Abstract
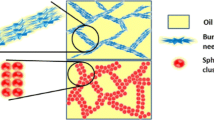
The mechanical properties of fat–oil mixtures are dictated by the morphology of the fat crystal network, which in turn is strongly affected by the processing conditions. In this work the effect of uniform (linear) cooling rates on the size and shape of tribehenin (fat) clusters in isopropyl myristate (oil) was studied. The state of the fat–oil mixtures was comprehensively analyzed by establishing pseudo-phase diagrams via visual observations, brightfield and polarization microscopy at various cooling rates and fat concentrations. The microstructure of the constituent fat clusters undergo a shape transition from bundles of needles at low cooling rates to spherical clusters at high cooling rates. The network structures formed with these different shaped fat clusters were examined using rheology. At a given cooling rate, the storage modulus (Gʹ) of fat–oil mixtures versus fat concentration followed a power law relationship. For a given fat concentration, the fat crystal networks comprising of bundles of needles showed higher Gʹ values as compared to fat networks made of spherical clusters. While the fractal dimension of networks cooled at different cooling rates varied over a small range of 2.78–2.86, considerable change in the pre-exponential factor (γ) was observed. The results show the critical importance of processing conditions on the shape and size of fat clusters and its impact on the rheological properties of the fat crystal network.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fat containing mixtures have wide applications in food, cosmetic and pharmaceutical industry. Some common examples of such fats are milk fat, tristearin, candelilla wax and so on. The properties of such systems are dependent on the structural characteristics of their fat crystal networks in oil or other complex fluids [1,2,3]. Many important functional properties of fat-containing products (i.e. hardness, spreadability, viscosity, gloss and smoothness) are dictated by the characteristics of the crystal network formed by the constituent triglyceride (TAG) species [4]. Structures of such crystal networks are a strong function of the concentration, structural and polymorphic nature of the fat along with the processing conditions [3]. Thus, the understanding of structure–property relationship of fat–oil mixtures will enable one to formulate products with desired characteristics [3, 4].
Crystallization of fat in oil occurs by nucleation and growth events that lead to the formation of particles usually in the micron size range. At sufficient fat concentration these particles further associate to form clusters and these clusters interconnect forming a continuous three dimensional network [5,6,7]. The clusters are held together by van der Waals forces and trapped inside this solid network structure is the oil phase. Furthermore, TAG also can crystallize in three major polymorphs, α, β′ and β in the ascending order of their melting point, density and stability. The different polymorphs are a result of different alignments of fatty acid chains of TAG molecules into different lattice structures [8]. The subcell structures of α, β’ and β forms have been associated with hexagonal, orthorhombic and triclinic arrangements, respectively with each having a characteristic X-ray diffraction (XRD) pattern [9]. It has been shown that rapid cooling of the fat–oil mixtures generally results in the formation of α polymorphs while slow cooling results in β and β′ [9,10,11].
Fat–oil mixtures can be liquid, plastic or solid at room temperature [12]. Therefore, it is essential to characterize the phase behavior of these fat–oil mixtures to formulate desired products. In some of the earlier reports, the porous structure of fat crystal networks were captured by removing the oil from the mixture using a cold solvent [17]. Many studies have characterized the structure and properties of model fat crystal networks by varying the processing conditions (i.e. varying the fat concentration and cooling conditions) [4, 13,14,15,16]. Further, the influence of network morphology on rheological properties has also been explored for the past few decades [4, 16, 18]. The understanding of the structure–property relationships of fat crystal networks was greatly enhanced by the application of fractal theory to analyze such networks [6, 7, 19,20,21]. In 1992, Vreeker et al. presented an interpretation of the rheological data of fat crystal networks based on the fractal theory [7]. They showed that the elastic modulus (Gʹ) of the network varied with fat concentration according to a power law, similar to models for the elasticity of colloidal gels [22]. Although strong correlations have been found between the fat concentration and rheological properties of fat–oil mixtures [23], the rheological properties dependence on shape and size of crystal clusters are not well understood [3].
In this study, a TAG that is used as a thickening agent in personal care products was used as a model system to study the fat crystal networks. This material is largely unexplored to the best of our knowledge. The primary purpose of this study is to understand the effect of fat concentration and cooling rate on cluster size, shape and rheological properties of fat–oil mixtures using tribehenin (fat)-isopropyl myristate (oil) as a model system. The cooling rates used were uniform (linear) ensuring homogeneous crystallizing conditions. The methodology of establishing phase diagrams which is commonly used in colloidal systems was adopted to study the evolution of fat–oil mixtures. Based on the visual observations across a range of cooling rates and fat concentrations, a macroscopic pseudo-phase diagram was first obtained. The microstructure of the constituent fat crystal clusters (at low fat concentration) and crystal network (at high fat concentration) were captured via brightfield and polarization optical microscopy. The properties of fat–oil mixtures were characterized by quantitative analysis of microscopy images and oscillatory rheology. The microstructure of the constituent fat clusters comprising these fat–oil mixtures showed a shape transition from bundles of needles to spherical clusters with increase in cooling rate.
Materials and Methods
Materials
The solid fat 2,3-di(docosanoyloxy)propyl docosanoate (tribehenin) was obtained from Croda chemicals (India) under the name of syncrowax-HRC which is commercially used in cosmetic applications. The purity of tribehenin in syncrowax-HRC as provided by the supplier is around 90–100 wt%. Tribehenin with a molecular formula of C69H134O6 is a triglyceride of glycerin and behenic acid, a naturally occurring long chain fatty acid. It is commonly used as an oil phase thickener. The melting point of tribehenin is 59.5 ± 2.5 °C. The oil phase isopropyl myristate (IPM) is an ester of isopropanol and myristic acid. IPM (C17H34O2) was purchased from Sigma–Aldrich at 98% purity (CAS Number 110-27-0). The density of IPM at 25 °C is 0.850 g/cm3 and density of tribehenin is 0.89 g/cm3. The melting and boiling points of IPM are 3 and 315 °C (at 760 mmHg), respectively.
Solubility
The solubility studies were carried out prior to fixing the cooling profile for the fat–oil mixtures. Fat–oil mixtures at various fat concentrations (0.5, 1, 2, 5, 10, 15, 25 wt%) were maintained at fixed temperatures for 24 h. After 24 h the maximum fat concentration at which the solution was clear without any turbidity (confirmed using a light source) was taken as the equilibrium fat solubility at that temperature. The solubility of tribehenin in isopropyl myristate is shown in Fig. 1. We observed complete crystallization at 20 °C and complete dissolution at 80 °C for the aforementioned fat concentrations. Hence, the temperatures for cooling were fixed at 80–20 °C.
Sample Preparation
The fat–oil mixtures were prepared in clean, dry glass vials. The total weight of each mixture was maintained at 10 grams. The mixtures were prepared at fat concentrations of 0.5, 1, 2, 5, 7.5, 10, 15, 20 and 25 wt%. The fat concentrations were converted to volume% for fractal dimension calculations using the densities of the fat and oil. These mixtures were placed in a clean environmental chamber (Mem-mert CTC256, Germany) maintained at 80 °C for 1 h to completely dissolve the fat in oil. After this, a homogeneous solution of fat–oil mixtures was obtained which was transparent as shown in Fig. 2a. These mixtures were then cooled to 2 °C at cooling rates of 0.1; 0.5; 1.0; 2.0 °C/min. The chamber was equipped with platinum temperature sensors along with microprocessor temperature controller. The temperature controller had an integrated timer which can be used to ramp temperatures linearly in a given time there by controlling the cooling rate. Hence, for crystallizing the samples at 0.1 °C/min the temperature was ramped down from 80 to 20 °C in 600 min. Similarly, the ramping down time was controlled to prepare samples at different cooling rates. After cooling the Samples were held at 20 °C for 24 h after which they which they were characterized at room temperature. Since the chamber could not be used to attain cooling rates higher than 2 °C/min owing to its size, higher cooling rates were attained by cooling the fat–oil mixtures in a constant temperature water bath. For the preparation of fat–oil mixtures at higher cooling rates, namely, 6 and 9 °C/min, water baths maintained at 20 and 5 °C, respectively were used. The cooling time for these samples was 2 h. Unlike lower cooling rates in an environment chamber, these cooling rates are not uniform as they were prepared in a constant temperature environment. The cooling profile of these mixtures was measured and the average cooling rate was calculated to be 9 and 6 °C/min. All the samples were prepared in duplicate to ensure consistency.
X-Ray Diffraction (XRD)
XRD patterns were recorded by illuminating the sample with a CuKα radiation of wavelength 1.54 Å. Approximately 1 g of 25 wt% fat–oil mixtures cooled at different cooling rates were analyzed. Samples were analyzed after 1 month giving enough time to reach a stable polymorphic form. The samples were analyzed in the 2θ range of 10–30°, which corresponds to d-spacing of 2.5–8.8 Å. All XRD patterns were obtained at a temperature of 25 °C and collected in triplicate.
Differential Scanning Calorimetry (DSC)
Thermal behavior of fat–oil mixtures were recorded using a TA-Q2000 instrument. Samples weighing 10–20 mg of 25 wt% fat–oil mixtures crystallized at different cooling rates were placed in a sealed Tzero alumina pan. Melting curves were recorded by heating the samples from 20 to 70 °C at the rate of 5 °C/min. All DSC melting curves were collected in triplicate.
Microstructure
The microstructure of the fat crystals were recorded using an inverted optical microscope from Leica, Germany. Brightfield and polarization modes were used at magnifications of 20 and 63×. Approximately 10 µl of fat–oil mixture was placed on a glass slide at room temperature and the crystals were viewed at different magnifications. Highly turbid mixtures were diluted with pure IPM to characterize their microstructures. For each sample, the size of more than 50 crystals was measured using ImageJ software. From the frequency distribution plot the mean and standard deviations were obtained.
Statistical Analysis
Size distribution of fat clusters are reported as mean values and standard deviations of various measurements. Regression analysis was performed to find the correlation between size of fat clusters and fat concentrations at each cooling rate using a level of significance of 0.05. Statistical analysis was performed using Microsoft Excel 2007 data analysis tool pack.
Rheology
Rheological measurements were done using a controlled stress rheometer MCR 301 from Anton Paar, Austria. A parallel plate geometry (rough) of diameter 25 mm was used at a gap of 0.8 mm. The geometry was roughened with sandpaper having a grit size of 46.2 µm. All the experiments were carried out at 25 °C. The limit of linear viscoelasticity was determined to be at strain of 0.05% or higher for all the samples that were studied. Frequency sweeps were performed at a constant strain amplitude of 0.01% over an angular frequency range of 0.1–100 rad/s. Three trials were carried out for each measurement.
Results and Discussion
Pseudo-Phase Diagram and Microstructure
Crystallization of fat could be achieved by cooling of fat–oil mixtures either in a constant temperature bath [4, 16] or at a desired cooling rate in a temperature controlled environment. Given the importance of cooling rate on fat crystallization, samples were crystallized at uniform cooling rates resulting in homogeneous crystallization. The study of macroscopic phase behavior by the formulation of a pseudo-phase diagram will help in identifying the state of fat–oil mixture and their stability. Therefore, the compositions where the mixtures are homogeneous, stable against sedimentation (caused possibly due to the large size of fat crystals and density difference) and gel-like properties can be qualitatively inferred. A pseudo-phase diagram of tribehenin in IPM was established based on visual observations at various fat concentrations and cooling rates. The samples were classified into three distinct phases, namely,
Settled Crystals
As the homogeneous fat–oil mixture maintained at 80 °C was cooled to a temperature below 40 °C, the fat started to crystallize and form a turbid dispersion. With further cooling to 20 °C, these fat crystals grew in size and eventually settled to the bottom of the vial forming a layer of settled fat crystals and clear oil at the top as shown in Fig. 2b. These settled crystals could be easily resuspended by gentle shaking of the glass vial implying that the fat crystals in the fat–oil mixtures did not form a continuous network.
Settled Gel
At intermediate concentrations of fat, the fat–oil mixtures eventually formed a two phase mixture consisting of settled wax crystals at the bottom and clear oil at the top, similar to the samples containing low fat content. However, the settled crystals could not be re-suspended back into the oil by shaking the vial as shown in Fig. 2c indicating that the fat crystals formed a continuous network. Thus, the settled network of crystals is gel-like.
Uniform Gel
At high fat concentrations, the fat–oil mixtures form a homogeneously turbid sample without any oil at the top as shown in Fig. 2d. The mixture was completely solid-like which was confirmed by tilting the glass vial upside down. Fat crystals formed a continuous porous network which encapsulates all the oil in the interstices. Hence, the sample was uniform with a no excess oil layer on top.
Fat–oil mixtures consisting of settled crystals represented by closed circles in Fig. 3 were observed at intermediate cooling rates of 0.5–2.0 °C/min at concentrations of 0.5–2.0 wt%. At a higher cooling rate of 9 °C/min, such a phase was observed up to a concentration of 5.0 wt%. The threshold concentrations at which mixtures consisted of settled gels (closed triangles in Fig. 3) were 0.5 wt% at 0.1 °C/min, 1 wt% at 0.5 °C/min, 5 wt% at 1.0, 2.0 °C/min and 7.5 wt% at 9 °C/min. The minimum fat concentration needed to form uniform gel (closed squares in Fig. 3) was found to be 7.5 wt% for samples cooled at 0.5; 1.0 and 2.0 °C/min. For samples cooled at 0.1 and 9 °C/min, threshold concentration for the formation of uniform gels were observed to be higher −15 and 10 wt%, respectively.
The height of the settled gels for the same concentration of fat can be compared to derive the relative porosity of the settled fat network. In other words, for the same concentration of fat lesser the height of the settled crystal network lesser is its porosity. Additionally, the suspend ability of the settled wax network indicates the strength of the interclustural bonds in them. Hence, for the same concentration of fat a settled gel indicates higher interclustural bond strength as compared to settled crystals. Based on these observations it can be seen from the pseudo-phase diagram that the crystal networks formed at 0.1 °C/min has the highest interclustural bond strength since it forms settled gels at fat concentrations as low as 0.5 wt%. This is followed by crystal networks formed at 0.5 °C/min which forms settled gels at a fat concentration of 1.0 wt%. Furthermore, it was also noted that the height of the settled fat network was the lowest for 0.1 °C/min indicating least porosity. This is the reason behind the formation of settled gels even at a high fat concentration such as 10 wt% where the unaccomodated oil is left on the top. Hence, preliminary information on interclustural bond strength and porosity of the network can be derived from the height of the settled fat crystal network and its suspend ability using the pseudo-phase diagram.
To analyze the microstructure of these fat–oil mixtures, brightfield and polarization optical microscopy were used. A pseudo-phase diagram was established based on the microstructure of the constituent crystal clusters as shown in Fig. 4. The microstructure was classified into 4 categories, namely individual needle-like crystals (Individual crystals), bundles of needle-like crystals (Bundles of needles), mixture of bundles of needles and spherical clusters and spherical clusters. Settled fat consisting of individual discrete needle-like crystals were observed at very low concentrations. A brightfield microscopy image of these crystals is shown in Fig. 5a and these crystals appeared bright under polarized light as shown in Fig. 5a1. At higher concentrations such crystals aggregated to form bundles of needles, wherein the needle-like crystals were stacked mostly along the length of the crystal as shown in Fig. 5b. These aggregate appeared bright under polarized light as shown in Fig. 5b1, confirming the crystalline nature. At a cooling rate of 1.0 °C/min, such bundles of needles were accompanied by aggregates of crystals connected together to give a spherical-like cluster as shown in Fig. 5c, c1. This appears to be a transition state as the formation of a spherical cluster with pointed needles at the periphery is dominant at higher cooling rates of 2.0 and 9 °C/min as shown in Fig. 5d. The crystalline nature of such clusters were confirmed under polarized light as shown in Fig. 5d1.
A complete pseudo-phase diagram based on the microstructure of fat–oil mixtures is shown in Fig. 4. Individual crystals were observed at a concentration of 0.5 wt% in the range of 0.1–2.0 °C/min cooling rates represented as open diamonds in Fig. 4. At 1 wt% individual crystals aggregated to form mixtures of bundles of needles and spherical clusters (closed diamonds) at cooling rates of 0.1–1.0 °C/min while, for cooling rate of 2.0 °C/min the network comprised of spherical clusters (closed circle). At 9 °C/min spherical clusters were seen from a very low concentration of 0.5 wt%. In the uniform gel region, bundles of needles (closed squares) were seen at cooling rates of 0.1 °C/min and 0.5 °C/min. At a cooling rate of 1.0 °C/min the network comprised of bundles of needles and spherical clusters. At higher cooling rates of 2.0 and 9 °C/min, the network comprised solely of spherical clusters. Hence, the formation of spherical clusters were favored at higher cooling rates such as 2.0 and 9 °C/min while the formation of bundles of needles were favored at 0.1 and 0.5 °C/min as shown in Fig. 4. The time scale over which the nucleation and growth events take place during crystallization is determined by the cooling rate. Therefore, the time given to form a network is longer in the case of 0.1 °C/min as compared to 2 °C/min. We speculate that this could be the reason for the formation of a more stable and less porous network of bundles of needles having strong interclustural bond strength. However, spherical clusters form less stable networks due to higher undercooling at higher cooling rates.
Polymorphism and Thermal Behavior
Typically, fats composed of TAG exist in different polymorphic forms and the form can be identified by their unique XRD peaks. The α form is typically identified by a single peak at a d-spacing of approximately 4.15 Å. Similarly, β′ form is identified by peaks at d-spacing values of 3.8 and 4.2 Å. The β form shows peaks in 3.5–4 Å range and at 4.6 Å. The XRD data showing different polymorphic forms in these fat–oil mixtures are reported in Fig. 6a. The α form was not seen in the cooling rates used in our study suggesting that it probably forms at higher cooling rates. The metastable β′ form was seen at higher cooling rates of 9–0.5 °C/min. At a very low cooling rate of 0.1 °C/min, the most stable β polymorph was observed. This is due to the fact that nucleation and growth of less stable polymorphs proceeds faster owing to lower surface free energy of such crystals. The metastable nucleation behavior of fat crystals arises due to the relatively large characteristic time required for the long fatty acid chains to align themselves. Hence, homotropic polymorphic phase transitions from a less stable β′ to a more stable β state is also possible. It must be noted from Fig. 6a that there is a slight shift in XRD peak values from expected values, the reason for this is not clear. This may probably be due to strain caused to the sample during preparation. Thermal analysis of each polymorph was also carried out using DSC. The melting curves of the 25 wt% fat–oil mixtures were studied as shown in Fig. 6b. The melting curves appear very similar for all the polymorphs except for a slight increase in the melting peak at lower cooling rates. This shows that there is no notable difference between the thermal behavior of β′ and β polymorphs of the tribehenin fat crystals in IPM.
Size Analysis of Fat Clusters
The microstructures of fat–oil mixtures observed via optical microscopy was further quantified by measuring the size of the constituent fat clusters using ImageJ. As the samples were opaque at high fat concentrations, their sizes were analyzed by diluting the sample with oil to ~5 wt%. The dilution was done only to improve the quality of optical microscopy images and did not affect the size and morphology of the fat crystals. In an effort to analyze the size of these clusters a characteristic length was defined. The characteristic length was taken as the longest dimension of the structure. This corresponds to length of the crystal in the case of individual crystals, length of bundles for bundles of needles and diameter for spherical clusters. At a fat concentration of 0.5 wt% the size of the individual crystals increased from 12.5 ± 5 µm at 2 °C/min (blue) to 45 ± 15 µm at 0.1 °C/min (black) as shown in Fig. 7. The decrease in size of crystals with high cooling rates can be explained by considering the degree of undercooling of the fat. During fast cooling rates the degree of undercooling is higher thereby forcing the nucleation and growth events to take place over a shorter period of time. Due to this undercooling, the thermodynamic driving force for the formation of crystal nuclei is also larger at high cooling rates. This leads to a high rate of nucleation and numerous smaller crystals. Furthermore, the size of bundles of needles increased significantly (P < 0.05) from 70 ± 30 µm at 5 wt% to 110 ± 22 µm at 25 wt% for a cooling rate of 0.1 °C/min. Similarly, at the cooling rate of 0.5 °C/min (red) the size of the bundles of needles increased significantly (P < 0.05) from 52 ± 20 µm at 5 wt% to 70 ± 21 µm at 25 wt%. On the other hand, the size of spherical clusters formed at 2 °C/min also increased significantly (P < 0.05) from 20 ± 10 µm at 5 wt% to 30 ± 15 µm at 25 wt% and for 9 °C/min (cyan) a similar significant (P < 0.05) increase in size was observed from 17.5 ± 5 µm at 5 wt% to 21 ± 5 µm at 25 wt%. The appreciable increase in size with increase in fat concentration for bundles of needles is shown in Fig. 8a, b and for spherical clusters is shown in Fig. 8c, d. The above data also suggest that long needles favor the formation of clusters in the form of bundles of needles while small needles favor the formation of spherical clusters.
Gel Microstructure and Rheology
In the gel phase observed at high fat concentrations, the constituent fat clusters of the crystal network were similar in shape as compared to the clusters observed at lower concentrations as shown in Fig. 8. The constituent spherical clusters (a) and bundles of needles (b) in the microstructure of gel containing 15 wt% fat cooled at 2.0 °C/min (c) and 0.1 °C/min (d) are shown in Fig. 9. In these fat crystal networks, the constituent clusters are held together by the van der Waals forces. The macroscopic properties of the crystal network are expected to depend on cluster size, shape and their interactions. Consequently, the same fat–oil mixture cooled under different conditions are expected to have different hardness, texture and strength. Few studies has been done in an effort to understand the impact of such parameters [24]. These studies have been done with different materials hence making it difficult to compare their properties. Since, there is a natural shape transition in case of tribehenin clusters, this will be a model system to study the effect of cluster shape and size on rheological properties of fat–oil mixtures.
The uniform gels were characterized using oscillatory rheology. A roughened geometry was used to eliminate wall slip. Frequency sweeps were carried out and Gʹ and G″ remained independent of frequency indicating gel-like behavior. The frequency sweeps of fat–oil mixtures containing initial fat concentrations of 25 wt% are shown in Fig. 10a. The average Gʹ values of fat–oil mixtures at given frequency of 10 rad/s for various cooling rates and initial fat concentrations are listed in Table 1.
For a given fat concentration the values of Gʹ were measured on fat network with constituent clusters of different shapes and sizes. The Gʹ values of 25 wt% mixtures show a non-monotonic dependency with cooling rate as shown in Fig. 10b. Firstly, it can be seen that the Gʹ of gels comprised of bundles of needles are higher than the gels comprised of spherical clusters. Secondly, the highest average storage modulus of 0.81 MPa was seen at 0.1 °C/min which decreases to 0.67 MPa at 0.5 °C/min. This suggests that the Gʹ decreases when the network is comprised of smaller sized bundles of needles. At the cooling rate of 2.0 °C/min where the network is solely made of spherical clusters, the average Gʹ is 0.34 MPa and this increases to 0.36 MPa at 6 °C/min and further increases to 0.45 MPa at 9 °C/min. Similar trends can be observed at other fat concentrations as shown in Table 1. Given that the size of the clusters at 9 °C/min are smaller than clusters formed at 2.0 °C/min, this increase in Gʹ could be due to the fact that the former has more number of spherical clusters than the latter. The above data also shows that for fat–oil mixtures of 25 wt% fat concentration, similar storage moduli can be achieved by two different cooling rates namely 1.0 and 9 °C/min. Hence, for a given fat concentration, similar Gʹ values can be obtained by achieving different microstructure states. Despite their similarity in Gʹ values, a detailed study is required to analyze other properties such as yielding, hardness.
It has been shown experimentally that the Gʹ of fat crystal networks are deter-mined by the fractal nature of the microstructure [7, 25]. To determine the fractal dimension of the fat crystal network, the concept of scaling behavior for elastic properties of colloidal gels suggested by Shih et al. [26] was used. They analyzed the scaling behavior under two regimes, based on the relative strength of the links between the clusters in a colloidal gel and the strength of the clusters themselves. The similarity between colloidal gels and fat crystal networks has led to the adaptation of this theory for fat crystal networks [3]. Among the two regimes discussed by Shih et al. the weak-link regime was considered in this study. In this regime, the elastic properties of the overall network is dominated by the intermicrostructural links as compared to intramicrostructural links. In other words, the elastic properties of the fat–oil mixtures are dominated by the weak links between the fat clusters which arise as a result of van der Waals forces. This was further supported by our preliminary results on yielding studies of these fat–oil mixtures, where the constituent fat clusters were found intact even after yielding as shown in Fig. 11.
At each cooling rate considered in this study, the storage modulus of the fat–oil mixtures as a function of fat concentration shows a power law relationship as depicted in Fig. 12. For the weak link regime the relationship described by Shih et al. [26] is:
where Gʹ is the storage modulus of the gel, φ is the particle concentration in the gel, D is the fractal dimension and d is the Euclidean geometry of the sample. Therefore, the storage modulus of the network follows a power law relationship with fat concentration with a slope of [(d − 2/d − 3)] or [(1/3 − D)]. Bremer et al. [27] suggested the equation can be written as,
where γ is the pre exponential factor that is independent of the fat concentration but dependent on size, shape and polymorphic nature of fat clusters. The values of D and γ derived from fitting the data is listed in Table 2. The fractal dimensions of the fat–oil mixtures was ~2.8 and showed very little variation while a considerable change in γ was observed. This is expected as γ is a function of size and shape of the fat clusters [3]. In other words, for materials of similar fractal dimensions the rheological properties can be modified by controlling the size and shape of fat clusters using processing conditions.
Conclusion
A comprehensive study on the phase behavior of model fat–oil mixtures was carried out. The microstructure and thermal behavior of these mixtures were also studied. It was found that the constitutive fat cluster shape of these fat–oil mixtures changed from bundles of needles at low cooling rates to spherical clusters at high cooling rates. Pseudo-phase diagram based on visual observations indicated that networks made of bundles of needles had higher interclustural bond strength as compared to spherical clusters. This was later confirmed using rheology, where networks made of bundles of needles exhibited higher Gʹ values than the networks made of spherical clusters. This study emphasized the importance of shape and size of fat clusters on the rheological properties of the fat network. Finally, we showed how the cooling rate and fat concentration could be used to crystallize fat–oil mixtures with definite crystal morphologies leading to specific rheological properties.
References
Sato K, Bayes-Garc´a L, Calvet T, Cuevas-Diarte MA, Ueno S (2013) External factors affecting polymorphic crystallization of lipids. Eur J Lipid Sci Technol 115(11):1224–1238
Hartel R (2013) Advances in food crystallization. Annu Rev Food Sci Technol 4:277–292
Narine SS, Marangoni AG (1999) Relating structure of fat crystal networks to mechanical properties: a review. Food Res Int 32(4):227–248
Campos R, Narine S, Marangoni A (2002) Effect of cooling rate on the structure and mechanical properties of milk fat and lard. Food Res Int 35(10):971–981
Narine SS, Marangoni AG (2002) Structure and mechanical properties of fat crystal networks. Adv Food Nutr Res 44:33–146
Marangoni AG, Rousseau D (1996) Is plastic fat rheology governed by the fractal nature of the fat crystal network? J Am Oil Chem Soc 73(8):991–994
Vreeker R, Hoekstra L, Den Boer D, Agterof W (1993) The fractal nature of fat crystal networks. Coll Surf 65(2–3):185–189
Hernqvist L (1990) Polymorphism of triglycerides a crystallographic review. Food Struct 9(1):5
D’Souza V, deMan JM, deMan L (1990) Short spacings and polymorphic forms of natural and commercial solid fats: a review. J Am Oil Chem Soc 67(11):835–843
Ten Grotenhuis E, Van Aken G, Van Malssen K, Schenk H (1999) Polymorphism of milk fat studied by differential scanning calorimetry and realtime X-ray powder diffraction. J Am Oil Chem Soc 76(9):1031–1039
Woodrow I, Deman J (1968) Polymorphism in milk fat shown by X-ray diffraction and infrared spectroscopy. J Dairy Sci 51(7):996–1000
Toro-Vazquez JF, Dibildox-Alvarado E, Herrera-Coronado V, Charo-Alonso MA (2001) Triacylglyceride crystallization in vegetable oils: application of models, measurements, and limitations. In: Widlak N, Hartel R, Narine SS (eds) Crystallization and solidification properties of lipids. AOCS Press, Champaign, IL, USA
DeMan J (1964) Effect of cooling procedures on consistency, crystal structure and solid fat content of milk fat. Dairy Ind 29:244–246
Herrera M, Hartel R (2000) Effect of processing conditions on crystallization kinetics of a milk fat model system. J Am Oil Chem Soc 77(11):1177–1188
Herrera M, Segura J, Rivarola G, Anon M (1992) Relationship between cooling rate and crystallization behavior of hydrogenated sunflower seed oil. J Am Oil Chem Soc 69(9):898–905
Irmscher SB, Gibis M, Herrmann K, Kohlus R, Weiss J (2015) Oil-fat mixtures with low solid fat concentration: in uence of fat concentration and cooling conditions. J Am Oil Chem Soc 92(9):1277–1291
Heertje I, Leunis M, Van Zeyl W, Berends E (1987) Product morphology of fatty products. Food Struct 6(1):2
Deman J, Beers A (1987) Fat crystal networks: structure and rheological properties. J Texture Stud 18(4):303–318
Narine SS, Marangoni AG (1999) Fractal nature of fat crystal networks. Phys Rev E 59(2):1908
Ball R (1989) Fractal colloidal aggregates: consolidation and elasticity. Phys D 38(1):13–15
Brown W, Ball R (1985) Computer simulation of chemically limited aggregation. J Phys A Math Gen 18(9):L517
Rueb C, Zukoski C (1997) Viscoelastic properties of colloidal gels. J Rheol 41(2):197–218
Haighton A (1976) Blending, chilling, and tempering of margarines and shortenings. J Am Oil Chem Soc 53(6):397–399
Liang B, Hartel R (2004) Effects of milk powders in milk chocolate. J Dairy Sci 87(1):20–31
Narine SS, Marangoni AG (1999) The difference between cocoa butter and salatrim R lies in the microstructure of the fat crystal network. J Am Oil Chem Soc 76(1):7–13
Shih WH, Shih WY, Kim SI, Liu J, Aksay IA (1990) Scaling behavior of the elastic properties of colloidal gels. Phys Rev A 42(8):4772
Bremer LG, van Vliet T, Walstra P (1989) Theoretical and experimental study of the fractal nature of the structure of casein gels. J Chem Soc Farad Trans 1 Phys Chem Condens Pha 85(10):3359–3372
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Ramamirtham, S., Shahin, A., Basavaraj, M.G. et al. Phase Behavior and Micro-Structure of Fat–Oil Mixtures: Engineering the Shape of Fat Clusters. J Am Oil Chem Soc 94, 121–132 (2017). https://doi.org/10.1007/s11746-016-2926-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-016-2926-2