Abstract
Global agriculturists have been looking for ways to mitigate the problem of increasing demand for food supply. Nanofertilizers serve as an effective alternative solution to this problem when compared to conventional fertilizers. Agricultural research on chitosan nanomaterials (NMs) as next-generation fertilizers has increased over the years, revealing their role in boosting agricultural productivity both as plant immune boosters and also by regulated, gradual, and targeted delivery of nutrients to plants. The presence of functional groups, the ability of surface modification, and the targeted delivery of nutrients to plant organelles have proven chitosan as an exceptional biopolymer for increased crop yield. The current review investigates the features of chitosan NMs as potential next-generation fertilizers and ways for formulating chitosan nanoforms and provides critical insights into future directions of chitosan-based next-generation nanofertilizers.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
An increase in urbanization and consumption has led to increasing demand for food supply, thereby leading to the necessity of increased yield and crop production. In order to satisfy this expanding worldwide demand, high dosages of synthetic fertilizers, herbicides, and agrochemicals are delivered to plants in an effort to boost production. Synthetic fertilizers enhance crop growth but do not exert any influence on plant nutrient uptake, and their hazardous effects on the environment continue to be the same (Adnan et al., 2020). They do not seem to hold an advantage when seen from an economic perspective either. Therefore, the search continues for alternatives that are not only well suited as fertilizers but are also highly efficient under various stressful conditions, are biocompatible, and are less toxic to the environment. The emerging application of nanotechnology in agriculture is seen to bring a breakthrough advancement to the field.
Nanofertilizers that serve the above purpose can be created utilizing specific chemical, physical, mechanical, and biological processes, either from standard bulk fertilizer ingredients or by extracting different plant parts. Nanoparticles are naturally produced in nature through various mechanisms such as physiochemical weathering, volcanic eruptions, neo-formation, etc., as well as from biological processes with the aid of mineral-producing microorganisms (Mura et al., 2013). They are naturally found in soil minerals. Moving colloids take these nanoparticles from soil micropores, enhance their mobility, and fix them in macropores. They may readily be hindered in their motion if taken up by stationary particles. In addition to generating a complicated system of soil sedimentation with macromolecules, nanoparticles may also add soil heterogeneity, posing some challenges to leaching and transport in soil (Ben-Moshe et al., 2010; Fang et al., 2009). Nanofertilizers release agrochemicals, decrease soil toxicity, provide a target delivery mechanism, and increase fertilizer nutritional efficiency. Because of their high surface area-to-volume ratio, target specificity, better solubility, tiny size resulting in excellent mobility, and low toxicity, nanofertilizers are highly beneficial.
Preference for nanofertilizers over conventional fertilizers has already resulted in improvements in a number of agricultural issues, including declining crop yield, decline in soil organic matter, lack of soil nutrients, lack of soil heterogeneity, and loss of soil biodiversity (Jakhar et al., 2022). Keeping in mind other environmental factors such as a changing climate, a reduction in the amount of arable land due to urbanization, freshwater inaccessibility, and a manpower shortage, new-age fertilizers need to be designed to combat existing issues and prevent new ones from surfacing.
Chitosan is also nontoxic, which eliminates any potential environmental concerns. Chitosan’s renewable food waste origin contributes to its biocompatibility and biodegradability. All of these characteristics combine to make chitosan nanoparticles an effective next-generation fertilizer for plant systems (Kashyap et al., 2015). The current review explores the properties of chitosan NMs as prospective next-generation fertilizers and methods for manufacturing chitosan nanoforms, as well as providing vital insights into the future directions of chitosan-based next-generation nanofertilizers (Fig. 11.1).
Chitosan NMs offer significant promise as next-generation fertilizers; nevertheless, more study is required to improve their characteristics and application methods for various crops and growth situations. Field trials and long-term environmental monitoring are needed to assess the effectiveness and safety of chitosan NMs as fertilizers. Furthermore, because of the complexity of their synthesis and processing, chitosan NMs may be more expensive than standard fertilizers in terms of cost-effectiveness. In conclusion, chitosan NMs have the potential to transform the fertilizer industry by offering environmentally benign, slow-release, and effective fertilizers. However, further research is required to fully understand their advantages and disadvantages as well as to design appropriate application techniques for various agricultural systems
2 Chitosan
As the need for sustainable agriculture becomes more prominent with each passing day, the search for novel candidates with the desired turnover has intensified. Materials that are nontoxic, biocompatible, and biodegradable are most suitable, particularly those that require low capital investment but produce high yields of crops. Chitosan ticks off all these requirements. As one of the most abundant naturally occurring amino polysaccharides derived from biological wastes, chitosan find numerous agricultural and biological applications (Kumaraswamy et al., 2018). There have been a variety of chitosan types derived with many biological activities, including antibacterial and antifungal properties, but no two types of chitosan share the exact same set owing to their differing structures and physiochemical properties. Nevertheless, it only results in a wider range of applications and novel findings to cater to different needs.
2.1 An Overview: Sources, Structure, and Medicinal Properties
Chitosan, a polycationic polymer, is essentially derived from chitin, the second most abundant polysaccharide available in nature, following cellulose. Chitin is an economic polymer that can be obtained from marine wastes; it is mainly found in crustacean exoskeletons and arthropods, although it is also spotted in the cell walls of fungi, yeast, and algae (Zargar et al., 2015). Chitosan and chitin are both made of numerous variations of the same two monomers: α,1-4 linked d-glucosamine and N-acetyl-d-glucosamine (Ibrahim & El-Zairy, 2015). Chitosan is essentially obtained from chitin through deacetylation (Fig. 11.2), and to date, three different crystallographic forms of chitin have been reported, namely: alpha (α), beta (β), and gamma (γ) chitosan, with alpha and gamma chitosan being more similar to each other in physiochemical terms (Kaya et al., 2017). These physiochemical characteristics are usually measured through Fourier-transform infrared spectroscopy, scanning electron microscopy, liquid-state nuclear magnetic resonance, etc.
Preparation of chitosan by deacetylation. (Created with BioRender.com)
Chitosan’s functionality is due to the amino and hydroxyl groups on its second, third, and sixth carbons, among which the hydroxyl group at C6 is the most active owing to the minimal steric hindrance it faces (Fig. 11.3). This allows free rotation of C6-OH when compared to the hydroxyl group at the third carbon. Due to the high availability of NH2 and OH for bonding, chitosan functions as a compound with high bioactivity (Wang et al., 2020a, b). Chitosan’s ability to adhere to plant surfaces comes from the amino group it owns, which gives chitosan a net positive charge as a result of which it is able to form interactions with anionic molecules on membrane layers (Jakhar et al., 2022).
Chitosan is a natural biopolymer formed from chitin, a polysaccharide found in crustaceans’ shells and fungi’s cell walls. Chitosan is created by deacetylating chitin, removing some of the acetyl groups, and converting it into a cationic polysaccharide. The resultant chitosan molecule has a linear structure with glucosamine and N-acetylglucosamine repeating units joined by (14) β glycosidic linkages. Chitosan’s chemical structure is represented by the molecular formula (C6H11NO4)n
Chitosan finds various applications in the biomedical field owing to its various properties, which make it medicinally compatible. In addition to having strong antibacterial, antiviral, and antifungal effects, it also has other characteristics like nontoxicity, hemocompatibility, and mucoadhesivity (Zhao et al., 2018). One of chitosan’s primary advantages as a biomedical agent is that it does not trigger a strong immune response. Its ability to adhere to mucus not only makes mucosal pathway delivery easy but also aids in the delivery of agents that lack affinity to mucus (Bugnicourt & Ladavière, 2016). Chitosan is seen to increase wound healing rates by interacting with platelets through its amino groups (Okamoto et al., 2003). It is also found to have potential antitumor activity along with its elevated antioxidant capacity (Tokoro et al., 1988; Younes & Rinaudo, 2015). On top of it all, chitosan’s biodegradability is what makes it a perfect next-generation candidate as an efficient biomedical agent.
2.2 Role of Chitosan in Agriculture
The presence of numerous amino and hydroxyl group in the chitosan made its use as adsorbents to remove organic and inorganic pollutants from water (Bandara et al., 2020). Chitosan was widely used as a flocculating agent to gather pollutants as a part of the wastewater treatment process (Lichtfouse et al., 2019). Magnetic chitosan nanoparticles were also seen as promising adsorbents because of the possibility of adsorbent recycling under a magnetic field (Lü et al., 2017). Following this, chitosan’s primary uses shifted from sewage and water treatment to extensive use as a fertilizer, plant growth stimulant, soil enricher, ant staling agent, etc. Chitosan not only has antibacterial and antifungal activity, but it also induces disease resistance in various plants by enhancing innate immunity (Babu et al., 2022). Chitosan application on plants reduced water loss in plant systems and restricted stomatal apertures, acting as an antitranspirant and limiting pathogen entry into plants through stomata (Bittelli et al., 2001). Chitosan is also proven to combat salinity and drought stress in plants. Several stresses occurring due to abiotic conditions are also tackled by chitosan through increased production of aldehydes, ketones, and phenols in plants, which aid in stress tolerance regulation (Bandara et al., 2020).
3 Chitosan as a Nanofertilizer
3.1 Chitosan as a Nanofertilizer: Properties and Function
Porous nanosized chitosan is considered one of the most effective candidates for micro- and macronutrient delivery in recent times. Although soil fertilization is one of the vital practices required to support crop growth, a variety of setbacks are still observed that hinder the proper functioning of said fertilizers. Conventional fertilizers used for prolonged periods in large quantities not only alter soil pH but also increase soil salinity. To combat such issues, chitosan-based nanofertilizers were researched owing to the promising properties they possess (Mujtaba et al., 2020).
Chitosan is an ideal choice for the formulation of nanofertilizers for various reasons, including its low cost, but there are certain distinct attributes that enhance its bioactivity (Yu et al., 2021). Chitosan’s ability to trigger the plant’s innate immune system guarantees plant redox homeostasis maintenance (Babu et al., 2022). The slow-release property of chitosan nanomaterials prolongs the availability of nutrients to the plants, thereby ensuring complete and efficient uptake of these materials (Prajapati et al., 2022). Chitosan nanomaterials possess a low dispersity index, which is essential to ensuring stability and consistent bioactivity. The zeta potential of nano-chitosan becomes extremely important due to its effect on the penetration ability of the nanoparticles and the possibility of surface interactions (Saharan et al., 2016). High zeta potential leads to high repulsion between nanoparticles, which prevents them from forming aggregates in the soil (Hu et al., 2020; Schwab et al., 2015). Size is, of course, a key consideration for elevated surface interactions. Chitosan nanoparticles with symmetric nanoarchitecture can infiltrate plant tissues with only moderate inhibition. The small size of chitosan nanofertilizer is said to facilitate its entry into plant leaf stomata, from where it is translocated throughout the plant system via phloem from the root to the shoot (Mujtaba et al., 2020). Nano-chitosan is seen to easily penetrate the stomata, stigma, cuticle, trichome, and even root connections and plant wounds (Eichert et al., 2008; Yu et al., 2021).
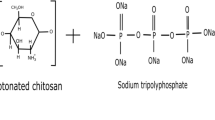
Structurally speaking, as a consequence of reduced steric hindrance, amino groups, and hydroxyl groups are made more available for bonding, which enables chitosan’s increased bioactivity (Wang et al., 2020a, b). Interactions of chitosan with membrane phospholipids are facilitated by the amino group, which imparts a net positive charge on chitosan. On the other hand, the hydroxyl group accelerates signal transduction due to its electron-accepting nature. This innate functionality of chitosan is elevated through cross-linking a cationic amino group containing linear chitosan with anionic tripolyphosphate (TPP) (Azmana et al., 2021). The resulting chitosan nanomaterials exhibit a higher surface area-to-volume ratio. This results in an increase in the number of surface functional groups, which ultimately increases the possibility of interactions with plant surfaces. These functional groups of chitosan nanomaterials covalently or electrostatically bond with various ingredients and form several conjugates with organic and inorganic materials. The porous structure of chitosan increases these interactions even more, leading to a higher load of active materials in the nano-chitosan (Kumaraswamy et al., 2018). In addition to all these characteristics, the cationic polymer is inherently anti-inflammatory and anti-hypercholesterolemic in nature, and this can be even more enhanced by conjugation with several compounds like urea (Bandara et al., 2020; Negm et al., 2020). Chitosan is also nontoxic, which eliminates any environmental concerns that might arise. Chitosan’s origin source being renewable food waste contributes to its biocompatibility and biodegradability. All these factors make chitosan nanomaterials an excellent next-generation fertilizer for plant systems (Kashyap et al., 2015).
3.2 Water Retention and Salinity Moderation Capacity of Chitosan
Stress in plants can be induced by various factors, including drought and salinity. Plant anatomy and physiology are heavily dependent on water availability, and a deficiency in this leads to a drastic decrease in yields. Water unavailability leads to the closure of plant stomata, which results in a reduced photosynthesis rate, decreased chlorophyll content in plants, and destruction of chloroplasts. High salinity levels have been proven to be detrimental to plant growth and development (Zayed et al., 2017). Chitosan nanomaterials are able to wonderfully combat these environmental stresses.
Foliar application of chitosan nanofertilizer reduced stomatal conductance and transpiration in plant systems, leading to water retention. Controlled release of nitric oxide (NO) by chitosan nanomaterials alleviates drought stress in plants more effectively than plants with free nitric oxide donor chemicals. Treated plants show high root biomass and photosynthesis rates in contrast to untreated controls. Chitosan is seen to increase the production of phenolic compounds, plant antioxidants, and osmoregulators, eventually increasing crop yield (Priyaadharshini et al., 2019; Rabêlo et al., 2019; Silveira et al., 2019).
Similar to drought stress, NO-releasing chitosan nanoparticles were more effective than free NO donors in tackling salt stress. The controlled release of NO by nano-chitosan increases the bioavailability of NO, thereby increasing chlorophyll content in treated plants. Gene coding for known detoxifying agents like superoxide dismutase (SOD) and jasmonic acid (JA) has been demonstrated in some cases to be upregulated by the application of chitosan nanofertilizer, mitigating any negative impacts of salinity stress on the plants (Hemantaranjan, 2014). Treated plants show high chlorophyll content, high protein levels, and improved metabolism, which ultimately aids better plant growth and development (Oliveira et al., 2016; Sen et al., 2020).
3.3 Chitosan Combats Temperature and Heavy Metal Stress
Extreme temperatures, constantly fluctuating temperatures, and the presence of toxic heavy metals in the cultivation soil are other environmental stresses that are induced in crops (Bandara et al., 2020). There has been a combinational use of bulk chitosan and zinc to suppress heat stress in plants (Ibrahim & Ramadan, 2015). Also, at low temperatures, priming seeds with chitosan at 15 °C did not only decrease germination time but also increase shoot height and root length (Guan et al., 2009). Additionally, chitosan is seen to possess the ability to complex heavy metal ions that might be present in the soil, making them unavailable to enter and affect plant systems, thereby preventing associated damage (Kamari et al., 2011).
4 Types of Chitosan-Based Nanofertilizers and Applications
Owing to their small size, nanoparticles have an increased ability to penetrate plant surfaces, which makes them a potential candidate for fertilizer delivery (Jakhar et al., 2022). Nanofertilizers can be classified into two types, mainly micronutrient and macronutrient nanofertilizers. Different macronutrients like calcium, phosphorus, potassium, magnesium, sulfur, nitrogen, and phosphorus that have been encapsulated with NPs minimize their overall requirements while providing the crops with the appropriate amount of nutrients (Zulfiqar et al., 2019). When combined with various metals and substances, chitosan is seen to show enhanced growth and development in plants, and the amine groups contained in chitosan that are available for bonding exhibit elevated affinity for metals; this trait is exploited for designing chitosan-based nanofertilizers (Adisa et al., 2019).
4.1 Chitosan–NPK Nanofertilizer
Chitosan is combined with nitrogen, phosphorous, and potassium by polymerization to synthesize CS-PMAA nanoparticles, to which urea, calcium phosphate, and potassium chloride are consecutively loaded (Abdel-aziz et al., 2016). When chitosan-NPK was delivered to plant systems through foliar spray, plant characteristics such as plant stem diameter and leaf area were seen to be enhanced when compared to untreated controls, which subsequently increased the overall harvest, mobilization, and crop index (Corradini et al., 2010; Khalifa & Hasaneen, 2018). α- and β-chitosan (CS) derived from shrimp wastes were characterized, produced into nano NPK fertilizers, and applied to Capsicum annum L. cv. The acquired results showed that, in comparison to the control and chemical fertilizer-treated plants, the nano-composite NPK with a 25% concentration considerably increased the growth, yield, and harvest of C. annuum (Abdel-Aziz et al., 2021). In addition to increased root and shoot height, higher starch content in the roots of treated plants was observed, and slow release of NPK was also detected with over 80% release of loaded materials through 168 h (Prajapati et al., 2022). Elevated carbon and phosphorous availability contribute to enhanced enzymatic activity of acid-alkaline phosphatases and glucosidases in the soil (Kubavat et al., 2020). A comparison drawn between nanomaterials based on their size and zeta potentials extends the conclusion that nanomaterials with greater zeta potential and smaller sizes tend to show enhanced nanofertilizer activity (Motakef Kazemi & Salimi, 2019). One notable advantage of chitosan-NPK fertilizers is that they have a strong affinity to the surface of plants due to a higher positive charge, thereby reducing runoff by a great percentage. This also contributes to a reduction in the interaction of chitosan-NPK nanofertilizer with other nutrients. These nanofertilizers additionally prove to have little to no adverse effects on plant systems (Prajapati et al., 2022).
4.2 Chitosan–Zinc Nanofertilizer
Zinc is an essential plant micronutrient and serves as a cofactor for over 300 enzymes in plants, all while playing a crucial role in maintaining cellular metabolism and homeostasis (Pereira et al., 2017; Deshpande et al., 2017). Hence, formulations of Zn-chitosan nanofertilizers were made to enhance the growth and development of plants. It was also found to have a positive effect on cellular stability and photosynthesis and to increase chlorophyll content in the plant system. The slow release of Zn is seen to contribute significantly to nanofertilizer efficiency (Kumar et al., 2021). Zinc nanoparticles and zinc nitrate were foliar sprayed at dosages of 0, 25, 50, and 100 ppm with and without chitosan. The findings show that zinc nitrate at 50 ppm and zinc nanoparticles at 25 ppm were the most effective dosages for promoting biomass production and accumulation. Particularly when mixed with zinc nitrate, the addition of chitosan improved biomass, production, and photosynthesis-related metrics (Palacio-Márquez et al., 2021). In addition to upregulating several enzymes, such as soluble starch synthase and invertase, which are crucial for plant development, Zn–chitosan NM also increases the availability of antioxidants, which in turn improves cellular stability and redox equilibrium. Due to these various factors, increased starch content in grains, along with enhanced plant survivability, can be achieved, by virtue of which crop development and yield can be significantly positively altered (Prajapati et al., 2022).
4.3 Chitosan–Urea Nanofertilizer
Nitrogen, an extremely essential macronutrient in plants, serves as a precursor for amino acids, which thereafter form various different plant proteins and enzymes. The most commonly used source of nitrogen, which is conventionally administered as a fertilizer for plants, is urea (Kalia et al., 2019). Therefore, chitosan–urea nanofertilizers will ultimately become a fertile field of research. Granular urea is encapsulated into chitosan nanomaterials and produces spherical urea–chitosan nano particles through numerous cross-links. On application, it was found that the release of urea occurs slowly over a period of one whole month, and this controlled release was seen to impact nitrogen interaction dynamics in the soil to a considerable extent (Kalia et al., 2019). When deployed on potato plants, chitosan-urea nanofertilizer was found to increase tuber size and root length, elevate levels of carbon and potassium, and alter ammonium-nitrogen and nitrate-nitrogen in the treated soil (Kondal et al., 2021). Additionally, it induced noticeable alterations in urease and dehydrogenase activity, especially decreasing the former to a large extent. Chitosan–urea nanofertilizers increased water intake in potato plants and induced improved seed germination, which ultimately led to higher potato yield (Wang et al., 2020a, b).
4.4 Chitosan–Copper Nanofertilizer
Although considered toxic for plants and the environment at higher concentrations, copper proves to be a helpful micronutrient when administered in limited quantities, and these effects are reflected in plant growth, development, and reproducibility (Zarb et al., 2002). Naturally, to exploit this effect of copper on plant metabolism, chitosan–copper nanofertilizers were proposed. Through ionic gelation, copper ions were encapsulated in the nanopores of the chitosan matrix; glutaraldehyde-cross-linked hydrogels of chitosan were prepared, which were then complexed with copper nanoparticles (Saharan et al., 2015; Juárez-Maldonado et al., 2016). Studies after application reveal that plants treated with chitosan–Cu nanofertilizers exhibited increased root length, plant height, and stem diameter. Apart from morphological enhancements, a boost in the content of various enzymes like catalase, amylase, protease, and a few defense enzymes was seen, in addition to accelerated antioxidant activity owing to an increase in the production of lycopene, superoxide dismutase, and peroxidase (Saharan et al., 2015). This increase in the growth and development of plants contributes to the controlled release of copper ions from the matrix, which extends the availability of copper for the plants over comparatively long periods of time. Also, when compared to standalone chitosan, chitosan–Cu nanofertilizers seem to have an elevated positive effect on photosynthesis and seedling development, which leads to higher protein content in seeds, thereby increasing crop yield overall (Choudhary et al., 2017; Sathiyabama & Manikandan, 2018). Rivera-Jaramillo et al. demonstrated that PVA-chitosan-nCu complex nanoparticles applied to tomato plants promoted their yield along with an increase in the number of fruits, average fruit weight, aerial fresh weight, and root fresh weight. The complex nanoparticle also improved the defense system by boosting the activity of the phenylalanine ammonia lyase (PAL) enzyme and PR1 gene overexpression (Rivera-Jaramillo et al., 2021).
4.5 Chitosan–Silicon Nanofertilizer
Even though it is not considered a conventional plant essential nutrient, silicon supplementation has proven to enhance various plant characteristics over a period of exposure. Simple silica treatment yielded stronger and thicker stems, better positioning of leaves with shorter internodes, and enhanced resistance to environmental stresses. All these benefits can be magnified if combined with the chitosan delivery system (Frew et al., 2018). Chitosan–Si nanofertilizers are produced by encapsulating silicon in chitosan–TPP matrix. As seen previously in chitosan–NPK nanofertilizers, chitosan–Si fertilizers also have a high value of zeta potential and greater affinity to plant surfaces, which contributes to their high performance. On foliar application of this nanofertilizer, it was observed that the treated plants displayed increased leaf surface area and chlorophyll content, which are naturally reflected in the plant’s photosynthesis capabilities. According to a study (Kumaraswamy et al., 2021), chitosan–Si nanofertilizer is seen to influence root length, root number, shoot length, seedling length, and fresh weight significantly. By adding nano-silicon and nano-chitosan to the soil, either individually or in combination, the bioavailability of mineral nutrients is increased, which minimizes the need for huge conventional fertilizer applications. This results in more robust crops and productive plant edaphic systems (Robledo-Olivo). Elevated levels of antioxidant activity and defense enzymes were also observed in the treated plants as compared to their controls. As chitosan and silica induce great plant growth-promoting activity individually, when combined, they give enhanced results in terms of plant development (Prajapati et al., 2022). By encapsulating additional crucial nutrients alongside Si, CS–Si NF may be further customized to expand its utility in treating multi-nutrient insufficiency (Kumaraswamy et al., 2021).
4.6 Chitosan-Copper-Salicylic Nanofertilizer
Another interesting example of the enhanced results of synergistic combinations is the case of chitosan-copper–salicylic nanofertilizers. This is achieved by co-encapsulating copper and salicylic acid inside a highly porous and symmetric chitosan nanomatrix. This symmetry and porosity caused due to a low PDI were attributed to the slow release of components into the soil from the nanomatrix (Sharma et al., 2020). On administration with chitosan–Cu–SA nanofertilizer, treated plants showed increased sucrose content in growing cobs. In a study conducted by (Choudhary et al., 2017), plant height, stem diameter, root length, and root number all demonstrated significantly higher values. Plant photosynthesis and oxidative stress resistance were both elevated as a result of reduced malondialdehyde (Prajapati et al., 2022). Foliar application of Cu–chitosan nanoparticles significantly increased the antioxidant/defense enzyme activity in maize leaves. These plant leaves had SOD activity that was four to six times greater than those treated with bulk chitosan. Apart from boosting plant development, chitosan–Cu nanoparticles also reduce the severity of diseases in plants (Choudhary et al., 2017). Chitosan–copper–salicylic is the best example to support the possibility of delivering both macro and micronutrients into plants while objectively enhancing plant characteristics through the delivery of nutrients, even while inducing high stress and disease resistance in them. Chitosan nanoparticles loaded with salicylic acid at a concentration of 200–400 ppm have been used to reduce Cassava leaf spot disease. Other concentrations of CS-NP-loaded SA improved Cassava plant growth with an increase in the number of shoots, root length, and weight (Hoang et al., 2022).
5 Methods of Formulation
Chitosan micro- and nanoparticles have been prepared using a variety of techniques. While choosing a method, it is important to take into account the particle size, stability of the active component and the finished product, residual toxicity present in the finished product, and the kinetics of the drug release profile (Agnihotri et al., 200). The molecular weight of chitosan, its chemical structure, specifically the degree of deacetylation, and the preparation method all have a significant impact on the size of the generated particles when creating chitosan particulate systems. Higher molecular weight chitosan typically results in larger-sized particles (Luangtana-anan et al., 2005). There are various ways to make chitosan micro-nanoparticles, and these particles often include a drug that is primarily attached to the chitosan through hydrogen bonding, electrostatic contact, or hydrophobic coupling (Table 11.1). Chitosan micro/nanoparticles can generally be loaded with a therapeutic agent either during the preparation process or after the particles have been created. The therapeutic drug is integrated and embedded in the chitosan matrix in the first case, whereas it is adsorbed on the surface of the particle in the second. The goal is often to achieve high entrapment efficiency, which can be done by incorporating the therapeutic agent into the matrix; however, the preparation method, additives, etc. may have an impact on the therapeutic agent (Ahmed & Aljaeid, 2016).
5.1 Precipitation
This technique relies on the chitosan’s physicochemical features, specifically its insolubility in an alkaline pH medium and the precipitate it produces as a result. As seen in Fig. 11.4, using a compressed air nozzle and a chitosan solution, coacervate droplets are created by blowing the chitosan solution into an alkali solution (Wang et al., 2016). The particles are then separated and purified by filtering or centrifugation, followed by multiple washings in hot and cold water. This method is used to make chitosan-DNA nanoparticles (Agnihotri et al., 2004). Allopurinol-loaded chitosan-coated magnetic nanoparticles have been used for the treatment of nephrolithiasis caused by hyperuricemic nephropathy (Kandav et al., 2019).
5.2 Sieving Method
Chitosan hydrogel containing the drug is first formed, and then a cross-linking agent, such as glutaraldehyde, is added to create a cross-linked chitosan hydrogel. This cross-linked chitosan hydrogel is then passed through a sieve of a specific size to obtain the drug-loaded microparticles (Fig. 11.5) (Ahmed & Aljaeid, 2016). The resulting microparticles are washed with sodium hydroxide to remove any excess glutaraldehyde and then heat-dried in an oven (Agnihotri et al., 2004).
5.3 Reverse Micelles
Mitra et al. (2001) were the first to report the production of chitosan nanoparticles from reverse micelles as a strategy for tumor-targeted delivery. In this process of reverse micellization, a lipophilic surfactant is dissolved in a suitable organic solvent, such as n-hexane, to create a W/O microemulsion. Due to the action of surfactants, reverse micelles are produced that are made up of water droplets that are dispersed in organic solvents in the nanometer range (1–10 nm) (Melo et al., 2001). These nanodroplets can be used as a reactor to create nanoparticles in their aqueous core. A cross-linking agent is added to ensure complete cross-linking. To obtain a dry bulk, the organic solvent is subsequently evaporated. The resultant dried mass is dissolved in water, a suitable salt is applied to precipitate the surfactant out, and then the drug-loaded chitosan nanoparticles are recovered by centrifugation to remove the surfactant, as shown in Fig. 11.6 (Mohammed et al., 2017). 5-Fluorouracil-loaded cross-linked chitosan nanoparticles formulated using the reverse micelles technique for effective drug delivery to a certain targeted area along with a reduction in oral toxicity and improved tolerability (Sethi et al., 2021).
5.4 Spray Drying
Spray drying has been used to produce dry powders and granules from drug-excipient mixes that are either in solution or in suspension (Chawla et al., 1994). This technique could create microparticles from various polymeric materials that were loaded with proteins, vaccine antigens, and medications (Ahmed & Aljaeid, 2016). For protein-loaded chitosan micro/nanoparticles, spray drying offers a simple, effective, one-step, and protein-friendly technique. To produce the necessary particles, an aqueous chitosan-protein solution is prepared and sprayed into a drying chamber via a nozzle (Fig. 11.7). Examples of proteins that can be loaded into chitosan microparticles using this technique include salmon calcitonin and bovine serum albumin (BSA) (He et al., 1999).
5.5 Ionotropic Gelation
Ionotropic gelation is based on the ability of polyelectrolytes to cross-link in the presence of counterions (Fan et al., 2012; Giri et al., 2012). The most commonly used technique for creating alginate nanoparticles is ionic gelation (Calvo et al., 1997). In a two-step process based on the ionotropic gelation of polyanion with calcium chloride and polycationic cross-linking, alginate-chitosan nanoparticles were created. Chitosan polysaccharide is dissolved in an acidic aqueous solution in the ionic gelation process to produce the cation of chitosan. The polyanionic tripolyphosphate solution is then gradually added while being constantly stirred (Fig. 11.8). Chitosan experiences ionic gelation and precipitates as spherical particles because of the complexation between species that have opposing charges. The lipid-chitosan hybrid nanoparticles fabricated by the single-step ionic gelation method can deliver cisplatin under regulated conditions and serve as a viable platform for the prospective delivery of cisplatin to tumors, according to the characterization and in vitro release profile (Khan et al., 2019).
5.6 Emulsion Cross Linking
In this technique, a cross-linking agent interacts chemically with the main amino groups of chitosan to produce chitosan micro-/nanoparticles. Glutaraldehyde, p-phthaldehyde, ascorbyl palmitate, and dehydroascorbyl palmitate are typical cross-linkers (Bugamelli et al., 1998). Chemical cross-linking can occur in one or two steps. The process comprises creating an aqueous water/oil (W/O) emulsion with the therapeutic drug and chitosan which is then emulsified with an external immiscible solvent before cross-linking is gradually added (Fig. 11.9). Centrifugation, numerous washing processes (with petroleum ether, acetone, sodium metabisulfite, and water), and vacuum- or freeze-drying are frequently used to separate NPs from the emulsion. As the external oil phase prevents the therapeutic agent from escaping, the formation of these particles in the interior water phase of a W/O emulsion promotes the trapping of the therapeutic agent (Jameela et al., 1998). This technique has been used to create chitosan microparticles that contain BSA. Due to toxicity and drug integrity issues associated with glutaraldehyde, this method is no longer used (Garg et al., 2019).
6 Emulsion-Droplet Coalescence
In order to create two emulsions, chitosan, and NaOH solutions were both emulsified into the same oil phase (paraffin oil). The two emulsions are combined and mixed while spinning at a rapid rate, allowing the emulsion droplets to coalesce randomly and precipitate (Fig. 11.10) (Tokumitsu et al., 1999). The advantage of this method over the emulsion cross-linking method is that it enables an electrostatic connection between the free amino groups of chitosan and the used anionic drug, allowing for increased drug loading (Agnihotri et al., 2004).
7 Controlled Release of Active Ingredients from Chitosan-Based Nanomaterials
The therapeutic effects of CSNPs are significantly influenced by the drug release from those particles. Due to their physicochemical characteristics, CSNPs come in a variety of forms and sizes, which affect how the drug is released. The ability of the components that make up the NPs to absorb water, the rate and speed of degradation, the chemical composition, MW, solubility, and crystallinity have an impact on the release of the NPs. Even interactions between drugs or between drugs and polymers seem to have a major impact on the drug’s release from the delivery system (Iacob et al., 2021). One of the following methods can control the release of the drug from the polymer: (a) erosion of the polymer matrix’s surface; (b) the breakdown of polymer bonds at the surface or in the bulk of the matrix; or (c) drug diffusion. Sometimes a combination of the three techniques can frequently be employed to release the drug (Herdiana et al., 2022). The release of the drug from CSNPs is also regulated by pH due to the solubility of CS. Drug release can be controlled using CS derivatives in accordance with the expected pharmacokinetic characteristics of the drug. Several mechanisms, including polymer swelling, drug diffusion via the polymeric matrix, drug diffusion through the adsorbed drug, polymer erosion or degradation, and a combination of both erosion and degradation, control the release of drugs from chitosan nanoparticles (Mohammed et al., 2017).
7.1 Diffusion-Controlled Release
The diffusion control mechanism is the most useful one for drug release. The drug or active substance flows through the polymer NP matrix, which serves as a controlled release device, and this induces the diffusion mechanism. When the active agent has a longer duration, the rate of drug release reduces (Herdiana et al., 2022). The molecule passes through the polymer matrix’s interior in the direction of the release medium. Polymer chains provide the diffusion barrier, which prevents the drug from moving. Diffusion may also be related to swelling or erosion. Diffusion is mathematically explained by Fick’s Law. The following assumptions must be made in order to derive the parameters of Fick’s law: sink conditions are always provided by the medium surrounding the nanoparticles; a pseudo-steady state is maintained during drug release; and the drug particle diameter is lower than the average distance of drug diffusion through the polymeric matrix (Siepmann & Siepmann, 2012). The release of Punica granatum L. extracts in a controlled manner for antibacterial applicability and topical delivery was controlled by a Fickian diffusion mechanism (Mohamady Hussein et al., 2021).
7.2 Swelling-Controlled Release
Water is absorbed into the polymer until the polymer dissolves, which causes the polymer to swell. The solubility of the polymer in water or the surrounding biological medium acts as a chief aspect of this medication release mechanism. The polymer chains untangle when it comes into contact with the surrounding medium and begin to swell. Drug release from that region of the polymer matrix occurs next. The drug release profile is often greatly influenced by the hydrophilicity of the polymer, the swelling velocity of the polymer, and the density of the polymer chains (Fonseca-Santos & Chorilli, 2017). By using a non-Fickian diffusion technique, the active ingredient is delivered to the polymer matrix simultaneously by erosion and diffusion. Relaxation constant plays a vital role in the matrix swelling device. The slower the drug is released from the matrix, the more significant the relaxation constant’s value. The release process seems to be best described by the Weibull model (Herdiana et al., 2022). This will consequently have an impact on how quickly the medication is made available for membrane transport or cellular uptake, which will have an impact on how quickly the drug is absorbed from the site of delivery in vivo.
7.3 Erosion and Degradation-Controlled Release
Polymer erosion and degradation have common characteristics. Sometimes, as bonds break due to polymer breakdown, physical erosion may result. Polymer erosion is a complicated process that includes swelling, diffusion, and disintegration. There are two types of erosion: homogeneous and heterogeneous. In contrast to heterogeneous erosion, which occurs from the surface toward the inner core, homogenous erosion occurs at the same pace throughout the matrix (Lee & Yeo, 2015). Enzymes or the medium in the region may be responsible for polymer breakdown. The copolymer composition, pH of the surrounding medium, and water uptake by the polymer are further factors that affect how quickly a polymer degrades. The type of polymer, internal bonding, additives (chitosan derivatives), the shape and size of the nanoparticles influences the drug release (Göpferich, 1996).
7.4 Oral Drug Delivery
For the development of new drugs, oral administration (OD) is the preferred dosage form due to its ease, safety, and patient tolerance. However, achieving oral delivery presents a number of difficulties, including a variation in pH (the stomach is highly acidic), the presence of enzymes, the first-pass action in the liver, and the intestinal barrier to drug absorption (Bowman & Leong, 2006). NPs are used as oral delivery vehicles for polynucleotides, proteins, and macromolecules due to their benefits over other drug delivery systems, including their small particle size, high surface area, and possibility for surface modification. Also, they make the acid-labile medicines more GIT stable (Palacio et al., 2016).
Tamoxifen is a mildly water-soluble anticancer medication that makes an excellent option for oral cancer treatment delivery. Tamoxifen was made into lecithin-chitosan nanoparticles to improve its ability to penetrate through the intestinal epithelium (Barbieri et al., 2015). Tamoxifen is more readily absorbed by the paracellular route due to the NPs’ mucoadhesive properties. Additionally, Feng et al. (2013) reported on a possible method for administering anticancer medications orally. Chitosan and carboxymethyl chitosan were used to create doxorubicin hydrochloride (DOX) nanoparticles. The small intestine’s ability to absorb DOX was found to be improved by these nanostructures (Feng et al., 2013).
7.5 Nasal Drug Delivery
A noninvasive method of drug administration to the brain, respiratory system, and/ or systemic circulation is nasal delivery. Moreover, due to their low permeability across the nasal epithelium, hydrophilic medications, proteins and peptides, nucleic acids, and polysaccharides pose challenges. Nasal absorption is essential for the medications to work properly. Molecular weight, lipophilicity, and charge are examples of the physical properties of drugs that control nasal absorption. Due to its use in nasal delivery, chitosan has mucoadhesion qualities as well as low toxicity, biodegradability, and biocompatibility, which can help address this issue. Nasal absorption can occur in three different ways: through the trigeminal nerves, the paracellular pathway, and the transcellular pathway. Liu et al., 2018 proposed that carbamazepine (an antiepileptic medicine) can be delivered intra-nasally with the help of carboxymethyl chitosan nanoparticles which bypass the blood–brain barrier. They created NPs that had good entrapment efficiency (80%) and a small particle size (218.76 ± 2.41 nm). They performed in vivo and in vitro testing, and the results demonstrated improved drug absorption and brain-targeting properties (Liu et al., 2018). Leuprolide-loaded chitosan and thiolated-chitosan nanoparticles were created by Shahnaz et al. (2012). When compared to the leuprolide solution, these chitosan and thiolated-chitosan nanoparticles boosted drug transport across the porcine nasal mucosa by twofold to fivefold, respectively (Shahnaz et al., 2012). Delivering drugs to the brain in case of any brain tumor has always been a major challenge. Another study emphasized the appropriateness of lipid-core nanocapsules coated with chitosan (LNCchit) as a promising method for administering simvastatin for the treatment of brain malignancies via a nose-to-brain approach (Bruinsmann et al., 2019).
7.6 Injection Drug Delivery
The term “injection administration” typically refers to administering medications intravenously, subcutaneously, intramuscularly, and intraarterially. Drugs can be administered intravenously, which ensures that they all enter the systemic circulation and start working right away. The systemic circulation is reached by intramuscular or subcutaneous injections, which have the drawbacks of low bioavailability and slow effectiveness. The primary use of arterial injection is blood transfusion for serious sickness. To provide therapeutic effects, the chitosan nanodrugs are administered via parenteral injection (Li et al., 2018).
To release doxorubicin when needed, pH-responsive nanoparticles were created that responded gradually. After being injected into the tumor-bearing animals, the DOX-loaded NPs could respond sequentially to extracellular and intracellular pH. The nanoparticles contained pH-responsive dimethylmaleic acid and urocanic acid. The tumor tissues significantly absorbed the DOX-loaded NPs in the somewhat acidic extracellular environment of the tumor. Then, the acidic endo/lysosome environment caused NPs to release DOX as necessary. The volume and pace of DOX accumulation in tumor tissue were significantly higher for the stepwise pH-responsive NPs than for free DOX. Additionally, they created gradual pH-/reduction-responsive nanoparticles for regulated DOX release by injection into the tail vein (Chen et al., 2017).
Since it treats severe or sudden sickness more quickly, injection administration has clear advantages in emergency situations. Another study evaluated the strong immunity against intranasal Chlamydia psittaci, which is induced by intranasal immunization with inactivated chlamydial elementary bodies formulated in VCG-chitosan nanoparticles (Zuo et al., 2021). However, the use of injections can have certain negative side effects, such as vascular injury, skin damage, and severe bacterial and viral infections (Kwon et al., 2017).
8 Mechanisms of Action
8.1 Plant Innate Immunity Booster
Over time, plants have evolved to defend themselves through various dynamic responses, such as the production of defense enzymes and antioxidants to combat stress and pathogen invasion. Despite not being specific to diseases and pathogens, they offer the plants broad protection (Iriti et al., 2006; Iriti & Faoro, 2009). This wonderful mechanism in plants is proven to be naturally elicited by chitosan through various means such as phenolic compound accumulation, synthesis of the cell wall, reactive oxygen species (ROS) generation, and gene regulation (Kumaraswamy et al., 2018).
It has been proven that negatively charged genetic materials exhibit elevated affinity for positively charged chitosan, which ultimately results in gene regulation at the chromatin level (Isaac et al., 2009; Liu et al., 2005; Xing et al., 2014). In the case of pathogen entry, chitosan competes with nuclear proteins for attachment to DNA (Hadwiger, 2008). The plant pattern recognition receptor recognizes chitosan as a pathogen-associated molecular pattern, and hence, chitosan induces receptor-like kinase and MAP kinase pathways. On recognition by receptors, chitosan further induces increased production of ROS against pathogens, upregulates pathogen-related genes and proteins such as β 1,3 glucanase and thaumatin, and increases the amounts and accumulation of NO, phenols, flavonoids, cytosolic Ca2+, jasmonic acid, and abscisic acid. NO in particular leads to enhanced expression of various antioxidants and defense enzymes, including superoxide dismutase (SOD), catalase (CAT), peroxidase (POD), phenylalanine ammonia lyase (PAL), and polyphenol oxidase (PPO), due to regulation of gene expression, which in turn regulates protein expression and function (Fig. 11.11) (Hadwiger, 2013; Iriti & Faoro, 2009; Iriti & Varoni, 2014). There was a twofold to fourfold increase in the above-mentioned enzyme production in treated plants in comparison to control plants (Kumaraswamy et al., 2018). Neutralization of ROS to reduce chances of oxidative stress in plants during pathogenic entry is owed to the increase in SOD, POD, and CAT due to increased gene expression, while cell wall reinforcement is seen due to elevated production of lignin, suberin, and melanin caused by the increased production of POD, PAL, and PPO (Bruce & West, 1989; Gómez-Vásquez et al., 2004; Kuźniak & Urbanek, 2000). Notably, chitin derived from fungal cell walls exhibited higher activity of chitinase (Sathiyabama & Charles, 2015). Therefore, chitosan ultimately boosts plant innate dynamic immunity in the plant systems on which it is applied through an increase in diverse reactive species scavenging enzymes, antioxidants, and cell wall thickening agents.
The effects of chitosan nanoparticles on plant cells are complex and vary depending on the nanoparticles’ concentration, size, and surface features, as well as the plant species and environmental conditions. Depending on the dose and length of exposure, chitosan NPs can have both helpful and negative effects on plant cells. As a result, it is critical to thoroughly assess the potential impacts of chitosan NPs on plant cells as well as optimize their concentration and administration methods for various crops and growing environments
8.2 Plant Growth Enhancer
Plant growth can be stimulated by various methodologies, including a few that are primarily enhanced by the application of chitosan to plants. Chitosan creates a suitable environment for plants by providing ample amounts of micronutrients and antioxidant enzymes for their development. The most extensively researched application techniques for chitosan are foliar application, soil amendment, and seed treatment (Bittelli et al., 2001; Choudhary et al., 2017; Guan et al., 2009).
Remarkably, the nanosize of chitosan nanomaterials in synergy with its net positive charge makes it easy for attachment onto plant surfaces and penetration into plant systems, which ultimately results in improved seed germination, although it is also contributed by the production of antioxidant enzymes during the germination stage to scavenge reactive species (Anusuya & Banu, 2016; Kumaraswamy et al., 2018; Saharan et al., 2015; Nguyen Van et al., 2013). Chitosan is seen to degrade and mobilize food reserves through the activation of hydrolytic enzymes such as α-amylase, which make nutrients required for plant growth more available and are supplemented by enhanced root cell division and the activation of auxin and cytokinin, which help in the uptake of those immobilized nutrients (Dzung et al., 2011; John et al., 1997). Chitosan shows the ability to enhance plant development and growth even under diseased conditions. Bulk chitosan demonstrates decreased solubility in aqueous medium and requires acidic conditions, but this in turn, may cause cytotoxic effects in plants (Saharan et al., 2016); but when coupled with copper (Cu), chitosan-Cu nanoparticles have been studied to improve plant growth by a noticeable extent owing to copper’s crucial role in electron transfer and its strong fungicidal activity (Mujtaba et al., 2020). In addition to these enhanced features, the slow release of Cu from chitosan prevents any possible toxicity occurring in seeds due to the Cu ions (Kumaraswamy et al., 2018). Chitosan also increases nitrogen, potassium, and phosphorous contents in plants after application. It also increases the osmotic pressure of the stomata, which leads to greater opening of stomata. In addition to this, chitosan also increases leaf area and chlorophyll content, which all synergistically increase photosynthesis in plants (Kumaraswamy et al., 2018).
These bioregulatory and bioenhancing activities make chitosan an excellent candidate for plant growth promotion, and in combination with other growth-enhancing factors, the effect on plants only seems to get stronger.
9 Future Directions and Challenges
Global agriculture has been facing numerous issues due to an increase in population, the use of more agrochemicals, nutrient deficiency, and climate change. An emerging alternative for maintaining food safety and the sustainability of agricultural production systems is biopolymer-based nano-delivery systems. One such approach that can fulfill the huge demand for food supply in the agricultural field involves chitosan-based nanofertilizers. This next-generation nanofertilizer exhibits a regulated, gradual release of encapsulated materials that can release their active components into the environment (Yahya, 2018). The higher surface charge property and the presence of functional groups on chitosan nanomaterials can be exploited for targeted delivery of nutrients in the subcellular organelles of plants. Delivery of macronutrients to various plant parts can also be possible via the targeted interaction of guiding peptides with the OH−/NH3+ groups in chitosan NMs (Santana et al., 2020). Another future prospect of chitosan nanofertilizers is complementing them with features such as supplying nutrients based on biotic and abiotic stress conditions. Therefore, chitosan nanofertilizers would help combat climate change while increasing crop yield, reducing carbon emissions, and leading to a balanced ecosystem.
Despite being both economically and environmentally sustainable, chitosan nanofertilizers face challenges to be delivered as next-generation fertilizers for agricultural applications. It is currently difficult to tailor chitosan biopolymer into useful nanoforms. The availability of raw materials (mainly chitin/chitosan) for the industrial-scale synthesis of chitosan NMs needs to be ensured, along with the scaling up of the process for large-scale distribution. Once these challenges are addressed, the production of commercially viable chitosan nanofertilizers using appropriate techniques can be done easily, leading to a future where even limited usage of such fertilizers would generate the desired higher agricultural yield.
10 Conclusion
The use of conventional fertilizers has led to harmful impacts on crop yield and on the environment. Several literature studies have proven that next-generation fertilizers, primarily chitosan-based nanofertilizers, have been able to fill this gap. Chitosan, a biopolymer that has biophysical properties that can be easily modified, has varied applications in agricultural, biomedical, pharmaceuticals, and other applied fields. Chitosan nanoparticles have gained prominence in various scientific sectors over the past two decades. Numerous techniques for the formulation of these nanoforms have emerged that prove to be more eco-friendly, easily biodegradable, and lacking any hazardous chemicals. In a variety of industries, including pharma and agriculture, chitosan nanoparticles are used primarily to achieve sustained release and high loading capacity of medications or active substances (Yanat & Schroën, 2021). Several studies have shown that chitosan nanofertilizers have led to an increase in crop yield, with enhanced leaf and shoot growth in number and size. It has also contributed to plant defense mechanisms with minimal quantity of usage. In addition to reducing nutrient runoff and inducing plant antioxidant responses for improved performance under environmental stress conditions, chitosan NMs can provide nutrients to plants in a dynamic way (Prajapati et al., 2022). Despite the difficulties involved in converting and scaling up chitosan biopolymer into commercially viable suitable nanoforms, the authors anticipate that chitosan NMs can be transformed into an effective next-generation nanofertilizer technology in the agricultural domain through further research in this area.
References
Abdel-Aziz, H. M. M., Hasaneen, M. N. A., & Omer, A. M. (2016). Nano chitosan-NPK fertilizer enhances the growth and productivity of wheat plants grown in sandy soil. Spanish Journal of Agricultural Research, 14(1), e0902. https://doi.org/10.5424/sjar/2016141-8205
Abdel-Aziz, H. M. M., Soliman, M. I., Abo Al-Saoud, A. M., El-Sherbeny G.A. (2021) Waste-Derived NPK nanofertilizer enhances growth and productivity of capsicum annuum L. Plants (Basel). 2021 Jun 4;10(6):1144. https://doi.org/10.3390/plants10061144.
Abdulla, N. A., Balata, G. F., El-ghamry, H. A., & Gomaa, E. (2021). Intranasal delivery of Clozapine using nanoemulsion-based in-situ gels: An approach for bioavailability enhancement. Saudi Pharmaceutical Journal, 29(12), 1466–1485. https://doi.org/10.1016/j.jsps.2021.11.006
Adisa, I. O., Pullagurala, V. L. R., Peralta-Videa, J. R., Dimkpa, C. O., Elmer, W. H., Gardea-Torresdey, J. L., & White, J. C. (2019). Recent advances in nano-enabled fertilizers and pesticides: A critical review of mechanisms of action. Environmental Science: Nano, 6(7), 2002–2030. https://doi.org/10.1039/c9en00265k
Adnan, M., Fahad, S., Zamin, M., Shah, S., Mian, I. A., Danish, S., & Datta, R. (2020). Coupling phosphate-solubilizing bacteria with phosphorus supplements improve maize phosphorus acquisition and growth under lime induced salinity stress. Plants, 9(7), 900.
Agnihotri, S. A., Mallikarjuna, N. N., & Aminabhavi, T. M. (2004). Recent advances on chitosan-based micro- and nanoparticles in drug delivery. Journal of Controlled Release, 100(1), 5–28. https://doi.org/10.1016/j.jconrel.2004.08.010
Ahmed, T., & Aljaeid, B. (2016). Preparation, characterization, and potential application of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Design, Development and Therapy, 10, 483–507. https://doi.org/10.2147/dddt.s99651
Al-Qadi, S., Grenha, A., Carrión-Recio, D., Seijo, B., & Remuñán-López, C. (2012). Microencapsulated chitosan nanoparticles for pulmonary protein delivery: In vivo evaluation of insulin-loaded formulations. Journal of Controlled Release, 157(3), 383–390. https://doi.org/10.1016/j.jconrel.2011.08.008
Anusuya, S., & Banu, K. N. (2016). Silver-chitosan nanoparticles induced biochemical variations of chickpea (Cicer arietinum L.). Biocatalysis and Agricultural Biotechnology, 8, 39–44. https://doi.org/10.1016/j.bcab.2016.08.005
Azmana, M., Mahmood, S., Hilles, A. R., Rahman, A., Arifin, M. A. B., & Ahmed, S. (2021). A review on chitosan and chitosan-based bionanocomposites: Promising material for combatting global issues and its applications. International Journal of Biological Macromolecules, 185, 832–848. https://doi.org/10.1016/j.ijbiomac.2021.07.023
Babu, S., Singh, R., Yadav, D., Rathore, S. S., Raj, R., Avasthe, R., Yadav, S., Das, A., Yadav, V., Yadav, B., Shekhawat, K., Upadhyay, P., Yadav, D. K., & Singh, V. K. (2022). Nanofertilizers for agricultural and environmental sustainability. Chemosphere, 292, 133451. https://doi.org/10.1016/j.chemosphere.2021.133451
Bandara, S., Du, H., Carson, L., Bradford, D., & Kommalapati, R. (2020). Agricultural and biomedical applications of chitosan-based nNanomaterials. Nanomaterials, 10(10), 1903. https://doi.org/10.3390/nano10101903
Barbieri, S., Buttini, F., Rossi, A., Bettini, R., Colombo, P., Ponchel, G., Sonvico, F., & Colombo, G. (2015). Ex vivo permeation of tamoxifen and its 4-OH metabolite through rat intestine from lecithin/chitosan nanoparticles. International Journal of Pharmaceutics, 491(1–2), 99–104. https://doi.org/10.1016/j.ijpharm.2015.06.021
Başaran, E., Yenilmez, E., Berkman, M. S., Büyükköroğlu, G., & Yazan, Y. (2013). Chitosan nanoparticles for ocular delivery of cyclosporine A. Journal of Microencapsulation, 31(1), 49–57. https://doi.org/10.3109/02652048.2013.805839
Ben-Moshe, T., Dror, I., & Berkowitz, B. (2010). Transport of metal oxide nanoparticles in saturated porous media. Chemosphere, 81(3), 387–393. https://doi.org/10.1016/j.chemosphere.2010.07.007
Bittelli, M., Flury, M., Campbell, G. S., & Nichols, E. J. (2001). Reduction of transpiration through foliar application of chitosan. Agricultural and Forest Meteorology, 107(3), 167–175. https://doi.org/10.1016/s0168-1923(00)00242-2
Bowman, K., & Leong, K. W. (2006). Chitosan nanoparticles for oral drug and gene delivery. International Journal of Nanomedicine, 1(2), 117–128. https://doi.org/10.2147/nano.2006.1.2.117
Bruce, R. J., & West, C. A. (1989). Elicitation of lignin biosynthesis and isoperoxidase activity by pectic fragments in suspension cultures of castor bean. Plant Physiology, 91(3), 889–897. https://doi.org/10.1104/pp.91.3.889
Bruinsmann, F. A., Pigana, S., Aguirre, T., Dadalt Souto, G., Garrastazu Pereira, G., Bianchera, A., Tiozzo Fasiolo, L., Colombo, G., Marques, M., Raffin Pohlmann, A., Stanisçuaski Guterres, S., & Sonvico, F. (2019). Chitosan-coated nanoparticles: Effect of chitosan molecular weight on nasal transmucosal delivery. Pharmaceutics, 11(2), 86. https://doi.org/10.3390/pharmaceutics11020086
Bugamelli, F., Raggi, M. A., Orienti, I., & Zecchi, V. (1998). Controlled insulin release from chitosan microparticles. Archiv der Pharmazie, 331(4), 133–138. https://doi.org/10.1002/(sici)1521-4184(199804)331:4
Bugnicourt, L., & Ladavière, C. (2016). Interests of chitosan nanoparticles ionically cross-linked with tripolyphosphate for biomedical applications. Progress in Polymer Science, 60, 1–17. https://doi.org/10.1016/j.progpolymsci.2016.06.002
Calvo, P., Remunan-Lopez, C., Vila-Jato, J. L., & Alonso, M. J. (1997). Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. Journal of Applied Polymer Science, 63(1), 125–132.
Chawla, A., Taylor, K., Newton, J., & Johnson, M. (1994). Production of spray dried salbutamol sulphate for use in dry powder aerosol formulation. International Journal of Pharmaceutics, 108(3), 233–240. https://doi.org/10.1016/0378-5173(94)90132-5
Chen, W. L., Li, F., Tang, Y., Yang, S. D., Li, J. Z., Yuan, Z. Q., Liu, Y., Zhou, X. F., Liu, C., & Zhang, X. N. (2017). Stepwise pH-responsive nanoparticles for enhanced cellular uptake and on-demand intracellular release of doxorubicin. International Journal of Nanomedicine, 12, 4241–4256. https://doi.org/10.2147/ijn.s129748
Choudhary, R. C., Kumaraswamy, R. V., Kumari, S., Sharma, S. S., Pal, A., Raliya, R., Biswas, P., & Saharan, V. (2017). Cu-chitosan nanoparticle boost defense responses and plant growth in maize (Zea mays L.). Scientific Reports, 7(1), 9754. https://doi.org/10.1038/s41598-017-08571-0
Corradini, E., de Moura, M. R., & Mattoso, L. H. C. (2010). A preliminary study of the incorparation of NPK fertilizer into chitosan nanoparticles. Express Polymer Letters, 4(8), 509–515. https://doi.org/10.3144/expresspolymlett.2010.64
Deshpande, P., Dapkekar, A., Oak, M. D., Paknikar, K. M., & Rajwade, J. M. (2017). Zinc complexed chitosan/TPP nanoparticles: A promising micronutrient nanocarrier suited for foliar application. Carbohydrate Polymers, 165, 394–401. https://doi.org/10.1016/j.carbpol.2017.02.061
Dzung, N. A., Khanh, V. T. P., & Dzung, T. T. (2011). Research on impact of chitosan oligomers on biophysical characteristics, growth, development and drought resistance of coffee. Carbohydrate Polymers, 84(2), 751–755. https://doi.org/10.1016/j.carbpol.2010.07.066
Eichert, T., Kurtz, A., Steiner, U., & Goldbach, H. E. (2008). Size exclusion limits and lateral heterogeneity of the stomatal foliar uptake pathway for aqueous solutes and water-suspended nanoparticles. Physiologia Plantarum, 134(1), 151–160. https://doi.org/10.1111/j.1399-3054.2008.01135.x
Fan, W., Yan, W., Xu, Z., & Ni, H. (2012). Formation mechanism of monodisperse, low molecular weight chitosan nanoparticles by ionic gelation technique. Colloids and Surfaces B: Biointerfaces, 90, 21–27. https://doi.org/10.1016/j.colsurfb.2011.09.042
Fang, J., Shan, X. Q., Wen, B., Lin, J. M., & Owens, G. (2009). Stability of titania nanoparticles in soil suspensions and transport in saturated homogeneous soil columns. Environmental Pollution, 157(4), 1101–1109. https://doi.org/10.1016/j.envpol.2008.11.006
Feng, C., Wang, Z., Jiang, C., Kong, M., Zhou, X., Li, Y., Cheng, X., & Chen, X. (2013). Chitosan/o-carboxymethyl chitosan nanoparticles for efficient and safe oral anticancer drug delivery: In vitro and in vivo evaluation. International Journal of Pharmaceutics, 457(1), 158–167. https://doi.org/10.1016/j.ijpharm.2013.07.079
Fonseca-Santos, B., & Chorilli, M. (2017). An overview of carboxymethyl derivatives of chitosan: Their use as biomaterials and drug delivery systems. Materials Science and Engineering: C, 77, 1349–1362. https://doi.org/10.1016/j.msec.2017.03.198
Frew, A., Weston, L. A., Reynolds, O. L., & Gurr, G. M. (2018). The role of silicon in plant biology: A paradigm shift in research approach. Annals of Botany, 121(7), 1265–1273. https://doi.org/10.1093/aob/mcy009
Garg, U., Chauhan, S., Nagaich, U., & Jain, N. (2019). Current advances in chitosan nanoparticles based drug delivery and targeting. Advanced Pharmaceutical Bulletin, 9(2), 195–204. https://doi.org/10.15171/apb.2019.023
Giri, T. K., Thakur, A., Alexander, A., Badwaik, H., & Tripathi, D. K. (2012). Modified chitosan hydrogels as drug delivery and tissue engineering systems: Present status and applications. Acta Pharmaceutica Sinica B, 2(5), 439–449.
Gómez-Vásquez, R., Day, R., Buschmann, H., Randles, S., Beeching, J. R., & Cooper, R. M. (2004). Phenylpropanoids, phenylalanine ammonia lyase and peroxidases in elicitor-challenged cassava (Manihot esculenta) suspension cells and leaves. Annals of Botany, 94(1), 87–97. https://doi.org/10.1093/aob/mch107
Göpferich, A. (1996). Mechanisms of polymer degradation and erosion. Biomaterials, 17(2), 103–114. https://doi.org/10.1016/0142-9612(96)85755-3
Guan, Y. J., Hu, J., Wang, X. J., & Shao, C. X. (2009). Seed priming with chitosan improves maize germination and seedling growth in relation to physiological changes under low temperature stress. Journal of Zhejiang University Science B, 10(6), 427–433. https://doi.org/10.1631/jzus.b0820373
Hadwiger, L. A. (2008). Pea–Fusarium solani interactions contributions of a system toward understanding disease resistance. Phytopathology, 98(4), 372–379. https://doi.org/10.1094/phyto-98-4-0372
Hadwiger, L. A. (2013). Multiple effects of chitosan on plant systems: Solid science or hype. Plant Science, 208, 42–49. https://doi.org/10.1016/j.plantsci.2013.03.007
He, P., Davis, S. S., & Illum, L. (1999). Chitosan microspheres prepared by spray drying. International Journal of Pharmaceutics, 187(1), 53–65.
Hemantaranjan, A. (2014). A future perspective in crop protection: Chitosan and its oligosaccharides. Advances in Plants & Agriculture Research, 1(1), 23–30. https://doi.org/10.15406/apar.2014.01.00006
Herdiana, Y., Wathoni, N., Shamsuddin, S., & Muchtaridi, M. (2022). Drug release study of the chitosan-based nanoparticles. Heliyon, 8(1), e08674. https://doi.org/10.1016/j.heliyon.2021.e08674
Ho, S. L., Yue, H., Tegafaw, T., Ahmad, M. Y., Liu, S., Nam, S. W., Chang, Y., & Lee, G. H. (2022). Gadolinium neutron capture therapy (GdNCT) agents from molecular to nano: Current status and perspectives. ACS Omega, 7(3), 2533–2553. https://doi.org/10.1021/acsomega.1c06603
Hoang, N. H., Le Thanh, T., Thepbandit, W., Treekoon, J., Saengchan, C., Sangpueak, R., Papathoti, N. K., Kamkaew, A., & Buensanteai, N. (2022). Efficacy of chitosan nanoparticle loaded-salicylic acid and -silver on management of cassava leaf spot disease. Polymers, 14(4), 660. https://doi.org/10.3390/polym14040660
Hu, P., An, J., Faulkner, M. M., Wu, H., Li, Z., Tian, X., & Giraldo, J. P. (2020). Nanoparticle charge and size control foliar delivery efficiency to plant cells and organelles. ACS Nano, 14(7), 7970–7986. https://doi.org/10.1021/acsnano.9b09178
Iacob, A. T., Lupascu, F. G., Apotrosoaei, M., Vasincu, I. M., Tauser, R. G., Lupascu, D., Giusca, S. E., Caruntu, I. D., & Profire, L. (2021). Recent biomedical approaches for chitosan based materials as drug delivery nanocarriers. Pharmaceutics, 13(4), 587. https://doi.org/10.3390/pharmaceutics13040587
Ibrahim, H., & El-Zairy, E. (2015). Chitosan as a biomaterial – Structure, properties, and electrospun nanofibers. In V. Bobbarala (Ed.), Concepts, compounds and the alternatives of antibacterials. InTech. https://doi.org/10.5772/61300
Ibrahim, E. A., & Ramadan, W. A. (2015). Effect of zinc foliar spray alone and combined with humic acid or/and chitosan on growth, nutrient elements content and yield of dry bean (Phaseolus vulgaris L.) plants sown at different dates. Scientia Horticulturae, 184, 101–105. https://doi.org/10.1016/j.scienta.2014.11.010
Iriti, M., & Faoro, F. (2009). Chitosan as a MAMP, searching for a PRR. Plant Signaling & Behavior, 4(1), 66–68. https://doi.org/10.4161/psb.4.1.7408
Iriti, M., & Varoni, E. M. (2014). Chitosan-induced antiviral activity and innate immunity in plants. Environmental Science and Pollution Research, 22(4), 2935–2944. https://doi.org/10.1007/s11356-014-3571-7
Iriti, M., Sironi, M., Gomarasca, S., Casazza, A., Soave, C., & Faoro, F. (2006). Cell death-mediated antiviral effect of chitosan in tobacco. Plant Physiology and Biochemistry, 44(11–12), 893–900. https://doi.org/10.1016/j.plaphy.2006.10.009
Isaac, J., Hartney, S. L., Druffel, K., & Hadwiger, L. A. (2009). The non-host disease resistance response in peas; alterations in phosphorylation and ubiquitination of HMG A and histones H2A/H2B. Plant Science, 177(5), 439–449. https://doi.org/10.1016/j.plantsci.2009.07.007
Jakhar, A. M., Aziz, I., Kaleri, A. R., Hasnain, M., Haider, G., Ma, J., & Abideen, Z. (2022). Nano-fertilizers: A sustainable technology for improving crop nutrition and food security. NanoImpact, 27, 100411. https://doi.org/10.1016/j.impact.2022.100411
Jameela, S., Kumary, T., Lal, A., & Jayakrishnan, A. (1998). Progesterone-loaded chitosan microspheres: A long acting biodegradable controlled delivery system. Journal of Controlled Release, 52(1–2), 17–24. https://doi.org/10.1016/s0168-3659(97)00187-9
John, M., Röhrig, H., Schmidt, J., Walden, R., & Schell, J. (1997). Cell signalling by oligosaccharides. Trends in Plant Science, 2(3), 111–115. https://doi.org/10.1016/s1360-1385(97)01005-4
Juárez-Maldonado, A., Ortega-Ortiz, H., Pérez-Labrada, F., Cadenas-Pliego, G., & Benavides-Mendoza, A. (2016). Cu nanoparticles absorbed on chitosan hydrogels positively alter morphological, production, and quality characteristics of tomato. Journal of Applied Botany and Food Quality, 89, 183–189. https://doi.org/10.5073/jabfq.2016.089.023
Kalia, A., Rohini, Luthra, K., Sharma, S., Singh Dheri, G., Sachdeva Taggar, M., & Gomes, C. (2019). Chitosan-urea nano-formulation: Synthesis, characterization and impact on tuber yield of potato. Acta Horticulturae, 1255, 97–106. https://doi.org/10.17660/actahortic.2019.1255.16
Kamari, A., Pulford, I., & Hargreaves, J. (2011). Chitosan as a potential amendment to remediate metal contaminated soil – A characterisation study. Colloids and Surfaces B: Biointerfaces, 82(1), 71–80. https://doi.org/10.1016/j.colsurfb.2010.08.019
Kandav, G., Bhatt, D. C., & Jindal, D. K. (2019). Targeting kidneys by superparamagnetic allopurinol loaded chitosan coated nanoparticles for the treatment of hyperuricemic nephrolithiasis. DARU Journal of Pharmaceutical Sciences, 27(2), 661–671. https://doi.org/10.1007/s40199-019-00300-4
Kashyap, P. L., Xiang, X., & Heiden, P. (2015). Chitosan nanoparticle based delivery systems for sustainable agriculture. International Journal of Biological Macromolecules, 77, 36–51. https://doi.org/10.1016/j.ijbiomac.2015.02.039
Kaya, M., Mujtaba, M., Ehrlich, H., Salaberria, A. M., Baran, T., Amemiya, C. T., Galli, R., Akyuz, L., Sargin, I., & Labidi, J. (2017). On chemistry of γ-chitin. Carbohydrate Polymers, 176, 177–186. https://doi.org/10.1016/j.carbpol.2017.08.076
Khalifa, N. S., & Hasaneen, M. N. (2018). The effect of chitosan–PMAA–NPK nanofertilizer on Pisum sativum plants. 3 Biotech, 8(4), 193. https://doi.org/10.1007/s13205-018-1221-3
Khan, M. M., Madni, A., Torchilin, V., Filipczak, N., Pan, J., Tahir, N., & Shah, H. (2019). Lipid-chitosan hybrid nanoparticles for controlled delivery of cisplatin. Drug Delivery, 26(1), 765–772. https://doi.org/10.1080/10717544.2019.1642420
Kondal, R., Kalia, A., Krejcar, O., Kuca, K., Sharma, S. P., Luthra, K., Dheri, G. S., Vikal, Y., Taggar, M. S., Abd-Elsalam, K. A., & Gomes, C. L. (2021). Chitosan-urea nanocomposite for improved fertilizer applications: The effect on the soil enzymatic activities and microflora dynamics in N cycle of potatoes (Solanum tuberosum L.). Polymers, 13(17), 2887. https://doi.org/10.3390/polym13172887
Kubavat, D., Trivedi, K., Vaghela, P., Prasad, K., Vijay Anand, G. K., Trivedi, H., Patidar, R., Chaudhari, J., Andhariya, B., & Ghosh, A. (2020). Characterization of a chitosan-based sustained release nanofertilizer formulation used as a soil conditioner while simultaneously improving biomass production of Zea mays L. Land Degradation & Development, 31(17), 2734–2746. https://doi.org/10.1002/ldr.3629
Kumar, A., Prajapati, D., Devi, K. A., Pal, A., Choudhary, U., Dashora, A., Choudhary, J., Harish, Joshi, A., & Saharan, V. (2021). Slow-release Zn application through Zn-chitosan nanoparticles in wheat to intensify source activity and sink strength. Plant Physiology and Biochemistry, 168, 272–281. https://doi.org/10.1016/j.plaphy.2021.10.013
Kumaraswamy, R., Kumari, S., Choudhary, R. C., Pal, A., Raliya, R., Biswas, P., & Saharan, V. (2018). Engineered chitosan based nanomaterials: Bioactivities, mechanisms and perspectives in plant protection and growth. International Journal of Biological Macromolecules, 113, 494–506. https://doi.org/10.1016/j.ijbiomac.2018.02.130
Kumaraswamy, R., Saharan, V., Kumari, S., Chandra Choudhary, R., Pal, A., Sharma, S. S., Rakshit, S., Raliya, R., & Biswas, P. (2021). Chitosan-silicon nanofertilizer to enhance plant growth and yield in maize (Zea mays L.). Plant Physiology and Biochemistry, 159, 53–66. https://doi.org/10.1016/j.plaphy.2020.11.054
Kuźniak, E., & Urbanek, H. (2000). The involvement of hydrogen peroxide in plant responses to stresses. Acta Physiologiae Plantarum, 22(2), 195–203. https://doi.org/10.1007/s11738-000-0076-4
Kwon, K. M., Lim, S. M., Choi, S., Kim, D. H., Jin, H. E., Jee, G., Hong, K. J., & Kim, J. Y. (2017). Microneedles: Quick and easy delivery methods of vaccines. Clinical and Experimental Vaccine Research, 6(2), 156. https://doi.org/10.7774/cevr.2017.6.2.156
Lee, J. H., & Yeo, Y. (2015). Controlled drug release from pharmaceutical nanocarriers. Chemical Engineering Science, 125, 75–84. https://doi.org/10.1016/j.ces.2014.08.046
Li, J., et al. (2018). Chitosan-based nanomaterials for drug delivery. Molecules, 23(10), 2661.
Lichtfouse, E., Morin-Crini, N., Fourmentin, M., Zemmouri, H., do Carmo Nascimento, I. O., Queiroz, L. M., Tadza, M. Y. M., Picos-Corrales, L. A., Pei, H., Wilson, L. D., & Crini, G. (2019). Chitosan for direct bioflocculation of wastewater. Environmental Chemistry Letters, 17(4), 1603–1621. https://doi.org/10.1007/s10311-019-00900-1
Liu, W., Sun, S., Cao, Z., Zhang, X., Yao, K., Lu, W. W., & Luk, K. (2005). An investigation on the physicochemical properties of chitosan/DNA polyelectrolyte complexes. Biomaterials, 26(15), 2705–2711. https://doi.org/10.1016/j.biomaterials.2004.07.038
Liu, S., Yang, S., & Ho, P. C. (2018). Intranasal administration of carbamazepine-loaded carboxymethyl chitosan nanoparticles for drug delivery to the brain. Asian Journal of Pharmaceutical Sciences, 13(1), 72–81. https://doi.org/10.1016/j.ajps.2017.09.001
Lü, T., Chen, Y., Qi, D., Cao, Z., Zhang, D., & Zhao, H. (2017). Treatment of emulsified oil wastewaters by using chitosan grafted magnetic nanoparticles. Journal of Alloys and Compounds, 696, 1205–1212. https://doi.org/10.1016/j.jallcom.2016.12.118
Luangtana-anan, M., Opanasopit, P., Ngawhirunpat, T., Nunthanid, J., Sriamornsak, P., Limmatvapirat, S., & Lim, L. Y. (2005). Effect of chitosan salts and molecular weight on a nanoparticulate carrier for therapeutic protein. Pharmaceutical Development and Technology, 10(2), 189–196. https://doi.org/10.1081/pdt-54388
Melo, E., Aires-Barros, M., & Cabral, J. (2001). Reverse micelles and protein biotechnology. Biotechnology Annual Review, 7, 87–129. https://doi.org/10.1016/s1387-2656(01)07034-x
Mitra, S., Gaur, U., Ghosh, P., & Maitra, A. (2001). Tumour targeted delivery of encapsulated dextran–doxorubicin conjugate using chitosan nanoparticles as carrier. Journal of Controlled Release, 74(1–3), 317–323. https://doi.org/10.1016/s0168-3659(01)00342-x
Mohamady Hussein, M. A., Ulag, S., Abo Dena, A. S., Sahin, A., Grinholc, M., Gunduz, O., El-Sherbiny, I., & Megahed, M. (2021). Chitosan/gold hybrid nanoparticles enriched electrospun PVA nanofibrous mats for the topical delivery of Punica granatum L. extract: Synthesis, characterization, biocompatibility and antibacterial properties. International Journal of Nanomedicine, 16, 5133–5151. https://doi.org/10.2147/IJN.S306526
Mohammed, M., Syeda, J., Wasan, K., & Wasan, E. (2017). An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics, 9(4), 53. https://doi.org/10.3390/pharmaceutics9040053
Motakef Kazemi, N., & Salimi, A. A. (2019). Chitosan nanoparticle for loading and release of nitrogen, potassium, and phosphorus nutrients. Iranian Journal of Science and Technology, Transactions A: Science, 43(6), 2781–2786. https://doi.org/10.1007/s40995-019-00755-9
Mujtaba, M., Khawar, K. M., Camara, M. C., Carvalho, L. B., Fraceto, L. F., Morsi, R. E., Elsabee, M. Z., Kaya, M., Labidi, J., Ullah, H., & Wang, D. (2020). Chitosan-based delivery systems for plants: A brief overview of recent advances and future directions. International Journal of Biological Macromolecules, 154, 683–697. https://doi.org/10.1016/j.ijbiomac.2020.03.128
Mura, S., Seddaiu, G., Bacchini, F., Roggero, P. P., & Greppi, G. F. (2013). Advances of nanotechnology in agro-environmental studies. Italian Journal of Agronomy, 8(3), 18. https://doi.org/10.4081/ija.2013.e18
Negm, N. A., Hefni, H. H., Abd-Elaal, A. A., Badr, E. A., & Abou Kana, M. T. (2020). Advancement on modification of chitosan biopolymer and its potential applications. International Journal of Biological Macromolecules, 152, 681–702. https://doi.org/10.1016/j.ijbiomac.2020.02.196
Nguyen Van, S., Dinh Minh, H., & Nguyen Anh, D. (2013). Study on chitosan nanoparticles on biophysical characteristics and growth of Robusta coffee in green house. Biocatalysis and Agricultural Biotechnology, 2(4), 289–294. https://doi.org/10.1016/j.bcab.2013.06.001
Okamoto, Y., Yano, R., Miyatake, K., Tomohiro, I., Shigemasa, Y., & Minami, S. (2003). Effects of chitin and chitosan on blood coagulation. Carbohydrate Polymers, 53(3), 337–342. https://doi.org/10.1016/s0144-8617(03)00076-6
Oliveira, H. C., Gomes, B. C., Pelegrino, M. T., & Seabra, A. B. (2016). Nitric oxide-releasing chitosan nanoparticles alleviate the effects of salt stress in maize plants. Nitric Oxide, 61, 10–19. https://doi.org/10.1016/j.niox.2016.09.010
Özbaş-Turan, S., & Akbuğa, J. (2011). Plasmid DNA-loaded chitosan/TPP nanoparticles for topical gene delivery. Drug Delivery, 18(3), 215–222. https://doi.org/10.3109/10717544.2010.544688
Palacio, J., Agudelo, N. A., & Lopez, B. L. (2016). PEGylation of PLA nanoparticle to improve mucus-penetration and colloidal stability for oral delivery systems. Current Opinion in Chemical Engineering, 11, 14–19. https://doi.org/10.1016/j.coche.2015.11.006
Palacio-Márquez, A., Ramírez-Estrada, C. A., Gutiérrez-Ruelas, N. J., Sánchez, E., Barrios, D. L. O., Chávez-Mendoza, C., & Sida-Arreola, J. P. (2021). Efficiency of foliar application of zinc oxide nanoparticles versus zinc nitrate complexed with chitosan on nitrogen assimilation, photosynthetic activity, and production of green beans (Phaseolus vulgaris L.). Scientia Horticulturae, 288, 110297. https://doi.org/10.1016/j.scienta.2021.110297
Pereira, A., Sandoval-Herrera, I., Zavala-Betancourt, S., Oliveira, H., Ledezma-Pérez, A., Romero, J., & Fraceto, L. (2017). γ-Polyglutamic acid/chitosan nanoparticles for the plant growth regulator gibberellic acid: Characterization and evaluation of biological activity. Carbohydrate Polymers, 157, 1862–1873. https://doi.org/10.1016/j.carbpol.2016.11.073
Prajapati, D., Pal, A., Dimkpa, C., Harish, Singh, U., Devi, K. A., Choudhary, J. L., & Saharan, V. (2022). Chitosan nanomaterials: A prelim of next-generation fertilizers; existing and future prospects. Carbohydrate Polymers, 288, 119356. https://doi.org/10.1016/j.carbpol.2022.119356
Priyaadharshini, M., Sritharan, N., Senthil, A., & Marimuthu, S. (2019). Physiological studies on effect of chitosan nanoemulsion in pearl millet under drought condition. Journal of Pharmacognosy and Phytochemistry, 8(3), 3304–3307. https://www.phytojournal.com/archives/2019/vol8issue3/PartAV/8-3-350-389.pdf
Rabêlo, V. M., Magalhães, P. C., Bressanin, L. A., Carvalho, D. T., Reis, C. O. D., Karam, D., Doriguetto, A. C., Santos, M. H. D., Santos Filho, P. R. D. S., & Souza, T. C. D. (2019). The foliar application of a mixture of semisynthetic chitosan derivatives induces tolerance to water deficit in maize, improving the antioxidant system and increasing photosynthesis and grain yield. Scientific Reports, 9(1), 8164. https://doi.org/10.1038/s41598-019-44649-7
Rivera-Jaramillo, Y. A., Cadenas-Pliego, G., Benavides-Mendoza, A., Sandoval-Rangel, A., & Cabrera-De la Fuente, M. (2021). Complejo PVA-quitosán-nCu mejora el rendimiento y la respuesta de defensa en tomate. Revista Mexicana De Ciencias Agrícolas, 12(6), 970–979. https://doi.org/10.29312/remexca.v12i6.3012
Saharan, V., Sharma, G., Yadav, M., Choudhary, M. K., Sharma, S., Pal, A., Raliya, R., & Biswas, P. (2015). Synthesis and in vitro antifungal efficacy of Cu–chitosan nanoparticles against pathogenic fungi of tomato. International Journal of Biological Macromolecules, 75, 346–353. https://doi.org/10.1016/j.ijbiomac.2015.01.027
Saharan, V., Kumaraswamy, R. V., Choudhary, R. C., Kumari, S., Pal, A., Raliya, R., & Biswas, P. (2016). Cu-chitosan nanoparticle mediated sustainable approach to enhance seedling growth in maize by mobilizing reserved food. Journal of Agricultural and Food Chemistry, 64(31), 6148–6155. https://doi.org/10.1021/acs.jafc.6b02239
Santana, I., Wu, H., Hu, P., & Giraldo, J. P. (2020). Targeted delivery of nanomaterials with chemical cargoes in plants enabled by a biorecognition motif. Nature Communications, 11(1), 2045. https://doi.org/10.1038/s41467-020-15731-w
Sathiyabama, M., & Charles, R. E. (2015). Fungal cell wall polymer based nanoparticles in protection of tomato plants from wilt disease caused by Fusarium oxysporum f.sp. lycopersici. Carbohydrate Polymers, 133, 400–407. https://doi.org/10.1016/j.carbpol.2015.07.066
Sathiyabama, M., & Manikandan, A. (2018). Application of copper-chitosan nanoparticles stimulate growth and induce resistance in finger millet (Eleusine coracana Gaertn.) plants against blast disease. Journal of Agricultural and Food Chemistry, 66(8), 1784–1790. https://doi.org/10.1021/acs.jafc.7b05921
Schwab, F., Zhai, G., Kern, M., Turner, A., Schnoor, J. L., & Wiesner, M. R. (2015). Barriers, pathways and processes for uptake, translocation and accumulation of nanomaterials in plants – Critical review. Nanotoxicology, 10(3), 257–278. https://doi.org/10.3109/17435390.2015.1048326
Sen, S. K., Chouhan, D., Das, D., Ghosh, R., & Mandal, P. (2020). Improvisation of salinity stress response in mung bean through solid matrix priming with normal and nano-sized chitosan. International Journal of Biological Macromolecules, 145, 108–123. https://doi.org/10.1016/j.ijbiomac.2019.12.170
Sethi, A., Ahmad, M., Huma, T., Khalid, I., & Ahmad, I. (2021). Evaluation of low molecular weight cross linked chitosan nanoparticles, to enhance the bioavailability of 5-flourouracil. Dose Response, 19(2), 15593258211025353. https://doi.org/10.1177/15593258211025353
Shahnaz, G., Vetter, A., Barthelmes, J., Rahmat, D., Laffleur, F., Iqbal, J., Perera, G., Schlocker, W., Dünnhaput, S., Augustijns, P., & Bernkop-Schnürch, A. (2012). Thiolated chitosan nanoparticles for the nasal administration of leuprolide: Bioavailability and pharmacokinetic characterization. International Journal of Pharmaceutics, 428(1–2), 164–170. https://doi.org/10.1016/j.ijpharm.2012.02.044
Sharma, G., Kumar, A., Devi, K. A., Prajapati, D., Bhagat, D., Pal, A., Raliya, R., Biswas, P., & Saharan, V. (2020). Chitosan nanofertilizer to foster source activity in maize. International Journal of Biological Macromolecules, 145, 226–234. https://doi.org/10.1016/j.ijbiomac.2019.12.155
Siepmann, J., & Siepmann, F. (2012). Modeling of diffusion controlled drug delivery. Journal of Controlled Release, 161(2), 351–362. https://doi.org/10.1016/j.jconrel.2011.10.006
Silveira, N. M., Seabra, A. B., Marcos, F. C., Pelegrino, M. T., Machado, E. C., & Ribeiro, R. V. (2019). Encapsulation of S-nitrosoglutathione into chitosan nanoparticles improves drought tolerance of sugarcane plants. Nitric Oxide, 84, 38–44. https://doi.org/10.1016/j.niox.2019.01.004
Singh, A., & Van den Mooter, G. (2016). Spray drying formulation of amorphous solid dispersions. Advanced Drug Delivery Reviews, 100, 27–50. https://doi.org/10.1016/j.addr.2015.12.010
Tokoro, A., Takewaki, N., Suzuki, K., Mikami, T., Suzuki, S., & Suzuki, M. (1988). Growth-inhibitory effect of hexa-N-acetylchitohexaose and chitohexaose against Meth-A solid tumor. Chemical and Pharmaceutical Bulletin, 36(2), 784–790. https://doi.org/10.1248/cpb.36.784
Tokumitsu, H., Ichikawa, H., Fukumori, Y., & Block, L. H. (1999). Preparation of gadopentetic acid-loaded chitosan microparticles for gadolinium neutron-capture therapy of cancer by a novel emulsion-droplet coalescence technique. Chemical and Pharmaceutical Bulletin, 47(6), 838–842. https://doi.org/10.1248/cpb.47.838
Wang, Y., Li, P., Truong-Dinh Tran, T., Zhang, J., & Kong, L. (2016). Manufacturing techniques and surface engineering of polymer based nanoparticles for targeted drug delivery to cancer. Nanomaterials, 6(2), 26. https://doi.org/10.3390/nano6020026
Wang, C., He, Z., Liu, Y., Zhou, C., Jiao, J., Li, P., Sun, D., Lin, L., & Yang, Z. (2020a). Chitosan-modified halloysite nanotubes as a controlled-release nanocarrier for nitrogen delivery. Applied Clay Science, 198, 105802. https://doi.org/10.1016/j.clay.2020.105802
Wang, W., Xue, C., & Mao, X. (2020b). Chitosan: Structural modification, biological activity and application. International Journal of Biological Macromolecules, 164, 4532–4546. https://doi.org/10.1016/j.ijbiomac.2020.09.042
Xing, K., Zhu, X., Peng, X., & Qin, S. (2014). Chitosan antimicrobial and eliciting properties for pest control in agriculture: A review. Agronomy for Sustainable Development, 35(2), 569–588. https://doi.org/10.1007/s13593-014-0252-3
Yahya, N. (2018). Efficacy of green urea for sustainable agriculture. In Green urea. Green energy and technology (pp. 99–123). Springer. https://doi.org/10.1007/978-981-10-7578-0_4
Yanat, M., & Schroën, K. (2021). Preparation methods and applications of chitosan nanoparticles; with an outlook toward reinforcement of biodegradable packaging. Reactive and Functional Polymers, 161, 104849. https://doi.org/10.1016/j.reactfunctpolym.2021.104849
Younes, I., & Rinaudo, M. (2015). Chitin and chitosan preparation from marine sources. Structure, properties and applications. Marine Drugs, 13(3), 1133–1174. https://doi.org/10.3390/md13031133
Yu, J., Wang, D., Geetha, N., Khawar, K. M., Jogaiah, S., & Mujtaba, M. (2021). Current trends and challenges in the synthesis and applications of chitosan-based nanocomposites for plants: A review. Carbohydrate Polymers, 261, 117904. https://doi.org/10.1016/j.carbpol.2021.117904
Zarb, J., Ghorbani, R., Juntharathep, P., Shotton, P., Santos, J., Wilcockson, S., Leifert, C., Litterick, A., Bain, R. A., & Wolfe, M. (2002). Control strategies for late blight in organic potato production. In UK Organic Research: Proceedings of the Coloquium of Organic Researchers Conference.
Zargar, V., Asghari, M., & Dashti, A. (2015). A review on chitin and chitosan polymers: Structure, chemistry, solubility, derivatives, and applications. ChemBioEng Reviews, 2(3), 204–226. https://doi.org/10.1002/cben.201400025
Zayed, M., Elkafafi, S., Zedan, A., & Dawoud, S. (2017). Effect of nano chitosan on growth, physiological and biochemical parameters of Phaseolus vulgaris under salt stress. Journal of Plant Production, 8(5), 577–585. https://doi.org/10.21608/jpp.2017.40468
Zhao, D., Yu, S., Sun, B., Gao, S., Guo, S., & Zhao, K. (2018). Biomedical applications of chitosan and its derivative nanoparticles. Polymers, 10(4), 462. https://doi.org/10.3390/polym10040462
Zulfiqar, F., Navarro, M., Ashraf, M., Akram, N. A., & Munné-Bosch, S. (2019). Nanofertilizer use for sustainable agriculture: Advantages and limitations. Plant Science, 289, 110270. https://doi.org/10.1016/j.plantsci.2019.110270
Zuo, Z., Zou, Y., Li, Q., Guo, Y., Zhang, T., Wu, J., He, C., & Eko, F. O. (2021). Intranasal immunization with inactivated chlamydial elementary bodies formulated in VCG-chitosan nanoparticles induces robust immunity against intranasal Chlamydia psittaci challenge. Scientific Reports, 11(1), 10389. https://doi.org/10.1038/s41598-021-89940-8
Acknowledgment
We gratefully acknowledge the support of Pratiksha Trust, Bangalore, towards this research.
Disclosure
The authors report no conflicts of interest in this work.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Nirmala, M.J., Nayak, M., Narasimhan, K., Rishikesh, K.S., Nagarajan, R. (2024). Chitosan-Based Nanofertilizer: Types, Formulations, and Plant Promotion Mechanism. In: Abd-Elsalam, K.A., Alghuthaymi, M.A. (eds) Nanofertilizers for Sustainable Agroecosystems. Nanotechnology in the Life Sciences. Springer, Cham. https://doi.org/10.1007/978-3-031-41329-2_11
Download citation
DOI: https://doi.org/10.1007/978-3-031-41329-2_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-41328-5
Online ISBN: 978-3-031-41329-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)