Abstract
In this pilot study we changed several pre-analytical variables of bone marrow trephine biopsy processing with the task to achieve not only a preservation of morphology and antigens but also of nucleic acids. The changes involved employment of a newly established decalcification solution in conjunction with a short fixation time (2 h after receiving the specimens) and performance of decalcification at 37 °C. The comparison of the obtained results from three specimens with those of our routinely established protocol unequivocally revealed that the novel decalcification solution results in a superior preservation of nucleic acids, with only slight differences in preservation of morphology and cellular antigens. These encouraging results imply that this novel decalcification solution will allow a widely accepted standardization of bone marrow trephine biopsy processing.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Bone Marrow Trephine
- Bone Marrow Trephine Biopsy
- Decalcification Solution
- Heavy Chain Rearrangement
- Bony Trabecules
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
A bone marrow trephine (BMT) biopsy is routinely carried out to assess and classify various diseases of the hematolymphoid system. It is also important for staging of malignant lymphomas, for the identification of tumor metastases in the bone marrow and of outstanding clinical value in cases where examination of bone marrow aspirate has been unsuccessful (“dry tap”). A BMT has the advantage of the simultaneous assessment of bone marrow cellularity, the spatial distribution of various cell types, and of alterations regarding bone, marrow stroma, and blood vessels. This complex diagnostic approach can only be accomplished by the combined use of conventional histology and immunophenotyping, and if necessary, by application of molecular techniques for examination of DNA and RNA (interphase fluorescence in situ hybridization, PCR based DNA analysis, in situ hybridization for RNA detection) [1, 2].
It is conceivable that the evaluation of morphology as well as the results of immunohistochemistry and molecular biology techniques depend on pre-analytical, analytical, and post-analytical parameters. The pre-analytical steps are of particular importance due to the peculiarities of the bone marrow specimen, which contains bony trabecules and the vulnerable intertrabecular spaces and thus represents a special challenge to the chosen fixation and decalcification protocols. These pre-analytical steps in BMT processing are however not standardized. There is an enormous variation in the processing protocols among hematopathology laboratories leading to the usage of different fixatives and decalcification procedures. All these procedures have several advantages and also disadvantages, in particular regarding preservation of antigens and nucleic acids, turnaround time, and also toxicity of some components [3–6].
Also we have established a decalcification protocol for our laboratory, which allows an excellent preservation of morphology and extensive immunohistochemical studies within a reasonable turnaround time, so that a comprehensive pathology report is accomplished within two working days after receipt of the BMT biopsy. However we have been confronted with frequent negative molecular pathology results due to major degradation of nucleic acids.
In the present study we wanted to investigate whether a recently available non-toxic decalcification procedure could bypass the disadvantages of our and of most other currently used protocols and allow a BMT processing that fulfills the requirements for contemporary bone marrow diagnostics.
2 Materials and Methods
2.1 Routine Pre-analytical Steps in Our Laboratory
Bone marrow trephines are sent to us in 10 % neutral buffered formalin. The trephines are kept in this solution for at least additional 6 h at room temperature without any agitation and are placed afterwards in the decalcification solution. The decalcification solution used by our laboratory contains EDTA as well as 10 % formaldehyde adjusted at pH 7.0. The advantage of this solution is that fixation and decalcification are performed in parallel. The trephines are incubated in this solution without any agitation at 65 °C for 18 h. After decalcification the specimens are embedded in paraffin using a rapid microwave tissue processor (KOS, Milestone, Sorisole, Italy). The blocks are cut and the obtained paraffin sections are subjected in parallel to conventional stains (Haematoxylin and Eosin, Giemsa, periodic-acid Schiff (PAS), iron stain and Gomori silver impregnation) and to immunohistochemistry using varying antibody panels according to the presumed clinical diagnosis and particular requests from the hemato-oncologists. This procedure allows a comprehensive histological and immunohistochemical analysis of a BMT within 30 h after reaching our laboratory.
2.2 Modifications of Pre-analytical Steps for this Study
For the purpose of this study we introduced an instrument (BoneSTATION, Milestone, Italy), which allows standardization of fixation, decalcification time and temperature. The BoneSTATION consists of two work platforms, one featuring a heating plate with infrared sensors for automatic temperature control up to 50 °C and another with built-in magnetic stirring only. The following changes were introduced in the pre-analytical steps:
-
I.
Shortened fixation time: the freshly obtained BMT included in this pilot study have been placed immediately in 10 % neutral buffered formalin. Once received in the laboratory the specimens were put into labelled cassettes, loaded in the rack of the BoneSTATION and remained under stirring for 1 h at room temperature. Afterwards the fixation was continued for another hour under stirring at 37 °C.
-
II.
Decalcifier agent used: for this study, the MOLDecal Solution (Milestone, Italy) was employed. This is a 10 % EDTA solution consisting of a proprietary mixture of EDTA salts resulting in an optimized pH of 7.2–7.4.
-
III.
Decalcification temperature and time: all BMT samples of the present study were decalcified at 37 °C in the BoneSTATION. One sample was decalcified for a shorter time period than used in our laboratory (4 h) and two others for 18 h as already established.
2.3 Bone Marrow Trephines Included in this Study
Due to the fact that the fixation/decalcification instrument has been delivered to our laboratory 1 month before, only a limited number of BMT biopsies could be analyzed. A prerequisite for inclusion in this pilot project has been the length of the trephine, which should exceed 20 mm. The trephines were then divided in two parts and processed in parallel. The following samples were investigated: one BMT of a patient presenting with anemia of unclear etiology, a further biopsy from a patient with a cutaneous marginal zone lymphoma and one biopsy from a patient with suspected multiple myeloma.
2.4 Conventional Histology and Immunohistochemistry
From the decalcified and paraffin-embedded BMT serial sections were cut at 2 µm and stained with H&E.
For immunostaining, paraffin sections were deparaffinized and subjected to a heat-induced epitope retrieval employing an EDTA-buffer prior to incubation with primary antibodies. Deparafinization as well as epitope retrieval and immunostainings were performed within the automated Leica BondMaxTM system (Leica Microsystems, Nussloch, Germany). For this study we selected three antibodies directed against antigens located in the cell membrane/cytoplasm i.e. against CD20 (clone L26, Dako, Denmark), CD3 (clone LN10, Leica Biosystems Novocastra, UK) and CD117 (clone MI15, Dako) as well as Ki67 (clone MIB1, Dako) located in the cell nucleus. For visualization of bound antibodies the detection kit Bond Polymer Refine Diaminobenzidine (DAB) was used, which was obtained from Leica Biosystems.
2.5 DNA Extraction and Quality Assessment
Five sections (10 µm each) were cut from the paraffin blocks of the decalcified bone marrow trephines and subjected to DNA extraction using the Maxwell 16 FFPE Plus LEV DNA Purification Kit on a Maxwell 16 instrument (both Promega, Germany) according to the manufacturer’s protocol for isolation of genomic DNA from paraffin embedded tissue specimens.
DNA sample concentration was measured by spectrophotometry.
The quality of the DNA extracted from paraffin-embedded samples was assessed by employing a set of control gene PCR primers designed to amplify products of exactly 100, 200, 300, and 400 bp applied in a multiplex PCR according to the Biomed-2 protocols. PCR products were analyzed on acrylamide gels.
2.6 Detection of Clonal Immunoglobulin Heavy Chain Rearrangements
Multiplex PCRs for the amplification of the immunoglobulin heavy chain genes were performed using the Biomed-2 primers and protocols except that 50 PCR cycles were used [7]. PCR products were separated on a Genetic Analyzer 3130 (Thermo Fisher USA) to assess the clonality status of the samples. In case of a polyclonal B-cell proliferation, this analysis results in a Gaussian distribution of multiple peaks representing many different PCR products, while a monoclonal B-cell population results in a single peak from one type PCR product.
2.7 Fluorescence In Situ Hybridization (FISH)
Interphase FISH was performed on tissue sections using a break apart probe for the BCL6 gene supplied by Abbott, Germany together with the Dako Hybridizer and the FISH Accessory Kit (all from Dako, Denmark). Signals were detected using an Axio Imager Z1 (Zeiss, Germany) and Isis software (version 5.3.1, MetaSystems, Germany).
3 Results
All variations in the pre-analytic steps were compared with our routine protocol regarding preservation of morphology, maintenance of antibody reactivity and ability to perform molecular studies (FISH and IgH clonality analysis).
3.1 Morphology
-
Decalcification for 4 h at 37 °C: Two differences to the conventionally decalcified BMT could be identified: the cells appeared shrunken and the bone trabecules were not sufficiently decalcified (Fig. 1a, b). This fact did not allow sectioning of the tissue at 2 µm leading to difficulties in the precise evaluation of cellular details at higher magnification (Fig. 1c, d).
Fig. 1 Morphological evaluation of two differently processed parts of a bone marrow trephine biopsy (H&E). a, c Fixation in 10 % neutral buffered formalin (NBF) at room temperature (RT) for at least 6 h followed by decalcification for 18 h at 65 °C in a solution containing formaldehyde and EDTA at pH 7.0 leads to sufficient bone decalcification and preservation of cellular details. b, d Fixation in 10 % NBF for 1 h at RT and 1 h at 37 °C, followed by decalcification in MOLDecal for 4 h at 37 °C leads to incomplete decalcification of the bone with fragmentation of trabecules during cutting of the block. The resulting section is thick hampering evaluation of cellular details
-
Decalcification for 18 h at 37 °C
These specimens showed a sufficient decalcification of bony trabecula, resulting in thin sections with good preservation of cellular details. There was also a slight shrinkage of the cells detectable (Fig. 2).
Morphological evaluation of two differently processed parts of a bone marrow trephine biopsy (H&E). a, c Fixation in 10 % neutral buffered formalin (NBF) at room temperature (RT) for at least 6 h followed by decalcification for 18 h at 65 °C in a solution containing formaldehyde and EDTA at pH 7.0 leading to sufficient bone decalcification and preservation of cellular details. b, d Fixation in 10 % NBF for 1 h at RT and 1 h at 37 °C, followed by decalcification in MOLDecal also for 18 h at 37 °C. There is only a slight shrinkage of the cells detectable, while bone trabecules are sufficiently decalcified and the cellular details easily evaluable
3.2 Immunohistochemistry
-
Decalcification for 4 h at 37 °C: The nuclear antigen detected by Ki67 was equally good preserved in sections from both protocols. Also the expression patterns of CD3 and CD138 were identical independent of fixation/decalcification protocol. Only the immunostains for CD20 and CD117 were weaker after decalcification with MOLDecal and the reaction product was also more granular. This weaker intensity resulted also in an incorrect estimation of the number of positive cells.
-
Decalcification for 18 h at 37 °C: Both specimens showed weaker and more granular immunostains for CD20 and CD138 (Fig. 3) after decalcification with MOLDecal, while the results for CD3, CD117, and Ki67 (Fig. 3) were largely identical in both protocols.
Fig. 3 Immunohistological evaluation of two differently processed parts of a bone marrow trephine biopsy. Following antigens have been detected: Ki67 (a, b), CD3 (c, d) and CD138 (e, f). Our current protocol (fixation at RT for at least 6 h followed by decalcification for 18 h at 65 °C in a solution containing formaldehyde and EDTA at pH 7.0) leads to good preservation of the various antigens (a, c, e). The novel protocol with shorter fixation (1 h at RT and 1 h at 37 °C) and decalcification in MOLDecal for 18 h at 37 °C leads also to similar results with the exception of CD138 detection where the labelling is weaker and more granular (b, d, f)
3.3 DNA Quality Assessment
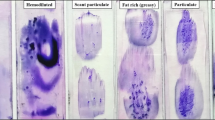
The multiplex control PCR amplification according to the Biomed-2 protocol resulted in a ladder of four fragments (100, 200, 300 and 400 bp) only in the specimens decalcified in the MOLDecal solution after acrylamide gel electrophoresis. In contrast, all samples decalcified with our current protocol showed only 100 bp fragments (Fig. 4).
Quality control of the extracted DNA by acrylamide gel electrophoresis: S corresponds to the control gene PCR products forming a ladder of 400, 300, 200, and 100 bp, T corresponds to tonsillar tissue, lanes 1, 3, 5 represent those parts of biopsy trephines processed with short fixation time and decalcifcation in MOLDecal revealing an intact DNA containing amplificates all four fragments, lanes 2, 4, 6 represent those parts of biopsies processed according to our routine protocol that leads only to 100 bp amplificates
3.4 Clonality Analysis
The highly degraded DNA after decalcification according to our routine protocol did not deliver evaluable PCR products with the primer sets for the framework regions (FR) 1 and 2 of the VH gene segments, while decalcification with MOLDecal led to sufficient PCR products with all 3 FR primer sets. The following results could be obtained:
-
Biopsy 1 (decalcification for 4 h at 37 °C): the evaluation of the PCR amplificates obtained from the routinely processed sample pointed out to an oligoclonal B-cell population, while the new protocol revealed the presence of a B-cell clone embedded in a polyclonal background.
-
Biopsies 2 and 3 (decalcification for 18 h at 37 °C): only the PCR with primers of the FR3 region could be evaluated from the routinely processed samples leading to the assessment of a polyclonal B-cell population. Decalcification with MOLDecal revealed the presence of a monoclonal B-cell population in both instances (Fig. 5).
Fig. 5 Evaluation of the immunoglobulin heavy chain gene (IgH) rearrangement results in a case with suspected manifestation of multiple myeloma. The left panel (a) corresponds to the results obtained from the trephine biopsy part processed according to our current protocol which indicate the presence of an oligoclonal B-cell population. The right panel (b) represents the results obtained after shorter formalin fixation and decalcification in MOLDecal at 37 °C for 18 h, which reveal the presence of a clear-cut monoclonal B-cell population
3.5 FISH
None of the samples decalcified with our routine protocol delivered evaluable FISH results. All samples exhibited high background staining with blurred BCL6-specific signals. In contrast, all specimens processed with the MOLDecal solution were characterized by an absent background staining and clear-cut BCL6-specific signals (Fig. 6).
Comparison of the FISH results using a break-apart probe for the BCL6 gene. Our current fixation and decalcification protocol (a) delivers a non-evaluable result with high background and no discernible BCL-6 specific signals, while shorter formalin fixation combined with MOLDecal at 37 °C for 18 h leads to clear specific signals without any background (b)
4 Conclusion and Outlook
The results of this small pilot study, demonstrate that introduction of modifications in pre-analytic steps can greatly influence the results of morphological, immunohistochemical and molecular evaluation of BMT biopsies. The novel decalcification solution tested leads to an excellent preservation of nucleic acids. A larger scale study is already planned to establish a protocol that will allow a universally applicable standardized BMT biopsy processing.
References
Fend F et al (2008) Modern techniques for the diagnostic evaluation of the trephine bone marrow biopsy: methodological aspects and applications. Prog Histochem Cytochem 42(4):203–252
Neat MJ et al (2013) Fluorescence in situ hybridisation analysis of bone marrow trephine biopsy specimens; an additional tool in the diagnostic armoury. J Clin Pathol 66(1):54–57
Mullink H et al (1985) Influence of fixation and decalcification on the immunohistochemical staining of cell-specific markers in paraffin-embedded human bone biopsies. J Histochem Cytochem 33(11):1103–1109
Naresh KN et al (2006) Optimal processing of bone marrow trephine biopsy: the Hammersmith protocol. J Clin Pathol 59(9):903–911
Torlakovic EE et al (2009) Call for a European programme in external quality assurance for bone marrow immunohistochemistry; report of a European Bone Marrow Working Group pilot study. J Clin Pathol 62(6):547–551
Bonds LA et al (2005) Acetic acid-zinc-formalin: a safe alternative to B-5 fixative. Am J Clin Pathol 124(2):205–211
van Dongen JJ et al (2003) Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia 17(12):2257–2317
Acknowledgments
The expert technical assistance of Vera Arnemann, Erika Berg, Franziska Gocht, Hedwig Lammert, Anita Lehman, Stefanie Mende and Hans-Henning Müller is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Anagnostopoulos, I., Lenze, D., Hummel, M., Dietel, M., Jöhrens, K. (2015). Bone Marrow Work-up: Report of a Pilot Study. In: Dietel, M., Wittekind, C., Bussolati, G., von Winterfeld, M. (eds) Pre-Analytics of Pathological Specimens in Oncology. Recent Results in Cancer Research, vol 199. Springer, Cham. https://doi.org/10.1007/978-3-319-13957-9_10
Download citation
DOI: https://doi.org/10.1007/978-3-319-13957-9_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-13956-2
Online ISBN: 978-3-319-13957-9
eBook Packages: MedicineMedicine (R0)