Abstract
The present investigation was attempted to reduce the toxic pollutants from the different mixtures of water samples (sewage, sea and well) using the freshwater alga, Chlorella vulgaris and the marine alga Chlorella salina. The results revealed that both algae species were highly efficient and having a potential to reduce pH, total dissolved solids (TDS), biological oxygen demand (BOD), chemical oxygen demand (COD), nitrate, ammonia, phosphate, sulphate, calcium, magnesium, sodium, potassium, heavy metals (Zn, Cu, Mn, Ni, Co, Fe and Cr) and the number of total Coli-form bacteria after 10 days of treatment compared to the untreated water samples. The removal efficiency of heavy metals was 13.61–100 %. In general, C. vulgaris shows higher removal efficiency in most of parameters than C. salina.
Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Bioremediation is a friendly approach for remediation of contaminated water and soil. Biological treatment can be accomplished by different ways, through use of mixed microbial culture such as bacteria, fungi and algae for the bioremediation of pollutants (Azab 2008).
Phycoremediation is the use of micro or macro algae for the removal of pollutants including xenobiotics and nutrients from wastewater and CO2 from emitted waste air (Olguín 2003). Bioremediation with microalgae is particularly effective because of their capabilities converting solar energy into useful biomasses and assimilate nutrients such as phosphorus and nitrogen which cause eutrophication in the process of photosynthesis (De la Nou¨e and De Pauw 1988). In tertiary wastewater treatment, microalgae offer effective low cost approach to remove contaminants and excess nutrients and produce potentially valuable biomass, because of its high ability for inorganic nutrient uptake (Bolan et al. 2004; Munoz and Guieyssea 2006). Zhang et al. (2008) found that Scendesmus sp. showed high ability to remove inorganic nutrients from artificial domestic secondary effluents.
Microalgae assimilate a significant amount of nutrients because they require high amounts of phosphorus and nitrogen for the synthesis of 45–60 % proteins of microalgal dry weight, nucleic acids and phospholipids. Nutrient removal can also be increased by NH3 stripping or phosphorus precipitation due to the rise in the pH associated with photosynthesis (Oswald 2012).
The removal of biochemical oxygen demand (BOD) in the wastewater treatment system, suspended solids, nutrients (NO3 −–N; NO2 −–N; NH4 +–N and PO −34 –P), coli-form bacteria, and toxicity are the main goal for getting purified wastewater. Biological oxygen demand (BOD) exploits the ability of microorganisms to oxidize organic material to CO2 and water using molecular oxygen as an oxidizing agent. Therefore, BOD can deplete the dissolved oxygen of receiving water leading to fish kills and anaerobiosis, hence its removal is a primary aim of waste water treatment. Suspended solids are removed principally by physical sedimentation (Choi and Lee 2012; Abdel-Raouf et al. 2012).
Algae take up metals through adsorption over the cell surface very quickly in a few seconds or minutes; this process is called physical adsorption. Then, these ions are transported slowly into the cytoplasm in a process called chemisorptions (Dwivedi 2012). Microalgae are efficient absorbers of heavy metals (Chaisuksant 2003; Akhtar et al. 2004). Therefore, this property of heavy metal absorption has application in wastewater and different mixtures of water samples (sewage, sea and well) treatment. Chlorella pyrenoidosa had a high protein content when grown on sewage sludge and the aqueous extract retained a comparatively low level of various heavy metals, i.e. Cu2+, Mn2+, Fe2+ and Zn2+ (Wong and Tam 1984). Cyanobacteria and algae were evaluated as biosorbents for removing nickel (Ni) at concentration of <20 ppm from a chemically complex wastewater effluent. Cyanobacteria and algae were chosen because they were easy to grow and could withstand processing into biosorbent materials (Corder and Reeves 1994).
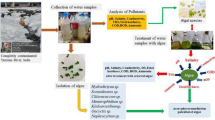
This work aimed to study the ability of two green microalgae Chlorella vulgaris and Chlorella salina for removal of pollutants from sewage and different mixtures of water samples (sewage, sea and well water) for eventual goal for recycling water.
2 Materials and methods
2.1 Water sampling
Waste water samples were collected from Al-Salhya sewage station, Qena, Egypt. Sea water samples were collected from the Red Sea at Hurghada region. Well water samples were collected from the wells of South Valley University.
2.2 Biological methods
2.2.1 Isolation and culturing of algae
Chlorella vulgaris (unicellular, green fresh microalga) was isolated from sewage water of El-Salhya sewage station, Qena, Egypt and Chlorella salina (unicellular, green marine microalga) was isolated from lake Marriott, Alexandria, Egypt. For cultivation and isolation of C. vulgaris, solid and liquid Beijernick’s nutritive medium (Stein 1966) were used. C. salina was cultivated and isolated by using solid and liquid F/2 nutritive medium (Guillard and Ryther 1962). The growth of different algal species was examined by means of a compound microscope (Leica DM500) and identified according to Prescott (1982).
2.2.2 Algal growth and culture conditions
Axenic algal samples of C. vulgaris and C. salina were grown separately in 500 mL culture flasks containing nutritive medium. The cultures were incubated at 25 ± 1 °C and illuminated with cool white fluorescent lamps at an intensity 100 µmol photons m−2 s−1. The algal cultures were supplied with dry air (Lorenzen 1964) to provide CO2 necessary for photosynthesis, to prevent the settling of the cells at the bottom of the containers and to maintain the algae in suspension without mechanical stress (Persoone et al. 1980). The algae were kept under optimum conditions and subcultured routinely.
2.3 Treatment of water samples by algae
2.3.1 Experimental layout
The cells of C. vulgaris and C. salina were inoculated in glass tanks with 10 liter capacity containing different mixtures of water samples; sewage (100 % sewage waste water), mixture 1 (70 % sewage:30 % sea water), mixture 2 (70 % sewage:30 % well water) and mixture 3 (70 % sewage:15 % sea:15 % well water). Preliminary experiments were carried out to determine the optimum concentration of water samples mixtures. Water samples were inoculated with algae by adding algae cell cultures to give a concentration of 5 %. Treatment of water samples with algae was carried out under illumination and aeration for 10 days. The treated water samples were centrifuged to precipitate the algal mats. The supernatant was used to determine the physico-chemical characteristics of water samples.
2.3.2 Physico-chemical characteristics of water samples
The initial physico-chemical analysis of water sample was made before inoculation of algae and at final stage, the total content in each flask was filtered to remove algae and then used for the analysis of various parameters (pH value, total dissolved salts (TDS), biological oxygen demand (BOD), chemical oxygen demand (COD), nitrate, ammonia and phosphate) (APHA 2005).
Calcium, magnesium, sodium, potassium and heavy metals were determined by using atomic absorption (Spectrometer: MESLO). Total coli-forms bacteria were determined by multiple tube fermentation method “Most Portable Number of bacteria (MPN method)” according to the standard methods for the examination of water and wastewater (APHA 1971).
2.4 Statistical analysis
The data were analyzed statistically by one way analysis of variance test using statistical computer program SPSS (version20).
3 Results and discussion
3.1 pH value
A marked reduction in pH values was noticed in water samples after the treatment with algae, where the algal treatment retained the pH value around neutral value (Fig. 1). C. vulgaris reduced the pH values in sewage, mixtures1,2&3 from 7.70, 7.87, 7.68 and 7.77 to 6.90, 7.01, 7.04 and 6.82, respectively, while C. salina reduced the pH values from 7.7 to 7.1 in sewage, 7.87 to 7.2 in mixture1, 7.68 to 7.36 in mixture2 and 7.77 to 7.37 in mixture3 water samples. Similar observation recorded by Aarti et al. (2008).
3.2 Total dissolved salts (TDS)
The TDS of water samples were significantly decreased with algal treatment. The removal percentages of TDS were 68.42, 38.52, 43.37 and 33.47 % in sewage, mixtures1,2&3, respectively, by C. vulgaris and 37.59, 34.40, 42.17 and 24.86 % in sewage, mixtures1,2&3, respectively, by C. salina compared to untreated water samples (Fig. 2). This reduction in TDS may be due to the utilization of various nutrients by algae (Rao et al. 2011; Ahmad et al. 2013). The unique mechanism of bioabsorption/adsorption of different types of dissolved solids in wastewater is responsible to reduce TDS to lowest level (Nanda et al. 2010; Azarpira et al. 2014).
3.3 Biological oxygen demand (BOD) and chemical oxygen demand (COD)
The algal treatment of water samples induced a progressive reduction in both BOD and COD values. C. salina was found to be more efficient than C. vulgaris in BOD and COD reduction. Where BOD removal efficiency was ranged from 83.17 to 90.63 % and from 87.01 to 90.75 % by C. vulgaris and C. salina, respectively, (Fig. 3). According to Ganapathy et al. (2011) the value of BOD indicates level of toxicity of wastewater and they further reported the reduction in BOD of distillery effluent by 53 % using Nostoc species. According to Abdel-Raouf et al. (2012) BOD indicates the respiratory demand of bacteria and algae metabolizing the organic matter present in wastewater and excess BOD usually depletes the dissolved oxygen. Sengar et al. (2011), Kshirsagar (2013), Azarpira et al. (2014) have reported very high reduction in BOD using different algal species and confirmed that microalgae are the best candidates for purification of wastewater and improvement in its physico-chemical parameters.
In addition, the reduction in COD values was ranged from 83.56 to 90.83 % by the treatment with C. vulgaris and ranged from 87.32 to 90.97 % in case of C. salina compared to untreated water samples (Fig. 3). Similar observation recorded by Sharma and Khan (2013), Elumalai et al. (2013), Azarpira et al. (2014). The chemical oxidations of carbon present in organic pollutants releasing carbon dioxide is responsible for reduction of COD value, similarly faster biodegradation and bioconversion of organic matter due to algae might be the additional reason Abdel-Raouf et al. (2012).
3.4 Removal of some nutrients from water samples
3.4.1 Nitrate
It is apparent from the data in Fig. 4 that algal treatment of water samples was accompanied by remarkable reductions in the nitrate content in water samples. Nitrate removal by C. vulgaris was 70.00, 60.00, 93.43 and 89.84 % in sewage and mixtures1,2&3, respectively, corresponding to 40.00, 53.33, 93.38 and 86.53 % by C. salina respectively, compared to untreated water samples. Similar observation recorded by Kshirsagar (2014).
3.4.2 Ammonia
The obtained results elucidated that remarkable decrease in ammonia concentration was observed in water samples after algal treatment. The removal efficiency ranged between 59.99 and 75.00 % by C. vulgaris treatment and 62.00 and 73.03 % by C. salina treatment compared to untreated water samples (Fig. 4).
Rao et al. (2011) reported that, C. vulgaris was able to reduce all forms of nitrogen substantially, and ammonia and nitrate levels, in particular. They revealed also that the phosphate removal efficiency of C. vulgaris was nearly 100 % in the wastewater.
3.4.3 Phosphate
From the data in Fig. 4, it is clear that the application of algal treatment reduced the phosphate contents in water samples after 10 days of treatment. The removal efficiency of C. vulgaris was ranged between 87.14 and 90.08 %. On the other hand, the removal efficiency of C. salina was ranged between 76.97 and 82.48 %. These results were in agreement with results obtained by Kshirsagar (2014).
The removal of P was mainly according to biological uptake mediated by the metabolic pathways of aerobic organisms (Maris et al. 1983). Phosphate removal by algae during phycoremediation is due to the utilization of phosphorus for growth. The phosphorus, which is used in the algal cells mainly for production of phospholipids, adenosine triphosphates (ATP) and nucleic acids, gets assimilated as inorganic orthophosphate and the uptake process is active, i.e. it requires energy (Becker 1994). Microalgae are able to assimilate phosphorus in excess, which is stored in the cells as polyphosphate granules, and magnesium and potassium are co-transported along with phosphate (Bitton 1990).
3.5 Removal of some minerals from water samples
3.5.1 Calcium
Application of C. vulgaris and C. salina treatment of water samples induced remarkable decrease in calcium contents (Fig. 5). C. vulgaris decreased the calcium contents from 105.23 to 2 mg/L in sewage, from 199.66 to 50 mg/L in mixture1, from 137.86 to 6 mg/L in mixture2 and from 168.76 to 26 mg/L in mixture3. C. salina decreased the calcium contents from 105.23 to 4 mg/L in sewage, from 199.66 to 70 mg/L in mixture1, from 137.86 to 30 mg/L in mixture2 and from 168.76 to 46 mg/L in mixture3 compared to untreated water samples.
3.5.2 Magnesium
The treatment with C. vulgaris induced a remarkable reduction in the magnesium contents amounted to 84.23, 58.46, 76.59 and 67.57 % in sewage and mixtures1,2&3, respectively. While C. salina reduced the magnesium contents by 58.75, 50.67, 55.40 and 40.20 %, respectively (Fig. 5).
3.5.3 Sodium
The data in Fig. 2 indicated that sodium content decreased markedly in water samples as a result of algal treatments. The percentage of reduction fluctuated between 3.38 and 54.39 % by C. vulgaris and 5.91 and 75.44 % by C. salina compared to untreated water samples.
3.5.4 Potassium
The treatment of water samples with algae was accompanied by remarkable decreases in the potassium content. The treatment with C. vulgaris reduced the potassium contents by 86.67, 50.00, 85.98 and 39.77 % in sewage and mixtures 1, 2& 3, respectively. On the other hand, the treatment with C. salina reduced potassium contents by 77.50, 48.05, 84.28 and 27.25 %, respectively (Fig. 5). This observation is in agreement with Azab (2002) who reported that the application of algae for wastewater treatment exhibited variable percentages of reduction in minerals. In this context Rao et al. (2011) reported a drastic reduction in magnesium levels and moderate decrease in potassium levels by using C. vulgaris in phycoremediation, although they observed that reduction in sodium levels and a significant reduction in calcium.
3.6 Removal of heavy metals from water samples
The ability of C. vulgaris and C. salina to remove toxic heavy metals from water samples after 10 days incubation was presented in Table 1. The data elucidated that the removal efficiency differed according to the types of heavy metal and microorganism used. It was obvious that the chlorophyte algae used here exhibited high ability to remove heavy metals from water samples.
3.6.1 Zinc
The water samples that treated with algae exhibited remarkable reductions in zinc concentrations. The highest removal efficiency of C. vulgaris (64.96 %) was recorded in mixture2. On the other hand, the removal efficiency of C. salina was ranged between 15.16 and 28.52 %.
3.6.2 Copper
The results showed that C. vulgaris was able to remove copper from sewage and mixtures1,2&3 by 98.64, 100,100 and 55.95 %, respectively, while the removal efficiency was 90.74, 100, 89.95 and 90.31 % by C. salina respectively.
3.6.3 Manganese
It is apparent from the results in Table 1 that, both C. vulgaris and C. salina showed a great ability to absorb manganese from the water samples. C. vulgaris absorbed all manganese contents in the water samples. In addition, the absorption of manganese by C. salina ranged between 89.94 and 93.71 %.
3.6.4 Nikel
From the data in Table 1, it can be noticed that there was a remarkable reduction in nickel contents after the application of biotreatment. The highest removal efficiency of C. vulgaris (100 %) was obtained in mixtures 1&2 and the absorption reached to 90.95 and 51.11 % in sewage and mixture3, respectively. while C. salina absorb 100 % of nickel contents in mixtures1&3 and 81.90 % in sewage. The lowest absorption value (13.61 %) of C. salina was recorded in mixture2 compared with control (initial concentration).
3.6.5 Cobalt
The removal of cobalt from the water samples ranged between 32.29 and 59.28 % by C. vulgaris and ranged between 47.92 and 100 % by C. salina compared to untreated water samples.
3.6.6 Iron
The data in Table 1 revealed that high removal efficiency of iron was recorded by both algal species. C. vulgaris and C. salina absorb all iron contents in the water samples, except C. salina absorbs the iron contents by 97.24 % in mixture3 after 10 days of incubation.
3.6.7 Chromium
The results indicated that C. vulgaris and C. salina show variable efficiencies in chromium removal. C. vulgaris absorb 21.74, 66.46 and 41.85 % of chromium content from mixtures1,2&3, respectively. While C. salina absorbs 5.13, 19.72 and 30.59 % of chromium contents from mixtures1,2&3, respectively. Chromium was not detected in the sewage water.
3.6.8 Cadmium and lead: cadmium and lead were not detected in the water samples
The results of our investigation are in agreement with Hamdy (2000), El-Sheekh et al. (2005) who found that the different metals uptake depended upon the type of biosorbent, which has different accumulation affinities towards the tested elements. In addition, Kaplan et al. (1986) found that the efficiency of absorption of metals depends on the nature and charge of the cell wall polysaccharides of C. stigmaphora which contain high amount of uronic acids and consequently, a high copper-complexing capacity compared to the less charged C. salina polysaccharides. Similar finding was concluded by Karamushka et al. (1994) who mentioned that the rate and magnitude of accumulation of heavy metals like gold substantially depended on the physiological state of the cells of Spirulina platensis. Moreover, the removal of heavy metal attributed to mechanisms other than the tendency of cells for bioaccumulation (Pena-Castro et al. 2004).
In our study, it was observed that green algal cells cultivated in water samples with high metal contents also accumulated higher metal contents. This observation has been emphasized by Priyadarshani et al. (2011) who mentioned that algal cells cultivated in the media with very high metal contents also accumulated higher metal contents. However, in few cases, the metal uptake was independent on the external metal concentration.
3.7 Removal of coli-form bacteria
The number to total Coli-form bacteria was >2400 cell/100 mL water samples before biotreatment in all water samples mixtures. The treatment with C. vulgaris and C. salina exerted considerable decrease in the total Coli-form bacteria. The maximum decrease was recorded in mixture2 by C. vulgaris treatment (4 cell/100 mL water samples). On the other hand, the highest effect of treatment with C. salina on total Coli-form bacteria was observed in mixture1 and mixture3 where the number of total Coli-form bacteria decreased to <2 cell/100 mL water samples. This ensured that the main removal mechanisms of pathogen and coli-forms are ruled by algal activity (Curtis 1994). Moawad (1968) observed that the environmental factors which were favorable for algal growth were unfavorable for the survival of coli-forms. A similar observation on the percent reduction of coli-forms and Salmonella was also made by Colak and Kaya (1988). Mezrioui et al. (1994) found that green algae reduce Vibrio cholerae abundance more than E. coli (Fecal contamination bacteria) and that the die off of E. coli appears to be more reduced in presence of cyanobacteria.
4 Conclusion
From the results, it is concluded that recycling and reusing of different mixtures of water samples is possible by Phycoremediation using different algal species like C. vulgaris and C. salina. The present investigation showed that both the algal species had very good potential to reduce the toxic level of all physico-chemical parameters. These experiments confirm that C. vulgaris and C. salina may be considered efficient nutrient removers.
References
Aarti N, Sumathi P, Subrahmanian V (2008) Phycoremediation to improve algal water quality. Ind Hydrobiol 11:173–184
Abdel-Raouf N, Al-Homaidan AA, Ibraheem IBM (2012) Microalgae and wastewater treatment. Saudi J Biol Sci 19:257–275
Ahmad F, Khan AU, Yasar A (2013) Comparative phycoremediation of sewage water by various species of algae. Proc Pak Acad Sci 50:131–139
Akhtar N, Iqbal J, Iqbal M (2004) Removal and recovery of nickel (II) from aqueous solution by loofa sponge-immobilized biomass of Chlorella sorokiniana: characterization studies. J Hazard Mater 108:85–94
American Public Health Association (APHA) (1971) Standard methods for examination of water and wastewater, 13th ed., American Public Health Association From G.J. tortora, B. R. Funke, and C. L. Case 1982 Microbiology Menlo Park, Calif: Benjamin/Cummings. p 724
American Public Health Association (APHA) (2005) Standard methods for examination of water and waste water, 21st edn. American public health association, Washington, D.C.
Azab YA (2002) The use of immobilized algae and algal biofilters for the treatment of industrial heavy metals pollution proceedings, plant and industrial pollution. The Egyptian Bot. Soc, Cairo, pp 135–148
Azab MS (2008) Waste-waste treatment technology and environmental management using sawdust bio-mixture. J Taibah Univ Sci 1:12–23
Azarpira H, Behdarvand P, Dhumal K, Pondhe G (2014) Comparative studies on phycoremediation of sewage water by using blue green algae. Int J Biosci 4:58–64
Becker EW (1994) Microalgae-biotechnology and microbiology. Cambridge University Press, Cambridge
Bitton G (1990) Wastewater microbiology. Wiley-Liss, New York
Bolan NS, Wong L, Adriano DC (2004) Nutrient removal from farm effluents. Bioresour Technol 94:251–260
Chaisuksant Y (2003) Biosorption of cadmium (II) and copper (II) by pretreated biomass of marine alga Gracilaria fisheri. Environ Technol 24:1501–1508
Choi H-J, Lee S-M (2012) Effects of microalgae on the removal of nutrients from wastewater: various concentrations of Chlorella vulgaris. Environ Eng Res 17(1):3–8
Colak O, Kaya Z (1988) A study on the possibilities of biological wastewater treatment using algae. Doga Biyoloji Serisi 12:18–29
Corder SL, Reeves M (1994) Biosorption of nickel in complex aqueous waste streams by cyanobacteria. Appl Biochem Biotechnol Part A Enzyme Eng Biotechnol 45:847–859
Curtis TP (1994) The effect of sunlight on mechanisms for the die-off of faecal coliform bacteria in waste stabilization ponds. Ph.D. Thesis. School of Civil Engineering. University of Leeds, UK. URL
De la Nou¨e J, De Pauw N (1988) The potential of microalgal biotechnology. A review of production and uses of microalgae. Biotechnol Adv 6:725–770
Dwivedi S (2012) Bioremediation of heavy metal by algae: current and future perspective. J Adv Lab Res Biol 3:195–199
El-Sheekh MM, El-Shouny WA, Osman MEH, El-Gammal EWE (2005) Growth and heavy metals removal efficiency of Nostoc muscorum and Anabaena subcylindrica in sewage and industrial wastewater effluents. Environ Toxicol Pharmacol 19:357–365
Elumalai S, Saravanan GK, Ramganesh S, Sakhtival R, Prakasam V (2013) Phycoremediation of textile dye industrial effluent from tirupur district, Tamil Nadu, India. Int J Sci Innov Discov 3:31–37
Ganapathy SG, Baskaran R, Mohan PM (2011) Microbial diversity and bioremediation of distilleries effluent. J Res Biol 3:153–162
Guillard RRL, Ryther JH (1962) Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt and Detonulaconfervaceae (Cleve) Gran. Can J Microbiol 8:229–239
Hamdy AA (2000) Biosorption of heavy metals by marine algae. Curr Microbiol 41(4):232–238
Kaplan D, Richmond AE, Dubinsky Z, Aaronson S (1986) Algal nutrition. In: Richmond A (ed) Handbook of microalgal mass culture. CRC Press, Boca Raton
Karamushka VI, Gruzina TG, Ul’berg ZR (1994) Accumulation of gold (III) by the cells of cyanobacterium Spirulina platensis. Microbiology 64:157–160
Kshirsagar AD (2013) Bioremediation of wastewater by using microalgae: an experimental study. Int. J. Life Sci Bt Pharm 2:140–146
Kshirsagar AD (2014) Remediation of domestic wastewater by using algal and fungal mixed culture: an experimental study. Int Interdiscip Res J. 4:166–173
Lorenzen H (1964) Synchronization of Chlorella with light dark changes and periodical dilution to a standard cell number. In: Zeiten E (Ed) Inersci. Publ., New York, pp 571
Maris GVR, Loewenthal RE, Siebritz IP (1983) Observations supporting phosphorus removal by biological excess uptake. A review. Water Sci Technol 15:15–41
Mezrioui N, Oudra B, Oufdous K, Hassani L, Loudiki M, Darley J (1994) Effect of microalgae growing on wastewater batch culture on Escherichia coli and Vibrio cholreae survival. Water Sci Technol 30:295–302
Moawad SK (1968) Inhibition of coliform bacteria by algal population in microoxidation ponds. Environ Health 10:106–112
Munoz R, Guieyssea B (2006) Algal–bacterial processes for the treatment of hazardous contaminants. A review. Water Res 40:2799–2815
Nanda S, Sarangi PK, Abraham J (2010) Cynobacterial remediation of industrial effluents II. Paper mill effluents. N Y Sci J. 3:37–41
Olguín EJ (2003) Phycoremediation: key issues for cost-effective nutrient removal processes. Biotechnol Adv 22:81–91
Oswald WJ (2012) My sixty years in applied algology. J Appl Phycol 15(2003):99–106
Pena-Castro JM, Martinez-Jeronimo F, Esparza-Garcia F, Canizares-Villanueva RO (2004) Heavy metals removal by the microalga Scenedesmus incrassatulus in continuous cultures. Bioresour Technol 94:219–222
Persoone G, Morales J, Verlet H, De Pauw N (1980) Air-lift pumps and the effect of mixing on algal growth. In: Shelef G, Soeder CJ (eds) Algae biomass production and use. Elsevier, North-Holand Academic Press, Amsterdam, pp 505–522
Prescott GW (1982) Algae of the western lakes area. W.M.C. Brown Publ., Dubuque, pp 1–977
Priyadarshani I, Sahu D, Rath B (2011) Microalgal bioremediation: current practices and perspectives. J Biochem Tech 3:299–304
Rao HP, Ranjith Kumar R, Raghavan BG, Subramanian VV, Sivasubramanian V (2011) Application of phycoremediation technology in the treatment of wastewater from a leather-processing chemical manufacturing facility. Water SA 37:7–14
Sengar RMS, Singh KK, Singh S (2011) Application of phycoremediation technology in the treatment of sewage water to reduce pollution load. Ind J Sci Res 2:33–39
Sharma GK, Khan SA (2013) Bioremediation of sewage wastewater using selective algae for manure production. Int J Environ Eng Manag. 4:573–580
Stein JR (1966) Growth and mating of Gonium pectoral (Volvocales) in defined media. J Phycol 2:23–28
Wong MH, Tam FY (1984) Sewage sludge for cultivating freshwater algae and the fate of heavy metals at higher trophic organisms. Arch Hydrobiol 100:287–318
Zhang E, Wang B, Wang Q, Zhang S, Zhao B (2008) Ammonia–nitrogen and orthophosphate removal by immobilized Scenedesmus sp. isolated from municipal wastewater for potential use in tertiary treatment. Bioresour Technol 99:3787–3793
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El-Sheekh, M.M., Farghl, A.A., Galal, H.R. et al. Bioremediation of different types of polluted water using microalgae. Rend. Fis. Acc. Lincei 27, 401–410 (2016). https://doi.org/10.1007/s12210-015-0495-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12210-015-0495-1