Abstract
The purpose of this work is to analyze the potential of two lignocellulosic wastes, namely peach and apricots stones, to adsorb 2,4-dichlorophenoxyacetic acid (2,4-D) pesticide from an aqueous solution. Both equilibrium and kinetic adsorption tests were performed. The maximum adsorption capacity achieved was 41.5 mg g−1 at 50 °C. The fitting of the experimental results to several empirical equilibrium models was studied, being Langmuir model the one that best fitted the adsorption data for both adsorbents. The thermodynamic analyses confirmed that 2,4-D adsorption on both lignocellulosic wastes was endothermic, spontaneous, and of a physisorption nature. For the kinetic study, the effects of multiple factors were examined including initial pH, adsorption temperature, 2,4-D initial concentration, and adsorbent dose. The results support the feasible use of these wastes as adsorbents in water treatment applications.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Extensive amounts of artificial organic contaminants, such as pesticides (Khan et al. 2021), dyes (Abbas 2020; Noreen et al. 2020), heavy metals, and pharmaceutical products (Bonilla-Petriciolet et al. 2017), are emitted daily into many kinds of wastewater. These chemicals can penetrate natural systems and infiltrate in the aquatic ecosystem and ultimately enter the food chain posing a potential risk to animal and human health. The majority of these chemicals are anthropogenic from different sources (Osagie et al. 2021), including agriculture (Salman et al. 2011) and/or industrial activities (Goscianska and Olejnik 2019). Pesticides, such as carbofuran, 2,4-dichlorophenoxyacetic acid and glyphosate, among many others, have been extensively documented in several studies to possess hazardous and toxic proprieties that can affect humans (Chen et al. 2021; Garabrant and Philbert 2016), animals (Goscianska and Olejnik 2019) and aquatic environments (Goscianska and Olejnik 2019; Saleh et al. 2020). Numerous methods have been studied to eliminate pesticide pollutants from water, including anaerobic sludge, ion exchange, ultrafiltration, reverse osmosis, or adsorption, and several others (Saleh et al. 2020). Adsorption is used by industries to remove hazardous pollutants, given its global recognition as a cost-effective, operationally efficient, and easily designed technique for pollutant elimination (Abbas 2020). In this study, the primary focus lies in the utilization of two biomass waste materials, namely peach, and apricot stones, for the adsorption of the 2,4-dichlorophenoxyacetic acid (2,4-D), a widely recognized and commonly used pesticide. This compound can show an anionic character (Bedia et al. 2018), displaying high solubility and poor biodegradability (Cabrera-Barjas et al. 2020; Hassan et al. 2020; Kul et al. 2019). The selection of these adsorbents is driven by the low cost and the abundant availability (in Tunisia, where more than 120,000 tons of peach stones are produced annually) and applied for providing a valorization alternative for these agricultural wastes (Bedia et al. 2018). The objective of this study is to compare the behavior of peach and apricot stones as adsorbents of 2,4-D pesticide. The adsorption isotherms have been determined at four different temperatures (25, 30, 40, and 50 °C) and obtained the corresponding thermodynamic parameters. The influence of pH, adsorbent doses, initial 2,4-D concentration, and temperature on the adsorption kinetics was analyzed. In the literature, there are numerous studies that concentrate on the use of bioadsorbents for the removal of pesticides. Cabrera-Barjas et al. (2020) investigated the removal of dimethoate pesticide using bioadsorbents from Aspergillus niger and Fusarium culmorum. Their findings revealed that the percentage of removal for the pesticide can achieve 100% at the optimal pH of 4. Moreover, Hassan et al. (2020) noticed that date pits proved to be highly effective for adsorbing an organophosphorus pesticide with total removal for concentrations of pesticide ranging from 2 to 10 ppm. Combined with the degradation, adsorption on starch was used by Chen et al. (2021) for the elimination of pesticides.
In recent studies, researchers have utilized peach stones, apricot stones, and their activated carbons as adsorbents to effectively remove pollutants from aqueous solutions. Kul et al. (2019) investigated the adsorption of a well-known basic dye (Acid Blue 25) by powdered peach seed. The findings indicate that the adsorption process was endothermic, spontaneous, and physisorption. In another research work, Maponga and Mahamadi (2019) studied the effect of different parameters on the synthesis of activated carbon from peach stones and its ability to remove aurocyanide. The results revealed that peach stones impregnated with ZnCl2 exhibited higher removal efficiency compared to those treated with H3PO4, with nearly 100% recovery for samples activated at 800 °C and treated for 2 hours. In the research, conducted by Abbas (2020), it was reported that the maximum adsorption capacity of methylene blue onto apricot stones was found to be 46.03 mg g−1 at 25 °C. They also determined that the adsorption process was spontaneous, endothermic, and involved a chemisorption mechanism. On a related note, Hashem et al. (2022) investigated the biosorption of acid blue 193 dye using an apricot seed shell. The highest quantity of dye adsorbed was recorded at 34.41 mg g−1 when the initial dye concentration was 40 mg L−1.
It is worth highlighting that this study marks the first instance of utilizing peach and apricot stones for the adsorption of the widely used pesticide 2,4-D, through equilibrium and kinetic adsorption tests performed at different conditions. This approach would allow the removal of this dangerous pesticide using a cheap and environmentally friendly methodology.
Materials and methods
Pre-treatment
The peach and apricot stones used in this work were collected from local farms in Tunisia. Following several water rinses, to eliminate surface impurities, the peach and apricot shell wastes were dehydrated at 105 °C for 24 h. Afterward, they were crushed in a mortar and sieved to obtain adsorbent particles with an approximate size of 250 µm.
Characterization
The major surface groups of the adsorbents were identified using Boehm titration. Experimentally, a convenient method involves placing 750 mg of the sample in a solution of Na2CO3 (0.05 mol L−1), NaHCO3 (0.05 mol L−1), and NaOH (0.05 mol L−1) accordingly. The solutions are, then, stirred at room temperature for 24 h and subsequently filtered. After obtaining the filtrates, a volume of 10 mL was taken. Then, 20 mL of HCl solution (0.05 mol L−1) was added to the NaOH and NaHCO3 filtrates. While 30 mL of HCl solution (0.05 mol L−1) was also added to the Na2CO3 filtrate. The neutralization points were determined by titrating NaOH solution (0.05 mol L−1). The surface charge was determined by the pH value at the point of zero charge (pHPZC). The pH was adjusted to the desired range of 2–12 by the introduction of solutions of NaOH or HC1 (0.1 M) and 150 mg of the adsorbent was introduced to each flask (50 mL). The suspensions are stirred at room temperature for 48 h, and the final pH (pHf) was determined. The figure of pHf as function of pHi (initial pH) was traced. The intersection with the first bisector represents the isoelectric point. Fourier transform infrared (FTIR) spectra were obtained using a Bruker Vertex 70 V spectrophotometer through transmission mode, in KBr pellets at wavenumbers ranging from 4000 to 400 cm−1 and employing a resolution of 2 cm−1 (Gómez-Avilés et al. 2022).
Adsorption tests
Table 1 summarizes some of the characteristics of 2,4-D herbicide, supplied by Sigma-Aldrich. According to the desired concentrations, specific quantities of the pesticide were dissolved in double-distilled water. Equilibrium adsorption tests were conducted in Erlenmeyer flasks of 250 mL maintaining a constant stirring speed for a duration of 3 h. The bioadsorbents (100 mg, with particle size under 250 µm) were introduced in 100 mL of an aqueous solution of 2,4-D pesticide at concentrations ranging from 4 to 80 mg L−1. Adsorption kinetic tests were conducted at 2,4-D concentrations ranging from 5 to 10 mg L−1. Various biomass weights were used ranging from 5 to 300 mg along with different stirring speeds of 300–600 rpm. The tests were carried out at various adsorption temperatures between 25 and 50 °C with an initial pH of 3. All the mentioned tests were conducted with 1 L in a double-walled reactor of 2 L volume controlled with a paddle stirrer. The 2,4-D concentration after adsorption was quantified with a UV–Vis spectrometer, specifically the Shimadzu UV-2600 model. It should be mentioned that the presented values are corresponding to the average points (each point is repeated three times) with an error always lower than 5%.
Results and discussion
Characterization of the adsorbents
The zero-charge point of the adsorbent (pHPZC) was determined using the pH derive method. Figure 1 represents the final pH of the solutions versus the initial pH values, indicating the point of intersection on diagonal of the pHPZC of adsorbent’s surface. The pHPCZ values obtained were 6.3 and 5.5 for apricot and peach stones, respectively. Both of these values indicate slightly acidic surfaces, with peach stones being more acidic. When the pH of the solution is below the pHPZC, the surface functional groups of the adsorbent become protonated due to an excess of H+ protons from the solution. As a result, the adsorbent carries a net positive charge, leading to electrostatic attraction with negatively charged molecules. On the other hand, if the pH of the solution is higher than the pHPZC of the adsorbent, the surface of the adsorbent will be negatively charged. This leads to electrostatic attraction for positively charged molecules and repulsion for negatively charged ones (Abbas 2020).
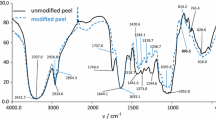
The surface chemistry of the lignocellulosic wastes is characterized by the diversity of heteroatoms (carboxyl, carbonyl, lactone, phenol, and other surface functional groups). Table 2 summarizes the amount of surface functional groups in peach and apricot kernels determined by the Boehm titration. The results confirm that both lignocellulosic wastes exhibit a predominantly acidic character, primarily due to the presence of acid groups such as carboxyl, carbonyl, and phenolic on their surfaces (Demiral et al. 2021; González-García 2018) .It is also confirmed the slightly higher surface acidity of peach than apricot stones (total acidities of 2.86 vs. 1.12 meq g−1, respectively). Indeed, it is noteworthy to mention the different surface group distributions of the two biomass adsorbents. Peach stones showed a much higher amount of carboxyl and carbonyl groups, while apricot stones display more lactone and phenol surface groups. Peach and apricot stones were equally analyzed using the FTIR technique. The FTIR spectra of both adsorbents are depicted in Fig. 2. The large bands at 3400 cm−1 indicate the presence of O–H groups, most likely attributed to the presence of chemisorbed water molecules. Additionally, the presence of asymmetric C–H groups is justified by the presence of the bands at a wavenumber of 2924 cm−1. Furthermore, the bands observed at around 1732 cm−1 and between 1600 and 1650 cm−1 confirm the presence of carboxylic and carbonylic groups, respectively. Phenolic groups corresponding to 1386 cm−1 is only observed in apricot stones. The presence of these groups confirms the results summarized in Table 2 obtained by Bohem titration. The bands at 1600 and 1460 cm−1 correspond to the presence of lignin while the bands at 1640, 1650 and 1030 cm−1 are associated to the existence of cellulose and hemicellulose.
Adsorption results
The pH of the solution significantly influences the uptake of 2,4-D pesticide by natural adsorbents (peach and apricot stones) (Salman et al. 2011). Figure 3 displays the equilibrium adsorption capacity of both peach and apricot stones at an adsorption temperature of 25 °C. The initial pHs values ranged from 2 to 12 with a constant concentration of 100 ppm. 2,4-D is a monobasic acid with moderate strength, as indicated by its pKa value of 2.64. Thus, at low pH values, the pesticide molecule exists in a neutral state, with no net charge. However, at pH values higher than 3, it is predominantly existing in a negative charged state (anionic state), as can be observed in the 2,4-D speciation diagram represented in Fig. 4. This agrees well with the evolution of the adsorbed amount with pH. The maximum amount is at pH equal to 3 and the suitable region of adsorption of 2,4-D in peach and apricot is between 3 and 6. At pH values lower than 3, the 2,4-D molecules are in their neutral state resulting in, no interaction with the adsorbent surfaces. However, at pH of 3, the 2,4-D molecules become negatively charged (in their anionic state), leading to an electrostatic attractive interaction between anions of 2,4-D and the positive charge of the surface of the adsorbent. Finally, in the basic zone, when the pH exceeds the pHPZC of the adsorbent, the adsorption capacity decreases due to the repulsion between the pesticide’s anions and the negative charge of the surface of the apricot and peach stones. This indicate that the main adsorption mechanism is primarily governed by the electrostatic interaction between the pesticide molecules and the surface of the bioadsorbents. Understanding the adsorption equilibrium data is crucial for the analysis of any adsorption study. Figure 5a and b represents the 2,4-D adsorption isotherms on peach and apricot stones at various adsorption temperatures, respectively. These isotherms reflect the interaction between the adsorbate and the adsorbent until reaching the equilibrium state (Kumar et al. 2013). As the adsorption temperature increases, the amount adsorbed also increases, indicating that the adsorption process is endothermic (Guiza 2017). The maximum adsorption capacities higher than 40 mg g–1 were obtained at 50 °C regardless of the adsorbent used. Several studies in the literature have focused on the adsorption of 2,4-D pesticides. For instance, Lazarotto et al. (2021) investigated the use of activated carbon derived from mushroom residue, chemically activated with ZnCl2, aimed at eliminating 2,4-D pesticide. They notably achieved a maximum adsorption of 241.7 mg g−1 which surpass the value obtained in the present work (41.5 mg g−1). However, it is essential to consider that the proposed approach avoids the need of high temperatures in a chemical activation (500 °C) and the use of chemicals like ZnCl2, making it cost effective and more environmentally friendly solution. Khan et al. (2021) reported significantly lower adsorption capacities of 2,4-D herbicide using polypyrrole and sugarcane bagasse adsorbents achieving 6.1 and 8.63 mg g−1, respectively. Hernandes et al. (2022) analyzed the adsorption of 2,4-D pesticide on biochars derived from cedar bark sawdust and concluded that none of the adsorbents were efficient in removing 2,4-D. The study conducted by Aswani and Kumar (2021) investigated the bioadsorption of this pesticide on acid-thermally modified Merremia vitifolia (a vigorous climbing plant). The reported maximum adsorption capacity was close to 150 mg g−1 but this result was obtained using a much more concentrated 2,4-D solution (200 mg L−1). When using an initial concentration similar to that used in this work (50 mg L−1), the maximum adsorption capacity was similar to the obtained in this study. However, it should be mentioned that the proposed bioadsorbents do not require any thermal or acidic treatment. Despite the straightforward preparation process and the absence of high temperatures or complex chemicals, the bioadsorbents proposed in the current study, peach and apricot stones demonstrated comparable or even higher 2,4-D adsorption capacities than those of other bioadsorbents reported in previous studies.
The equilibrium data of Fig. 5a and b were fitted to some of the most relevant isotherm models: Langmuir (1918), Freundlich (1907), and Dubinin–Radushkevich (Dubinin 2002). The Langmuir adsorption isotherm has been applied with considerable success in many adsorption processes. This model assumes that all adsorption sites are uniform and that each adsorption site can capture only one molecule. The adsorption occurs in a monolayer and homogenous surface. The linearized form of the Langmuir model is expressed by the equation below:
where Ce is the adsorbate equilibrium concentration (mg L−1), Qe is the amount adsorbed in the equilibrium (mg g−1), Qmax is the maximum adsorption capacity in monolayer (mg g−1), KL is the Langmuir equilibrium constant in (L mg−1) and C0 is the initial adsorbate concentration (mg L−1) (Chair et al. 2017; Semerjian 2018). The Freundlich isotherm model assumes a limited number of adsorption sites on a heterogonous surface. In this case, the adsorption process can proceed in multiple layers of adsorbate. This involves that the adsorbate molecules can be adsorbed on each other, creating an interfacial zone, that enables mutual interactions. The linear equation of Freundlich is represented as follows:
where Kf and nf are the distribution coefficient and the Freundlich affinity factor, respectively.
Finally, the Dubinin–Radushkevich isotherm model is commonly employed to describe the adsorption phenomenon on a heterogeneous surface with Gaussian energy distribution. This model is specifically designed to estimate the apparent free energy of the porosity and the features of the adsorption. It is calculated based on Eqs. (4) and (5):
where ε is the constant of the isotherm; Qm represents the maximum amount adsorbed per unit mass of the adsorbent (mg g‒1); and Kad is the Dubinin–Radushkevich isotherm constant (mol2/J2).
The results of fitting the equilibrium data to different models are summarized in Table 3. Among the models considered, the Langmuir model best fitted the experimental data, although a very good fitting is also obtained with the Dubinin-Radushkevich isotherm model. For the Langmuir model, the values of RL are between 0 and 1 suggesting that 2, 4-D adsorption is favorable onto both supports (Abbas 2020). For both adsorbents, peach and apricot stones, the value of nf is higher than 1 which indicates the high affinity between the solute and the adsorbent (Hidayat et al. 2021). For, Dubinin–Radushkevich model, the adsorption energy (E) onto peach and apricot stones ranges from 1.29 to 2.5 kJ mol−1 and from 1.29 to 1.58 kJ mol−1, respectively.
Thermodynamic studies
The thermodynamic study aimed to investigate the temperature effect on the adsorption of the 2,4-D pesticide onto peach and apricot stones. In other terms, this study helps anticipate the method of retention established between the adsorbate (2,4-D) and the adsorbent (peach and apricot stones) under different temperature conditions.
The distribution coefficient Kd was determined using the following equation:
where Qe is the solid phase concentration (mg g−1) and Ce is the liquid phase concentration (mg L−1) at equilibrium.
The enthalpy change (ΔH0), the free energy change (ΔG0), and the entropy change (ΔS0) of 2, 4-D pesticide adsorption were calculated using the Van't Hoff formula: (Gómez-Avilés et al. 2022)
The plots of Ln (Kd) in the function of 1/T (K−1) permit to obtain the values of entropies (ΔS0) and enthalpies (ΔH0) for the adsorption of the anionic pesticide 2,4-D onto both peach and apricot stones while the values of free energies (ΔG0) are calculated using the Eq. (8). The main findings are tabulated in Table 3. Both are endothermic (ΔH0 > 0), spontaneous (ΔG0 < 0) and with a high degree of heterogeneity between the liquid and the solid phases. These values agree to those previously reported by Aksu and Kabasakal (2004) when analyzing the adsorption of 2,4-D onto a granular activated carbon and Vinayagam et al (2023) for the adsorption of the same pesticide on algal magnetic activated carbon nanocomposite. Higher 2,4-D adsorption enthalpies were obtained by Almahri et al (2023) on coffee waste biochar, although with agreement on the spontaneous and endothermic nature of the adsorption process.
Adsorption kinetics
To elucidate the rate of adsorption, several models have been developed. In this work, the most frequent ones were used, namely pseudo-first order, pseudo-second order, and Elovich considering various initial 2,4-D concentrations (5, 7, 8, and 10 mg L−1). Table 4 sums up the empirical expressions and their findings for this effect. The coefficient of correlation (very close to the unit) shows that the Elovich model achieved the best fitting to the experimental data for both adsorbents (mentioned in Fig. 6a, b for peach and apricot stones, respectively). Raíssa et al. (2021) also concluded that the adsorption of 2,4-D on the H2SO4-modified bark from Campomanesia guazumifolia followed the Elovich model. On the contrary, Kirbiyik et al (2017) stated that the pseudo-second order kinetic model best fitted the adsorption kinetic of 2,4-D on biomass-based activated carbon, and Njoku et al (2015) confirmed the Avrami model as that that best fit the kinetic data when analyzing the adsorption of this same pesticide on coconut shell-activated carbon. The concentration of the 2,4-D pesticide, the amount of adsorbent, and the temperature can significantly influence the adsorption process. As the concentration of aqueous solution of 2,4-D pesticide is increased, the adsorption capacity also increased for both apricot and peach stones, aligning with the isotherm curves (Fig. 5a, b). In addition, the increase in the adsorbent dose led to a higher percentage of pesticide removal, as expected.
In order to assess the regeneration of the adsorbents, the peach and apricot stones were regenerated with hot water at 80 °C under vigorous stirring for 2 h in order to desorb the pollutant molecules and reuse them in successive adsorption-regeneration cycles. Figure 7 represents the evolution of the adsorption capacities of both peach and apricot stones (pH = 3, 50 °C, mads = 0.1 g and 2,4-D initial concentration of 10 mg/L) on four successive adsorption-regeneration cycles. As can be seen, the equilibrium adsorption capacities remain rather stable even after the fourth cycle, with a negligible decrease, for both peach and apricot stones.
Conclusion
In this study, simple, eco-friendly, and low-cost bioadsorbents were employed successfully for eliminating 2,4-D pesticide from an aqueous solution. Experiments were conducted to characterize both bioadsorbents, namely peach and apricot stones.
It was observed that the percentage of pesticide removal increased with contact time and reached equilibrium after 120 minutes.
Both adsorbents, peach, and apricot stones, exhibited an optimal pH of 3 for the removal of the 2,4-D pesticide. This pH value was found to be favorable due to enhanced electrostatic interactions.
The maximum amount adsorbed is almost equal to 41.5 mg g−1 for a temperature equal to 50 °C, for both adsorbents, confirming the endothermic nature of the adsorption process. This observation was confirmed by the thermodynamic parameter ΔH0 (> 0).
These results provide strong evidence for the practical applicability of these waste materials as effective adsorbents in water treatment applications.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Abbas M (2020) Performance of apricot stone to remove dyes from aqueous solutions—equilibrium, kinetics, isotherms modeling and thermodynamic studies. Mat Today Proc 31:437–443. https://doi.org/10.1016/j.matpr.2020.03.821
Aksu Z, Kabasakal E (2004) Batch adsorption of 2,4-dichlorophenoxy-acetic acid (2,4-D) from aqueous solution by granular activated carbon. Sep Pur Technol 35:223–240. https://doi.org/10.1016/S1383-5866(03)00144-8
Almahri A, Abou-Melha KS, Katouah HA et al (2023) Adsorption and removal of the harmful pesticide 2,4-dichlorophenylacetic acid from an aqueous environment via coffee waste biochar: synthesis, characterization, adsorption study and optimization via Box-Behnken design. J Mol Struct 1293:136238. https://doi.org/10.1016/j.molstruc.2023.136238
Aswani MT, Kumar MVP (2021) Acid-thermally modified Merremia vitifolia for the removal of 2,4-dichlorophenoxyacetic acid. Chem Eng and Technol 44:875–883. https://doi.org/10.1002/ceat.202000524
Bedia J, Belver C, Ponce S et al (2018) Adsorption of antipyrine by activated carbons from FeCl3-activation of Tara gum. Chem Eng J 333:58–65. https://doi.org/10.1016/j.cej.2017.09.161
Bonilla-Petriciolet A, Mendoza-Castillo DI, Reynel-Ávila HE (2017) Adsorption processes for water treatment and purification. Springer, Cham. https://doi.org/10.1007/978-3-319-58136-1
Cabrera-Barjas G, Gallardo F, Nesic A et al (2020) Utilization of industrial by-product fungal biomass from Aspergillus niger and Fusarium culmorum to obtain biosorbents for removal of pesticide and metal ions from aqueous solutions. J Environ Chem Eng 8:104355. https://doi.org/10.1016/j.jece.2020.104355
Chair K, Bedoui A, Bensalah N et al (2017) Combining bioadsorption and photoelectrochemical oxidation for the treatment of soil-washing effluents polluted with herbicide 2,4-D. J Chem Technol Biotechnol 92:83–89. https://doi.org/10.1002/jctb.5001
Chen X, Zhou Q, Liu F et al (2021) Performance and kinetic of pesticide residues removal by microporous starch immobilized laccase in a combined adsorption and biotransformation process. Environ Technol Innov 21:101235. https://doi.org/10.1016/j.eti.2020.101235
Demiral İ, Samdan C, Demiral H (2021) Enrichment of the surface functional groups of activated carbon by modification method. Surf Interface 22:100873. https://doi.org/10.1016/j.surfin.2020.100873
Dubinin M (2002) The potential theory of adsorption of gases and vapors for adsorbents with energetically nonuniform surfaces. Chem Rev 60:235–241. https://doi.org/10.1021/cr60204a006
Freundlich H (1907) Über die Adsorption in Lösungen. Z Phys Chem 57:385–470. https://doi.org/10.1515/zpch-1907-5723
Garabrant DH, Philbert MA (2016) Epidemiology and toxicology. https://doi.org/10.1080/20024091064237
Gómez-Avilés A, Peñas-Garzón M, Belver C et al (2022) Equilibrium, kinetics and breakthrough curves of acetaminophen adsorption onto activated carbons from microwave-assisted FeCl3-activation of lignin. Sep Purif Technol 278:119654. https://doi.org/10.1016/j.seppur.2021.119654
González-García P (2018) Activated carbon from lignocellulosics precursors: a review of the synthesis methods, characterization techniques and applications. Renew Sust Energy Rev 82:1393–1414. https://doi.org/10.1016/j.rser.2017.04.117
Goscianska J, Olejnik A (2019) Removal of 2,4-D herbicide from aqueous solution by aminosilane-grafted mesoporous carbons. Adsorption 25:345–355. https://doi.org/10.1007/s10450-019-00015-7
Guiza S (2017) Biosorption of heavy metal from aqueous solution using cellulosic waste orange peel. Ecol Eng 99:134–140. https://doi.org/10.1016/j.ecoleng.2016.11.043
Hashem A, Aniagor CO, Morsy OM, Abou-Okeil Aly AA (2022) Apricot seed shell: an agro-waste biosorbent for acid blue193 dye adsorption. Biomass Conv Bioref. https://doi.org/10.1007/s13399-022-03272-9
Hassan SS, Al-Ghouti MA, Abu-Dieyeh M, McKay G (2020) Novel bioadsorbents based on date pits for organophosphorus pesticide remediation from water. J Environ Chem Eng 8:103593. https://doi.org/10.1016/j.jece.2019.103593
Hernandes PT, Franco DSP, Georgin J et al (2022) Adsorption of atrazine and 2,4-D pesticides on alternative biochars from cedar bark sawdust (Cedrella fissilis). Environ Sci Poll Res 29:22566–22575. https://doi.org/10.1007/s11356-021-17590-4
Hidayat ARP, Sulistiono DO, Murwani IK et al (2021) Linear and nonlinear isotherm, kinetic and thermodynamic behavior of methyl orange adsorption using modulated Al2O3@UiO-66 via acetic acid. J Environ Chem Eng 9:106675. https://doi.org/10.1016/j.jece.2021.106675
Khan MM, Khan A, Bhatti HN et al (2021) Composite of polypyrrole with sugarcane bagasse cellulosic biomass and adsorption efficiency for 2,4-dicholrophonxy acetic acid in column mode. J Mat Res Technol 15:2016–2025. https://doi.org/10.1016/j.jmrt.2021.09.028
Kirbiyik C, Pütün AE, Pütün E (2017) Equilibrium, kinetic, and thermodynamic studies of the adsorption of Fe(III) metal ions and 2,4-dichlorophenoxyacetic acid onto biomass-based activated carbon by ZnCl2 activation. Surf Interfaces 8:182–192. https://doi.org/10.1016/j.surfin.2017.03.011
Kul AR, Aldemir A, Elik H (2019) Adsorption of acid blue 25 on peach seed powder: isotherm, kinetic and thermodynamic studies. Environ Res Technol 2:233–242. https://doi.org/10.35208/ert.650398
Kumar V, Pathania D, Sharma S et al (2013) Remediation and recovery of methyl orange from aqueous solution onto acrylic acid grafted Ficus carica fiber: isotherms, kinetics and thermodynamics. J Mol Liq 177:325–334. https://doi.org/10.1016/j.molliq.2012.10.007
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403. https://doi.org/10.1021/ja02242a004
Lazarotto JS, da Boit Martinello K, Georgin J et al (2021) Preparation of activated carbon from the residues of the mushroom (Agaricus bisporus) production chain for the adsorption of the 2,4-dichlorophenoxyacetic herbicide. J Environ Chem Eng 9:106843. https://doi.org/10.1016/J.JECE.2021.106843
Maponga TC, Mahamadi C (2019) Efficient Au(CN)2–1 adsorption using peach stone-derived granular activated carbon. Sci Rep 9:1–12. https://doi.org/10.1038/s41598-019-39964-y
Njoku VO, Asif M, Hameed BH (2015) 2,4-Dichlorophenoxyacetic acid adsorption onto coconut shell-activated carbon: isotherm and kinetic modeling. Desalinat Water Treat 55:132–141. https://doi.org/10.1080/19443994.2014.911708
Noreen S, Mustafa G, Ibrahim SM et al (2020) Iron oxide (Fe2O3) prepared via green route and adsorption efficiency evaluation for an anionic dye: kinetics, isotherms and thermodynamics studies. J Mat Res Technol 9:4206–4217. https://doi.org/10.1016/j.jmrt.2020.02.047
Osagie C, Othmani A, Ghosh S et al (2021) Dyes adsorption from aqueous media through the nanotechnology: a review. J Mat Res Technol 14:2195–2218. https://doi.org/10.1016/j.jmrt.2021.07.085
Raíssa C, Bevilacqua RC, Preigschadt IA, Netto MS et al (2021) One step acid modification of the residual bark from Campomanesia guazumifolia using H2SO4 and application in the removal of 2,4-dichlorophenoxyacetic from aqueous solution. J Environl Sci Health Part B 56:995–1006. https://doi.org/10.1080/03601234.2021.1997283
Saleh IA, Zouari N, Al-Ghouti MA (2020) Removal of pesticides from water and wastewater: chemical, physical and biological treatment approaches. Environ Technol Inn 19:101026. https://doi.org/10.1016/j.eti.2020.101026
Salman JM, Abd FM, Muhammed AA (2011) Adsorption of carbofuran insecticide from aqueous solution using commercial activated carbon. Int J Chem Sci 9:557–564
Semerjian L (2018) Removal of heavy metals (Cu, Pb) from aqueous solutions using pine (Pinus halepensis) sawdust: equilibrium, kinetic, and thermodynamic studies. Environ Technol Innov 12:91–103. https://doi.org/10.1016/j.eti.2018.08.005
Vinayagam R, Ganga S, Murugesan G et al (2023) 2,4-Dichlorophenoxyacetic acid (2,4-D) adsorptive removal by algal magnetic activated carbon nanocomposite. Chemosphere 310:136883. https://doi.org/10.1016/j.chemosphere.2022.136883
Zaini MAA, Zhi LL, Hui TS et al (2020) Effects of physical activation on pore textures and heavy metals removal of fiber-based activated carbons. Mater Today Proc 39:3–7. https://doi.org/10.1016/j.matpr.2020.03.815
Author information
Authors and Affiliations
Contributions
SG and MB contributed to conceptualization and supervision. JB and CB contributed to methodology. SH and JB contributed to writing—original draft preparation and writing—review and editing. CB, SG and MB contributed to funding acquisition. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Editorial responsibility: P.F. Rupani.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Harabi, S., Guiza, S., Bedia, J. et al. Biosorption of 2,4-dichlorophenoxyacetic acid pesticide on powdered peach and apricot stones. Int. J. Environ. Sci. Technol. 21, 6823–6832 (2024). https://doi.org/10.1007/s13762-023-05443-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-023-05443-1