Abstract
Organophosphate compounds, as most pesticides and chemical warfare agents, first appeared in the US after 1930s and became widespread after World War II. At present, enzymatic detoxification of organophosphate compounds represents an important issue worldwide, due to their permanent and excessive use that has led in many places to the contamination of soil and water. In the last years our research group focused the attention on the enzymes belonging to amidohydrolase superfamily. In particular, a new family of lactonases with promiscuous phosphotriesterase activity, dubbed PTE-like Lactonases (PLLs), has been discovered. We report here an overview of the actual use of organophosphate compounds and the hydrolytic enzymes able to degrade them. In the PLL family there are enzymes that hydrolyze pesticides, show high thermal resistance and, therefore, are very attractive from a biotechnology point of view. The combination of different in vitro evolution methods represents a successful approach to increase their promiscuous phosphotriesterase activity in order to obtain efficient detoxification enzymatic tools.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Organophosphate compounds (OPs) constitute the core of pesticides (i.e. paraoxon, parathion, coumaphos, diazinon, dimethoate and chlorpyrifos) and many nerve-based warfare agents (i.e. sarin, tabun, soman and VX) (Table 16.1).
Pesticides are commonly used in agriculture to obtain high productivity, thanks to the control of plants or animals life (pests). However, although they certainly improve the quality and quantity of the agricultural production, their extensive application has caused pollution of aquatic systems, currently posing risks also to human health.
OPs were introduced only in the last century but their use has increased rapidly and now they represent about 38 % of the total pesticides applied worldwide (Singh 2009). In 2008, China produced about 1.74 million tons of 300 different types of pesticides, which has made this country the largest producer and user of such compounds worldwide (Jin et al. 2010).
The latest estimate of the Environmental Protection Agency (EPA) on the annual application of pesticides is more than five million pounds worldwide and one million pounds in the USA (Gavrilescu 2005; Grube et al. 2011). Currently, it is estimated that 200,000 t of OPs are stocked in the world (Singh 2009) and that self-poisoning of workers is very frequent in agriculture settings (Aardema et al. 2008; Calvert et al. 2008; Eddleston et al. 2005).
The fate of the pesticides in the environment depends on several aspects, including soil physico-chemical properties, topography, weather, agricultural management practices and, ultimately, chemical properties of each pesticide (water solubility, tendency to adsorb to the soil, and pesticide persistence) (Tiryaki and Temur 2010).
Usually, the OPs have low persistence in soil because sunlight exposure, water and microbial hydrolysis cause their rapid degradation (Caceres et al. 2010; Ragnarsdottir 2000). However, their over-use, together with the storage of more than half a million tons of obsolete, prohibited or outdated pesticides (Ortiz-Hernàndez et al. 2013), as well as the limitation or absence in many developing countries of exposure-control programs, led to their anomalous accumulation in soil and water runoff and their magnification through the food chain. The high toxicity of these compounds led the Food and Agriculture Organization of the United Nations (FAO) and World Health Organization (WHO) to establish international food standards and guidelines codes for food safety (Codex Alimentarius) (FAO 2014).
In addition to their use as pesticides, in the early twentieth century extremely poisonous OPs were synthesized and used as chemical warfare agents in World War II (Raushel 2002) and, recently, in terroristic attacks (Tokio subway 1995) and poisoning of civilians (Syria 2013).
OPs are esters of phosphorus with various combinations of oxygen, carbon, sulphur and nitrogen attached, resulting in six different subclasses: phosphates, phosphonates, phosphorothioates, phosphorodithioates, phosphorothiolates and phosphoramidates (Can 2014). They have a general structure in which the phosphorus is linked by a double bond to either an oxygen atom (oxon-OPs), or to a sulphur atom (thion-OPs), and by ester linkages to alkoxy or amino groups, and to another group (halogen, aliphatic, aromatic, or heterocyclic groups).
Based on how the subgroups are oriented around the central phosphorus atom, they have either an RP or SP chiral configuration, where the SP one usually represents the more toxic isomer.
The most studied toxic effect of OPs is the irreversible inhibition of a key enzyme in cholinergic transmission, acetylcholinesterase (AChE), by the covalent phosphorylation of the serine residue within the active site. Such inhibition causes the accumulation of the neurotransmitter acetylcholine in the neuron-neuron or neuron-muscle junctions, determining lacrimation, hypersalivation, bronchial hypersecretion and bronchoconstriction, skeletal muscle fasciculation and twitching, ataxia, respiratory failure, convulsions, hypothermia and, ultimately, death (Carey et al. 2013).
Recent studies on the toxicological properties of OPs also reported gene mutations, chromosomal aberrations, DNA damage (Ojha and Gupta 2014), alteration of semen quality and sperm chromatin (Salazar-Arredondo et al. 2008), and insulin resistance (Lasram et al. 2014). Moreover, the involvement of OPs in cancerogenesis and endocrine disorders has been reported (Gupta 2005).
Since OPs pose hazards to human health and wildlife, it is necessary to realize strategies for the assessment of the respective risk, for their environmental monitoring, and for the treatment of waste and remediation of contaminated sites.
The traditional chromatography and spectroscopy analytical techniques, usually employed in environmental monitoring, show high sensitivity and selectivity, but require a lot of expertise and time, and are very expensive. Detection via biosensors represents a good complement or alternative to the classical methods by simplifying or eliminating some limiting steps, such as sample preparation and making field-testing easier and faster with significant decrease in cost per analysis (Marrazza 2014).
Many of the classical methods used in the OP decontamination field, including chemical treatment, electrochemical oxidation and reduction, volatilization, incineration or photodecomposition (Kiss and Viràg 2009), are expensive, not eco-friendly, and inefficient to remove low contaminant concentrations (LeJeune et al. 1998). The presence of a robust complement of metabolic pathways and enzymes necessary to use different xenobiotic compounds makes the microbial organisms possible candidate in several promising biotechnology platforms (biomonitoring and bioremediation, destruction of nerve agent stockpiles, use as bioscavengers in medical prophylaxis). However, different drawbacks, including strict dependence of growth on temperature, oxygen, and supply of nutrients, stability under harsh environmental conditions, inhibition of growth in the presence of different chemicals, have limited their application (Singh and Walker 2006).
Therefore enzymes, which hydrolyze phosphoester or phosphothiol bonds in organophosphates, are ideal candidate to be used in the above-discussed applications; indeed, they show stable half-lives, broad substrate ranges, high specificity, work very rapidly and are effective against racemic mixtures and individual stereoisomers (Tsai et al. 2010a, b). These enzymes, generally named phosphotriesterases (PTEs; EC 3.1.8.1), were isolated and characterized from different microorganisms (Islam et al. 2010; Singh 2009; Zhang et al. 2005).
To improve upon desirable traits (stability, activity, stereoselectivity and substrate specificity) of several well-known organophosphatases, many researchers have combined different mutational strategies (directed evolution and rational design) (Goldsmith et al. 2012; Iyer and Iken 2015; Tsai et al. 2012). In particular, rational design methods have provided numerous successful solutions but, over the last years, directed evolution, especially when combined with rational approaches (protein structure analysis and bioinformatics tools) has become the best method to design focused libraries (Cobb et al. 2013a, b; Denard et al. 2015). Directed evolution consists of iterative cycles of random mutagenesis and/or DNA recombination to generate diversity in a target gene sequence and allows the identification of the desired protein variants by high-throughput screening (Cobb et al. 2012). Over the years, directed evolution has enabled the engineering and improvement of known protein features, leading to the generation of optimized biocatalysts to employ in several industrial applications. Moreover, it has also helped the design and optimization of biocatalysts with activities not previously encountered in Nature (Denard et al. 2015).
This review will describe some of the recent achievements in the engineering of OP-hydrolyzing biocatalysts, with a special focus on the evolution of the promiscuous activities of Phosphotriesterase-like Lactonases, which represent the new appealing candidates in the OP remediation.
Promiscuous functions are of great importance because they represent a repertoire of enzymatic activities that can be recruited when the environment changes and a new activity becomes important for fitness. Moreover, they provide excellent opportunities for in vitro evolution of new functions to employ in biotechnology and drug development.
2 Organophosphorus Hydrolases
To date, many enzymes able to hydrolyze OP compounds have been identified, such as organophosphorus hydrolases (OPHs), methyl parathion hydrolases (MPHs), serum paraoxonases (PONs) and microbial prolidases/OP acid anhydrolases.
2.1 OPH Hydrolases
A variety of genes different in terms of form, location or host o rganism involved in OP degradation has been identified so far. The first and best characterized opd (‘OP degradation’) genes and their associated protein products (PTEs) were identified in Sphingobium fuliginis ATCC 27551 (previously, Flavobacterium sp.) (Kawahara et al. 2010; Singh and Walker 2006) and Brevundimonas diminuta GM (previously, Pseudomonas diminuta GM) (Harper et al. 1988; Segers et al. 1994); these enzymes showed a strong hydrolytic activity towards OP compounds, in particular, paraoxon (Dumas et al. 1989a).
The protein products of the first identified opd genes were initially designated as parathion hydrolases and then classified as OPHs.
OPH from B. diminuta (named bdPTE in this review) belongs to the amidohydrolase superfamily (subtype I) (Seibert and Raushel 2005) and derives from a gene included in a transposable element of a large plasmid. Structural data showed that bdPTE is a homodimer with (β/α)8-barrel structural fold including a catalytic binuclear centre at the C-terminal end (Benning et al. 1994, 1995, 2001; Vanhooke et al. 1996), containing two metal ions with zinc or cobalt as the favorite cofactors (Dumas et al. 1989a, b; Benning et al. 2001). The residues involved in the catalytic site are: four histidines (H55, H57, H201, and H230) that directly interact with the two divalent cations, an aspartate (D301) and a carboxylated lysine (K169) (Benning et al. 2000).
In bdPTE structure , as well as in the structure of other members of the amidohydrolase superfamily, two loops, one (loop 7) involved in the substrate binding and hydrolytic mechanism and the other (loop 8) responsible for the substrate specificity, have been identified (Seibert and Raushel 2005).
bdPTE hydrolyzes different organophosphate nerve agents, such as sarin, soman and VX that were used in recent years as chemical warfare agents (Caldwell and Raushel 1991). Moreover, to date the enzyme is the most efficient in the paraoxon hydrolysis (k cat/KM = 4 × 107 M−1s−1) (Caldwell et al. 1991) among the characterized OP degrading genes. Interestingly, different promiscuous activities (carboxylesterase and lactonase activities) have been detected for bdPTE (Roodveldt and Tawfik 2005), but the natural substrate is not yet identified; this suggests that its catalytic activity could have recently evolved from a preexisting enzyme, due to the widespread use of high levels of organophosphate insecticides throughout the world (Raushel and Holden 2000).
In a recent review of Jackson et al., the structural determinants of the high catalytic efficiency towards paraoxon of bdPTE were deeply investigated. Two dominant conformational sub-states (‘closed’ and ‘open’) with a low-energy structural transition were described for bdPTE. The ‘closed’ state seems to facilitate the substrate recognition and the lowering of the reaction activation energy but not the fast diffusion of the substrate/product; conversely, the ‘open’ state seems to facilitate mainly the enzyme/product complex dissociation rather than the rate of hydrolysis reaction (Jackson et al. 2008).
This study demonstrated the importance of the conformational changes in the optimization of enzyme catalysis, a concept that has also been revealed for GkaP in the work of Zhang et al. (2015).
A close homolog of bdPTE showing organophosphatase activity was isolated from Agrobacterium radiobacter P230 and designated as OpdA (Horne et al. 2002, 2003). OpdA was able to hydrolyze diethyl OPs (paraoxon, coumaphos, parathion, diazinon, coroxon) and, at a higher rate than bdPTE, dimethyl substrates (methyl-parathion, phosmet, fenthion, dMUP) (Horne et al. 2002).
The presence of opd-like genes has also been demonstrated in several bacterial genome sequencing projects, although these sequences are much more distant (30–40 % identity at the amino acid level) from those found in Flavobacterium, B. diminuta and Agrobacterium radiobacter.
In particular, an OPD-like protein, designated as PHP (phosphotriesterase homology protein), has been isolated from Escherichia coli, purified and characterized (Buchbinder et al. 1998). Biochemical analysis showed that PHP of E. coli (ePHP) is a monomer with two zinc ions; its structure is similar to that of bdPTE and the residues that coordinate the metal ions are conserved (excluding K169) (Buchbinder et al. 1998). The native function of this OPD homolog is not known. As for an ePHP mutant, only weak esterase and phosphotriesterase activities have been reported (Roodveldt and Tawfik 2005).
A protein with features similar to PHP is the product of gene mll7664 from Mesorhizobium loti. Similarly to ePHP, this enzyme has a glutamate bridging the two metals (by similarity) and the main activity observed is the carboxylesterase activity, with promiscous phosphotriesterase and phosphodiesterase activity. Accordingly this enzyme has been dubbed PLC, phosphotriesterase-like carboxylesterase. By mutation of the glutamate to a lysine, the carboxylesterase activity was completely abolished whereas the phosphodiesterase activity became the main activity (Mandrich and Manco 2009).
In these years many efforts have focused on the improvement of the catalytic efficiency of the known OPH enzymes. For example, several directed evolution strategies have been employed to improve bdPTE hydrolytic activity against phosphorothioate substrates, such as methyl parathion and chlorpyrifos, which are hydrolyzed at rates 30 and 1000-fold slower respect to paraoxon (Cho et al. 2002, 2004; Dumas et al. 1989a). By a mutagenesis strategy based on DNA shuffling, a variant of bdPTE (22A11) showing an improvement of the methyl parathion hydrolytic activity (25-fold) was identified (Cho et al. 2002).
By using the evolved variant (22A11) as template, a new mutagenesis approach was combined with a chlorpyrifos screening and led to the identification of a variant (B3561) able to hydrolyze chlorpyrifos at a rate 725-fold higher than the wild-type (k cat/K M value of 2.2 × 108 M−1s−1) (Cho et al. 2004). Some substitutions (A80V, I274N, and K185R), found in both variants (B3561 and 22A11), cause conformational changes resulting in a widening of the active site and in a stabilization of the enzymatic metal-free state (Cho et al. 2004, 2006).
Many reports have focused on modifying the stereoselectivity of bdPTE, in order to make the enzyme more active on the toxic SP isomers of OP compounds. Structural studies identified three binding pockets (small, large, and leaving group) of bdPTE as responsible for the interaction with the substituents of the phosphorus center of the substrates (Chen-Goodspeed et al. 2001a, b). A deep study on the determinants of the bdPTE stereoselectivity has demonstrated that the preference for chiral substrates is determined by the small sub-site size. Indeed, the substitution of a glycine residue with an alanine causes an increase of the SP enantiomer preference by reducing the small subsite size (Chen-Goodspeed et al. 2001b). On the contrary, mutations (I106A, F132A and S308A) that cause a widening of the small sub-site, decrease the SP preference (Chen-Goodspeed et al. 2001b).
Preferential mutations collected from several studies (K185R, H254Q, H254G, H257F, H257W, I274N and L303T) were chosen to design mutagenic libraries and to create highly effective bdPTE variants. From the screening of these libraries, GWT-F5 variant has emerged, which showed catalytic efficiencies for the SP analogs of nerve agents (soman, tabun, sarin, cyclosarin, VX) ranging between 102 M−1 s−1 and 106 M−1 s−1 (Benning et al. 1995; Madej et al. 2012; Tsai et al. 2012).
The power of the in vitro evolution was also highlighted by a recent work in which nine substitutions in Flavobacterium OPH (A80V, I106V, F132D, K185R, D208G, H257W, I274N, S308L, and R319S) were used as scaffold for the creation of a synthetic construct (M9), which showed an increase of the rate of catalysis on VX of 35-fold respect to wild type (Jeong et al. 2014). A prediction of M9 structure was obtained and used in a docking simulation with VX substrate. From this analysis two additional critical residues (L271 and Y309) were identified, which, if substituted with alanines, showed an activity fivefold higher than M9 one (Jeong et al. 2014).
In vitro evolution efforts have been also focused on other OP-degrading enzymes. For example, in order to increase the catalytic activity of Agrobacterium OpdA on malathion, a combinatorial active site saturation testing (CASTing) was used (Naqvi et al. 2014). In this mutagenesis approach the protein tridimensional structure is analyzed in order to choose groups of few amino acids in the binding pocket which can be randomized simultaneously to generate small mutagenic libraries easy to screen. The CASTing strategy, used for the evolution of the malathion hydrolytic activity of OpdA, led to the identification of a double mutant (S308L/Y309A) which showed a widening of the active site and an increase of 5000-fold in catalytic efficiency towards malathion, reaching the highest value reported for this substrate (Naqvi et al. 2014).
2.2 MPH Hydrolases
A distinct OP-degrading pathway is represented by mpd (methyl parathion degradation) genes which confer hydrolytic activity towards methyl paraoxon, methyl parathion, and chlorpyrifos. Based on their similar metallo-β-lactamase domains, the protein products of mpd genes were designated as methyl parathion hydrolases (MPHs ) . The mpd genes were found in Achromobacter, Ochrobactrum, Stenotrophomonas and Pseudomonas but none of these shows a significant homology to opd or other OP-degrading gene (Cui et al. 2001; Yang et al. 2006; Zhang et al. 2005).
Recently, thanks to whole genome sequencing projects, many putative mpd homologues were discovered and cloned; a phylogenetic analysis revealed a separated evolution of these genes from opd genes.
The isolation of many mpd genes from soil bacteria of China (Liu et al. 2005; Zhang et al. 2005, 2006; Zhongli et al. 2001), suggested there is an environmental influence on mpd evolution. An AHL lactonase from Bacillus thuringiensis, belonging to the β-lactamase superfamily, showed some promiscuous organophosphatase activities, suggesting that it is possible an evolution of OPH and MPH from different lactonase enzymes (Afriat et al. 2006).
MPH enzyme from Pseudomonas sp. WBC-3 is a dimer containing a mixed binuclear zinc centre per subunit, where one of the zinc ions is usually replaced by cadmium (Dong et al. 2005). It has been demonstrated that three aromatic residues at the entry of the active site have a crucial role in the determination of affinity towards methyl parathion; indeed, any substitution in these positions results in a significant loss of catalytic activity on the tested substrate (Dong et al. 2005).
Moreover, a newly identified MPH from Pseudomonas pseudoalcaligenes (named OPHC2) showed an unexpected thermal resistance and a broad substrate activity spectrum. OPHC2 catalyzes the hydrolysis of a lactone and different phosphotriesters and esters (Gotthard et al. 2013 ). Its high Tm (97.8 °C) is probably due to the presence of an extended dimerization surface and an intramolecular disulfide bridge (typical of thermostable proteins) and makes OPHC2 a good candidate for OP decontamination (Gotthard et al. 2013).
2.3 Serum Paraoxonases (PONs)
Another interesting group that showed a critical role in OP metabolism is represented by mammalian lactonase/arylesterase enzymes, usually named as serum paraoxonases (Draganov 2010). These enzymes are components of the high-density lipoproteins, which seem to be implicated in the inactivation of toxic by-products of lipid oxidation (Blum et al. 2006; Mackness et al. 2000). The structure of the PON proteins is similar to two lactonases exhibiting promiscuous organophosphatase activity: squid diisopropyl fluorophosphatase (DFPase) and the human senescence marker protein-30 (Belinskaya et al. 2012).
In humans the following PON variants were found: PON1, PON2 and PON3; extensive reports have demonstrated that only PON1 is able to hydrolyze the P–O bond in insecticides, such as paraoxon or chlorpyrifos oxon, and G-series nerve agents (Draganov 2010; Josse et al. 2001; Rochu et al. 2007).
Given its importance as bioscavenger in pharmacological applications, PON1 enzyme was used as template of many evolution attempts. PON proteins are not soluble when produced in recombinant expression systems, therefore, human, mouse and rabbit PON1 genes were mutagenized by DNA shuffling to obtain highly soluble, recombinant enzymes (Aharoni et al. 2004). An interesting variant, named rePON1, was identified and employed as start point for protein engineering studies (Aharoni et al. 2004; Harel et al. 2004; Madej et al. 2012). Functional analysis on rePON1 identified L69, V346, and H115 in the active site as key residues in the catalysis (Harel et al. 2004). Substitutions of these residues were used as target to change the enzymatic stereoselectivity. L69V and V346A substitutions enhance SP catalysis of soman and diisopropylfluorophosphate (DFP), and H115W substitution increases the specificity for the P–S bond (Amitai et al. 2006; Rochu et al. 2007).
In another study a combinatorial saturation mutagenesis of well-characterized PON1 catalytic sites was used to obtain variants with higher catalytic efficiency on cyclosarin (107 M−1 min−1). This value is consistent with an appropriate prophylactic protection in vivo; indeed, protection assays in mice demonstrated that, in the presence of these variants, the survivability of mice increases up to 6 h after acute exposure to cyclosarin (Gupta et al. 2011). A chimeric PON1 mutant with high catalytic efficiency towards soman and cyclosarin was also obtained by protein engineering but, however, its activity is still very low on the phosphoramidate tabun and absent on VX (Worek et al. 2014). Other achievements in the evolution of the interesting properties of PON1 are well-described in a recent review (Iyer and Iken 2015).
2.4 Microbial Prolidases/OP Acid Anhydrolases
Despite their main function, many enzymes able to hydrolyze P–O and P–F bonds in G-type nerve agents, were identified and designated as OP acid anhydrases (OPAAs) (EC 3.1.8.2). In particular, prolidase/OPAA enzymes with hydrolytic activity towards G-series nerve agents were isolated from Alteromonas species (Theriot et al. 2011; Vyas et al. 2010).
These enzymes, belonging to the prolidase family (Theriot et al. 2011), were found in all domains of life (from bacteria to humans) and represent novel thermostable templates for directed evolution studies. Despite the success achieved in bdPTE and PON1 evolution, mutagenesis strategies addressed to engineer efficient prolidases for organophosphorus degradation were underutilized.
OPAA proteins are similar to prolidase enzymes; indeed, they are able to hydrolyze dipeptides that have a proline at the carboxyl-terminal position but are also active on many substrates of OPH and MPH enzymes. They show an high similarity of their structure and their catalytic and stereoselective mechanisms with OPH or MPH (Cheng and DeFrank 2000) but not significant sequence homology; it is now suggested that OPAA could have evolved from an ancestral prolidase (Theriot and Grunden 2011; Vyas et al. 2010).
Two prolidases were identified in hyperthermophilic archaeons Pyrococcus furiosus (Pfprol) and Pyrococcus horikoshii (Ph1prol); they were characterized and used as templates for directed evolution (Theriot et al. 2010a, b, 2011; Theriot and Grunden 2011).
In order to obtain thermophilic enzymes with high activity at lower temperatures, many mutagenesis efforts were employed; indeed, the first studies on prolidases from P. furiosus (Pfprol) have focused on the increasing of its activity at 35 °C, 50 °C, and 70 °C (Theriot et al. 2010a). By random mutagenesis of Ph1prol from P. horikoshii, four mutants (A195T/G306S, Y301C/K342N, E127G/E252D, and E36V) with high thermostability and increased prolidase and phosphotriesterase activity in a wider range of temperatures were obtained (Theriot et al. 2011). The mutations responsible for the catalytic improvement were located mainly in the loops and linker regions of the enzyme and seem to cause structural changes in regions away from the active site (Madej et al. 2012; Theriot et al. 2010b, 2011).
To date, bdPTE from Pseudomonas/Brevundiminas, OpdA from Agrobacterium and PON1 from mammalian are the best studied organophosphate-degrading enzymes. These enzymes and their evolved mutants showed significant activity against OPs and nerve gases but their low stability in solution limits their application in OP biosensing and remediation.
At this purpose, in recent years more researches were focused on a new family of OP-degrading enzymes (Phosphotriesterase-Like Lactonases) of which many extremophilic members were identified.
3 Phosphotriesterase-Like Lactonases (PLLs)
Phosphotriesterase-Like Lactonase (PLL) family includes a group of enzymes that have main lactonase activity on lactones and acyl-homoserin lactones (AHLs) and, in addition, low promiscuous phosphotriesterase activity towards organophosphate compounds (OPs) (Table 16.1).
PLLs have first been identifi ed in the hyperthermophilic crenearchaeon S. solfataricus (SsoPox) and S. acidocaldarius (SacPox) (Afriat et al. 2006; Merone et al. 2005; Porzio et al. 2007) and also in mesophilic organisms such as Mycobacterium tuberculosis (PPH), Rhodococcus erythropolis (AhlA), Deinococcus radiodurans (DrOPH/Dr0930) (Afriat et al. 2006; Hawwa et al. 2009b) and Mycobacterium avium subsp. Paratuberculosis K-10 (MCP) (Chow et al. 2009). Then new thermostable PLLs from Geobacillus stearothermophilus (GsP) (Hawwa et al. 2009a) and from Geobacillus kaustophilus (GKL/GkaP) (Chow et al. 2010) have been reported. Very recently other PLLs have been characterized from Sulfolobus islandicus (SisLac) (Hiblot et al. 2012b), and from Vulcanisaeta moutnovskia (VmoLac/VmutPLL) (Kallnik et al. 2014) (Table 16.2).
The PLL family is related to bacterial phosphotriesterases (PTEs) from a structural and biochemical point of view (Afriat et al. 2006; Elias et al. 2008). Most of PLL members shows a low sequence identity (about 30 %) with the best characterized phosphotriesterase bdPTE. At the beginning most of them has been identified as putative phosphotriesterase and were called “Paraoxonases” (Pox) because able to degrade pesticides such as paraoxon (Merone et al. 2005; Porzio et al. 2007).
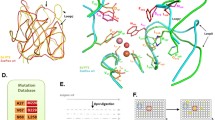
However, further structural, phylogenetic, and biochemical studies have revealed that these enzymes have a proficient lactonase activity, beside the weak phosphotriesterase activity (Afriat et al. 2006). Therefore these new enzymes were designed as PLLs and they share the same (β/α)8 barrel fold of bdPTE with a conserved binuclear metal center, essential for the catalysis (Seibert and Raushel 2005). Both PTE and PLL families belong to the amidohydrolase superfamily, but some extremophilic PLLs are structurally different from the mesophilic PTEs, in particular for loop 7, that is practically absent in the extremophilic enzymes, and for loop 8 where the few sequence differences are more important from a structural point of view. We performed a structural alignment by using the known 3D structure of these PLLs, and it confirms that, except for AhlA from R. erythropolis, all the PLLs have major differences respect to bdPTE in the loop 7 (Fig. 16.1).
Structured-based sequence alignment among representatives of PLLs and bdPTE from Fig. 16.2. SsoPox, VmoLac (from hyperthermophilic archaea); GsP, GkaP (from extremophilic bacteria); PPH, Ahla (from mesophilic bacteria). The structured-based alignment was performed by using Swiss-PdbViewer 4.1.0. Conserved residues are labeled by asterisks. The loop 7 and loop 8 are indicated by black square frames. Conserved residues that constitute the binuclear metal center are labeled in gray
In fact, thanks to the first solved 3D structure of a PLL (SsoPox), very important structural differences emerged (Elias et al. 2008). The structure of the archaeal PLL shows an atypical active site topology and a single hydrophobic channel that perfectly accommodates lactone s ubstrates such as AHLs (Elias et al. 2008). In Fig. 16.2, the superimposition of the structure of bdPTE with those of six PLLs (two from hyperthermophilic archaea, SsoPox and VmoLac two from extremophilic bacteria, GsP and GkaP; two from mesophilic bacteria, PPH and Ahla) indicates that the proteins are homologous. It’s worth noting that the length of loop 7 in all PLLs, except for AhlA (pink), is shorter than bdPTE (gray). Loop 8 of all PLLs has almost the same length as in bdPTE, but its topology is different for GkaP (green) and GsP (blue), where it is more detached from the protein core than that of bdPTE, and for VmoPLL; in the latter case loop 8 is rigid and structured to form an α-helix, differently in comparison with the thermostable PLL SsoPox (Fig. 16.2). Previously, some studies have supposed that the elongation of loop 7 in bdPTE forms a short α-helix, which may provide an active “cap” that narrows the active site mouth and may thereby increase phosphotriesterase activity toward the substrate paraoxon (Afriat et al. 2006).
Structural superposition of bdPTE with six PLLs. The structural superposition was performed by using Swiss-PdbViewer 4.1.0. bdPTE from B. diminuta (gray), SsoPox from S. solfataricus (red); VmoLac from V. moutnovskia (magenta), GsP from G. stearothermophilus (blue), GkaP from G. kaustophilus (green), PPH from M. tuberculosis (cyan), Ahla from R. erythropolis (pink); metal cations are represented as gray spheres
All the PLLs possesses a homo- or hetero-binuclear metal center which generates a catalytic hydroxide ion involved in the hydrolysis of both OPs and lactones. The main difference between the two hydrolytic activities is the transition state geometry: sp3 coordination for phosphotriesters and pentacoordinated state for lactones (Elias et al. 2008). The coexistence of the two activities in the same active site suggests the use of the same catalytic machinery with the optimization for substrate specificity linked to environmental adaptation.
The active site of PLLs contains three sub-sites that are very well adapted for the lactone binding : a small sub-site, a large sub-site and a hydrophobic channel (Elias et al. 2008). The aliphatic chain of the lactones binds within the hydrophobic channel, the large sub-site accommodates the amide group of the N-acyl chain, and the small sub-site positions the lactone ring. Moreover, biochemical analyses confirmed that PLL enzymes are natural lactonases because they have a high activity on acyl-homoserine lactones. Thus it was supposed that PTE has evolved from a yet unknown PLL, whose primary activity was the hydrolysis of quorum sensing homoserine lactones (HSLs) (Afriat et al. (2006). Recently it has been reported that a nine-amino-acid deletion alongside an adjacent point mutation of bdPTE gave an intermediate (PTEΔ7-2/254R) that exhibits both the ancestral acyl homoserine lactonase activity (k cat/KM values of up to 2 × 104), (undetectable in bpPTE), and low paraoxonase activity (Afriat et al. 2012). Bifunctional intermediates with changed specificity have a powerful role in the divergence of new enzymatic functions that are also due to a very important structural changes of loop remodeling.
The bifunctional activity profile of PTEΔ7-2/254R is compatible with the expected properties of an evolutionary intermediate along a trajectory leading from an HSL with a promiscuous phosphotriesterase activity to a highly efficient phosphotriesterase with a weak, promiscuous lactonase activity (Afriat et al. 2012). The SsoPox lactonase activity was tested with synthetic substrates (thiolactones) (Fig. 16.3) but also with real acyl-homoserine lactones (Afriat et al. 2006). However, docking studies and structural analyses demonstrated that the mode of binding is likely to be different (Merone et al. 2010). Starting from this observation, we found a trend of activity on thiolactones quite different from that on AHL lactones, because the specificity constant decreases with the increase of the lateral acyl chain (Fig. 16.3).
Lactonase activity of SsoPox on thiolactones with different acyl chain length. The assays were performed at 70 °C as reported in Afriat et al. (2006). SsoPox shows a k cat/KM of 7 × 105, 3 × 105 and 0.8 × 105 M−1 s−1 on TEBL, TBBL and THBL respectively
AHLs, also named as autoinducer 1 (AI-1), are small signal molecules, produced mainly by gram-negative bacteria, and mediate cell to cell communication known as quorum sensing (QS). When these molecules accumulate in the extracellular environment until a critical threshold, the transcriptional profile of the bacteria is altered (Hentzer et al. 2003).
It is reported that the virulence and the biofilm formation of some pathogens are regulated by QS (Costerton et al. 1999; Dickschat 2010; Jones et al. 2010; Popat et al. 2008), suggesting that the quenching of this mechanism (quorum quenching) could be an interesting strategy against multi-resistant pathogen bacteria which use AHL based QS like P. aeruginosa (Amara et al. 2011; Dong et al. 2000, 2001; Ma et al. 2009; Reimmann et al. 2002). Therefore, as lactonases, PLLs can hydrolyze AHLs and quench the QS mechanism, as seen for AiiA, human paraoxonases (Dong et al. 2001; Ma et al. 2009), and SsoPox, SacPox and Dr0930 that, as reported, are able to reduce swarming PAO motility (Mandrich et al. 2011). Moreover SsoPox has also recently been shown to decrease virulence factors expression and biofilm formation in vitro and to reduce rat mortality in a rat pulmonary infection model (Hraiech et al. 2014).
By considering they have both lactonase and phosphotriesterase activity, PLLs represent a very interesting candidate for biotechnological application as quorum quenching agent (Dong et al. 2000) or OPs biodecontaminant (Singh 2009).
However, at present it is still unclear which is (are) their biological function(s), also because in many cases PLLs were found in microorganisms that do not produce or are regulated by AHLs; therefore, probably they could act on the mechanism of communication of other microrganisms. Furthermore, some members of the PLL family efficiently hydrolyze gamma and/or delta oxo-lactones, but not AHLs (Chow et al. 2010; Xiang et al. 2009). Anyway the interest in these enzymes, in particular for biotechnological applications in decontamination field, lies in exploring phosphotriesterase activity. Most of the extremophilic PLLs are able to hydrolyze different OP pesticides, even if at different extent. As representative compound it is reported the catalytic efficiency on ethyl-paraoxon of the described PLLs (Table 16.2). Among the archaeal PLLs, the SsoPox promiscuous catalytic activity against paraoxon is 3.75 × 103 s−1M−1, tenfold lower than that of SacPox (2.66 × 104 s−1M−1) at 70 °C (Merone et al. 2010; Porzio et al. 2013). These values are higher than SisLac catalytic efficiency (6.98 × 102 s−1M−1) at 70 °C (Hiblot et al. 2012b).
SsoPox and SacPox are both in dimeric form, which is consistent with the crystal structures of SisLac, Dr0930, GsP and GkaP (Table 16.2).
The recent discovered hypertermophilic PLL from V. mountnovski (VmoLac) shows a low catalytic efficiency against paraoxon of 1.86 s−1 M−1 (Hiblot et al. 2015), comparable to those of Dr0930 (1.39 s−1 M−1) (Hawwa et al. 2009b; Mandrich et al. 2013) and GkaP (4.5 s−1 M−1 at 35 °C; 1.1 × 102 at 75 °C), even if all of them are highly stable enzymes. Among the others extremophilic bacterial PLLs, GsP from G. stearothermophilus has the highest value of k cat/KM on paraoxon that is 3.28 × 103 s−1 M−1 at the same comparable temperature (35 °C) (Hawwa et al. 2009a). All the mesophilic PLLs, PPH, AhlA and MCP, exhibit a weak phosphotriesterase activity. For hydrolysis of paraoxon by AhlA, only the k cat /KM ratio was estimated (0.5 s−1 M−1), while MCP and PPH exhibited a higher value of 4.1 and 8.6 M−1 s−1, respectively (Afriat et al. 2006; Chow et al. 2009).
4 In Vitro Evolution of Extremophilic PLLs
The promiscuous phosphotriesterase activity of PLL enzymes on OP compounds suggests that they may constitute a “generalist” intermediate from which PTE may have arisen. However, the possibility that M. loti PLC (more similar to ePHP) represents such starting point could not be discarded (Fig. 16.4).
Representation of likely evolution paths among members of the Amidohydrolase Superfamily. Schematic representation of the possible evolution of three different members of the amidohydrolase superfamily: PLL Phosphotriesterase-Like Lactonase, PTE PhosphoTriestErase, PLC Phosphotriesterase-Like Carboxylesterase, enzymes. PLPD Phosphotriesterase-Like PhosphoDiesterase was generated from PLC by a single mutation
Recently, various mutagenesis strategies, such as site-directed mutagenesis and directed evolution, have been used to enhance the promiscuous OP-degrading activity of several PLLs; this approach efficiently reshapes enzymatic proficiency and specificity, and provides a powerful evidence of the evolvability of PLL enzymes for OP decontamination.
An important difference between PLL and PTE enzymes is that loop 7 and loop 8 have a different length and topology. As reported above, both the loops in the amidohydrolase superfamily are often found to interact with the substrates and to play important role in determination of substrate specificity (Seibert and Raushel 2005). The association of protein and solvent engineering for SsoPox has led to kinetics parameters (k cat and specificity constant) more similar to those of the PTE, but it was exceptionally more stable and so it represent an interesting detoxification device (Merone et al. 2010). The active site structure of SsoPox shows a low elasticity to changes afforded by temperature because various mutations have led to different activity/temperature profiles (Merone et al. 2010).
This means that in the active site a compromise is required between the rigidity necessary to withstand high temperatures assuring at the same time specificity, and the flexibility which is a prerequisite for catalytic activity (Tehei et al. 2005). In detail, variants Y97W, I98F, Y97W/I98F showed profiles very similar to wild type even though with different degrees of activation. As already reported in Elias et al. (2008), single mutant Y97W suggests an involvement of position 97 in the correct orientation of the substrate for catalysis. The single mutant W263F emerged as the best variant, but activating effects were observed for all mutants (Table 16.3). W263F is sixfold more efficient towards ethyl-paraoxon and it also shows a little improvement of rate of hydrolysis towards the warfare agent cyclosarin (Merone et al. 2010).
It has also been reported that the residue W263 plays an important role in substrate binding in the SsoPox active site, at the dimer interface in loop 8, and influence the protein stability, enzymatic activity and promiscuity (Hiblot et al. 2013). Recent structural analyses of SsoPox show that all the 19 different substitutions of the W263 residue increase the SsoPox paraoxonase activity, by inducing very subtle changes in the loop 8 positioning with no effect on the very short loop 7 (Hiblot et al. 2013) (Table 16.3).
Hiblot et al. (2012b) reported that, on the basis of SisLac and SsoPox structural analysis , variations at the positions 14 and 34 seem to have a big impact between the two structures, with a possible effect on dimerization changes (position 34 of SisLac) or stability (positions 14, 34 of SisLac). The analysis of Tm of the mutants E14K and Y34Q shows that single substitution destabilize SisLac, but the combination of both of them helps to restore partially the stability (Hiblot et al. 2012b). Additionally, the rational-designed mutants activity have been tested on different substrates, and in particular it was observed an increase of until 17-fold (E14K) the wild type SisLac (Hiblot et al. 2012b) (Table 16.3).
To improve the organophosphatase activity of Dr0930, in particular on ethyl and methyl paraoxon, site-directed mutagenesis, random mutagenesis, and site-saturation mutagenesis were also used. More than 30,000 potential mutants were screened, and a total of 26 mutated enzymes were purified and characterized kinetically (Hawwa et al. 2009a). In total, all the mutagenesis efforts raised the specificity for paraoxon until 559-fold respect to wt (Table 16.3).
This study underline that by using an iterative approach to mutagenesis, it’s possible to achieve a large rate enhancements when mutations are made in already active mutants. Moreover, the effect of new mutations on kinetic parameters allows to have important structural and functional information of the enzyme (Hawwa et al. 2009b). In addition other authors described rational protein design and random mutagenesis methodologies that were combined with an efficient in vitro screening to identify highly efficient hydrolase variants with enhanced degradation ability (Meier et al. 2013). The best mutant displayed a catalytic activity more than two orders of magnitude higher than the wild-type Dr0930 (Table 16.3).
As regards the Geobacillus sp, by combining rational and random mutagenesis strategies, new variants were successfully obtained after four rounds of screening for GkaP from G. kaustophilus. The catalytic efficiency (k cat /KM) of the best variant against ethyl-paraoxon was about 40-fold higher than that of the D71G/E101G/V235L Dr0930 variant, and comparable to that of the W263FSsoPox variant (Zhang et al. 2012) (Table 16.3).
Eight amino acid changes in GkaP have enhanced the catalytic efficiency for ethyl-paraoxon by nearly two orders of magnitude. Among the eight mutations (F28I, Y99L, T171S, F228L, N269S, V270G, W271C, and G273D), some of these substitutions were located far from the catalytic β-metal, whereas others were within the vicinity of the catalytic site. Tyrosine 99, which is located at the entrance of the binuclear metal center and is thought to reside in the leaving group sub-site, is a conserved residue in the PLL family that appears to play a critical role in stabilizing the lactone ring in a favorable state (Elias et al. 2008). The GkaP variants showed a strong inverse relationship between thermostability and catalytic activity, even if they were still remarkably thermostable (Zhang et al. 2012).
Beside the in vitro evolution approach, it is worth noting that metals in the active site have an important role in influencing the kinetic parameters, as reported in bdPTE (Rochu et al. 2004). Recently we reported that promiscuous paraoxonase activity of SacPox seems to be more sensitive than the main lactonase activity to the metal species in the binuclear metal centre (Porzio et al. 2013). In fact the enzyme prepared in presence on Mn2+ cations (SacPox-Mn2+) shows about 30- and 19-fold increase in paraoxon or me-paraoxon efficiency respectively, with respect to the enzyme prepared with Cd2+ (SacPox-Cd2+). This finding suggest a new interesting approach to be used to increase phosphotriesterase promiscuous activity for biotechnological application.
Finally, the functional and structural homologies suggest that PTE evolved from an as yet unknown PLL, using its promiscuous paraoxonase activity as an essential starting point. As demonstrated by the properties of SsoPox, the paraoxonase activity of PLLs can be considerably high, even without compromising the lactonase activity, and perhaps while acquiring other activities such as aryl esterase. In that respect, SsoPox resembles a “generalist” intermediate from which PTE may have emerged (Aharoni et al. 2005; Afriat et al. 2006).
The recent specialization as a phosphotriesterase, as seen in bdPTE , probably through an insertion into loop 7, did not completely eliminate the ancestor’s lactonase activity (Afriat et al. 2006). The promiscuous carboxylesterase and phosphodiesterase activities observed in bdPTE and PLLs in turn paved the way for the prediction of the existence in Nature of PTE-like enzymes with main carboxylesterase or phosphodiesterase activities. It has been discovered that an homologue of ePHP, from Mesorhizobium loti, is an efficient carboxylesterase with promiscuous phosphotriesterase activity, and therefore it was called MloPLC (Phosphotriesterase-Like Carboxylesterase) (Mandrich and Manco 2009). It shows also a low promiscuous phosphodiesterase and lactonase activity, and we suppose it may be originated from either an ancestral PLL-like or PTE-like enzyme, considering that it maintains both these promiscuous activities (Fig. 16.4). Moreover the discovery of MloPLC supports the theory on the enzyme evolution starting from promiscuous activities in the amidohydrolase superfamily (Afriat et al. 2006; Aharoni et al. 2005; Khersonsky et al. 2006). However, the conversion of MloPLC into an excellent phosphodiesterase by means of a single mutation, in parallel with the complete loss of carboxylesterase activity, is a rare case of substrate assisted gain-of-function via stabilization of the binuclear metal center (Mandrich and Manco 2009). This scenery let us to speculate the existence of a new intermediate, PLC-like, between PTE and PLL, from which, during evolution by sequence/structural rearrangements, phosphodiesterase activity could have been arising (Fig. 16.4). The mutant obtained shows a complete loss of carboxylesterase and lactonase activities (Mandrich and Manco 2009), suggesting that a Phosphotriesterase-like Phosphodiesterase enzyme (PLPD) may represent a final point in this evolutionary route, supposing probably other interesting intermediates after PLC to be discovered and explored.
5 Potentiality of Evolved OP-Degrading Enzymes in Biotechnological Applications
The rapid accumulation of data on evolved OP-degrading enzymes led to renewed interest in developing new strategies for several biotechnological applications, such as therapy and prophylaxis of OP poisoning, OP biomonitoring and OP remediation.
5.1 Therapy and Prophylaxis of OP Poisoning
The human PON1 provides the organism with a natural protection from low-dose intake of some organophosphates, such as paraoxon, but is not efficient in the case of assumption of high doses of deadly organophosphates, such as chemical warfare agents. Therefore, mutants of bdPTE, OpdA and PON1 have been obtained and assayed for in vivo prophylaxis of OP pesticides and nerve agents (Masson and Rochu 2009). Despite the achievements of these studies, their promising therapeutic application requires a deep study of efficient transport and delivery methods in order to reduce the decrease or the full loss of the enzymatic activity. Furthermore, others important issues regard their expression, pharmacokinetics and humanization.
An improvement of the thermal stability and an extension of the mean residence time in mice (from minutes to days) was reached by PEGylation of these enzymes (Trovaslet-Leroy et al. 2011) (for a recent review, see Nachon et al. 2013).
5.2 OP Biomonitoring
While ELISA kits are commercially available for the monitoring of few OPs, in the recent years, enzymatic sensors were deeply investigated for the rapid OP detection (Carullo et al. 2015).
OP biosensors used today are inhibition-based rather than catalysis-based. Enzyme inhibition requires regular replacement of the enzyme part and is measured via amperometric detection of thiocholine, hydrogen peroxide, or p-aminophenol (Wanekaya et al. 2008). These compounds are produced by hydrolysis of acetylcholine (thiocoline) or aminophenyl acetate (p-aminophenol) by AChE, or choline oxidation (hydrogen peroxide) by choline oxidase.
Other OP biosensors use a potentiometric transduction based on the pH variation caused by acetic acid reduction.
AChE-based biosensors are sensitive but suffer of many drawbacks, such as the limited selectivity since AChE is inhibited by several neurotoxins (carbamates, heavy metals), the impossibility to be reused without regeneration with reactivators such as pyridine 2-aldoxime, and the unsuitability for real-time monitoring. Also stability over time is a problem. Recently we investigated the opportunity to use the thermostable EST2 from Alicyclobacillus acidocaldarius as substitute of AChE (Febbraio et al. 2011).
The use of catalysis-based systems (typically using OPHs) is extremely attractive for OP biosensing because they have broad substrate recognition, and the hydrolysis product (p-nitrophenol) can be also quantitatively detected by electrochemical and optical methods (Wanekaya et al. 2008).
The use of higher ionic strength buffers (in which the enzymes show the maximum activity for all time course of the measures) represents the main advantage of the use of optical biosensors rather than the potentiometric ones (Wanekaya et al. 2008).
An example of the discussed application is represented by the combination of a mutant of bdPTE (H254R/H257L), showing an increased catalytic rate for P–S bond hydrolysis, with carbon nanotubes for the sensitive, rapid and selective amperometric detection of V-type nerve agents and demeton-S insecticide (Joshi et al. 2006).
5.3 OP Remediation
In recent years, a strong public opposition to the use of polluting chemical methods has led to the research and development of alternative eco-friendly devices to remediate OPs. The use of microorganisms has limited applicability because poses many issues (dependence of growth on temperature, presence of growth inhibitors such as toxic compounds and some metabolites, and so forth). Therefore enzymes represent a feasible and good alternative.
Many examples of the application of OP-degrading enzymes in the remediation were reported.
The presence of a permeability barrier represented by the outer membrane hinders the OP/OP degrading enzymes interaction in the cell (Richins et al. 1997); to overcame this problem, different surface anchoring motifs, such as Lpp-OmpA chimera, ice nucleation protein (INP), or autotransporter have been used to address these enzymes to the cell surface (Li et al. 2008; Richins et al. 1997; Shimazu et al. 2001).
By using an INP anchoring motif, Yang et al. (2013) reported the functional display of the PTE-GFP fusion on the cell surface of Sphingobium japonicum UT26. The strain containing the fusion showed the ability to hydrolyze OPs and allowed to track the fluorescence during the time course of bioremediation.
The same anchoring motif was successfully used to display an improved mutant of OPH from B. diminuta on the cell surface of E. coli (Tanga et al. 2014). This engineered biocatalysts showed the highest paraoxonase activity among the already reported OPH-displayed strains, an optimal temperature of 55 °C and an high stability (100 % activity over 1 month at room temperature) (Tanga et al. 2014).
Pinjari et al. (2013) have heterologously expressed bdPTE on the membrane of Pseudomonas sp. Ind01, a strain isolated from the activated sludge of a pesticide-manufacturing sites (Pinjari et al. 2012). The engineered strain was very efficient in the remediation of different commercially-available OP pesticides (Pinjari et al. 2013).
Many strategies have been used to immobilize, encapsulate or entrap OP-degrading enzymes to create materials with preserved organophosphatase activity. In a recent paper, mesoporous thin films carried over glass slides were used as host matrices for enzymatic remediators. These bio-catalysts were active and very sensitive (down to 15 μM concentration for paraoxon) and, moreover, they were easly prepared, and reused several times with no significant loss in catalytic activity (Francic et al. 2014).
Gao et al. (2014) reported the covalent immobilization of OpdA enzyme on highly porous nonwoven polyester fabrics for OP degradation. First, these materials were activated with ethylenediamine; the enzyme was, then, immobilized by glutaraldehyde, a bifunctional crosslinker. Following the enzymatic immobilization, the KM for methyl parathion was increased, the pH profile was widened, and the enzymatic stability was enhanced. In batch mode, this remediation system could hydrolyze 20 μM methyl parathion in un-buffered water (Gao et al. 2014).
Despite these applications, many data report a gradual inactivation of bdPTE at temperatures up to 35 °C until a full loss of catalytic activity at 60 °C (Caldwell and Raushel 1991) and, moreover, all attempts to immobilize the enzyme were linked to short-term stability, hi gh costs and possible changes of the kinetic properties (Braatz 1994; Caldwell et al. 1991; Gill and Ballesteros 2000; Grimsley et al. 2001; LeJeune et al. 1997).
Therefore it is crucial to find alternative highly resistant and stable enzymes. As already described, phosphotriesterase activity has been found as promiscuous component in several hydrolases such as prolidase, PLL and MBL enzymes isolated from different extremophilic hosts. The exceptional thermal stability of the extremophilic bacterial and archaeal enzymes offers many biotechnological advantages in industrial processes such as high resistance to harsh conditions (presence of solvents or detergents), minimized contamination potential, extreme stability and increased enzymatic longevity. Furthermore, the purification costs of the enzymes expressed in mesophilic hosts are affordable, indeed, the contaminating proteins deriving from the host can be precipitated by simple thermal fractioning.
Thanks to the link between protein stability and evolvability (Bloom et al. 2006), many reports used highly stable scaffold for next-generation engineering technologies.
For this purpose, many efforts have been performed to use extremophilic PLL enzymes as remediation devices. To date, only two examples of practical applications of PLLs have been reported, even if one of them was not properly applied in OP remediation. In fact, the first report deals with the employment of the lactonase activity of SsoPox on acyl-homoserine lactones (Ng et al. 2011). The enzyme was absorbed onto nanoalumina membranes; in order to interfere with quorum sensing signaling, bacterial cultures were treated with the immobilized enzyme. The results open up new scenarios of biotechnological applications, for example, it could be possible to use lactonases immobilized on filtration membranes for the control of undesirable microbial activities in water purification systems (Ng et al. 2011).
In the second report, the advantage of a mutant of SsoPox (W263F) as detoxification tool was assessed. The enzyme was dissolved in different buffered aqueous solvents (30 % ethanol, 30 % or 50 % methanol and 0.1 % sodium-dodecyl-sulphate) to analyze its activity under stressing denaturing environment, typical, for example, of the conditions usually employed in the toxin extraction from contaminated soils (Merone et al. 2010; Hiblot et al. 2012a). In one case the results were compared with those obtained with bdPTE from B. diminuta. W263F outperforms bdPTE under most of the tested conditions; in 15 min at room temperature about 99.5 % of paraoxon was hydrolized in 30 % methanol and ethanol and 0.1 % sodium-dodecyl-sulphate (Merone et al. 2010).
6 Concluding Remarks and Future Perspectives
It is generally accepted the theory that thermostable enzymes are the best candidate for the in vitro evolution since they well-accept destabilizing mutations, which can lead to new or improved enzymatic functions. The investigation reported here outlines the in vitro evolution potential of PLL enzymes and their successful employment in OP detoxification. It is well-known that also p-nitrophenol produced by paraoxon/parathion hydrolysis is toxic (120-fold less than the original OP) (Munnecke 1979), but, in the last years, different soil bacteria able to use p-nitrophenol as carbon source were studied offering the opportunity to completely remove the toxicity of such compounds (Spain and Gibson 1991).
We have described many examples of OP-degrading enzymes with considerably improved activities that can be used in combination with PNP-utilizing bacteria for a full degradation of the OP compounds (Shimazu et al. 2001).
References
Aardema H, Meertens JH, Ligtenberg JJ, Peters-Polman OM, Tulleken JE, Zijlstra JG (2008) Organophosphorus pesticide poisoning: cases and developments. Neth J Med 66(4):149–153
Afriat L, Roodveldt C, Manco G, Tawfik DS (2006) The latent promiscuity of newly identified microbial lactonase is linked to a recently diverged phosphotriesterase. Biochemistry 45:13677–13686
Afriat-Jurnou L, Jackson CJ, Tawfik DS (2012) Reconstructing a missing link in the evolution of a recently diverged phosphotriesterase by active-site loop remodeling. Biochemistry 51:6047–6055
Aharoni A, Gaidukov L, Yagur S, Toker L, Silman I, Tawfik DS (2004) Directed evolution of mammalian paraoxonases PON1 and PON3 for bacterial expression and catalytic specialization. Proc Natl Acad Sci USA 101(2):482–487
Aharoni A, Gaidukov L, Khersonsky O, Mc QGS, Roodveldt C, Tawfik DS (2005) The ‘evolvability’ of promiscuous protein functions. Nat Genet 37:73–76
Amara N, Krom BP, Kaufmann GF, Meijler MM (2011) Macromolecular inhibition of quorum sensing: enzymes, antibodies, and beyond. Chem Rev 111:195–208
Amitai G, Gaidukov L, Adani R, Yishay S, Yacov G, Kushnir M et al (2006) Enhanced stereoselective hydrolysis of toxic organophosphates by directly evolved variants of mammalian serum paraoxonase. FEBS J 273(9):1906–1919
Belinskaya T, Pattabiraman N, diTargiani R, Choi M, Saxena A (2012) Differences in amino acid residues in the binding pockets dictate substrate specificities of mouse senescence marker protein-30, human paraoxonase1, and squid diisopropylfluorophosphatase. Biochim Biophys Acta 1824(5):701–710
Benning MM, Kuo JM, Raushel FM, Holden HM (1994) Three-dimensional structure of phosphotriesterase: an enzyme capable of detoxifying organophosphate nerve agents. Biochemistry 33:15001–15007
Benning MM, Kuo JM, Raushel FM, Holden HM (1995) Three-dimensional structure of the binuclear metal center of phosphotriesterase. Biochemistry 34:7973–7983
Benning MM, Hong SB, Raushel FM, Holden HM (2000) The binding of substrate analogs to phosphotriesterase. J Biol Chem 275:30556–30560
Benning MM, Shim H, Raushel FM, Holden HM (2001) High resolution X-ray structures of different metal-substituted forms of phosphotriesterase from Pseudomonas diminuta. Biochemistry 40:2712–2722
Bloom JD, Labthavikul ST, Otey CR, Arnold FH (2006) Protein stability promotes evolvability. Proc Natl Acad Sci USA 103(15):5869–5874
Blum MM, Löhr F, Richardt A, Rüterjans H, Chen JC (2006) Binding of a designed substrate analogue to diisopropyl fluorophosphatase: implication for the phosphotriesterase mechanism. J Am Chem Soc 128:12750–12757
Braatz JA (1994) Biocompatible polyurethane-based hydrogels. J Biomater Appl 9:71–96
Buchbinder JL, Stephenson RC, Dresser MJ, Pitera JW, Scanlan TS, Fletterick RJ (1998) Biochemical characterization and crystallographic structure of an Escherichia coli protein from the phosphotriesterase gene family. Biochemistry 37:5096–5106
Bzdrenga J, Hiblot J, Gotthard G, Champion C, Elias M, Chabriere E (2014) SacPox from the thermoacidophilic crenarchaeon Sulfolobus acidocaldarius is a proficient lactonase. BMC Res Notes 7:333
Cáceres T, Megharaj M, Venkateswarlu K, Sethunathan N, Naidu R (2010) Fenamiphos and related organophosphorus pesticides: environmental fate and toxicology. Rev Environ Contam Toxicol 205:117–162
Caldwell SR, Raushel FM (1991) Detoxification of organophosphate pesticides using a nylon based immobilized phosphotriesterase from Pseudomonas diminuta. Appl Biochem Biotechnol 31:59–63
Caldwell SR, Newcomb JR, Schlecht KA, Raushel FM (1991) Limit of diffusion in the hydrolysis of substrate by the phosphotriesterase from Pseudomonas diminuta. Biochemistry 30:7438–7444
Calvert GM, Karnik J, Mehler L, Beckman J, Morrissey B, Sievert J, Barrett R, Lackovic M, Mabee L, Schwartz A, Mitchell Y, Moraga-McHaley S (2008) Acute pesticide poisoning among agricultural workers in the United States, 1998–2005. Am J Ind Med 51(12):883–898
Can A (2014) Quantitative structure-toxicity relationship (QSTR) studies on the organophosphate insecticides. Toxicol Lett 230(3):434–443
Carey JL, Dunn C, Gaspari RJ (2013) Central respiratory failure during acute organophosphate poisoning. Respir Physiol Neurobiol 189(2):403–410
Carullo P, Cetrangolo GP, Mandrich L, Manco G, Febbraio F (2015) Fluorescence spectroscopy approaches for the development of a real-time organophosphate detection system using an enzymatic sensor. Sensors (Basel) 15(2):3932–3951
Cheng TC, DeFrank JJ (2000) Hydrolysis of organophosphorus compounds by bacterial prolidases. In: Zwanenburg B, Mikolajczyk M, Kielbasinski P (eds) Enzymes in action: green solutions for chemical problems, vol 33. Kluwer, Dordrecht, pp 243–262
Chen-Goodspeed M, Sogorb MA, Wu F, Hong SB, Raushel FM (2001a) Structural determinants of the substrate and stereochemical specificity of phosphotriesterase. Biochemistry 40(5):1325–1331
Chen-Goodspeed M, Sogorb MA, Wu F, Raushel FM (2001b) Enhancement, relaxation, and reversal of the stereoselectivity for phosphotriesterase by rational evolution of active site residues. Biochemistry 40(5):1332–1339
Cho CMH, Mulchandani A, Chen W (2002) Bacterial cell surface display of organophosphorus hydrolase for selective screening of improved hydrolysis of organophosphate nerve agents. Appl Environ Microbiol 68(4):2026–2030
Cho CMH, Mulchandani A, Chen W (2004) Altering the substrate specificity of organophosphorus hydrolase for enhanced hydrolysis of chlorpyrifos. Appl Environ Microbiol 70(8):4681–4685
Cho CMH, Mulchandani A, Chen W (2006) Functional analysis of organophosphorus hydrolase variants with high degradation activity towards organophosphate pesticides. Protein Eng Des Sel 19(3):99–105
Chow JY, Wu L, Yew WS (2009) Directed evolution of a quorum-quenching lactonase from Mycobacterium avium subsp. paratuberculosis K-10 in the amidohydrolase superfamily. Biochemistry 48:4344–4353
Chow JY, Xue B, Lee KH, Tung A, Wu L, Robinson RC, Yew WS (2010) Directed evolution of a thermostable quorum-quenching lactonase from the amidohydrolase superfamily. J Biol Chem 285(52):40911–40920
Cobb RE, Si T, Zhao H (2012) Directed evolution: an evolving and enabling synthetic biology tool. Curr Opin Chem Bio 16:285–291
Cobb RE, Chao R, Zhao H (2013a) Directed evolution: past, present, and future. AIChE J 59:1432–1440
Cobb RE, Sun N, Zhao H (2013b) Directed evolution as a powerful synthetic biology tool. Methods 60:81–90
Costerton JW, Stewart PS, Greenberg EP (1999) Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322
Cui ZL, Li SP, Fu GP (2001) Isolation of methyl parathion-degrading strain M6 and cloning of the methyl parathion hydrolase gene. Appl Environ Microbiol 67:4922–4925
Denard CA, Ren H, Zhao H (2015) Improving and repurposing biocatalysts via directed evolution. Curr Opin Chem Biol 25:55–64
Dickschat JS (2010) Quorum sensing and bacterial biofilms. Nat Prod Rep 27(3):343–69
Dong YH, Xu JL, Li XZ, Zhang LH (2000) AiiA, an enzyme that inactivates the acylhomoserine lactone quorum-sensing signal and attenuates the virulence of Erwinia carotovora. Proc Natl Acad Sci USA 97:3526–3531
Dong YH, Wang LH, Xu JL, Zhang HB, Zhang XF et al (2001) Quenching quorum-sensing-dependent bacterial infection by an N-acyl homoserine lactonase. Nature 411:813–817
Dong YJ, Bartlam M, Sun L, Zhou YF, Zhang ZP, Zhang CG et al (2005) Crystal structure of methyl parathion hydrolase from Pseudomonas sp. WBC-3. J Mol Biol 353:655–663
Draganov DI (2010) Lactonases with organophosphatase activity: structural and evolutionary perspectives. Chem Biol Interact 187(1):370–372
Dumas DP, Caldwell SR, Wild JR, Raushel FM (1989a) Purification and properties of the phosphotriesterase from Pseudomonas diminuta. J Biol Chem 264(33):19659–19665
Dumas DP, Wild JR, Raushel FM (1989b) Diisopropylfluorophosphate hydrolysis by a phosphotriesterase from Pseudomonas diminuta. Biotechnol Appl Biochem 11:235–243
Eddleston M, Eyer P, Worek F, Mohamed F, Senarathna L, von Meyer L, Juszczak E, Hittarage A, Azhar S, Dissanayake W, Sheriff MH, Szinicz L, Dawson AH, Buckley NA (2005) Differences between organophosphorus insecticides in human self-poisoning: a prospective cohort study. Lancet 366(9495):1452–1459
Elias M, Dupuy J, Merone L, Mandrich L, Porzio E, Moniot S, Rochu D, Lecomte C, Rossi M, Masson P, Manco G, Chabriere E (2008) Structural basis for natural lactonase and promiscuous phosphotriesterase activities. J Mol Biol 379:1017–1028
FAO (2014) Tools for the implementation of the international code of conduct on pesticide management. http://www.fao.org/agriculture/crops/thematic-sitemap/theme/pests/code/toolkits/en/. Accessed 12 May 2014
Febbraio F, Merone L, Cetrangolo GP, Rossi M, Nucci R, Manco G (2011) Thermostable esterase 2 from Alicyclobacillus acidocaldarius as biosensor for the detection of organophosphate pesticides. Anal Chem 83(5):1530–1536. doi:10.1021/ac102025z
Francic N, Bellino MG, Soler-Illia GJAA, Lobnik A (2014) Mesoporous titania thin films as efficient enzyme carriers for paraoxon determination/detoxification: effects of enzyme binding and pore hierarchy on the biocatalyst activity and reusability. Analyst 139:3127–3136
Gao Y, Truong YB, Cacioli P, Butler P, Kyratzis IL (2014) Bioremediation of pesticide contaminated water using an organophosphate degrading enzyme immobilized on nonwoven polyester textiles. Enzyme Microb Technol 54:38–44
Gavrilescu M (2005) Fate of pesticides in the environment and its bioremediation. Eng Life Sci 6:497–526
Gill I, Ballesteros A (2000) Bioencapsulation within synthetic polymers (part 2): non-sol–gel protein-polymer composites. Trends Biotechnol 18:469–479
Goldsmith M, Ashani Y, Simo Y, Ben-David M, Leader H, Silman I et al (2012) Evolved stereoselective hydrolases for broad-spectrum G-type nerve agent detoxification. Chem Biol 19(4):456–466
Gotthard G, Hiblot J, Gonzalez D, Elias M, Chabriere E (2013) Structural and enzymatic characterization of the phosphotriesterase OPHC2 from Pseudomonas pseudoalcaligenes. PLoS One 8, e77995. doi:10.1371/journal.pone.0077995
Grimsley JK, Singh WP, Wild JR, Giletto A (2001) A novel, enzyme-based method for the wound-surface removal and decontamination of organophosphorous nerve agents. Bioact Fibers Polymers 742:35–49
Grube A, Donaldson D, Kiely T, Wu L (2011) Pesticides industry sales and usage 2006–2007 market estimates. US Environmental Protection Agency, Washington, DC. http://www.epa.gov/opp00001/pestsales/07pestsales/market_estimates2007.pdf
Gupta RC (2005) Toxicology of organophosphate and carbamate compounds. Academic, London
Gupta RD, Goldsmith M, Ashani Y, Simo Y, Mullokandov G, Bar H et al (2011) Directed evolution of hydrolases for prevention of G-type nerve agent intoxication. Nat Chem Biol 7(2):120–125
Harel M, Aharoni A, Gaidukov L, Brumshtein B, Khersonsky O, Meged R et al (2004) Structure and evolution of the serum paraoxonase family of detoxifying and anti-atherosclerotic enzymes. Nat Struct Mol Biol 11(5):412–419
Harper LL, McDaniel CS, Miller CE, Wild JR (1988) Dissimilar plasmids isolated from Pseudomonas diminuta MG and a Flavobacterium sp. (ATCC 27551) contain identical opd genes. Appl Environ Microbiol 54(10):2586–2589
Hawwa R, Aikens J, Turner RJ, Santarsiero BD, Mesecar AD (2009a) Structural basis for thermostability revealed through the identification and characterization of a highly thermostable phosphotriesterase-like lactonase from Geobacillus stearothermophilus. Arch Biochem Biophys 488:109–120
Hawwa R, Larsen SD, Ratia K, Mesecar AD (2009b) Structure-based and random mutagenesis approaches increase the organophosphate-degrading activity of a phosphotriesterase homologue from Deinococcus radiodurans. J Mol Biol 393:36–57
Hentzer M, Wu H, Andersen JB, Riedel K, Rasmussen TB et al (2003) Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J 22:3803–3815
Hiblot J, Gotthard G, Chabriere E, Elias M (2012a) Characterisation of the organophosphate hydrolase catalytic activity of SsoPox. Sci Rep 2:779
Hiblot J, Gotthard G, Chabriere E, Elias M (2012b) Structural and enzymatic characterization of the lactonase SisLac from Sulfolobus islandicus. PLoS ONE 7(10), e47028
Hiblot J, Gotthard G, Elias M, Chabriere E (2013) Differential active site loop conformations mediate promiscuous activities in the lactonase SsoPox. PLoS ONE 8(9), e75272
Hiblot J, Bzdrenga J, Champion C, Chabriere E, Elias M (2015) Crystal structure of VmoLac, a tentative quorum quenching lactonase from the extremophilic crenarchaeon Vulcanisaeta moutnovskia. J Sci Rep 5:8372
Horne I, Sutherland TD, Harcourt RL, Russell RJ, Oakeshott JG (2002) Identification of an opd (organophosphate degradation) gene in an Agrobacterium isolate. Appl Environ Microbiol 68(7):3371–3376
Horne I, Qiu X, Russell RJ, Oakeshott JG (2003) The phosphotriesterase gene opdA in Agrobacterium radiobacter P230 is transposable. FEMS Microbiol Lett 222(1):1–8
Hraiech S, Hiblot J, Lafleur J, Lepidi H, Papazian L, Rolain JM, Raoult D, Elias M, Silby MW, Bzdrenga J, Bregeon F, Chabriere E (2014) Inhaled lactonase reduces Pseudomonas aeruginosa quorum sensing and mortality in rat pneumonia. PLoS ONE 9(10), e107125
Islam SM, Math RK, Cho KM, Lim WJ, Hong SY, Kim JM, Yun MG, Cho JJ, Yun HD (2010) Organophosphorus hydrolase (OpdB) of Lactobacillus brevis WCP902 from kimchi is able to degrade organophosphorus pesticides. J Agric Food Chem 58(9):5380–5386
Iyer R, Iken B (2015) Protein engineering of representative hydrolytic enzymes for remediation of organophosphates. Biochem Eng J 94:134–144
Jackson CJ, Foo JL, Kim HK, Carr PD, Liu JW, Salem G, Ollis DL (2008) In crystallo capture of a Michaelis complex and product-binding modes of a bacterial phosphotriesterase. J Mol Biol 375(5):1189–1196
Jeong YS, Choi JM, Kyeong HH, Choi JY, Kim EJ, Kim HS (2014) Rational design of organophosphorus hydrolase with high catalytic efficiency for detoxifying a V-type nerve agent. Biochem Biophys Res Commun 449(3):263–267
Jin F, Wang J, Shao H, Jin M (2010) Pesticide use and residue control in China. J Pestic Sci 35:138–142
Jones MB, Peterson SN, Benn R, Braisted JC, Jarrahi B et al (2010) Role of luxS in Bacillus anthracis growth and virulence factor expression. Virulence 1:72–83
Joshi KA, Prouza M, Kum M, Wang J, Tang J, Haddon R, Chen W, Mulchandani A (2006) V-type nerve agent detection using a carbon nanotube-based amperometric enzyme electrode. Anal Chem 78:331
Josse D, Lockridge O, Xie W, Bartels CF, Schopfer LM, Masson P (2001) The active site of human paraoxonase (PON1). J Appl Toxicol 21(S1):S7–S11
Kallnik V, Bunescu A, Sayer C, Bräsen C, Wohlgemuth R, Littlechild J, Siebers B (2014) Characterization of a phosphotriesterase-like lactonase from the hyperthermoacidophilic crenarchaeon Vulcanisaeta moutnovskia. J Biotechnol 190:11–17
Kawahara K, Tanaka A, Yoon J, Yokota A (2010) Reclassification of a parathion degrading Flavobacterium sp. ATCC 27551 as Sphingobium fuliginis. J Gen Appl Microbiol 56(3):249–255
Khersonsky O, Roodveldt C, Tawfik DS (2006) Enzyme promiscuity: evolutionary and mechanistic aspects. Curr Opin Chem Biol 10:498–508
Kiss A, Virág D (2009) Photostability and photodegradation pathways of distinctive pesticides. J Environ Qual 38(1):157–163
Lasram MM, Dhouib IB, Annabi A, El Fazaa S, Gharbi N (2014) A review on the molecular mechanisms involved in insulin resistance induced by organophosphorus pesticides. Toxicology 322:1–13
LeJeune KE, Mesiano AJ, Bower SB, Grimsley JK, Wild JR, Russell AJ (1997) Dramatically stabilised phosphotriesterase polymers for nerve agent degradation. Biotechnol Bioeng 54:105–114
LeJeune KE, Wild JR, Russell AJ (1998) Nerve agents degraded by enzymatic foams. Nature 395(6697):27–28
Li C, Zhu Y, Benz I, Schmidt MA, Chen W, Mulchandani A, Qiao C (2008) Presentation of functional organophosphorus hydrolase fusions on the surface of Escherichia coli by the AIDA-I autotransporter pathway. Biotechnol Bioeng 99:485–490
Liu H, Zhang JJ, Wang SJ, Zhang XE, Zhou NY (2005) Plasmid-borne catabolism of methyl parathion and p-nitrophenol in Pseudomonas sp. strain WBC-3. Biochem Biophys Res Commun 334(4):1107–1114
Ma F, Wang Y, Zhang Y, Xiong N, Yang B et al (2009) Heterologous expression of human paraoxonases in Pseudomonas aeruginosa inhibits biofilm formation and decreases antibiotic resistance. Appl Microbiol Biotechnol 83:135–141
Mackness B, Durrington PN, Mackness MI (2000) The paraoxonase gene family and coronary heart disease. Curr Opin Lipidol 13:357–363
Madej T, Addess KJ, Fong JH, Geer LY, Geer RC, Lanczycki CJ et al (2012) MMDB: 3D structures and macromolecular interactions. Nucleic Acids Res 40(Database issue):D461–D464
Mandrich L, Manco G (2009) Evolution in the amidohydrolase superfamily: substrate-assisted gain of function in the E183K mutant of a phosphotriesterase-like metal-carboxylesterase. Biochemistry 48(24):5602–5612
Mandrich L, Porzio E, Merone L, Febbraio F, Nucci R, Manco G (2011) Exploring thermostable quorum quenching lactonases to counteract bacterial infections in cystic fibrosis. In: Mendez-Vilas A (ed) Science and technology against microbial pathogens. Research, development and evaluation. Proceedings of the international conference on antimicrobial research (ICAR2010). World Scientific, pp 150–154
Mandrich L, Di Gennaro S, Palma A, Manco G (2013) A further biochemical characterization of DrPLL the thermophilic lactonase from Deinococcus radiodurans. Protein Pept Lett 20(1):36–44
Marrazza G (2014) Piezoelectric biosensors for organophosphate and carbamate pesticides: a review. Biosensors 4:301–317
Masson P, Rochu D (2009) Catalytic bioscavengers against toxic esters, an alternative approach for prophylaxis and treatments of poisonings. Acta Nat 1(1):68–79
Meier MM, Rajendran C, Malisi C, Fox NG, Xu C, Schlee S, Barondeau DP, Hocker B, Sterner R, Raushel FM (2013) Molecular engineering of organophosphate hydrolysis activity from a weak promiscuous lactonase template. J Am Chem Soc 135:11670–11677
Merone L, Mandrich L, Rossi M, Manco G (2005) A thermostable phosphotriesterase from the archaeon Sulfolobus solfataricus: cloning, overexpression and properties. Extremophiles 9(4):297–305
Merone L, Mandrich L, Porzio E, Rossi M, Müller S, Reiter G, Worek F, Manco G (2010) Improving the promiscuous nerve agent hydrolase activity of a thermostable archaeal lactonase. Bioresour Technol 101(23):9204–9212
Munnecke DM (1979) Hydrolysis of organophosphate insecticides by an immobilized-enzyme system. Biotechnol Bioeng 21:2247–2261
Nachon F, Brazzolotto X, Trovaslet M, Masson P (2013) Progress in the development of enzyme-based nerve agent bioscavengers. Chem Biol Interact 206:536–544
Naqvi T, Warden AC, French N, Sugrue E, Carr PD, Jackson CJ et al (2014) A 5000-fold increase in the specificity of a bacterial phosphotriesterase for malathion through combinatorial active site mutagenesis. PLoS ONE 9(4), e94177
Ng FSW, Wright DM, Seah SYK (2011) Characterization of a phosphotriesterase-like lactonase from Sulfolobus solfataricus and its immobilization for disruption of quorum sensing. Appl Environ Microbiol 77(4):1181–1186
Ojha A, Gupta Y (2014) Evaluation of genotoxic potential of commonly used organophosphate pesticides in peripheral blood lymphocytes of rats. Hum Exp Toxicol pii, 0960327114537534. [Epub ahead of print]
Ortiz-Hernández ML, Sánchez-Salinas E, Castrejón Godínez ML, Dantan González E, Popoca Ursino EC (2013) Mechanisms and strategies for pesticide biodegradation: opportunity for waste, soils and water cleaning. Rev Int Contam Ambie 29:85–104
Pinjari AB, Novikov B, Rezenom YH, Russell DH, Wales ME, Siddavattam D (2012) Mineralization of acephate, a recalcitrant organophosphate insecticide is initiated by a Pseudomonad in environmental samples. PLoS One 7, e31963
Pinjari AB, Pandey JP, Kamireddy S, Siddavattam D (2013) Expression and subcellular localization of organophosphate hydrolase in acephate-degrading Pseudomonas sp. strain Ind01 and its use as a potential biocatalyst for elimination of organophosphate insecticides A.B. Lett Appl Microbiol 57:63–68
Popat R, Crusz SA, Diggle SP (2008) The social behaviours of bacterial pathogens. Br Med Bull 87:63–75
Porzio E, Merone L, Mandrich L, Rossi M, Manco G (2007) A new phosphotriesterase from Sulfolobus acidocaldarius and its comparison with the homologue from Sulfolobus solfataricus. Biochimie 89(5):625–636
Porzio E, Di Gennaro S, Palma A, Manco G (2013) Mn2+ modulates the kinetic properties of an archaeal member of the PLL family. Chem Biol Interact 203(1):251–256
Ragnarsdottir KV (2000) Environmental fate and toxicology of organophosphate pesticides. J Geol Soc 157:859–876
Raushel FM (2002) Bacterial detoxification of organophosphate nerve agents. Curr Opin Microbiol 5(3):288–295
Raushel FM, Holden HM (2000) Phosphotriesterase: an enzyme in search of its natural substrate. Adv Enzymol Relat Areas Mol Biol 74:51–93
Reimmann C, Ginet N, Michel L, Keel C, Michaux P et al (2002) Genetically programmed autoinducer destruction reduces virulence gene expression and swarming motility in Pseudomonas aeruginosa PAO1. Microbiology 148:923–932
Richins RD, Kaneva I, Mulchandani A, Chen W (1997) Biodegradation of organophosphorus pesticides by surface-expressed organophosphorus hydrolase. Nat Biotechnol 15:984–987
Rochu D, Viguie N, Renault F, Crouzier D, Froment MT, Masson P (2004) Contribution of the active site metal cation to catalytic activity and to conformational stability of phosphotriesterase: a thermo- and pH-dependence study. Biochem J 380:627–633
Rochu D, Chabriere E, Masson P (2007) Human paraoxonase: a promising approach for pre-treatment and therapy of organophosphorus poisoning. Toxicology 233(1):47–59
Roodveldt C, Tawfik DS (2005) Shared promiscuous activities and evolutionary features in various members of amidohydrolase superfamily. Biochemistry 44:12728–12736
Salazar-Arredondo E, Solis-Heredia MJ, Rojas-Garcia E, Ochoa IH, Quintanilla Vega B (2008) Sperm chromatin alteration and DNA damage by methyl-parathion, chlorpyrifos and diazinon and their oxon metabolites in human spermatozoa. Reprod Toxicol 25:455–460
Segers P, Vancanneyt M, Pot B, Torck U, Hoste B, Dewettinck D et al (1994) Classification of Pseudomonas diminuta Leifson and Hugh 1954 and Pseudomonas vesicularis Büsing, Döll, and Freytag 1953 in Brevundimonas gen. nov. as Brevundimonas diminuta comb. nov. and Brevundimonas vesicularis comb. nov., respectively. Int J Syst Bacteriol 44(3):499–510
Seibert CM, Raushel FM (2005) Structural and catalytic diversity within the amidohydrolase superfamily. Biochemistry 44(17):6383–6391
Shimazu M, Mulchandani A, Chen W (2001) Simultaneous degradation of organophosphorus pesticides and p-nitrophenol by a genetically engineered Moraxella sp. with surface-expressed organophosphorus hydrolase. Biotechnol Bioeng 76:318–324
Singh BK (2009) Organophosphorus-degrading bacteria: ecology and industrial applications. Nat Rev Microbiol 7(2):156–164
Singh BK, Walker A (2006) Microbial degradation of organophosphorus compounds. FEMS Microbiol Rev 30(3):428–471
Spain JC, Gibson DT (1991) Pathway for biodegradation of p-nitrophenol in a Moraxella sp. Appl Environ Microbiol 57:812–819
Tanga X, Lianga B, Yi T, Manco G, Palchetti I, Liua A (2014) Cell surface display of organophosphorus hydrolase for sensitive spectrophotometric detection of p-nitrophenol substituted organophosphates. Enzyme Microb Technol 55:107–112
Tehei M, Madern D, Franzetti B, Zaccai G (2005) Neutron scattering reveals the dynamic basis of protein adaptation to extreme temperature. J Biol Chem 280:40974–40979
Theriot CM, Grunden AM (2011) Hydrolysis of organophosphorus compounds by microbial enzymes. Appl Microbiol Biotechnol 89(1):35–43
Theriot CM, Du X, Tove SR, Grunden AM (2010a) Improving the catalytic activity of hyperthermophilic Pyrococcus prolidases for detoxification of organophosphorus nerve agents over a broad range of temperatures. Appl Microbiol Biotechnol 87(5):1715–1726
Theriot CM, Tove SR, Grunden AM (2010b) Characterization of two proline dipeptidases (prolidases) from the hyperthermophilic archaeon Pyrococcus horikoshii. Appl Microbiol Biotechnol 86(1):177–188
Theriot CM, Semcer RL, Shah SS, Grunden AM (2011) Improving the catalytic activity of hyperthermophilic Pyrococcus horikoshii prolidase for detoxification of organophosphorus nerve agents over a broad range of temperatures. Archaea 2011:5651271–5651279
Tiryaki O, Temur C (2010) The fate of pesticide in the environment. J Biol Environ Sci 4(10):29–38
Trovaslet-Leroy M, Musilova L, Renault F, Brazzolotto X, Misik J, Novotny L, Froment MT, Gillon E, Loiodice M, Verdier L, Masson P, Rochu D, Jun D, Nachon F (2011) Organophosphate hydrolases as catalytic bioscavengers of organophosphorus nerve agents. Toxicol Lett 206:14–23
Tsai PC, Bigley A, Li Y, Ghanem E, Cadieux CL, Kasten SA et al (2010a) Stereoselective hydrolysis of organophosphate nerve agents by the bacterial phosphotriesterase. Biochemistry 49(37):7978–7987
Tsai PC, Fan Y, Kim J, Yang L, Almo SC, Gao YQ, Raushel FM (2010b) Structural determinants for the stereoselective hydrolysis of chiral substrates by phosphotriesterase. Biochemistry 49(37):7988–7997
Tsai PC, Fox N, Bigley AN, Harvey SP, Barondeau DP, Raushel FM (2012) Enzymes for the homeland defense: optimizing phosphotriesterase for the hydrolysis of organophosphate nerve agents. Biochemistry 51(32):6463–6475
Vanhooke JL, Benning MM, Raushel FM, Holden HM (1996) Three dimensional structure of the zinc containing phosphotriesterase with the bound substrate analog diethyl 4-methylbenzylphosphonate. Biochemistry 35:6020–6025
Vyas NK, Nickitenko A, Rastogi VK, Shah SS, Quiocho FA (2010) Structural insights into the dual activities of the nerve agent degrading organophosphate anhydrolase/prolidase. Biochemistry 49(3):547–559
Wanekaya AK, Chenb W, Mulchandani A (2008) Recent biosensing developments in environmental security. J Environ Monit 10:703–712
Worek F, Seeger T, Goldsmith M, Ashani Y, Leader H, Sussman JS, Tawfik D, Thiermann H, Wille T (2014) Efficacy of the rePON1 mutant IIG1 to prevent cyclosarin toxicity in vivo and to detoxify structurally different nerve agents in vitro. Arch Toxicol 88(6):1257–1266
Xiang DF, Kolb P, Fedorov AA, Meier MM, Fedorov LV et al (2009) Functional annotation and three-dimensional structure of Dr0930 from Deinococcus radiodurans, a close relative of phosphotriesterase in the amidohydrolase superfamily. Biochemistry 48:2237–2247
Yang C, Liu N, Guo X, Qiao C (2006) Cloning of mpd gene from a chlorpyrifos-degrading bacterium and use of this strain in bioremediation of contaminated soil. FEMS Microbiol Lett 265:118–125
Yang C, Liu R, Yuan Y, Liu J, Cao X, Qiao C, Song C (2013) Construction of a green fluorescent protein (GFP)-marked multifunctional pesticide-degrading bacterium for simultaneous degradation of organophosphates and γ-hexachlorocyclohexane. J Agric Food Chem 61(6):1328–1334
Zhang R, Cui ZL, Jiang J, He J, Gu X, Li S (2005) Diversity of organophosphorus pesticide-degrading bacteria in a polluted soil and conservation of their organophosphorus hydrolase genes. Can J Microbiol 51(4):337–343
Zhang R, Cui ZL, Zhang X, Jiang J, Gu JD, Li SP (2006) Cloning of the organophosphorus pesticide hydrolase gene clusters of seven degradative bacteria isolated from a methyl parathion contaminated site and evidence of their horizontal gene transfer. Biodegradation 17(5):465–472
Zhang Y, An J, Ye W, Yang G, Qian ZG, Chen HF, Cui L, Feng Y (2012) Enhancing the promiscuous phosphotriesterase activity of a thermostable lactonase (GkaP) for the efficient degradation of organophosphate pesticides. Appl Environ Microbiol 78(18):6647–6655
Zhang Y, An J, Yang GY, Bai A, Zheng B, Lou Z, Wu G, Ye W, Chen HF, Feng Y, Manco G (2015) Active site loop conformation regulates promiscuous activity in a lactonase from Geobacillus kaustophilus HTA426. PLoS ONE 10(2), e0115130
Zhongli C, Shunpeng L, Guoping F (2001) Isolation of methyl parathion-degrading strain M6 and cloning of the methyl parathion hydrolase gene. Appl Environ Microbiol 67(10):4922–4925
Acknowledgments
This research was supported from the Italian Ministry for University and Research (MIUR) (project PON01_01585 to G.M.).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Porzio, E., Del Giudice, I., Manco, G. (2016). Engineering of Extremophilic Phosphotriesterase-Like Lactonases for Biotechnological Applications. In: Rampelotto, P. (eds) Biotechnology of Extremophiles:. Grand Challenges in Biology and Biotechnology, vol 1. Springer, Cham. https://doi.org/10.1007/978-3-319-13521-2_16
Download citation
DOI: https://doi.org/10.1007/978-3-319-13521-2_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-13520-5
Online ISBN: 978-3-319-13521-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)