWF-5A (Ampion®) is an injectable, low MW fraction of 5% human serum albumin, of which constituent, aspartyl-alanyl diketopiperazine, modulated the inflammatory immune response in vitro through a molecular pathway implicated in T-lymphocyte anergy [134]. Comparing to saline injection, LMWF-5A injection group showed significantly better WOMAC pain scores at week 12 (estimated difference from control −0.25, P = 0.004). LMWF-5A effect on pain was more pronounced in severe knee OA patients. AEs were generally mild and similar in vehicle control group (47%) and LMWF-5A group (41%) [135]. Another RCT resulted that 71% of severe knee OA injected LMWF-5A met the OMERACT-OARSI responder criteria, exceeding the 30% threshold (p < 0.001) and at week 12, there were significantly more responders in the LMWF-5A group than saline control group (65% vs. 43%, p < 0.001). There were no reported drug-related serious AEs. Overall, the available data suggest that the short-term effects of LMWF-5A may be non-inferior (although not likely to be superior) to currently used IA treatment modalities. However , the long-term effects of LMWF-5A have not yet been determined [136].
10.4.3.1.2 Growth Factor Therapy
10.4.3.1.2.1 rhFGF18 (Sprifermin)
Sprifermin (recombinant human fibroblast growth factor 18; rhFGF18) specifically binds to fibroblast growth factor receptor 3 (FGFR-3) in cartilage and activates promoting chondrogenesis and cartilage matrix production in vitro. Post hoc analyses of the phase I data showed that the patellofemoral joint had less worsening from baseline to 12 months, and bone marrow lesions showed further improvement in the whole knee joint from 6 to 12 months [137]. A 5 years FGF-18 Osteoarthritis Randomized Trial with Administration of Repeated Doses (FORWARD) study was conducted with 40–85 years of symptomatic radiological knee OA patients were selected as eligible participants and KL grade 2 or 3. Five groups were randomized into IA injections of 100 μg of sprifermin every 6 or 12 months, 30 μg of sprifermin every 6 or 12 months, or placebo every 6 months. Each treatment consisted of weekly injections over 3 weeks. After 2 years, compared with placebo, there was a significant increase in total tibiofemoral cartilage thickness for 100 μg of sprifermin injection every 6 months group (0.05 mm) and every 12 months group (0.04 mm). There was no statistically significant difference in the mean absolute change from baseline in the total WOMAC score compared to placebo. Arthralgia was the most frequently reported AEs. Sprifermin is a potential anabolic IA disease-modifying OA drug but of uncertain clinical importance; the durability of response also was uncertain [138].
10.4.3.1.3 Cytokine Antagonist
10.4.3.1.3.1 Interleukin (IL)-1β Antagonist
IL-1β is a key mediator of the inflammation and catabolic processes that lead to cartilage degradation and destruction of joint tissues. It might directly mediate the erosive processes that lead to OA [139]. Systemic administration of IL-1 receptor antagonist (IL-1Ra), anakinra, may reduce joint inflammation and slow down the erosive process of the disease, such as rheumatoid arthritis. IA anakinra injection can have beneficial effects on symptoms and structural alterations in canine OA model [140]. In a randomized, multicenter, double-blind, placebo-controlled trial, pharmacokinetic profile of anakinra showed that the mean terminal half-life of it in serum after IA injection was ~4 h. The mean WOMAC score improvement from baseline to 4 weeks was not statistically different between the placebo group and injecting anakinra 50 or 150 mg group. Moreover, anakinra was well tolerated [141].
10.4.3.1.3.2 Tumor Necrotizing Factor (TNF) Antagonist
TNF is known to play an important role in cartilage matrix degradation in OA. It has the function of inducing the production of cytokines such as IL-6, matrix metalloproteinase and prostaglandins, and inhibiting the synthesis of proteoglycans and type II collagen [142]. Lindsley et al. [143] reported IA infliximab for knee OA showed significant improvement in the total WOMAC score by 8 weeks and baseline synovial cellularity and CRP also correlated with improvement. Comparing to single IA HA injection, single injection of IA etanercept in moderate-to-severe knee OA showed that VAS and WOMAC scores did not differ significantly between groups, but significant pain relief was shown in the etanercept injection group at 1 and 2 weeks by VAS [144]. Single IA injection of 10 mg adalimumab compared to 25 mg single IA injection of HA in moderate-to-severe knee OA showed improvements in VAS and WOMAC scores [145]. In 2019 ACR guideline, TNF inhibitors and IL-1 receptor antagonists are strongly recommended against in knee OA patients [2]. Their efficacy was not proved and its risk was known .
10.4.3.2 Cellular Therapy
There has been many limitations in treating chronic diseases such as OA using standard drug. There have been attempts to overcome these limitations with regenerative medicine, such as cell therapy. Cell therapy is one of the fields of regenerative medicine, and there has been a lot of interest and research recently. However, cell therapy has a variety of unresolved issues in cell sources, manufacturing processes, administration methods, bioavailability, clinical results, etc. IA Cell therapy includes cell concentrates, MSCs, and gene therapy. Cell therapies can be classified by cell sources or by specific cell type from tissues or by cell processing methods [9] (Fig. 10.7). Cell sources maybe autologous avoiding immune response issues and disease transmission or allogeneic to eliminate donor-site morbidity and to maximize availability. Cells can be used as point-of care therapy with non- or minimal manipulated cell concentrates or as cell therapy with manipulated in ex vivo (expanded in culture) [146]. The most utilized IA injection cell therapies for knee OA include the use of concentrates of peripheral blood, bone marrow aspirate concentrates (BMAC), and stromal vascular fraction (SVF) from adipose tissue which comprise stem cells, growth factors, and cytokines. They can be obtained with minimal manipulation in the clinics, without the need to isolate and expand the cells, and immediately implanted in the patient as point-of-care cell therapies [146]. Because the number of MSCs extracted directly from tissues is too small to be used and it is difficult to distinguish MSCs, they are cultivated to distinguish cells with MSC characteristics and used for clinical use. Cell concentrates are the least processed and most heterogeneous therapies, whereas MSCs are the most processed and least heterogeneous. Within each treatment category, therapies (and the regulations that apply to clinical use) tend to differ significantly [9].
Intra-articular cell therapies. Cell therapies can be classified by the method used to produce them or by their relative heterogeneity compared with the source tissue. Cellular therapy includes cell concentrates as autologous point-of-care cell therapy, expanded cell therapy as mesenchymal stromal (or stem) cells, and gene therapy. Autologous point-of-care cell therapies are heterogeneous mixtures containing cells (or cell products) that are derived from autologous blood, bone marrow, or adipose tissue. Expanded cell therapies are the cultivation and use of cells with MSC characteristics under ex vivo culture. MSCs must be plastic-adherent, express or lack specific cell surface markers, and be capable of trilineage differentiation into osteoblasts, adipocytes, and chondrocytes in vitro. BMAC bone marrow aspirate concentrate, MSC mesenchymal stem/stromal cell, PRP platelet-rich plasma, SVF stromal vascular fraction
10.4.3.2.1 Stromal Vascular Fraction
Stromal vascular fractions (SVF) are derived from lipoaspirate and harvested with liposuction devices in the abdomen or flank under local anesthesia. The lipoaspirate is washed, processed with collagenases, and then placed into a centrifuge. Centrifugation allows for identification and collection of the SVF, which contains a heterogeneous cell population including MSCs, endothelial cells, and endothelial precursors. Alternative systems can use mechanical rather than enzymatic processes to break down adipose tissue or can use filtration rather than centrifugation to isolate the SVF. The resultant SVF ideally contains no adipocytes and has only low concentrations of leukocytes and extracellular matrix [147]. However, SVF is highly heterogeneous and only ~15–30% of the cellular content is stromal cells. Moreover, although adipose-derived stromal cells can be purified from SVF, be aware of the difference between SVF and adipose-derived stromal cells [148].
There are a few clinical research evaluating the efficacy of SVF on treatment for knee OA. Pak et al. [149] conducted a case series of treating knee OA patients with autologous adipose SVF and regenerating cartilage-like tissue. That showed the VAS, functional rating index (FRI), and ROM were improved and MRI evidence of cartilage regeneration was observed after 3 months. Koh et al. [150] conducted a case series for evaluating the effectiveness of SVF on knee OA using clinical outcome and second look arthroscopy. This study showed clinical parameters significantly improved at 2-year follow-up. Furthermore, 87.5% of elderly patients improved or maintained cartilage status at least 2 years postoperatively. Michalek et al. [151] performed a multicenter case-control study involving 1114 knee and hip OA patients from four different countries. The clinical outcomes such as pain, use of nonsteroidal analgesics , limping, joint movement and stiffness, all improved at 12 months after treatment. Garza et al. [152] performed a DB RCT for evaluating whether IA SVF injection improves knee OA symptom and this improvement would be dose dependent. They reported IA SVF injections can significantly decrease knee OA symptoms and pain for at least 12 months, dose dependently. There were no changes in cartilage thickness on MRI evaluation and no serious AEs occurred. However, one of the major drawbacks relating to autologous adipose SVF in the treatment of knee OA is the absence of accessibility of RCTs. Due to these limitations, despite successful results by these reports , it is not yet easily accepted as mainstream treatment [153].
10.4.3.2.2 Bone Marrow Aspirate Concentrate
Bone marrow aspirate (BMA) contains a mixture of cellular components including platelets, WBC, RBC, adipose cells, hematopoietic and non-hematopoietic precursors. Bone marrow aspirate concentrates (BMAC) is a centrifugation form of BMA and contains platelets, growth factors, and multipotent MSCs. However, multipotent MSCs in the BMAC comprise merely 0.001–0.01% of mononuclear cells within bone marrow aspirate. Numerous growth factors, such as TGF-β, PDGF, IL-1β, insulin growth factor-1 (IGF-1), fibroblast growth factor-18, bone morphogenetic protein-2 (BMP2) and BMP-7, have been identified in BMAC. BMA taken usually from the posterior iliac crest has the highest concentration of multipotent MSCs. The aspirate then undergoes centrifugation. After that, resultant concentrates were injected into the joint as a fluid or delivery of cells through an implantable scaffold. The wide variation in BMAC preparation protocols and lack of standardization methods make comparisons difficult because the true biological potential of each product in a patient is unknown [133].
Rodriguez-Fontan et al. [154] performed a case series to assess the clinical outcomes of IA injection of BMAC for the treatment of early knee OA. IA injections of BMAC for the treatment of early knee were safe and demonstrated satisfactory results in 63.2% of patients. Kim et al. [155] conducted a case series study evaluating the clinical efficacy of the IA injection of autologous BMAC with adipose tissue. This study showed improvement in the VAS pain scores, IKDC scores, Lysholm scores, and SF 36 at post-injection 12 months. The improvement of VAS pain score was less in grade IV OA patients, which means that BMAC is more effective in mild to moderate OA. Centeno et al. [156] reported improvement in Lower Extremity Functional Scale scores and lower mean NRS scores, and low rate of AEs in BMAC with and without adipose grafts. Addition of an adipose graft to the BMAC did not provide a detectible benefit over BMAC alone. Shapiro et al. [157] conducted a single-blind, saline injection placebo-controlled, RCT for evaluating the efficacy and safety of BMAC in knee OA. Their results showed that the VAS pain and OARSI Intermittent and Consistent Osteoarthritis Pain scores improved in both knees at 1 week, 3 and 6 months from baseline. However, there are no differences between BMAC and placebo injections. There were no serious AEs from the BMAC procedure. The same group of authors also conducted a DB RCT to evaluate cartilage appearance using MRI T2 quantitative mapping following these patients up to 12 months . No significant differences were found on T2 quantitative MRI mapping between the saline control and BMAC knees [158]. From these clinical studies, although the use of BMAC in the treatment of symptomatic OA demonstrates promising early clinical outcomes, no clear regenerative benefits have been showed to date.
10.4.3.2.3 Platelet-Rich Plasma
Platelets are small, nonnucleated cells in peripheral blood and its main function is a role in hemostasis. Platelets contain a number of proteins, cytokines, and other bioactive factors and normal platelet counts in blood range from 150,000 to 350,000/μL [159]. Plasma is the liquid part of the blood including clotting factors, other proteins, and ions. PRP, with a platelet concentration of at least 1,000,000 platelets/μL in 5 mL plasma, has a positive effect on tissue healing and regeneration. PRP includes a high concentration of platelets, which has more than 1100 proteins like growth factors. PRP increases growth factor concentration 3–5 times [160]. Platelets play an important role in the initiation of healing as they form scaffolds for the formation of clot, leading to the chemotaxis of appropriate cytokines. Platelet α-granules include growth factors and anti-inflammatory cytokines such as IGF-1, IGF-2, vascular endothelial growth factor, TGF-β, FGF, endothelial growth factor, and PDGF. These are released at the healing site and have been found to help promote the growth of autologous chondrocytes, MSCs, and extracellular matrix components like proteoglycans, type I and II collagen [161].
For PRP preparation, autologous venous blood was centrifuged to enrich platelets above the levels normally found in serum. Because the density of whole blood components is different, spinning the whole blood by density gradient centrifugation can separate each component into different layers: platelet-poor plasma, buffy coat, and RBCs. A buffy coat is located between the platelet-poor plasma and RBCs and it contains the highest concentration of platelets. PRP can be obtained in two forms: plasma based and buffy coat preparations. Plasma-based methods of PRP work to isolate only plasma and platelets while excluding leukocytes. The buffy coat-based method separates the platelet-poor plasma layer and the buffy coat layer, containing both white and red blood cells. The blood composition and humoral factors of PRP vary depending on the method used, and according to the leukocyte content, PRP is classified as pure PRP, leukocyte-poor PRP (LP-PRP), and leukocyte-rich PRP (LR-PRP) [162]. Although contrasting scientific evidence exists for PRP injections for knee OA from several studies, the efficacy of IA PRP injections has been widely reported. Dai et al. [163] conducted a meta-analysis to assess the efficacy and safety of PRP for symptomatic knee OA. They reported PRP and HA had similar effects with regard to pain relief and functional improvement. However, at 12 months after injection, PRP had better pain and functional improvement than HA, and the improvement effect of WOMAC pain and functional score exceeded Minimal Clinically Important Difference (MCID) (−0.79 for WOMAC pain and −2.85 for WOMAC functional score). Compared to saline injection, PRP was more effective in pain and functional improvement at 6 and 12 months after injection, and the improvement effect of WOMAC pain and functional score exceeded MCID. PRP did not have a higher risk of AEs compared to HA and saline. Tang et al. [164] performed a meta-analysis study to evaluate the clinical efficacy of PRP injection compared with HA injection for knee OA. They concluded that IA PRP injection appeared to be more efficacious than HA injection for the treatment of knee OA in terms of short-term functional recovery. Moreover, PRP injection was superior to HA injection in terms of long-term pain relief and functional improvement. In addition, PRP injection did not increase the risk of AEs. The level of evidence was moderate or low due to the heterogeneity and/or study design limitations. So even though profits are conclusive, the degree of benefit must be studied. Filardo et al. [165] conducted a meta-analysis study to evaluate effectiveness of PRP injections for knee OA compared to placebo and other IA treatments. This study resulted in WOMAC score favored PRP, with a statistically and clinically significant difference versus placebo at 12 months follow-up (P = 0.02) and versus HA at 6 months (P < 0.001) and 12 months (P < 0.001) follow-ups. A clinically significant difference favoring PRP versus steroids was documented for VAS pain (P < 0.001), KOOS pain (P < 0.001), function in daily activities (P = 0.001), and quality of life (P < 0.001) at 6 months follow-up. However, superiority of PRP did not reach the MCID for all outcomes, and quality of evidence was low.
The different preparation of PRP leads to different concentrations of platelets, WBCs, and RBCs, which might affect clinical outcomes. Riboh et al. [166] performed a meta-analysis to compare the clinical outcomes between HA, LP-PRP, and LR-PRP for treatment of knee OA. The authors reported clearly better WOMAC scores in LP-PRP than the HA or placebo, but no such difference was observed with LR-PRP. Both PRP preparations resulted in higher incidences of AEs than HA but there was no difference in AEs between LP-PRP and LR-PRP. Belk et al. conducted a meta-analysis study to compare the efficacy of PRP and HA injections for the treatment of knee OA and evaluate the clinical outcomes according to leukocyte concentration. They concluded IA PRP injection showed better outcomes, such as WOMAC score, VAS and subjective IKDC score, at 11 months post-injection. Moreover, LP-PRP was associated with significantly better subjective IKDC scores versus LR-PRP.
The results of the meta-analysis studies show that the use of PRP can improve the short- and mid-term (6–12 months) pain scale over other IA treatments, such as HA injection. However, despite the apparent positive effects of PRP use, the methodological quality among studies is low, there is considerable heterogeneity between studies, and the diversity of PRP formulations confuses the clear demonstration of clinical efficacy [167]. Large-scale RCTs are needed to further evaluate the efficacy and duration of PRP treatment in patients with knee OA. The number and frequency of injections, the method of activation (for anticoagulant PRP), aspects of storage, plasma separation time, and concomitant therapy currently vary greatly from group to group and must be considered when planning or analyzing [168]. In 2019 OARSI and ACR guideline, IA PRP injection for knee OA is strongly recommended against because the quality of evidence supporting these treatments is extremely low, and there is a lot of the heterogeneity and lack of standardization in preparations [2, 75].
10.4.3.2.4 Autologous Protein Solution
An autologous protein solution (APS) is a kind of cell concentrates made from whole blood. The APS consists of WBCs which contain anti-inflammatory proteins, platelets which contain anabolic growth factors, and concentrated plasma that contains both. This solution produced by combination of WBCs, platelets, and concentrated plasma has properties of increased concentrations of anti-inflammatory cytokines and anabolic growth factors [169]. Conceptually, APS and LR-PRP are very similar. However, unlike traditional PRP systems, commercially available APS kit passes the concentrated plasma through a dried polyacrylamide gel that leads to a high level of anti-inflammatory cytokines while ensuring low levels of proinflammatory molecules, preferentially concentrates anti-inflammatory cytokines including IL-1 receptor antagonist and TNF receptor inhibitor [9]. Kon et al. [170] performed a multicenter, saline controlled, DB RCT to investigate if single IA injection of APS is able to reduce pain and improve function in knee OA. The results showed a statistically significant improvement in WOMAC pain score at 12 months for APS compared with placebo. However, improvements from baseline to 2 weeks and 1, 3, 6 months were similar between treatments. Additionally, this study failed to show any significant differences in VAS pain improvement between groups as primary outcome. The same group of authors also conducted 3 years follow-up study to investigate if the positive effects of APS, previously documented at 1 year in a clinical trial, last up to 3 years [171]. In the APS cohort, WOMAC pain improved from 11.5 to 4.3 at 1 year and to 5.7 at 3 years (P < 0.0001 vs baseline). The APS cohort also showed a statistically significant improvement in its KOOS pain score from 39 to 70 at 1 year and to 64 at 3 years (P < 0.0001 vs baseline) and VAS pain scores from 5.5 to 2.6 at 1 year and to 3.4 at 3 years (P = 0.0184 vs baseline). However, VAS pain score significantly worsened from 12 to 36 months (P = 0.0411). MRI Osteoarthritis Knee Score findings showed no statistically significant differences. Patients with better cartilage had greater WOMAC pain improvement when their baseline scores were worse, whereas the trend was reversed for patients with cartilage loss at baseline. IA injection of APS for mild to moderate knee OA was safe , and significant pain improvement was documented 3 years after a single injection.
10.4.3.2.5 Mesenchymal Stromal (or Stem) Cells
MSC is defined when it has the following cellular characteristics: First, MSC must be plastic-adherent when maintained under standard culture conditions. Second, MSC must express specific cell surface marker. Third, MSC must be differentiated into osteoblasts, adipose cells, and chondroblasts in vitro [172]. There have been many controversies about the action mechanism of MSCs in vivo if regenerative process occurs either by implanted MSC differentiation or by endocrine and paracrine activity on host cells, or both [173]. Although the in vivo mechanisms of MSCs are still unclear, the release of chemical mediators is thought to be important [174]. Exogenously administered MSCs induce to produce soluble growth factors and cytokines in the injury site and exert immunomodulatory, anti-inflammatory, and nourishing (regenerative) effects on the patient’s resident stem cells to form the new tissue [175].
MSC can be obtained from any tissue, autogenous or allogenic source. Autogenous BMAC or SVF is most commonly used for injectable MSCs in knee OA, that components are heterogeneous. MSC proportion in BMAC or SVF is very low, for example, 0.001%–0.01% of mononuclear cells within BMAC and ~15–30% within SVF. Therefore, ex vivo isolation and expansion of cell concentrates might be needed to obtain clinically useful amount of MSCs. However, ex vivo expanded cell therapy might increase the risk of AEs. Although no major AEs have been reported on IA MSCs injection for knee OA, malignant transformation remains a potential risk for ex vivo expanded cell therapy [176]. Ex vivo expanded MSCs are classed as drugs that require government regulatory approval before clinical application. Although minimally manipulated cell product such as BMAC or SVF might contain MSCs, they might claim to be exempt from government regulation as point-of-care therapy. Autologous point-of-care cell therapy should not be confused with true MSC therapies because they tend to include more heterogeneous cells than those in true MSC therapies and have effects which are not mainly attributed to their pharmacological immunomodulatory and/or immunosuppressive capacity or differentiation potential [9]. In this section, only clinical research results on IA injection therapy for knee OA using ex vivo expanded MSCs will be reviewed. Recently, many clinical studies have been carried out in the hope that MSCs will heal damaged tissues. In spite of hopeful expectations, RCT studies are rare and the results of those studies have not reached MCID. In 2019 OARSI and ACR guideline, IA MSCs injection for knee OA is strongly recommended against because the quality of evidence supporting of these treatments is extremely low, and there is a lot of the heterogeneity and lack of standardization in preparations [2, 75].
10.4.3.2.6 Bone Marrow-Derived Mesenchymal Stromal Cells
Soler Rich et al. [177] conducted a case series to assess the efficacy and safety of autologous expanded BM-MSCs for KL 2–4 knee OA. This study showed the IA infusion of a dose of 40 × 106 expanded BM-MSC suspended in an 8 mL solution of Ringer-lactate and albumin has no local or systemic AEs and can significantly improve symptoms due to joint inflammation in a short period of time. Also, there is a report that the OA cartilage tissue evaluated through T2 mapping MRI was improved. Soler R et al. [178] reported a phase I–II trial using ex vivo expanded autologous MSCs for the treatment of KL 2–3 knee OA. Patients were injected with 10 mL of saline solution supplemented with 2% human serum albumin suspended with 40 × 106 expanded BM-MSC and followed up to 12 months post-injection. There were few reported AEs (mild joint and low back pain). Pain intensity was decreased since day 8 after the injection, which was maintained after 12 months. The SF-36 showed improvement in parameters including joint pain and physical function at month 12. Moreover, T2 mapping MRI showed cartilage regeneration in all patients 12 months after treatment. Emadedin et al. [179] performed a randomized, triple-blind, placebo-controlled phase 1/2 clinical trial to evaluate the safety and efficacy of IA implantations of autologous BM-MSCs in patients with knee OA. 43 patients enrolled this study and study group were injected with 40x106 ex vivo expanded MSCs in 5 ml saline supplemented with 2% human serum albumin and followed up for 6 months after the implantations. Patients who injected MSC had significantly improved in overall WOMAC scores, WOMAC pain and function subscales, and pain-free walking distance compared to those who received placebo. There were no major AEs attributed to the MSC treatment.
Doyle et al. [180] reported a narrative review that evaluated the efficacy of IA injections of BM-MSCs for the treatment of knee OA. This study showed clinical applications of IA injections of BM-MSCs are steadily increasing, with most studies demonstrating a decrease in poor cartilage index, improvements in pain, function and Quality of Life (QoL); with moderate-to-high level evidence regarding safety for therapeutic administration. A moderate number of cells (40 × 106) were identified as most likely to achieve optimal responses in individuals with KL grade ≥ 2 knee OA. However, low confidence in clinical efficacy remains due to a plethora of heterogenous methodologies used, resulting in need for comparative RCT.
10.4.3.2.7 Adipose-Derived Mesenchymal Stromal Cells (AD-MSCs)
Jo et al. [181] conducted a case series clinical trial to assess the mid-term safety and efficacy of AD-MSCs for knee OA. Patients in each group received low-dose (1.0 × 107 cells), mid-dose (5.0 × 107 cells), and high-dose (1.0 × 108 cells) injections and followed up to 2 years post-injection. IA injections of autologous AD-MSCs improved knee functional scores as measured by WOMAC, KSS, and KOOS and decreased VAS pain scores for up to 2 years in all cell dosage groups. However, statistical significance was mainly seen in the high-dose group. Clinical outcomes tended to be worsen after 1 year in the low-dose and medium-dose groups, while lasting up to 2 years in the high-dose groups. The structural outcomes evaluated by MRI showed similar patterns. There were no treatment-related AEs during the 2-year period. Freitag et al. [182] reported an RCT to evaluate the efficacy of autologous AD-MSC on pain, function, and disease modification in knee OA. 30 participants were randomly divided into three groups. Two groups received IA AD-MSC injection consisting of either a single (100 × 107 cells) or two injections (100 × 107 cells at baseline and 6 months). The third group served as a control group, with only conservative treatment. Both treatment groups showed clinically significant pain and functional improvement at 12 months. Radiological analysis with MRI showed modification of disease progression. 2 IA injections of AD-MSCs at 6-month intervals achieved more consistent OA stabilization than single injection. Lee et al. [183] conducted a Phase IIb, saline controlled, RCT to evaluate the efficacy and safety of a single IA injection of AD-MSCs for knee OA patients. Patients received a single injection of 1 × 108 cells of ADMSCs in 3 mL of saline and followed up to 6 months. This study resulted in a significant improvement in WOMAC scores at 6 months with a single injection of AD-MSC. No serious AEs were observed in both groups during follow-up period. When measured by MRI, cartilage defects at 6 months after injection were not significantly changed in AD-MSC group, whereas defects increased in control group. Song et al. [184] reported a randomized phase I/IIa clinical trial with a 96-week follow-up to evaluate the safety and therapeutic potential of different dose and repeated injection of AD-MSCs in patients with knee OA. 18 participants were divided into three dose groups: the low-, mid-, and high-dose group (1 × 107, 2 × 107 and 5 × 107 cells, respectively), injected three times and followed up for 96 weeks. They concluded IA injections of AD-MSCs reduced pain, improved function and cartilage volume without treatment-related AEs. Also, this improvement was more pronounced when repeated injections were combined with a 5 × 107 cell dose.
10.4.3.2.8 Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells
MSCs can be separated from umbilical cord Wharton’s jelly, perivascular tissue, and blood using various techniques. Those cells are characterized by phenotypic similarities with BM-MSCs but their differentiation into the osteogenic and chondrogenic lineage is not as consistent as for BM-MSCs [185]. Human umbilical cord blood-derived mesenchymal stem cells (hUCB-MSCs) have biological benefits such as longer culture, large-scale expansion, retardation of senescence, and high anti-inflammatory effect compared to bone marrow or adipose tissue [186]. Park et al. [187] reported 7 years of follow-up case series study. The stem cell-based medicinal product (a composite of culture-expanded allogeneic hUCB-MSCs and HA hydrogel [Cartistem]) was surgically applied to the lesion site, not IA injection. The improved clinical results were stable for 7 years follow-up period. The histological findings at 1 year showed hyaline-like cartilage. Regenerated cartilage maintained persistence on MRI at 3 years. Mata et al. [188] conducted a controlled randomized phase I/II trial to evaluate the safety and efficacy of the IA injection of single or repeated allergenic hUCB-MSCs in knee OA. Patients with symptomatic knee OA were randomized to receive HA at baseline and 6 months, single-dose (20 × 106) hUCB-MSC at baseline, or repeated hUCB-MSC doses at baseline and 6 months (20 × 106 × 2: MSC-2) and followed up for 12 months. No severe AEs were reported. Only MSC-treated patients experienced significant pain relief and functional improvements from baseline (p = 0.001). At 12 months, WOMAC pain subscale significantly decreased in the MSC-2-treated group as compared with the HA group. For total WOMAC score, MSC-2 was lower than HA at 12 months. No differences in MRI Osteoarthritis Knee scores were detected. The author concluded repeated UC-MSC therapy is safer and better than active comparator in knee OA at 1 year follow-up.
10.4.3.2.9 Gene Therapy
Gene transfer has been performed in two ways, directly in vivo injection into the joint or cell harvesting from the patient, ex vivo exposure to vectors and return of modified cells to the joint. Gene therapy with genes encoding cartilage growth factor and anti-inflammatory cytokines is effective in treating OA [146]. TissueGene-C (TG-C) was developed as a cell-based growth factor expression strategy and contains human allogeneic chondrocytes and genetically modified chondrocytes engineered to express TGF-b1 in a 3:1 ratio. TGF-b1 expressing cells were irradiated with low-dose gamma rays to prevent proliferation within the knee joint after injection. TG-C was administered by a single IA injection [189] (Fig. 10.8). Cherian et al. [190] conducted a phase II randomized study to evaluate the efficacy and safety of genetically engineered allogeneic human chondrocytes expressing TGF-b1 in KL 3 knee OA patients. Improvement of IKDC subjective or VAS scores showed statistically significant differences at 12 weeks, 52 weeks and overall. Kim et al. [189] reported a multicenter, DB, phase III clinical trial to access the efficacy and safety of gene therapy in knee OA. In this study, 163 patients with KL grade III OA were randomly assigned to receive a single IA injection of TG-C or saline. TG-C showed statistically significant improvements over placebo in total IKDC scores and individual categories, VAS scores at 26, 39, and 52 weeks. Patients treated with TG-C were not statistically significant, but tended to have thicker cartilage and slower growth of the subchondral bone surface area in the joint (p > 0.05). There were minor AEs in the TG-C group, such as peripheral edema (9%), joint pain (8%), joint swelling (6%), and injection site pain (5%). Lee et al. [191] reported a phase II study to determine the 24 months efficacy and safety of genetically engineered allogeneic human chondrocytes expressing TGF-β1 in patients with KL III knee OA. 102 patients were 2:1 randomized to TG-C at a dose of 3.0 × 107 cells, or placebo injection. There were significant improvements in the IKDC and VAS scores in the TG-C cohort, comparing with the placebo cohort at 12, 52, 72, and 104 weeks (p < 0.05). MRI at 12 months after treatment showed fewer findings of cartilage damage, infrapatellar fat pad synovitis, and effusion synovitis in the TG-C cohort. No severe AEs were observed. Common AEs were arthralgia, inflammation, and effusion of joint which were similar between both cohorts. Although TG-C has a long-term safety issues due to viral transfected cells, TG-C appears to be an effective modality for the treatment of KL grade III knee OA, so far.
Schematic process for gene therapy with TissueGene-C. Gene therapies with genes encoding for chondrogenic growth factors and anti-inflammatory cytokines are of interest to treat OA. TissueGene-C (TG-C) was developed as a cell-based growth factor expression strategy and involves a 3:1 ratio of human allogeneic chondrocytes and genetically modified 293 cells engineered to express TGF-b1. TGF-b1 expressing cells were irradiated with a low dose of gamma-ray so that they could not proliferate within the knee joint after injection. TG-C was administered by a single intra-articular injection. TGF transforming growth factor
10.4.4 Summary
Articular cartilage is avascular structure. Oral or parenteral administered drugs used to treat knee OA must go through complicated processes, such as the drug passing through the wall of capillaries, then diffusing to the synovial interstitium, and then passing through the synovial membrane into the joint cavity. In this process, drugs with low MW enter the joint relatively easily, but high MW makes it difficult to enter the joint due to the capillary sieving effect. IA injection therapy has the advantage of bypassing this process and avoiding systemic AEs of drugs by oral or parenteral administration. In addition, the low MW drugs in the joints are absorbed through veins and the large through lymphatics. In case of joint inflammation, the residence time of drugs becomes shorter because of increased vascularity and lymphatic flow. In OA treatment, it is important to maintain the IA concentration of the therapeutic drug for enhancing its efficacy.
For IA injection therapy to work effectively, drugs must be injected accurately into the joints. This is because if a drug is injected into an extra-synovial area, the effect of the drug not only decreases but also the discomfort of the patient increases. Image guided injection using US is more useful than blind method for accurate IA injection. US images of anatomical structures around the joints should be understood when attempting IA injections under US guidance, and the ultrasonic artifact produced by needles should be differentiated.
There are CSs and HAs in the standard therapeutic agents used for IA injections. CS is effective in improving symptoms of knee OA because it has a strong anti-inflammatory effect, but the duration of the effect is only 4 weeks or less. However, systemic and local AEs such as articular cartilage destruction should be considered. According to the guideline of the non-profit organizations, IA CS injection recommends limited use in treating symptomatic knee OA that do not respond to non-pharmacological or pharmacologic treatment. HA not only acts as a viscosupplement that increases the viscosity of the synovial fluid but also has some anti-inflammatory effects. HA relieves symptoms over 3–6 months after injection, which is relatively longer than CS. However, most non-profit organization guideline does not recommend IA HA injection or recommends limited use in treating knee arthritis with symptoms that do not respond to non-pharmacological or pharmacologic treatment. Although PDRN or hypertonic dextrose injection is known to help wound healing and tissue regeneration, much research is still needed to prove a clinical efficacy in the treatment of knee OA. Recently, small molecule drug therapy for treatment of knee OA has been introduced. SM04690 is a novel small molecule Wnt-β-catenin signaling pathway inhibitor, which has potential as a disease-modifying OA drug (DMOAD). CNTX-4975 is an injectable, high-purity trans-capsaicin targeted the capsaicin receptor (TRPV1), which has a potent analgesic effect.
OA results from an imbalance between catabolic and anabolic factors, and biologic agents either target specific catabolic proinflammatory mediators or affect anabolism. Attempts to treat knee OA with biologics have been made relatively recently. Biologics used for treatment of knee OA are categorized into non-cellular therapy or cell therapy. Non-cellular therapy includes human serum albumin, growth factors, cytokine antagonists. In particular, the rhFGF 18 and the Wnt receptor inhibitor have an anabolic effect, drawing attention for their role as DMOAD along with the symptom improvements. However, non-cellular therapeutic agents of various substances are not yet widely used in clinical practice because of lacking of sound scientific evidence. Cell therapy includes cell concentrates, MSCs, and gene therapy. Although the composition of cell concentrates is heterogenous, it has recently drawn much attention as a kind of autologous point-of-care cell therapy, with the advantage of less government regulation because it can be extracted through minimal manipulation and injected into the patient immediately. SVF is a cell concentrate extracted from adipose tissue and has advantages as 15–30% of the extracted cells consist of stromal cells. The therapeutic effect of SVF in knee OA has shown good results in several studies but has not reached MICD. BMAC is a cell concentrates derived from bone marrow, and only 0.001%–0.01% of the cells extracted contain stromal cells. There is little research on the therapeutic effect of BMAC in knee OA. PRP is cell concentrates separated from blood. PRP is a plasma that contains high content of platelets. Platelet α-granules contain a variety of growth factors and anti-inflammatory cytokines, which help wound healing and regeneration. The therapeutic effect using PRP in knee OA is known to be maintained for 6–12 months, but the efficacy does not reach MCID. MSCs have cellular characteristics such as plastic-adherent, express or lack specific cell surface markers and be capable of trilineage differentiation into osteoblasts, adipocytes, and chondrocytes in vitro. MSCs are grown from cell concentrates in vitro and separated with only cells with MSCs characteristics and used to treat knee OA. Government regulation is needed in its use because the cell’s properties change in this process and in vitro manipulation is required. MSCs used in the treatment of knee OA include bone marrow-derived MSCs and adipose-derived MSCs. Despite the possibility of tissue healing and regeneration in MSCs, clinical efficacy in knee OA treatment is limited. According to 2019 OARSI and ACR guideline, PRP and MSCs injections are strongly recommended against in patients with knee OA because the evidence in support of these treatments is of extremely low quality and there is a lot of heterogeneity and lack of standardization in preparations of PRP and MSCs.
References
Silverwood V, Blagojevic-Bucknall M, Jinks C, Jordan J, Protheroe J, Jordan K. Current evidence on risk factors for knee osteoarthritis in older adults: a systematic review and meta-analysis. Osteoarthr Cartil. 2015;23(4):507–15.
Kolasinski SL, Neogi T, Hochberg MC, Oatis C, Guyatt G, Block J, et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheumatol. 2020;72(2):220–33.
Nahhas CR, Fuller BC, Hannon CP, Gerlinger TL, Nam D, Della Valle CJ. Which nonsurgical treatments do patients believe are most effective for hip and knee arthritis? J Am Acad Orthop Surg. 2020;4(5):e20.
Jackson DW, Evans NA, Thomas BM. Accuracy of needle placement into the intra-articular space of the knee. J Bone Joint Surg Am. 2002;84(9):1522–7.
Evans CH, Kraus VB, Setton LA. Progress in intra-articular therapy. Nat Rev Rheumatol. 2014;10(1):11.
Nguyen C, Rannou F. The safety of intra-articular injections for the treatment of knee osteoarthritis: a critical narrative review. Expert Opin Drug Saf. 2017;16(8):897–902.
Bannuru RR, McAlindon TE, Sullivan MC, Wong JB, Kent DM, Schmid CH. Effectiveness and implications of alternative placebo treatments: a systematic review and network meta-analysis of osteoarthritis trials. Ann Intern Med. 2015;163(5):365–72.
Saltzman BM, Leroux T, Meyer MA, Basques BA, Chahal J, Bach BR Jr, et al. The therapeutic effect of intra-articular normal saline injections for knee osteoarthritis: a meta-analysis of evidence level 1 studies. Am J Sports Med. 2017;45(11):2647–53.
Jones IA, Togashi R, Wilson ML, Heckmann N, Vangsness CT. Intra-articular treatment options for knee osteoarthritis. Nat Rev Rheumatol. 2019;15(2):77–90.
Paoloni M, Bernetti A, Belelli A, Brignoli O, Buoso S, Caputi AP, et al. Appropriateness of clinical and organizational criteria for intra-articular injection therapies in osteoarthritis: a Delphi method consensus initiative among experts in Italy. Ann Ist Super Sanita. 2015;51:131–8.
Oo WM, Liu X, Hunter DJ. Pharmacodynamics, efficacy, safety and administration of intra-articular therapies for knee osteoarthritis. Expert Opin Drug Metab Toxicol. 2019;15(12):1021–32.
Simkin PA, Pizzorno JE. Synovial permeability in rheumatoid arthritis. Arthritis Rheum. 1979;22(7):689–96.
Knight A, Levick J. Morphometry of the ultrastructure of the blood-joint barrier in the rabbit knee. Q J Exp Physiol. 1984;69(2):271–88.
Kushner I, Somerville JA. Permeability of human synovial membrane to plasma proteins. Relationship to molecular size and inflammation. Arthritis Rheum. 1971;14(5):560–70.
Simkin PA. Synovial perfusion and synovial fluid solutes. Ann Rheum Dis. 1995;54(5):424.
Larsen C, Østergaard J, Larsen SW, Jensen H, Jacobsen S, Lindegaard C, et al. Intra-articular depot formulation principles: role in the management of postoperative pain and arthritic disorders. J Pharm Sci. 2008;97(11):4622–54.
Scanzello CR, Goldring SR. The role of synovitis in osteoarthritis pathogenesis. Bone. 2012;51(2):249–57.
Jones A, Regan M, Ledingham J, Pattrick M, Manhire A, Doherty M. Importance of placement of intra-articular steroid injections. BMJ. 1993;307(6915):1329–30. https://doi.org/10.1136/bmj.307.6915.1329.
Glattes RC, Spindler KP, Blanchard GM, Rohmiller MT, McCarty EC, Block J. A simple, accurate method to confirm placement of intra-articular knee injection. Am J Sports Med. 2004;32(4):1029–31.
Qvistgaard E, Kristoffersen H, Terslev L, Danneskiold-Samsøe B, Torp-Pedersen S, Bliddal H. Guidance by ultrasound of intra-articular injections in the knee and hip joints. Osteoarthr Cartil. 2001;9(6):512–7.
Waddell D, Estey D, Bricker D, Marsala A. Viscosupplementation under fluoroscopic control. Am J Sports Med. 2001;3(4):327–241. 9
Tarhan S, Unlu Z. Magnetic resonance imaging and ultrasonographic evaluation of the patients with knee osteoarthritis: a comparative study. Clin Rheumatol. 2003;22(3):181–8.
Esenyel C, Demirhan M, Esenyel M, Sonmez M, Kahraman S, Senel B, et al. Comparison of four different intra-articular injection sites in the knee: a cadaver study. Knee Surg Sports Traumatol Arthrosc. 2007;15(5):573–7.
Luc M, Pham T, Chagnaud C, Lafforgue P, Legre V. Placement of intra-articular injection verified by the backflow technique. Osteoarthr Cartil. 2006;14(7):714–6.
Maricar N, Parkes MJ, Callaghan MJ, Felson DT, O’Neill TW. Where and how to inject the knee—a systematic review. Semin Arthritis Rheum. 2013;43(2):195–203.
Toda Y, Tsukimura N. A comparison of intra-articular hyaluronan injection accuracy rates between three approaches based on radiographic severity of knee osteoarthritis. Osteoarthr Cartil. 2008;16(9):980–5.
Im SH, Lee SC, Park YB, Cho S-R, Kim JC. Feasibility of sonography for intra-articular injections in the knee through a medial patellar portal. J Ultrasound Med. 2009;28(11):1465–70.
Park Y, Lee SC, Nam H-S, Lee J, Nam SH. Comparison of sonographically guided intra-articular injections at 3 different sites of the knee. J Ultrasound Med. 2011;30(12):1669–76.
Myung JS, Lee JW, Lee JY, Choi J-Y, Kim SH, Jun WS, et al. Usefulness of fluoroscopy-guided intra-articular injection of the knee. J Korean Radiol Soc. 2007;56(6):563–7.
Chavez-Chiang CE, Sibbitt WL, Band PA, Chavez-Chiang NR, DeLea SL, Bankhurst AD. The highly accurate anteriolateral portal for injecting the knee. Sports Med Arthros Rehabil Ther Technol. 2011;3(1):6.
Hussein M. An accurate full-flexion anterolateral portal for needle placement in the knee joint with dry osteoarthritis. J Am Acad Orthop Surg. 2017;25(7):e131–e7. https://doi.org/10.5435/jaaos-d-16-00338.
Lee SY, Gn KK, Chung BJ, Lee SW, Kim TK. Anterolateral portal is less painful than superolateral portal in knee intra-articular injection. Knee Surg Relat Res. 2015;27(4):228–32. https://doi.org/10.5792/ksrr.2015.27.4.228.
Shortt CP, Morrison WB, Roberts CC, Deely DM, Gopez AG, Zoga AC. Shoulder, hip, and knee arthrography needle placement using fluoroscopic guidance: practice patterns of musculoskeletal radiologists in North America. Skelet Radiol. 2009;38(4):377–85.
Douglas RJ. Aspiration and injection of the knee joint: approach portal. Knee Surg Relat Res. 2014;26(1):1.
Telikicherla M, Kamath SU. Accuracy of needle placement into the intra-articular space of the knee in osteoarthritis patients for viscosupplementation. J Clin Diagn Res. 2016;10(2):RC15.
Chavez-Chiang NR, Sibbitt WL, Band PA, DeLea SL, Park KS, Bankhurst AD. The outcomes and cost-effectiveness of intraarticular injection of the rheumatoid knee. Rheumatol Int. 2012;32(2):513–8.
Hermans J, Bierma-Zeinstra SM, Bos PK, Verhaar JA, Reijman M. The most accurate approach for intra-articular needle placement in the knee joint: a systematic review. Semin Arthritis Rheum. 2011;41:106–15.
Curtiss HM, Finnoff JT, Peck E, Hollman J, Muir J, Smith J. Accuracy of ultrasound-guided and palpation-guided knee injections by an experienced and less-experienced injector using a superolateral approach: a cadaveric study. PM&R. 2011;3(6):507–15.
Bum Park Y, Ah Choi W, Kim YK, Chul Lee S, Hae LJ. Accuracy of blind versus ultrasound-guided suprapatellar bursal injection. J Clin Ultrasound. 2012;40(1):20–5.
Sibbitt W Jr, Kettwich L, Band P, Chavez-Chiang N, DeLea S, Haseler L, et al. Does ultrasound guidance improve the outcomes of arthrocentesis and corticosteroid injection of the knee? Scand J Rheumatol. 2012;41(1):66–72.
Cunnington J, Marshall N, Hide G, Bracewell C, Isaacs J, Platt P, et al. A randomized, double-blind, controlled study of ultrasound-guided corticosteroid injection into the joint of patients with inflammatory arthritis. Arthritis Rheum. 2010;62(7):1862–9.
Ihnatsenka B, Boezaart AP. Ultrasound: basic understanding and learning the language. Int J Shoulder Surg. 2010;4(3):55.
Sites BD, Brull R, Chan VW, Spence BC, Gallagher J, Beach ML, et al. Artifacts and pitfall errors associated with ultrasound-guided regional anesthesia: Part I: Understanding the basic principles of ultrasound physics and machine operations. Reg Anesth Pain Med. 2007;32(5):412–8.
Sites BD, Brull R, Chan VW, Spence BC, Gallagher J, Beach ML, et al. Artifacts and pitfall errors associated with ultrasound-guided regional anesthesia: part II: a pictorial approach to understanding and avoidance. Reg Anesth Pain Med. 2007;32(5):419–33.
Chevalier X, Eymard F, Richette P. Biologic agents in osteoarthritis: hopes and disappointments. Nat Rev Rheumatol. 2013;9(7):400.
MacMahon PJ, Eustace SJ, Kavanagh EC. Injectable corticosteroid and local anesthetic preparations: a review for radiologists. Radiology. 2009;252(3):647–61. https://doi.org/10.1148/radiol.2523081929.
Creamer P. Intra-articular corticosteroid injections in osteoarthritis: do they work and if so, how? Ann Rheum Dis. 1997;56(11):634–5.
Pekarek B, Osher L, Buck S, Bowen M. Intra-articular corticosteroid injections: a critical literature review with up-to-date findings. Foot. 2011;21(2):66–70.
Schumacher HR, Chen LX. Injectable corticosteroids in treatment of arthritis of the knee. Am J Sports Med. 2005;118(11):1208–14.
Østergaard M, Halberg P. Intra-articular corticosteroids in arthritic disease. BioDrugs. 1998;9(2):95–103.
Derendorf H, Möllmann H, Grüner A, Haack D, Gyselby G. Pharmacokinetics and pharmacodynamics of glucocorticoid suspensions after intra-articular administration. Clin Pharmacol Ther. 1986;39(3):313–7.
Kraus V, Conaghan P, Aazami H, Mehra P, Kivitz A, Lufkin J, et al. Synovial and systemic pharmacokinetics (PK) of triamcinolone acetonide (TA) following intra-articular (IA) injection of an extended-release microsphere-based formulation (FX006) or standard crystalline suspension in patients with knee osteoarthritis (OA). Osteoarthr Cartil. 2018;26(1):34–42.
Hepper CT, Halvorson JJ, Duncan ST, Gregory AJ, Dunn WR, Spindler KP. The efficacy and duration of intra-articular corticosteroid injection for knee osteoarthritis: a systematic review of level I studies. J Am Acad Orthop Surg. 2009;17(10):638–46.
Pyne D, Ioannou Y, Mootoo R, Bhanji A. Intra-articular steroids in knee osteoarthritis: a comparative study of triamcinolone hexacetonide and methylprednisolone acetate. Clin Rheumatol. 2004;23(2):116–20.
Yavuz U, Sökücü S, Albayrak A, Öztürk K. Efficacy comparisons of the intraarticular steroidal agents in the patients with knee osteoarthritis. Rheumatol Int. 2012;32(11):3391–6.
Lomonte ABV, de Morais MGV, de Carvalho LO, de Freitas Zerbini CA. Efficacy of triamcinolone hexacetonide versus methylprednisolone acetate intraarticular injections in knee osteoarthritis: a randomized, double-blinded, 24-week study. J Rheumatol. 2015;42(9):1677–84.
Buyuk AF, Kilinc E, Camurcu IY, Camur S, Ucpunar H, Kara A. Compared efficacy of intra-articular injection of methylprednisolone and triamcinolone. Acta Ortop Bras. 2017;25(5):206–8.
Popma JW, Snel FW, Haagsma CJ, Brummelhuis-Visser P, Oldenhof HG, van der Palen J, et al. Comparison of 2 dosages of intraarticular triamcinolone for the treatment of knee arthritis: results of a 12-week randomized controlled clinical trial. J Rheumatol. 2015;42(10):1865–8.
Benzon Honorio T, Chew T-L, McCarthy Robert J, Benzon Hubert A, Walega DR. Comparison of the particle sizes of different steroids and the effect of dilution: a review of the relative neurotoxicities of the steroids. Anesthesiology. 2007;106(2):331–8. https://doi.org/10.1097/00000542-200702000-00022.
Chu CR, Coyle CH, Chu CT, Szczodry M, Seshadri V, Karpie JC, et al. In vivo effects of single intra-articular injection of 0.5% bupivacaine on articular cartilage. J Bone Joint Surg Am. 2010;92(3):599–608.
Chakravarty K, Pharoah P, Scott D. A randomized controlled study of post-injection rest following intra-articular steroid therapy for knee synovitis. Rheumatology. 1994;33(5):464–8.
Charalambous C, Paschalides C, Sadiq S, Tryfonides M, Hirst P, Paul A. Weight bearing following intra-articular steroid injection of the knee: survey of current practice and review of the available evidence. Rheumatol Int. 2002;22(5):185–7.
Bellamy N, Campbell J, Welch V, Gee TL, Bourne R, Wells GA. Intraarticular corticosteroid for treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;2
Bannuru RR, Natov NS, Obadan IE, Price LL, Schmid CH, McAlindon TE. Therapeutic trajectory of hyaluronic acid versus corticosteroids in the treatment of knee osteoarthritis: a systematic review and meta-analysis. Arthritis Care Res. 2009;61(12):1704–11.
Jüni P, Hari R, Rutjes AW, Fischer R, Silletta MG, Reichenbach S, et al. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst Rev. 2015;10
Paik J, Duggan ST, Keam SJ. Triamcinolone acetonide extended-release: a review in osteoarthritis pain of the knee. Drugs. 2019;79(4):455–62.
Conaghan PG, Hunter DJ, Cohen SB, Kraus VB, Berenbaum F, Lieberman JR, et al. Effects of a single intra-articular injection of a microsphere formulation of triamcinolone acetonide on knee osteoarthritis pain: a double-blinded, randomized, placebo-controlled, multinational study. J Bone Joint Surg Am. 2018;100(8):666.
Maricar N, Callaghan MJ, Felson DT, O’Neill TW. Predictors of response to intra-articular steroid injections in knee osteoarthritis—a systematic review. Rheumatology. 2013;52(6):1022–32.
Matzkin EG, Curry EJ, Kong Q, Rogers MJ, Henry M, Smith EL. Efficacy and treatment response of intra-articular corticosteroid injections in patients with symptomatic knee osteoarthritis. J Am Acad Orthop Surg. 2017;25(10):703–14.
Maricar N, Parkes MJ, Callaghan MJ, Hutchinson CE, Gait AD, Hodgson R, et al. Structural predictors of response to intra-articular steroid injection in symptomatic knee osteoarthritis. Arthritis Res Ther. 2017;19(1):88.
Jevsevar DS, Brown GA, Jones DL, Matzkin EG, Manner PA, Mooar P, et al. the American Academy of Orthopaedic Surgeons evidence-based guideline on: treatment of osteoarthritis of the knee, 2nd edition. J Bone Joint Surg Am. 2013;95(20):1885–6.
McAlindon TE, Bannuru RR, Sullivan M, Arden N, Berenbaum F, Bierma-Zeinstra S, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthr Cartil. 2014;22(3):363–88.
Carlson VR, Ong AC, Orozco FR, Hernandez VH, Lutz RW, Post ZD. Compliance with the AAOS guidelines for treatment of osteoarthritis of the knee: a survey of the American Association of Hip and Knee Surgeons. J Am Acad Orthop Surg. 2018;26(3):103–7. https://doi.org/10.5435/jaaos-d-17-00164.
Meiyappan KP, Cote MP, Bozic KJ, Halawi MJ. Adherence to the American Academy of Orthopaedic Surgeons clinical practice guidelines for nonoperative management of knee osteoarthritis. J Arthroplast. 2020;35(2):347–52.
Bannuru RR, Osani M, Vaysbrot E, Arden N, Bennell K, Bierma-Zeinstra S, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr Cartil. 2019;27(11):1578–89.
Bruyère O, Honvo G, Veronese N, Arden NK, Branco J, Curtis EM, et al. An updated algorithm recommendation for the management of knee osteoarthritis from the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Semin Arthritis Rheum. 2019;49(3):337–50.
McAlindon TE, LaValley MP, Harvey WF, Price LL, Driban JB, Zhang M, et al. Effect of intra-articular triamcinolone vs saline on knee cartilage volume and pain in patients with knee osteoarthritis: a randomized clinical trial. JAMA. 2017;317(19):1967–75.
Raynauld JP, Buckland-Wright C, Ward R, Choquette D, Haraoui B, Martel-Pelletier J, et al. Safety and efficacy of long-term intraarticular steroid injections in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2003;48(2):370–7.
Cancienne JM, Werner BC, Luetkemeyer LM, Browne JA. Does timing of previous intra-articular steroid injection affect the post-operative rate of infection in total knee arthroplasty? J Arthroplast. 2015;30(11):1879–82.
Richardson SS, Schairer WW, Sculco TP, Sculco PK. Comparison of infection risk with corticosteroid or hyaluronic acid injection prior to total knee arthroplasty. J Bone Joint Surg Am. 2019;101(2):112–8.
McGarry JG, Daruwalla ZJ. The efficacy, accuracy and complications of corticosteroid injections of the knee joint. Knee Surg Sports Traumatol Arthrosc. 2011;19(10):1649–54.
Bird HA, Ring EF, Bacon PA. A thermographic and clinical comparison of three intra-articular steroid preparations in rheumatoid arthritis. Ann Rheum Dis. 1979;38(1):36–9.
Balazs EA, Watson D, Duff IF, Roseman S. Hyaluronic acid in synovial fluid. I. Molecular parameters of hyaluronic acid in normal and arthritic human fluids. Arthritis Rheum. 1967;10(4):357–76.
Strauss EJ, Hart JA, Miller MD, Altman RD, Rosen JE. Hyaluronic acid viscosupplementation and osteoarthritis: current uses and future directions. Am J Sports Med. 2009;37(8):1636–44.
Balazs EA, Denlinger JL. Viscosupplementation: a new concept in the treatment of osteoarthritis. J Rheumatol Suppl. 1993;39:3–9.
Rydell N, Balazs EA. Effect of intra-articular injection of hyaluronic acid on the clinical symptoms of osteoarthritis and on granulation tissue formation. Clin Orthop Relat Res. 1971;80:25–32.
Scale D, Wobig M, Wolpert W. Viscosupplementation of osteoarthritic knees with hylan: a treatment schedule study. Curr Ther Res. 1994;55(3):220–32.
Altman R, Bedi A, Manjoo A, Niazi F, Shaw P, Mease P. Anti-inflammatory effects of intra-articular hyaluronic acid: a systematic review. Cartilage. 2019;10(1):43–52.
Moreland LW. Intra-articular hyaluronan (hyaluronic acid) and hylans for the treatment of osteoarthritis: mechanisms of action. Arthritis Res Ther. 2003;5(2):54.
Smith MM, Ghosh P. The synthesis of hyaluronic acid by human synovial fibroblasts is influenced by the nature of the hyaluronate in the extracellular environment. Rheumatol Int. 1987;7(3):113–22.
Bowman S, Awad ME, Hamrick MW, Hunter M, Fulzele S. Recent advances in hyaluronic acid based therapy for osteoarthritis. Clin Transl Med. 2018;7(1):6.
Altman R, Hackel J, Niazi F, Shaw P, Nicholls M. Efficacy and safety of repeated courses of hyaluronic acid injections for knee osteoarthritis: a systematic review. Semin Arthritis Rheum. 2018;48(2):186–75.
Brockmeier SF, Shaffer BS. Viscosupplementation therapy for osteoarthritis. Sports Med Arthrosc Rev. 2006;14(3):155–62.
Reichenbach S, Blank S, Rutjes AW, Shang A, King EA, Dieppe PA, et al. Hylan versus hyaluronic acid for osteoarthritis of the knee: a systematic review and meta-analysis. Arthritis Care Res. 2007;57(8):1410–8.
Rutjes AW, Jüni P, da Costa BR, Trelle S, Nüesch E, Reichenbach S. Viscosupplementation for osteoarthritis of the knee: a systematic review and meta-analysis. Ann Intern Med. 2012;157(3):180–91.
Jevsevar D, Donnelly P, Brown GA, Cummins DS. Viscosupplementation for osteoarthritis of the knee: a systematic review of the evidence. J Bone Joint Surg Am. 2015;97(24):2047–60.
Bowman EN, Hallock JD, Throckmorton TW, Azar FM. Hyaluronic acid injections for osteoarthritis of the knee: predictors of successful treatment. Int Orthop. 2018;42(4):733–40.
Wang C-T, Lin J, Chang C-J, Lin Y-T, Hou S-M. Therapeutic effects of hyaluronic acid on osteoarthritis of the knee: a meta-analysis of randomized controlled trials. J Bone Joint Surg Am. 2004;86(3):538–45.
Levy DM, Petersen KA, Vaught MS, Christian DR, Cole BJ. Injections for knee osteoarthritis: corticosteroids, viscosupplementation, platelet-rich plasma, and autologous stem cells. Arthroscopy. 2018;34(5):1730–43.
Hunter DJ. Viscosupplementation for osteoarthritis of the knee. N Engl J Med. 2015;372(11):1040–7.
Altman R. Intra-articular sodium hyaluronate (Hyalgan®) in the treatment of patients with osteoarthritis of the knee: a randomized clinical trial. J Rheumatol. 1998;25:2203–12.
Adams ME, Atkinson MH, Lussier AJ, Schulz JI, Siminovitch KA, Wade JP, et al. The role of viscosupplementation with hylan GF 20 (Synvisc®) in the treatment of osteoarthritis of the knee: a Canadian multicenter trial comparing hylan GF 20 alone, hylan GF 20 with non-steroidal anti-inflammatory drugs (NSAIDs) and NSAIDs alone. Osteoarthr Cartil. 1995;3(4):213–25.
Caborn D, Rush J, Lanzer W, Parenti D, Murray C, Group SS. A randomized, single-blind comparison of the efficacy and tolerability of hylan GF 20 and triamcinolone hexacetonide in patients with osteoarthritis of the knee. J Rheumatol. 2004;31(2):333–43.
Ong KL, Anderson AF, Niazi F, Fierlinger AL, Kurtz SM, Altman RD. Hyaluronic acid injections in medicare knee osteoarthritis patients are associated with longer time to knee arthroplasty. J Arthroplast. 2016;31(8):1667–73.
Altman R, Lim S, Steen RG, Dasa V. Hyaluronic acid injections are associated with delay of total knee replacement surgery in patients with knee osteoarthritis: evidence from a large US health claims database. PLoS One. 2015;10(12):e0145776.
Jubb R, Piva S, Beinat L, Dacre J, Gishen P. A one-year, randomised, placebo (saline) controlled clinical trial of 500-730 kDa sodium hyaluronate (Hyalgan) on the radiological change in osteoarthritis of the knee. Int J Clin Pract. 2003;57(6):467.
Wang Y, Hall S, Hanna F, Wluka AE, Grant G, Marks P, et al. Effects of Hylan GF 20 supplementation on cartilage preservation detected by magnetic resonance imaging in osteoarthritis of the knee: a two-year single-blind clinical trial. BMC Musculoskelet Disord. 2011;12(1):195.
Divine JG, Zazulak BT, Hewett TE. Viscosupplementation for knee osteoarthritis: a systematic review. Clin Orthop Relat Res. 2007;455:113–22.
Bannuru R, Natov N, Dasi U, Schmid C, McAlindon T. Therapeutic trajectory following intra-articular hyaluronic acid injection in knee osteoarthritis–meta-analysis. Osteoarthr Cartil. 2011;19(6):611–9.
Smith C, Patel R, Vannabouathong C, Sales B, Rabinovich A, McCormack R, et al. Combined intra-articular injection of corticosteroid and hyaluronic acid reduces pain compared to hyaluronic acid alone in the treatment of knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2019;27(6):1974–83.
Colangelo MT, Galli C, Guizzardi S. The effects of polydeoxyribonucleotide on wound healing and tissue regeneration: a systematic review of the literature. Regen Med. 2020; https://doi.org/10.2217/rme-2019-0118.
Squadrito F, Bitto A, Irrera N, Pizzino G, Pallio G, Minutoli L, et al. Pharmacological activity and clinical use of PDRN. Front Pharmacol. 2017;8:224.
Vanelli R, Costa P, Rossi SM, Benazzo F. Efficacy of intra-articular polynucleotides in the treatment of knee osteoarthritis: a randomized, double-blind clinical trial. Knee Surg Sports Traumatol Arthrosc. 2010;18(7):901–7. https://doi.org/10.1007/s00167-009-1039-y.
Bitto A, Polito F, Irrera N, D'Ascola A, Avenoso A, Nastasi G, et al. Polydeoxyribonucleotide reduces cytokine production and the severity of collagen-induced arthritis by stimulation of adenosine A((2)A) receptor. Arthritis Rheum. 2011;63(11):3364–71. https://doi.org/10.1002/art.30538.
Gennero L, Denysenko T, Calisti GF, Vercelli A, Vercelli CM, Amedeo S, et al. Protective effects of polydeoxyribonucleotides on cartilage degradation in experimental cultures. Cell Biochem Funct. 2013;31(3):214–27. https://doi.org/10.1002/cbf.2875.
Kim MS, Cho RK, In Y. The efficacy and safety of polydeoxyribonucleotide for the treatment of knee osteoarthritis: Systematic review and meta-analysis of randomized controlled trials. Medicine. 2019;98(39):e17386.
Zazgyva A, Gergely I, Russu OM, Roman C, Pop TS. Polynucleotides versus sodium hyaluronate in the local treatment of knee osteoarthritis. Acta Medica Transilvanica. 2013;2(2):260–3.
Giarratana LS, Marelli BM, Crapanzano C, De Martinis SE, Gala L, Ferraro M, et al. A randomized double-blind clinical trial on the treatment of knee osteoarthritis: the efficacy of polynucleotides compared to standard hyaluronian viscosupplementation. Knee. 2014;21(3):661–8.
Yoon S, Kang JJ, Kim J, Park S, Kim JM. Efficacy and safety of intra-articular injections of hyaluronic acid combined with polydeoxyribonucleotide in the treatment of knee osteoarthritis. Ann Rehabil Med. 2019;43(2):204.
Dallari D, Sabbioni G, Del Piccolo N, Carubbi C, Veronesi F, Torricelli P, et al. Efficacy of intra-articular polynucleotides associated with hyaluronic acid versus hyaluronic acid alone in the treatment of knee osteoarthritis: a randomized, double-blind, controlled clinical trial. Clin J Sport Med. 2018;30(1):1–7.
Hauser RA, Lackner JB, Steilen-Matias D, Harris DK. A systematic review of dextrose prolotherapy for chronic musculoskeletal pain. Clin Med Insights Arthritis Musculoskelet Disord. 2016;9:139–59. https://doi.org/10.4137/CMAMD.S39160.
Park Y-S, Lim S-W, Lee I-H, Lee T-J, Kim J-S, Han JS. Intra-articular injection of a nutritive mixture solution protects articular cartilage from osteoarthritic progression induced by anterior cruciate ligament transection in mature rabbits: a randomized controlled trial. Arthritis Res Ther. 2007;9(1):R8.
Topol GA, Podesta LA, Reeves KD, Giraldo MM, Johnson LL, Grasso R, et al. Chondrogenic effect of intra-articular hypertonic-dextrose (prolotherapy) in severe knee osteoarthritis. PM&R. 2016;8(11):1072–82.
Reeves KD, Hassanein K. Randomized prospective double-blind placebo-controlled study of dextrose prolotherapy for knee osteoarthritis with or without ACL laxity. Altern Ther Health Med. 2000;6(2):68–80.
Dumais R, Benoit C, Dumais A, Babin L, Bordage R, de Arcos C, et al. Effect of regenerative injection therapy on function and pain in patients with knee osteoarthritis: a randomized crossover study. Pain Med. 2012;13(8):990–9.
Rabago D, Zgierska A, Fortney L, Kijowski R, Mundt M, Ryan M, et al. Hypertonic dextrose injections (prolotherapy) for knee osteoarthritis: results of a single-arm uncontrolled study with 1-year follow-up. J Altern Complement Med. 2012;18(4):408–14.
Sit RW, Chung VC, Reeves KD, Rabago D, Chan KK, Chan DC, et al. Hypertonic dextrose injections (prolotherapy) in the treatment of symptomatic knee osteoarthritis: a systematic review and meta-analysis. Sci Rep. 2016;6:25247.
Wang Y, Fan X, Xing L, Tian F. Wnt signaling: a promising target for osteoarthritis therapy. Cell Commun Signal. 2019;17(1):1–14.
Yazici Y, Mcalindon T, Gibofsky A, Lane N, Lattermann C, Skrepnik N, et al. THU0458 Efficacy and safety from a phase 2B trial of SM04690, a novel intra-articular wnt pathway inhibitor for the treatment of osteoarthritis of the knee. BMJ. 2019:591–19.
Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21(3):531–43.
Simone DA, Nolano M, Johnson T, Wendelschafer-Crabb G, Kennedy WR. Intradermal injection of capsaicin in humans produces degeneration and subsequent reinnervation of epidermal nerve fibers: correlation with sensory function. J Neurosci. 1998;18(21):8947–59.
Stevens RM, Ervin J, Nezzer J, Nieves Y, Guedes K, Burges R, et al. Randomized, double-blind, placebo-controlled trial of intraarticular trans-capsaicin for pain associated with osteoarthritis of the knee. Arthr Rheumatol. 2019;71(9):1524–33.
Cotter EJ, Frank RM, Mandelbaum B. Management of osteoarthritis-biological approaches: current concepts. J ISAKOS. 2020;5(1):27–31.
Shimonkevitz R, Thomas G, Slone DS, Craun M, Mains C, Bar-Or D. A diketopiperazine fragment of human serum albumin modulates T-lymphocyte cytokine production through rap1. J Trauma Acute Care Surg. 2008;64(1):35–41.
Bar-Or D, Salottolo KM, Loose H, Phillips MJ, McGrath B, Wei N, et al. A randomized clinical trial to evaluate two doses of an intra-articular injection of LMWF-5A in adults with pain due to osteoarthritis of the knee. PLoS One. 2014;9(2):e87910.
Salottolo K, Cole B, Bar-Or D. Intra-articular injection of the anti-inflammatory compound LMWF-5A in adults with severe osteoarthritis: a double-blind prospective randomized controlled multi-center safety and efficacy trial. Patient Saf Surg. 2018;12(1):11.
Roemer FW, Aydemir A, Lohmander S, Crema MD, Marra MD, Muurahainen N, et al. Structural effects of sprifermin in knee osteoarthritis: a post-hoc analysis on cartilage and non-cartilaginous tissue alterations in a randomized controlled trial. BMC Musculoskelet Disord. 2016;17(1):267.
Hochberg MC, Guermazi A, Guehring H, Aydemir A, Wax S, Fleuranceau-Morel P, et al. Effect of intra-articular Sprifermin vs placebo on femorotibial joint cartilage thickness in patients with osteoarthritis: the FORWARD randomized clinical trial. JAMA. 2019;322(14):1360–70.
Jacques C, Gosset M, Berenbaum F, Gabay C. The role of IL-1 and IL-1Ra in joint inflammation and cartilage degradation. Vitam Horm. 2006;74:371–403.
Caron JP, Fernandes JC, Martel-Pelletier J, Tardif G, Mineau F, Geng C, et al. Chondroprotective effect of intraarticular injections of interleukin-1 receptor antagonist in experimental osteoarthritis. Suppression of collagenase-1 expression. Arthritis Rheum. 1996;39(9):1535–44.
Chevalier X, Goupille P, Beaulieu A, Burch F, Bensen W, Conrozier T, et al. Intraarticular injection of anakinra in osteoarthritis of the knee: a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Care Res. 2009;61(3):344–52.
Stannus O, Jones G, Cicuttini F, Parameswaran V, Quinn S, Burgess J, et al. Circulating levels of IL-6 and TNF-α are associated with knee radiographic osteoarthritis and knee cartilage loss in older adults. Osteoarthr Cartil. 2010;18(11):1441–7.
Lindsley H, Schue J, Tawfik O, Bolce R, Smith D, Hinson G, et al. FRI0304 treatment of knee osteoarthritis with intra-articular infliximab improves total womac score. High baseline levels of synovial cellularity predict improvement. Ann Rheum Dis. 2013;71(Suppl 3):417.
Ohtori S, Orita S, Yamauchi K, Eguchi Y, Ochiai N, Kishida S, et al. Efficacy of direct injection of etanercept into knee joints for pain in moderate and severe knee osteoarthritis. Yonsei Med J. 2015;56(5):1379–83.
Wang J. Efficacy and safety of adalimumab by intra-articular injection for moderate to severe knee osteoarthritis: an open-label randomized controlled trial. J Int Med Res. 2018;46(1):326–34.
Roseti L, Desando G, Cavallo C, Petretta M, Grigolo B. Articular cartilage regeneration in osteoarthritis. Cell. 2019;8(11):1305.
Aronowitz JA, Ellenhorn JDI. Adipose stromal vascular fraction isolation: a head-to-head comparison of four commercial cell separation systems. Plast Reconstr Surg. 2013;132(6):932e–9e. https://doi.org/10.1097/PRS.0b013e3182a80652.
Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy. 2013;15(6):641–8.
Pak J. Regeneration of human bones in hip osteonecrosis and human cartilage in knee osteoarthritis with autologous adipose-tissue-derived stem cells: a case series. J Med Case Rep. 2011;5(1):1–8.
Koh Y-G, Choi Y-J, Kwon S-K, Kim Y-S, Yeo J-E. Clinical results and second-look arthroscopic findings after treatment with adipose-derived stem cells for knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2015;23(5):1308–16.
Michalek J, Moster R, Lukac L, Proefrock K, Petrasovic M, Rybar J, et al. Autologous adipose tissue-derived stromal vascular fraction cells application in patients with osteoarthritis. Cell Transplant. 2015;20:1–36.
Garza JR, Campbell RE, Tjoumakaris FP, Freedman KB, Miller LS, Santa Maria D, et al. Clinical efficacy of intra-articular mesenchymal stromal cells for the treatment of knee osteoarthritis: a double-blinded prospective randomized controlled clinical trial. Am J Sports Med. 2020;48(3):588–98.
Pak J, Lee JH, Park KS, Park M, Kang L-W, Lee SH. Current use of autologous adipose tissue-derived stromal vascular fraction cells for orthopedic applications. J Biomed Sci. 2017;24(1):9.
Rodriguez-Fontan F, Piuzzi NS, Kraeutler MJ, Pascual-Garrido C. Early clinical outcomes of intra-articular injections of bone marrow aspirate concentrate for the treatment of early osteoarthritis of the hip and knee: a cohort study. PM&R. 2018;10(12):1353–9.
Kim J-D, Lee GW, Jung GH, Kim CK, Kim T, Park JH, et al. Clinical outcome of autologous bone marrow aspirates concentrate (BMAC) injection in degenerative arthritis of the knee. Eur J Orthop Surg Traumatol. 2014;24(8):1505–11.
Centeno C, Pitts J, Al-Sayegh H, Freeman M. Efficacy of autologous bone marrow concentrate for knee osteoarthritis with and without adipose graft. Biomed Res Int. 2014;2014
Shapiro SA, Kazmerchak SE, Heckman MG, Zubair AC, O’Connor MI. A prospective, single-blind, placebo-controlled trial of bone marrow aspirate concentrate for knee osteoarthritis. Am J Sports Med. 2017;45(1):82–90.
Shapiro SA, Arthurs JR, Heckman MG, Bestic JM, Kazmerchak SE, Diehl NN, et al. Quantitative T2 MRI mapping and 12-month follow-up in a randomized, blinded, placebo controlled trial of bone marrow aspiration and concentration for osteoarthritis of the knees. Cartilage. 2019;10(4):432–43.
Senzel L, Gnatenko DV, Bahou WF. The platelet proteome. Curr Opin Hematol. 2009;16(5):329.
Foster TE, Puskas BL, Mandelbaum BR, Gerhardt MB, Rodeo SA. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med. 2009;37(11):2259–72.
Southworth TM, Naveen NB, Tauro TM, Leong NL, Cole BJ. The use of platelet-rich plasma in symptomatic knee osteoarthritis. J Knee Surg. 2019;32(01):037–45.
DeLong JM, Beitzel K, Mazzocca AD, Shepard D, Roller BL, Hanypsiak BT. Update on platelet-rich plasma. Curr Orthop Pract. 2011;22(6):514–23.
Dai W-L, Zhou A-G, Zhang H, Zhang J. Efficacy of platelet-rich plasma in the treatment of knee osteoarthritis: a meta-analysis of randomized controlled trials. Arthroscopy. 2017;33(3):659–70.e1.
Tang JZ, Nie MJ, Zhao JZ, Zhang GC, Zhang Q, Wang B. Platelet-rich plasma versus hyaluronic acid in the treatment of knee osteoarthritis: a meta-analysis. J Orthop Surg Res. 2020;15(1):403. https://doi.org/10.1186/s13018-020-01919-9.
Filardo G, Previtali D, Napoli F, Candrian C, Zaffagnini S, Grassi A. PRP injections for the treatment of knee osteoarthritis: a meta-analysis of randomized controlled trials. Cartilage. 2020;1947603520931170
Belk JW, Kraeutler MJ, Houck DA, Goodrich JA, Dragoo JL, McCarty EC. Platelet-rich plasma versus hyaluronic acid for knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Am J Sports Med. 2021;49(1):249–60.
Delanois RE, Etcheson JI, Sodhi N, Henn RF III, Gwam CU, George NE, et al. Biologic therapies for the treatment of knee osteoarthritis. J Arthroplast. 2019;34(4):801–13.
Kon E, Di Matteo B, Delgado D, Cole BJ, Dorotei A, Dragoo JL, et al. Platelet-rich plasma for the treatment of knee osteoarthritis: an expert opinion and proposal for a novel classification and coding system. Expert Opin Biol Ther. 2020:1–14.
O’Shaughnessey K, Matuska A, Hoeppner J, Farr J, Klaassen M, Kaeding C, et al. Autologous protein solution prepared from the blood of osteoarthritic patients contains an enhanced profile of anti-inflammatory cytokines and anabolic growth factors. J Orthop Res. 2014;32(10):1349–55.
Kon E, Engebretsen L, Verdonk P, Nehrer S, Filardo G. Clinical outcomes of knee osteoarthritis treated with an autologous protein solution injection: a 1-year pilot double-blinded randomized controlled trial. Am J Sports Med. 2018;46(1):171–80.
Kon E, Engebretsen L, Verdonk P, Nehrer S, Filardo G. Autologous protein solution injections for the treatment of knee osteoarthritis: 3-year results. Am J Sports Med. 2020;48(11):2703–10.
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7.
Galipeau J, Krampera M. The challenge of defining mesenchymal stromal cell potency assays and their potential use as release criteria. Cytotherapy. 2015;17(2):125–7.
Stappenbeck TS, Miyoshi H. The role of stromal stem cells in tissue regeneration and wound repair. Science. 2009;324(5935):1666–9.
Caplan AI. Mesenchymal stem cells: time to change the name! Stem Cells Transl Med. 2017;6(6):1445–51.
Pas HI, Winters M, Haisma HJ, Koenis MJ, Tol JL, Moen MH. Stem cell injections in knee osteoarthritis: a systematic review of the literature. Br J Sports Med. 2017;51(15):1125–33.
Soler Rich R, Munar A, Soler Romagosa F, Peirau X, Huguet M, Alberca M, et al. Treatment of knee osteoarthritis with autologous expanded bone marrow mesenchymal stem cells: 50 cases clinical and MRI results at one year follow-up. J Stem Cell Res Ther. 2015;5(285):2.
Soler R, Orozco L, Munar A, Huguet M, López R, Vives J, et al. Final results of a phase I–II trial using ex vivo expanded autologous mesenchymal stromal cells for the treatment of osteoarthritis of the knee confirming safety and suggesting cartilage regeneration. Knee. 2016;23(4):647–54.
Emadedin M, Labibzadeh N, Liastani MG, Karimi A, Jaroughi N, Bolurieh T, et al. Intra-articular implantation of autologous bone marrow–derived mesenchymal stromal cells to treat knee osteoarthritis: a randomized, triple-blind, placebo-controlled phase 1/2 clinical trial. Cytotherapy. 2018;20(10):1238–46.
Doyle EC, Wragg NM, Wilson SL. Intraarticular injection of bone marrow-derived mesenchymal stem cells enhances regeneration in knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2020:1–16.
Jo CH, Chai JW, Jeong EC, Oh S, Shin JS, Shim H, et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a 2-year follow-up study. Am J Sports Med. 2017;45(12):2774–83.
Freitag J, Bates D, Wickham J, Shah K, Huguenin L, Tenen A, et al. Adipose-derived mesenchymal stem cell therapy in the treatment of knee osteoarthritis: a randomized controlled trial. Regen Med. 2019;14(3):213–30.
Lee WS, Kim HJ, Kim KI, Kim GB, Jin W. Intra-articular injection of autologous adipose tissue-derived mesenchymal stem cells for the treatment of knee osteoarthritis: a phase IIb, randomized, placebo-controlled clinical trial. Stem Cells Transl Med. 2019;8(6):504–11.
Song Y, Du H, Dai C, Zhang L, Li S, Hunter DJ, Lu L, Bao C. Human adipose-derived mesenchymal stem cells for osteoarthritis: a pilot study with long-term follow-up and repeated injections. Regenerative medicine. 2018;13(3):295–307.
Klontzas ME, Kenanidis EI, Heliotis M, Tsiridis E, Mantalaris A. Bone and cartilage regeneration with the use of umbilical cord mesenchymal stem cells. Expert Opin Biol Ther. 2015;15(11):1541–52.
Jin HJ, Bae YK, Kim M, Kwon S-J, Jeon HB, Choi SJ, et al. Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. Int J Mol Sci. 2013;14(9):17986–8001.
Park YB, Ha CW, Lee CH, Yoon YC, Park YG. Cartilage regeneration in osteoarthritic patients by a composite of allogeneic umbilical cord blood-derived mesenchymal stem cells and hyaluronate hydrogel: results from a clinical trial for safety and proof-of-concept with 7 years of extended follow-up. Stem Cells Transl Med. 2017;6(2):613–21.
Matas J, Orrego M, Amenabar D, Infante C, Tapia-Limonchi R, Cadiz MI, et al. Umbilical cord-derived mesenchymal stromal cells (MSCs) for knee osteoarthritis: repeated MSC dosing is superior to a single MSC dose and to hyaluronic acid in a controlled randomized phase I/II trial. Stem Cells Transl Med. 2019;8(3):215–24.
Kim M-K, Ha C-W, In Y, Cho S-D, Choi E-S, Ha J-K, et al. A multicenter, double-blind, phase III clinical trial to evaluate the efficacy and safety of a cell and gene therapy in knee osteoarthritis patients. Hum Gene Ther Clin Dev. 2018;29(1):48–59.
Cherian J, Parvizi J, Bramlet D, Lee K, Romness D, Mont M. Preliminary results of a phase II randomized study to determine the efficacy and safety of genetically engineered allogeneic human chondrocytes expressing TGF-β1 in patients with grade 3 chronic degenerative joint disease of the knee. Osteoarthr Cartil. 2015;23(12):2109–18.
Lee B, Parvizi J, Bramlet D, Romness DW, Guermazi A, Noh M, et al. Results of a phase ii study to determine the efficacy and safety of genetically engineered allogeneic human chondrocytes expressing TGF-β1. J Knee Surg. 2020;33(02):167–72.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Seo, SS., Lee, IS., Lee, GH. (2021). Intra-articular Injection Therapy and Biologic Treatment. In: Seo, SS. (eds) A Strategic Approach to Knee Arthritis Treatment. Springer, Singapore. https://doi.org/10.1007/978-981-16-4217-3_10
Download citation
DOI: https://doi.org/10.1007/978-981-16-4217-3_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-4216-6
Online ISBN: 978-981-16-4217-3
eBook Packages: MedicineMedicine (R0)