Abstract
Successful drug delivery using dry powder inhaler (DPI) technology is based on knowledge of pulmonary deposition, targeting and its relationship to aerodynamic particle size distribution. DPI technologies consist of three notable elements, the formulation, the metering system and the mechanism of dispersion as an aerosol. Each of these is discussed below but emphasis is placed on powder formulation, the forces of interaction between particles that must be overcome to disperse them and the means whereby energy is imparted to achieve this objective. Efficient and reproducible drug delivery with respect to aerosol properties and dose are the objectives of product development and a requirement for regulatory approval for satisfactory disease therapy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Drug Particle
- Fine Particle Fraction
- Aerosol Performance
- Aerodynamic Particle Size Distribution
- Salmeterol Xinafoate
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
10.1 Introduction
Powdered drugs can be administered to the respiratory system for effective relief of ailments such as asthma and chronic obstructive pulmonary disease. Good design of the particle size distribution is essential for adequate delivery of the drug to the required region. Dry powder inhalers (DPIs) have proven their efficacy in this application.
The purpose of DPIs is to generate aerosols of drug particles in doses sufficient to treat a specific disease when inhaled. Many considerations that have to be addressed in order to develop a DPI for drug administration are presented in the following sections.
DPI’s consist of three important elements: the drug formulation, the metering system and the aerosol dispersion mechanism each of which requires attention for the drug product to achieve efficient and reproducible drug delivery to ensure efficacy and safety.
10.2 Pulmonary Deposition and Targeting: The Clinical Considerations
The efficacy of aerosol dry powder depends upon the deposition of the drug particles in the required dosage to the desired location. An understanding of the physical mechanisms of deposition and the pharmacological mechanisms of action of the drugs are crucial for the successful pulmonary drug delivery.
10.2.1 Deposition
The patient interface with the inhalation platform dictates the path the particles take through the oropharynx to the upper airways and, ultimately, the rest of the respiratory tract. Once in the airways, deposition of aerosol particles in the respiratory tract depends on their aerodynamic particle size distribution (APSD), the width of the respiratory channels, and the mode of inhalation (breathing rate, inhaled volume and pause between inhalation and exhalation etc.).
The main mechanisms of deposition, depending on the particle size, include inertial impaction, gravitational sedimentation, diffusion (Brownian motion), and interception (Fig. 10.1). Either of them dominates in defined parts of the respiratory tract. Inertial impaction occurs mainly in the tracheobronchial airways (i.e. airway generations 1–8 in the Weibel lung model [1]), and it is proportional to the particle size squared and airflow rate [2, 3]. Sedimentation is a time-dependent process. It primarily occurs in the lung periphery including the bronchioles and terminal bronchioles (generations 8–16 in the Weibel model [1]) where the linear velocity of the airflow is low and settling distances are relatively small [2, 3]. Diffusion mainly occurs in the alveoli region. It is inversely proportional to the particle size (<1 μm). Interception refers to elongated particles when they are captured even if they remain aligned in the streamline [3].
The optimal size distribution for regional respiratory deposition differs substantially depending upon a balance of APSD and inter-particulate forces, the mode of inhalation, and the disease state [4]. Generally speaking, it may be concluded that drug particles having an aerodynamic size range of 1–5 μm show optimum deposition in the deep lungs [2]. Those larger than 10 μm are generally deposited in the oropharyngeal region, while smaller than 1 μm are prone to be exhaled [2, 3]. The APSDs of drugs in a DPI are often represented by log-normal distributions because they fit the observed distributions reasonably well and can be expressed in only two parameters [5]. They may be described in terms of mass median aerodynamic diameter and geometric standard deviation [2, 6].
For most pharmaceutical aerosols, the dynamics of particles fall in the Stokes’ regime of curvilinear motion and, the aerodynamic diameter is derived from Stokes’ law. The same law predicts that low-density (porous or hollow) particles of a particular geometric size behave as if they are smaller and elongated particles perform as if they have a size close to their cross-sectional area size rather than their length [6]. Both physical properties allow particles to penetrate deeper into the lungs. Density can be controlled through particle manufacturing methods. However, elongated particles are more difficult to manufacture reproducibly.
10.2.2 Pulmonary Targeting
Targeted delivery of pharmaceutical aerosols to desired respiratory regions is essential to more effectively exert their therapeutic effect while minimizing side effects of unwanted aerosol exposure. For instance, the location of adrenergic and cholinergic receptors in the lungs responsible for bronchomotor tone through the parasympathetic and sympathetic nervous system are distributed differently. Adrenergic receptors are more localized in the lung periphery and cholinergic receptors are more centrally located [7]. This should guide the target particle size to achieve the therapeutic effect for beta-adrenergic agonists such as albuterol, or for anticholinergics such as tiotropium. Corticosteroids are delivered to the whole lung to treat inflammation, the underlying cause of asthma and a corollary of COPD. Inflammation occurs throughout the airways in chronic disease [8]. For drugs which are intended for systemic delivery, such as insulin, targeting to the alveolar region is desired for enhanced bioavailability [9]. There is a large supply of blood arriving to the large surface area of alveolar epithelium for oxygenation, which would facilitate rapid drug uptake [10]. However, clearance mechanisms such as mucociliary escalation and phagocytosis by alveolar macrophage should also be considered. The upper airways are supplied from a different arterial system and take the blood back to the vena cava. Consequently, both site of delivery and clearance mechanism play a role in achieving the desired therapeutic effect. The target for delivery of aerosol particles may also vary for the disease state being addressed.
10.2.3 Computational Modeling
Complementary to the effort of clinical lung deposition assessment using gamma scintigraphy technology, advances have been seen in the prediction of total and regional respiratory deposition using more accurate modeling, owing to the high resolution imaging technology, refined physical airway replicas, and the more powerful computational simulation capabilities [11]. Several reviews of these advances have been published recently that cover the lung deposition prediction over a broad range of particle size and breathing pattern using correlated curve fitting, and computational fluid dynamics using simulated respiratory tract [12–15]. These studies represent the progress in the fundamental understanding of targeted pulmonary delivery that may not be observed experimentally. However, further refinement and validation work remains to be done. Uncertainties of the predictions may still exist due to the facts such as the heterogeneous nature of pharmaceutical aerosol, the changes in the oropharyngeal opening due to flow resistance, and the diversity of dynamic breathing regime due to different disease state [14].
10.3 Analysis of Size Distribution of Dry Powder Aerosols
The most important property of a therapeutic aerosol related to clinical effect is particle size distribution. More rapid inspiration through DPIs results in higher flow rate, elevated aerodynamic shear stress, pressure drop, and Reynolds’ number, which corresponds to enhanced fine particle dose, but at the same time increases the tendency for throat deposition by inertial impaction. It is clear that the powder dispersion is influenced by flow profiles and more than one flow rate should be adopted for the performance evaluation. Since the aerosol delivered from a passive dry powder inhaler does not exist independently of the inspiratory flow, shifts in the agglomerate state of drug, which can be monitored through light scattering particle size analysis or inertial impaction method at different flow rates.
10.3.1 Inertial Impaction Particle Size Analysis
Current compendia methods for dry powder aerosol testing are based on inertial impaction methods for aerodynamic sizing at fixed airflow rate (30–100 L/min), and these methods are considered the most relevant for the in vitro description of pharmaceutical aerosols [16]. According to the United States Pharmacopoeia standard, any inertial sampling apparatus can be selected for DPI testing, including Marple-Miller impactor (MMI), Andersen cascade impactor (ACI), multi-stage liquid impinger (MSLI), and next generation impactor (NGI). The correct operation of these apparatus in terms of measurement accuracy and reproducibility at established flow profile is important [17].
An induction port standardized for all systems is used. Surface coating of stages to avoid bouncing and re-entrainment is a necessary step to all inertial samplers except MSLI that uses water in each stage. The collected mass must be within 75–125 % of expected, based on delivered dose. The theory of inertial sampling and the apparatus were reviewed before [18–21], so they are not reiterated here.
From a quality control and regulatory perspective, the amount of drug sampled at each stage of the impactor is plotted against the cut-off size for the stage. The particle size distribution represented as cumulative percent undersize can be further analyzed to calculate the mass median aerodynamic diameter and geometric standard deviation [18]. A drawback of impactors is the small number of given size classes. Furthermore, how to interpret the specifications is not yet part of the compendia.
10.3.2 Light Scattering Analysis
Particle de-agglomeration during aerosolization can be seen as changes in particle size distribution. The most commonly used method for volume particle size distribution is laser diffraction (Fig. 10.2). This method is based, depending on particle size, on Mie scattering or Fraunhofer diffraction theory for spherical particles [22]. The fine particle fraction can be obtained by analyzing differential or cumulative distribution data. The volume fine fraction can be converted to aerodynamic fine fraction under the assumption that the sizing results are the same as those obtained by sedimentation (based on the Stokes’ law), or a shape factor is available, and the particle density is known (see also Sect. 10.3.1). The advantage of the laser diffraction method is its efficiency in real time measurement of size distribution at different flow rates, which is valuable for accessing the de-agglomeration [23–25]. This method is mainly used for carrier-free formulations because of the skewed distribution and difficulty in differentiating drug and fine particles. But the measurement of carrier-based formulation can still be achieved by eliminating the inner detector element data which relate to the larger carrier particles [25].
Flow rate dependence of dispersion of dry powder aerosols [141]. Particle size distributions of aerosolised plume of lactohale 300 measured by laser diffraction and aerosolised from Rotahaler, Monodose Inhaler and Handihaler® at: (a) 60 l min−1 showing typical variability; the mean of five replicates of lactohale 300 aerosolised at 45, 90 and 120 l min−1 through (b) Rotahaler, (c) Monodose Inhaler, and (d) Handihaler (120 l min−1 on secondary y-axis)
Laser Doppler velocimetry, also known as laser Doppler anemometry, measures the velocity of particles passing through the intersecting point of two laser beams [26]. It is also used to indirectly evaluate the de-agglomeration by measuring the turbulent flow field [27]. Other related laser Doppler methods, such as Phase Doppler analysis (PDA) that measures both particle size and velocity characteristics of irregular shaped particles, have been reviewed previously [18]. PDA optically splits a laser beam, uses the interference of particles with one element of the laser and then converges both portions of the beam to indicate a phase shift proportionate to the size of the particles.
The aerodynamic particle size distribution can be measured by time-of-flight particle sizing systems such as Aerosizer. An aerodisperser generates an aerosol, accelerates the particles which pass two subsequent laser beams. Smaller particles are accelerated faster and reach higher velocities than larger particles due to inertia. The time-of-flight of these particles between the two beams is then converted into corresponding particle size distribution. A shift in the aerodynamic particle size distribution can be achieved by changing the shear force applied for the aerodisperser, and used for evaluation of agglomeration strength of carrier-free powders [28, 29]. The advantage of this method is its in situ generation of aerodynamic data. But it may not be used for formulation blends because it is not possible to differentiate drug and carrier.
10.4 Drug Formulation
10.4.1 Static Powder Properties
Drug particles are ordinarily prepared in micrometer sizes to be respirable, typically an aerodynamic size of less than 5 μm [30]. These small sizes are subject to a variety of forces of interaction that lead to particle clustering. The major fundamental underlying forces of interaction are van der Waals forces, which are inherently important for particles in this size range. They dictate the bulk behavior in dry and uncharged fine powders. The magnitude of the van der Waals forces is a function of the particle separation distance and the molecular properties of the materials involved [31, 32]. They are typically effective within a separation distance less than 100 nm, so they are sensitive to changes in properties including particle size, shape, surface roughness, and contact deformation [33, 34]. From the point of view of thermodynamic work of adhesion, they are also influenced by the surface chemistry and interfacial energy of the powder [35]. It should be noted that van der Waals forces are a manifestation of combining relations of the intermolecular forces and should be operative between molecules (or extended surfaces), nanostructures such as colloids, and microparticles [31].
Electrostatic forces are mainly generated during pharmaceutical powder processing (such as milling, mixing, and filling), when electrostatic charges are accumulated by means of contact, coulombic interaction, and induced charging [36, 37]. They are long range forces compared to van der Waals forces. The magnitude and polarity of electrostatic forces are related to the nature of powder and container wall. Electrostatic charges decay when relative humidity increases [38, 39]. Their effects become negligible at elevated relative humidity (e.g. RH > 65 %), at which the capillary forces become dominant due to capillary condensation of water vapor between neighboring particles. High tensile liquid bridges are formed because of the Laplace pressure difference at the contact interface [31]. The magnitude of capillary forces is a function with respect to the water meniscus and the surface tension between the solid and water interface [40]. The reciprocal but non-linear relationship between capillary and electrostatic forces is a major conundrum in predicting the behavior of dry powders.
In addition, other sources of particle interaction including mechanical interlocking and frictional forces are considered important, though not frequently studied. The magnitude of the mechanical interlocking is dependent on surface roughness and can be enhanced with prolonged mixing [18]. The inter-particulate friction may play an important role at the initiation of powder aerosolization [41]. The special features and implications of dry powder friction forces have been explored previously [32, 42]. Many factors influence and add complexity to the particulate interaction, and they are described in the next section [43].
10.4.2 Powder Dynamics
The aerodynamic fluid phase and the solid phase interacting in the DPI device, play crucial roles in powder dynamics. The powder aerosolization may be considered in terms of static powder, dilation, fluidization, and de-agglomeration (Fig. 10.3). Fluidization is the mobilization of the bulk powder by interaction with air molecules. Depending on the DPI metering system and device design, the fluidization mechanisms include shear force, gas-assisted, and capillary for aerodynamic fluidization, and vibration and impaction for mechanical fluidization [18]. De-agglomeration is the second stage interaction of fluidized powder in air when drug particles are stripped from the carrier surfaces or drug-drug agglomerates and dispersed into primary respirable particles. Turbulent airstreams are generally recognized as the major source for particle de-agglomeration. The primary mechanisms responsible for de-agglomeration are turbulent shear stress and inertial separation such as that achieved by collision, centrifugal and vibration [18] (Fig. 10.4). Powder entrainment and de-agglomeration within a DPI are dynamic processes with rapid expansion of system volume. However, it is enlightening to consider a related situation when deposition-re-suspension equilibrium is established at a fixed system volume. Two theoretical models, force balance and energy accumulation, were developed for particle re-suspension in turbulent flows [44].
When a turbulent airflow entrains through a DPI device, it exerts aerodynamic forces or moments on the static dry powder to overcome the inter-particulate or surface forces. Once fluid forces reach a critical value to balance adhesive forces, particle detachment occurs. For example, the critical diameter for the separation of two identical spheres can be estimated by equating the viscous shear force to the adhesive van der Waals force [18]. The aerodynamic lift forces could be smaller than adhesion forces by several orders of magnitude because of rolling and sliding [45–47]. Once re-suspended, the particles move/rotate along the downstream airflow or collide with other particles or walls by momentum transfer. The mechanism of turbulent “burst” within the viscous sub-layer has been used to explain particle detachment, but its contribution is controversial [47, 48].
Alternatively, the flow transfers the turbulent energy into the powder bed. Particles can be re-suspended when sufficient energy is accumulated to overcome the adhesion potential resulting in entrainment [49]. There are several advantages in using the energy accumulation model. It accounts for the re-suspension below the critical flow velocity. It also takes into account of the time scale and explains the time dependence of particle re-suspension. Furthermore, it implies that particle re-suspension is analogous to the desorption process that occurs on a molecular level [50].
10.4.3 Carrier-Based Formulation and Aerosol Performance
The most common DPI formulation consists of interactive physical mixtures of micronized drugs, coarse carriers (and surface fines) maintained by thermodynamically favorable interfacial interactions at the solid-solid interface (Fig. 10.5a). A balance of cohesive and adhesive forces between these components is formed during mixing. The principle involved in this approach is the uniform distribution of micronized drug particles at the surface of a larger carrier (typically 60–90 μm), such as α-lactose monohydrate (abbr. as “lactose” in this chapter) which achieves ease of dose filling, where the lactose is a diluent to facilitate dose metering and a carrier to enhance aerosol dispersion properties, since drug particles can be removed more readily from the surface of lactose than separated from agglomerates with other drug particles. However, this represents a simplified scenario when drug-carrier adhesive forces are much stronger than drug-drug cohesive and drug-fine adhesive forces (so-called ordered mixture [51]). Other scenarios such as the formation of drug-drug or drug-fine agglomerates are common with respect to the heterogeneity and multi-component nature of the powder mixtures. Besides this common carrier-based formulation, topics of specialized forms such as composite micro-particles with coarse carriers [52], or composite carriers [53, 54] are observed but will not be discussed here.
Schematic illustration of DPI powders: (a): Carrier based formulation (2 % w/w Albuterol sulfate – Trehalose interactive physical mixture); (b): Carrier-free agglomerates (Albuterol sulfate); and (c) Low density porous particles generated by spray-drying [6]
For the purpose of efficient drug particle dispersion, manipulation of the adhesive/cohesive properties and their force balance by a variety of approaches, such as modification of particle size [55], morphology and surface roughness [56, 57], surface coating [58], ternary component inclusion [59], alternative carrier [60, 61], or particle engineering [62, 63], to alter bulk or surface properties of the mixing components has been extensively reviewed [64–71]. It is evident that there are many methods to improve aerosol performance, but the improved performance may be confounded with additional complexities at the heterogeneous solid mixture interface. Previous studies focused on a few featured variables that may or may not be the key influences. For example, increased drug aerosolization was often observed with smaller carrier sizes because they may effectively disrupt the drug-drug cohesion [72]. However, it was also reported that increasing the carrier size had little influence [41] or increased aerosolization [73] in some formulation.
A few recent well-controlled studies on specific formulation variables influencing the aerosolization performance may be highlighted. In one study, a series of model polystyrene spheres used as carriers with different size ranges (volume median size = 82.8; 277.5; 582.9 μm) were studied [55]. Using these carriers, the variables, including carrier shape, morphology, and drug-carrier adhesion, were eliminated. The model drug employed was micronized albuterol sulfate. Selection of these model spheres eliminated other formulation variables while only focusing on the carrier size effect. The aerosol performance increased as carrier size decreased. The decreased carrier size corresponded to increased particle number and surface area, which were presumably attributed to the increased impaction events and associated frictional and rotational collision [55]. A similar study focused on drug loading (varying carrier to drug ratio from 5:1 to 85:1) showed that, as the drug loading increased, the aerosol performance with respect to the fine particle fraction increased after a threshold was reached, at which point, the formulation transitioned from ordered mixture to agglomerated mixture transition [74].
10.4.4 Carrier-Free Formulation and Aerosol Performance
Loose agglomerates of respirable drug (Fig. 10.5b) have also been employed to facilitate aerosol dispersion. These carrier-free formulations avoid possible drug-carrier chemical reaction and are suitable for high drug payload. They are used in commercial DPI formulations such as Pulmicort® [75] and Bricanyl® [23]. Micronized drugs are often very cohesive and have poor aerosolization properties [71]. In order to achieve satisfactory flowability for accurate filling and aerosolization, this type of formulation generally requires well-controlled processes to form loose microparticle agglomerates of different size, a processing technique referred to as pelletization [76]. Special carrier-free formulations such as that inhaled insulin (Exubera®) will not be discussed here.
Increased fluidization and aerosol performance can be achieved by reducing drug-drug cohesive forces. This is typically performed by modification of surface physicochemical properties or particle engineering. For the former method, micronized drugs are often coated with low surface energy additives, known as force control agents (e.g. leucine, lecithin or magnesium stearate), using a variety of coating techniques such as mechanofusion [77–80]. and physical vapor deposition [81, 82]. It has been proposed that the increase in aerosol performance is due to formation of a low surface energy disruption layer to reduce drug-drug cohesion and attrition [58]. Decreasing inter-particulate interactions may also reduce the dependency of aerosolization on the flow rate and inhaler [83]. It should be noted that this method may be limited by the kind of substance that is approved for pulmonary delivery [84]. If this is the case, particle manufacture by spray drying has often been employed to prepare drug particles [62, 85, 86]. Spray dried particles can be prepared having lower bulk density, which decreases cohesive forces. Moreover, they show greater susceptibility to shear in the fluid dynamic environment of the airflow channel of the inhaler because of their larger volumes and lower density [9, 87] (Fig. 10.5c).
10.5 Theories for Dry Powder Aerosol Dispersion
The theories for dispersion of drug from dry powder beds differ for carrier-based versus drug particle alone (carrier-free) formulations (see above). The dispersion of carrier-based formulations will be considered as it has been more extensively studied. The reader interested in theoretical studies of carrier-free formulations is referred to the literature [28, 80, 81, 88–90].
10.5.1 “Active Site” Versus “Agglomeration” Theory
Two complementary theories exist for the improved aerosol performance by introducing ternary fine particle components into carrier-based formulations, namely the “active site” and the “agglomeration” hypotheses [59] (Fig. 10.6).
Schematic of ‘Active Site’ (above) and ‘Agglomerate’ mechanisms (below) [66]
The former theory proposes that both geometric (asperities larger than drug particles providing shelter) and energetic (raised surface energy) features exist on the coarse carrier surface which are more adhesive than other areas, namely “active” site. The addition of fine particles preferentially binds to these sites, forcing drug particles to the weaker binding site, thus drug particles are more easily liberated from the surface of carrier particles after actuation [91, 92]. The more adhesive sites were shown to exist as was probed by atomic force microscopy [93] and nitrogen adsorption isotherm [94]. The influence of drug payload had little effect on the performance of an albuterol sulfate/lactose formulation until the occupancy of “active” sites was presumably saturated [95]. However, introducing fines of higher energy resulted in an overall increase in surface energy and may have increased the drug-carrier adhesion [96]. The influence of blending sequence studies [91] on aerosol performance have often been used to support “active” site theory, but time-dependent redistribution may occur during mixing [97], and the results may be confounded by other variables such as drug concentrations [98]. Furthermore, the “active” site has a vague definition similar to surface heterogeneity that covers both surface geometric and energetic features. A categorical comparison of “active” vs. “non-active” may be an over-simplified treatment of the complex features on the carrier surface [66].
The latter theory, of particle agglomeration, proposed that drug and fine particles can form loose agglomerates, and can be dispersed more easily than drug particles alone [99]. An investigation of the mechanism by AFM, employing the cohesive-adhesive balance approach suggested that increasing drug-fine adhesion may give rise to larger agglomerates that experience greater separation forces for de-agglomeration [99]. Surface energy analysis using inverse gas chromatography showed that increasing surface energy interactions between drug and carrier resulted in improved aerosol performance [100]. According to several studies, an optimum drug and lactose fines ratio exists when studying the salmeterol xinafoate blends [101–103]. These findings support the agglomeration theory.
In these theories, it is presumed that carrier lactose helps dilate the powder bed in response to a driving airflow and particles coated with drug are carried into the airstream. In the airstream, impaction with the walls of the inhaler and with other particles in the turbulent environment and surface shear strips drug particles from lactose surfaces, and entrains them towards the patient. The efficiency and reproducibility of the dose delivered is dependent on the forces of interaction between the lactose and drug particles. Theories for this phenomenon have focused on cohesive and adhesive balance of forces. These are thought to underpin the ‘active’ site theory of binding of drug to lactose and the agglomerate dispersion interpretation both of which explain the behavior of drug in response to airflow parameters.
10.5.2 Powder Aerosol De-agglomeration Equation
Recently, we have proposed a theory for drug dispersion involving a powder aerosol de-agglomeration equations, or PADE (Fig. 10.7), which directly correlate aerosol performance with an airflow parameter of the standardized entrainment tubes (SETs [104]) (see Sect. 10.6.4) using an algebraic equivalence of Langmuir adsorption equation, namely, the fine particle fraction (FPF, %) is correlated with shear stress (τ s , N/m2). Increasing τ s corresponds with increasing FPF, until it approaches invariant region (FPF max , %) (Eqs. 10.1 and 10.2) [105]:
The rationale for the development of the PADE is based on the analogous physical phenomena and meaningful interpretation based on a comprehensive investigation of experimental data [106–108].
Firstly, the behavior of heterogeneous energy distribution and de-agglomeration in a particulate system is considered to be analogous to the surface coverage described in molecular surface adsorption and the number of surface binding sites in colloidal protein binding. During powder mixing, micronized drug particles thermodynamically prefer to adhere onto higher energy sites. When these high energy sites are gradually occupied, the drug particles adhere onto progressively lower energy sites, resulting in a decreased heat of adhesion (or from the guiding analogy, adsorption) with loading. When a certain shear stress is applied, drug particles at lower energy sites are preferentially de-agglomerated while those at higher energy sites remain associated unless higher shear stress is applied [105]. Secondly, it is the notion that the models of molecular surface dissociation described by an adsorption expression may be adapted to fit drug de-agglomeration caused by shear displacement. This is based on the fundamental understanding that inter-molecular and particulate forces that cover these analogous phenomena are essentially the same. Thirdly and most importantly, this theory has shown excellent correlations between experimental data and mathematical approximations using the PADE method.
The adoption of PADE theory has profound potential implications (Fig. 11.7). Expressions describing surface adsorption were intended to allow elucidation of mechanisms including monolayer adsorption heterogeneity (e.g. Freudlich expression [109]), lateral interaction (e.g. Fowler-Guggenheim expression [110]), and multi-layer adsorption (e.g. Brunauer-Emmett-Teller expression [111–113]), the analogy of which may be used to account for heterogeneity, drug-drug cohesion, and multi-layer drug agglomeration, respectively. It should be noted that such an approach may circumvent debate surrounding “active” site and agglomeration theories, and mechanistically evaluates the underlying particle-particle (drug-drug, drug-fines) and particle-surface (drug-carrier, fine-carrier) interactions. In addition, the PADE method can be readily used for fast formulation screening/optimization and robust aerosol performance prediction in a given shear stress range [106].
10.6 Device Technology
10.6.1 Principles of Turbulent Pipe Flow
The development of an efficient DPI device requires fundamental understanding of fluid dynamics. Turbulent airflow is generally recognized as the major source for aerosol dispersion. The airflow paths of DPIs are complex, but it is a useful simplification to consider airflow through a cylindrical pipe, which consists of three regimes: an inviscid turbulent core, a viscous laminar sublayer, and a buffer layer where transition from turbulent to laminar flow occurs [114]. The large-scale motions of airflow are influenced by the boundary conditions, while the small-scale motion is determined by the rate of energy received from the large scales and the fluid viscosity. Eddies carry turbulent kinetic energy distributed over a broad range of scales. The airflow path of the pipe flow can be characterized by several closely related airflow parameters including Reynolds’ number (Re), shear stress (τ s ), pressure drop (ΔP), and power, which may be interpreted as indirect reflections of the energy experienced by the powder during dispersion. Among these parameters, Re is the ratio of inertial forces to the viscous forces. The value of Re is often used for prediction of turbulence. It is dependent upon device geometry, time scales and initial disturbance [18]. The viscous τ s is characterized by the energy cascade and Kolmogorov theory [115]. Briefly, the larger scales (eddies) transfer kinetic energy to successively smaller scales until reaching the smallest dissipative scale (Kolmogorov scale), and the energy is dissipated by viscous action into heat. The value of τ s can be approximated by relating the dynamic and kinematic viscosity of the air, and the energy dissipation rate (ε) which is a function of the nozzle velocity and diameter [116]. The kinetic energy dissipation can also cause ΔP. The value of ΔP measured using manometer up- and downstream of a device yields a combined value of both viscous and inviscid contribution [117], but either contribution can also be measured separately [118]. The power is the rate of work done or inspiratory effort during inhalation, so its value is related to the patient condition and inhalation capability [119]. In general these airflow parameters are positively correlated, and they have a close relationship with powder flow and de-agglomeration. In addition, specific resistance (R D ), an airflow independent parameter, is an intrinsic value that is a function of the device internal geometry and dimension. For a given device (determined R D ), inhaled flow rate is proportional to the square root of the ΔP [117].
10.6.2 DPI Device Technologies
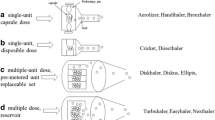
The goals of device innovation include design of efficient aerosolization mechanisms, reproducible dose metering, reduced dependence on inspiratory flow rates, and user friendliness. Each type of DPI device has unique airflow paths and internal geometries, which have been designed to help disperse the drug. The airflow path can be characterized in term of the aforementioned four airflow parameters. The DPI device design and innovation features can be classified into aerosolization, dose metering, and actuation methods (Table 10.1). The aerosolization methods include a variety of baffles, impellers, or tortuous channels through which particles will experience collisions of sufficient magnitude to dislodge drug particles and entrain them in the airstream. The dose metering can further be classified into single unit dose (capsule), multiunit dose (capsules, blisters, strips) and multiple doses (reservoir). The actuation can be either passive (breath-actuated) or energy assisted actuation. These features have been extensively reviewed [18, 68, 120–123]. The following discussion focuses on two technologies that can be used to facilitate device design. They include the incorporation of computational fluid dynamics (CFD) for device design and the standardized entrainment tubes (SETs) for the selection of proper airflow parameter for specific formulations.
10.6.3 Device Development Using CFD Technologies
Despite the many DPI products in the market, CFD has only recently gained its momentum for the study of DPI fluid flow, powder de-agglomeration, and device design, owning to the high speed computer technology. The turbulent flow around each particle can be analyzed using the Reynolds averaged Navier–Stokes equation [124]. The integral scale strain rate (γ i ) of the turbulence flow can be obtained as a function of the rate of energy dissipation (ε) and the rate of turbulence kinetic energy (k) [26]. The magnitude of the γ i is related to the effectiveness of particle de-agglomeration. By using CFD method, Coates et al. studied the influencing factors to the particle de-agglomeration in the Aerolizer device design by varying grid structure and mouthpiece length [26], capsule size [125], airflow rate [126], mouthpiece geometry [127]. The CFD results were coupled with experimental data using spray dried mannitol agglomerates to gain insight in aerosolization. More recently, a similar computer-aided design using rapid-prototyping 3-D print and CFD have been used for the rational design of devices [128]. This provided a quicker and simpler method for inhaler design than trial and error [128].
It should be noted that current CFD simulation capability is still limited with regard to the particle number (~10,000), the model formulation (carrier free agglomerates more often studied than carrier-based formulation [129]), and devices (specific de-agglomeration mechanism required for simulation).
10.6.4 Standardized Entrainment Tubes (SETs)
It is clear that mechanistic study of powder aerosolization requires that each contributing factor such as turbulence and impaction be studied separately [130]. This led to the design of specialized dispersion/entrainment tubes for specific mechanism study, such as the standardized entrainment tube that specialized for the study of the particle de-agglomeration influenced by grid structure [27, 131], turbulence [132], and impaction [133], respectively.
We have conducted a number of experiments in which the intention was to control the airflow path and describe it accurately in terms of defined airflow properties to evaluate formulation performance independently of unique device geometries. A series of standardized entrainment tubes with specific resistance range covering commercial DPI devices were built for these purposes [104] (Fig. 10.8). The PADE was first developed when the fine particle fraction of the carrier-based formulation performance was correlated with airflow parameter τ s [105]. For a specific carrier-based formulation, it can easily estimate by interpolation of the τ s to the performance invariable region [105]. In addition, two portions of the entrained dry powders: a major portion that involves in particle de-agglomeration and a minor portion remains in the entrainment tubes without de-agglomeration were differentiated, which is a useful feature of the entrainment tubes since it points exclusively to formulation performance in the absence of specific inhaler design characteristics [106]. This is a novel method of assessing the mass of powder delivered on a portion of the delivered airstream, which is important in assessing where the drug particles would be delivered effectively on the inspiratory flow.
Standardized entrainment tubes (with I. peripherally-located dosage table and II. centrally-located dosage table [104])
10.7 DPI Product Development
The development of a successful DPI is a challenging task which includes a comprehensive exploration with respect to the formulation/pre-formulation, device innovation, and patient interfaces, let alone the regulatory and marketing aspects. A myriad of factors and considerations for a DPI product are illustrated in Fig. 10.9. However, to be successful and competitive, simple approaches to formulation (minimal processes and excipients) and device development (minimum number of components) are adopted for rapid and efficient product development. For example, the pharmacopeial induction port (throat) was known to be a simplified treatment, but it is recommended for use in product quality control, for the reproducible in vitro measurement [17]. The major variables that influence the product development are often highlighted by the experimental design while any further optimization might not be considered. By minimizing the potential for confounded errors arising from complex strategies the likelihood of achieving manufacturing and performance specifications with sufficient control to achieve limited batch failures is more likely [134, 135]. The recent US FDA interest in quality by design is achieved by adopting engineering practices that allow for control and monitoring that with adequate risk management ensures the product quality. This also serves the commercial needs of the pharmaceutical industry as it ensures a rapid time to market.
10.8 Regulatory Considerations
Characterization of dry powder inhalers involves unique tests that are not employed for active aerosol dispersion systems such as propellant based metered dose inhalers or nebulized solutions/suspensions. For example, the delivered dose uniformity, fine particle mass over patient flow rate range, the airflow resistance are required for the DPI development but not for pMDI or nebulizer [136]. The responsiveness to airflow conditions is an important part of DPI aerosol performance. This is because:
-
1.
The DPI can only be used by a patient capable of inhaling adequately through it;
-
2.
The dose and particle size distribution of the delivered dose determined in vitro may be dependent upon the airflow rate chosen to test the system [137].
There is no clear path to generic equivalence for DPIs [138, 139]. This has resulted in a number of exploratory studies by the Product Quality Research Institute (PQRI) and the International Pharmaceutical Aerosol Consortium on Regulatory Science (IPAC-RS) to address the specific question of appropriate multivariate statistical approaches to the comparison of both particle size distributions and delivered dose uniformity to allow for specifications on in-vitro equivalence [140].
10.9 Conclusion
The foregoing discussion has presented the underlying scientific and engineering principles on which dry powder inhalers are based. There are unique performance variables for these devices, which require assessment and impact on the important measures biological effect delivered dose and particle size distribution. It is important to note that some knowledge of pharmacology and physiology is required to adequately understand the likely outcome of delivering aerosol with defined dose and size properties. Pharmaceutical development requires adequate engineering and process controls to ensure the final product quality. While regulatory guidance exists for the development of DPIs there is currently inadequate direction on statistical methods for comparison of in vitro equivalence.
10.10 Definitions, Abbreviations and Symbols
Aerodynamic particle size | Diameter of a sphere with density 1,000 kg/m3 having the same aerodynamic property as the particle |
Aerosol | Dispersion of liquid or solid particles in a gas (usually air), which is typically stable over long periods of time |
Agglomerate | Assemblage of primary particles with intermediate attractive forces (typically by point contacts; sometimes named aggregate) |
Aggregate | Assemblage of primary particles with strong attractive forces (typically by face contacts; sometimes named aggregate) |
Cluster | Generic name for the total group of agglomerated, aggregated and flocculated particles |
De-agglomeration | Formation of primary particles from agglomerates |
Fraunhofer diffraction | Diffraction of light at the contour of the particle (assumed to be spherical) |
Laser diffraction | Technique for estimating the particle size distribution from the measured scattering pattern by an ensemble of dispersed particles using laser light |
Power | The rate of work done or inspiratory effort during inhalation, an airflow parameter |
Primary particle | Basic particle, which cannot be separated unless by breakage |
Stokes’ diameter | Diameter of a sphere that has the same density and settling rate as the particle under conditions of Stokes’ law (viscous flow conditions) |
ACI | Andersen cascade impactor |
AFM | Atomic force microscopy |
APSD | Aerodynamic particle size distribution |
BET | Brunauer-Emmett-Teller expression |
CFD | Computation fluid dynamics |
COPD | Chronic obstructive pulmonary disease |
ΔP | Pressure drop, an airflow parameter |
DPI | Dry powder inhaler |
FPF | Fine particle fraction, the proportion of a nominal dose below a certain cutoff size |
FPFmax | Maximum fine particle fraction, a constant in the powder aerosol deaggregation equation |
GSD | Geometric standard deviation |
IPAC-RS | International Pharmaceutical Aerosol Consortium on Regulatory Science |
MMAD | Mass median aerodynamic diameter |
MMI | Marple-Miller impactor |
MSLI | Multi-stage liquid impinger |
NGI | Next generation impactor |
PADE | Powder aerosol deaggregation equation |
PDA | Phase Doppler analysis |
pMDI | Pressurized metered dose inhaler |
PQRI | Product Quality Research Institute |
PSD | Particle size distribution |
Re | Reynolds’ number of a device |
SET | Standardized entrainment tube |
VMD | Volume median diameter |
k | Turbulence kinetic energy |
k d | Deaggregation constant |
R D | Specific resistance |
γ i | Integral scale strain rate |
ε | Energy dissipation rate |
τ s | Shear stress, an airflow parameter |
References
Weibel, E.R.: Morphometry of the human lung: the state of the art after two decades. Bull. Eur. Physiopath. Res. 15, 999–1013 (1979)
Carvalho, T.C., Peters, J.I., Williams 3rd, R.O.: Influence of particle size on regional lung deposition–what evidence is there? Int. J. Pharm. 406, 1–10 (2011)
Sbirlea-Apiou, G., Katz, I., Caillibotte, G., Martonen, T., Yang, Y.: Deposition mechanics of pharmaceutical particles in human airways. In: Hickey, A.J. (ed.) Inhalation Aerosols: Physical and Biological Basis for Therapy, vol. 221, pp. 1–30. Informa Healthcare USA, New York (2007)
Byron, P.R.: Prediction of drug residence times in regions of the human respiratory tract following aerosol inhalation. J. Pharm. Sci. 75, 433–438 (1986)
http://www.mmadcalculator.com/andersen-impactor-mmad.html. Accessed 22 Jan 2012
Crowder, T.M., Rosati, J.A., Schroeter, J.D., Hickey, A.J., Martonen, T.B.: Fundamental effects of particle morphology on lung delivery: predictions of Stokes’ law and the particular relevance to dry powder inhaler formulation and development. Pharm. Res. 19, 239–245 (2002)
Gardenhire, D.S.: Airway pharmacology. In: Wilkins, R.L., Stoller, J.K., Kacmarek, R.M. (eds.) Egan’s Fundamentals of Respiratory Care, pp. 667–692. Mosby/Elsevier Inc., St. Louis (2009)
Gardenhire, D.S.: Corticosteroids in respiratory care. In: Gardenhire, D.S. (ed.) Rau’s Respiratory Care Pharmacology, pp. 204–225. Mosby/Elsevier Inc., St. Louis (2008)
Weers, J.G., Tarara, T.E., Clark, A.R.: Design of fine particles for pulmonary drug delivery. Expert Opin. Drug Deliv. 4, 297–313 (2007)
Raltiere, R.J., Thompson, D.C.: Physiology and pharmacology of the airways. In: Hickey, A.J. (ed.) Inhalation Aerosols: Physical and Biological Basis for Therapy, vol. 221, pp. 83–126. Informa Healthcare USA, Inc., New York (2007)
Byron, P.R., Delvadia, R.R., Longest, P.W., Hindle, M.: Stepping into the trachea with realistic physical models: Uncertainties in regional drug deposition from powder inhalers. Respir. Drug Deliv. 1, 215–224 (2010)
Finlay, W.H., Martin, A.R.: Recent advances in predictive understanding of respiratory tract deposition. J. Aerosol Med. Pulm. Drug Deliv. 21, 189–206 (2008)
Rostami, A.A.: Computational modeling of aerosol deposition in respiratory tract: A review. Inhal. Toxicol. 21, 262–290 (2009)
Byron, P.R., Hindle, M., Lange, C.F., Longest, P.W., McRobbie, D., Oldham, M.J., Olsson, B., Thiel, C.G., Wachtel, H., Finlay, W.H.: In vivo-in vitro correlations: Predicting pulmonary drug deposition from pharmaceutical aerosols. J. Aerosol Med. Pulm. Drug Deliv. 23(Suppl 2), S59–S69 (2010)
Longest, P.W., Holbrook, L.T.: In silico models of aerosol delivery to the respiratory tract – development and applications. Adv. Drug Deliv. Rev. 64, 296–311 (2012)
Physical test and determinations, aerosols, nasal sprays, metered-dose inhalers, and dry powder inhalers, USP <601>, pp. 220–240 (2007)
Byron, P.R.: Selection and validation of cascade impactor test methods. Respir. Drug Deliv. IX, 169–178 (2004)
Dunbar, C.A., Hickey, A.J., Holzner, P.: Dispersion and characterization of pharmaceutical dry powder aerosols. KONA 16, 7–45 (1998)
Marple, V.A., Olson, B.A., Santhanakrishnan, K., Mitchell, J.P., Murray, S.C., Hudson-Curtis, B.L.: Next generation pharmaceutical impactor (a new impactor for pharmaceutical inhaler testing). Part II: Archival calibration. J. Aerosol Med. 16, 301–324 (2003)
Marple, V.A., Olson, B.A., Santhanakrishnan, K., Roberts, D.L., Mitchell, J.P., Hudson-Curtis, B.L.: Next generation pharmaceutical impactor: a new impactor for pharmaceutical inhaler testing. Part III. Extension of archival calibration to 15 L/min. J. Aerosol Med. 17, 335–343 (2004)
Marple, V.A., Roberts, D.L., Romay, F.J., Miller, N.C., Truman, K.G., Van Oort, M., Olsson, B., Holroyd, M.J., Mitchell, J.P., Hochrainer, D.: Next generation pharmaceutical impactor (a new impactor for pharmaceutical inhaler testing). Part I: Design. J. Aerosol Med. 16, 283–299 (2003)
Merkus, H.G.: Laser diffraction. In: Particle size measurements: Fundamentals, practice, quality, pp. 259–286. Springer (2009). http://www.amazon.com/Particle-Size-Measurements-Fundamentals-Technology/dp/904818052X
Martin, G.P., MacRitchie, H.B., Marriott, C., Zeng, X.M.: Characterisation of a carrier-free dry powder aerosol formulation using inertial impaction and laser diffraction. Pharm. Res. 23, 2210–2219 (2006)
Marriott, C., MacRitchie, H.B., Zeng, X.M., Martin, G.P.: Development of a laser diffraction method for the determination of the particle size of aerosolised powder formulations. Int. J. Pharm. 326, 39–49 (2006)
Zeng, X.M., MacRitchie, H.B., Marriott, C., Martin, G.P.: Correlation between inertial impaction and laser diffraction sizing data for aerosolized carrier-based dry powder formulations. Pharm. Res. 23, 2200–2209 (2006)
Coates, M.S., Fletcher, D.F., Chan, H.K., Raper, J.A.: Effect of design on the performance of a dry powder inhaler using computational fluid dynamics. Part 1: Grid structure and mouthpiece length. J. Pharm. Sci. 93, 2863–2876 (2004)
Voss, A., Finlay, W.H.: Deagglomeration of dry powder pharmaceutical aerosols. Int. J. Pharm. 248, 39–50 (2002)
Begat, P., Morton, D.A., Staniforth, J.N., Price, R.: The cohesive-adhesive balances in dry powder inhaler formulations II: Influence on fine particle delivery characteristics. Pharm. Res. 21, 1826–1833 (2004)
Das, S., Larson, I., Young, P., Stewart, P.: Influence of storage relative humidity on the dispersion of salmeterol xinafoate powders for inhalation. J. Pharm. Sci. 98, 1015–1027 (2009)
Hickey, A.J.: Pharmaceutical Inhalation Aerosol Technology. Marcel Dekker, New York (2004)
Israelachvili, J.N.: Intermolecular and Surface Forces. Academic, London (1992)
Podczeck, F.: Particle-Particle Adhesion in Pharmaceutical Powder Handling. Imperial College Press, London (1998)
Visser, J.: An invited review van der Waals and other other cohesive forces affecting powder fluidization. Powder Technol. 58, 1–10 (1989)
Visser, J.: Particle adhesion and removal: A review. Particul. Sci. Technol. 13, 169–196 (1995)
Derjaguin, B.V.: Friction and adhesion. IV. The theory of adhesion of small particles. Kolloid Z. 69, 155–164 (1934)
Matsusaka, S., Maruyama, H., Matsuyama, T., Ghadiri, M.: Triboelectric charging of powders: A review. Chem. Eng. Sci. 65, 5781–5807 (2010)
Crowder, T.M., Hickey, A.J., Louey, M.D., Orr, N.: A Guide to Pharmaceutical Particulate Science. Interpharm Press/CRC, Boca Raton (2003)
Elajnaf, A., Carter, P., Rowley, G.: The effect of relative humidity on electrostatic charge decay of drugs and excipient used in dry powder inhaler formulation. Drug Dev. Ind. Pharm. 33, 967–974 (2007)
Elajnaf, A., Carter, P., Rowley, G.: Electrostatic characterisation of inhaled powders: Effect of contact surface and relative humidity. Eur. J. Pharm. Sci. 29, 375–384 (2006)
Hooton, J.C., German, C.S., Allen, S., Davies, M.C., Roberts, C.J., Tendler, S.J., Williams, P.M.: An atomic force microscopy study of the effect of nanoscale contact geometry and surface chemistry on the adhesion of pharmaceutical particles. Pharm. Res. 21, 953–961 (2004)
Podczeck, F.: The relationship between physical properties of lactose monohydrate and the aerodynamic behaviour of adhered drug particles. Int. J. Pharm. 160, 119–130 (1998)
Podczeck, F., Newton, J.M.: Development of an ultracentrifuge technique to determine the adhesion and friction properties between particles and surfaces. J. Pharm. Sci. 84, 1067–1071 (1995)
Hickey, A.J., Mansour, H.M., Telko, M.J., Xu, Z., Smyth, H.D., Mulder, T., McLean, R., Langridge, J., Papadopoulos, D.: Physical characterization of component particles included in dry powder inhalers. I. Strategy review and static characteristics. J. Pharm. Sci. 96, 1282–1301 (2007)
Ziskind, G., Fichman, M., Gutfinger, C.: Resuspension of particulates from surfaces to turbulent flows – review and analysis. J. Aerosol Sci. 26, 613–644 (1995)
Ziskind, G., Fichman, M., Gutfinger, C.: Adhesion moment model for estimating particle detachment from a surface. J. Aerosol Sci. 28, 623–634 (1997)
Wang, H.C.: Effects of inception motion on particle detachment from surfaces. Aerosol Sci. Technol. 13, 386–393 (1990)
Ibrahim, A.H., Dunn, P.F., Brach, R.M.: Microparticle detachment from surfaces exposed to turbulent air flow: Controlled experiments and modeling. J. Aerosol Sci. 34, 765–782 (2003)
Gradon, L.: Resuspension of particles from surfaces: Technological, environmental and pharmaceutical aspects. Adv. Powder Technol. 20, 17–28 (2009)
Reeks, M.W., Reed, J., Hall, D.: On the resuspension of small particles by a turbulent flow. J. Phys. D Appl. Phys. 21, 574–589 (1988)
Wenand, H.Y., Kasper, G.: On the kinetics of particle reentrainment from surfaces. J. Aerosol Sci. 20, 483–498 (1989)
Hersey, J.A.: Ordered mixing: A new concept in powder mixing practice. Powder Technol. 11, 41–44 (1975)
Corrigan, D.O., Corrigan, O.I., Healy, A.M.: Physicochemical and in vitro deposition properties of salbutamol sulphate/ipratropium bromide and salbutamol sulphate/excipient spray dried mixtures for use in dry powder inhalers. Int. J. Pharm. 322, 22–30 (2006)
Young, P., Roberts, D., Chiou, H., Rae, W., Chan, H.K., Traini, D.: Composite carriers improve the aerosolisation efficiency of drugs for respiratory delivery. J. Aerosol Sci. 39, 82–93 (2008)
Young, P.M., Kwok, P., Adi, H., Chan, H.K., Traini, D.: Lactose composite carriers for respiratory delivery. Pharm. Res. 26, 802–810 (2009)
Ooi, J., Traini, D., Hoe, S., Wong, W., Young, P.M.: Does carrier size matter? A fundamental study of drug aerosolisation from carrier based dry powder inhalation systems. Int. J. Pharm. 413, 1–9 (2011)
Adi, H., Traini, D., Chan, H.K., Young, P.M.: The influence of drug morphology on aerosolisation efficiency of dry powder inhaler formulations. J. Pharm. Sci. 97, 2780–2788 (2008)
Donovan, M.J., Smyth, H.D.: Influence of size and surface roughness of large lactose carrier particles in dry powder inhaler formulations. Int. J. Pharm. 402, 1–9 (2010)
Zhou, Q.T., Morton, D.A.: Drug-lactose binding aspects in adhesive mixtures: Controlling performance in dry powder inhaler formulations by altering lactose carrier surfaces. Adv. Drug Deliv. Rev. (2011)
Jones, M.D., Price, R.: The influence of fine excipient particles on the performance of carrier-based dry powder inhalation formulations. Pharm. Res. 23, 1665–1674 (2006)
Hooton, J.C., Jones, M.D., Price, R.: Predicting the behavior of novel sugar carriers for dry powder inhaler formulations via the use of a cohesive-adhesive force balance approach. J. Pharm. Sci. 95, 1288–1297 (2006)
Jones, M.D., Harris, H., Hooton, J.C., Shur, J., King, G.S., Mathoulin, C.A., Nichol, K., Smith, T.L., Dawson, M.L., Ferrie, A.R., Price, R.: An investigation into the relationship between carrier-based dry powder inhalation performance and formulation cohesive-adhesive force balances. Eur. J. Pharm. Biopharm. 69, 496–507 (2008)
Vehring, R.: Pharmaceutical particle engineering via spray drying. Pharm. Res. 25, 999–1022 (2008)
York, P.: Strategies for particle design using supercritical fluid technologies. Pharm. Sci. Technol. Today 2, 430–440 (1999)
Pilcer, G., Wauthoz, N., Amighi, K.: Lactose characteristics and the generation of the aerosol. Adv. Drug Deliv. Rev. 64, 233–256 (2012)
Pilcer, G., Amighi, K.: Formulation strategy and use of excipients in pulmonary drug delivery. Int. J. Pharm. 392, 1–19 (2010)
Xu, Z., Mansour, H.M., Hickey, A.J.: Particle interactions in dry powder inhaler unit processes: A review. J. Adhes. Sci. Technol. 25, 451–482 (2011)
Chan, H.K.: What is the role of particle morphology in pharmaceutical powder aerosols? Expert Opin. Drug Deliv. 5, 909–914 (2008)
Frijlink, H.W., De Boer, A.H.: Dry powder inhalers for pulmonary drug delivery. Expert Opin. Drug Deliv. 1, 67–86 (2004)
Telko, M.J., Hickey, A.J.: Dry powder inhaler formulation. Respir. Care 50, 1209–1227 (2005)
Chan, H.K.: Dry powder aerosol delivery systems: Current and future research directions. J. Aerosol Med. 19, 21–27 (2006)
Smythand, H., Hickey, A.J.: Carriers in dry powder delivery: Implications for inhalation system design. Am. J. Drug Deliv. 3, 117–132 (2005)
Steckeland, H., Muller, B.W.: In vitro evaluation of dry powder inhalers II: Influence of carrier particle size and concentration on in vitro deposition. Int. J. Pharm. 154, 31–37 (1997)
Vanderbist, F., Wery, B., Moyano-Pavon, I., Moes, A.J.: Optimization of a dry powder inhaler formulation of nacystelyn, a new mucoactive agent. J. Pharm. Pharmacol. 51, 1229–1234 (1999)
Young, P.M., Wood, O., Ooi, J., Traini, D.: The influence of drug loading on formulation structure and aerosol performance in carrier based dry powder inhalers. Int. J. Pharm. 416, 129–135 (2011)
Wetterlin, K.: Turbuhaler: A new powder inhaler for administration of drugs to the airways. Pharm. Res. 5, 506–508 (1988)
Edwards, A.M., Chambers, A.: Comparison of a lactose-free formulation of sodium cromoglycate and sodium cromoglycate plus lactose in the treatment of asthma. Curr. Med. Res. Opin. 11, 283–292 (1989)
Zhou, Q.T., Qu, L., Larson, I., Stewart, P.J., Morton, D.A.: Improving aerosolization of drug powders by reducing powder intrinsic cohesion via a mechanical dry coating approach. Int. J. Pharm. 394, 50–59 (2010)
Zhou, Q.T., Armstrong, B., Larson, I., Stewart, P.J., Morton, D.A.: Understanding the influence of powder flowability, fluidization and de-agglomeration characteristics on the aerosolization of pharmaceutical model powders. Eur. J. Pharm. Sci. 40, 412–421 (2010)
Zhou, Q.T., Denman, J.A., Gengenbach, T., Das, S., Qu, L., Zhang, H., Larson, I., Stewart, P.J., Morton, D.A.: Characterization of the surface properties of a model pharmaceutical fine powder modified with a pharmaceutical lubricant to improve flow via a mechanical dry coating approach. J. Pharm. Sci. 100, 3421–3430 (2011)
Begat, P., Morton, D.A., Shur, J., Kippax, P., Staniforth, J.N., Price, R.: The role of force control agents in high-dose dry powder inhaler formulations. J. Pharm. Sci. 98, 2770–2783 (2009)
Raula, J., Lahde, A., Kauppinen, E.I.: Aerosolization behavior of carrier-free L-leucine coated salbutamol sulphate powders. Int. J. Pharm. 365, 18–25 (2009)
Raula, J., Thielmann, F., Naderi, M., Lehto, V.P., Kauppinen, E.I.: Investigations on particle surface characteristics vs. dispersion behaviour of L-leucine coated carrier-free inhalable powders. Int. J. Pharm. 385, 79–85 (2010)
Chew, N.Y., Shekunov, B.Y., Tong, H.H., Chow, A.H., Savage, C., Wu, J., Chan, H.K.: Effect of amino acids on the dispersion of disodium cromoglycate powders. J. Pharm. Sci. 94, 2289–2300 (2005)
Hickey, A.J.: Pulmonary drug delivery: Pharmaceutical chemistry and aerosol technology. In: Wang, B., Siahaan, T., Soltero, R.A. (eds.) Drug Delivery: Principles and Applications, pp. 341–361. Wiley, New Jersey (2005)
Van Oort, M., Sacchetti, M.: Spray-drying and supercritical fluid particle generation techniques. In: Hickey, A.J. (ed.) Inhalation Aerosols: Physical and Biological Basis for Therapy, vol. 1, pp. 307–346. Informa Healthcare, New York (2007)
Seville, P.C., Li, H.Y., Learoyd, T.P.: Spray-dried powders for pulmonary drug delivery. Crit. Rev. Ther. Drug Carrier Syst. 24, 307–360 (2007)
Edwards, D.A., Hanes, J., Caponetti, G., Hrkach, J., Ben-Jebria, A., Eskew, M.L., Mintzes, J., Deaver, D., Lotan, N., Langer, R.: Large porous particles for pulmonary drug delivery. Science 276, 1868–1871 (1997). New York, NY
Tay, T., Das, S., Stewart, P.: Magnesium stearate increases salbutamol sulphate dispersion: What is the mechanism? Int. J. Pharm. 383, 62–69 (2010)
Young, P.M., Tobyn, M.J., Price, R., Buttrum, M., Dey, F.: The use of colloid probe microscopy to predict aerosolization performance in dry powder inhalers: AFM and in vitro correlation. J. Pharm. Sci. 95, 1800–1809 (2006)
Pilcer, G., Vanderbist, F., Amighi, K.: Spray-dried carrier-free dry powder tobramycin formulations with improved dispersion properties. J. Pharm. Sci. 98, 1463–1475 (2009)
Zeng, X.M., Martin, G.P., Tee, S.K., Ghoush, A.A., Marriott, C.: Effects of particle size and adding sequence of fine lactose on the deposition of salbutamol sulphate from a dry powder formulation. Int. J. Pharm. 182, 133–144 (1999)
Zeng, X.M., Pandhal, K.H., Martin, G.P.: The influence of lactose carrier on the content homogeneity and dispersibility of beclomethasone dipropionate from dry powder aerosols. Int. J. Pharm. 197, 41–52 (2000)
Traini, D., Young, P.M., Jones, M., Edge, S., Price, R.: Comparative study of erythritol and lactose monohydrate as carriers for inhalation: Atomic force microscopy and in vitro correlation. Eur. J. Pharm. Sci. 27, 243–251 (2006)
Sacchetti, M.: The nitrogen adsorption isotherm of alpha-lactose monohydrate. Pharm. Dev. Technol. 11, 351–358 (2006)
Young, P.M., Edge, S., Traini, D., Jones, M.D., Price, R., El-Sabawi, D., Urry, C., Smith, D.C.: The influence of dose on the performance of dry powder inhalation systems. Int. J. Pharm. 296, 26–33 (2005)
Ho, R., Muresan, A.S., Hebbink, G.A., Heng, J.Y.: Influence of fines on the surface energy heterogeneity of lactose for pulmonary drug delivery. Int. J. Pharm. 388, 88–94 (2010)
Louey, M.D., Stewart, P.J.: Particle interactions involved in aerosol dispersion of ternary interactive mixtures. Pharm. Res. 19, 1524–1531 (2002)
Jones, M.D., Santo, J.G., Yakub, B., Dennison, M., Master, H., Buckton, G.: The relationship between drug concentration, mixing time, blending order and ternary dry powder inhalation performance. Int. J. Pharm. 391, 137–147 (2010)
Jones, M.D., Hooton, J.C., Dawson, M.L., Ferrie, A.R., Price, R.: An investigation into the dispersion mechanisms of ternary dry powder inhaler formulations by the quantification of interparticulate forces. Pharm. Res. 25, 337–348 (2008)
Clineand, D., Dalby, R.: Predicting the quality of powders for inhalation from surface energy and area. Pharm. Res. 19, 1274–1277 (2002)
Adi, H., Larson, I., Chiou, H., Young, P., Traini, D., Stewart, P.: Role of agglomeration in the dispersion of salmeterol xinafoate from mixtures for inhalation with differing drug to fine lactose ratios. J. Pharm. Sci. 97, 3140–3152 (2008)
Adi, H., Larson, I., Stewart, P.J.: Adhesion and redistribution of salmeterol xinafoate particles in sugar-based mixtures for inhalation. Int. J. Pharm. 337, 229–238 (2007)
Adi, H., Larson, I., Chiou, H., Young, P., Traini, D., Stewart, P.: Agglomerate strength and dispersion of salmeterol xinafoate from powder mixtures for inhalation. Pharm. Res. 23, 2556–2565 (2006)
Louey, M.D., VanOort, M., Hickey, A.J.: Standardized entrainment tubes for the evaluation of pharmaceutical dry powder dispersion. J. Aerosol Sci. 73, 1520–1533 (2006)
Xu, Z., Mansour, H.M., Mulder, T., McLean, R., Langridge, J., Hickey, A.J.: Heterogeneous particle deaggregation and its implication for therapeutic aerosol performance. J. Pharm. Sci. 99, 3442–3461 (2010)
Xu, Z., Mansour, H.M., Mulder, T., McLean, R., Langridge, J., Hickey, A.J.: Dry powder aerosols generated by standardized entrainment tubes from drug blends with lactose monohydrate: 1. Albuterol sulfate and disodium cromoglycate. J. Pharm. Sci. 99, 3398–3414 (2010)
Xu, Z., Mansour, H.M., Mulder, T., McLean, R., Langridge, J., Hickey, A.J.: Dry powder aerosols generated by standardized entrainment tubes from drug blends with lactose monohydrate: 2. Ipratropium bromide monohydrate and fluticasone propionate. J. Pharm. Sci. 99, 3415–3429 (2010)
Mansour, H.M., Xu, Z., Hickey, A.J.: Dry powder aerosols generated by standardized entrainment tubes from alternative sugar blends: 3. Trehalose dihydrate and D-mannitol carriers. J. Pharm. Sci. 99, 3430–3441 (2010)
Freundlich, H.M.F.: Over the adsorption in solution. J. Phys. Chem. 57, 385–470 (1906)
Do, D.D.: Adsorption Analysis: Equilibria and Kinetics. Imperial College Press, London (1998)
Giles, C.H., Smith, D., Huitson, A.: A general treatment and classification of the solute adsorption isotherm I. Theoretical. J. Colloid Interface Sci. 47, 755–765 (1974)
Giles, C.H., Smith, D., Huitson, A.: A general treatment and classification of the solute adsorption isotherm II. Experimental interpretation. J. Colloid Interface Sci. 47, 766–778 (1974)
Brunauer, S., Emmett, P.H., Teller, E.: Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 60, 309–319 (1938)
Hickey, A.J., Ganderton, D.: Fluid flow. In: Hickey, A.J., Ganderton, D. (eds.) Pharmaceutical Process Engineering, pp. 4–30. Informa Healthcare USA, New York (2010)
Pope, S.B.: Wall flows. In: Pope, S.B. (ed.) Turbulent Flows, pp. 264–332. Cambridge University Press, Cambridge, UK (2000)
Shekunov, B.Y., Feeley, J.C., Chow, A.H., Tong, H.H., York, P.: Aerosolization behavior of micronized and supercritically-processed powders. J. Aerosol Sci. 34, 553–568 (2003)
Clarkand, A.R., Hollingsworth, A.M.: The relationship between powder inhaler resistance and peak inspiratory conditions in healthy volunteers – implications for in vitro testing. J. Aerosol Med. 6, 99–110 (1993)
Mendes, P.J., Pinto, J.F., Sousa, J.M.M.: A non-dimensional functional relationship for the fine particle fraction produced by dry powder inhalers. J. Aerosol Sci. 38, 612–624 (2007)
de Boer, A.H., Winter, H.M.I., Lerk, C.F.: Inhalation characteristics and their effects on in vitro drug delivery from dry powder inhalers part 1. Inhalation characteristics, work of breathing and volunteers’ preference in dependence of the inhaler resistance. Int. J. Pharm. 130, 231–244 (1996)
Islamand, N., Gladki, E.: Dry powder inhalers (DPIs)–a review of device reliability and innovation. Int. J. Pharm. 360, 1–11 (2008)
Newman, S.P., Peart, J.: Dry powder inhalers. In: Newman, S.P. (ed.) Respiratory Drug Delivery: Essential Theory and Practice, pp. 257–307. Respiratory Drug Delivery Online/VCU, Richmond (2009)
Hickey, A.J., Crowder, T.M.: Next generation dry powder inhalation delivery systems. In: Hickey, A.J. (ed.) Inhalation Aerosols: Physical and Biological Basis for Therapy, vol. 221, pp. 445–460. Informa Healthcare USA, New York (2007)
Smith, I.J., Parry-Billings, M.: The inhalers of the future?A review of dry powder devices on the market today. Pulm. Pharmacol. Ther. 16, 79–95 (2003)
Menter, F.R.: Two-equation eddy-viscosity models for engineering applications. AIAA J. 32, 1598–1605 (1994)
Coates, M.S., Fletcher, D.F., Chan, H.K., Raper, J.A.: The role of capsule on the performance of a dry powder inhaler using computational and experimental analyses. Pharm. Res. 22, 923–932 (2005)
Coates, M.S., Chan, H.K., Fletcher, D.F., Raper, J.A.: Influence of air flow on the performance of a dry powder inhaler using computational and experimental analyses. Pharm. Res. 22, 1445–1453 (2005)
Coates, M.S., Chan, H.K., Fletcher, D.F., Chiou, H.: Influence of mouthpiece geometry on the aerosol delivery performance of a dry powder inhaler. Pharm. Res. 24, 1450–1456 (2007)
Wong, W., Adi, H., Traini, D., Chan, H.K., Fletcher, D.F., Crapper, J., Young, P.: Use of rapid prototyping and computational fluid dynamics in the design the DPI devices. Respir. Drug Deliv. 3, 879–882 (2010)
Donovan, M.J., Kim, S.H., Raman, V., Smyth, H.D.: Dry powder inhaler device influence on carrier particle performance. J. Pharm. Sci. 101, 1097–1107 (2012)
Calvert, G., Ghadiri, M., Tweedie, R.: Aerodynamic dispersion of cohesive powders: A review of understanding and technology. Adv. Powder Technol. 20, 4–16 (2009)
Wong, W., Fletcher, D.F., Traini, D., Chan, H.K., Crapper, J., Young, P.M.: Particle aerosolisation and break-up in dry powder inhalers: Evaluation and modelling of the influence of grid structures for agglomerated systems. J. Pharm. Sci. 100, 4710–4721 (2011)
Wong, W., Fletcher, D.F., Traini, D., Chan, H.K., Crapper, J., Young, P.M.: Particle aerosolisation and break-up in dry powder inhalers 1: Evaluation and modelling of venturi effects for agglomerated systems. Pharm. Res. 27, 1367–1376 (2010))
Wong, W., Fletcher, D.F., Traini, D., Chan, H.K., Crapper, J., Young, P.M.: Particle aerosolisation and break-up in dry powder inhalers: Evaluation and modelling of impaction effects for agglomerated systems. J. Pharm. Sci. 100, 2744–2754 (2011)
USP: The United States Pharmacopeia. U.S. Pharmacopeial Convention, Rockville (2004)
Physical test and determinations, <601 > Aerosols, nasal sprays, metered-dose inhalers, and dry powder inhalers, USP, pp. 220–240
Guidance_for_industry.: Pharmaceutical quality of inhalation and nasal products, pp. 1–32. Canada or EU (2006). http://www.hc-sc.gc.ca/dhp-mps/alt_formats/hpfb-dgpsa/pdf/prodpharma/inhalationnas-eng.pdf
Committee_for_proprietary_medicinal_products.: Note for guidance on dry powder inhalers, pp. 1–6. London (1998)
Persson, G., Ankerst, J., Gillen, M., Bengtsson, T., Thorsson, L.: Relative systemic availability of budesonide in patients with asthma after inhalation from two dry powder inhalers. Curr. Med. Res. Opin. 24, 1511–1517 (2008)
Taki, M., Ahmed, S., Marriott, C., Zeng, X.M., Martin, G.P.: The ‘stage-by-stage’ deposition of drugs from commercial single-active and combination dry powder inhaler formulations. Eur. J. Pharm. Sci. 43, 225–235 (2011)
Shiand, S., Hickey, A.J.: Multivariate data analysis as a semi-quantitative tool for interpretive evaluation of comparability or equivalence of aerodynamic particle size distribution profiles. AAPS Pharm. Sci. Technol. 10, 1113–1120 (2009)
Behara, S.R., Larson, I., Kippax, P., Morton, D.A., Stewart, P.: The kinetics of cohesive powder de-agglomeration from three inhaler devices. Int. J. Pharm. 421, 72–81 (2011)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Hickey, A.J., Xu, Z. (2014). Dry Powder Inhalers. In: Merkus, H., Meesters, G. (eds) Particulate Products. Particle Technology Series, vol 19. Springer, Cham. https://doi.org/10.1007/978-3-319-00714-4_10
Download citation
DOI: https://doi.org/10.1007/978-3-319-00714-4_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-00713-7
Online ISBN: 978-3-319-00714-4
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)