Abstract
Prior to the identification of diagnostic criteria for adenomyosis using modern imaging, the condition could only be diagnosed in hysterectomy specimens. The use of Magnetic Resonance Imaging and modern Ultrasound created the conditions for less radical treatment. This may include symptomatic treatment for heavy bleeding or dysmenorrhea, gonadotropin-releasing hormone agonists, levonorgestrel-releasing intrauterine device, continuous administration of the oral contraceptive pill, progestogens, danazol or aromatase inhibitors but the evidence base for these options is limited and the disease recurs following discontinuation. Uterine sparing adenomyomectomy, hysteroscopic endomyometrial ablation or uterine artery embolisation may have a role. But these options remain limited because of the often diffuse nature of adenomyosis and because of uncertainty about the effect on future pregnancy
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- GnRH agonists
- Levonorgestrel-releasing intrauterine device
- Oral contraceptive pill
- Progestogens
- Danazol
- Aromatase inhibitors
- Valproic acid
- Bromocriptine
- Hysterectomy
- Uterus-sparing surgery
- Hysteroscopic
- Endomyometrial ablation
Introduction
Recent progress has enabled adenomyosis to be diagnosed in women not undergoing a hysterectomy and it is no longer believed that adenomyosis only affects parous women.
Awareness and knowledge about adenomyosis have grown considerably in recent years. Preoperative diagnosis has become increasingly feasible following the advent of transvaginal ultrasound scanning (TVS) and Magnetic Resonance Imaging (MRI) [1]. As a result, it is now possible to develop individual patient management plans in advance. This has the advantage of being able to take into consideration all available medical and surgical treatment options.
Factors That Influence the Choice of Treatment
There are many important factors to be considered when counseling a patient with adenomyosis.
A critical question is whether to remove or preserve the uterus. Hysterectomy remains the standard treatment for symptomatic adenomyosis if fertility is not an issue. More challenging are cases where women decline hysterectomy or wish to pursue future pregnancy.
Planned intervention should take into consideration the indication for treatment. Symptoms more frequently associated with adenomyosis are dysmenorrhea and menorrhagia, less commonly women present with dyspareunia or chronic pelvic pain, but intervention may not be required in as many as 35 % of women as they are asymptomatic [2]. Adenomyosis identified in the course of infertility investigations creates a challenging dilemma whether or not there are other associated symptoms. These cases are likely to become increasingly common as women delay their first pregnancy into their late 30s or early 40s. Adenomyosis is known to be more common in the fourth and fifth decade of life [3]. A recent review [4] linked adenomyosis to subfertility in women seeking conception after surgery for severe rectovaginal and colorectal endometriosis. In women seeking pregnancy, 7/59 (11.9 %) women with concomitant adenomyosis conceived, compared with 74/172 (43.0 %) in those without adenomyosis. Adenomyosis was associated with a 68 % reduction in the likelihood of pregnancy in women seeking conception after surgery for rectovaginal and colorectal endometriosis [4]. In another meta-analysis of published literature women with adenomyosis had a 28 % reduction in pregnancy rate after IVF/ICSI compared to women without adenomyosis. This reduction is a factor of reduced clinical pregnancy and implantation and increased early pregnancy loss [5].
Adenomyosis may be diffuse involving all or a large part of the myometrium or localized to a limited portion of the myometrium. The extent of adenomyosis is critical to the choice of uterus-sparing excision for adenomyosis.
Also important is the question of coexisting pelvic disease. It is estimated that endometriosis is present in 6–22 % of patients with adenomyosis, and that myomas are present in 35–55 % [6]. Treatment plans may need to be tailored in the presence of coexisting pathology.
Medical Treatment
Traditionally, treatment for heavy periods and dysmenorrhoea has not been tailored to adenomyosis because of the lack of reliable nonsurgical diagnosis. Treatments still include the anti-fibrinolytic agent -tranexamic acid-, non-steroidal anti-inflammatory agents or the combined oral contraceptive pill. Yet, there is no research that specifically addresses the effectiveness of these agents in adenomyosis. Women may have also undergone endometrial destructive procedures (resection, or ablation) only for the condition to be diagnosed in a proportion of those who do not have favourable outcomes.
Medical treatments that specifically target adenomyosis may rely on the observation that the disease is hormone dependent and on the similarities to endometriosis [7]. Although adenomyosis and endometriosis are distinct conditions with different epidemiological features, ectopic endometrium can be a common target for hormonal therapy. Therapies that affect endometriosis can potentially be useful for adenomyosis.
The main limitation of medical treatments is that they are not cytoreductive and available compounds induce regression but not eradication of ectopic endometrium. Consequently, symptoms may recur after treatment is discontinued. Moreover, medical treatments induce atrophy of eutopic endometrium and frequently inhibit ovulation, which are problematic in women seeking pregnancy.
Medical treatments for adenomyosis include gonadotropin-releasing hormone (GnRH) agonists, levonorgestrel-releasing intrauterine device (LNG-IUD), continuous administration of the oral contraceptive pill, progestogens, danazol or aromatase inhibitors.
Experimental medical treatments include valproic acid, which has an antiproliferative action on endometrial and endometriotic cells and bromocriptine, which can prevent prolactin-induced adenomyosis in mice.
GnRH Agonists
GnRH agonists bind to GnRH receptor in the pituitary gland resulting in a reversible hypo-estrogenic hormonal state. The pronounced lowering of estrogen levels explains the efficacy as well as the primary limitation of GnRH agonists. Adverse effects of GnRH agonists include hot flushes and reduced bone mineral density with prolonged treatment. These side effects may be diminished using add-back oral contraceptive pill or hormone replacement therapy [8]. GnRH agonists can be administered via intramuscular or subcutaneous injections, nasal spray and, more recently, orally.
GnRH agonists were among the first drugs used for adenomyosis. Since 1991, case reports or small case series reported that GnRH agonists reduce uterine volume and resolve symptoms associated with adenomyosis [9–11]. Other studies reported successful pregnancies within 6 months of discontinuing GnRH agonists in infertile patients with adenomyosis [12–14]. GnRH agonists have also been administered prior to surgical excision of adenomyosis in an attempt to improve outcome, reduce menstrual blood loss and improve preoperative hematological indices. The reduction of adenomyosis burden may make surgery technically easier, but may also render smaller foci difficult to identify. Postoperative GnRH agonists may have a role in relation to smaller adenomyotic foci that cannot be removed surgically. In one small retrospective study, the use of GnRH agonist in women with adenomyosis (n = 37) was compared to a group (n = 28) who were treated by conservative surgery with or without GnRH agonists. The live birth rate in the group who underwent conservative surgery was higher (32 %) compared to those who received GnRH agonist alone (8 %), the difference was statistically significant (p = 0.022) [15].
Levonorgestrel-Releasing Intrauterine Device
The use of the levonorgestrel intrauterine device (LNG-IUD) which released 20 μg of levonorgestrel per day induces marked atrophy of endometrial glands and stromal decidualization and significantly reduces menstrual blood loss [16]. LNG-IUD improves dysmenorrhea by reduction of prostaglandin within the endometrium [17]. LNG-IUD acts directly on adenomyotic deposits resulting in down-regulation of the oestrogen receptors at least for the first year of use [18], as a result, adenomyotic deposits and the uterus reduce in size. The LNG-IUD is licensed for idiopathic menorrhagia where it can be used for 5 years. Serum levonorgestrel varies in women using the device but may be in the region of 276 ± 119 pg/ml –177 ± 70 pg/ml. A disadvantage of the device is prolonged duration of bleeding and frequent and variable intermenstrual spotting and bleeding especially during the first months of use.
Fedele et al. [19] evaluated the use of LNG-IUD in 25 women with recurrent menorrhagia (blood loss >80 ml) for at least 6 months. Adenomyosis was diagnosed by transvaginal ultrasound and MRI in 9 cases. At the end of the first year of follow-up, all women had reduced blood loss and a rise in haemoglobin. There was a moderate but significant reduction in uterine volume during treatment. Only two patients discontinued treatment prematurely; one due to expulsion of the LNG-IUD and the other had persistent irregular bleeding.
Sheng et al. [20] reported 85 % reduction in the visual analogue scale for dysmenorrhea from 77.9 at baseline to 11.8 after 36 months of the LNG-IUD insertion in women with adenomyosis. In another study, a significant decrease in pain score was reported 3–6 months after LNG-IUD insertion [21].
LNG-IUD has also been used to improve the clinical outcome of endometrial resection in women with adenomyosis-associated menorrhagia. In a retrospective study, 53 women who had a LNG-IUD inserted following endometrial resection were compared to 42 women who underwent a resection and no further treatment. After a 1-year follow-up, all women in the LNG-IUD group compared to only 9 % of women in the control group were amenorrhoeic and no women in the LNG-IUD group and 8 women in the control group required a second endometrial resection. Women in the LNG-IUD group reported a significant reduction in pain symptoms [22]. The data remain limited but the combination of endometrial ablation and LNG-IUD may have a place as an alternative to hysterectomy, while LNG-IUD is a possible option for women who wish to preserve fertility [23].
Oral Contraceptives and Progestogens
The combined oral contraceptive pill (OCP), especially when taken continuously, results in symptom control and inhibits disease progression in women with endometriosis [24], but no studies have evaluated the effectiveness in adenomyosis.
Oral progestogens have also been reported to be effective in the treatment of endometriosis [25]. The mechanism of action may involve modulation of mitotic activity, local growth factors and their receptors or modulation of paracrine and anti-inflammatory reactions [26, 27]. Muneyyrci-Delale et al. [26] evaluated the role of norethindrone acetate (norethisterone, NA), a synthetic progestogen, in the management of adenomyosis. In this study, 28 women with adenomyosis-associated moderate or severe pelvic pain and abnormal uterine bleeding received a low dose of 5 mg of norethisterone based on “three weeks on, one week off” treatment protocol advocated to minimize breakthrough bleeding. There was an 82 % reduction in dysmenorrhea and a 71 % reduction in bleeding score at 3-months and no breakthrough bleeding after 2 months of therapy. This suggests that norethisterone can be a useful medical treatment option but the long-term use requires careful consideration.
Danazol
Danazol is a derivative of ethisterone with progestin-like effects. It exerts its uterine action through induction of a hypogonadotropic state and by a direct interaction with endometrial androgens and progesterone receptors [7] causing atrophy. However, there is limited research on the use of danazol in patients with adenomyosis. For the treatment of endometriosis, danazol is now superseded by GnRH analogues – in part because of the androgenic side effects and because of its association with ovarian cancer [28]. Ishihara et al. demonstrated that systemic treatment with danazol improves adenomyosis-related symptoms and reduces uterine size [29]. A recent study compared the use of low-dose danazol with low-dose dienogest for the long-term treatment of adenomyosis [30], the study suggested that both treatments are effective and safe for the long-term management but details of this small size study are not available. Takebayashi et al. [31] injected 10 mg of danazol into the cervix in patients (n = 22) affected by adenomyosis at 2-week intervals for a period of 12 weeks. They observed a significant improvement in pelvic pain and uterine bleeding and a reduction in uterine size, without significant adverse effects. Igarashi et al. [32] described the effects of the insertion of an intrauterine device loaded with 300–400 mg of danazol in 14 women with symptomatic adenomyosis. The results were encouraging with a reduction in pain and bleeding in the majority of the enrolled patients. In addition, three of the four infertile women conceived after removal of the device. Serum levels of danazol remained below the limit of detection and no systemic side-effects were noted. Despite encouraging preliminary results, it is unlikely that danazol will have a major role in the management of adenomyosis because of the recognised risk profile.
Aromatase Inhibitors
Both eutopic and ectopic endometrium in women affected by endometriosis, adenomyosis, and leiomyomas express aromatase P450, an enzyme implicated in the conversion of C19 androgens to C18 estrogenic steroids [33, 34]. Inhibition of aromatase P450 results in reduction in local estrogens and thus interferes with an important mechanism implicated in the development of the disease [7]. Badawy et al. compared the efficacy of aromatase inhibitor (letrozole) and GnRH agonists in the treatment of adenomyosis. Thirty-three patients were randomly allocated to receive oral letrozole (2.5 mg/day) or a subcutaneous GnRH agonist for 12 weeks. GnRH agonist was more effective than letrozole in relieving chronic pelvic pain, dysmenorrhea, menorrhagia, metrorrhagia and dyspareunia, but the difference was not significant. They claimed that no women in the letrozole group had hot flushes. Worryingly, two women in the letrozole group became pregnant whilst on treatment [35].
Experimental Drugs
Valproic Acid
Valproic acid (VPA) is a specific and potent histone deacetylase inhibitor (HDI), approved by the US Food and Drug Administration for the treatment of epilepsy. In 2010 Liu et al. [36] published a case series on the efficacy of the off-label use of VPA in the treatment of adenomyosis, with encouraging results. They recruited 12 patients with confirmed adenomyosis, dysmenorrhea and an enlarged uterus. All patients received VPA for 3 months and afterwards they were randomly assigned to either the insertion of a LNG- IUD or to no further treatment. There was a significant reduction in uterine size and dysmenorrhea at 6 months in both groups. VPA efficacy could be related to its antiproliferative action on endometrial and endometriotic cells or to its ability to impair myogenesis. In addition HDIs have been reported to suppress growth factors, like vascular endothelial growth factor which play a key role in regulating uterine bleeding and could be involved in adenomyosis related menorrhagia. Studies on the effectiveness of VPA for adenomyosis remain very limited, and no information is available on the medium- and long- term effects.
Bromocriptine
In a mouse, anterior pituitary isografting results in raised prolactin and adenomyosis [37]. Pretreatment with bromocriptine-mesilate (a suppressor of pituitary prolactin) between 4 and 8 weeks of age decreased the incidence of adenomyosis to the level of controls. This led to speculation of a possible role in the human, but no studies have been published that explored the putative effect.
Surgical Treatment
Hysterectomy
Hysterectomy is the definitive cure for women affected by adenomyosis-related dysmenorrhea and menorrhagia who do not desire a future pregnancy and who accept the operation. Hysterectomy should be performed laparoscopically or vaginally whenever technically feasible. Factors to be considered are uterine size, parity and the surgeon’s experience. However, vaginal hysterectomy in women with adenomyosis has been associated with an increased rate of bladder injuries when compared to women with uterine fibroids [38]. This may be due to difficult dissection of the vesicovaginal and vesicuterine planes in the anterior adenomyosis or to the presence of deep infiltrating endometriosis of the vesico-uterine pouch [39]. Hysterectomy for uterine adenomyosis may be technically challenging in cases associated with pelvic disease such as rectovaginal endometriosis and adhesions. In these cases, laparoscopic hysterectomy may be feasible.
However, although laparoscopic hysterectomy seems to be effective in reducing the rate of bladder injuries, it is associated with a higher ureteric injury rate compared to vaginal hysterectomy [40]. No studies have evaluated the possible role of preoperative administration of GnRH agonists in reducing intraoperative complications.
Uterus-Sparing Surgery for Adenomyosis
Uterus-Sparing Surgery for Focal Adenomyosis
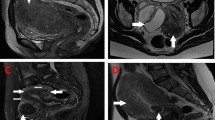
Focal adenomyosis is characterized by localized lesions that are distinct from the surrounding normal myometrium [41]. Focal adenomyosis has been referred to as adenomyoma reflecting some similarity to leiomyoma. The surgical technique for adenomyomectomy can be more challenging than myomectomy because of the lack of a demarcation plane in adenomyosis. The first series of adenomyomectomies were performed through laparotomy [42–44] but more recent series demonstrated that most cases can be safely and effectively performed laparoscopically. The surgical steps, as shown in Fig. 12.1, are as follows: (1) an incision is made along the uterine wall overlying the adenomyoma; (2) the adenomyotic lesion is dissected from the surrounding myometrium by gentle sharp dissection. This step requires the use of sharp dissection or monopolar cautery to separate the adenomyoma from the healthy myometrium (Fig. 12.1b–d). This can be more readily achieved in cases of focal adenomyosis but in more diffuse disease some affected tissue has to be preserved to allow satisfactory uterine reconstruction; (3) Closure of the uterine cavity if it was opened during dissection; (4) Closure of the uterine incision (Fig. 12.1f). An alternative technique to adenomyomectomy involves wedge resection of the adenomyoma. In published surgical series, wedge resection is less frequently performed compared to adenomyomectomy as it may not be sufficiently effective in removing lesions. One study reported comparable outcomes in terms of symptom control, but wedge resection was associated with a significantly higher rate of sonographic recurrences (69.2 % vs. 15.0 %; P < 0.001) [45].
(a) Laparoscopic view of the uterus with a posterior adenomyoma of 6 cm. (b, c) Sharp excision of the adenomyoma. Typically, a cleavage plane from healthy myometrium is absent. (d) After the removal of the adenomyoma, residual adenomyotic tissue is removed in order to minimize residual disease. (e) Adenomyoma is removed from the abdominal cavity. (f) Uterine myometrium and serosa are closed with multiple interrupted suture
Table 12.1 summarizes the outcome of adenomyomectomy in published medium sized and large series, excluding individual case reports and small series of less than five cases. The five studies reviewed here included 497 patients [45–49]. Overall, reduction of dysmenorrhea was achieved in 97 % of cases and the mean pain reduction was 70 %. Reduction of menorrhagia was achieved in 93 % of cases with a mean reduction of bleeding of 65 %. At a mean follow-up of 23 months, relapse of symptoms occurred in 16 % of cases. At a mean follow up of 27 month, there were 75 (34 %) conceptions and the live birth rate was 28 % (Table 12.2) amongst the 221 women wishing to conceive following adenomyomectomy [45–48, 50].
Uterus-Sparing Surgery for Diffuse Adenomyosis
Diffuse adenomyosis typically involves the myometrium with digitations and irregularly shaped lesions with unclear borders. Consequently, complete excision of adenomyotic tissue is not technically possible and in most instances there will be some loss of healthy myometrium. Excision of diffuse adenomyosis is best performed via laparotomy because digital palpation of the uterus is fundamental to the identification of affected areas. This allows selective and piecemeal removal of lesions whilst sparing unaffected myometrium. A few surgical techniques have been described which differ mainly in the type of uterine incision used and in the approach used for uterine reconstruction. Most cases can be done through a transverse laparotomy. A tourniquet placed to compress the uterine arteries reduces blood loss. Adenomyosis lesions can be exposed by bisecting the uterus longitudinally in the midline from the fundus to the endometrial cavity. The index finger is introduced into the endometrial cavity to protect and guide the excision of the lesion [51]. But whilst some authors reported routinely opening the uterine cavity [52, 53], others count breach of the endometrium as a surgical complication [54]. Adenomyotic tissue is resected with the aid of digital palpation. Preservation of at least 1–1.5 cm of myometrial thickness is needed for uterine reconstruction. The endometrial cavity is then closed with interrupted sutures and the myometrium is re-approximated. Reconstruction following extensive ademyomectomy can be particularly challenging. Multiple layers of interrupted sutures are always required for a good repair and particular attention should be paid to avoid intramural dead spaces which can lead to a weak scar with implications on future pregnancies.
Modification of dysmenorrhea and menorrhagia was evaluated in six studies involving 296 women who underwent excision of diffuse adenomyosis [15, 52–56]. Overall, dysmenorrhea and menorrhagia were reduced in 46 % and 69 % of cases respectively with a mean improvement of 60 %. Symptoms relapsed in 5 % of cases after a mean follow-up of 22 months (Table 12.3). Fertility outcomes were reported in seven studies [15, 43, 44, 52–55] involving 219 women wishing to conceive after excision of diffuse adenomyosis. The conception and live birth rates were 36 % and 28 % respectively over a mean follow up of 25 month (Table 12.4).
Comparison of Outcomes of Uterus Sparing Surgery for Focal or Diffuse Adenomyosis
Available evidence suggests that excision of adenomyosis is associated with a 60–70 % reduction in dysmenorrhea and menorrhagia. However, the proportion of women experiencing such postoperative symptomatic relief at 2 years is higher (90 %) in women with focal adenomyosis, as compared to the lower symptomatic relief of dysmenorrhea (46 %) and of menorrhagia (69 %) in women with diffuse adenomyosis. As for fertility outcome, there was no difference between women who underwent excision of focal or diffuse adenomyosis, with an identical live birth rate of 28 % in both groups. In comparison, Grimbizis et al., reported a delivery rate of 50 % (74 of 147) for total adenomyomectomy and 34 % (11 of 32) for partial adenomyomectomy [41]. Maheshwari et al. reported an overall live birth rate of 36 % (21 of 58) following conservative surgery for adenomyosis [57]. There is a wide variation in fertility outcome reported by studies of conservative surgical treatment, with live birth rates ranging from 1 % [48] to 73 % [46]. It is difficult to ascertain if this reflects differences in surgical technique or in patient characteristics.
The potential risks of uterine-sparing surgery for adenomyosis include uterine rupture, Asherman syndrome, uterine deformities and reduced uterine capacity. However, the incidence of these complications is unknown. There thus remains a need for well-designed, prospective clinical trials.
It is possible that decidualization of residual adenomyotic fragments further weakens the scar leading to uterine rupture during pregnancy [58]. Attention to myometrial reconstruction may reduce the risk of rupture. Recently, Saremi et al. reported a series of 103 women who underwent excision of diffuse adenomyosis through laparotomy, 70 of whom wished to conceive. There were 21 pregnancies including 16 that reached term. The residual myometrial thickness was at least 0.5 mm [53]. There were 2 (9.5 %) cases of uterine rupture which occurred at 32 and 37 weeks. Postoperative Asherman syndrome was diagnosed in 4 out of 103 (3.9 %) patients. Osada et al. described a similar surgical technique, but preserved at least 1 cm of myometrial thickness. They reported no uterine rupture among 14 women who subsequently had a term delivery [52]. Until more data becomes available, it is advisable to preserve at least 1 cm of myometrial thickness for uterine reconstruction.
In conclusion, the decision to proceed with surgical excision of extensive adenomyosis in women desiring future pregnancy should only be taken after extensive counseling and consideration of alternatives and there should be a low index of suspicion with regards to uterine rupture in women who conceive after uterine sparing surgery [59].
Hysteroscopic Endomyometrial Ablation/Resection
Hysteroscopic endomyometrial resection has been, perhaps also inadvertently, performed for the treatment of adenomyosis-related menorrhagia in women not desiring future pregnancy. The rationale for hysteroscopic treatment is based on the assumption that adenomyosis located at the endomyometrial junction is responsible of excessive uterine bleeding. McCausland and McCausland [60] demonstrated that the success rate of rollerball endometrial resection was inversely correlated with the depth of penetration of endometrium within the myometrium. Patients with superficial adenomyosis with penetration <2 mm experienced postoperative amenorrhea or light menses, whereas patients with deep adenomyosis (penetrating >2 mm) experienced poor outcomes and ultimately required a hysterectomy. Endometrial ablation >3 mm is contraindicated due to the presence of significant myometrial arteries approximately 5 mm deep within the myometrium [61]. Wood (1998) reported that 10 out of 18 (55 %) patients who underwent endometrial ablation for adenomyosis-related menorrhagia were free of symptoms at 24 months follow-up [61]. In another study evaluating hysteroscopic roller-ball ablation in 190 patients with adenomyosis, 98 % of treated women reported improved symptoms and 3 % required a hysterectomy [62]. The cause of failure of hysteroscopic endomyometrial ablation may be that deeper ectopic endometrial glands persist and eventually regrow causing recurrent menorrhagia or new onset dysmenorrhea. Alternative treatment options for women who had failed ablation include repeat ablation [63], insertion of LNG-IUD [22] or hysterectomy.
Uterine Artery Embolization
Fifteen studies published between 1999 and 2010, evaluated the outcomes of uterine artery embolization (UAE) in the treatment of symptomatic adenomyosis. These included 511 affected women [64]. Overall, 387 patients (75.7 %) had symptomatic improvement in bleeding, pain and bulk-related discomfort in long term follow-up (median follow-up of 26.9 months). Recent studies have confirmed these findings. Froeling et al. [65] and Smeets et al. [66] reported similar outcomes: 29 out of 40 (72.5 %) women treated with UAE for symptomatic adenomyosis were free of symptoms after a follow up of 40 and 65 months. Ten patients underwent hysterectomy because of failed UAE in the study by Froeling et al. (2012) and 7 needed a hysterectomy in the study by Smeets et al., (2012). Similarly, Nijenhuis et al. [67] reported that 22 out of 29 (76 %) patients with severe adenomyosis-related symptoms, were asymptomatic 37 months after UAE.
No data are available regarding fertility among women with adenomyosis following UAE. However, a recent review evaluated existing evidence about fertility after UAE for uterine fibroids. Out of 738 women affected by uterine fibroids who expressly wished to conceive at the time of UAE, fertility, miscarriage and take-home baby rates were 36.3, 29.7 and 19.6 %, respectively [68]. This study by Torre et al., also prospectively evaluated pregnancy rates in a cohort of 66 women who desired future pregnancy and who were treated with UAE for symptomatic fibroids. Fibroid symptoms including menorrhagia, metrorrhagia, pain and bulk syndrome were significantly improved after UAE. However, in spite of seeking pregnancy of 33.4 ± 14.5 months, only 1 out of 31 women became pregnant and she finally miscarried. In this series, 5 patients resorted to in-vitro fertilization and 22 embryos were transferred, including 8 embryos from oocyte donation. No pregnancy was achieved, suggesting deficient implantation. Based on the low pregnancy rates, the authors concluded that UAE might have had a negative impact on fertility and suggested that UAE should not be performed routinely in young women of childbearing age with extensive fibroids.
In conclusion, UAE may improve symptoms in more than 70 % of women with adenomyosis but future fertility rates and obstetric outcomes are unknown and need to be assessed by means of randomized controlled trials.
Conclusions
Medical treatments for uterine adenomyosis remain poorly investigated. Most existing medical treatment options have been evaluated only in short-term studies involving small populations. However, what we know from the little data available and from more extensive experience with hypo-estrogenizing drugs for the treatment of endometriosis, is that medical therapies are effective for as long as they are administered, and symptoms may promptly recur after treatment is discontinued. Therefore, although adenomyosis manifests at a more advanced age compared to endometriosis, medical treatment of adenomyosis may need to be administered long term, hypothetically from onset of symptoms to the time when affected women seek pregnancy or to the onset of the menopause.
Hypo-estrogenic status can be induced by GnRH analogues, which are reportedly very effective in the treatment of adenomyosis-related menorrhagia and dysmenorrhea. However, the use of these drugs in the long term is limited by significant adverse effects including hot flushes and osteoporosis as well as by high costs. In comparison, inexpensive and well-tolerated drugs such as progestogens, if proven effective and safe in the long term, might represent an alternative. Prospective studies are needed to evaluate the efficacy of progestogens and estro-progestogens in the long term. Finally, a promising option is the LNG-IUD, which reportedly decreases or eliminates symptoms of dysmenorrhea and menorrhagia, with a low adverse effects profile.
Although endometrial ablation have been reported to improve symptoms in a high proportion of women with adenomyosis [62], this technique has the major disadvantages of being indicated only for superficial disease (penetrating < 2 mm) and of precluding the possibility of a future pregnancy.
In light of the availability of effective medical treatment options, uterus sparing surgery in patients not wanting children has a very limited place. In fact, postoperative symptom relief may be comparable to medical treatment alone. Adenomyomectomy or wedge resection may be as burdensome for patients as a hysterectomy, but with a lower short and long term success rates. In women not desiring a pregnancy, when medical and possibly hysteroscopic treatments have failed, hysterectomy should be considered as the definitive treatment.
Uterus-sparing surgery has a role in women affected by adenomyosis who desire future pregnancy. The only study comparing surgery plus GnRH agonists and GnRH agonists alone showed higher pregnancy rates in the surgical group [15]. Nowadays, the demand for conservative, fertility-enhancing surgical treatment of adenomyosis is increasing because more women delay their first pregnancy until 30 or 40 years of age. Results are difficult to compare between surgical series but it seems that live birth rate after excision of adenomyosis may be around 30 %. It has to be kept in mind that surgical excision of adenomyosis is technically demanding, especially in cases of diffuse disease, and that myometrial reconstruction has to be performed meticulously, leaving at least 1 cm of myometrial thickness and no intramural dead spaces. Finally, women have to be extensively counseled about the risk of uterine rupture in a future pregnancy.
References
Luciano DE, Exacoustos C, Albrecht L, LaMonica R, Proffer A, Zupi E, Luciano AA. Three-dimensional ultrasound in diagnosis of adenomyosis: histological correlation with ultrasound targeted biopsies of the uterus. J Minim Invasive Gynecol. 2013;20:803–10.
Garcia L, Isaacson K. Adenomyosis: review of the literature. J Minim Invasive Gynecol. 2011;18:428–37.
Azzi R. Adenomyosis: current perspectives. Obstet Gynecol Clin North Am. 1989;16:221–35.
Vercellini P, Consonni D, Barbara G, Buggio L, Frattaruolo MP, Somigliana E. Adenomyosis and reproductive performance after surgery for rectovaginal and colorectal endometriosis: a systematic review and meta-analysis. Reprod Biomed Online. 2014;28(6):704–13.
Vercellini P, Consonni D, Dridi D, Bracco B, Frattaruolo MP, Somigliana E. Uterine adenomyosis and in vitro fertilization outcome: a systematic review and meta-analysis. Hum Reprod. 2014;29:964–77.
Ferenczy A, Bronsen I. Pathophysiology of adenomyosis. Hum Reprod Update. 1998;4:312–22.
Fedele L, Bianchi S, Frontino G. Hormonal treatments for adenomyosis. Best Pract Res Clin Obstet Gynaecol. 2008;22:333–9.
Wu D, Hu M, Hong L, Hong S, Ding W, Min J, Fang G, Guo W. Clinical efficacy of add-back therapy in treatment of endometriosis: a meta-analysis. Arch Gynecol Obstet. 2014;290(3):513–23.
Grow DR, Filer RB. Treatment of adenomyosis with long-term GnRH analogues: a case report. Obstet Gynecol. 1991;78:538–9.
Nelson J, Corson S. Long term management of adenomyosis with a gonadotropin releasing hormone agonist: a case report. Fertil Steril. 1993;59:441–3.
Huang F, Kung F, Chang S, et al. Effects of short-course buserelin therapy on adenomyosis: a report of two cases. J Reprod Med. 1999;44:741–4.
Hirata J, Moghissi K, Ginsburg K. Pregnancy after medical therapy of adenomyosis with gonadotropin-releasing hormone agonist. Fertil Steril. 1993;59:444–5.
Silva P, Perkins H, Schauberger C. Live birth after treatment of severe adenomyosis with a gonadotropin-releasing hormone agonist. Fertil Steril. 1994;61:171–2.
Ozaki T, Takahashi K, Okada M, et al. Live birth after conservative surgery for severe adenomyosis following magnetic resonance imaging and gonadotropin-releasing hormone agonist therapy. Int J Fertil Womens Med. 1999;44:260–4.
Wang PH, Fuh JL, Chao HT, Liu WM, Cheng MH, Chao KC. Is the surgical approach beneficial to subfertile women with symptomatic extensive adenomyosis? J Obstet Gynaecol Res. 2009;35:495–502.
Bergholt T, Eriksen L, Berendt N, Jacobsen M, Hertz JB. Prevalence and risk factors of adenomyosis at hysterectomy. Hum Reprod. 2001;16:2418–21.
Cameron IT, Leask R, Kelly RW, Baird DT. The effects of danazol, mefenamic acid, norethisterone and a progesterone-impregnated coil on endometrial prostaglandin concentrations in women with menorrhagia. Prostaglandins. 1987;34:99–110.
Critchley HO, Wang H, Kelly RW, Gebbie AE, Glasier AF. Progestin receptor isoforms and prostaglandin dehydrogenase in the endometrium of women using a levonorgestrel-releasing intrauterine system. Hum Reprod. 1998;13:1210–7.
Fedele L, Bianchi S, Raffaelli R, Portuese A, Dorta M. Treatment of adenomyosis-associated menorrhagia with a levonorgestrel-releasing intrauterine device. Fertil Steril. 1997;68:426–9.
Sheng J, Zhang Q, Zhang J. The LNG-IUS study on adenomyosis: a 3 year follow-up study on the efficacy and side effects of the use of levonorgestrel intrauterine system for the treatment of dysmenorrhea associated with adenomyosis. Contraception. 2009;79:189–93.
Bragheto A, Caserta N, Bahamondes L, et al. Effectiveness of levonorgestrel-releasing intrauterine system in the treatment of adenomyosis diagnosed and monitored by MRI. Contraception. 2007;76:195–9.
Maia Jr H, Maltez A, Coelho G, et al. Insertion of Mirena after endometrial resection in patients with adenomyosis. J Am Assoc Gynecol Laparosc. 2003;10:512–6.
Farquhar C, Brosens I. Medical and surgical management of adenomyosis. Best Pract Res Clin Obstet Gynecol. 2006;20:603–16.
Vercellini P, Trespidi L, Colombo A, Vendola N, Marchini M, Crosignani PG. A gonadotropin-releasing hormone agonist versus a low-dose oral contraceptive for pelvic pain associated with endometriosis. Fertil Steril. 1993;60(1):75–9.
Vercellini P, Fedele L, Pietropaolo G, Frontino G, Somigliana E, Crosignani PG. Progestogens for endometriosis: forward to the past. Hum Reprod Update. 2003;9(4):387–96.
Muneyyirci-Delale O, Chandrareddy A, Mankame S, Osei-Tutu N, Hv G. Norethindrone acetate in the medical management of adenomyosis. Pharmaceuticals (Basel). 2012;22:1120–7.
Schweppe KW. The place of dydrogesterone in the treatment of endometriosis and adenomyosis. Maturitas. 2009;65:23–7.
Cottreau CM, Ness RB, Modugno F, Allen GO, Goodman MT. Endometriosis and its treatment with danazol or lupron in relation to ovarian cancer. Clin Cancer Res. 2003;9:5142–4.
Ishihara H, Kitawaki J, Kado N. Gonadotropin-releasing hormone agonist and danazol normalize aromatase cytochrome P450 expression in eutopic endometrium from women with endometriosis, adenomyosis, or leiomyomas. Fertil Steril. 2003;79:735–42.
Sasa H, Imai K, Suzuki A, Makimura N, Furuva K. Comparison of low-dose dienogest with low-dose danazol for long-term treatment of adenomyosis. Obstet Gynecol. 2014;123 Suppl 1:97S–8.
Takabeyashi T, Funjino Y, Umeski N, Ogita S. Danazol suspension injected into the uterine cervix of patients with adenomyosis and myoma. Preliminary study. Gynecol Obstet Invest. 1995;39:207–11.
Igarashi M, Abe Y, Fukada N, Ando A, Miyasaka M, Yoshida M, Shawki OA. Novel conservative medical therapy for uterine adenomyosis with a danazol-loaded intrauterine device. Fertil Steril. 2000;74:412–3.
Noble LS, Simpson ER, Johns A, Bulun SE. Aromatase expression in endometriosis. J Clin Endocrinol Metab. 1996;81:174–9.
Bulun SE, Fang Z, Imir G, Gurates B, Tamura M, Yilmaz B, et al. Aromatase and endometriosis. Semin Reprod Med. 2004;22:45–50.
Badawy AM, Elnashar AM, Mosbah AA. Aromatase inhibitors or gonadotropin-releasing hormone agonists for the management of uterine adenomyosis: a randomized controlled trial. Acta Obstet Gynecol Scand. 2012;91:489–95.
Liu X, Yuan L, Guo SW. Valproic acid as a therapy for adenomyosis: a comparative case series. Reprod Sci. 2010;17:904–12.
Mori T, Kawashima S, Nagasawa H. Induction of uterine adenomyosis by pituitary grafting and retardation of its development by bromocriptine-mesilate (CB-154) in BALB/c mice. In Vivo. 1991;5:107–9.
Furuhashi M, Miyabe Y, Katsumata Y, et al. Comparison of complications of vaginal hysterectomy in patients with leiomyomas and in patients with adenomyosis. Arch Gynecol Obstet. 1998;262:69–73.
Fedele L, Piazzola E, Raffaelli R, Bianchi S. Bladder endometriosis: deep infiltrating endometriosis or adenomyosis? Fertil Steril. 1998;69:972–5.
Garry R, Gountain J, Mason S, et al. The eVALuate study: two parallel randomized trials, one comparing laparoscopic with abdominal hysterectomy, the other comparing laparoscopic with vaginal hysterectomy. BMJ. 2004;8:494–591.
Grimbizis GF, Mikos T, Tarlatzis B. Uterus-sparing operative treatment for adenomyosis. Fertil Steril. 2014;101:472–87.
Fedele L, Bianchi S, Zanotti F, Marchini M, Candiani GB. Fertility after conservative surgery for adenomyomas. Hum Reprod. 1993;8:1708–10.
Strizhakov AN, Davydov AI. Myometrectomy–a method of choice for the therapy of adenomyosis patients in the reproductive period. Akush Ginekol (Mosk). 1995;5:31–3.
Tadjerouni A, Henry-Suchet J, Loysel T, Bergeron N. Adenomyosis and infertility surgical treatment. Gynecol Rev Gynecol. 1995;3:380–6.
Sun AJ, Luo M, Wang W, Chen R, Lang JH. Characteristics and efficacy of modified adenomyomectomy in the treatment of uterine adenomyoma. Chin Med J (Engl). 2011;124:1322–6.
Wang PH, Liu WM, Fuh JL, Cheng MH, Chao HT. Comparison of surgery alone and combined surgical-medical treatment in the management of symptomatic uterine adenomyoma. Fertil Steril. 2009;92:876–85.
Takeuchi H, Kitade M, Kikuchi I, Shimanuki H, Kumakiri J, Kitano T, et al. Laparoscopic adenomyomectomy and hysteroplasty: a novel method. J Minim Invasive Gynecol. 2006;13:150–4.
Dai Z, Feng X, Gao L, Huang M. Local excision of uterine adenomyomas: a report of 86 cases with follow-up analyses. Eur J Obstet Gynecol Reprod Biol. 2012;161:84–7.
Liu M, Cheng Z, Dai H, Qu X, Kang L. Long-term efficacy and quality of life associated with laparoscopic bilateral uterine artery occlusion plus partial resection of symptomatic adenomyosis. Eur J Obstet Gynecol Reprod Biol. 2014;176:20–4.
Al Jama FE. Management of adenomyosis in subfertile women and pregnancy outcome. Oman Med J. 2011;26:178–81.
Fedele L, Bianchi S, Zanconato G, Carinelli S, Berlanda N. Conservative treatment of diffuse uterine leiomyomatosis. Fertil Steril. 2004;82(2):450–3.
Osada H, Silber S, Kakinuma T, Nagaishi M, Kato K, Kato O. Surgical procedure to conserve the uterus for future pregnancy in patients suffering from massive adenomyosis. Reprod Biomed Online. 2011;22:94–9.
Saremi A, Bahrami H, Salehian P, Hakak N, Pooladi A. Treatment of adenomyomectomy in women with severe uterine adenomyosis using a novel technique. Reprod Biomed Online. 2014;28(6):753–60.
Fujishita A, Masuzaki H, Khan KN, Kitajima M, Ishimaru T. Modified reduction surgery for adenomyosis: a preliminary report of the transverse H incision technique. Gynecol Obstet Invest. 2004;57:132–8.
Nishida M, Takano K, Arai Y, Ozone H, Ichikawa R. Conservative surgical management for diffuse uterine adenomyosis. Fertil Steril. 2010;94:715–9.
Kim JK, Shin CS, Ko YB, Nam SY, Yim HS, Lee KH. Laparoscopic assisted adenomyomectomy using double flap method. Obstet Gynecol Sci. 2014;57(2):128–35.
Maheshwari A, Gurunath S, Fatima F, Bhattacharya S. Adenomyosis and subfertility: a systematic review of prevalence, diagnosis, treatment and fertility outcomes. Hum Reprod Update. 2012;18:374–92.
Ukita S. Total uterine rupture during pregnancy after adenomyomectomy. Am J Case Rep. 2011;12:106–9.
Pepas L, Deguara C, Davis C. Update on the surgical management of adenomyosis. Curr Opin Obstet Gynecol. 2012;24(4):259–64.
McCausland AM, McCausland VM. Depth of endometrial penetration in adenomyosis helps determine outcome of rollerball ablation. Am J Obstet Gynecol. 1996;174:1786–94.
Wood C. Surgical and medical treatment of adenomyosis. Hum Reprod Update. 1998;4:323–36.
Preutthipan S, Herabutya Y. Hysteroscopic rollerball endometrial ablation as an alternative treatment for adenomyosis with menorrhagia and/or dysmenorrhea. J Obstet Gynaecol Res. 2010;36:1031–6.
Wortman M, Daggett A. Reoperative hysteroscopic surgery in the management of patients who fail endometrial ablation and resection. J Am Assoc Gynecol Laparosc. 2001;8:272–7.
Popovic M, Puchner S, Berzaczy D, Lammer J, Bucek RA. Uterine artery embolization for the treatment of adenomyosis: a review. J Vasc Interv Radiol. 2011;22(7):901–9.
Froeling V, Scheurig-Muenkler C, Hamm B, Kroencke TJ. Uterine artery embolization to treat uterine adenomyosis with or without uterine leiomyomata: results of symptom control and health-related quality of life 40 months after treatment. Cardiovasc Intervent Radiol. 2012;35(3):523–9.
Smeets AJ, Nijenhuis RJ, Boekkooi PF, Vervest HA, van Rooij WJ, Lohle PN. Long-term follow-up of uterine artery embolization for symptomatic adenomyosis. Cardiovasc Intervent Radiol. 2012;35(4):815–9.
Nijenhuis RJ, Smeets AJ, Morpurgo M, Boekkooi PF, Reuwer PJ, Smink M, van Rooij WJ, Lohle PN. Uterine artery embolisation for symptomatic adenomyosis with polyzene F-coated hydrogel microspheres: three-year clinical follow-up using UFS-QoL questionnaire. Cardiovasc Intervent Radiol. 2014;2.
Torre A, Paillusson B, Fain V, Labauge P, Pelage JP, Fauconnier A. Uterine artery embolization for severe symptomatic fibroids: effects on fertility and symptoms. Hum Reprod. 2014;29:490–501.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Berlanda, N., Buggio, L., Vercellini, P. (2016). Current Treatment for Adenomyosis. In: Habiba, M., Benagiano, G. (eds) Uterine Adenomyosis. Springer, Cham. https://doi.org/10.1007/978-3-319-13012-5_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-13012-5_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-13011-8
Online ISBN: 978-3-319-13012-5
eBook Packages: MedicineMedicine (R0)