Abstract
This paper reports the experimental results of processes used for the formation of whipped oils composed of vegetable oils (salad oil) and high-melting fat crystals [fully hydrogenated rapeseed oil rich in behenic acid (FHR-B)]. No emulsifier was added to form this whipped oil. Microprobe FT-IR spectroscopy, synchrotron radiation microbeam X-ray diffraction (SR-μ-XRD), polarized optical microscopy, and differential scanning calorimetry (DSC) were employed to observe fine fat crystal particles of the most stable polymorph of β (β-fat crystal), FHR-B, and their adsorption at the air–oil surface before, during, and after the formation of the whipped oil. The results obtained revealed the following: (1) The preparation of an organogel composed of salad oil and small fibrous β-fat crystals using a special tempering procedure was a prerequisite for forming whipped oil. (2) The β-fat crystals were adsorbed at the air–oil surface to encapsulate the air bubbles during the formation process of whipped oil. (3) The values of overrun of the whipped oil reached >200 % after an aeration time of 30 min at 20 °C. (4) The SR-μ-XRD experiments demonstrated that the lamellar planes of the β-fat crystals near the air–oil surface were arranged almost parallel to the air–oil surface plane. The present study provides the first evidence that tiny fat crystal particles may cause aeration in liquid oils without the addition of other whip-assisting substances such as emulsifier crystals.
Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aerated colloidal systems, namely foams, are widely applied in the production of cosmetics, foods, and porous materials. In personal care products, food materials, and cosmetic formulations, foams have significant advantages with regard to shape retention, soft texture, thermal barrier, and low calorie content. Either aqueous or non-aqueous foams are formed depending on whether air bubbles are incorporated into aqueous or non-aqueous (oil) phases. Aqueous foams are most widely used in applications such as cosmetics, foods, pharmaceuticals, and paper products [1]. Non-aqueous foams are important for foamed plastics and transport of crude oils [2]. In foods, whipped oils have already applied to whipped butter and confections such as aerated chocolate bars, where micrometer- or millimeter-sized air bubbles are incorporated into continuous semi-solid fat phases, revealing such functionality such as shape retention, soft body and taste, and fewer calories.
Foam-forming systems are thermodynamically unstable because of the excess surface energy of air bubbles, which range from several to several tens of micrometers in diameter, between the air phase and the surrounding water or oil phases. Therefore, two important factors must be clarified concerning the formation and stabilization of whipped systems [3–8]. The first involves the thermodynamic and kinetic nature of aeration, necessitating the reduction of surface energy between air cells and continuous phases. The second involves the stabilization mechanisms of the air bubbles over long storage periods before use, preventing gas diffusion in order to retard disproportionation and coalescence of the air bubbles.
In aqueous foams, the air–water surface energy is reduced by the adsorption of amphiphilic substances (e.g., proteins, surfactants, polysaccharides, and lipids) at the air–water surface. To prevent Ostwald ripening and coalescence of the air bubbles, protein aggregates and partially coalesced fat globules may act as a mechanical barrier to stabilize the air bubbles [9–15]. In particular, foamed emulsions in dairy cream containing a wide range of compounds including fats, proteins, emulsifiers, hydrocolloids, and colloidal particles are aerated and sometimes gelled. Inorganic materials such as partially hydrophobic silica nanoparticles can also form and stabilize aqueous foam systems [16–22].
In contrast, research on non-aqueous foams has been quite limited, and systematic research is needed, as the formation mechanisms of non-aqueous foams are quite different from those of aqueous foams in terms of the forces driving aeration, surface structures between the air and oil phases, and stabilization of air bubbles dispersed in the oil phase during long-term storage.
Recent studies have investigated the stabilization of non-aqueous foams with lamellar liquid crystals in diglycerol mono-saturated fatty acids (lauric acid and myristic acid) [23, 24]. In these studies, amphiphilic substances were employed for forming non-aqueous foams in which air bubbles were entrapped by lamella liquid crystal layers. Further studies have reported on non-aqueous foam constructed by liquid oil and air-stabilizing materials using solid particles [25–27] and surfactant crystals [28, 29]. However, no study of whipped oil using fat crystals has been done.
In the present study, we found that whipped oil systems with overrun values exceeding 200 % were formed when tiny crystals of high-melting fat were prepared in liquid oil by special crystallization and tempering processes before aeration. A prerequisite for the formation of the whipped oil was the preparation of an organogel constructed from high-melting fat, fully hydrogenated rapeseed oil rich in behenic acid (FHR-B), and liquid oil. FHR-B contains different triacylglycerols (TAGs) whose major fatty acid moieties are stearic acid (C18:0), arachidic acid (C20:0), and behenic acid (C22:0), as shown below. FHR-B reveals polymorphic behavior in a crystalline state, having α, βʹ and β forms, similar to typical TAG crystals. The α form is the least stable polymorph of TAG, having the lowest melting temperature, whereas βʹ and β are metastable and the most stable polymorphs, respectively. We observed that rapid cooling of FHR-B liquid oil mixtures led to the crystallization of FHR-B in α, which transformed to β during thermal annealing either by isothermal treatment or by rapid heating to temperatures below the melting temperature of β (melt-mediated α–β transformation). Organogels were formed by the application of such thermal treatments [30–32], after which aeration was performed. To gain a precise understanding of the formation mechanisms of whipped oils, we employed microprobe Fourier transform infrared spectroscopy (FT-IR, beam width of 80 × 50 μm2) and synchrotron radiation microbeam X-ray diffraction (SR-μ-XRD, beam width 5 × 5 μm2) experiments. In our work thus far, we have applied SR-μ-XRD to colloidal dispersion systems, including lipids such as spherulite structures of lipid crystals [33, 34], oil-in-water emulsion droplets [35], granular crystal formation in water-in-oil emulsions [36], fat crystals near water droplets in water-in-oil emulsions [37], and palm oil with an emulsifier additive [38].
Materials and Methods
Materials
FHR-B was supplied by ADEKA Corporation (Tokyo, Japan). Salad oil (mixture of soybean oil and rapeseed oil) was purchased from The Nisshin OilliO Group., Ltd. (Tokyo, Japan). The fatty acid composition of FHR-B and salad oil and the carbon number of TAGs in FHR-B are shown in Table 1.
Preparation of β-Fat Gel and Whipped Oil
The β-fat gel was formed by mixing FHR-B and salad oil at 80 °C followed by cooling to form the crystallization of FHR-B after proper tempering procedures. The concentration ratios of FHR-B to salad oil ranged from 4:96 to 10:90. Soon after the formation of the organogel, aeration was carried out using a rotating-type aeration machine, the KENMIX KM-600 (Kenwood; purchased from Aicohsha Manufacturing Co., Ltd., Toda, Japan), with an attached whipper at a rotation speed of 200 rpm. The temperature of the aeration machine was kept constant at room temperature with circulating water connected to a water bath. Overrun values is defined as [(volume after aeration − volume before aeration)/volume before aeration] × 100. The concentrations of FHR-B in the whipped oils varied from 4 to 30 wt%, depending on the experimental method. For example, moderate concentrations of FHR-B of 4–10 wt% were chosen for optical, FT-IR, and SR-m-XRD measurements, whereas 30 wt% FHR-B was needed to clearly detect the polymorphic transformation with macroscopic SR-XRD measurements (see below).
In order to examine the effects of different tempering processes on the morphology of β-fat crystals and the overrun properties of the whipped oil, the FHR-B and salad oil mixture was subjected to four tempering processes, ranging from high to ambient temperatures; details will be presented later.
Differential Scanning Calorimetry (DSC) Measurements
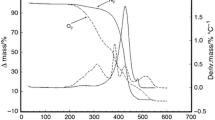
The DSC experiments were conducted for the mixtures of FHR-B and liquid oil (not for the whipped oil samples) using the Thermo Plus 8240 (Rigaku Co., Tokyo, Japan). The sample (15 mg) was sealed in an aluminum pan (50 mL), and Al2O3 was used as a reference material for calibration. The samples were cooled from 80 to 10 °C at a rate of 5 °C/min, maintained for 5 min at 10 °C, and then heated to 80 °C at a rate of 2 °C/min.
Optical Microscopy Observations
Whipped oil was subjected to polarized optical microscopy (POM) by taking a sample from the middle of the foam and sealing by gently pressing into a thin space (around 50 μm) with two cover slides. The POM observations were carried out at ambient temperature using the CX31-P POM (Olympus Co., Tokyo, Japan) with a DP-12 digital camera (Olympus Co., Tokyo, Japan). Magnification of the objective lens was ×20, and that of the eye lens was ×10. Polarized and non-polarized images were acquired to observe the distributions of β-fat crystals and air bubbles at different durations of aeration up to 60 min.
X-Ray Diffraction Analysis
Laboratory-scale conventional XRD measurement was carried out to determine the polymorphic forms of β-fat crystals using the RINT-TTR powder X-ray diffractometer (Rigaku Co., Tokyo, Japan) (power 1 kWh, wavelength 0.154 nm).
Small-angle and wide-angle diffraction measurements using synchrotron radiation (SR-XRD) were performed with the synchrotron radiation source at the Photon Factory (BL-15A) in the High Energy Accelerator Research Organization (KEK) laboratory, Tsukuba, Japan. The methods used for the SR-XRD experiments are fully described elsewhere [39, 40]. In situ SR-XRD measurements were carried out on the crystallization processes during cooling from 80 to 10 °C at a rate of 10 °C/min, holding for 5 min at 10 °C, and then heating to 80 °C at a rate of 2 °C/min.
The SR-μ-XRD measurements were performed at BL-4A of the Photon Factory in the KEK. Full details of the SR-μ-XRD method are reported elsewhere [33]. The X-ray microbeam wavelength was 0.11 nm, and the beam area was 5 × 5 μm2. A whipped oil sample subjected to the SR-μ-XRD measurements was selected from the middle of the foam and placed in a sample cell (thin space less than 50 μm) made of thin polyethylene terephthalate (PET) films, and sealed by gentle pressing. Because of the difficulty in simultaneously measuring small-angle and wide-angle X-ray scattering, we examined only small-angle X-ray diffraction in the SR-μ-XRD measurements. Keeping the microbeam position fixed, the measured sample was moved using an x–y–z stepping motor for observation by optical microscopy (magnification ×200). The sample was moved within a two-dimensional (2D) plane in 5-μm steps. The SR-μ-XRD experiment was difficult, particularly in fixing the air bubble within sub-micrometers and avoiding irradiation damage by focused microbeam X-ray while searching for the air bubble. However, we obtained ten successful results that indicated no irradiation damage and no deformation of the scanning SR-μ-XRD experiment over the entire range of the air bubble.
Microprobe FT-IR Analysis
The distribution and concentration of FHR-B crystals in the oil phase of the whipped oil were quantitatively measured by changes in the intensity of the FT-IR absorption band using the μIR-8000 instrument (Shimadzu Co., Kyoto, Japan). The intensity of the FT-IR spectrum reflects the quantity of fat crystals in the oil phase in the area of the incident IR beam. We applied microprobe FT-IR using a narrow beam of several tens of micrometers at different time points of aeration of the whipped oil. The whipped oil was placed within KBr windows with a constant sample thickness (29 μm), so that changes in the relative concentration of the FHR-B crystals in the oil phase during aeration were detected. We observed the FT-IR absorption spectra of the fat crystals only from the oil phase, not from areas including the oil–air surface. The incident IR microbeam was carefully focused on the oil phase during observation by optical microscopy. For comparison, we observed the absorption from inside the air bubble.
Results and Discussion
Phase Behavior of FHR-B/Salad Oil Mixtures and Tempering Processes
Figure 1a shows the crystallization temperatures (T c) of the α form and the melting temperatures (T m) of the β form of FHR-B crystals. T c (α) and T s (β) were obtained by observing the exothermic DSC peaks during cooling (5 °C/min) and the endothermic DSC peaks during heating (2 °C/min), respectively. The DSC thermopeaks are shown in the supporting information (Fig. S1). Since FHR-B is a mixture of different TAGs with different fatty acid moieties (Table 1), DSC melting peaks of β were broad or split because of the eutectic mixing behavior of multicomponent TAGs. Therefore, we show the peak top temperatures for broad melting peaks and average temperatures for split melting peaks in Fig. 1.
T c (α) and T m (β) increased with increasing FHR-B concentration in salad oil, and T m (β) was always higher than T c (α) by 15 ± 5 °C. For example, T c (α) = 27.1 °C and T m (β) = 47.3 °C with an FHR-B concentration of 2 wt%, and T c (α) = 48.5 °C and T m (β) = 63.2 °C with an FHR-B concentration of 50 wt%. This result is reasonable, since α is a metastable form and β is the most stable form of FHR-B crystals [30]. Although not shown here, the β form occurred through α-melt-mediated transformation, in which the melting of α caused rapid crystallization of the β form at every FHR-B concentration during heating of the first crystallized α form, as confirmed by SR-XRD measurements (see below).
Based on the mixing phase behavior of FHR-B and salad oil, we tested four tempering processes that differed in cooled temperature, cooling rate, and subsequent heating process (Fig. 1b). Tempering A was conducted by rapidly cooling (10 °C/min) the liquid mixtures to the final temperature (T final) of the tempering process between T c (α) and T m (β), where isothermal crystallization was carried out. Tempering B was performed by rapidly cooling (10 °C/min) the liquid mixtures to T c, which was set far below Tc (α) so that α crystals were formed, and the temperature was kept constant at T c to cause solid-state transformation of FHR-B crystals from α to βʹ and/or β forms. In this case, T c = T final. Tempering C was a modification of tempering B, such that the α crystals of FHR-B formed by rapid cooling were transformed to β forms through melt-mediated transformation by heating (2 °C/min) from T c to T final. Tempering D was a modification of tempering C, as the initial cooling of the liquid mixture to T c was done slowly (2 °C/min), followed by heating to T final.
As will be explained below, the four tempering procedures resulted in substantial differences in aeration properties, basically because of differences in the crystallization characteristics of FHR-B in terms of crystal size and morphology.
Formation of Whipped Oil
Figure 2 depicts the overrun values of whipped oils containing FHR-B (4 wt%) in salad oil prepared by four different tempering processes. It is clear that the overrun values are significantly influenced by the tempering process. Tempering B actually did not form whipped oil at all, as the overrun value reached only 30 % after aeration for 60 min. The main cause of the very poor whipping properties produced by this process was the loss of polymorphic control of FHR-B crystallization, as will be demonstrated by the XRD study. Tempering A increased the overrun values to 158 % and tempering D to 215 % after aeration for 60 min. The best aeration performance was exhibited by tempering C, whose overrun value reached 211 % after aeration for 30 min, probably because of the collapse and/or coalescence of air bubbles under long-term shear.
Overrun values of whipped oils containing FHR-B (4 wt%) in salad oil after four different tempering processes denoted in Fig. 1b: tempering A (filled diamond), tempering B (filled square), tempering C (filled triangle) and tempering D (filled circle)
The common property among the last three tempering procedures was that crystallization of the FHR-B/salad oil mixture was finally achieved at T final, indicating that the crystallization of β fat may be a necessary condition for optimized aeration. Tempering C and D caused α-melt-mediated transformation into βʹ; however, with tempering A, β crystals were formed from the beginning and grew to large crystals which did not form an organogel.
As to the relationship between crystal sizes of FHR-B and tempering procedure, our previous paper [31] precisely reported the size and morphology of FHR-B crystals obtained by different tempering procedures using liquid oils of sal fat olein. For example, tempering C formed tiny crystals of β ranging in size from 1 to 3 μm, whereas large aggregates of β, around 20 μm in size, were formed after tempering D. The results obtained in the present study were quite similar: with tempering A, B, and D, the FHR-B crystals formed soon after the tempering process before aeration ranged in size from 10 to 30 μm, whereas tiny crystals 2–6 μm in size were obtained after tempering C.
In Fig. 2, the effects of tempering on the variation in overrun values over time are revealed in the morphology of the FHR-B crystals in the whipped oil. Figure 3 presents polarized optical micrographs of whipped oils containing FHR-B (4 wt%) and salad oil (96 wt%) aerated at 20 °C for 20 min, prepared by four tempering processes. The size of air bubbles did not vary greatly among processes (20–35 μm); however, the number of air bubbles and the distribution of FHR-B crystal particles differed significantly. The number of air bubbles was smallest in tempering B, intermediate in tempering A and D, and largest in tempering C. Many FHR-B crystals were randomly present in the continuous oil phase in the whipped oil prepared by tempering B. Tempering A and D formed whipped oil in which fewer FHR-B crystals were distributed in the oil phase. However, few FHR-B crystals were also observed in the oil phase in the whipped oil prepared by tempering C, and air bubbles surrounded by thick bright layers (~10 μm) were observed.
Polarized optical micrographs of whipped oils containing FHR-B (4 wt%) and salad oil (96 wt%) aerated at 20 °C for 20 min after four tempering processes shown in Fig. 1b. Scale bar 100 μm
It is worth noting that in Fig. 3, some bubbles reveal a non-spherical elongated shape, and all bubbles are surrounded with layers showing bright POM images. This is evidence of the presence of crystal layers adsorbed at the air–oil surface that hinder shape relaxation.
Polymorphic Behavior of FHR-B Crystals
The polymorphic forms of the FHR-B crystals formed by the four tempering processes were determined with the laboratory-scale RINT XRD instrument (Fig. 4). Because of the abundance of liquid salad oil (96 wt%), small-angle XRD (SAXD) patterns in the diffraction angles (2θ) of 2~12º could not be detected, whereas wide-angle XRD (WAXD) patterns appearing in 2θ = 18~24º were observed. A peak of 0.46 nm was characteristic of the β form, and peaks of 0.42 and 0.37 nm were characteristic of the β’ form. Using these peaks, we can compare the relative occurrence of β and βʹ forms of the FHR-B crystals among the four tempering processes. Tempering A predominantly formed β form; tempering B crystallized βʹ > β; and tempering C and D crystallized β > βʹ.
X-ray diffraction patterns of FHR-B crystals (4 wt%) in salad oil after four different tempering processes denoted in Fig. 1b taken at 20 °C. Unit, nm
Among the four tempering processes, the overrun value of the whipped oils prepared by tempering C was highest after an aeration period of 30 min (Fig. 2). Therefore, we focused on only the whipped oil prepared by tempering C for studies with SR-XRD, polarized optical microscopy, FT-IR, and SR-μ-XRD methods.
We observed polymorphic transformation processes of FHR-B in the liquid mixture (FHR-B 30 %) that occurred during tempering C processes using the SR-XRD method presented in Fig. 5. The SAXD peak of 5.81 nm appeared at 46 °C during cooling from 80 to 10 °C at a rate of 10 °C/min. The WAXD peak of 0.42 nm appeared at the same temperature. These two peaks are characteristic of long and short spacing values of the α form of FHR-B crystals [30]. Heating from 10 to 80 °C caused α-melt-mediated transformation into β in such a manner that the WAXD peak of β of 0.46 nm began to appear at 30 °C, and its peak intensity suddenly increased as soon as the WAXD peak of α disappeared by melting at 43 °C (denoted by an arrow). After the transformation was complete, additional WAXD peaks of β appeared at 0.39 and 0.38 nm. Correspondingly, the SAXD peak of α shifted to that of β (5.17 nm) at 43 °C. Further heating caused the β form to melt at 60 °C, as revealed by the disappearance of the SAXD and WAXD peaks. The crystallization of α and subsequent transformation from α to β through melt mediation corresponded well to the phase behavior of FHR-B in salad oil (Fig. 1), and the same process as that observed by the SR-XRD study of the mixture of FHR-B 30 wt% should occur during tempering process C in the mixture containing 4 wt% FHR-B at lower temperature ranges, following the phase behavior of Fig. 1.
Synchrotron radiation X-ray diffraction patterns of FHR-B crystals (30 wt%) in salad oil obtained during the tempering C process denoted in Fig. 1b. Unit, nm
Optical Microscopy Observations and Microprobe FT-IR Analyses
The aeration processes and distribution characteristics of the FHR-B crystals were carefully observed by polarized optical microscopy (Fig. 6). A droplet of the whipped oil mixture of FHR-B (6 wt%) and salad oil aerated at 20 °C was not spherical after an aeration period of 10 min (Fig. 6a), indicating an insufficient whipped state. This result was supported by the bright-field optical micrograph depicting a small number of air bubbles (Fig. 6b) and a high concentration of FHR-B crystal aggregates in the bulk oil phase (Fig. 6c). However, as the aeration period increased to 30 min, a droplet of whipped oil exhibited a sharp tail on the top, indicating increased viscosity (Fig. 6d). Optical images indicated an increase in air bubbles (bright images) and the disappearance of large aggregates of crystal particles (crossed-Nicols image). Instead, the whipped oil was filled with air bubbles (dark circles) with thin films around them, as indicated in the crossed-Nicols image (Fig. 6e, f). The polarized crossed-Nicols micrographs presented in Fig. 6c and f strongly indicate changes in the distribution of the FHR-B crystal particles as aeration proceeded, such that FHR-B crystals were distributed in the liquid oil phase at the initial stage of aeration; however, the particles may be adsorbed at the air–oil surface with a longer aeration period. This tendency was confirmed by microprobe FT-IR analysis.
Figure 7 depicts microprobe FT-IR absorption spectra from whipped oil containing 6 wt% FHR-B. We chose FT-IR spectra at 1463 cm−1 as a reference absorption band in order to evaluate the relative concentrations of the FHR-B crystals in the oil phase due to the CH2 scissoring band of fat crystals [41]. The microprobe FT-IR bands were acquired at whipping periods of 0, 5, 10, 30, and 60 min by irradiating the FT-IR microbeam in only the oil phase, while avoiding positions at the air bubbles and air–oil surface. For comparison, the FT-IR spectra acquired from inside the air bubble are also depicted. The relative intensities of the six absorption bands denoted in Fig. 7b were normalized at the position of 1490 cm−1, where no absorption was expected, for comparison purposes.
No absorption peak was detectable from inside the air bubble, indicating the absence of FHR-B crystals. It was evident that the intensity of the absorption band decreased with increasing length of aeration periods, reaching a minimum value at 30 and 60 min of whipping (Fig. 7a). For example, the intensity of the FT-IR band observed before whipping (0 min) did not change appreciably after whipping for 5 min. However, it decreased by 15 % for a whipping period of 10 min and by 30 % for whipping periods of 30 and 60 min. The absorption intensity of the CH2 scissoring band at 1463 cm−1 was directly proportional to the relative concentration of FHR-B crystals in the area of the oil phase under microbeam irradiation. We can therefore conclude that the relative concentration of the FHR-B crystals decreased with increasing length of aeration. We may also reasonably assume that the disappearance of FHR-B crystals may be compensated by the increased adsorption of FHR-B crystals at the oil–air surface during aeration. Figure 6f indicates such adsorption in thick membranes around every air bubble after aeration for 30 min.
Synchrotron Radiation Microbeam X-Ray Diffraction Analyses
We next performed SR-μ-XRD experiments to determine the polymorphic crystallization and the arrangement of their lamellar planes with respect to the air–oil surface of the FHR-B crystals very close to the air bubbles. Despite difficulty in the scanning SR-μ-XRD experiments, we obtained ten successful results, one of which is presented here.
Figure 8 depicts scanning SR-μ-XRD patterns around an air bubble 35 μm in diameter in whipped oil containing 10 wt% FHR-B crystals. Every SR-μ-XRD pattern was observed from a distance of 5 μm, and 100 original 2D SR-μ-XRD patterns were obtained. We observed the β form of FHR-B crystals around the air bubble and performed a precise analysis of the three-dimensional (3D) images and the azimuthal angle extension for every 2D SR-μ-XRD pattern. In Fig. 8a, a total of 10 × 10 2D patterns obtained at various positions over the entire area within and outside of an air bubble are shown. Figure 8b shows two typical distribution patterns of SR-μ-XRD peaks at varying azimuthal angles (χ) at positions 1 and 2, which were close to and a bit distant from an air bubble, respectively, as denoted in Fig. 8a.
Scanning SR-μ-XRD patterns around an air bubble with a diameter of 35 μm in whipped oil containing 10 wt% FHR-B crystals. a Total 2D patterns obtained over the entire area, and b two selected positions shown in a. A solid line in a denotes the air–oil boundary, and solid arrows in b denote strong peaks of SR-μ-XRD in a range of 0º ≤ χ ≤ 180º at each position
In both images in Fig. 8b, the outside ring patterns and the innermost black ring patterns are not the SR-μ-XRD patterns from the FHR-B crystals, but from PET films and direct beam cutting, and the SR-μ-XRD patterns from the FHR-B crystals are denoted by solid arrows. Position 1 exhibited two sharp patterns, whereas position 2 exhibited discretely dotted patterns. The 2θ extension analysis of the two patterns provided long spacing values of 5.18 nm for position 1 and 5.22 nm for position 2, both of which correspond to the 5.17-nm spacing of the β form (Fig. 5) within experimental errors. The azimuthal angle χ was calculated from the right position in Fig. 8b. The χ extension of the SR-μ-XRD patterns of the two positions exhibited very different behaviors in terms of lamellar arrangements.
Figure 9 depicts 3D images and the χ extension patterns of SR-μ-XRD data acquired at the two positions presented in Fig. 8b. The 3D image of position 1 reveals two sharp peaks (Fig. 9a) whose azimuthal angles (χ) are 25° and 205°, which are superimposed by a 180° rotation. The two peaks correspond to the symmetric diffraction peaks from crystal lattices within the lamellar planes of the crystals aligned in the same direction. Therefore, we refer to such a combination of χ extension peaks as a single peak. This result means that the lamellar planes of the TAG molecules in the FHR-B crystals present within the area of the X-ray microbeam are aligned in a direction along χ = (25° + 205°)/2 = 115°. This direction is almost parallel to the surface plane of the air bubble and the oil phase close to position 1 (dotted line in Fig. 8b).
a Three-dimensional images and b azimuthal angle (χ) extension patterns of SR-μ-XRD data obtained at two positions around an air bubble shown in Fig. 8. In b, solid arrows note strong peaks of SR-μ-XRD in a range of 0º ≤ χ ≤ 180º at each position
In contrast, the 3D images of the SR-μ-XRD pattern at position 2 (Fig. 9a, position 2) are composed of at least four peaks, as denoted by solid arrows. Further detailed structures of the SR-μ-XRD pattern of this position exhibited in the χ extension revealed four large peaks (χ = 30°, 90°, 210°, and 270°) and two small peaks (χ = 150° and 330°). From these results, we may conclude that the lamellar planes in the FHR-B crystals at this position are aligned along more than four directions. However, such an arrangement may actually be called random orientation.
Figure 10 depicts the behavior of the χ extension of the SR-μ-XRD patterns acquired at the positions denoted by A through L moved by 30° at positions in the oil phase, quite close to the air bubble boundary (circle in Fig. 8a). Two sharp peaks appear at the χ values of 15° and 195° at position A, and two peaks also appeared at χ = 45° and 225° at position B, which are rotated by 30° from position A. At position C, the χ values of the center positions of the four peaks are 75° and 255°, which are rotated by 30° from B. Similar situations were observed in the relationship of χ values among the other positions with respect to the rotation of the microbeam-irradiated positions around the air bubble depicted in Fig. 9. For purposes of clarity, the positions of sharp peaks or the central positions of the split peaks of the χ extension of the SR-μ-XRD patterns observed at positions A through L are connected by dotted lines in Fig. 10. The peak positions of every χ extension pattern are placed along a straight line, indicating that the lamellar planes of the FHR-B crystals present at the areas of the microbeam-irradiated positions near the air bubble boundary are almost parallel to the air–oil surface plane. The same results were obtained in different cases around the air bubbles, as shown in the supporting information (Figs. S2 and S3).
Azimuthal angle (χ) extension patterns of SR-μ-XRD data taken at 12 positions around an air bubble shown in Fig. 8
Figure 11 summarizes the lamellar planes, either one-way directed or randomly directed, of all 2D SR-μ-XRD patterns acquired around the air bubble depicted in Figs. 8 and 11a. There is an air bubble at the upper-left position and at the lower-left position close to the bubble in Fig. 11a and b. The lamellar planes at all positions surrounding the air bubble surface are largely aligned in one way, parallel to the air–oil surface plane. The randomly oriented positions are distant from the air–oil surface, except for a few positions close to the air–oil surface at the bottom of the bubble in Fig. 11b.
The present study of the aeration properties of liquid oil containing small amounts of FHR-B crystals can be summarized as follows.
-
(1)
The overrun values reached 200 %, which is high enough to produce nicely whipped oils comparable to aqueous whipped systems such as dairy whipped cream [42].
-
(2)
SR-XRD and DSC analyses of the FHR-B crystals revealing whipped oil with high overrun values indicated that the formation of FHR-B β crystals by a special tempering process was a prerequisite for forming whipped oil.
-
(3)
Microprobe FT-IR analysis indicated that adsorption of the FHR-B crystals into the air–oil surface occurred in accordance with aeration through the diffusion of FHR-B crystals from the oil phase to the oil–air surface.
-
(4)
SR-μ-XRD analysis unveiled the arrangement of the lamellar planes of the FHR-B crystals parallel to the air–oil surface planes only around the air bubbles.
Based on the observations summarized above, we may now discuss the mechanisms underlying the crystallization processes and microstructures of the FHR-B crystals that enabled the formation of well-whipped oil systems containing only fat crystal particles.
Our previous study of organogel formation using FHR-B crystals and various liquid oils demonstrated that tiny fibrous β-form FHR-B crystals were formed by rapid cooling of the liquid oil and FHR-B mixture below the T c of α, and subsequent heating to temperatures between T m of α and T m of β [30–32]. During this temperature variation, α crystals were formed initially, and were rapidly transformed from α to β through melt-mediated transformation. In contrast to this tempering process, slow crystallization of FHR-B did not form organogels, because the large bulky FHR-B β crystals thus formed did not entrap the liquid oil. The differences in the morphological characteristics of the FHR-B crystals between slow crystallization and melt-mediated transformation are due to the difference in the rate of nucleation of α and its transformation into β through melt mediation. These results can be explained by the crystallization processes presented in Fig. 12; essentially the same results were confirmed in the present cases of whipped oil, as demonstrated by the SR-XRD measurements presented in Fig. 5.
The nucleation rate of α under rapid cooling is so high that numerous crystal nuclei of FHR-B are formed in the liquid oil. Rapid heating soon after the rapid crystallization of α may cause the melt-mediated transformation that occurs by the melting of α followed by nucleation/crystal growth of β. Therefore, β crystals created from the precursors of α crystals are distributed in the same manner as α crystals. Tiny fibrous FHR-B β crystals then construct a fat crystal network through crystal–crystal contact in the liquid oil (Fig. 12a). In contrast, large crystals are formed by slow crystallization, and they do not form a tightly connected fat crystal network (Fig. 12b). We posit that tempering A and B (Fig. 1) may correspond to the process depicted in Fig. 12b, although T final in tempering A was between T m of β and α, and that of tempering B was below T c of α. Tempering A led to the direct formation of β crystals of FHR-B. Tempering B caused the formation of βʹ and β by a transformation from α to βʹ and β without melt mediation, as it occurred below the T c of α. Tempering C and D may correspond to the processes depicted in Fig. 12a, although the rate of cooling was lower in tempering D. The size and morphology of the FHR-B crystals formed by melt-mediated transformation (tempering C and D) must be quite different from those formed by tempering A and B.
We may assume that the tiny crystals formed by the melt-mediated transformation from α to β were able to be adsorbed at the oil–air surface, tightly packing with each other and thus entrapping air bubbles. Therefore, the overrun values of the whipped oil formed by tempering C and D were quite large. The fact that the increase in overrun values with time occurred more rapidly with tempering C than with tempering D may be due to the much smaller crystals of FHR-B, as the nucleation rate of α during the first cooling process was higher in tempering C than in tempering D because of a higher rate of cooling.
The next problem in the processes of whipped oil formation involves the mechanisms of adsorption and arrangement of FHR-B crystals at the oil–air surface. It was evident that the concentration of FHR-B crystals in the continuous oil phase decreased with increased whipping time, as observed by microprobe FT-IR measurement (Fig. 7). The polarized optical microscopy observations indicated that the FHR-B crystals were adsorbed at the oil–air surface, as the thickness of the layers surrounding the air bubbles increased with increasing whipping period length (Fig. 6).
We assume that the driving force for the diffusion of FHR-B crystals from the oil phase to the air–oil surface may be the effects of reduced surface energy of the salad oil–air interface, as salad oil contains hydrophilic minor components that may increase surface energy when in contact with the air phase. Although not shown here, we were unable to form whipped oil when we employed n-dodecane (instead of salad oil) and FHR-B crystals even through tempering C. As n-dodecane is extremely hydrophobic, there may be no driving force for FHR-B crystals to reduce the oil–air surface energy in this combination. To prove this, it is necessary to obtain precise measurements of the surface energy values of the salad oil and air bubble with and without the FHR-B crystals. Aramaki’s group measured surface energy values of air and olive oil with and without diglycerol monolaurate [24], and found that the surface energy was 32.1 mN/m without diglycerol monolaurate, decreasing to 26.9 mN/m with the addition of 1 wt% diglycerol monolaurate, and to 26.2 mN/m with the addition of 3 wt% diglycerol monolaurate.
In the present study, SR-μ-XRD demonstrated that the lamellar planes of the FHR-B crystals were parallel to the surface (Fig. 13). This means that the lamellar planes composed of methyl end groups (–CH3) were facing the air phase, and the lateral planes composed of glycerol groups connected to each other through the crystals adsorbed at the air–oil surface. Such a surface configuration connected by oil–FHR-B crystal–air phases may reduce the total surface energy compared to that with direct contact between the air and oil phases, because of the contribution of the lamellar plane of the FHR-B crystal that is facing the air surface. Calculation of surface energy values of β-form stearic acid indicated that the surface energy of the lateral (100) plane (γ 100) was 86.5 mN/m, whereas the surface energy of the basal (100) plane (γ 001) was 43.5 mN/m [43]. The reason for this difference is that van der Waals forces of stearic acid molecules along the normal to the (001) plane are much weaker than those within the lamellar plane along the (100) direction. Although the surface energy values of the TAG crystals were not available, we may reasonably assume that the surface energy value of the lamellar plane was much lower than that of the lateral plane. This is because the lamellar plane was composed of methyl end groups (–CH3), and the lateral plane was composed of glycerol groups, whose van der Waals interactions should be much stronger than those between the methyl end group. Therefore, if the TAG crystals were able to be adsorbed at the air–oil surface, the stable arrangement of the crystals should be as illustrated in Fig. 13, because of the effects of surface energy reduction.
It was quite difficult to determine the precise number of adsorbed crystal layers around the air bubbles in the POM and SR-μ-XRD experiments. However, we may assume a crystal layer thickness of a few tens of micrometers, based on POM images and X-ray microbeam scanning data (diameter of 5 μm). This indicates not single but multiple crystal layers.
The next problem is determining why the FHR-B crystals are adsorbed at the air–oil surface in the whipped oil containing salad oil. The most likely reason is the reduction in the γ values of salad oil due to the adsorption of the FHR-B crystals, as shown in olive oil with the addition of diglycerol monolaurate [24]. To prove this, we carried out a preliminary study of surface tension measurement of salad oil in the absence and presence of FHR-B crystals (0.5 wt%) after the tempering C procedure at 25 °C, using a pendant drop method. We confirmed that the addition of 0.5 wt% FHR-B crystal resulted in a reduction in the surface tension of salad oil from 33.17 dyne/cm without FHR-B to 32.40 dyne/cm (unpublished data).
Finally, it is worth noting that whipped oil with an overrun value of 200 % containing 6 wt% FHR-B is stable, without leakage of liquid oil from the whipped oil, over several days at ambient temperature, since the coalescence of air bubbles and the separation of air and oil phases do not occur. Such coalescence may be retarded by crystal–crystal contact of tiny FHR-B crystals surrounding the air bubbles, as depicted in Fig. 13. However, future studies should investigate the stabilization of whipped oil over longer periods.
Conclusion
In the present study, we have reported the processes used for the formation of whipped oils composed of vegetable oils and high-melting fat crystals of FHR-B by applying appropriate crystallization and tempering procedures. We have shown that the most determinative factor for the formation of the whipped oil was the creation of tiny β-fat crystals which could be adsorbed at the air–oil surface to encapsulate the air bubbles. The present study provides the first evidence that tiny fat crystal particles may cause aeration in liquid oils without the addition of other whip-assisting substances such as solid or liquid crystalline emulsifiers. To obtain a thorough understanding of fat crystal-based whipped oils using FHR-B, further studies are needed on the following subjects:
-
Effects of oil types with different polarity and origin (mineral, vegetable, or animal) on aeration and stabilization.
-
Morphological changes, such as coalescence of air bubbles (Ostwald ripening) or air-and-oil draining with time and varying temperature.
-
Direct and precise observation of morphology and arrangement and adsorption kinetics of fat crystal particles around air bubbles, particularly with scanning electron microscopy.
-
Precise measurement of the surface tension of different liquid oils with the addition of FHR-B crystals whose sizes and morphology are modified by varying tempering procedures.
Finally, we may extend the present work toward the formation of whipped oils using organogel or oleogel systems [44, 45]. Organogels are highly viscoelastic mixtures, since large amounts of liquid oil are immobilized by the presence of solid particles or fibers (organogelators). We conclude that oil-binding mechanisms may be applied for the formation and stabilization of air bubbles in the oil phase such that whipped oil can be formed by applying aeration processes to various organogel systems.
References
Eisner MD, Jeelani SAK, Bernhard L, Windhab E (2007) Stability of foams containing proteins, fat particles and nonionic surfactants. Chem Eng Sci 62:1974–1987
Friberg SE, Chang S, Greene WB, Gilder RV (1984) A nonaqueous foam with excellent stability. J Colloid Interface Sci 101:593–595
Prins A (1988) Principles of foam stability. In: Dickinson E, Stainsby G (eds) Advances in food emulsions and foams. Elsevier Applied Science Publishers LTD, Crown House, pp 91–122
Bisperink CGJ, Ronteltap AD, Prins A (1992) Bubble-size distributions in foams. Adv Colloid Interface Sci 38:13–32
Garret CG (1993) Recent development in the understanding of foam generation and stability. Chem Eng Sci 48:367–392
Tanaka T, Kaneko Y, Ohyama M (1995) Dynamic surface tension and foaming properties of aqueous polyoxyethylene n-dodecyl ether solutions. J Colloid Interface Sci 173:493–499
Zuniga RN, Aguilera JM (2008) Aerated food gels: fabrication and potential applications. Trends Food Sci Technol 19:176–187
Murray BS, Ettelaie R (2004) Foam stability: proteins and nanoparticles. Curr Opin Colloid Interface Sci 9:314–320
Murray BS (2007) Stabilization of bubbles and foams. Curr Opin Colloid Interface Sci 12:232–241
Murray BS, Dickinson E, Wang Y (2009) Bubble stability in the presence of oil-in-water emulsion droplets: influence of surface shear versus dilatational rheology. Food Hydrocoll 23:1198–1208
Shrestha LK, Shrestha RG, Solans C, Aramaki K (2007) Effect of water on foaming properties of diglycerol fatty acid ester-oil systems. Langmuir 23:6918–6926
Kalnin D, Gamaud G, Amenitsch H, Ollivon M (2002) Monitoring fat crystallization in aerated food emulsions by combined DSC and time-resolved synchrotron X-ray diffraction. Food Res Int 35:927–934
Boode K, Walstra P (1993) Partial coalescence in oil-in-water emulsions 1. Nature of the aggregation. Colloids Surf A 31:121–137
Leser M, Michel M (1999) Aerated milk protein emulsions––new microstructural aspects. Curr Opin Colloid Interface Sci 4:239–244
Tual A, Bourles E, Barey P, Houdoux A, Desprairies M, Courthaudon JL (2006) Effect of surfactant sucrose ester on physical properties of dairy whipped emulsions in relation to those of O-W interfacial layers. J Colloid Interface Sci 295:495–503
Gonzenbach UT, Studart AR, Tervoort E, Gauckler LJ (2006) Stabilization of foams with inorganic colloidal particles. Langmuir 22:10983–10988
Areyard R, Binks BP, Fretcher PDI, Peck TG, Rutherford CE (1994) Aspects of aqueous foam stability in the presence of hydrocarbon oils and solid particles. Adv Colloid Interface Sci 48:93–120
Du Z, Bilbao-Montaya MP, Binks BP, Dickinson E, Ettelaie R, Murray BS (2003) Outstanding stability of particle-stabilized bubbles. Langmuir 19:3106–3108
Shirtcliffe NJ, McHale G, Newton M, Perry CC (2003) Intrincically superhydrophobic organosillica sol-gel foams. Langmuir 19:5626–5631
Binks BP, Horozov TS (2005) Aqueous foams stabilized solely by silica nanoparticles. Angew Chem Int Ed 44:3722–3725
Dickinson E, Ettelaie R, Kostakis T, Murray BS (2004) Factors controlling the formation and stability of air bubbles stabilized by partially hydrophobic silica nanoparticles. Langmuir 20:8517–8525
Dickinson E (2010) Food emulsions and foams: stabilization by particles. Curr Opin Colloid Interface Sci 15:40–49
Shrestha LK, Shrestha RG, Sharma SC, Aramaki K (2008) Stabilization of nonaqueous foam with lamellar liquid crystal particles in diglycerol monolaurate/olive oil system. J Colloid Interface Sci 328:172–179
Shrestha RG, Shrestha LK, Solans C, Gonzalez G, Aramaki K (2010) Nonaqueous foam with outstanding stability in diglycerol monomyristate/olive oil system. Colloids Surf A 353:157–165
Binks BP, Davies CA, Fletcher PDI, Sharp EL (2010) Non-aqueous foams in lubricating oil systems. Colloids Surf A 360:198–204
Binks BP, Rocher A, Kirkland M (2011) Oil foams stabilized solely by particles. Soft Matter 7:1800–1808
Binks BP, Sekine T, Tyowua AT (2014) Dry oil powders and oil foams stabilized by fluorinated clay platelet particles. Soft Matter 10:578–589
Brun M, Delample M, Harte E, Lecompte S, Leal-Calderon F (2015) Stabilization of air bubbles in oil by surfactant crystals: a route to produce air-in-oil foams and air-in-oil-in-water emulsions. Food Res Int 67:366–375
Binks BP, Garvey EJ, Vieira L (2016) Whipped oil stabilised by surfactant crystals. Chem Sci 7:2621–2632
Higaki K, Sasakura Y, Koyano T, Hachiya I, Sato K (2003) Physical analyses of gel-like behavior of binary mixtures of high- and low-melting fats. J Am Oil Chem Soc 80:263–270
Higaki K, Koyano T, Hachiya I, Sato K (2004) In situ optical observation of microstructure of β-fat gel made of binary mixtures of high-melting and low-melting fats. Food Res Int 37:2–10
Higaki K, Koyano T, Hachiya I, Sato K, Suzuki K (2004) Rheological properties of β-fat gel made of binary mixtures of high-melting and low-melting fats. Food Res Int 37:799–804
Ueno S, Nishida T, Sato K (2008) Synchrotron radiation microbeam X-ray analysis of microstructures and the polymorphic transformation of spherulite crystals of trilaurin. Cryst Growth Des 8:751–754
Bayes-Garcia L, Calvet T, Cuevas-Diarte MA, Ueno S, Sato K (2011) Heterogeneous microstructures of spherulites of lipid mixtures characterized with synchrotron radiation microbeam X-ray diffraction. Cryst Eng Comm 13:6694–6705
Arima S, Ueno S, Ogawa A, Sato K (2009) Scanning microbeam small-angle X-ray diffraction study of interfacial heterogeneous crystallization of fat crystals in oil-in-water emulsion droplets. Langmuir 25:9777–9784
Tanaka L, Tanaka K, Yamato S, Ueno S, Sato K (2009) Microbeam X-ray diffraction study of granular crystals formed in water-in-oil emulsion. Food Biophys 4:331–339
Wassel P, Okamura A, Young NWG, Bonwick G, Smith C, Sato K, Ueno S (2012) Synchrotron radiation macrobeam and microbeam X-ray diffraction studies of interfacial crystallization of fats in water-in-oil emulsions. Langmuir 28:5539–5547
Verstringe S, Dewettinck K, Ueno S, Sato K (2014) Triacylglycerol crystal growth: templating effects of partial glycerols studied with synchrotron radiation microbeam X-ray diffraction. Cryst Growth Des 14:5219–5226
Sonoda T, Takata Y, Ueno S, Sato K (2004) DSC and synchrotron radiation X-ray diffraction studies on crystallization and polymorphic behavior of palm stearin in bulk and oil-in-water emulsion states. J Am Oil Chem Soc 8:365–373
Sonoda T, Takata Y, Ueno S, Sato K (2006) Effect of emulsifiers on crystallization behavior of lipid crystals in nanometer-size oil-in-water emulsion droplets. Cryst Growth Des 6:306–312
Yano J, Sato K (1999) FT-IR studies on polymorphism of fats: molecular structures and interactions. Food Res Int 32:249–259
Brooker BE (1990) The adsorption of crystalline fat to the air-water interface of whipped cream. Food Struct 9:223–229
Beckmann W, Boistelle R (1984) Calculations of the intermolecular potential and surface energies of stearic acid. J Cryst Growth 67:271–277
Marangoni AG, Garti N (eds) (2011) Edible oleogels: structure and health implications. AOCS Press, Urbana, Illinois, pp 1–352
Dassanayake LSK, Kodali DR, Ueno S (2011) Formation of oleogels based on edible lipid materials. Curr Opin Colloid Interface Sci 16:432–439
Acknowledgments
This work is supported in part by the following two projects: the Ministry of Education, Culture, Sports, Science, and Technology (Grant-in-Aid, 20540397) and the Food Nanotechnology Project of the Ministry of Agriculture, Forestry and Fisheries of Japan. The experiments were performed with the approval of the Photon Factory Program Advisory Committee (Proposal Nos. 2008G201 and 2008G623). The authors acknowledge Mr. Y. Shinohara, University of Tokyo, for kind help with the microbeam SAXS experiment. The authors also appreciate the help of Prof. A. Iida, station manager of beam line 4A at Photon Factory, KEK Institute, Tsukuba, Japan. The authors also acknowledge Prof. Y. Furukawa, Institute of Low Temperature Science, Hokkaido University, and Dr. Y. Uda, Sumitomo Chemical Co., Ltd, for kind help with the FT-IR experiment.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Mishima, S., Suzuki, A., Sato, K. et al. Formation and Microstructures of Whipped Oils Composed of Vegetable Oils and High-Melting Fat Crystals. J Am Oil Chem Soc 93, 1453–1466 (2016). https://doi.org/10.1007/s11746-016-2888-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-016-2888-4