Abstract—
Adsorption and desorption reactions at the solid phase–solution interface, play a significant role in controlling metal concentrations in soil solution and metal translocation to plants. Five predominant benchmarks soils of Pakistan were characterized for their lead (Pb), cadmium (Cd), and nickel (Ni) adsorption parameters by constructing equilibrium adsorption isotherms. The adsorption parameters (maximum adsorption capacity and affinity coefficient) were calculated by fitting measured adsorption data to Langmuir, Freundlich, and Redlich–Peterson adsorption models. Thermodynamic properties (Gibbs free energy—ΔG°, enthalpy—ΔH°, and entropy—ΔS°) were measured by equilibrating soils with Pb, Cd, and Ni solution at 25 ± 2 and 45 ± 2°C. The results revealed that the Langmuir and Redlich–Peterson adsorption models described metal adsorption data equally good. Langmuir’s predicted Pb, Cd, and Ni adsorption demonstrated better correlation with measured adsorption than Freundlich’s model. According to the maximum Pb, Cd, and Ni adsorption calculated by Langmuir and Redlich–Peterson models and Freundlich sorbate affinity to sorbent, the studied soils were formed the following sequence: Kotli > Miranpur > Gujranwala > Shahdara > Rasulpur. The magnitude of metal adsorption (b and qmon) and affinity (Kf) was highly correlated with the clay, soil organic matter, and iron and aluminum contents and cation exchange capacity. The measured metal adsorption in different soils followed the order: Pb > Cd > Ni. The magnitude of maximum Pb sorption (calculated by Langmuir and Redlich–Peterson models) was approximately two and four times higher than that for Cd and Ni, respectively. Similarly, the Freundlich’s sorbate affinity was also higher for Pb than for Cd and Ni. Thermodynamic parameters revealed that the Pb, Cd, and Ni adsorption reactions were spontaneous and exothermic in nature, and the process was dominated by physical adsorption.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Heavy metals are continuously increasing in terrestrial environment mainly due to anthropogenic activities such as disposal of municipal/industrial discharges to arable land and water bodies without any pretreatment—a common practice in developing countries. Long-term application of untreated municipal/industrial discharges to arable land elevate the metal concentrations in surface and subsurface horizons [29]. Majority of Pakistani soils have been developed from alluvial deposits [42] and are low in organic carbon and cation exchange capacity; therefore, have low metal retention capacity. When the metal ions retaining capacity of a soil is overloaded/diminished due to repeated application of untreated discharges, soils start releasing metal ions into soil solution which can either be leached to shallow groundwater or taken up by plants.
The fate and mobility of heavy metals in soil rhizosphere is mainly governed by sorption processes, i.e., adsorption, surface precipitation, and fixation [36]. The adsorption is an intermolecular interaction between metal in the soil solution and the soil solid phase. It is directly related to metal concentrations in the soil solid phase and soil solution. Metal adsorption by soils generally involves nonspecific and specific interactions and depends on the amounts of carbonates, phosphorus, sulfur, iron, and manganese compounds in soil. Soil properties, i.e., pH, amount and type of clay minerals, and soil organic carbon content also play important role in metal adsorption. In nonspecific sorption, relatively weak electrostatic forces of negatively charged soil particles attract metal cations, while in specific sorption reaction heavy metal cations exchange with surface ligands and also make partial covalent bonding with lattice ions. These reactions are highly pH dependent and increase with increasing pH. Typically, bioavailability of heavy metals decreases as pH shifts from acidic to neutral and alkaline conditions [17]. Predominantly, soils of Pakistan are alkaline calcareous (pH < 7) and hence high metal adsorption can be expected [46] because at high pH, OH-groups present at the clay mineral surface edges, are negatively charged [10]. Soil organic matter is another factor affecting heavy metals adsorption; however, soils having low organic matter (<1%), as Pakistani soils [5], so its role cannot be over emphasized. Additionally, clay content and cation exchange capacity (CEC) have direct relation with soils metal adsorption capacity; the larger the clay content/CEC value, the more the chances of metal retention.
Metal adsorption affinity of soil colloids is increased with the increase in valence of metal ions (M+ < M2+ < M3+) and also depends upon the hydrated radius of metal ions [7]. The results of seven Brazilian soils [33] showed that in a competitive situation Cr, Pb, and Cu were adsorbed most strongly, whereas Cd, Ni, and Zn were the least adsorbed cation in the competitive situation. They observed that the sequences of metals adsorption of the same valence did not exactly follow the order of electronegativity. Results of another metal adsorption study [13] reported that the metal adsorption affinity of calcareous soils (using the ratio between the amount of metal adsorbed and the amount of metal in solution, as an indicator of metal sorption affinity) was in the order of Pb > Cu > Zn > Ni=Cd. Lead, Cr and Cd also depicted similar metal adsorption trend (Pb > Cr > Cd) when equilibrated with lignocellulose materials in a competitive adsorption system [30].
The capacity of soils to retain and release metals is an important factor to predict environmental impact of the applied untreated municipal/industrial discharges to arable land [25]. In calcareous alkaline soils, metals are adsorbed either by clay minerals or carbonates [10]. In these soils, metal adsorption processes are mainly controlled by hydrous oxide and aluminosilicate (characterized by a permanent structural charge) minerals, organic matter and density of functional groups [10]. The surfaces of these soils display a variety of hydroxyl groups having different reactivities. For example, the terminal –OH group, on deprotonation, become negatively charged in alkaline condition and hence bonds more strongly to metals cations than that of acidic condition [22].
Isotherm studies of metals adsorption by soils, sediment and clay minerals provide an effective way to determine the relationships of metals in solution and on solid phase. Further, sorption isotherms provide useful information about the soil metal retention capacity and the strength by which the metals are held on to the soil phase. Metal adsorption intensity and affinity can be predicted using different models, i.e., Langmuir, Freundlich, and Redlich–Peterson equations [26, 28]. The Langmuir and Freundlich are two parameters equations; while Redlich–Peterson (R–P) isotherm equation is a three parameters equation including β being an adjustable parameter (1 < β < 0) [6]. Although, enough information on adsorption of plant nutrients, i.e., phosphorus, potassium, and zinc are available for Pakistani soils, however, information on heavy metals is lacking. Hence, in present study, Pb, Cd and Ni sorption behavior of different calcareous soils was investigated and sorption parameters, calculated using different empirical equations, were correlated with major soil properties.
MATERIALS AND METHODS
Soils
Surface (0–15 cm) soil samples of five benchmark soil series were collected from the reference locations described by the Soil Survey of Pakistan [42]; Gujranwala series (fine-loamy, mixed, hyperthermic Ustic Haplustalf) was collected from 32.1878 N and 74.1485 E, Miranpur (fine, mixed, hyperthermic Ustertic Camborthid) from 31.883 N and 73.783 E, Kotli (fine, mixed, hyperthermic, Entic Chromustert) from 32.2169 N and 74.2452 E, Rasulpur (coarse-loamy, mixed, hyperthermic Ustochreptic Camborthid) from 31.7671 N and 73.7519 E and Shahdara (coarse-silty, mixed, hyperthermic Typic Torrifulvent) from 31.689 N and 74.379 E. All the sites fall under arid and semi-arid tropical continental climate. The samples were air dried, crushed, and passed through a 2-mm sieve. Subsequently, portions of 2‑mm sieved soil samples were further ground to 0.5 mm. The samples were analyzed for basic physicochemical characteristics. Particle size distribution was analyzed by hydrometer method [8], and US classification was used for textural class. The equivalent calcium carbonate content was measured by neutralizing carbonates with HCl and back titration of excess acid with NaOH [38]. Organic carbon was determined using the Walkley–Black method [3], pH with the standard glass electrode [9], electrical conductivity with glass electrode [16], and cation exchange capacity by ammonium acetate method [31]. Aluminum and Fe contents were extracted using AB-DTPA extractant [35] and then analyzed by flame atomic absorption spectrometry (Perkin Elmer AAnalyst 700). The soils were alluvial, non-saline [ECe (1 : 1), 0.45–0.54 dS m–1], low in organic matter (0.45–0.72%), alkaline–calcareous (pH 7.2–8.2; CaCO3 1.25–6.51%), and CEC ranged from 3.56 to 29.2 cmol kg–1 (Table 2).
Metal Sorption Isotherms
Multi-point Pb, Cd, and Ni adsorption isotherms were constructed, separately, by equilibrating 3 grams of soil (in triplicate) with 30 mL 0.01 M Ca(NO3)2 solution having different graded concentrations (0–100 mg L–1) of Cd, and Ni (0–100 mg L–1) as Cd(NO3)2 and Ni(NO3)2, respectively. While, the concentration of Pb [as Pb(NO3)2] equilibrium solution was kept relatively high (based on previous studies) ranging from 0 to 300 mg L–1. The suspensions were agitated at 175 rpm and 25 ± 2°C for 24 h [9]. Similarly, in other sets of experiments, the soils and metals solution suspensions were equilibrated at 45°C. The suspensions were centrifuged at 5000 rpm for 5 minutes (J2-21, Beckman) and supernatant was filtered through Whatman No. 42 filter paper. The concentrations of Pb, Cd, and Ni in the filtrates were determined using graphite atomic adsorption spectrometer (Perkin Elmer AAnalyst 800). Detection limits of the graphite atomic adsorption spectrometer for Pb, Cd, and Ni are 0.45, 0.028, and 0.10 mg L–1 respectively. The difference between the amount of metal concentration in equilibrium solution before and after equilibration was taken as the absorbed amount and calculated using amass balance equation:
where, x is the amount of Pb, Cd, and Ni adsorbed (mg kg–1), Ci and Ce are the initial and final metal concentrations (mg L–1) of equilibrium solution, respectively, w is the weight of soil used (g) and V is the volume (L) of equilibrium metal solution. Sorption data were fitted to the linear forms of the Langmuir and Freundlich and non-linear form of Redlich–Peterson models for calculation of the metal’s adsorption parameters.
Statistical Analysis
Statistical analysis of variance was accomplished using computer software Statistica 8.1 (Analytical Software, USA) and significant of difference between means were computed by performing Duncan’s multiple range test at 5% probability level [37]. Microsoft Excel (2016) derived Pearson correlation coefficient (r) values were used for comparison of some parameters.
RESULTS AND DISCUSSION
Surface soil of five benchmark series, i.e., Gujranwala (silt loam), Miranpur (silty clay), Kotli (silty clay), Rasulpur (sandy loam) and Shahdara (silt loam) were used in these metal adsorption studies (Table 1). The soils are developed on alluvium deposit in the late Pleistocene in Indus river valley (between Chenab and Ravi rivers). All the soils fall in hyperthermic temperature regime and arid and semi-arid tropical continental climate. The soils are the most dominant soils in the rice-wheat cropping sequence. Soil organic matter contents were less than 1.0% in the Gujranwala, Shahdara and Rasulpur soil series and were higher than 1.0% in the Kotli and Miranpur soil series (Table 2). The soils were alkaline calcareous in nature. The cation exchange capacity (CEC) varies greatly; the Kotli soil series had highest CEC (29.2 cmol kg–1) and Rasulpur series had lowest (3.6 cmol kg–1). The CEC decreased in the order of Kotli > Miranpur > Gujranwala > Shahdara > Rasulpur and was analogue to clay contents.
Metal Adsorption Batch Experiment
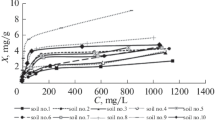
The Cd, Ni, and Pb adsorption isotherms constructed using different soils are shown in Figs. 1a, 1b and 1c. Traditionally, with increase in the Cd, Ni, and Pb concentrations in the equilibrium solution, the metal adsorption by different soils was also increased. There was a rapid increase in the metal adsorption of all the metals by all the soils at lower concentrations and then followed by a gradual increase as proceeded to higher metal concentration of equilibrium solution. The rapid increase in adsorption at initial stage of the batch experiment could be due to the easy accessibility to the negatively charged surface of the solid phase by the positively charged metal ions present in the equilibrium solution. The initial high adsorption rate may be controlled chemically and/or by hydrogen (H) bonding between divalent metal ions and the surface hydroxyls of the adsorbents [19]. While the subsequent gradual adsorption phase increase could be due to electrostatic hindrance caused by already occupied surface sites [34] and ions diffusion into the clay mineral [20]. Although, same metal adsorption trend was followed by all the soils for all metals, the magnitudes of the metal adsorption isotherms were different for each soil. The magnitude of the metal adsorption isotherms of different soils were in the order of Kotli > Miranpur > Gujranwala > Shahdara > Rasulpur. This analogy can be related to their CEC and clay content as metal adsorption depends significantly upon the specific surface area of the soils (Table 2) [32, 44]. Further, soil particle-size distribution play an important role in metal adsorption and increases with decreasing particle size because of its attribution to the high surface area and binding sites [40]. They reported that soils having high clay fraction and higher CEC and clay content adsorbed much more Cd and Pb than those having low CEC and clay content. Similarly, this high metal adsorption by soils with high CEC and clay fraction also observed in another study [47].
Langmuir and Freundlich and Redlich-Peterson Adsorption Isotherms
Soil Pb, Cd, and Ni adsorption data were fitted in two classical linear models, i.e. Langmuir and Freundlich and in one non-linear model, i.e., Redlich–Peterson for description and prediction of the heavy metals adsorption behavior by the different soils.
Langmuir Isotherm
A conventional form of the Langmuir equation is:
where Ce is the Cd, Ni, or Pb concentration of equilibrium solution, x/m is the amount of metal adsorbed by soil (mg/kg), K is the binding energy, and b is the maximum adsorption capacity of soils (mg/kg).
Rearranged form of equation 2 in linear form is:
A plot of C/x/m versus Ce yields a straight line. Maximum adsorption (b) is reciprocal of slope and 1/Kb is the intercept.
The Langmuir adsorption parameters for Pb, Cd, and Ni of all five soils are presented in Table 1, and linearized isotherms are shown in Fig. 2. The Langmuir model described the adsorption data satisfactorily irrespective of soils and metals as indicated by Pearson regression coefficient (r2). However, goodness of fitness of the Langmuir model to the observed data was equally good as the Redlich-Peterson and was slightly better than the Freundlich model. The r2 values of the Langmuir and Redlich-Peterson models were between 0.98 and 0.99; for the Freundlich model, it was between 0.89 and 0.98. The high goodness of fit of both the formerly mentioned models conformed the earlier observations that the Langmuir and Redlich-Peterson models describe metal adsorption data precisely than the Freundlich model [6, 47]. The maximum adsorption (b) predicted by the Langmuir model was the highest in the case of Pb (1971. 5 mg kg–1—mean for all five soils) followed by Cd (928.4 mg kg–1) and was the lowest in the case of Ni (525.9 mg kg–1). The sequences did not follow the order of electronegativity of metal ions (Pb—1.87, Cd—1.69, and Ni—1.91) and support the sequence of other authors [24] reported earlier. However, the maximum adsorption was directly proportional to the ionic radii of the metals, i.e. Pb (119), Cd (97), and Ni (72 pm) and was inversely proportional to the hydrated ionic radii, i.e., Pb (401), Cd (426), and Ni (404 pm). The higher Pb adsorption was exhibited by all the soils than the Cd and Ni, which could be credited to the smaller hydrated ionic radius size of the Pb and hydration energy, i.e., Pb—1481, Cd—1807, and Ni—2106 kJ mol–1 [15, 24, 45]. During the sorption process, metal ions having higher charge bounded strongly to soil particles than smaller charges. However, metal ions having the same charges, unhydrated and hydration ionic radii, and hydration energy play an important role in the metal adsorption. Metal ions having larger radii emit weaker electric field and resultantly less hydrated than those having smaller radii (as they emit stronger electrical fields). This mechanism showed that ions having larger radii are preferably adsorbed by soil particles [45].
The favorableness and nature of the adsorption isotherms curve can be narrated using dimensionless parameter, separation factor (RL) [27] as expressed:
where Ci is the initial concentration of metal ions in solution. The values of RL depict the nature of the adsorption reaction; whether the reaction was irreversible (RL = 0), favorable (0 < RL < 1), linear (RL = 1), and unfavorable (RL > 1). The calculated RL values were between 0.12–0.95, 0.10–0.48, and 0.04–0.31 for Pb, Cd, and Ni, respectively indicating that the adsorption reaction was favorable [18].
A good agreement between the measured and the predicted Langmuir metal adsorption (R ≤ 0.99), at each equilibrium metal concentration, was observed for all the three metals, i.e., Pb, Ni, and Cd (soils data was pooled for each metal). The high correlation coefficient value suggested that Langmuir model is valid for the estimation of metal adsorption for all the soils used in this study. The Langmuir adsorption prediction of Ni and Cd, over all the equilibrium concentration range, followed the 1 : 1 line (Fig. 4). However, in case of Pb, the Langmuir predicted adsorption values were slightly diverged from the experimentally measured values at high equilibrium concentrations. This observation is contradictory to authors [44] reported that the conventional Langmuir equation model predicts 56–64% less Pb adsorption than that of measured.
Freundlich Isotherm
The adsorption data was also fitted to the Freundlich model. This is an empirical model and depict the relationship between amounts of heavy metal ions adsorbed per unit mass of absorbent (qe, mg/kg) and equilibrium concentration (Ce, mg/L) as under:
where the Kf (measure of sorbate affinity to sorbent) and the 1/n (measure of heterogeneity of binding sites of sorbent) are empirical adsorption constants.
The model implies that the energy of adsorption decreases logarithmically as a fraction of surface increases. The linear form of the above equation (5) is as under:
The Kf (intercept) and the 1/n (slope) were calculated by plotting log qe versus log Ce. The Freundlich adsorption isotherm is an empirical model and does not include adsorption maximum as Langmuir equation.
The Freundlich isotherm constants, Kf (measure of sorbate affinity to sorbent) and then (measure of heterogeneity of binding sites of sorbent) and the respective r2 values are presented in Table 4 and linearized isotherms in Fig. 2. The r2 values (0.89–0.98) represent a fairly good linearity and goodness of fitness. The magnitude of the n value is considered an indicator of adsorption isotherms’ favorability. Depending upon the n value, equilibrium behavior can be categorized as good (2–10), moderate (1–2) and poor (<1) [4]. The calculated n values showed that all the soils were good adsorbent of Pb (2.040 < n > 2.141), Cd (4.174 < n > 3.106), and Ni (2.277 < n > 2.098) (Table 4). The Freundlich sorbate affinity (Kf) values were constantly less than those of Langmuir maximum adsorption capacity (b) and Redlich–Peterson parameter (qmon) values. The differences were maximum in case of Pb (for all soils) and were minimum in case of Ni. This difference was similar to results reported by other authors [52]. The order of Freundlich Kf values for all the soils was similar to that of Langmuir b values.
Unlike Langmuir, the correlation coefficient values were less (0.97 < R > 0.95) when the estimated Pb, Ni and Cd adsorption capacity values were compared with the corresponding experimental data at all equilibrium concentrations, irrespective of soils (average of the five soils). The Freundlich model slightly overestimated Pb adsorption at both initial and extreme ends of the equilibrium solution concentrations and underestimated it in between (Fig. 5). Nevertheless, this model is equally good for the estimation of Ni and Cd adsorption at different concentrations of equilibrium solution.
Redlich–Peterson Isotherm
The Redlich–Peterson (known as a three-parameter isotherm) is also commonly used to describe the metal ions adsorption parameters and is based on a kinetic principle [6]. It is assumed that the adsorbing sites increase exponentially with increase in adsorption and suggests a multilayer adsorption. Empirical expression of the equation is:
where qe is the metal adsorption (mg kg–1) at corresponding equilibrium concentration – Ce (mg L–1), qmon (L/g) and bRP (L/mg) are R–P isotherm parameters and exponent β lies between 0 and 1.
Two linear forms [6, 47, 43], i.e., logarithmic and exponential linear forms of the R–P equation are:
and
In this study, exponential linear form of the R–P equation was employed (using SciDAVis—a Windows-based computer code) for calculation of the Pb, Cd and Ni adsorption parameter on to different soil series.
The R–P isotherm became to Langmuir isotherm in case of β = 1 and in case of β = 0, it is inclined to Freundlich isotherm. The calculated β values, closed to one, revealed that the isotherms were most likely Langmuir (Table 5). The high r2 values showed that the description of Redlich–Peterson model for the Pb, Cd, and Ni adsorption data (for different soil series) was equally good as Langmuir model. Several studies [6, 47, 43] have shown that the Redlich–Peterson models gave a relatively better representation than those of the Langmuir and the Freundlich models as it contains three parameters and β being an adjustable parameter. The obtained values of adsorption capacity (qmon) of different soils series for Pd, Cd, and Ni were agreed well with those predicted by Langmuir (b) [47]. While, qmon values were consistently higher than those of Freundlich’s Kf values [14].
Soil Properties and Adsorption of Heavy Metals
Correlation coefficients (R) between soils properties and the Pb, Cd and Ni adsorption are given in Table 6.The order of the metal adsorption by the different soil series was: Kotli > Miranpur > Gujranwala > Shahdara > Rasulpur. The Pb, Cd and Ni maximum adsorption capacity of different soils obtained from Langmuir, Redlich–Peterson and Freundlich models showed significant positive correlation with soil CEC (R = 0.97–0.98), organic carbon (R = 0.81–0.93), Fe (R = 0.93–0.97), Al (0.90–0.98) and clay contents (0.93–0.98) (Table 6). The metal adsorption capacity was significantly affected by soil CEC and clay contents as maximum metal sorption was exhibited by Kotli soil and minimum by Rasulpur soil. The CEC has direct relation with the soils metal adsorption capacity [31, 41]. They reported that clayey soils displayed the highest metal adsorption, while sandy soils showed lowest adsorption. The greater the CEC values, the more exchange sites on soil minerals will be available for the metal retention [39, 21]. The Kotli soil adsorbed 15, 47, 119 and 182% more Pb than those of Miranpur, Gujranwala, Shahdara and Rasulpur, respectively. The adsorption of Cd (9–38%) and Ni (5–117%) by Kotli soils was also higher than that by other soils. Although, all the soil series have mix mineralogy, the Kotli soil series has highest fraction of montmorillonite and vermiculite minerals and the Rasulpur soil series has minimum [42]. These minerals are 2:1 type hydrous aluminosilicate with the octahedral sheet sandwiched between two tetrahedral sheets. Cation substitution in tetrahedral and in octahedral sites provides permanent negative surface charges [48]. The soils, dominated with these minerals, have a high cation exchange capacity and hence high metal adsorption capacities [38]. Similarly, significant positive correlation of Pb, Ni, and Cd adsorption with organic matter showed by all soils and same has been observed by other workers [44].
METAL ADSORPTION THERMODYNAMIC PARAMETERS
The Pb, Cd and Ni metal adsorption related thermodynamic parameters, i.e., ΔG°—change in standard free energy (kJ mol−1), ΔH°—change in standard enthalpy (kJ mol−1) and ΔS° change in standard entropy (kJ mol−1 K–1) were measured at 25 and 45°C [12]:
where Kd is the adsorption equilibrium constant and was computed by multiplying the Langmuir constant KL (L mg–1) with molar mass of the adsorbate [1, 50, 51]
Where, R is the universal gas constant and T is the temperature (Kelvin). The change in ΔH° and ΔS° at constant temperature were calculated by the following van’t Hoff equation.
The thermodynamic parameters of Pb, Cd and Ni adsorption are presented in Table 7. The negative values of ΔG° showed that the Pb, Cd, and Ni adsorption reactions were spontaneous and exothermic in nature for all soils [2]; increase in ΔG° with increase in temperature made reactions more spontaneous and hence favored the metal ions adsorption [49]. The > –15.4 kJ mol–1 experimental values of ΔG° for the Pb, Cd and Ni revealed that the reactions between adsorbing sites and metal ions were static and physical adsorption mechanism dominated sorption process [23]. The high metal ions adsorption also confirmed that more negative values of ΔG° at high temperature favored the metal adsorption [44]. Similarly, high Pb and Cd adsorption at more negative ΔG° energy because of high temperature is also reported by other authors [44]. Further, the obtained negative ΔH° value also confirms that the Pb, Cd, and Ni adsorption reactions were exothermic in nature. The magnitude ΔH° can be used to explain the type of metal adsorption, i.e., physical or chemical. The values between 0 and −20 kJ mol–1 generally describe the dominance of physical adsorption and values between −80 and −400 kJ mol–1 relate to chemical dominated adsorption [11]. Therefore, the obtained values of ΔH° for Pb were between –11.57 and –10.9 kJ mol–1, Cd between °11.13 and ‒6.25 kJ mol–1 and Ni between ‒17.38 and –10.80 kJ mol–1 may be attributed to a physical adsorption process in all soils. The negative value of ΔS° also in supported that the adsorption process was enthalpy driven.
CONCLUSIONS
Results of the Pb, Cd, and Ni adsorption isotherms showed that the investigated alkaline calcareous soils had fairly high adsorption capacity and was analogue to some physical and chemical properties, i.e., clay content, organic carbon, CEC and iron & aluminum contents. The metal adsorption reaction followed Langmuir and Redlich–Peterson adsorption isotherms as experimental data fitted better than the Freundlich adsorption isotherm. The Langmuir predicted metal adsorption and experimental measured adsorption values (at each equilibrium metal concentration) were very closely distributed around 1 : 1 line. While, slightly deviation was noticed in case of the Freundlich’s sorbate affinity. The Langmuir separation factor (RL) and Freundlich affinity coefficient (n) values indicated that metal adsorption reactions were favorable and soils were good adsorbents. The thermodynamic results illustrate that the metals adsorption reactions were spontaneous and exothermic in nature and predominantly controlled by physical adsorption.
REFERENCES
A. Soenmezay, M. S. Oncel and N. Bektas, “Adsorption of lead and cadmium ions from aqueous solutions using manganoxide minerals,” Trans. Nonferrous Met. Soc. China 22, 3131–3139 (2012).
D. G. Strawn, H. L. Bohn and G. A. O’Connor, Soil Chemistry (Wiley, Chichester, 2015).
D. W. Nelson and L. E. Sommers, “Total carbon, organic carbon, and organic matter,” in Methods of Soil Analysis, Part 3: Chemical Methods, SSSA Book Series no. 5, Ed. by D. L. Sparks, P. A. Helmke, A. L. Page, et al. (Soil Science Society of America, Madison, WI, 1996), pp. 961–1010.
R. E. Treybal, Mass Transfer Operations (McGraw-Hill, New York, 1968).
F. Azam, M. Iqbal, C. Inayatullah and K. Malik, Technologies for Sustainable Agriculture (Nuclear Institute for Agriculture and Biology, Faisalabad, 2001).
F.-C. Wu, B.-L. Liu, K.-T. Wu, and R.-L. Tseng, “A new linear form analysis of Redlich–Peterson isotherm equation for the adsorptions of dyes,” Chem. Eng. J. 162, 21–27 (2010).
G. Ji and H. Li, Electrostatic Adsorption of Cations (Oxford University Press, New York, 1997).
G. W. Gee and D. Or, “2.4 particle-size analysis,” in Methods of Soil Analysis, Part 4: Physical Methods (Soil Science Society of America, Madison, 2002), pp. 255–293.
G. W. Thomas, “Soil pH and soil acidity,” in Methods of Soil Analysis, Part 3: Chemical Methods, SSSA Book Series no. 5, Ed. by D. L. Sparks, P. A. Helmke, A. L. Page, et al. (Soil Science Society of America, Madison, WI, 1996), pp. 475–490.
H. B. Bradl, “Adsorption of heavy metal ions on soils and soils constituents,” J. Colloid Interface Sci. 277, 1–18 (2004).
H. Hu, B. Jiang, J. Zhang and X. Chen, “Adsorption of perrhenate ion by bio-char produced from acidosasa edulis shoot shell in aqueous solution,” RSC Adv. 5, 104769–104778 (2015).
H. K. Boparai, M. Joseph and D. M. O’Carroll, “Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano zerovalent iron particles,” J. Hazard. Mater. 186, 458–465 (2011).
H. Veeresh, S. Tripathy, D. Chaudhuri, B. Hart and M. Powell, “Competitive adsorption behavior of selected heavy metals in three soil types of India amended with fly ash and sewage sludge,” Environ. Geol. 44, 363–370 (2003).
H. Yavuz, A. Denizli, H. Gungunes, M. Safarikova and I. Safarik, “Biosorption of mercury on magnetically modified yeast cells,” Sep. Purif. Technol. 52, 253–260 (2006).
Mobasherpour, E. Salahi, and M. Pazouki, “Comparative of the removal of Pb2+, Cd2+ and Ni2+ by nano crystallite hydroxyapatite from aqueous solutions: adsorption isotherm study,” Arab. J. Chem. 5, 439–446 (2012).
J. D. Rhoades, “Salinity: electrical conductivity and total dissolved solids,” in Methods of Soil Analysis, Part 3: Chemical Methods, SSSA Book Series no. 5, Ed. by D. L. Sparks, P. A. Helmke, A. L. Page, et al. (Soil Science Society of America, Madison, WI, 1996), pp. 417–435.
J. E. Lim, M. Ahmad, S. S. Lee, C. L. Shope, Y. Hashimoto, K. R. Kim, A. R. Usman, J. E. Yang and Y. S. Ok, “Effects of lime-based waste materials on immobilization and phytoavailability of cadmium and lead in contaminated soil,” Clean Soil, Air, Water 41, 1235–1241 (2013).
K. R. Hall, L. C. Eagleton, A. Acrivos and T. Vermeulen, “Pore- and solid-diffusion kinetics in fixed-bed adsorption under constant-pattern conditions,” Ind. Eng. Chem. Fundam. 5, 212–223 (1966).
K. S. Low, C. K. Lee and S. C. Liew, “Sorption of cadmium and lead from aqueous solutions by spent grain,” Process Biochem. 36, 59–64 (2000).
K. S. Smith, “Metal sorption on mineral surfaces: An overview with examples relating to mineral deposits,” Environ. Geochem. Miner. Deposits Part B 6, 161–182 (1999).
M. Alamgir, “The effects of soil properties to the extent of soil contamination with metals,” in Environmental Remediation Technologies for Metal-Contaminated Soils (Springer-Verlag, New York, 2016).
M. B. McBride, Environmental Chemistry of Soils (Oxford University Press, New York, 1994).
M. Jnr. Horsfall and A. I. Spiff, “Effects of temperature on the sorption of Pb2+ and Cd2+ from aqueous solution by Caladium bicolor (wild cocoyam) biomass,” Electron. J. Biotechnol. 8, 43–50 (2005).
M. Khani, “Dynamics and thermodynamics studies on the lead and cadmium removal from aqueous solutions by Padina sp. algae: studies in single and binary metal systems,” Sep. Sci. Technol. 48, 2688–2699 (2013).
M. L. A Silveira, L. R. F. Alleoni and L. R. G. Guilherme, “Biosolids and heavy metals in soils,” Sci. Agric. 60, 793–806 (2003).
M. Mahmood-Ul-Hassan, M. Akhtar and G. Nabi, “Boron and zinc transport through intact columns of calcareous soils,” Pedosphere 18, 524–532 (2008).
M. Mahmood-Ul-Hassan, M. Yasin, M. Yousra, R. Ahmad and S. Sarwar, “Kinetics, isotherms, and thermodynamic studies of lead, chromium, and cadmium bio-adsorption from aqueous solution onto Picea smithiana sawdust,” Environ. Sci. Pollut. Res. Int. 25 (13), 12570–12578 (2018).
M. Mahmood-Ul-Hassan, V. Suthar, R. Ahmad and M. Yousra, “Biosorption of metal ions on lignocellulosic materials: Batch and continuous-flow process studies,” Environ. Monit. Assess. 190, 287 (2018).
M. Mahmood-Ul-Hassan, V. Suthor, E. Rafique, R. Ahmad and M. Yasin, “Metal contamination of vegetables grown on soils irrigated with untreated municipal effluent,” Bull. Environ. Contam. Toxicol. 88, 204–209 (2012).
M. Mahmood-Ul-Hassan, V. Suthar, E. Rafique, R. Ahmad and M. Yasin, “Kinetics of cadmium, chromium, and lead sorption onto chemically modified sugarcane bagasse and wheat straw,” Environ. Monit. Assess. 187, 470 (2015).
M. Sumner and W. Miller, “Cation exchange capacity and exchange coefficients,” in Methods of Soil Analysis, Part 3: Chemical Methods, SSSA Book Series no. 5, Ed. by D. L. Sparks, P. A. Helmke, A. L. Page, et al. (Soil Science Society of America, Madison, WI, 1996), pp. 1201–1229.
N. Sangiumsak and P. Punrattanasin, “Adsorption behavior of heavy metals on various soils,” Pol. J. Environ. Stud. 23, 853–865 (2014).
P. C. Gomes, M. P. Fontes, A. G. da Silva, E. de S. Mendonca, and A. R. Netto, “Selectivity sequence and competitive adsorption of heavy metals by Brazilian soils,” Soil Sci. Soc. Am. J. 65, 1115–1121 (2001).
P. K. Pandey, S. Sharma and S. Sambi, “Kinetics and equilibrium study of chromium adsorption on zeolitenax,” Int. J. Environ. Sci. Technol. 7, 395–404 (2010).
P. N. Soltanpour, and A. P. Schwab, “A new soil test for simultaneous extraction of macro-, and micro-nutrients in alkaline soils,” Commun. Soil Sci. Plant Anal. 8, 195–207 (1977).
R. Apak, “Adsorption of heavy metal ions on soil surfaces and similar substances: theoretical aspects,” in Encyclopedia of Surface and Colloid Science, Ed. by A. Hubbard (Marcel Dekker, New York, 2002), p. 385.
R. G. Steel and J. Torrie, Principles and Procedures of Statistics (McGraw-Hill, New York, 1960).
R. Loeppert and D. Suarez, “Carbonate and gypsum,” in Methods of Soil Analysis, Part 3: Chemical Methods, SSSA Book Series no. 5, Ed. by D. L. Sparks, P. A. Helmke, A. L. Page, et al. (Soil Science Society of America, Madison, WI, 1996), pp. 437–474.
R. Malandrino, O. Abollino, A. Giacomino, M. Aceto, and E. Mentasti, “Adsorption of heavy metals on vermiculite: Influence of pH and organic ligands,” J. Colloid Interface Sci. 299, 537–546 (2006).
S. Mandzhieva, T. Minkina, D. Pinskiy, T. Bauer and S. Sushkova, “The role of soil’s particle-size fractions in the adsorption of heavy metals,” Eurasian J. Soil Sci. 3, 197–205 (2014).
S. Singh, L. Ma, and W. Harris, “Heavy metal interactions with phosphatic clay,” J. Environ. Qual. 30, 1961–1968 (2001).
“Soil survey of Pakistan,” in Proceedings of XII International Forum on Soil Taxonomy and Agrotechnology Transfer, Ed. by M. Ahmad, M. Akram, and M. Baig (U.S. Department of Agriculture, Washington, DC, 1986), p. 268.
T. A. Benhammou, A. Yaacoubi, L. Nibou and B. Tanouti, “Adsorption of metal ions onto Moroccan Steven site: kinetic and isotherm studies,” J. Colloid Interface Sci. 282, 320–326 (2005).
T. Adhikari and M. Singh, “Sorption characteristics of lead and cadmium in some soils of india,” Geoderma 114, 81–92 (2003).
T. Bohli, N. Fiol, I. Villaescusa and A. Ouederni, “Adsorption on activated carbon from olive stones: Kinetics and equilibrium of phenol removal from aqueous solution,” J. Chem. Eng. Process Technol. 4, 1–5 (2013).
V. Antoniadis and E. Golia, “Sorption of Cu and Zn in low organic matter-soils as influenced by soil properties and by the degree of soil weathering,” Chemosphere 138, 364–369 (2015).
V. M. Boddu, K. Abburi, J. L. Talbott, E. D. Smith and R. Haasch, “Removal of arsenic (III) and arsenic (V) from aqueous medium using chitosan-coated biosorbent,” Water Res. 42, 633–642 (2008).
V. V. Krupskaya, S. V. Zakusin, E. A. Tyupina, O. V. Dorzhieva, A. P. Zhukhlistov, P. E. Belousov and M. N. Timofeeva, “Experimental study of montmorillonite structure and transformation of its properties under treatment with inorganic acid solutions,” Minerals 7, 49 (2017). https://doi.org/10.3390/min7040049
X. Ma, C. Liu, D. P. Anderson, and P. R. Chang, “Porous cellulose spheres: preparation, modification and adsorption properties,” Chemosphere 165, 399–408 (2016).
Y. Liu, “Is the free energy change of adsorption correctly calculated?” J. Chem. Eng. Data 54, 1981–1985 (2009).
Y. Liu, “Some consideration on the Longmuir isotherm equation,” Colloids Surf. A 274, 34–36 (2006).
Z. Melichova and L. Hromada, “Adsorption of Pb2+ and Cu2+ ions from aqueous solutions on natural bentonite,” Pol. J. Environ. Stud. 22, 457–464 (2013).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Munazza Yousra, Muhammad Mahmood-ul-Hassan, Sarwar, S. et al. Adsorption of Lead, Cadmium, and Nickel on Benchmark Soils of Pakistan. Eurasian Soil Sc. 52, 1063–1074 (2019). https://doi.org/10.1134/S1064229319090126
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1064229319090126