Abstract
This chapter summarizes recent advances in oleofoam systems, the current understanding of their structure, and also proposes new characterization methods, which could give new insights into these systems. Despite their potential for food, cosmetic, and pharmaceutical products, in comparison to aqueous foam systems, oleofoam research is still in its infancy. Oleofoams could be used as food products combining a reduced fat content with a new appealing texture and sensorial properties due to the presence of gas bubbles. Oleofoams are produced by adding a high-melting fat component to a vegetable oil in the molten state, followed by cooling—to induce the formation of fat crystals—and aeration. The fat crystals stabilize both the air bubbles and the continuous phase between the bubbles. Oleofoams are multiscale hierarchical systems, for which relevant scales span from nanometers up to millimeters; as a consequence, a combination of characterization methods is required to understand the full structure and stabilization mechanisms, of which X-ray and neutron scattering and tomography offer unique opportunities.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Oleofoam
- Oleogel
- Organogel
- Whipped oil
- Crystalline particles
- Pickering stabilization
- X-ray scattering
- Neutron scattering
- Tomography
15.1 Introduction
Oleogelation of edible oils is a good alternative for the replacement of saturated fat in food formulations. To achieve oleogelation, gelator molecules are added to an edible oil, which convert the liquid oil into a gel (see previous chapters on oleogels). While this strategy may significantly improve the nutritional profile of full-fat products, due to the use of large amounts of oils within the formulation, the calorific content remains similar to the conventional solid fat product. Oleofoams (oil foams) are based on the incorporation of air bubbles inside an oleogel [1]. Oleofoams are a promising option for food products combining both a reduced fat content and a new appealing texture due to the presence of air bubbles [2]. Oleofoams have been described in several patents to partially replace butter in food products such as cakes, biscuits, laminated pastry, and mayonnaise [3,4,5,6]. Oleofoams were shown to decrease the fat content while still retaining a similar taste to the original products [3,4,5,6]. In the cosmetic and pharmaceutical industries, oleofoams could also be a good alternative for oily products, since oleofoams are easier for consumers to apply on the skin and hair due to their rheological properties [7,8,9]. Oleofoams more broadly exhibit further advantages such as long-term stability (for months even above room temperature), reduction in microbial spoilage (as water is absent and so preservatives are not needed). In addition, only one or even no additive is required to produce them; therefore, oleofoams could enter in the category of “clean label” products in various industries such as in food, cosmetics, and pharmaceuticals. However, the literature and understanding of oleofoam systems is still scarce in comparison to the aqueous foams despite their wide potential [10]. The main reason is that oleofoams are much more difficult to stabilize as the choice of foam stabilizer is very limited compared to their aqueous counterparts, explaining also why they are less studied in the literature [9].
The main difference between aqueous foams and oleofoams comes from the relatively large difference in the surface tension of the solvents. Water has a very high surface tension of 71.9 mN⋅m−1 at 25 °C, whereas most of the edible oils have surface tensions relatively lower between ca. 15 and 35 mN⋅m−1. In aqueous foams, a large variety of hydrocarbon-based surfactants, proteins, polymers, and particles can adsorb at the interface decreasing the air-water surface tension. For systems based on edible oils, the low surface tension between air and oil makes the adsorption of the most common hydrocarbon-based surfactants used to produce aqueous foams energetically unfavorable, leading to no adsorption at the air-oil surface [11]. Most of the suitable oil-soluble surfactants need to form crystalline particles to adsorb at the air-oil surface and allow foam formation and stabilization [11]. Indeed, most of the research on oleofoams has focused on finding a suitable foam stabilizer based on the oleogel literature and on trying to understand the relationship between crystalline properties inside the oleogels and the resulting foaming properties (foamability and foam stability) [12]. However, in common with aqueous foams, oleofoams are multiscale materials with relevant structural length scales spanning from a few nanometers up to millimeters. Different characterization methods need to be combined to obtain a comprehensive understanding of the oleofoam structure, which is the prerequisite to link formulation, processing, and the resulting macroscopic properties. This understanding is essential to control and adjust the properties of oleofoams in terms of rheological properties and their stability with respect to time and temperature, and improve the process for their manufacture [13]. As for aqueous foams, the characterization of oleofoams is challenging since they are turbid systems; the direct optical observation of bubbles and foam structure is limited to a small quantity of sample and thus with limited numbers of bubbles leading to a restricted understanding of some three-dimensional features. Moreover, oleofoams deform under low levels of shear and are highly sensitive to temperature fluctuations. In oleofoams, the measure of the liquid fraction is also much more challenging in comparison to aqueous foams. For aqueous foams, electrical conductivity is widely used to measure the liquid fraction in the foam, but for nonconducting fluids, measurements are much more complicated [14].
Powerful techniques based on the X-ray and neutron could be used to better understand the structure of oleofoams at the different relevant scales of these systems as it is already the case for aqueous foams [15,16,17,18]. In small-angle scattering (SAS) techniques, the intensity of scattered radiation versus the magnitude of the scattering vector (q) is measured. These techniques are commonplace in the study for soft matter, since they are noninvasive and without the need for special sample preparation methods (e.g., staining, exposure to vacuum) leading to a minimization of sample artifacts in contrary to microscopy techniques [16]. However, the reader needs to keep in mind that SAS yields nonvisual information unlike microscopy and provides structural information in the so-called reciprocal space. Thus, to extract information with SAS, the scattering data have to be either inverted back to real direct space or fitted with reciprocal space models available [19]. Since neutrons are an isotopically sensitive probe of structure, one advantage of small-angle neutron scattering (SANS) in the case of oleofoams is the potential for contrast variation by using deuterated components [15]. The aim of this chapter is to highlight the techniques and the corresponding structural information, which could be obtained by X-ray (SAXS) and neutron (SANS) scattering as well as by tomography on oleofoams in order to stimulate the interest of the readers to apply them in the future. That is why we also recommend the readers to have a look to reviews on SAS applied to food science if they want to go further on the understanding of these techniques and how to apply them in details [16, 19, 20].
15.2 Oleofoams: Formulation and Process
15.2.1 Formulation of Oleofoams
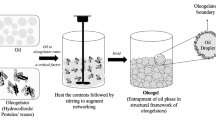
Oleofoams are composed of air bubbles dispersed in a continuous edible oil phase (Fig. 15.1). The process to produce oleofoams is based on two distinct steps. The first is to produce an oleogel; that is to say that an edible vegetable oil is used as the continuous phase into which a high-melting edible component is added. This mixture needs to be heated above the solubility boundary of the mixture to dissolve the high-melting edible component. By cooling the mixture below the solubility boundary, the high-melting edible component crystallizes leading to an oleogel. In the second step, the mixture is whipped to introduce air bubbles leading to oleofoams (Fig. 15.1a). Crystalline particles from various systems have been used to obtain oleofoams: mono-, di- and/or triglycerides, fatty alcohols, fatty acids, mixtures of phytosterols and mono- or diglycerides, and cocoa butter [8, 21,22,23,24,25,26,27]. Another approach to produce oleofoams is to use an oil or fat containing a high proportion of saturated medium-length fatty acid chains in order to have additive-free edible oleofoams such as coconut oil or shea butter [28, 29]. By controlling the temperature, the triglycerides with a high melting point crystallize while the other triglycerides with a lower melting point remain in a liquid state.
Schematic showing the two oleofoam production strategies. (a) Crystalline particles need first to be formed to stabilize air bubbles produced by whipping. (b) Surfactant molecules can stabilize air bubbles produced by whipping but, for long-term stabilization, cooling is required to produce crystalline particles. The resulting oleofoam is stabilized by both crystalline particles at the bubbles surface and in the oil continuous phase as observed by optical light microscopy
15.2.2 Emerging Systems to Produce Oleofoams
Recently, a new approach to produce oleofoams has been described [30]. Oleofoams can be stabilized directly by the addition of molecular surfactants before crystallization has occurred by using sucrose ester surfactant or sorbitan monoester [30, 31] (Fig. 15.1b). Oleofoams can be produced from the one-phase molecular solution because the triglycerides molecules form H-bonds with the hydroxyl groups of the surfactant [30]. In this case, the aeration process is first performed in the one-phase molecular region above the solubility boundary of the mixture. The prerequisite is that the oil-soluble surfactant should contain hydroxyl groups and that the alkyl chain lengths of the triglycerides and surfactant should be comparable to promote interfacial crystallization on cooling. These molecular complexes can adsorb at the air-oil surface, rendering efficient foam formation upon whipping above the solubility boundary. However, an efficient oleofoam stabilization is obtained only after rapid cooling of the foam to induce crystal formation in situ. In comparison to all the previous studies on oleofoams described above, this foaming strategy has the advantage of achieving much greater overrun (foam quantity).
15.2.3 Production Methods of Oleofoams
For all the systems cited above, the most popular foaming process is the whipping technique (electric beater, rotor-stator homogenizer, or kitchen food mixer). The whipping can take place during or after the cooling process, when the foam is stabilized only by crystalline particles (Fig. 15.1a). When foams are first stabilized by the surfactant layer at the air-oil surface, the whipping need to be first applied before cooling to increase the quantity of foam produced in the crystallized state (Fig. 15.1b). In whipping, the production of foam is based on air entrainment and progressive bubble breakup under shear [32]. During shearing, the gas fraction increases and the average bubble size decreases. Both the rheological properties of the oil dispersion and the whipping speed control the characteristic gas fraction and bubble size of the foaming mixture. To produce edible oleofoams on an industrial scale, high shear rotor-stator mixers such as Mondomix VL (Bühler company) are frequently used in the food industry for aqueous foam as well as oleofoam production [26]. It is important to point out that whatever the foaming process for these systems, the foam formation and stabilization is only possible when crystals are present in solution. The melting and crystallization temperatures of crystalline particles are linked to the solubility boundary, and temperature is therefore a crucial control parameter [9]. It is important to notice that oleofoams have also been produced by another foaming technique using depressurization but, thus far, only for cosmetic applications [33].
15.3 Structure and Properties of Oleofoams: State-of-the-Art of the Current Knowledge
15.3.1 Oleofoam Stabilization Mechanisms
For oleofoams, the presence of crystals is crucial for long-term stabilization. Each foam bubble is covered by adsorbed crystalline particles alone or below a surfactant monolayer, which prevents the relaxation of the bubble to a spherical shape [9]. This very high foam stability is due to the dense layer of adsorbed crystals at bubble surfaces that considerably reduce both coarsening and coalescence [9]. Moreover, the fraction of nonadsorbed crystals, which increases with crystal concentration, leads to gel network formation in the continuous oil phase reducing buoyancy-driven creaming of bubbles within the foam. The aging process is thus arrested leading to long-term stability. The presence of crystals both in bulk and at the interfaces is easily seen by using microscopy techniques such as polarized light microscopy, confocal Raman microscopy, or cryo-scanning electron microscopy (cryo-SEM) (Fig. 15.1).
15.3.2 Crystalline Particle Properties Leading to Oleofoams
The key criterion for crystals to adsorb at the air/oil interface is to exhibit a suitable contact angle below 90° [34]. An elegant study by Mishima and coworkers used synchrotron radiation microbeam X-ray diffraction (XRD) to understand the arrangement of triacylglycerol crystals around the air bubbles within an oleofoam [35]. They demonstrated that the lamellar planes of crystals near the air-oil surface are arranged almost parallel to the surface, that is, the lamellar planes composed of methyl end groups are facing the air phase, whereas the lateral planes composed of glycerol groups are connected to each other through crystals adsorbed at the air-oil surface [35].
For oleofoams stabilized by crystalline particles, both the foamability and foam stability depend on the size, shape, and concentration of particles. Needle-like and platelet crystals lead to efficient adsorption, whereas spherulitic particles seem less favorable for the adsorption [26, 36]. For oleogels, it is well known that the simplest way to tune the size and shape of crystalline particles is to use different cooling and shearing rates and also introduce tempering (cooling followed by slight warming) [37]. A clear link has been drawn between the properties of oleogels and those of foams in the case of oleogels based on fatty alcohol and fatty acid in sunflower oil forming platelet-shaped crystals [38]. Oleogel properties such as hardness and stability against oil loss are correlated with their resulting foaming properties in terms of foamability, foam firmness, and stability [7, 8]. For example, the foamability and foam stability of oleofoams based on fatty alcohol or fatty acid in sunflower oil were increased by increasing the cooling rate during the oleogel formation before whipping. Such enhancement in terms of oleofoam properties was shown to be linked to an increase of crystal quantity and a decrease in size inside the oleogel [7, 8]. However, there was no direct measurement on the crystal quantity and their size inside the foam after the oleogel whipping.
Another easy way to tune the quantity of crystals and their size without tuning the cooling rate or the shearing rate described in the oleogel field is to use a mixture of fatty alcohols and fatty acids with the same hydrocarbon chain length but at a specific molar ratio [39]. The optimal molar ratio of these two components is 3:1 of fatty alcohol:fatty acid for which co-crystallization occurs forming mixed crystals of small size and in high quantity [8, 40]. However, for other systems in which tempering was applied, the effect of polymorph type was not so clear and was not always observed on the foam properties [26, 35, 41]. These different conclusions could result from the fact that the tempering process not only influences the type of polymorph but also affects the quantity of crystals and the bulk rheological properties, which have a strong impact on the oleofoam properties [9].
15.3.3 Rheological Properties of Oleofoams
The main requirement to produce oleofoams from oleogels, from rheological perspective, is the need to behave as viscous dispersions once the whipping or mixing process starts in order to allow air incorporation; this implies specific rheological properties (such as yielding) for the base oleogels [42]. The rheological properties of oleofoams are still far from understood, since it results from both the bubbles coated by crystals and the rheology of the gelled continuous phase made of crystals [42, 43]. However, the rheological properties also strongly depend on the amount of incorporated air bubbles and their size. All these intermediate-scales parameters then control the macroscopic stability and rheology of the produced oleofoams. For the industrial applications of oleofoams in terms of the scale-up, it is very important that the oleofoam can be easily pumped through pipes and mixed with other components and it is directly linked to the rheological properties [2].
15.4 Multiscale Characterization of Oleofoams
15.4.1 Foam Structure and Rheological Properties
The structure of oleofoams is usually studied by using microscopy techniques or by indirect measurements such as oil drainage and texture profile analysis [8]. The main problem with microscopy techniques is sample preparation leading to artifacts in the microstructure, and which provide only two-dimensional (2D) information and a limited understanding of the three-dimensional (3D) foam structure [13]. X-ray tomography is a much better technique than microscopy to study the structure of oleofoams in three dimensions since, in contrast to visible light, X-rays are much less scattered by foams. By using X-ray tomography, the 3D oleofoam structure can be obtained in a matter of seconds with micrometric resolution [44]. Only one recent study used X-ray tomography to study oleofoam based on cocoa butter [13]. By using this technique, the authors quantitatively determine the number, size, and shape of the bubbles, as well as the thickness of the continuous phase. As a result, they could compare the effect of the process and time on the oleofoam structure (Figs. 15.2 and 15.3). For example, it is possible by looking at slices of the reconstructed volumes in a 2D plane to see a clear distinction between the gas phase (dark gray pixels) and the continuous phase (light gray pixels) enabling the thickness of the oleogel between the bubbles to be calculated and compared. By using X-ray tomography, it is possible to determine the thickness of oleogels present between the bubbles (around 10μm) and the remaining large domains of unwhipped oleogels as highlighted by the red narrow in Fig. 15.2.
Tomographic slice of an oleofoam based on 15 wt.% of cocoa butter crystals (15S) in a high oleic sunflower oil after (a) 5 or (b) 30 minutes of aeration by whipping the oleogel. The distribution of the oleogel thickness in yellow is overlaid on the respective images. The large oleogel fragments are highlighted with a red arrow. (Reproduced from [13] with permission from the American Chemical Society. This article is available under the terms of the creative commons attribution license (http://creativecommons.org/licenses/by/4.0/))
3D renderings of representative volume of interest of two oleofoam samples: (a) 15 wt.% of cocoa butter (15S) and (b) 30 wt.% of cocoa butter (30 S) in high oleic sunflower oil after 5 minutes of aeration by whipping. The equivalent bubble diameter distribution for both samples is represented: (c) 15 wt.% of cocoa butter (15S) and (d) 30 wt.% of cocoa butter (30S). The corresponding scatter plots with bubble size and sphericity (e, f). The color bar shows the volume fraction occupied by each bubble. (Reproduced from [13] with permission from the American Chemical Society. This article is available under the terms of the creative common attribution license (http://creativecommons.org/licenses/by/4.0/))
The dispersed gas phase can be described in terms of bubble size and shape distribution as shown in Fig. 15.3. The size distribution of the air bubbles inside oleofoams can be estimated and compared for different processes and oleofoam formulations as shown in Fig. 15.3. Another advantage of this technique, in contrast to microscopy techniques, is the examination of the shape of the bubbles in 3D, that is, the extent of bubble sphericity can be analyzed (Fig. 15.3); indeed, the nonspherical shape of the bubbles in oleofoams is clearly seen in Fig. 15.3e–f.
Small-angle scattering techniques, and indeed its lower q analog ultra-small-angle scattering (USAXS and USANS), are also valuable characterization tools for aqueous liquid foams, but unfortunately still not used for oleofoams. Specific setups have been designed to study aqueous foams produced by bubbling by SANS [18, 45]. Aqueous foams produced by other techniques such as hand-shaking and double-syringes techniques can also be studied by SANS without using specific measurement devices [46, 47]. SAXS and SANS have the advantages of being both sensitive to the foam structure but also to the foam stabilizer structure (crystalline particles in the case of oleofoams) [15]. Neutrons offer the opportunity to take advantage of isotopic sensitivity enabling different components within oleofoams to be specifically studied via the so-called contrast variation methods. There are few studies on the use of SANS for aqueous foams [18, 45,46,47,48]. However, emulsions have been studied extensively by this method [16, 49]. From the use of SANS, one could expect to extract different information on the oleofoam structure. Most of the oleofoam systems described in the literature are wet foams with high liquid fraction (above 36%). In oleofoams most of the bubbles are thus not in contact and thus the presence of the so-called thin films is limited. By knowing the liquid fraction, it would be possible to estimate a mean bubble size quantitatively with a high statistical precision inside the oleofoam due to the scattering occurring at the air/oil surface without the need of any sample preparation. The size of bubbles would be determined directly by using the q−4 decay at low q as described by the Porod law [15] (Fig. 15.4). For more information on how to extract the bubble size from SANS measurements, the reader is directed to the review by Mikhailovskaya et al. [15]. Temperature control is routinely conducted during SANS experiments, thus providing the potential to dynamically follow changes of bubbles size at different temperatures close to the melting temperatures of crystals. Rheo-SAXS and rheo-SANS could also give important information by measuring simultaneously both foam structure and rheological properties, and better characterize what happens for the foam structure under deformation [50]. Rheo-SAXS has already been used successfully to characterize the behavior of fat during crystallization and shear and the resulting network formation [51].
15.4.2 Characterization Methods of the Crystalline Phase Within the Foam
Oleofoams are specific foam systems since crystalline particles are present both at the interface and in bulk, and they are sensitive to temperature. Based on the literature, what is currently missing is the characterization in situ of crystals inside oleofoams in the bulk oleogel phase between the air bubbles. Information on the size, shape, polymorph type, and quantity is needed to better understand the oleofoam properties. Only wide-angle X-ray scattering (WAXS) characterization has been performed until now giving rise to information on the polymorph inside the foam [23, 24]. However, different techniques need to be combined to study these crystals in the continuous phase of the oleofoam. The best way to gain a better understanding of these foams at different length scales is to use and adapt the characterization techniques established to study classical aqueous liquid foams, emulsions, and/or oleogels. For example, low-field solid-state nuclear magnetic resonance (NMR) and differential scanning calorimetry (DSC) are best suited to determine the quantity of crystals. The size, aggregation state, shape, and polymorph of crystalline particles would require the combination of different microscopy techniques with scattering techniques such as SAXS and/or SANS as already used to study oleogels and aqueous foams stabilized by solid particles [15, 52, 53]. For example, SAXS and ultra-small-angle scattering (USAXS) have been shown to be very important techniques to describe the triglyceride (TAG) structure in liquid oils from the type of polymorph, to the packing of the lamellae, and also the shape and size of the TAG platelet as shown in Fig. 15.5 [54, 55]. SANS has already been used also to characterize oleogels [56,57,58]. For example, the self-assembled structures giving rise to oleogel formation in the mixtures of β-sitosterol and γ-oryzanol have been revealed by using SANS combined with solvent contrast variation [58]. By using decane as solvent in its hydrogenated form and deuterated form in different proportions, and keeping the oleogelator components in their hydrogenated forms (β-sitosterol and γ-oryzanol), it was possible to precisely determine the structure at the nanoscale at the origin of the gelling behavior. The oleogel structure is formed by both β-sitosterol/γ-oryzanol tubules, forming larger structures both trough intertubule aggregation and interconnection via junction zones leading to a space-filling network of fibrils [58]. This study is a good example to describe the value of SANS in comparison to SAXS, since the use of manipulation of contrast variation helps to test different structural models in order to be sure about the fitting of the scattering curves. SANS and USANS have also been applied to understand the gelling behavior of oleogels based on oleic acid and sodium oleate [56, 57]. In this case the solvent contrast variation method was used with two solvents: decane and hexadecane, which are easy to buy in deuterated forms. Inverse micellar structures and lamellar phases were measured before the formation of a crystalline space-filling network leading to oleogel formation. At low q by USANS, a mass fractal-like behavior was observed. SANS could also be used with the contrast variation method by using deuterated triglycerides oil, deuterated fatty acids, deuterated fatty alcohols, etc. The use of SANS for oleogel characterization is just at the beginning. The same approach could be used to characterize the structure, shape, and aggregation of crystalline particles within the oleofoam by using SAS and USAS techniques. There may also be opportunities to conduct simultaneous SAS and DSC studies [59, 60].
USAXS and SAXS data for a system made from tristearin as TAG into triolein as liquid oil. The red line shows the fitting using the unified fit model while solid black lines show the different slopes obtained [55]. At the smallest length scale, the morphology of the surface of the crystalline nanoplatelets is obtained from the slope P1 in the Porod region, while the size is obtained from the radius of gyration (Rg1) in the Guinier region. At intermediate q, from the Guinier-Porod model (P2 and Rg2), the presence of cylinders was observed. The crystalline nanoplatelets aggregate into cylinders due to the stacking of the platelets as shown in the figure. 𝑃2 and mass fractal values can be determined in the intermediate q region. In the smallest q region, the Porod exponent P3 and Rg3 can be also determined giving information on the surface fractal dimension. (Reproduced from [55] with permission from Elsevier)
15.4.3 Crystals at the Interface
In terms of interfacial characterization of crystals, not only is the contact angle important but also the structure of crystals at the interface inside oleofoams. One aim in these foam systems is to separate the structure and the interfacial effects of crystals from their effects in the bulk continuous phase. Experiments on planar model surfaces are needed. For example, by using interfacial rheology, Mishra et al. studied the crystal layer formation of tripalmitin and monopalmitin at the planar air-oil surface to gain insight into the adsorption of crystals at the interface [36]. They studied crystallization in situ by cooling the vessel below the crystallization temperature; they showed that monopalmitin initially adsorbs molecularly and, upon crystallization, an elastic network is formed rapidly. Tripalmitin however formed β crystals in bulk, which adsorbed to the surface forming a plastic deformable network of high absolute strength. Tripalmitin crystals stabilize the air-oil surface by a Pickering-type mechanism, whereas monopalmitin exists as both molecules and subsequent crystals [36]. However, there are significant opportunities to study model planar surfaces, to determine the kinetics of adsorption of species and the resulting layer thickness and structure using neutron and X-ray reflectivity techniques. Such techniques could be helpful in gaining further insight into the structure of the layer during cooling and crystallization but also to capture changes during heating. X-ray and neutron reflectivity have already applied to characterize a wide range of complex structures at the air/water interface but also at the oil/water surface [49, 61, 62].
15.5 Conclusions
Oleofoams are very promising for the food industry since the benefits include long-term stability above room temperature, healthier food products with low fat content, and new sensorial properties. In recent years, the main building blocks explaining oleofoam stability have been identified, allowing a first picture to be developed based on contributions from interfaces and bulk. While crystals govern the entire foam stability, a deeper understanding on the stabilization mechanisms and the foam structure is still required; this, in turn, offers the possibility to design new food products as well as oil foams for cosmetic and pharmaceutical applications. In the future, it will be important to continue the recent works to establish the detailed link between the foam structure and their resulting rheological properties depending on the crystal concentration, their size, and shape, both in bulk between the air bubbles and at the interfaces. The main problem remains to separate bulk and interfacial effects, which could be discriminated by combining techniques at different length scales such as NMR, microscopy techniques, and especially neutron and X-ray scattering techniques [15,16,17, 52, 63, 64]. For the readers interested in the use of SAS techniques after reading this chapter to characterize oleofoams but also oleogels, a review by Gilbert [16] describes the method as well as how it has been applied across a wide range of food-relevant colloidal systems. For general information on SAS, the SAS portal is an excellent resource for with comprehensive tutorials, software, books, and articles [19]. Similarly, for the application of neutron methods more broadly, including reflectivity, to food materials, the reader is directed to the review by Lopez-Rubio and Gilbert [20].
Abbreviations
- DSC:
-
Differential scanning calorimetry
- NMR:
-
Nuclear magnetic resonance
- SANS:
-
Small-angle neutron scattering
- SAS:
-
Small-angle scattering
- SAXS:
-
Small-angle X-ray scattering
- USANS:
-
Ultra-small-angle neutron scattering
- USAXS:
-
Ultra-small angle X-ray scattering
- WAXS:
-
Wide-angle X-ray scattering
References
Fameau A-L, Cousin F, Saint-Jalmes A (2017) Morphological transition in fatty acid self-assemblies: a process driven by the interplay between the chain-melting and surface-melting process of the hydrogen bonds. Langmuir 33. https://doi.org/10.1021/acs.langmuir.7b02651
Heymans R, Tavernier I, Dewettinck K, Van der Meeren P (2017) Crystal stabilization of edible oil foams. Trends Food Sci Technol 69:13–24
Gunes ZD, Schafer O, Chisholm H, Deyber H, Pelloux C, Binks BP (2018) Lipid based foam. WO 2016/150978
Gehin-Delval C, Chisholm H, Gunes ZD, Deyber H, Pelloux C, Schafer O, Nouzille CA, Destribats MJ (2019) Food composition comprising gas bubbles. US 10 , 383 , 352 B2
Chisholm H, Gunes ZD, Gehin-Delval C, Nouzille CA, Garvey E, Destribats MJ, Chandrasekaran SN, Vieira JB, German J, Binks BP (2018) Aerated confectionery material. US 2018 / 0064127 A1
Gehin-Delval C, Chisholm H, Chung W, Deyber H, Destribats MJ, Gunes ZD, Pelloux C (2019) Method for forming a laminated pastry. US 2019 / 0200625 A1
Callau M, Jenkins N, Sow-Kébé K, Levivier C, Fameau A-L (2021) The effect of vegetable oil composition on the structural properties of oleogels based on behenyl alcohol/behenic acid oleogelator system. J Cosmet Sci 72:399–405
Callau M, Sow-Kébé K, Jenkins N, Fameau A-L (2020) Effect of the ratio between fatty alcohol and fatty acid on foaming properties of whipped oleogels. Food Chem 333:127403. https://doi.org/10.1016/j.foodchem.2020.127403
Fameau A-L, Binks BP (2021) Aqueous and oil foams stabilized by surfactant crystals: new concepts and perspectives. Langmuir 37:4411–4418. https://doi.org/10.1021/acs.langmuir.1c00410
Dickinson E (2020) Advances in food emulsions and foams: reflections on research in the neo-Pickering era. Curr Opin Food Sci 33:52–60
Binks BP, Vishal B (2021) Particle-stabilized oil foams. Adv Colloid Interf Sci 291:102404. https://doi.org/10.1016/j.cis.2021.102404
Fameau A-L, Saint-Jalmes A (2020) Recent advances in understanding and use of oleofoams. Front Sustain Food Syst 4. https://doi.org/10.3389/fsufs.2020.00110
Metilli L, Storm M, Marathe S, Lazidis A, Marty-Terrade S, Simone E (2022) Application of X-ray microcomputed tomography for the static and dynamic characterization of the microstructure of Oleofoams. Langmuir 38:1638–1650
Salonen A, Lhermerout R, Rio E, Langevin D, Saint-Jalmes A (2012) Dual gas and oil dispersions in water: production and stability of foamulsion. Soft Matter 8:699–706
Mikhailovskaya A, Zhang L, Cousin F, Boue F, Yazhgur P, Muller F, Gay C, Salonen A (2017) Probing foam with neutrons. Adv Colloid Interf Sci 247:444–453
Gilbert EP (2019) Small-angle X-Ray and neutron scattering in food colloids. Curr Opin Colloid Interface Sci 42:55–72
Metilli L, Francis M, Povey M, Lazidis A, Marty-Terrade S, Ray J, Simone E (2020) Latest advances in imaging techniques for characterizing soft, multiphasic food materials. Adv Colloid Interf Sci 279:102154
Lamolinairie J, Dollet B, Bridot J-L, Bauduin P, Diat O, Chiappisi L (2022) Probing foams from the nanometer to the millimeter scale by coupling small-angle neutron scattering, imaging, and electrical conductivity measurements. Soft Matter 18:8733
SAS portal (n.d.). http://smallangle.org/. Accessed 29 Nov 2022
Lopez-Rubio A, Gilbert EP (2009) Neutron scattering: a natural tool for food science and technology research. Trends Food Sci Technol 20:576–586
Qiu C, Lei M, Lee WJ, Zhang N, Wang Y (2021) Fabrication and characterization of stable oleofoam based on medium-long chain diacylglycerol and β-sitosterol. Food Chem 350:129275
Metilli L, Lazidis A, Francis M, Marty-Terrade S, Ray J, Simone E (2021) The effect of crystallization conditions on the structural properties of oleofoams made of cocoa butter crystals and high oleic sunflower oil. Cryst Growth Des 21:1562–1575
Fameau A-L, Lam S, Arnould A, Gaillard C, Velev OD, Saint-Jalmes A (2015) Smart nonaqueous foams from lipid-based Oleogel. Langmuir 31:13501–13510
Binks BP, Garvey EJ, Vieira J (2016) Whipped oil stabilised by surfactant crystals. Chem Sci 7:2621–2632. https://doi.org/10.1039/C6SC00046K
Shrestha RG, Shrestha LK, Solans C, Gonzalez C, Aramaki K (2010) Nonaqueous foam with outstanding stability in diglycerol monomyristate/olive oil system. Colloids Surfaces A Physicochem Eng Asp 353:157–165
Gunes DZ, Murith M, Godefroid J, Pelloux C, Deyber H, Schafer O, Breton O (2017) Oleofoams: properties of crystal-coated bubbles from whipped Oleogels-evidence for pickering stabilization. Langmuir 33:1563–1575
Brun M, Delample M, Harte E, Lecomte S, Leal-Calderon F (2015) Stabilization of air bubbles in oil by surfactant crystals: a route to produce air-in-oil foams and air-in-oil-in-water emulsions. Food Res Int 67:366–375
Binks BP, Marinopoulos I (2017) Ultra-stable self-foaming oils. Food Res Int 95:28–37
Liu Y, Binks BP (2021) Foams of vegetable oils containing long-chain triglycerides. J Colloid Interface Sci 583:522–534
Liu Y, Binks BP (2021) A novel strategy to fabricate stable oil foams with sucrose ester surfactant. J Colloid Interface Sci 594:204–216
Liu Y, Binks BP (2022) Fabrication of stable Oleofoams with Sorbitan Ester Surfactants. Langmuir 38:14779–14788
Drenckhan W, Saint-Jalmes A (2015) The science of foaming. Adv Colloid Interf Sci 222:228–259
Shrestha LK, Aramaki K, Kato H, Takase Y, Kunieda H (2006) Foaming properties of monoglycerol fatty acid esters in nonpolar oil systems. Langmuir 22:8337–8345
Fameau A-L, Saint-Jalmes A (2017) Non-aqueous foams: current understanding on the formation and stability mechanisms. Adv Colloid Interf Sci 247:454–464. https://doi.org/10.1016/j.cis.2017.02.007
Mishima S, Suzuki A, Sato K, Ueno S (2016) Formation and microstructures of whipped oils composed of vegetable oils and high-melting fat crystals. J Am Oil Chem Soc 93:1453–1466. https://doi.org/10.1007/s11746-016-2888-4
Mishra K, Bergfreund J, Bertsch P, Fischer P, Windhab EJ (2020) Crystallization induced network formation of tri-and monopalmitin at the MCT oil/air interface. Langmuir 36:7566–7572
Marangoni AG (2004) Fat crystal networks. CRC Press
Callau M, Sow-Kébé K, Nicolas-Morgantini L, Fameau AL (2020) Effect of the ratio between behenyl alcohol and behenic acid on the oleogel properties. J Colloid Interface Sci 560:874–884
Blach C, Gravelle AJ, Peyronel F, Weiss J, Barbut S, Marangoni AG (2016) Revisiting the crystallization behavior of stearyl alcohol: stearic acid (SO: SA) mixtures in edible oil. RSC Adv 6:81151–81163. https://doi.org/10.1039/C6RA15142F
Shah DO (1971) Significance of the 1: 3 molecular ratio in mixed surfactant systems. J Colloid Interface Sci 37:744–752
Heymans R, Tavernier I, Danthine S, Rimaux T, Van der Meeren P, Dewettinck K (2018) Food-grade monoglyceride oil foams: the effect of tempering on foamability, foam stability and rheological properties. Food Funct 9:3143–3154. https://doi.org/10.1039/C8FO00536B
Mishra K, Dufour D, Windhab EJ (2020) Yield stress dependent foaming of edible crystal-melt suspensions. Cryst Growth Des 20:1292–1301
Saha S, Saint-Michel B, Leynes V, Binks BP, Garbin V (2020) Stability of bubbles in wax-based oleofoams: decoupling the effects of bulk oleogel rheology and interfacial rheology. Rheol Acta 59:1–12
Meagher AJ, Mukherjee M, Weaire D, Hutzler S, Banhart J, Garcia-Moreno F (2011) Analysis of the internal structure of monodisperse liquid foams by X-ray tomography. Soft Matter 7:9881–9885
Kühnhammer M, Widmann T, Kreuzer LP, Schmid AJ, Wiehemeier L, Frielinghaus H, Jaksch S, Bögershausen T, Barron P, Schneider H (2021) Flexible sample environments for the investigation of soft matter at the European Spallation Source: part III—the macroscopic foam cell. Appl Sci 11:5116
Fameau A-L, Saint-Jalmes A, Cousin F, Houinsou Houssou B, Novales B, Navailles L, Nallet F, Gaillard C, Boué F, Douliez J-P (2011) Smart foams: switching reversibly between ultrastable and unstable foams. Angew Chemie Int Ed 50:8264–8269. https://doi.org/10.1002/anie.201102115
Arnould A, Cousin F, Salonen A, Saint-Jalmes A, Perez A, Fameau A-L (2018) Controlling foam stability with the ratio of Myristic acid to choline hydroxide. Langmuir 34:11076–11085. https://doi.org/10.1021/acs.langmuir.8b02261
Zhang L, Mikhailovskaya A, Yazhgur P, Muller F, Cousin F, Langevin D, Wang N, Salonen A (2015) Precipitating sodium dodecyl sulfate to create ultrastable and stimulable foams. Angew Chemie Int Ed 54:9533–9536
Penfold J, Thomas RK (2014) Neutron reflectivity and small angle neutron scattering: an introduction and perspective on recent progress. Curr Opin Colloid Interface Sci 19:198–206
Eberle APR, Porcar L (2012) Flow-SANS and Rheo-SANS applied to soft matter. Curr Opin Colloid Interface Sci 17:33–43
Nikolaeva T, Den Adel R, Velichko E, Bouwman WG, Hermida-Merino D, Van As H, Voda A, Van Duynhoven J (2018) Networks of micronized fat crystals grown under static conditions. Food Funct 9:2102–2111
Fameau A-L, Salonen A (2014) Effect of particles and aggregated structures on the foam stability and aging. Comptes Rendus Phys 15:748–760
Marangoni AG, Van Duynhoven JPM, Acevedo NC, Nicholson RA, Patel AR (2020) Advances in our understanding of the structure and functionality of edible fats and fat mimetics. Soft Matter 16:289–306
Ramel PR, Co ED, Acevedo NC, Marangoni AG (2016) Structure and functionality of nanostructured triacylglycerol crystal networks. Prog Lipid Res 64:231–242
Peyronel F, Pink DA, Marangoni AG (2014) Triglyceride nanocrystal aggregation into polycrystalline colloidal networks: ultra-small angle X-ray scattering, models and computer simulation. Curr Opin Colloid Interface Sci 19:459–470
Nikiforidis CV, Gilbert EP, Scholten E (2015) Organogel formation via supramolecular assembly of oleic acid and sodium oleate. RSC Adv 5:47466–47475. https://doi.org/10.1039/c5ra05336f
Cornet S, de Campo L, Martínez-Sanz M, Scholten E, Gilbert EP (2021) Small-angle neutron scattering reveals basis for composition dependence of gel behaviour in oleic acid-sodium oleate oleogels. Innov Food Sci Emerg Technol 73:102763
Gilbert EP (2022) Building blocks of β-sitosterol-γ-oryzanol gels revealed by small-angle neutron scattering and real space modelling. Food Funct 13:7123–7131
Pullen SA, Booth N, Olsen SR, Day B, Franceschini F, Mannicke D, Gilbert EP (2014) Design and implementation of a differential scanning calorimeter for the simultaneous measurement of small angle neutron scattering. Meas Sci Technol 25:55606
Gilbert EP, Nelson A, Sutton D, Terrill N, Martin C, Lal J, Lang E (2005) Phase separation in the organic solid state: the influence of quenching protocol in unstable n-alkane blends. Mol Cryst Liq Cryst 440:93–105
Campbell RA (2018) Recent advances in resolving kinetic and dynamic processes at the air/water interface using specular neutron reflectometry. Curr Opin Colloid Interface Sci 37:49–60
Skoda MWA (2019) Recent developments in the application of X-ray and neutron reflectivity to soft-matter systems. Curr Opin Colloid Interface Sci 42:41–54
Marangoni AG, Garti N (2018) Edible oleogels: structure and health implications. AOC Press, San Diego
Low LE, Siva SP, Ho YK, Chan ES, Tey BT (2020) Recent advances of characterization techniques for the formation, physical properties and stability of Pickering emulsion. Adv Colloid Interf Sci 277:102–117
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Fameau, AL., Gilbert, E.P. (2024). Oleofoams: Formulation Rules and New Characterization Methods Based on X-Rays and Neutrons to Advance Current Understanding. In: Palla, C., Valoppi, F. (eds) Advances in Oleogel Development, Characterization, and Nutritional Aspects. Springer, Cham. https://doi.org/10.1007/978-3-031-46831-5_15
Download citation
DOI: https://doi.org/10.1007/978-3-031-46831-5_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-46830-8
Online ISBN: 978-3-031-46831-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)