Abstract
Soil contamination by hazardous metals such as Pb and Cd is a serious environmental problem. Adsorption isotherms are required for understanding mobility and fate in soils. In this chapter, the adsorption equilibrium of hazardous metals was examined, and how various environmental factors (pH, humic substances, and salt concentration) influence the adsorption and elution of hazardous metals from contaminated soil was investigated. The adsorption of metal ions to soil can be represented by the ion-exchange isotherms. Moreover, the influence of pH, humic substances, and salt concentration on the solubility of aqueous metal ions is also described.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Total Organic Carbon
- Humic Substance
- Adsorption Isotherm
- Inductively Couple Plasma Mass Spectrometry
- Adsorption Equilibrium
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Soil contamination by hazardous metals such as lead and cadmium is a serious environmental problem. To understand this problem, an investigation into heavy metal contamination of soil and possible countermeasures was carried out. In Japan, soil contamination is typically assessed by elution tests, which evaluate the extent to which hazardous metals pollute groundwater after permeating through soil, and by content tests, which evaluate how hazardous metals are absorbed by humans through direct ingestion of soil or via inhalation of airborne dust. There is often considerable variation in the elution tests, because the ease with which hazardous metals elute from soil is greatly influenced by the states of metals in soil. Insoluble lead compounds such as lead oxides and lead carbonate seldom cause groundwater contamination. However, water-soluble lead can be dissolved in pore water and attains the adsorption equilibrium with the soil particles. And the elute property is greatly changed by the various environmental factors, such as pH, ORP, and coexisting substances.
This article investigates the adsorption equilibrium of hazardous metals, including lead, and examines how various environmental factors (pH, humic substances, and salt concentration) influence the adsorption and elution of hazardous metals from contaminated soil.
2 The Adsorption Equilibrium
2.1 The Adsorption Equilibrium Equation
The relationship between the metal concentration in groundwater and the amount of metals adsorbed onto the soil is often analyzed by adsorption isotherms such as the Freundlich and Langmuir equations (Anghinoni et al. 1996; Atenassova and Okazaki 1997; Houng and Lee 1988; Ramachandran and Souza 1999; Kinniburgh et al. 1999; Limousin et al. 2007; Febrianto et al. 2009). However, these adsorption isotherms are only applicable over a limited concentration range and cannot account for the influence of pH and coexisting substances, which differ according to soil type. Therefore, parameters for the adsorption isotherms have to be determined for each environmental condition.
Urano et al. demonstrated experimentally that the adsorption equilibrium of a metal ion to chelating resin, when the system contains another coexisting ion, can be represented by an adsorption isotherm shown in Eq. (5.1) (a competitive ion-exchange equation) (Urano et al. 1981). As described in the next section, this equation holds for the adsorption of metals to soil:
where C 1 and C 2 are the concentrations of metal ion 1 and coexisting ion 2 in the adsorption equilibrium, respectively; Q 1 is the amount of ion 1 exchanged in equilibrium (the adsorbed amount of the ion 1 in equilibrium); n 1 and n 2 are ion-exchange constants, which represent slopes of the ion isotherms; K 12 is the selectivity coefficient of ion 1 to ion 2; and Q max is the maximum ion-exchange capacity of the soil, which is equivalent to the cation-exchange capacity of soil when the metal ion is cationic. Each substance is expressed by equivalent weights.
When multiple coexisting ions are involved, a multicomponent adsorption isotherm expressed by Eq. (5.2) can be applied:
Here, C k is the equilibrium concentration of the coexisting ion k, n k is the ion-exchange constant of the coexisting ion k, and K 1 k is the selectivity coefficient of the target ion to the coexisting ion. When C 1 is small enough, Eq. (5.2) can be approximated by Eq. (5.3). This means that the relationship between the amount exchanged in equilibrium, Q 1, and the equilibrium concentration, C 1, can be linearly approximated on a logarithmic scale, which approaches a Freundlich-type adsorption isotherm:
2.2 The Adsorption Equilibrium Experiment
Figure 5.1 outlines the adsorption isotherm experiments (Kameya et al. 2004; Liu et al. 2011). Wet soil and sample solution were prepared at a given solid–liquid ratio and shaken at 25 °C for 24 h, adjusting the pH as appropriate. After checking the final pH, the liquid was filtered through a 0.45 μm membrane filter to separate solid and liquid phases. The metal concentration of the filtrate was quantified by inductively coupled plasma mass spectrometry (ICP-MS). The amount adsorbed in equilibrium was calculated by subtracting the equilibrium concentration of the filtrate from the initial concentrations of the metals. Diluted nitric acid was added to the filtrate to prevent deposition and precipitation of the metals.
3 The Examples of Adsorption Equilibrium of Cd
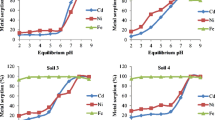
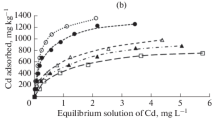
Figure 5.2 is an example of adsorption isotherms of metals. This particular example is an adsorption equilibrium experiment performed with cadmium and andosol at pH 5–7. The abscissa represents the concentration of an equilibrated metal ion aqueous solution, and the ordinate represents the amount of the ions exchanged in equilibrium. Solid lines are obtained through curve fitting by varying the selectivity coefficient K in Eq. (5.1). In this experiment, the cation-exchange capacity (CEC) was used as Q max, and n Cd and n H were parameters independent of the soil type. The parameters for andosol, Kanto loam, and sandy soil are summarized in Table 5.1. It was found that pH variation, which can be regarded as coexistence of hydrogen ions, can be analyzed by Eq. (5.1). It is apparent that Q max and the selectivity coefficient to hydrogen ions vary greatly according to soil type.
4 The Presence of Dissolved Humic Substances
Figure 5.3 shows an example of adsorption isotherms of lead on andosol at pH 7 and 5. In the adsorption experiment at pH 7, humus extract solution was added to the sample solution to obtain adsorption isotherms at various organic carbon concentrations (Liu et al. 2011, 2013). When the total organic carbon (TOC), that is, concentration of humic substances, is low, it was found that the adsorption isotherm can be expressed by Eq. (5.1). The parameters are summarized in Table 5.1. At lower equilibrium concentrations (approx. 1 × 10−3 mEq/L or lower) at pH 7 (neutral range), soils with high organic carbon content tended to show slightly higher lead concentrations in the liquid phases. When andosol is used in the experiment, humic substances are eluted into the filtered sample solutions, which are clear, pale-yellow solutions, at pH 7 or higher. Because lead exists in a form that is bound to the humic substances, and given the relative abundance of humic substances in soil, lead bound to the humic substances and dissolved in the water phase becomes impossible to ignore. Accordingly, when adsorption isotherms were obtained with addition of humic substances, the higher the TOC, the higher the lead concentration was in the liquid phase. It is thought that in soil with high levels of organic carbon, lead is bound to humic substances and elutes from the soil with the humic substances when the pH is high.
This is critical to understanding the underground permeation behavior in basic soils. In addition, lead bound to humic substances can, upon solid–liquid separation, exist in both solid and liquid phases, which contributes to the variation of the measured elution test values.
5 The Adsorption Equilibrium of Pb(II) in the Presence of Dissolved Salts
Figure 5.4 shows an example of adsorption isotherms of lead with high salt concentration. The salt concentration was adjusted with artificial seawater. EC = 4,500 indicates a salt concentration similar to that of seawater. This experiment demonstrated that the coexistence of hydrogen ions and lead ions can be analyzed by Eq. (5.2). In addition, when the concentration of equilibrated ions is low and the salt concentration is high, the adsorbed amount in equilibrium will be small. This is because sodium ions act as coexisting ions and compete with the lead ions. This influence is significant at higher pH. Moreover, the influence is greater in sandy soil than in andosol, because the Q max of sandy soil is smaller than that of andosol.
Moreover, the underground permeation of lead was investigated in areas of Japan affected by tsunami. Although there was very little data, the underground permeation of lead into soil with high salt concentration tended to be high.
6 Conclusion
This article described how the adsorption of metal ions such as lead and cadmium onto soil can be represented by the ion-exchange isotherms. The influence of pH, coexisting humic substances, and salt concentration on the solubility of aqueous metal ions is also described. Although this article only addresses cations, anions such as chromium and arsenic can be analyzed in a similar way. Preparing a database of Q max and selectivity coefficients for hydrogen ions and hydroxide ions of various soils will be helpful in analyzing the permeability of contaminated groundwater and understanding the contamination states.
References
Anghinoni I, Baligar VC, Wright RJ (1996) Phosphorus sorption isotherm characteristics and availability parameters of Appalachian acidic soils. Commun Soil Sci Plant Anal 27:2033–2048
Atenassova I, Okazaki M (1997) Adsorption-desorption characteristics of high levels of copper in soil clay fractions. Water Air Soil Pollut 98:213–228
Febrianto J, Kosasih AN, Sunarso J, Ju YH, Indraswati N, Ismadji S (2009) Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: a summary of recent studies. J Hazard Mater 162:616–645
Houng KH, Lee DY (1988) Comparisons of linear and nonlinear langmuir and Freundlich curve-fit in the study of Cu, Cd, and Pb adsorption on Taiwan soils. Soil Sci 163:115–121
Kameya T, Ohashi Y, Kobayashi T, Urano K (2004) Exchange isotherm of toxic metal cations on soils in consideration of coexistence in wide concentration range for metal contamination survey. J Jpn Soc Civil Eng 776(VII-33):29–37
Kinniburgh DG, van Riemsdijk WH, Koopal LK, Borkovec M, Benedetti MF, Avena MJ (1999) Ion binding to natural organic matter: competition, heterogeneity, stoichiometry and thermodynamic consistency. Colloids Surf A 151:147–166
Limousin G, Gaudet JP, Charlet L, Szenknect S, Barthes V, Krimissa M (2007) Sorption isotherms: a review on physical bases, modeling and measurement. Appl Geochem 22:249–275
Liu Y, Kobayashi T, Kameya T, Takahashi Y, Ohashi Y (2011) Simulation of adsorption equilibrium of heavy metal cations on soils in circumneutral aqueous solution: influences of solution pH and dissolved humus substances. Adv Mater Res 287–290:2822
Liu Y, Kobayashi T, Takahashi Y, Kameya T, Urano K (2013) A simple simulation of adsorption equilibrium of Pb(II) on Andosols in the presence of dissolved humic substances for monitoring soil contamination. J Environ Sci Health A 48:1694–1699
Ramachandran VD, Souza TJ (1999) Adsorption of cadmium by Indian soils. Water Air Soil Pollut 111:225–234
Urano K, Chanyasak V, Fujii N (1981) Adsorption isotherm equation of single heavy metal ion in acidic solution on chelating resin. J Colloid Interface Sci 82(1):251–253
Acknowledgment
This work was partially supported by the Environmental Research and Technology Development Fund (B-0914) of the Ministry of the Environment, Government of Japan, and JSPS KAKENHI Grant Number 25550077.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Kobayashi, T., Liu, Y., Takahashi, Y., Kameya, T., Yamada, Y. (2015). The Adsorption Equilibrium of Pb and Cd on Several Soils in Various pH Solutions or in the Presence of Dissolved Humic Substances. In: Sherameti, I., Varma, A. (eds) Heavy Metal Contamination of Soils. Soil Biology, vol 44. Springer, Cham. https://doi.org/10.1007/978-3-319-14526-6_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-14526-6_5
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-14525-9
Online ISBN: 978-3-319-14526-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)