Abstract
This research presents experimental studies of wastewater treatment by microalgae. Discharge of insufficiently treated wastewater from nutrients into water bodies is one of the most common causes of eutrophication. Aquatic ecosystems respond to nutrient enrichment primarily by the intensive development of cyanobacteria. Their rapid reproduction causes “blooming” of water, which leads to oxygen deficiency and mass death of fish and other aquatic organisms. Removal of nitrogen and phosphorous compounds from sewage water in a culture of Euglena gracilis was investigated. The ability of Euglena gracilis for 7 days almost completely to remove ammonium nitrogen and phosphorus phosphates from the studied wastewater was established.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
It is truism nowadays to recognize that pollution associated problems are a major concern of society. Environmental laws are given general applicability and their enforcement has been increasingly stricter. So, in terms of health, environment and economy, the fight against pollution has become a major issue. Today, although the strategic importance of fresh water is universally recognized more than ever before, and although issues concerning sustainable water management can be found almost in every scientific, social, or political agenda all over the world, water resources seem to face severe quantitative and qualitative threats. The pollution increase, industrialization and rapid economic development, impose severe risks to availability and quality of water resources, in many areas worldwide [1].
A major report by the World Health Organization (WHO) has recently been released, according to which a polluted environment is one of the leading causes of high mortality in the world. According to the organization, almost a quarter of the world's population dies due to poor environmental conditions: environmental risks cause more than 100 of the most dangerous diseases, and every year they kill 12.6 million people, which is 23% of all deaths in the World [2].
2 Analysis of Literature Data and Problem Statement
Water resources of any country are a source of drinking water for the population, and therefore require their rational use and protection from pollution. One of the biggest problems of river pollution is the poor quality of wastewater treatment. In many regions there is no complete set of treatment facilities and sanitary protection zones at all.
Due to the high rate of urban development there is an increase in wastewater. This situation leads to the fact that existing treatment systems become inefficient, and the quality of treated wastewater often does not meet the required standards [3]. Discharge of insufficiently treated wastewater from nutrients into water bodies is one of the most common causes of eutrophication. Aquatic ecosystems respond to nutrient enrichment primarily by the intensive development of cyanobacteria. Their rapid reproduction causes “blooming” of water, which leads to oxygen deficiency and mass death of fish and other aquatic organisms. Favorable conditions are created for the development of pathogenic microflora and pathogens [4]. This problem is especially relevant for small rivers and lakes. “Water blooming”, makes impossible to use water for recreation, fishing and domestic needs. The phytoplankton toxins can cause health problems through exposure to the human body after contact with the skin or the use of contaminated water for drinking. Therefore, there is a problem of more efficient wastewater treatment from nutrients because these compounds are removed in low amounts which usually do not exceed 10% [5].
The main goal of this paper is to study the metabolism of Euglena gracilis in relation to nitrogen and phosphorus compounds that enter wastewater due to human activity.
The supply of nutrients to surface waters occurs as due to natural factors (leaching from the layer of soil, precipitation, intra-reservoir processes), and also because of anthropogenic (receipts from industrial and economic domestic wastewater, agricultural land runoff and animal complexes). The most surface water bodies in Ukraine are classified as polluted or much polluted. This condition significantly increases environmental risks. It is the use of water that does not meet quality requirements that causes and spreads many infectious and non-infectious diseases [6]. The consequences of water pollution by nutrients are regularly described in national reports on drinking water quality in Ukraine. Watercourses receive pollution from many different sources, which vary both in strength and volume. As known the main sources of water pollution are: wastewater, farms, oil spills, solid municipal waste, agriculture, atmospheric precipitations etc. The composition of wastewater is a reflection of the life styles and technologies practiced in the producing society. It is a complex mixture of natural organic and inorganic materials as well as man-made compounds. Three quarters of organic carbon in sewage are present as carbohydrates, fats, proteins, amino acids, and volatile acids. The inorganic constituents include large concentrations of sodium, calcium, potassium, magnesium, chlorine, sulphur, phosphate, nitrates, bicarbonate, ammonium salts and heavy metals [7].
Total nitrogen represents the sum of organic and inorganic nitrogen compounds. For wastewater Kjeldahl-nitrogen is generally applied as a measure. The Total Kjeldahl Nitrogen value (TKN) represents a total nitrogen concentration, which is the sum of organic nitrogen compounds and ammonium nitrogen (TKN = org–N + NH4−N [mg/L]). Nitrogen mainly occurs in wastewater in this form. After biological wastewater treatment, it mainly occurs as oxidized nitrite [8].
Municipal wastewaters may contain from 5 to 20 mg/L of total phosphorous, of which 1–5 mg/l is organic and the rest in inorganic. The individual contribution tends to increase, because phosphorous is one of the main constituent of synthetic detergents. The individual phosphorous contribution varies between 0.65 and 4.80 g/inhabitant per day with an average of about 2.18 g. The usual forms of phosphorous found in aqueous solutions include:
-
Orthophosphates: available for biological metabolism without further breakdown.
-
Polyphosphates: molecules with 2 or more phosphorous atoms, oxygen and in some cases hydrogen atoms combine in a complex molecule. Usually polyphosphates undergo hydrolysis and revert to the orthophosphate forms. This process is usually quite slow.
Normally secondary treatment can only remove 1–2 mg/L, so a large excess of phosphorous is discharged in the final effluent, causing eutrophication in surface waters. New legislation requires a maximum concentration of P discharges into sensitive water of 2 mg/L [9]. Figure 1 demonstrates phosphorous compounds presented in wastewater.
Diehard wastewater engineers understand the value of wastewater, which they view as an asset rather than a waste. That’s why some of them call it “used water” instead, and refer to what most people call wastewater treatment plants as water resource recovery facilities.
In fact, wastewater can contain more than three times the amount of energy needed to treat it. One simple and mature technique for recovering part of this energy is anaerobic digestion, a natural process in which microorganisms feed on grease and other organic materials in wastewater and produce biogas, just as yeast can eat up barley and spit out beer. Biogas contains roughly 50% methane, which can be used as a renewable fuel for boilers, furnaces and heating systems or to turn turbines and generate electricity [10].
More advanced techniques, such as hydrothermal processes, take sewage sludge—the solids removed from wastewater during treatment—and convert it into biobased fuels that can be used to replace gasoline and diesel fuel. This process is currently at the demonstration stage.
In additional to sewage sludge, many researchers—including us—are very interested in microalgae. Microalgae are promising feedstock for biofuels, and some of them can grow in wastewater.
Researches of scientists in many countries of the world are focused on the problems of wastewater treatment. There are many methods and technologies to purify waste water. Biological wastewater treatment, with application of microalgae, originating from a variety of industries has been regarded as an economically and technologically justified process.
Microalgae constitute a diversified group of eukaryotic photosynthetic microorganisms which colonize both the marine and freshwater environments. Microalgae are among the fastest developing photosynthetic organisms. Their photosynthetic mechanism is similar to that of terrestrial plants. Microalgae do not compete for cultivated land. With access to water, carbon dioxide and biogenic compounds such as nitrogen and phosphorus, offer higher biomass yields than terrestrial plants. Algae have the ability to produce 50 times more biomass than higher plants [11, 12]. Different varieties of algae are able to develop in a wide variety of environments, even in degraded or contaminated areas [13]. Such cultures bring a positive effect to the natural environment because algae may be produced using communal, agricultural or industrial waste-water containing carbon dioxide which is required for their growth CO2 [14]. The molar ratio of the algae biomass main components proposed by Grobbelaar [15] is as follows: CO–0.48; H–1.83; N–0.11; P–0.01. In the course of microalgae population development, four following phases can be distinguished: adaptation phase, growth phase, stationary phase and decline phase [16, 17]. The importance and interest in algae has been increasing with time.
Thus, the scientists from Uttaranchal University (India), have developed a method for purifying wastewater using microalgae and successfully tested it on the waters of the Indian Bindal River [18]. As part of the first phase of the experiment, scientists used several tens of liters of contaminated water by placing microalgae in it. The result was a significant reduction in total bacteria and E. coli (by 90%), organic and inorganic compounds (by 90%) and total organic carbon, as well as a decrease in alkalinity and water hardness (by 70%). The content of heavy metals in water also significantly decreased: for example, the content of zinc, lead, copper, iron, nickel and other metals decreased by about 90%. Biodiesel was obtained from algal biomass used to purify polluted water.
It is not the first year that there have been discussions around the topic of banning the use of phosphates in detergents. And at a time when the countries of the world legally limited or completely banned the use of phosphates in detergents back in the 80s and 90s of the last century, outdated technical regulations continue to operate in Ukraine.
But so far we have disappointing statistics regarding phosphate discharges into surface water bodies with wastewater. For example, the analysis of data from the state water use accounting for 2019 shows that last year almost 6 thousand tons of phosphates were dumped into the water bodies of Ukraine. The largest polluter of water bodies is the housing and communal economy—5354 tons [19].
In order to solve this global world problem, in the 1970s, under the auspices of the UN, an international agreement on the protection of the world's oceans and freshwater resources was concluded. It defines the main areas of activity to reduce pollution. And the first of them is the reduction and complete cessation of pollution of water resources by biogenic substances, especially phosphorus. However, experiments conducted in some countries have shown that phosphate fertilizers are slow-moving compounds and remain in the soil for up to 5–8 years. The share of contamination of water bodies with mineral phosphate fertilizers is from 10 to 30% of the total input of phosphorus. More than 60% of phosphates enter water bodies due to the use of phosphate washing powders.
In the early 1970s, some US states began to restrict and ban the use of phosphates in laundry detergents. In 1994, they were banned throughout the country. In July 2010, 17 states also banned the use of phosphates in dishwasher detergents. In 1976, the use of phosphates in washing powders was stopped in Japan. In 2011, the European Parliament banned phosphates in laundry detergents from June 2013 and in dishwasher detergents from January 2017. Manufacturers of washing powders began to replace phosphates with substances that are biodegradable. At the same time, in many countries of the world, the construction of special treatment facilities for the removal of phosphorus and nitrogen from wastewater began. As scientists explain, the optimal concentration of phosphates in washing powder should not exceed five percent. In this case, the use of detergent is not harmful to humans. Moreover, at such a concentration, phosphates are completely rinsed from the fabric with water [20].
After washing with dirty water, tripolyphosphate directly enters the soil, and from there into rivers and lakes. Its quantity accumulates, and then it begins to act as a fertilizer. That is, the water system of rivers and lakes is being increasingly fed with harmful discharges from cities and industrial waste. Such feeding of water first causes rapid flowering, and then the inevitable aging of the reservoir. In places where the dangerous substance accumulates on the surface of the water, a “harvest” of blue-green algae is observed, which has the ability to reproduce with terrible force: 1 g of sodium tripolyphosphate stimulates the formation of 5–10 kg of algae, which, when decomposed, poison water and fish. More than 220.000 tons of washing powders are sold in Ukraine every year, most of which contain sodium tripolyphosphate. Thus, more than 25 thousand tons of this dangerous compound are dumped into the water, which can lead to a large-scale environmental disaster. Phosphates are not only produced by algae. Plankton is also actively growing. And the more any kind of suspension is in the water, the less opportunity there is to use rivers and reservoirs as sources of drinking water. But since we have no other water, we have to use this poisoned water. The circle closed. We began to poison the environment in which we live with phosphates—we get back through food and drink all that we poisoned ourselves. An excess of phosphorus initiates the following chain: rapid growth of plants—death of plants—decay—impoverishment of water bodies with oxygen—deterioration of the life of organisms [21].
There is a hypothesis that the main supplier of phosphates into the environment is the use of household chemicals. Studies of leading brands of detergents have shown that the vast majority of them contain a large amount of phosphates. We present the research data in Table 1.
An excessive number of microorganisms poisons the water with waste products, higher animals (fish, crayfish, etc.) begin to die due to a lack of oxygen in the water. In addition, blue-green algae not only reduce the level of oxygen in the water but also accumulate toxic substances. When they decompose, these accumulations enter the environment.
Together with water, phosphates enter the human body. A person comes into contact with all the toxic components of washing powders through the skin because it is almost impossible to rinse them from linen and clothes.
Phosphates contribute to increased degreasing of the skin, active destruction of cell membranes, sharply reduce the barrier function of the skin, and can also cause manifestations of various types of allergic reactions.
An excess of sodium phosphate can be perceived by the body as a laxative, and an excess of potassium phosphate can cause a disturbance of the intestinal microflora. In addition, phosphates can lead to the development of allergies, negatively affect the work of the kidneys and liver [23].
The association ‘Ukrvodokanalekologia’ has submitted a petition to the President of Ukraine to submit to the Verkhovna Rada for consideration a draft law on a complete ban on the production, import, sale and use of phosphate-containing detergents in Ukraine. Phosphates entering water bodies due to the use of synthetic detergents are one of the main sources of their pollution and the development of such negative processes as water bloom (eutrophication).
According to the data of the state record of water use, in the last four years, the total content of phosphates in waste water of water supply and sewerage and, to a lesser extent, other enterprises of the Mykolaiv region has increased 3 times.
As stated in the petition, the problem of removing phosphorus compounds from wastewater does not have an optimal solution. Since the existing biological methods do not allow to achieve the necessary degree of purification of waste water from phosphorus compounds, and physical and chemical methods require significant costs and additionally create the problem of the need to treat sediments formed during reagent treatment.
Most of the world's countries have long since abandoned the use of phosphate-based detergents in order not to pollute their water bodies with them. The European Union has adopted a number of legislative acts on reducing the negative impact of detergents on human health and the environment [24].
Foam separation, chemical precipitation in the form of sparingly soluble compounds and destructive destruction are used to reduce the concentration of surfactants, and ion exchange and sorption are used to further purify water. The processing of information on methods of purification of wastewater from surfactants and phosphates makes it possible to highlight a number of the latest methods of purification.
In recent years, data on the issue of reduction of nitrogen and phosphorus in wastewater discharged into open water. These publications mainly advertise the own developments of private firms, but the theoretical foundations and mechanism of biological processes of nitrification–denitrification and dephosphorization of wastewater remain unresolved. So far, there are no reliable and theoretically sound methods of technological calculations of aeration tanks, which carry out anaerobic and aerobic processes of deep wastewater treatment from nitrogen and phosphorus. Researches of authors [25, 26] indicate that the growth of microhydrogen rates is a superior and effective biological method for purifying waste water from nitrogen and phosphorus. As a matter of fact, micro-hydrophilic growths are used to regulate their metabolism, to reduce their concentration in water [25]. Besides, micro-hydrophilic growth is a byproduct of photosynthesis, which can be used as aerobic bacteria for biological distribution of organic bacteria present in cold waters. The purification of waste water from the microwells is recognized ecologically without baking, and there are no examples of secondary flows, such as the use of an active mule, which requires disposal [1]. Most often for the purification of sewage waters, nitrogen and phosphorus are used to produce green micro-growth, sprouts are representatives of the genus Chlorella, Scenedesmus and Chlamydomonas. Protein, it appears [27].
Scientific references [28] provide many examples of algae-based systems in wastewater treatment used algae cultures of Botryococcus braunii species as the third phase of wastewater treatment in closed systems. Such a technological solution allowed for efficient removal of both nitrogen and phosphorus from communal wastewater discharged from activated sludge reservoirs. The use of wastewater resulted in algae biomass production with a high concentration of carbohydrates. Authors [27] presented data and a detailed description of the application of varied waste-water in Chlorella sp. algae culture. They distinguish three main wastewater sources: communal, agricultural and industrial, containing a wide variety of components. Researchers indicate that nutrients such as nitrogen and phosphorus contained in wastewater may successfully be used as a culture medium for an intensive biomass culture.
Wastewater rich in organic carbon, nitrogen and phosphorus may serve as a convenient source of carbon and nutrients for a year-long microalgae production [29]. Scientific reports indicate that some single-cell microalgae such as Chlorella and Scenedesmus are highly tolerant to wastewater environments and efficiently remove biogenic compounds [30].
It follows from the literature that to deepen the wastewater from nitrogen and phosphorus in existing treatment plants must primarily use biological agents and techniques, rather than chemicals, which greatly impair operating costs and may create additional difficulties with the disposal of sewage sludge.
3 Research Methodology
Strain Euglena gracilis Klebs HPDP-114 was obtained from the collection of microalgae cultures of the Institute of Hydrobiology of the National Academy of Sciences of Ukraine. Euglena are characterized by an elongated cell (15–500 µm [1 µm = 10−6 m], or 0.0006–0.02 inch) with one nucleus, numerous chlorophyll-containing chloroplasts (cell organelles that are the site of photosynthesis), a contractile vacuole (organelle that regulates the cytoplasm), an eyespot, and one or two flagella. Unlike plant cells, Euglena lacks a rigid cellulose wall and has a flexible pellicle (envelope) that allows them to change shape. Though they are photosynthetic, most species can also feed heterotrophically (on other organisms) and absorb food directly through the cell surface via phagocytosis (in which the cell membrane entraps food particles in a vacuole for digestion). Food is often stored as a specialized complex carbohydrate known as paramylon, which enables the organisms to survive in low-light conditions. Euglena reproduces asexually by means of longitudinal cell division, in which they divide down their length, and several species produce dormant cysts that can withstand drying.
The experiments used synthetic domestic wastewater, which was prepared by adding to the culture medium different concentrations of ammonium nitrogen (N–NH4+) and phosphorus phosphates (P–PO43−) (Table 2). NH4Cl was used as a source of N–NH4+, KH2PO4– as a source of P–PO43−. Algae were kept at a temperature of 24 ± 2 °C and illumination of 3500 lx (with alternating light and dark periods 16:8). The duration of cultivation of Euglena gracilis in wastewater was 7 days. Materials for analysis were selected on 0 and 7 days of the experiment.
The concentration of ammonium nitrogen and phosphorus phosphates in wastewater was investigated by conventional methods in hydrochemistry.
Dry mass of the culture was determined by weight [31, 32]. The algal cell suspension was filtered through a pre-dried and weighed membrane filter (pore diameter 0.45 μm). Filters with precipitated algae were dried in a thermostat at a temperature of 105 °C to constant weight.
Biomass productivity was calculated by the formula:
where N—the dry matter content at the end of cultivation, (mg L−1 day−1); N0—dry matter content at the beginning of cultivation, (mg L−1 day−1); T—duration of cultivation (7 days).
The content of chlorophyll a and the sum of carotenoids was determined by extract-spectrophotometric method [31]. For extraction, the algae filter was placed in a porcelain mortar and thoroughly ground with the addition of quartz sand and 90% acetone. The obtained extract was separated by centrifugation during 15 min at 5000 thousand rpm. Spectrophotometry was performed at wavelengths of 664, 647 and 480 nm, corresponding to the maxima of light absorption in 90% acetone, respectively, chlorophyll a, b and carotenoids. Non-specific absorption of the light extract at a wavelength of 750 nm was also measured.
The chlorophyll a content was calculated by the formula [33, 34]:
where V1—extract volume, cm3; V2—the volume of the filtered sample, dm3; E 664 and E 647—experimentally determined the optical densities of the extract at wavelengths 664 and 647 nm.
The total carotenoid content was calculated by the formula [34, 35]:
where V1—extract volume, cm3; V2—the volume of the filtered sample, dm3; 1—the thickness of the spectrophotometric cell, sm; and E 480 and E 750—optical densities of the extract at wavelengths 480 and 750 nm.
The content of photosynthetic pigments was expressed in terms of dry weight of algae.
All measurements were performed in triplicate. Statistical processing of the results was performed using IBM SPSS Statistics Base v.22.
4 Results and Disscusion
Typical forms of phosphorus in munitipal sewage waters are the next.
-
Orthophosphates—salts of phosphoric acids, in particular orthophosphoric acid H3PO4, with one phosphorus atom, for example sodium orthophosphate Na3PO4. They are simple molecules that are suitable for using in biological metabolism without prior decomposition.
-
Polyphosphates—polymethyl phosphates with the general formula M–O–[P(OM)(O)–O]n–M, where M is any metal. Their chains pass between other chemical groups, for example, sodium-3-phosphate (Na5P3O10)n. They are complex molecules with two or more phosphorus atoms, which before assimilation by organisms require preliminary hydrolysis with decomposition into orthophosphates. It takes a long time;
-
Organic phosphates—orthophosphoric acid esters with the general formula (RO)nP(O)(OH)3-n, where RO is a hydrocarbon radical, for example, methyl phosphate (methylphosphoric acid (CH3O)P(O)(OH)2).
According to [36], municipal sewage waters can contain from 5 to 20 mg/dm3 of total phosphorus (in terms of phosphates for PO4, it is from 15.7 to 62.7 mg/dm3). Organic compounds make up from 1 to 5 mg/dm3 (3.1 … 15.7 mg/dm3) out of it. All the rest are inorganic compounds. The individual contribution of phosphorus to the sewage network from one inhabitant of a residential area is estimated from 0.65 to 4.80 g/day. The average value is 2.18 g/day. This contribution tends to increase due to the increased use of detergents.
According to [37], municipal sewage waters can contain from 4 to 16 mg/dm3 of total phosphorus (12.5–50.1 g/m3 of phosphates). According to [38], these waters may contain 10 mg/dm3 of total phosphorus (31.3 g/m3 of phosphates).
Secondary (biological) treatment of sewage waters, as a rule, reduces the content of phosphates by 1 … 2 g/m3. It's not enough.
According to the data from the Bortnytska Aeration Station [39], the content of phosphates in the sewage waters of Kyiv city is 26 g/m3 at the standard level of 8 g/m3. That is, there is an excess of more than three times. In terms of total phosphorus, this value can be 8.48 g/m3. With a phosphorus removal efficiency of 0.2 at traditional sewage treatment plants, its residual concentration in treated sewage waters is 6.78 g/m3. The plant discharges about 0.6 × 106 m3 of treated sewage into the Dnipro river. Under such conditions, 4,068 tons of phosphorus is supplied to the Dnipro with municipal sewage waters. It is known that each gram of phosphate compounds, under the remaining favorable conditions, causes an increase of 5 … 10 kg of cyanobacteria in the water body.
The typical forms of nitrogen in municipal sewage waters are the next.
-
Nitrogen nitrites—salts of nitric acid (HNO2), for example, NaNO2.
-
Ammonium nitrogen (general formula NH4+).
-
Nitrogen nitrates—salts of nitric acid (HNO3), for example, 2NaNO3.
-
Nitrogen in organic compounds.
The most toxic among these compounds are nitrites, and the least toxic are nitrates. Ammonium nitrogen occupies an intermediate position.
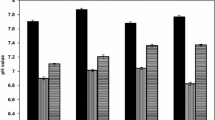
According to the results of the studies, Euglena gracilis for 7 days almost completely removes ammonium nitrogen and phosphorus phosphates from the studied wastewater (Table 3). It has been shown [25] that the ability of microalgae to remove nitrogen and phosphorus compounds from the aquatic environment is determined primarily by the physiological characteristics of species, including metabolic rate and the need for nutrients to maintain their viability. In the process of bioremediation of microalgae, a large amount of nitrogen and phosphorus is used for the synthesis of proteins, nucleic acids, phospholipids and other important organic compounds.
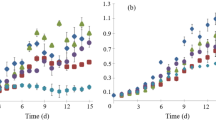
The growth intensity of Euglena gracilis was assessed by the accumulation of dry mass (Table 4). At concentrations of N–NH4+ 30 mg L−1 and P–PO43− 7 mg L−1 the increase in biomass was 120 mg L−1 day−1, at concentrations of ammonium nitrogen 50–90 mg L−1 and phosphorus phosphate 14–26 mg L−1 it was 151–156 mg L−1 day−1, which indicates a positive dynamics of dry mass accumulation during the experiment. Researches [26] obtained similar results on Euglena sp. During short-term cultivation of this alga in domestic wastewater, 98% removal of N–NH4+ (initial concentration 25 mg L−1) and 85% removal P–PO43− (initial concentration 16 mg L−1) was achieved, the biomass productivity index was 132 mg L−1 day−1.
The photosynthetic activity of Euglena gracilis under the conditions of the experiment was evaluated by the change in the content of pigments—chlorophyll and the sum of carotenoids (Table 5). During the cultivation of Euglena gracilis in wastewater with a concentration of N–NH4+ and P–PO43− 30 and 7 mg L−1, respectively, the content of chlorophyll in the biomass doubled. At the concentration of ammonium nitrogen 50–90 mg L−1 and phosphorus phosphate 14–26 mg L−1 at the end of the experiment, the content of basic photosynthetic was 2.5 times higher than the initial values. A similar trend was observed in the case of carotenoids, their content increased more than 2 times, indicating high photosynthetic activity of algae. However, it should be noted that the dry weight changed to a greater extent than the content of photosynthetic pigments. During seven days of cultivation, the dry weight increased 13–16 times. According to [26], an important feature that allows Euglena algae to develop intensively in wastewater is the ability to mixotrophic type of food.
Biomass Euglena gracilis is considered a promising raw material for biofuel production, as it is characterized by a high content of lipids [40]. Cultivation of Euglena gracilis in domestic wastewater can provide not only an environmentally friendly way to extract nutrients from them, but also a cost-effective way to obtain biomass of microalgae for biodiesel production.
5 Conclusions
The efficiency of microalgae application in the processes of contaminated wastewater treatment is analyzed. It is established that the use of microalgae is a reliable and effective biological method of wastewater treatment from nitrogen and phosphorus compounds. It is known that microalgae absorb these substances to maintain their metabolism, thereby reducing their concentration in water. In addition, microalgae produce oxygen as a by-product of photosynthesis that can be used by aerobic bacteria to biodegrade organic pollutants present in wastewater.
Wastewater treatment using microalgae is recognized as environmentally friendly because it does not generate secondary waste, such as spent activated sludge, which requires disposal. Eugene algae is most often used to treat wastewater from nitrogen and phosphorus compounds.
The peculiarities of Euglena gracilis metabolism, which are characterized by resistance to high concentrations of nitrogen and phosphorus, have been studied.
Experimental studies have been performed and the ability of Euglena gracilis to reduce the concentration of nutrients in wastewater has been established. Due to experimental sessions we can conclude that after 7 days ammonium nitrogen and phosphorus phosphates from the studied wastewater was almost completely removed.
It is shown that the ability of microalgae to remove nitrogen and phosphorus compounds from the aquatic environment is determined primarily by the physiological characteristics of the species, in particular the intensity of metabolism and the need for nutrients to maintain their vital functions. In the process of bioremediation of microalgae, a large amount of nitrogen and phosphorus is used for the synthesis of proteins, nucleic acids, phospholipids and other important organic compounds.
It is established that the biomass of Euglena gracilis is considered a promising raw material for biofuel production, as it is characterized by a high content of lipids. Cultivation of Euglena gracilis in domestic wastewater can provide not only an environmentally friendly method of extracting nutrients from them, but also a cost-effective way to obtain biomass of microalgae for biodiesel production.
References
Abdel-Raouf, N., Al-Homaidan, A.A., Ibraheem, I.B.M.: Microalgae and wastewater treatment. Saudi J. Biol. Sci. 19(3), 257–275 (2012). https://doi.org/10.1016/j.sjbs.2012.04.005
Shamanskyi, S.I., Boichenko, S.V.: Energy efficient and environmentally friendly technology of stabilizing airline enterprises’ wastewater sludges. East.-Eur. J. Enterp. Technol. 5(8(77), 39–45 (2015). https://doi.org/10.15587/1729-4061.2015.52264
Shamanskyi, S., Boichenko, S., Pavliukh, L.: Estimated efficiency of biogenic elements removal from waste water in the ideal displacement photobioreactor. In: Zaporozhets, A., Artemchuk, V. (eds.) Systems, Decision and Control in Energy II. Studies in Systems, Decision and Control, vol. 346. Springer, Cham (2021). https://doi.org/10.1007/978-3-030-69189-9_21
Jędrzejewska-Cicińska, M., Krzemieniewski, M.: Effect of corrosion of steel elements on the treatment of dairy wastewater in a UASB reactor. Environ. Technol. 31(6), 585–589 (2010). https://doi.org/10.1080/09593331003616821
Pavliukh, L., Shamanskyi, S., Boichenko, S., Jaworski, A.: Evaluation of the potential of commercial use of microalgae in the world and in Ukraine. Aircr. Eng. Aerosp. Technol. ahead-of-print No. ahead-of-print (2020). https://doi.org/10.1108/AEAT-08-2020-0181
Lim, S., Chu, W., Phang, S.: Use of Chlorella vulgaris for bioremediation of textile wastewater. J. Bioresour. Technol. 101, 7314–7322 (2010)
Nezbrytska, I., Shamanskyi, S., Pavliukh, L., Kharchenko, G.: Assessment of inorganic nitrogen and phosphorus compounds removal efficiency from different types of wastewater using microalgae cultures. Ocean. Hydrobiol. Stud. 51(1), 45–52 (2022). https://doi.org/10.26881/oahs.2022.1.05
Shamanskyi, S., Boichenko, S.: Development of environmentally safe technological water disposal scheme of aviation enterprise. East.-Eur. J. Enterp. Technol. 6(10(84)), 49–57 (2016). https://doi.org/10.15587/1729-4061.2016.86053
Li, Y., Horsman, M., Wu, N., Dubois-C, L.C.Q., Alero, N.: Biofuels from microalgae. Biotechnol. Prog. 24(4), 815–820 (2008)
Apt, K.E., Behrens, P.W.: Commercial developments in microalgae biotechnology. J. Phycol. 35(2), 215–226 (2002). https://doi.org/10.1046/j.1529-8817.1999.3520215
Mata, T.M., Martins, A.A., Caetano, N.S.: Microalgae for biodiesel production and other applications: a review. Renew. Sust. Energ. Rev. 14, 217–232 (2010). https://doi.org/10.1016/j.rser.2009.07.020
Chisti, Y.: Biodiesel from microalgae. Biotechnol. Adv. 25(3), 294–306 (2007). https://doi.org/10.1016/j.biotechadv.2007.02.001
Grobbelaar, J.U.: Algal Nutrition—Mineral Nutrition (2003).https://doi.org/10.1002/9780470995280.ch6
Barsanti, L., Gualtieri, P.: Algal Culturing. Algae: Anatomy, Biochemistry and Biotechnology. CRC Press, Boca Ranton, pp. 209–250 (2006)
Singh, B., Guldhe, A., Rawat, I., Bux, F.: Towards a sustainable approach for development of biodiesel from plant and microalgae. Renew. Sust. Energ. Rev. 29, 216–245 (2014). https://doi.org/10.1016/j.rser.2013.08.067. https://www.rfbr.ru/rffi/ru/press_about/o_2106600
Solovchenko, A.E., Lukjjanov, A.A., Vasylj'eva S.G. y dr.: Vozmozhnosty byotekhnologhycheskoj pererabotky seljskokhozjajstvennыkh otkhodov s yspoljzovanyem mykrovodoroslej. Vestnyk Mosk. un-ta. Ser. 16. Byol. No. 1, pp. 38–49 (2014)
Shamanskyi, S., Boichenko, S., Khrutba, V., Topilnyckyi, P., Pavliukh, L.: Improving the photobioreactor operation efficiency in the technological scheme of wastewater treatment. East.-Eur. J. Enterp. Technol. 6(10–114), 6–15 (2021)
http://journals.nubip.edu.ua/index.php/Energiya/article/viewFile/3660/3581
The negative impact of household chemicals and harmful substances on humans indoors. http://ukrref.com.ua/?id=MjQxMg%3D%3D
https://vspu.edu.ua/faculty/geogr/chemistry/art25_03/3_1.pdf
Shamanskyi, S., Boichenko, S.: Environment-Friendly Technology of Airport’s Sewerage. In: Karakoç, T., Colpan, C., Şöhret, Y. (eds.) Advances in Sustainable Aviation. Springer, Cham (2018). https://doi.org/10.1007/978-3-319-67134-5_11.
Madkour, A.G., Rasheedy, S.H., Dar, M.A., et al.: The differential efficiency of Chlorella vulgaris and Oscillatoria sp. to treat the municipal wastewater. J. Biol. Agric. Healthcare 7(22), 83–94 (2017)
Mahapatra, D.M., Chanakya, H.N., Ramachandra, T.V.: Euglena sp. as a suitable source of lipids for potential use as biofuel and sustainable wastewater treatment. J. Appl. Phycol. 25(3), 855–865 (2013). https://doi.org/10.1007/s10811-013-9979-5
Sawayama, S., Inoue, S., Dote, Y., Yokoyama, S.Y.: CO2 fixation and oil production through microalgae. Energy Convers. Manag. 36(69), 729–731 (1995). https://doi.org/10.1016/0196-8904(95)00108
Schenk, P.M., Thomas-Hall, S.R., Stephens, E., Marx, U.C., Mussgnug, J.H., Posten, C., Hankamer, B.: Second generation biofuels: high-efficiency microalgae for biodiesel production. Bioenerg. Res. 1(1), 20–43 (2008). https://doi.org/10.1007/s12155-008-9008-8
Nezbrytskaya, I.N., Kureyshevich, A.V., Yarovoy, A.A., et al.: Peculiarities of the influence of high concentrations of ammonium on the functioning of some species of cyanoprokaryota, chlorophyta, and euglenophyta. Hydrobiol. J. 55(2), 69–82 (2019)
Chiu, S.Y., Kao, C.Y., Chen, T.Y., Chang, Y.B., Kuo, C.M., Lin, C.S.: Cultivation of microalgal Chlorella for biomass and lipid production using wastewater as nutrient resource. Bioresour. Technol. 184, 179–189 (2015). https://doi.org/10.1016/j.biortech.2014.11.080
Ruiz-Marin, A., Mendoza-Espinosa, L.G., Stephenson, T.: Growth and nutrient removal in free and immobilized green algae in batch and semi-continuous cultures treating real wastewater. Bioresour. Technol. 101(1), 58–64 (2010). https://doi.org/10.1016/j.biortech.2009.02.076
Shkilniuk, I., Boichenko, S.: Biological risk of aviation fuel supply. In: Babak, V., Isaienko, V., Zaporozhets, A., (eds.) Systems, Decision and Control in Energy I. Studies in Systems, Decision and Control. Springer, Cham, Switzerland, vol. 298, pp. 179–199 (2020)
Nezbrytskaya, I.N., Kureyshevich, A.V.: Changes in the content of photosynthetic pigments in representatives of chlorophyta and cyanoprokaryota at a high temperature. Hydrobiol. J. 51(4), 46–56 (2015)
Jeffrey, S.W., Humphrey, F.H.: New spectrophotometric equations for determining chlorophyll a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem. Physiol. Pflanz. 167, 171–194 (1975)
Parsons, T.R., Strickland, J.D.H.: Discussion of spectrophotometric determination of marineplant pigments and carotinoids. J. Marine. Res. 21, 155 –163 (1963)
Lenntech. Phosphorous removal from wastewater (2017). https://www.lenntech.com/phosphorous-removal.htm
Tchobanoqlous, G., Burton, F.L., Stensel, H.D.: Wastewater Engineering: Treatment and Reuse. 4th edn. Metcalf&Eddy Inc., 1819 p. (2003)
Kroiss, H.: Betrieb von Klaeranlagen, vol. 202. Technische Universitat Wien, Wien, Band, 626 p. (2007)
Toyama, T., Hanaoka, T., Yamada, K., et al.: Enhanced production of biomass and lipids by Euglena gracilis via co-culturing with a microalgae growth-promoting bacterium, Emticicia sp. EG3. Biotechnol. Biofuels 12, pp. 205 (2019). https://doi.org/10.1186/s13068-019-1544-2
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Nezbrytska, I., Shamanskyi, S., Pavliukh, L., Gorbunova, Z., Horbachova, O., Repeta, V. (2024). Removal of Biogenic Compounds from Sewage Water in a Culture of Euglena Gracilis (EUGLENOPHYTA). In: Boichenko, S., Zaporozhets, A., Yakovlieva, A., Shkilniuk, I. (eds) Modern Technologies in Energy and Transport. Studies in Systems, Decision and Control, vol 510. Springer, Cham. https://doi.org/10.1007/978-3-031-44351-0_9
Download citation
DOI: https://doi.org/10.1007/978-3-031-44351-0_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-44350-3
Online ISBN: 978-3-031-44351-0
eBook Packages: EngineeringEngineering (R0)