Abstract
Purpose
To evaluate the clinical outcome for uterine adenomyosis with or without uterine leiomyomata 40 months after uterine artery embolization (UAE).
Methods
Forty women aged 39–56 years (median 46 years) with symptomatic uterine adenomyosis and magnetic resonance imaging findings of uterine adenomyosis with or without combined uterine leiomyomata underwent UAE. Self-perceived changes in clinical symptoms were assessed, and residual symptom severity and health-related quality of life (HRQOL) after UAE were evaluated. Clinical failure was defined as no symptomatic improvement or second invasive therapy after UAE. Results were stratified by the extent of uterine adenomyosis at baseline magnetic resonance imaging.
Results
Patients were followed for a median of 40 months (range 5–102 months). UAE led to symptomatic control after UAE in 29 (72.5%) of 40 patients while 11 women underwent hysterectomy (n = 10) or dilatation and curettage (n = 1) for therapy failure. No significant difference between women with pure uterine adenoymosis and women with uterine adenomyosis combined with uterine leiomyomata was observed. Best results were shown for UAE in uterine adenomyosis with uterine leiomyomata predominance as opposed to predominant uterine adenomyosis with minor fibroid disease (clinical failure 0% vs. 31.5%, P = 0.058). Throughout the study group, HRQOL score values increased and symptom severity scores decreased after UAE. Least improvement was noted for women with pure adenomyosis.
Conclusions
UAE is clinically effective in the long term in most women with uterine adenomyosis. Symptomatic control and HRQOL were highest in patients with combined disease of uterine adenomyosis but leiomyomata predominance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Adenomyosis of the uterus is defined as the presence of ectopic endometrial glands and stroma within the myometrium [1]. This benign disease has either a focal or diffuse distribution and occurs alone or, more frequently, in up to 55% of cases, in combination with uterine leiomyomata [2]. Clinical symptoms include menorrhagia, dysmenorrhea, and dyspareunia. The clinical manifestations of uterine adenomyosis are similar to those caused by uterine leiomyomata [3]. Magnetic resonance imaging (MRI) is highly reliable for the detection and differentiation of these two entities, with a sensitivity and specificity ranging 70–86% and 77–92% [4–7]. Although clinical manifestations of uterine leiomyomata and uterine adenomyosis are similar, their treatment may differ. In addition to medical treatment and hysterectomy, uterine-sparing treatment options such as myomectomy, hysteroscopic resection, and uterine artery embolization (UAE) are well-established treatment options for symptomatic leiomyomata [8].
The clinical success rate of UAE for uterine leiomyomata with respect to symptomatic improvement of associated menorrhagia and pelvic pain ranges from 85–95% to 80–90% [9, 10]. According to the literature, there is a 25% chance of failure of symptom control or recurrence after UAE for uterine leiomyomata over the course of a 5-year follow-up period [11].
Uterine adenomyosis, on the other hand, usually requires hysterectomy because of poor results of hormone treatment or endometrial ablation [12]. Several studies have reported symptomatic improvement after UAE in women with uterine leiomyomata and uterine adenomyosis, so investigators have proposed UAE for managing symptomatic uterine adenomyosis. Despite reports of successful short-term and midterm results of UAE for symptomatic uterine adenomyosis, there is still a paucity of longer follow-up results [13–17].
The purpose of our retrospective study was to evaluate the clinical outcome and health-related quality of life (HRQOL) of women undergoing UAE for uterine adenomyosis with or without uterine leiomyomata after 40 months.
Methods
Study Design and Study Population
This study was a retrospective review of data in a prospectively collected database. The institutional review board approved the entire study, and each patient provided written informed consent. At our institution, all patients who are potential candidates for UAE are evaluated with MRI. Women were eligible for the study if they had dominant menorrhagia, dysmenorrhea with or without bulk symptoms, and a MRI diagnosis of pure (isolated) uterine adenomyosis or uterine adenomyosis coexisting with uterine leiomyomata. A previous attempt of surgical or medical therapy was not a prerequisite, but symptoms had to be severe enough to warrant consideration of hysterectomy. Eligibility was restricted to premenopausal women not desiring to become pregnant. Patients were ineligible for the study if they had an active pelvic inflammatory disease or an undiagnosed pelvic mass, were pregnant, or had laboratory findings consistent with renal insufficiency.
A total of 64 women with symptomatic adenomyosis (menorrhagia, dysmenorrhea, or bulk symptoms) who were treated by UAE in the years of 2001 to 2009 were identified, and 40 women could be followed up with a validated questionnaire for evaluating symptom severity and HRQOL by a telephone interview performed especially for this study. There was no baseline evaluation of the HRQOL at the same time point before UAE. The presence of clinical symptoms was noted at baseline before UAE on a yes/no basis. The median age of the 40 women further evaluated in this study was 46 years (range 39–56 years). At the time of UAE, all women were premenopausal. At the time of treatment, the symptoms were menorrhagia (n = 36), dysmenorrhea (n = 34), and bulk symptoms (n = 30). Demographics, clinical symptoms at baseline, and MRI findings before UAE are listed in Table 1.
Imaging before Embolization
All patients underwent MRI before UAE with a 1.5-T scanner (Magnetom Vision or Symphony, Siemens Medical Systems, Erlangen, Germany) using a phased-array body coil. Breath-hold T2-weighted half-Fourier acquisition single-shot turbo spin echo sequences (T2 HASTE, TR ∞,TE 65 ms, flip angle 150°) in the sagittal, coronal, and axial planes as well as turbo spin echo sequences (T2-TSE, TR 5200 ms, TE 115 ms) in the transaxial and sagittal planes were performed. To ensure optimal image quality, butylscopolamine (30 mg) was provided intramuscularly for bowel relaxation.
Magnetic resonance (MR) images were analyzed independently by two reviewers experienced in MRI of the female pelvis. Disagreements in interpretation were resolved by consensus. For the diagnosis of uterine adenomyosis, the established criterion of a junctional zone thickness exceeding 12 mm in maximum diameter had to be present. The presence of foci of high signal intensity within the myometrium constituted an additional but not a mandatory criterion. Findings of adenomyosis were further subclassified as focal or diffuse (Fig. 1).
On the basis of MRI findings, three different groups of uterine adenomyosis with and without combined uterine leiomyomata were identified: pure uterine adenomyosis, uterine adenomyosis with leiomyomata predominance, and uterine leiomyomata with adenomyosis predominance. We defined the subgroups of adenomyosis and coexisting uterine leiomyomata by MRI and clinical criteria as follows.
Pure adenomyosis was defined as adenomyosis in the absence of uterine leiomyomata. If leiomyomata were present, these cases of combined pathology were further subdivided in those with adenomyosis or leiomyomata predominance. If leiomyomata were larger than 5 cm in size and/or exhibited extensive (two-thirds of the cavital surface area) contact with the uterine cavity and patients presented with dominant bulk symptoms in the presence of adenomyosis, these cases were defined as combined disease of adenomyosis with leiomyomata predominance. If the combined leiomyomata were smaller than 5 cm in size and/or covered less than two-thirds of the cavital surface area, these cases were defined as combined disease of predominant adenomyosis (Fig. 2).
A A 49-year-old woman with leiomyomata predominance. Axial T2-weighted MR image (T2-TSE) shows an intramural leiomyoma (black arrow) and focal adenomyosis (white arrow) of the posterior uterine wall before embolization. B A 50-year-old woman with pure adenomyosis. Axial T2-weighted MR (T2-TSE) image shows a broadened junctional zone with high signal-intensity spots in the posterior uterine wall, diagnostic of focal adenomyosis (white arrow). C A 53-year-old woman with adenomyosis predominance. Axial T2-weighted MR image (T2-HASTE) shows a subserous leiomyoma in the posterior wall (black arrow) and focal adenomyosis (white arrow) within the anterior uterine wall before embolization
Embolization Procedure
Bilateral UAE was carried out under local anesthesia after establishing arterial access to the common femoral artery. Direct superselective catheterization of both uterine arteries was performed with a 4F or 5F cobra- or hook-type catheter under fluoroscopic imaging. A microcatheter was used at the operator’s discretion. Embolization was performed by deploying a particulate embolic agent in a diameter range of 355–900 μm (Embosphere, 500–700 μm, Biosphere Medical, Rockland, MA; BeadBlock 700–900 μm, Biocompatibles, Farnham, Great Britain; Contour 355–510 μm, Boston Scientific/Medi-Tech, Natick, MA) suspended in a dilute solution of an iodinated contrast agent (Imeron 300, Schering Bayer Health Care Pharmaceuticals, Berlin, Germany). The angiographic end point of embolization was stasis of flow in both uterine arteries for all embolic agents used. The stability of the end point was regularly checked after 5 min and additional particles injected if the end point was not reached. Intravenous narcotics and nonsteroidal anti-inflammatory drugs were provided for pain control, and patients were admitted to the hospital for observation.
Clinical Follow-up
Women were followed for a median of 40 months (range 5–102 months) after UAE by evaluating the self-perceived changes in symptoms and HRQOL in February 2010. There was no evaluation of symptom severity and HRQOL based on routine follow-up in the time between. The clinical follow-up symptom severity score were assessed by a questionnaire offering the following options for each symptom category: resolved, markedly improved, improved, unchanged, worsened, markedly worsened. For evaluating residual symptom severity and HRQOL after UAE, the Uterine Fibroid Symptom and Quality-of-Life Questionnaire (UFS-QOL) was used.
The questionnaire comprises eight questions pertaining to the type and severity of symptoms and 29 questions on how the disease affects different aspects of the patient’s HRQOL. The questions refer to the 3 preceding months. The eight symptom items (questions 1–8) are summarized in a symptom severity scale. The 29 HRQOL items are grouped in six subscales pertaining to Concern, Activities, Energy/mood, Control, Self-consciousness, and Sexual function, and together they represent the HRQOL total score. The mode of calculation of the symptom severity score and HRQOL score is described in detail elsewhere [18].
All secondary treatments either for symptomatic failure or possible complications of UAE were recorded. Clinical failure was defined as no improvement in symptom severity (all categories) or second invasive therapy to control symptoms after UAE.
Statistical Analysis
Demographic data are provided with median and range or upper and lower quartile (25th and 75th percentile), according to nonparametric distribution, or total numbers for all patients in general and for the three identified subgroups.
The Kruskal–Wallis test for multiple unpaired samples was used. Significance was tested with the use of Pearson’s χ2 test.
Kaplan–Meier analysis (KMA) was performed to determine freedom from second intervention including all patients with reinterventions and with the occurrence of a negative clinical event. In a first step, all patients with combined uterine leiomyomata and uterine adenomyosis were compared as one group to all women with pure uterine adenomyosis. In a second step, patients with combined disease were separated into two groups with either uterine leiomyomata or uterine adenomyosis predominance and compared to each other. The results are illustrated graphically, and mean event-free follow-up times with the according 95% confidence interval are provided in the text. Differences among groups were tested for significance using the associated log-rank test.
The results of the symptom severity and HRQOL total score of the UFS-QOL questionnaire at follow-up are provided as median with range and the Kruskal–Wallis test for multiple unpaired samples was used to test for differences between the subgroups.
Subgroup analysis of patients with pure adenomyosis included Pearson’s χ2 test and Mann–Whitney test for unpaired samples to identify interrelations of uterine adenomyosis type (focal, diffuse) and thickness of the junctional zone on the outcome regarding clinical failure as defined above for the KMA and HRQOL.
Statistical significance was accepted at P < 0.05. Statistical analysis was performed by SPSS software, version 11.5 (SPSS, Chicago, IL).
Results
Embolization Results
Bilateral UAE was technically successful in all 40 women. No complications occurred during treatment or hospital stay.
In 31 (77.5%) of 40 tris-acryl gelatin microspheres (Embosphere), in 5 (12.5%) of 40 nonspherical polyvinyl alcohol particles (Contour), and in 4 (10.0%) of 40 acryl-amido poly-vinyl alcohol microspheres (BeadBlock) were used for embolization.
Eleven (27.5%) of 40 women had disease that clinically failed to respond to therapy, resulting in unchanged, worsened, or markedly worsened symptoms, or resulting in the need for surgical reintervention (hysterectomy n = 10, dilatation and curettage n = 1). None of the women with clinical failure underwent a second UAE as a second intervention.
MRI Stratified Results
On the basis of MRI before UAE pure uterine adenomyosis and adenomyosis combined with uterine leiomyomata were identified before UAE and results were stratified retrospectively: 11 women with pure uterine adenomyosis and 28 women with uterine adenomyosis associated with uterine leiomyomata were identified. The latter group consisted of women with uterine adenomyosis with uterine leiomyomata predominance (n = 9) and uterine leiomyomata with uterine adenomyosis predominance (n = 19).
In the group of patients with uterine leiomyomata predominance none had a clinical failure. According to Kaplan–Meier analysis the mean cumulative survival free from reintervention for patients with pure adenomyosis reaches 48% (standard error: 17) and for patients with predominant uterine adenomyosis 58% (standard error: 14) after 6 years (Fig. 3).
A Cumulative survival free from reintervention according to Kaplan–Meier analysis. Pure uterine adenomyosis (A) vs. uterine adenomyosis with coexisting leiomyomata (B). B Cumulative survival free from reintervention according to Kaplan–Meier analysis. Uterine adenomyosis with uterine leiomyomata predominance (A) vs. adenomyosis predominance (B)
Women with uterine leiomyomata and predominant uterine adenomyosis had a mean event-free survival time (time to second intervention) of 68 months (range, 48–88 months), women with pure uterine adenomyosis a mean event-free survival time of 80 months (range, 65–95 months).
Differences between groups regarding to clinical failure were nearly significant comparing patients with uterine leiomyomata predominance and patients with uterine adenomyosis predominance (P = 0.058).
Results for Symptom Severity and HRQOL
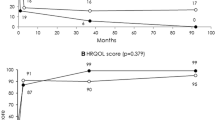
Thirty-six patients complained about menorrhagia before UAE. Three (8.3%) of 36 patients with menorrhagia before UAE had clinical failure. In 28 (77.7%) of 36 patients, menorrhagia resolved, improved markedly in 4 women (11.1%) and improved in 1 woman (2.7%). Thirty-four (85.0%) of 40 patients had dysmenorrhea before UAE. Three (8.8%) of 34 experienced clinical failure with no symptomatic improvement. Twenty-three (73.5%) of 34 patients had resolution of dysmenorrhea after UAE; 6 (17.6%) of 34 were markedly improved and 2 (5.9%) of 34 improved. Thirty (75.0%) of 40 patients had bulk symptoms before UAE, 2 (6.6%) of 30 had disease that failed to clinically respond to therapy, in 22 (73.3%) of 30 bulk symptoms resolved after UAE, 4 (13.3%) of 30 experienced marked improvement, and 2 (6.7%) of 30 experienced improvement. One (3.3%) of 30 experienced no improvement in bulk symptoms but menorrhagia and dysmenorrhea improved. Results for symptom severity and HRQOL after UAE stratified by the extent of uterine adenomyosis at baseline MRI is shown in Table 2.
HRQOL and symptom severity as measured by the scoring system of the UFS-QOL questionnaire showed improvement irrespective of the pattern of uterine adenomyosis (focal or diffuse) or junctional zone thickness. Treatment failure was not associated with either junctional zone thickness or pattern of uterine adenomyosis (focal or diffuse). With regard to the concomitant presence of uterine leiomyomata, the HRQOL and the symptom severity after UAE was better if adenomyosis and uterine leiomyomata were present. Patients with pure uterine adenomyosis reached the least HRQOL score of 94.83 (range, 71.55–100) and had the best symptom severity score of 3.13 (0–28.13) after UAE. Women with uterine leiomyomata with predominant uterine adenomyosis showed a better HRQOL score of 99.14 (93.97–100) and a lower symptom severity score of 0 (0–6.25). Women with uterine adenomyosis and predominant uterine leiomyomata showed the highest HRQOL after UAE with a HRQOL score of 100 (95.26–100) and near complete elimination of clinical symptoms with a symptom severity score of 0 (0–9.38) (Table 2).
Discussion
Different patterns of uterine adenomyosis exist pathoanatomically, according to the depth of myometrial invasion and the distribution within the uterus classified as diffuse or focal [19]. Also there are no standardized definitions to classify subgroups with coexisting uterine leiomyomata of the uterus by imaging. Therefore, our definition of subgroups was based on the synopsis of clinical symptoms and MRI findings. MRI is considered the most accurate imaging modality for the diagnosis of uterine adenomyosis with a reported sensitivity and specificity ranging from 70–77% and 86–92%, respectively [6, 7, 20]. Although minimally invasive therapies such as implantation of a levonorgestrel intrauterine system (LNG-IUS) and endometrial ablation or resection have been used to manage uterine adenomyosis their success rates vary with the depth of uterine adenomyosis infiltration, and long-term results remain unclear [12, 21, 22]. Studies showing the effect of UAE for uterine adenomyosis mainly describe successful short-term results [13–17]. Midterm results of UAE for uterine adenomyosis are reported by some authors as being disappointing with a high rate of recurrence of clinical symptoms [23, 24].
We found UAE to be clinically effective in the majority (72.5%) of patients with uterine adenomyosis. Our results compare to those of Kim et al. with a symptom control in 57.4% of patients at 4.9 years’ follow-up and Pelage et al., who reported symptom control in 56.0% of patients at 2 years’ follow-up [24, 25]. The difference may be explained by the fact that we included women with pure uterine adenomyosis as well as patients with uterine adenomyosis and coexisting uterine leiomyomata. Our study population reflects the common scenario encountered in daily clinical practice, where a combination of uterine adenomyosis and uterine leiomyomata is seen in 35–55% of patients [2]. In the group of patients with uterine adenomyosis and uterine leiomyomata predominance in our study none had a clinical failure. On the contrary for patients with uterine leiomyomata and predominant uterine adenomyosis clinical failure rate was high (31.5%) and clinical failure requiring hysterectomy nearly reached significance compared to women with predominant uterine leiomyomata (P = 0.058). On the basis of a higher number of cases this difference would supposably be significant. However, UAE was successful even in patients with predominant uterine adenomyosis and with pure adenomyosis and led to symptomatic control and improvement in HRQOL. This must be seen in the context of alternative uterine sparing treatments for adenomyosis such as hormonal treatment and endometrial ablation for whom poor results for extensive disease have been reported [12]. None of the women who failed clinically underwent repeat UAE as a second intervention because in case of clinical failure the clinical symptoms were so intense that the patients preferred a surgical therapy. On the basis of the results of our study, patients with a diagnosis of uterine adenomyosis should be counselled appropriately about the differences in success rates of UAE for adenomyosis with predominant leiomyomata vs. leiomyomata with predominant adenomyosis or pure adenomyosis. Interestingly, there was no significant correlation of clinical outcome after UAE and the depth of uterine adenomyosis measured by the junctional zone thickness or with respect to the different patterns of uterine adenomyosis such as focal or diffuse at baseline MRI. This is partially in line with observations of Kitamura et al., who saw no differences in clinical and imaging outcome after UAE with respect to the pattern of uterine adenomyosis [15]. A major limitation of this study was the small sample size as a result of cases lost to follow-up and a study population with different morphological extent of disease with or without associated uterine leiomyomata. The use of three different embolic agents may be seen as another limitation of this study. However, the angiographic end point of embolization was the same for all agents and great care was taken to ensure a stable angiographic end point. No statistically significant difference in clinical outcome was seen using different embolic agents.
Being an underdiagnosed disease entity, no validated health-related quality-of-life and symptom severity questionnaire for uterine adenomyosis exists [26]. We decided to use the UFS-QOL to assess self-perceived residual symptom severity and HRQOL after UAE because it may represent a good approximation of the residual symptomatic burden in this group of women. Paralleling the observed frequency of reinterventions and reported symptom changes after UAE, we found the lowest symptom severity and the highest HRQOL in women who presented with uterine adenomyosis and uterine leiomyomata predominance. Further comparative studies that use MRI and the UFS-QOL for assessing the outcome of different treatment options are warranted.
In conclusion, UAE provides sustained control of menorrhagia, dysmenorrhea, bulk symptoms and an improvement in HRQOL in the majority of patients with uterine adenomyosis. Although the pattern of adenomyosis as well as depth of infiltration does not influence clinical outcome, the results for patients with concomitant leiomyomata seem to be better than for patients with predominant or isolated uterine adenomyosis.
References
Matalliotakis IM, Kourtis AI, Panidis DK (2003) Adenomyosis. Obstet Gynecol Clin North Am 30:63–82
Ferenczy A (1998) Pathophysiology of adenomyosis. Hum Reprod Update 4:312–322
Bergeron C, Amant F, Ferency A (2006) Pathology and physiopathology of adenomyosis. Best Pract Res Clin Obstet Gynaecol 20:511–521
Dueholm M, Lundorf E, Hansen ES et al (2001) Magnetic resonance imaging and transvaginal ultrasonography for the diagnosis of adenomyosis. Fertil Steril 76:588–594
Bazot M, Cortez A, Darai E et al (2001) Ultrasonography compared with magnetic resonance imaging for the diagnosis of adenomyosis: correlation with histopathology. Hum Reprod 16:2427–2433
Reinhold C, McCarthy S, Bret PM et al (1996) Diffuse adenomyosis: comparison of endovaginal US and MR imaging with histopathologic correlation. Radiology 199:151–158
Ascher SM, Arnold LL, Patt RH et al (1994) Adenomyosis: prospective comparison of MR imaging and transvaginal sonography. Radiology 190:803–806
Dumousset E, Chabrot P, Rabischong B et al (2008) Preoperative uterine artery embolization (PUAE) before uterine myomectomy. Cardiovasc Intervent Radiol 31:512–520
Goodwin SC, Spies JB (2009) Uterine fibroid embolization. N Engl J Med 361:690–697
Katsumori T, Kasahara T, Kin Y et al (2007) Magnetic resonance angiography of uterine artery: changes with embolization using gelatine sponge particles alone for fibroids. Cardiovasc Intervent Radiol 30:398–404
Spies JB, Bruno J, Czeyda-Pommersheim F et al (2005) Long-term outcome of uterine artery embolization of leiomyomata. Obstet Gynecol 106(5 Pt 1):933–939
McCausland V, McCausland A (1998) The response of adenomyosis to endometrial ablation/resection. Hum Reprod Update 4:350–359
Jha RC, Takahama J, Imaoka I et al (2003) Adenomyosis: MRI of the uterus treated with uterine artery embolization. AJR Am J Roentgenol 181:851–856
Kim MD, Won JW, Lee DY et al (2004) Uterine artery embolization for adenomyosis without fibroids. Clin Radiol 59:520–526
Kitamura Y, Allison SJ, Jha RC et al (2006) MRI of adenomyosis: changes with uterine artery embolization. AJR Am J Roentgenol 186:855–864
Siskin GP, Tublin ME, Stainken BF et al (2001) Uterine artery embolization for the treatment of adenomyosis: clinical response and evaluation with MR imaging. AJR Am J Roentgenol 177:297–302
Lohle PN, De Vries J, Klazen CA et al (2007) Uterine artery embolization for symptomatic adenomyosis with or without uterine leiomyomas with the use of calibrated tris-acryl gelatin microspheres: midterm clinical and MR imaging follow-up. J Vasc Interv Radiol 18:835–841
Spies JB, Coyne K, Guaou Guaou N et al (2002) The UFS-QOL, a new disease-specific symptom and health-related quality of life questionnaire for leiomyomata. Obstet Gynecol 99:290–300
Gordts S, Brosens JJ, Fusi L et al (2008) Uterine adenomyosis: a need for uniform terminology and consensus classification. Reprod Biomed Online 17:244–248
Bazot M, Lafont C, Rouzier R et al (2009) Diagnostic accuracy of physical examination, transvaginal sonography, rectal endoscopic sonography, and magnetic resonance imaging to diagnose deep infiltrating endometriosis. Fertil Steril 92:1825–1833
Farquhar C, Brosens I (2006) Medical and surgical management of adenomyosis. Best Pract Res Clin Obstet Gynaecol 20:603–616
Fukunishi H, Funaki K, Sawada K et al (2008) Early results of magnetic resonance-guided focused ultrasound surgery of adenomyosis: analysis of 20 cases. J Minim Invasive Gynecol 15:571–579
Bratby MJ, Walker WJ (2009) Uterine artery embolisation for symptomatic adenomyosis—mid-term results. Eur J Radiol 70:128–132
Pelage JP, Jacob D, Fazel A et al (2005) Midterm results of uterine artery embolization for symptomatic adenomyosis: initial experience. Radiology 234:948–953
Kim MD, Kim S, Kim NK et al (2007) Long-term results of uterine artery embolization for symptomatic adenomyosis. AJR Am J Roentgenol 188:176–181
Daraï E, Coutant C, Bazot M et al (2009) Relevance of quality of life questionnaires in women with endometriosis. Gynecol Obstet Fertil 37:240–245
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Froeling, V., Scheurig-Muenkler, C., Hamm, B. et al. Uterine Artery Embolization to Treat Uterine Adenomyosis with or without Uterine Leiomyomata: Results of Symptom Control and Health-Related Quality of Life 40 Months after Treatment. Cardiovasc Intervent Radiol 35, 523–529 (2012). https://doi.org/10.1007/s00270-011-0254-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-011-0254-3