Abstract
Fruit processing industries generate millions of tons of organic waste annually, often improperly disposed of at open landfills. Based on the circular economy and waste management concepts, reusing these bio-waste materials is one of the future sustainable demands. Furthermore, recent investigations have shown that this material type can be reused as high-quality sorbents, with certain modifications that should be applied. Considering this, we have investigated the possible application of lignocellulosic waste-peach stones (Prunus Persica L.), immobilized in sodium alginate, as heavy metals sorbent. The immobilized particles (IPS) were utilized to remove metals from synthetic water solutions. Among all metals (Pb, Cu, Cd, and Zn), IPS has shown superior performance in Pb removal, governing further investigations. Dried IPS spheres were characterized by FTIR, SEM/EDX, and TG techniques. The batch reaction system investigated the effects of the contact time, initial Pb concentration, and mass-to-volume ratio. Optimized operational parameters were used in kinetic and isotherm studies. Obtained data were modeled using a nonlinear form of pseudo-first, pseudo-second, Elovich, Freundlich, and Langmuir equations. The results showed pseudo-second-order kinetics with Freundlich isotherm fitting Pb removal, indicating a heterogeneous IPS surface with the multilayer adsorption and adsorbed molecule interaction. As obtained from Langmuir isotherm, IPS particles have removed Pb by saturation capacity of 80.40 mg Pb/g. These preliminary results indicate that IPS can be applied to purify waters contaminated with lead metal.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
The growth of industrial activity and uncontrolled or improper release of pollutants into the environment induced the need for new methods and materials that might be used to solve environmental pollution issues. Many techniques have been developed for different pollutant removal. Still, most of them are often ineffective, economically or technically demanded, especially if the pollutant concentration (e.g., metals) falls below 100 mg/L (Abdolali et al., 2014). Sorption techniques using low-cost, abundant lignocellulosic waste (LCW) material might be applied for that purpose. Although LCWs pose some disadvantages concerning their direct applications as purification agents (Abdi & Kazemi, 2015; Abdolali et al., 2014), their properties might be improved by different modifications and immobilization in various polymer matrices (Chatterjee & Schiewer, 2014).
This paper investigates the possibility of lead (Pb) removal by immobilized LCW peach stone particles. Lead was chosen as a high-toxicity heavy metal, resistant to chemical or biological degradation, presenting a hazard to living and non-living environments (Check & Marteel-Parrish, 2013). On the other hand, recent investigations have shown that LCW generated by the fruit processing industry represents a stable matrix that might be efficiently used in wastewater purification (Lopičić et al., 2017). Therefore, to improve the sorption properties of the raw material as well as the overall separation process, we have immobilized mechanically treated peach stones (Prunus Persica L.) in the sodium alginate and applied it as an efficient sorbent in lead removal.
2 Materials and Methods
All the chemicals used in this study were of high purity. Peach stones (PS) were obtained as waste from a local juice factory. After washing, grinding, and sieving (to a diameter less than 0.1 mm), mechanically treated PS particles were immobilized in Na-alginate according to the method described by Yuan and Viraraghavan (2001). Formed alginate beads (IPS) were further used for the characterization and sorption experiments.
Dried IPS spheres were characterized by FTIR (Thermo Fisher Scientific Nicolet IS-50 spectrophotometer, ATR mode), SEM/EDX (model JEOL JSM-6610LV), and TG (Netzsch STA 409 EP) technique.
Sorption experiments were done in triplicate in a batch reactor with mixing (200 rpm) at constant temperature (25 °C) with optimization of operational parameters. Obtained values were further used in kinetic and isothermal studies. The analytical Pb(II) measurements were done using AAS (Perking Elmer AAS Analyst 300). The percent of Pb(II) removal, R (%), as well as the amount of Pb(II), sorbed per unit mass of IPS, q (mg/g), was calculated as described in Lopičić et al. (2017).
3 Results
3.1 IPS Sorbent Characterization
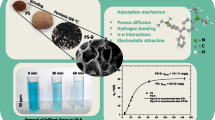
Figure 1a, b shows SEM images of the IPS sphere. IPS represents a regular sphere of 5 mm average diameter, with PS evenly entrapped within the matrix, regularly exposed to a highly developed surface. Opposite to native material (Lopičić et al., 2019), the presence of macro pores is not evident. EDX analyses showed typical LCW composition (Lopičić et al., 2019) with higher Ca picks than in native material, while after Pb(II) sorption, reduced peaks of Ca, as well as a new peak of Pb(II), are observed in the EDX spectrum (image not shown). Figure 1c presents a TG/DTA analysis of the IPS sample. The first negative peak at 86 °C on the DTA curve is assigned to the loss of free water and water linked through hydrogen bonds. The second mass loss corresponds to the thermal decomposition of IPS composite with the fracture of glycosidic bonds and release of H2O, with a degradation peak moved from lower (pure alginate) to a higher temperature (333 °C). This result suggests the composite sorbent poses high thermal stability than the alginate itself. The final step of mass loss with a peak at 443 °C may be attributed to the carbonate formation, partial lignin, and cellulose degradation.
All bands’ characteristics for raw PS have been seen on the FTIR spectrum of IPS (not shown), but due to the presence of the alginate with reduced intensity. The identified chemically active IPS groups were characterized as polysaccharides, cellulose, hemicellulose, and lignin, all present in composite material.
3.2 Batch Sorption Results
The immobilized particles of Prunus Persica L. waste biomass (IPS) were utilized to remove heavy metals from the synthetic water solutions. Among all metals (Pb, Cu, Cd, and Zn), IPS has shown superior performance in Pb(II) removal, so further sorption experiments were performed only with this pollutant. According to the literature review, the initial pH was set to 5.00 and was not adjusted during the sorption process. A significant decrease in pH (up to 3.98) is observed by the end of the process, owing to the presence of carboxylic groups as the main cation exchangers. The effect of the sorbent dose on the amount of Pb(II) sorbed by IPS and its corresponding removal percentage was investigated in the range 0.25–20 g/L. The results have shown an increase in percentage in Pb(II) removal (from 27.4 up to 90.8%) with an increase in IPS dose from 0.25 to 2.0 g/L. Further, an increase in sorbent dose increased R but significantly decreased Pb(II) sorbed. The influence of contact time revealed slow kinetics (equilibrium up to 24 h), which was the best fit by the pseudo-second-order model (Fig. 2a). Isotherm data showed the best correlation with the Freundlich model (Fig. 2b), indicating a multilayer sorption mechanism onto complex IPS surfaces.
4 Discussion
IPS characterization revealed a spherical sorbent shape with a high surface area and plenty of functional groups suitable for lead removal. This sorbent type posed higher thermal stability than the alginate itself and higher ash content than the raw PS, owing to crosslinked Ca in its matrix. Lead removal was superior to other sorbates investigated (thanks to ion exchange Ca–Pb). The typical overall adsorption efficiency (R(%)) was between 27% (for 0.25 mg/L of 1 M Pb solution) and 99% for the final value of 20 g of IPS/L. This removal efficiency did not appreciably increase with an increase in the mass-to-volume ratio higher than 2 g/L, and this operating parameter was selected for kinetic and isothermal investigations. Investigating the influence of contact time showed slow kinetics best fitted by a pseudo-second-order equation. The results of isotherm investigations have demonstrated that the Freundlich isotherm fits better with the removal of Pb, indicating a heterogeneous IPS surface with multilayer adsorption and interaction between adsorbed molecules. Calcium alginate-immobilized PS particles have removed Pb by a saturation capacity of 80.40 mg Pb/g, as obtained from Langmuir isotherm. This value is much higher than the one for raw PS, which was calculated as 28.64 mg Pb/g under the same operational conditions. During the sorption process, calcium, added as a crosslinking agent, was exchanged with lead due to the affinity of the weak acid cation exchanger, the carboxyl group, which has a higher selectivity toward the lead.
5 Conclusions
Although LCW often has limited sorption capacity compared to current commercial sorbents, a good sorbent selection with a proper modification can considerably improve its sorption properties. Furthermore, based on the circular economy and waste management concepts, the reuse of LCW materials is one of the future demands in achieving economic and environmental sustainability. Preliminary results presented in this paper indicate that IPS can be applied to purify waters contaminated with lead metal. Thus, further investigations into the IPS application should be conducted.
References
Abdi, O., & Kazemi, M. A. (2015). Review study of biosorption of heavy metals and comparison between different biosorbents. Journal of Materials and Environmental Science, 6(5), 1386–1399.
Abdolali, A., Guo, W. S., Ngo, H. H., Chen, S. S., Nguyen, N. C., & Tung, K. L. (2014). Typical lignocellulosic wastes and by-products for biosorption process in water and wastewater treatment: A critical review. Bioresource Technology, 160, 57–66.
Chatterjee, A., & Schiewer, S. (2014). Multi-resistance kinetic models for biosorption of Cd by raw and immobilized citrus peels in batch and packed-bed columns. Chemical Engineering Journal, 244, 105–116.
Check, L., & Marteel-Parrish, A. (2013). The fate and behavior of persistent, bioaccumulative, and toxic (PBT) chemicals: Examining lead (Pb) as a PBT metal. Reviews on Environmental Health, 28(2–3), 85–96.
Lopičić, Z., Stojanović, M., Kaluđerović Radoičić, T., Milojković, J., Petrović, M., Mihajlović, M., & Kijevčanin, M. (2017). Optimization of the process of Cu(II) sorption by mechanically treated Prunus persica L.—contribution to sustainability in food processing industry. Journal of Cleaner Production, 156, 95–105.
Lopičić, Z., Stojanović, M., Marković, S., Milojković, J., Mihajlović, M., Kaluđerović Radoičić, T., & Kijevčanin, M. (2019). Effects of different mechanical treatments on structural changes of lignocellulosic waste biomass and subsequent Cu(II) removal kinetics. Arabian Journal of Chemistry, 12(8), 4091–4103.
Yan, G., & Viraraghavan, T. (2001). Heavy metal removal in a biosorption column by immobilized M. rouxii biomass. Bioresource Technology, 78, 243–249.
Acknowledgements
These results are part of the investigations supported by the Ministry of Education and Science of the Republic of Serbia (Grant number 451-03-9/2021-14/200023).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Lopičić, Z. et al. (2023). Lead Removal from Water Solutions Using Alginate-Immobilized Peach Stone Particles. In: Çiner, A., et al. Recent Research on Environmental Earth Sciences, Geomorphology, Soil Science, Paleoclimate, and Karst. MedGU 2021. Advances in Science, Technology & Innovation. Springer, Cham. https://doi.org/10.1007/978-3-031-42917-0_8
Download citation
DOI: https://doi.org/10.1007/978-3-031-42917-0_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-42916-3
Online ISBN: 978-3-031-42917-0
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)