Abstract
Biopolymer chitosan is presently one of the most favorable natural polymers for use in micro- and nanotechnology, and it is very effective for use in agricultural sector when combined with natural functional compounds or metal nanoparticles to eliminate problems associated with the waste of destructive chemicals. In the current chapter, the primary uses of nanochitosan in agriculture and its potential uses in plant protection control are reviewed. Nanochitosan has been reported to possess antifungal and antibacterial activity and shown to be effective against seed-borne pathogens when applied as seed treatment. Chitosan behaves as a resistance elicitor inducing both local and systemic plant defense responses even when applied to the seeds. The chitosan used as soil improvement was shown to provide many benefits to different plant species by reducing pathogen attack and infection and promoting growth. The authors outline the plant protection and growth regulatory applications of chitosan nanomaterials. Current and possible utilization of chitosan nanomaterials in plant nutrition, abiotic stress management, pesticides remediation, plant transformation, and post-harvest application is also highlighted.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
13.1 Introduction
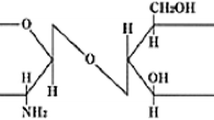
Chitosan is a nontoxic and biodegradable natural biopolymer resulting from the deacetylation of chitin. Chitosan is a linear semicrystalline polysaccharide obtained by deacetylation of chitin and composed by N-acetyl D-glucosamine and D-glucosamine units, linked through β (1→4) glycosidic bonds, Chitin or poly(β-(1→4)-N-acetyl-D-glucosamine) is prepared by some living organisms, being the structural component of the shells of crustaceous, cell walls of fungi, and exoskeletons of insects (Alves and Mano 2008; Rinaudo 2006; Mano 2008). Most of the naturally occurring polysaccharides are acidic in nature, but chitosan is a one of the naturally occurring basic polymers (Prasad et al. 2017a). Chitosan can be dissolved in water under acidic conditions after the amino protonation to discuss positive charges, gelations, and membrane-forming properties (Berscht et al. 1994). The physical and chemical properties of chitosan depend mainly on its molecular weight and degree of deacetylation (Shukla et al. 2013). Based on molecular weight chitosan can be classified into three types including; low molecular weight (LMWC), medium molecular weight (MMWC), and high molecular weight (HMWC). Some studies indicated that chitosan with shorter oligomers, such as LMWC, has greater antifungal activity (Rhoades and Roller 2000; Park et al. 2008). Chitosan-based nanoparticles (CHT NPs) can be easily prepared through self-aggregation. The appliction of nanotechnology is less discovered in agriculture in general, but some substantial research has been done in crop protection (Park et al. 2006; Jo et al. 2009; Nair et al. 2010; Sharon et al. 2010; Ghormade et al. 2011; He et al. 2011; Lamsal et al. 2011; Kim et al. 2012; Perez-de-Luque et al. 2012; Jayaseelan et al. 2013; Wani and Ahmad 2013; Saharan et al. 2013; Prasad 2014; Saharan et al. 2015; Wang et al. 2016; Saharan et al. 2016; Abd-Elsalam et al. 2017; Rubina et al. 2017; Prasad et al. 2014, 2017a; Sangeetha et al. 2017). Chitosan has garnered extensive interest because of its antimicrobial and antifungal properties (Kendra and Hadwiger 1984; Aziz et al. 2015, 2016). In future, there is a urgently need to discover chitosan biopolymer usage not only for its innovative antimicrobial agent but also for its plant defense supporter property to combat future challenges of mutating plant pathogenic population under climate change (Saharan et al. 2014; Prasad et al. 2017b). This is because nanochitosan as compared to bulk chitosan has superior physico-chemical characteristics that provide enhanced biological activities (Saharan et al. 2013; Van et al. 2013). This fact has promoted the study of CHT NPs at using them as new antimicrobial agents (Qi et al. 2004; Wazed et al. 2011). Copper, zinc, and other metal hybrids with chitosan are active components of many agrochemicals like fertilizers and pesticides and serve as cofactors of several enzymes in plant. Thus, Cu and Zn ultimately lead to higher plant growth and contribute in plant disease control (Gornik et al. 2008; Cabrera et al. 2013; Saharan et al. 2016; Rubina et al. 2017). Chitosan-based nanomaterials hold enormous promise with regard to their application in plant protection and growth (Shukla et al. 2013). Although, specific reports has been introduced to evaluate chitosan biopolymer/nanoparticle for use in plant growth and protection by using nanotechnology tools (Shukla et al. 2013; Saharan et al. 2014; Abd-Elsalam et al. 2017: Choudhary 2017; Prasad et al. 2017b). Some pioneering uses of CHT polymer include synthesis of CHT NPs as a valuable delivery system for fertilizers, herbicides, pesticides, and micronutrients for crop growth promotion by a balanced and sustained nutrition (Siddiqui et al. 2015). In addition, CHT NPs can safely deliver genetic material for plant transformation.
13.2 Synthesis Chitosan Nanomaterials
13.2.1 Synthesis Chitosan Nanoparticles
However, several methods have been used to produce chitosan nanoparticulate. Choice of method depends on the shape and particle size requirements (Agnihotri et al. 2004). Chitosan is a hydrophilic polymer with positive charge that comes from weak basic groups, which provides it specific characteristics from the technological point of view (Lopez-Leon et al. 2005). Recently, chitosan nanoparticles have received great attention in numerous fields because of their physicochemical and biological properties (Racovita et al. 2008; Shi et al. 2011). Some advantages and disadvantages of the methods used for synthesis of nanochitosan are summarized in Table 13.1.
13.2.1.1 Emulsion Cross-Linking Method
This method utilizes the reactive functional amine group of chitosan cross links with aldehyde groups of the cross-linking agent. Actually, chitosan solution is emulsified in oil phase (water-in-oil emulsion) and the aqueous droplets are stabilized using an appropriate surfactant. The stable emulsion was reacted with an appropriate cross-linking agent such as glutaraldehyde to stabilize the chitosan droplets. The nanoparticles are then washed and dried (Agnihotri et al. 2004).
13.2.1.2 Ionotropic Gelation Method
Ionotropic gelation method is the modified method linking multiphase for the production of biopolymeric oil-core microcapsules due to its nontoxic and slight processing conditions. This technique is based on the electrostatic interactions between the chitosan amine group and a polyanion such as tripolyphosphate. In this method, chitosan is dissolved in water or in weak acidic medium. This solution is then added dropwise under continuous stirring to the solutions containing other counterions. Due to the complexation between oppositely charged species, chitosan goes through ionic gelation and precipitate to produce spherical CHT NPs (Racovita et al. 2008).
13.2.1.3 In Coacervation/Precipitation Method
In the coacervation/precipitation method , chitosan solution is sprayed into sodium hydroxide, NaOH methanol, or ethanediamine alkaline solutions using compressed air, which in turn creates coacervated chitosan droplets in the form of nanoparticles (Shi et al. 2011). Emulsion–bead combination strategy incorporates both emulsion cross-connecting and precipitation. A stable emulsion containing the aqueous chitosan solution in oil and a second emulsion containing an NaOH solution are formed. By mixing the two emulsions under high magnetic stir, droplets of each emulsion collide at random, coalesce, and lastly precipitate as small size particles (Shikata et al. 2002).
13.2.1.4 In the Reverse Micelles Technique
In the reverse micelles technique , a surfactant is dissolved in organic solvent to formulate reverse micelles. The aqueous phase containing the chitosan is mixed to this emulsion with constant vortexing and the chitosan nanoparticles forms in the core of the reverse micelles (Agnihotri et al. 2004).
13.2.1.5 Self-Assembly
Self-assembly strategy depends on cationic and hydrophobic properties of chitosan, cationic chitosan derivatives can be simply adsorbed onto the colloid surface of anionic inorganic (bentonite) suspensions because of electrostatic attraction. This strategy is described by dispersion took after by particular relationship of atoms through noncovalent interactions, including electrostatic as well as hydrophobic connections (Ichikawa et al. 2005).
13.2.2 Synthesis Chitosan Nanocomposites
Chitosan nanocomposite generally refers to chitosan polymer containing dispersed nanofillers having an average particles size less than 100 nm. Thus this composite retains exceptionally enhanced properties pertaining to both of polymer and nanoparticles. By and large, regular polymers are considered as great facilitating materials for nanoparticles especially for natural applications , because of their maintainability, eco-accommodating property, nontoxicity, biodegradability, and biocompatibility. The natural inorganic half-breed nanocomposites have been seen to be valuable in numerous fields like medication conveyance, tissue building, bundling material, covering, and sensors (Rhim et al. 2006; Di Carlo et al. 2012).The primary methods that have been utilized to deliver chitosan nanocomposites, in particular dissolvable throwing are solidify drying, layer-by-layer, and electrospinning (Moura et al. 2016).
13.2.2.1 Solvent Casting
Dissolvable throwing is a standout amongst the most widely recognized strategies for planning of CHI nanocomposites films and membranes (Caridade et al. 2013; Zheng et al. 2015). The polymer is broken down into a dissolvable and then cast onto a surface, for example, glass Petri dish. The dissolvable is accordingly permitted to dissipate at room temperature or in air stove, and from that point onward, the films/membranes are separated (Ambrosio 2009).
13.2.2.2 Freeze Drying
Stop drying method was utilized for the arrangement of exceedingly porous scaffolds by inducing thermal phase separation. Usually, the solution temperature is brought down until solid– fluid demixing happens, forming two unique stages: solidified dissolvable and polymer stage. At this point, the solidified dissolvable, through sublimation, leaves the polymeric structure framing a pore. The resultant structure can be controlled by changing the kind of polymer and its concentration (Sarmento and Neves 2012).
13.2.2.3 Layer-by-Layer Assembly
Layer-by-layer (LbL) meeting , proposed by way of Iler in 1966, is a technique able to enhance surfaces and fabricate especially ordered polymeric films and nanocomposites over extraordinary varieties of substrates (Decher 1997; Borges et al. 2014). The current technique is based on the sequential adsorption of different macromolecular components, which are attracted to each other due to electrostatic interactions, hydrogen bonding, van der Waals forces, and electron exchange, among others (Borges et al. 2014; Borges and Mano 2014). Various LbL tactics can be used to build up a multilayer film, including dip coating, spin coating, and spraying coating (Richardson et al. 2015). Due to its versatility and notable availability of building blocks (e.g., CNTs, clays, NPs, polymers), this technology permits fabrication of multilayered gadgets of any nature, length, shape, and chemical composition , assuring the improvement of nanostructures with favored geometries and functionalities (Costa and Mano 2014). Furthermore, the properties of multilayered devices may be tuned through solution pH, temperature, or ionic electricity (Borges and Mano 2014).
13.2.2.4 Electrospinning
Electrospinning represents an appropriate technique to produce fibers with diameters in the nm–μm period scale, since it permits morphology, porosity, and composition to be managed by the use of relatively unsophisticated equipment. Typically, the electrospinning process uses an electric field created between the polymer solution and the collector, which generates internal repulsive forces in the polymer solution and, at a critical point, causes the expulsion of the polymer solution in shape of fibers towards the collector (Martins et al. 2008; Teo and Ramakrishna 2006). There are three special kinds of electrospinning: wet–dry electrospinning, moist–wet electrospinning, and coaxial electrospinning , The main distinction between the first two techniques is that the moist–dry approach makes use of a risky solvent that evaporates because the fibers are spun via the collector, while the moist–wet approach spins a nonunstable solvent to a collector with a 2D solvent. Regarding the final technique, it is possible to achieve fibers with a center-sheath shape, as two exclusive components may be spun at the same time (Zheng et al. 2015).
13.2.2.5 Template Polymerization
In the present method , chitosan is firstly dissolved in an acrylic monomer solution below magnetic stirring. Due to the electrostatic interplay, the negatively charged acrylic monomers align along the chitosan molecules. After whole dissolution of chitosan, the polymerization is commenced through adding potassium persulfate initiator (K2S2O8) underneath stirring at 70 °C. The entire polymerization ends in the appearance of an opalescent answer, indicating the nanoparticles formation (Fang et al. 2009; Shi et al. 2011).
13.3 Nanochitosan Characterization
13.3.1 X-Ray Powder Diffraction
X-ray Powder Diffraction (XRPD) analysis was performed to confirm the crystalline structures of magnetite present in chitosan/Fe3O4 nanocomposites, The samples were analyzed by X-ray powder diffractometer Xpert Pro MPD (Panalytical) using Bragg–Brentano geometry in the range 15–70° with a rate of 1° min−1. CuK _ radiation (k = 1.54059 Å) was used and the tube operated at 40 kV and 30 mA (Freire et al. 2016).
13.3.2 Fourier Transform Infrared Spectroscopy
Fourier Transform Infrared Spectroscopy (FTIR) evaluation was carried out in a PerkinElmer 2000 spectrophotometer used to report spectra within the range between 4000 and 400 cm−1. In previous measurements, the samples were dried and level-headed to be powdered and pressed (∼10 mg of sample to 100 mg of KBr) in disk format (Freire et al. 2016).
13.3.3 Transmission Electron Microscopy (TEM)
In Transmission Electron Microscopy (TEM) , the snap shots were recorded through a JEOL JEM-1400 electron microscope operating at an accelerating voltage of 120 kV. The samples had been prepared by way of diluting nanoparticles dispersion in distillated water. Then, one droplet of the sample was placed on 300-mesh carbon-covered copper grids and dried overnight under ambient conditions. The size distribution was decided by means of 50 randomly selected debris in exclusive regions of the increasing TEM micrograph (Freire et al. 2016).
13.3.4 Thermogravimetric Analysis
Thermogravimetric analysis (TGA) is a useful method for examining thermal stability of materials as well as for compositional information. Five miligram of nanoparticles were executed in nitrogen atmosphere by employing a Thermogravimetric Analyzer Q50 V20. The lack of mass was monitored heating up samples from 25 to 900 °C in a fee of 10 °C min−1. The zero time for the thermal degradation observe became taken after temperature stabilization (Freire et al. 2016).
13.3.5 Dynamic Light Scattering
Dynamic light scattering (DLS) was used for the size of particle length, polydispersity index (PDI), and zeta capacity of NPs via Zetasizer model PSS0012–22 (Malvern, UK). The evaluation was completed at a scattering angle of 90° at RT. Sample was accurately diluted with distilled water prior to size (Kheiri et al. 2016).
13.4 Application of Nanochitosan in Plant Protection
Natural defense reaction of plants against pathogenesis depends upon early detection of pathogens. In fact, during the process of evolution, plant researchers have evolved numerous techniques to combat some plant pathogens. This induction of herbal protection response consists of over expression of various defense-associated genes and enzymes, increased accumulation of phenolic compounds, cellular wall synthesis, etc. (Sánchez et al. 2009; Chandra et al. 2015). Recently, chitosan nanoparticles have shown significant antimicrobial activity against fungal plant pathogens (Saharan et al. 2013). Chitosan nanoparticles serve as the precise choice for the control of pests which might eliminate the toxic pesticide use and additionally enhances the yield of soybean (Kendra and Hadwiger 1984). Application of chitosan-based nanostructures in plant protection is shown in Fig. 13.1 and Table 13.2.
13.4.1 Antimicrobial
Chitosan nanoparticles specific extra affinity toward pathogen’s outer membrane and therefore without difficulty enter into the pathogens’ cell (Van et al. 2013). Several research confirmed that chitosan isn’t best an antimicrobial agent however additionally an effective elicitor of plant systemic received resistance to pathogens (Sharp 2013; Katiyar et al. 2015; Xing et al. 2014), enhancer and regulator of plant growth, development and increase crop yield (Gornik et al. 2008; Cabrera et al. 2013; Wang et al. 2016). Chitosan NPs treatment of leaves and seeds produced significant improvement in the plant growth and innate immune response through induction of defense enzyme activity, upregulation of defence related genes including that of several antioxidant enzymes as well as elevation of the levels of total phenolics (Chen et al. 2014; Chandra et al. 2015).
However, very few reports have been conducted on the biological activity of chitosan nanoparticles to control of plant pathogenic bacteria. An in vitro observe in which chitosan nanoparticles and chitosan nanocomposites with lime essential oil and thyme critical oil was accomplished. The effects indicated an inhibition of the Pectobacterium carotovorum via the treatment the usage of chitosan nanocomposites with thyme essential oil (Sotelo-Boyás et al. 2016). Concentration 1000 ppm of Cu-chitosan NPs have antibacterial effect against Pseudomonas syringae pv. glycinea that cause bacterial blight of soybean (Swati et al. 2017).
Chitosan was proven to inhibit the systemic propagation of viruses and viroids throughout the plant and to enhance the host’s hypersensitive response to infection (Pospieszny et al. 1991; Faoro et al. 2001; Chirkov 2002). The degree of suppression of viral infections diverse based on the type of chitosan molecular weight (Kulikov et al. 2006). However, not one of the studies that investigated this effect has in reality proven the capacity of chitosan in absolutely inactivating viruses or viroids. Most literature i.e, (Kulikov et al. 2006) Reported on the inactivation of replication, which cause the stoppage of multiplication and spread. This will be linked to the reality that upon penetration into plant tissues, chitosan nanoparticles tightly bind nucleic acids and purpose a ramification of damages and selective inhibitions. Diverse time requirements by means of chitosan remedies for improvement of most appropriate defense stimulation ability was recorded in advance research in specific plant-pathogen systems wherein tobacco-TMV interactions (Zhao et al. 2007).
13.4.2 Antifungal
Chitosan NP were stated to have antifungal activity against specific plant pathogens. Nanoparticles on their own can negotiate mobile partitions and membranes at some distance more effectively than compared to the middle molecules they are organized from. This partially explains why chitosan NPs were discovered to demonstrate higher immune stimulation in comparison to chitosan itself (Chandra et al. 2015). Chitosan can effectively inhibit the development of phytopathogenic fungi at different life cycle ranges. For instance, chitosan completely inhibited spore germination, germ tube elongation, and mycelial growth of Alternaria kikuchiana Tanaka and Physalospora piricola Nose at 5 g/ L in vitro assay (Meng et al. 2010). In the pear fruit, treatments with chitosan reduced the ailment prevalence and inhibited the lesion growth as a result of the two aforementioned phytopathogenic fungi (Meng et al. 2010). The antifungal effect of rhodamine-labeled chitosan on Fusarium oxysporum was greater than on Pochonia chlamydosporia. Chitosan penetrated cell membranes of F. oxysporum by plasma membrane permeabilization, resulting in cell lysis (Palma-Guerrero et al. 2010). In commercial wine grapes, chitosan efficaciously inhibited growth of Botrytis cinerea in liquid and suppressed gray mold on detached grapevine leaves and bunch rot (Reglinski et al. 2010). The chitosan TPP NPs with sizes between 80 nm and 20 nm were evaluated for their antifungal efficacy against Aspergillus parasiticus, and an improved antifungal potential was found in comparison to the chitosan solution (Cota-Arriola et al. 2013). The antifungal activity of chitosan nanoparticles in controlling both A. niger and F. solani was recorded (Ing et al. 2012). Chitosan–silver nanoparticles were investigated for control of seed-borne pathogens in chickpea. Significant antifungal activity against Aspergillus flvus, R. solani, and A. alternate was evaluated (Kaur et al. 2012). Chitosan has strong antifungal activity against Rhizoctonia solani in rice. Two kinds of acid-soluble chitosan (with different degrees of deacetylation) caused a 60–91% inhibition in mycelial growth, 31–84% inhibition of disease incidence, and 66–91% inhibition in lesion length (Liu et al. 2012). The antifungal impact of chitosan on fungal growth of Rhizopus sp., C. capsici, C. gloeosporioides, and A. niger in seeds of chilli pepper (Capsicum sp.) was confirmed, showing at the least mycelial increase of 2.8, 2.2, 2.4, and 5.5 mm, respectively (Chookhongkha et al. 2013).
The antifungal properties of chitosan nanoparticles with silver functionalized with 4(E)-2-(3-hydroxynaphthalene-2-yl) diazen-1-yl) and benzoic acid for controlling A. flavus and Aspergillus terreus was evaluated. The fungal increase inhibition was proven between 20.2 and 27.0 mm, respectively (Mathew and Kuriakose 2013). Chitosan nanoparticles displayed robust inhibition of mycelial increase of M. phaseolina. A maximum 87.6% inhibition degree was recorded at 0.1% of chitosan nanoparticles, so specifically chitosan and Cu-chitosan nanoparticles proved their uniform size and stability which may additionally make a contribution to their higher antifungal efficacy against both of A. Alternata, M. phaseolina and R. solani in vitro assay. Cu-chitosan nanoparticles also confirmed maximum inhibition rate of spore germination of A. alternate (Saharan et al. 2013). Chitosan nanoemulsion was used for controlling C. gloeosporioides in vitro; the effects showed that the low molecular weight chitosan at a concentration of 1.0% had the best results in phrases of inhibition of conidial germination of the fungus (Zahid et al. 2013). The effect of peppermint vital oil (Mentha piperita) encapsulated in chitosan nanogels with cinnamic acid on A. flavus was studied. Inhibitory effect at the mycelial growth of the fungus at a concentration of 800 mg/mL was observed in vitro (Beyki et al. 2014). The antifungal impact of chitosan combined with silver for controlling C. gloeosporioides was evaluated using mango fruit, the varied concentrations was decreased the anthracnose by means of 45.7 and 71.3%, respectively (Chowdappa et al. 2014).
The polyethylene terephthalate punnets containing thyme oil and wrapped with chitosan/boehmite nanocomposite lidding films drastically reduced the incidence and severity of brown rot because of Monilinia laxa in artificially inoculated peach fruit (cv. Kakawa) held at 25 °C for 5 days and considerably reduced brown rot occurrence additionally at decrease temperatures (Cindi et al. 2015). Encapsulation of thyme EO in chitosan–benzoic acid nanogel has greater antimicrobial property in A. flavus for the protection of tomatoes (Khalili et al. 2015). As a broad-spectrum fungicide , primarily chitosan-based nanoparticles have been showed to be fungicidal against many pathogenic fungi. Cu–chitosan nanoparticles at 0.12% concentration caused 70.5 and 73.5% inhibition of mycelia growth in Alternaria solani and Fusarium oxysporum, respectively (Saharan et al. 2015). The management of Fusarium head blight (FHB) by using chitosan (CS) and chitosan nanoparticles (CS/NPs) has been studied. CS/NPs prevented fungal growth and CS/NPs may be a beneficial organic pesticide for controlling FHB. Spikelets treated with CS and CS/NPs showed gradual infection (Kheiri et al. 2016). The results confirmed that the percentage of disease severity reduced when CS and CS/NPs were employed before the fungus inoculation on the host (Fig. 13.2). Thus, CS and specifically CS/NPs, synthetized via an appropriate technique, can be used as biological pesticide in controlling fungal plant pathogens . Mycelial growth showed that there were considerable differences in tolerance to primarily chitosan-based nanoparticles among the various fungi examined. Plant pathogenic fungi including; N. sphaerica, B. dothidea, N. oryzae, and A. tenuissima was sensetive to O-chitosan nanoparticles, whilst G. zeae and F. culmorum was resistant. The antifungal activity of nanoparticles on four chitosan-sensitive fungi was concentration-dependent. The maximum value of antifungal index was discovered in medium containing nanoparticles at 2.0 mg/mL for each fungus (Xing et al. 2016). Synthesize and describe Cu-chitosan NCPs and evaluates its antifungal activities against Fusarium verticillioids causing publish flowering stalk rot (PFSR) disorder of maize. All collectively Cu-chitosan NCs has exquisite potential as antifungal agent against PFSR of maize in pot condition as well as in field condition (Choudhary 2017). The antifungal activity of α-chitin nanoformulations with sizes ranging from 80–100 nm diameters became evaluated on A. niger fungal increase, and they showed 87% of fungal growth inhibition (Salaberria et al. 2017).
Effect of different treatments on disease severity of FHB , 4 weeks after fungus inoculation. (a) Control (distilled water), (b) CS/NPs, (c) Control (0.5% v/v acetic acid aqueous solution), (d) CS, (e) Positive control (Tilt fungicide). (Reprinted from Kheiri et al. 2016)
Chitosan NPs have been synthesized from low-molecular-weight chitosan having better degree of acetylation was evaluated for thier efficacy aganist downy mildew disease of pearl millet resulting from Sclerospora graminicola (Siddaiah et al. 2018). This report confirmed that pearl millet seed remedy with nanochitosan particles was appreciably promoted seed germination and seedling vigor and, in addition, effectively induced systemic and durable resistance against downy mildew disease under greenhouse conditions. Nanochitosan has a broad-spectrum activity and an extensive antifungal activity on plant pathogenic fungi (Table 13.2).
13.4.3 Postharvest
Plant pathogenic bacteria negatively affect a wide variety of vital fruit and vegetables during the growing season and the duration of postharvest storage (Li et al. 2009a, b, 2010).
Traditional antimicrobials have been extensively used for decades. Recently, chitosan coating has emerged as a super alternative to chemically synthesized pesticides. In postharvest production , to prevent unwanted losses, chitosan biopolymer has emerged as a promising coating agent for end-product and vegetables due to its antimicrobial and defense eliciting properties. Some studies confirm that the in vitro growth of Pseudomonas fluorescens causing bacterial head rot of Broccoli (Li et al. 2009a, b), and Burkholderia seminalis inflicting bacterial fruit rot of apricot (Lou et al. 2011), have been markedly inhibited by chitosan under various environmental conditions. In addition, the antibacterial agent comprising 1–2%, also chitosan has accurate results in inhibiting the bacteria in the fruit and vegetable in reserving process and has inhibiting effect to the rot of fruit and vegetable. Besides, it has simple processing technology and low fee and is in accordance with the developmental path of modern antibacterial agent (Feng et al. 2009). The most efficient treatment to reduce disease occurrence has been the ones based on Cinnamomum zeylanicum essential oil (CEO)-loaded chitosan NPs. This treatment become not simplest powerful in controlling cucumber decay, however additionally in delaying disease symptoms and slowing down P. drechsleri increase during the storage stage. Uncoated cucumbers began to decay from the fourth day of storage (Mohammadi et al. 2015). Chitosan can also help to preserve the safety of fit to be eaten products. The protection of fresh cut broccoli aganist E. coli and Listeria monocytogenes was confirmed with the aid of chitosan alongside bioactive components which includes bee pollen and extracts from propolis and pomegranate. Coating of chitosan on fruits and greens induces various defense enzymes, together with phenylalanine ammonia lyase (PAL), chitinase, and glucanase. Introduction of chitosan nanofilm composites made from chitosan and other antimicrobial agents could be more powerful in controlling postharvest losses of end-product and vegetables.
13.4.4 Food Preservation
Food packaging nanoparticles with antimicrobial interest which include chitosan (Qi et al. 2004; Tan et al. 2013), chitosan nanoparticles, and different inorganics. Combination of silver nanoparticles to the polymer matrix made the nanocomposites more appealing to be used in packaging (Duncan 2011; Fernández-Saiz and Lagaron 2011; Molina and Mejía 2016). Incorporation of those substances into the polymer matrix renders it lighter, stronger, fire resistance, better thermal properties, much less permeable to gases. Development of nanocomposites (up to 5% w/w nanoparticles) has been reported (Llorens et al. 2012). Chitosan nanoparticles (89 nm) and pectin (used as plasticizer) have been added into banana puree films (Martelli et al. 2013). The chitosan derivative coating was effective in reducing the decay of green asparagus caused by F. concentricum. In vitro assay, L-chitosan and H-chitosan inhibited the radial growth of F. concentricum, with an extremely good impact at concentration of 4 mg/ml, and totally inhibited spore germination at a concentration of 0.05 mg/ml, indicating that chitosan derivatives have been either fungistatic or fungicidal. The found inhibition of degradation and augmented lignification became attributed to the fungistatic activity of chitosan and its ability to induce a protection response (Qiu et al. 2014). The use of nanoparticles might assist in manufacturing of recent meals packaging materials with advanced mechanical, barrier and antimicrobial properties to growth shelf life (Chaudhry et al. 2008; Mihindukulasuriya and Lim 2014). Incorporated ZnO nanomaterial at diverse concentrations in a chitosan polymer with neem critical oil to enhance the properties of the bionanocomposite films (Sanuja and Agalya 2015).
13.4.5 Antimicrobial Mechanism
Chitosan has been validated to be a natural molecule that induces numerous organic responses in plant life, dependent on its structure, concentration, and on species and developmental stage of the plant (Malerba and Cerana 2016). Chitosan NPs remedy of leaves and seeds produced giant development inside the plant increase and innate immune reaction via induction of defense enzyme interest, up-regulation of defense-associated genes together with that of several antioxidant enzymes as well as elevation of total phenolics (Chen et al. 2014; Chandra et al. 2015). Natural protection response of plant life depends upon early detection of pathogens during the pathogenesis. Chitosan, whilst realised to plant tissues, often agglutinate across the penetration sites and has two main effects. The first one is the isolation of the penetration plant host via the formation of a physical barrier stopping the pathogen from spreading and invading other wholesome tissues. This phenomenon resembles the abscission zones regularly found on leaves stopping several necrotrophic pathogens from spreading further. It is widely found on potato tubers for instance (El Hadrami et al. 2009).
13.4.6 Induce Resistance
Furthermore, El Hassni et al. (2004) observed the impact of chitosan in date palm in reaction to Fusarium oxysporum f. sp. albedinis , the causal agent of a fusarium wilt on this crop. Beside an instantaneous toxicity of the molecule on the fungus, the authors confirmed an enhancement of critical additives of the host resistance. When injected into the roots at different concentrations, chitosan elicited date palm peroxidase and polyphenoloxidase activities, and increased the extent of phenolic compounds. Among the accrued phenolics, there was growth in content of unique nonconstitutive hydroxycinnamic acid derivatives, regarded to be of great importance in the resistance of this plant to this vascular fusariosis. Similarly, remedy of wheat seeds with chitosan found a growth in hydroxycinnamic (i.e., p-coumaric, caffeic, and ferulic) and benzoic (i.e., benzoic, protocatechuic, and gallic) acid derivatives, key to an increase in lignin synthesis and accumulation (Reddy et al. 1999). Chitosan is thought to behave as powerful inducer, enhancing a battery of plant responses each regionally around the infection sites and systemically to alert healthy elements of the plant. These encompass early signaling events in addition to the buildup of defense-related metabolites and proteins including phytoalexins and PR-proteins (Reddy et al. 1999; El Hadrami et al. 2009; Hammerschmidt 1999; Vander et al. 1998; Wang et al. 2008). Hence, as an exogenous elicitor, chitosan can stimulate resistance in plant host by way of increasing a few defense-related enzymes activities, together with phenylalanine ammonia-lyase (PAL), peroxidase(POD), catalase (CAT), superoxide dismutase (SOD), and polyphenol oxidase (PPO) activity (Xing et al. 2015). Finally, Chandra et al. (2015) have mentioned that accumulation of chitosan NP will increase plant protection through increasing the stages of SOD and CAT. Chitosan NP binds extracellularly across the cellular wall of leaves. One of the most crucial signaling molecules is nitric oxide (NO), which is also related to a variety of physiological procedures including induction of defense mechanism in plants. Plants treated with chitosan NP showed increased degrees of NO, in comparison to nontreated plants with chitosan NP (Raho et al. 2011; Malerba et al. 2012).
Chitosan NP treated sets resulted in up-regulation of PAL leading to a higher level of phenolic compound accumulation. These accumulated compounds help in alteration to distinct environmental conditions and offer resistance against pathogen. In the presence of NADPH, ANR uses anthocyanidins as substrates to synthesize EC. EC, sooner or later, transforms into proanthocyanidins, which are broadly dispensed as plant defense compounds having great toxicity toward pathogens. High levels of flavonoid accumulation are probably an illustration of superior resistance to flowers. Chitosan NP-treated flora, higher expression of SOD and CAT was observed, resulting in increase of these enzymes. SOD and CAT are the important antioxidant enzymes involved with ROS scavenging (Chandra et al. 2015). Polyphenol oxidase, catalyzing the phenolic materials to provide lignin, is everywhere among angiosperms and said to be involved in plant protection by means of helping the formation of lignin that contributes to the reinforcement of the cell wall structure heading off the penetration of pathogen (Li and Zhu 2013). ROS, Ca2+, nitric oxide (NO), ethylene (ET), jasmonic acid (JA), salicylic acid (SA), and abscisic acid (ABA) are all engaged in chitosan-mediated sign pathway (Xing et al. 2015). More details related to antimicrobial mechanism for nanochitosin was reviewed in Chap. 11.
13.4.7 Anti-insects
Chitosan has been found to expose robust insecticidal activity in a few plant pests (Zheng et al. 2005; Rabea et al. 2005). Chitosan (i.e., N-alkyl-, N-benzylchitosans) are made to be had through chemical synthesis, their insecticidal activities are being reported using an oral larvae feeding bioassay (Rabea et al. 2005; Badawy et al. 2005). Encapsulated microcrystals of the insecticide imidacloprid (IMI) with the aid of LbL assembly the use of chitosan and sodium alginate accompanied by way of addition of photocatalytic NPs (Guan et al. 2008). The insecticide etofenprox was encapsulated the use of a nanosized chitosan carrier in three types according to a difference in release patterns by adjusting the molecular weight and concentration of chitosan. Release properties of etofenprox and its organic activity aganist Spodoptera litura suggested that such managed-release method is used as a technique for preventing loss of etofenprox, increasing its activity against the target pest (Hwang et al. 2011).
The entomopathogenic fungi Nomuraea rileyi were investigated against S. Litura, and chitosan nanoparticle coated fungal metabolite (CNPCFM) showed better pesticidal activity, as compared with Uncoated Fungal Metabolite (UFM) and Fungal Spores (FS) (Chandra et al. 2013). Chitosan nanoparticles integrated insecticidal protein beauvericin (CSNp-BV) became organized by using ionic gelation technique to enhance insecticidal activity against S. litura. Pesticidal interest found out that all lifestyles stages have been susceptible to the CSNp-BV formulation and the maximum mortality was recorded in early larval instars. CSNp-BV treatment reduced pupal and adult emergence (Bharani et al. 2014).
Chitosan (CS)-g-poly (acrylic acid) PAA nanoparticles reduced egg laying of Aphis gossypii (20.9 ± 9.1 and 28.9 ± 9.2 eggs/woman for laboratory and below semi-discipline situations, respectively) than manage (97.3 ± 4.9 and 90.34. Nine eggs/female for laboratory and below semi-subject situations, respectively (Sahab et al. 2015). Chitosan nanoparticles decreased egg laying of Callosobruchus maculatus (10.9 ± 9.9 and 19.9 ± 9. Nine eggs/female laboratory and under semi-storage conditions, respectively (Sahab et al. 2015). Under semi subject situations, the wide variety of Schistocerca gregaria had been notably reduced after the chitosan and nanochitosan remedy, the quantity of infestations with S. gregaria reduced to 29 ± 3.6 and 8 ± 1.1 individuals after 120 days of remedies (Sabbour 2016). Chapter 1 was tested the application information of nanotechnology for insect’s management.
13.4.8 Growth Promotions
During the past 100 years, chemical fertilizers and pesticides were used to face these problems and increase yield. The huge utilization of these products raised productivity significantly, but it also led to reduced biological diversity and degraded natural and agricultural systems. In addition, residue accumulation led to environmental pollution and public health problems, with development of resistant pests (Sun et al. 2012). Therefore, alternative techniques that include nanomaterials are important to address the issues of lowering the environmental impact without affecting agricultural productivity and economic viability for farmers (Ghormade et al. 2011; Kah and Hofmann 2014).
Micro-chitosan remedies have plant growth promoting activities, resulting in increased yields and plant health in numerous plants and fruits. The activation of defensive mechanisms in plant tissues with chitosan inhibited the growth of taxonomically unique pathogens (Vasyukova et al. 2001). Therefore, radicles, after penetrating the seed coatings may want to contact the NPs directly. The presence of chitosan NPs in huge quantities on the root floor should adjust the surface chemistry of the root and block the root openings and both hydraulic and nutrient uptake in roots is inhibited. Therefore, plant growth is negatively affected because of NPs (Behboudi et al. 2017). In the case of tomato seeds, Saharan et al. (2016) observed that Cu-chitosan NPs at high concentration showed more inhibitory effect on seedling growth. The seed quality of minitubers derived from chitosan treatments in vitro was improved, giving rise to field plants with increased tuber numbers and yields (Kowalski et al. 2006). Chitosan and chitooligosaccharides are as molecular signals to induce plant promoter and develop disease resistance system in plants (Bueter et al. 2013). Chitosan oligomer has large influences on biophysical feature, growth, and improvement of coffee such as increasing nutrient uptake, content material of chlorophyll and information of growth and yield (Dzung et al. 2011). Chitosan has remarkable film-forming property making it a clean agent to form a semipermeable coat at the seed surface that may keep the seed moisture and take in soil moisture which hence can promote seed germination. Moreover, it restrains the seed respiration through preventing oxygen entry, proscribing loss of CO2 and keeping high concentration of CO2 inside the film (Furbank et al. 2004). Chitosan also can increase soluble sugar content and improves the activity of protease to increase loose amino acid content (Zeng et al. 2012).
The effect of chitosan NPs on seed germination and seedling vigor has been evaluated in diverse vegetation for promotion of plant growth through increasing the uptake of vitamins and water through adjusting cellular osmotic pressure (Guan et al. 2009; Katiyar et al. 2015). Insufficient studies were carried out on using Cu/Zn chitosan NPs in seed germination and seedling growth. Wheat growth was promoted in terms of germination ability, root period, and seedling top by way of the motion of oligochitosan (Ma et al. 2014). Positive response of micro-chitosan on seed germination and seedling increase results in improvement of nanoformulation of chitosan. Chitosan nanoparticles have nanometer size and they can inter into the cells of the plants simply and improve their better bioactivities (Van et al. 2013). Chitosan nanoparticles effected strongly on nutrient uptake of the coffee seedlings in the green house condition. In the same cultivation condition, application of chitosan nanoparticles enhanced significantly the uptake of nitrogen, phosphorus, potassium, calcium and magnesium compared to the control (Van et al. 2013). In Robusta coffee nanochitosan substantially expanded chlorophyll content material, photosynthetic depth, nutrient uptake, and seedling growth (Van et al. 2013). Zn–chitosan formulation has been evaluated for growth and development in dry bean (Ibrahima and Ramadan 2015). The effect of foliar application of nanochitosan NPK fertilizer at the chemical composition of wheat grains. Foliar application of nanofertilizers showed a sizable increase in total saccharide, while induced significant decrease in protein content and nitrogen content of the wheat grains (Abdel-Aziz et al. 2018).
The growth promoting impact of micro-chitosan has been verified to be significantly lower as compared to chitosan NPs. The growth promotory effect of bulk chitosan has been recorded signifiantly lower as compared to chitosan NPs. Similarly, as compared to control and CuSO4, bulk chitosan has been reported to have higher value for all parameters except for percent germination (Saharan et al. 2016). Similar efforts are needed for nanomaterials intended for use as fertilizers, so that nanofertilizers are advanced from the current mostly pristine products easily manipulated by the test environment to more functional products. To this quit, upgrades to this point made to nanoscale nutrients to generate improved nanofertilizers consist of the ones already stated in following sections concerning surface modifications along with alginate and chitosan (Saharan et al. 2016; Abdel-Aziz et al. 2016). There is an pressing neediness to make the most chitosan-based nanoparticles alone or in conjugation with various organic and inorganic compounds. Chitosan biopolymer based totally nanoparticles have large plant growth stimulatory activity (Table 13.2).
Very few studies have implemented chitosan particles in the agricultural context including incorporation of NPK fertilizer into the chitosan nanoparticles to make fertilizer consumption more efficient (Corradini et al. 2010; Wu et al. 2008; Hasaneen et al. 2014). Nanoparticles composed with an internal coating of CHT , an outer coating of cross-linked poly(acrylic acid)/diatomite-containing urea, and a middle of water-soluble granular nitrogen (N), phosphorus (P), and potassium (K) (NPK) fertilizer showed controlled release of the nutrients with no adverse impact on the soil (Wu et al. 2008). Recently, biodegradable polymeric chitosan NPs (~78 nm) were used for controlled release of the NPK fertilizer sources, which includes urea, calcium phosphate, and potassium chloride (Corradini et al. 2010), while Hussain et al. (2012) encapsulated urea in CHT microspheres acquiring a controlled release of the nutrient.
Chitosan NPs of the mean diameter 20 nm for loading NPK fertilizers were prepared and showed that the stability of the colloidal chitosan nanoemulsion was better with the addition of nitrogen and potassium than with the addition of phosphorus because of the higher anion rate from the calcium phosphate than the anion charges from the potassium chloride and urea (Hasaneen et al. 2014). Biodegradable and biocompatible nanoparticles in a green manner originated from locally available raw materials and natural excipients addressing the said risks which will ultimately lead to development of eco-friendly nanofertilizers to release nutrients gradually in a controlled manner was produced. A herbal pass linker, Genipin was extracted from tender fruit of Gardenia jasminoides (Shantha Siri et al. 2017).
13.4.9 Pesticides Delivery and Remediation
Nano-pesticide transport systems have a series of critical advantages over their classical bulk counterparts. Chitosan has emerged as one of the most promising polymers for the green transport of agrochemicals along with micronutrients, insecticides, and herbicides in nanoparticles. Chitosan simply absorbs to plant surfaces (e.g., leaves and stems), which helps to prolong the touch time between agrochemicals and the goal absorptive surface. Chitosan nanoparticles are seen to facilitate active molecule or compound uptake through the cellular membrane. The absorption improving impact of chitosan nanoparticles improves the molecular bioavailability of the active elements contained inside the nanoparticles (Tiyaboonchai 2003).
Polyvinylpyridine and polyvinylpyridine-co-styrene nanoparticles were investigated to control release of tebuconazole and chlorothalonil fungicides for better wood maintenance (Liu et al. 2001). Boehm et al. (2003) developed strong polymeric nanospheres (135 nm) with 3.5% encapsulation rate, and apart from the low active ingredient content, this formulation yielded substantial upgrades in the bioavailability of the insecticide (RPA 107382) to crops. It has additionally been reported that aluminosilicate-crammed nanotubes stick to plant surfaces whilst the nanoscale aluminosilicate particles leach from the nanotubes and in the end stick to the surface hair of insect pests. These particles ultimately enter the body and influence certain physiological functions (Bhattacharyya et al. 2010; Kashyap et al. 2013). A new type from amphiphilic derivative of chitosan, N-(octadecanol-1-glycidyl ether)-O-sulfate chitosan was produced by Lao et al. (2010). Nanoparticulate structures based totally on herbal polysaccharides (chitosan and cyclodextrin) have been prepared for use in the ionic gelation technique and have been used by companies for botanical pesticides, including carvacrol and linalool (Campos et al. 2018).
Some authors synthesized and tested changed CHT nanoparticles to hold paraquat, the most widely used herbicide. Cea et al. (2010) included atrazine, a herbicide used for broadleaf weed control, into ethyl cellulose managed release formulations (CRFs) with the aid of solvent evaporation. Allophanic clays and nano-clay changed the matrix. All CRFs multiplied the atrazine activity and decreased leaching loss. Silva et al. (2011) verified that alginate/CHT nanoparticles regulate the discharge of the herbicide and its interaction with the soil. In addition, CHT/tripolyphosphate nanoparticles decreased paraquat toxicity (Grillo et al. 2014), improved herbicidal activity against Eichhornia crassipes was observed when paraquat was encapsulated in silver/CHT nanoparticles (Namasivayam et al. 2014). Decomposable chitosan-lactide copolymer was implemented as a hydrophobic provider for pyraclostrobin, a wide-spectrum foliar fungicide . In comparison with 25% pyraclostrobin emulsifiable listen, the NPs confirmed higher fungicidal interest against Colletotrichum gossypii (Xu et al. 2014). More recently, nanoparticles of CHT and sodium tripolyphosphate have been prepared and evaluated for suppression of Pyricularia grisea (Manikandan and Sathiyabama 2016) and oleoyl-chitosan nanoparticles have been synthesized and used to disperse antifungal products (Xing et al. 2016).
The authors concluded that these novel absorbent materials may be implemented as an alternative biocompatible and green method for pesticide removal, and also applied in water remedy systems. Bin Hussein et al. (2009) had already evolved a unique nanohybrid pesticide controlled release machine from 4-(2, four-dichlorophenoxy) butyrate and a Zn–Al-layered double hydroxide inorganic interlayer with the aid of specific methods. The bioavailability of the chiral herbicide dichlorprop to the green alga Chlorella pyrenoidosa, in the absence and presence of chitosan nanoparticles was reported by Wen et al. (2010). The capacity of debris primarily based on polymers and cyclodextrin to adsorb insecticides is indicative of their capability for use in water remediation (Liu et al. 2011). Chitosan complexes and nanoparticles of chitosan containing the chiral herbicide dichlorprop were synthesized and characterized with the aim of decreasing potential for leaching and contamination of subterranean waters (Wen et al. 2011). Celis et al. (2012) used specific methods to prepare a bionanocomposite cloth based totally on chitosan and clay (montmorillonite), used as an adsorbent for the herbicide clopyralid found in an aqueous solution or in a mixture of water and soil. A nanocomposite material of CHT and montmorillonite was used to adsorb and dispose of the herbicide clopyralid existing in water and soil (Grillo et al. 2014). Chitosan–zinc oxide (CS–ZnO) nanoparticles as an absorbent for the elimination of the pesticide permethrin from water. ZnO nanoparticles possessed an nearly spherical morphology with a size of 58 nm. Researchers investigated the influence of the quantity of absorbent, agitation time, pesticide initial concentration, and pH on the sorption of the pesticide by using CS–ZnO absorbents (Dehaghi et al. 2014). In addition, CHT/tripolyphosphate nanoparticles decreased paraquat toxicity (Grillo et al. 2014), (Namasivayam et al. 2014). Alginate/chitosan and chitosan/tripolyphosphate nanoparticles were developed for encapsulation of the herbicides imazapic and imazapyr. Comparison was made between the nanoparticle systems and the free herbicides in phases of their cytotoxicity and genotoxicity that allows to verify that the encapsulation procedure led to a reduction in toxicity (Maruyama et al. 2016). These observations delivered a clear suggestion that chitosan was able to modify the enantioselective bioavailability of the pesticide and herbicide, which could be safe for agricultural ecology and environment (Table 13.3).
13.4.10 Plant Genetic Transformation
Nanotechnology will possibly play a critical role in the improvement of genetically modified plants. The improvement of biotic and abiotic kinds of crop plant involves the transport of genetic cloth of both DNA and RNA resulting in the alteration of gene expression (Palerice and Gatehouse 2008). There are many obstacles for gene transfer to transform plant (Ghormade et al. 2011). Gene transfer is a technique for plant transformation using nanoparticle through nonviral-mediated transport vehicles including chitosan nanoparticle. And it’s better than traditional methods in plants such as Agrobacterium-mediated gene transfer, electroporation, PEG-mediated gene transfer, particle gun bombardment, etc., are costly, labor intensive and cause significant perturbation to the growth of cells. In addition, these methods have very low efficiency (0.01–20% performance). However, it has been particularly a success for genetic transformation of dicots (Sivamani et al. 2009). Biodegradable nanoparticles with common diameters of approximately 0.2 μm can be a product of chitosan and polyglutamic acid and used as microprojectiles (Lee et al. 2008). These particles had been effective for encapsulating and protecting DNA for transdermal gene transport by way of acceleration with a low-strain gene gun. Yu-qin et al. (2012) have screened the benefits of using nanoparticles over conventional companies. Firstly, nanoparticles are applicable to both monocotyledons and dicotyledonous plants and any types of organs. Secondly, this type of gene carriers can effectively overcome transgenic silencing via controlling the copies of DNA combined to nanoparticles. Thirdly, nanoparticles can be easily functionalized so as to further enhance transformation efficiency. Finally, nanoparticles-mediated multigene transformation can be achieved without involving traditional building method of complex carrier.
Biodegradable polymers like chitosan can report an advantageous charge on DNA conjugate nanomaterial surface (Li et al. 2011). Chitosan is a polymer that has been used extensively both in nucleic acid delivery and tissue engineering packages (Raftery et al. 2013). CHT nanoparticles can appropriately deliver genetic material for plant transformation (Malerba and Cerana 2016). Chitosan/DNA nanoparticles can be effectively formed by using coacervation among the definitely charged amine corporations on chitosan and negatively charged phosphate groups on DNA. However, the transfection performance of chitosan is low. The transfection performance has been shown to rely on the chitosan molecular weight, degree of deacetylation, pH of the transfecting medium, and cell kind (Mao et al. 2010). A pH of 6.8–7.0 is vital for transfection (Sailaja et al. 2013), and proof shows that DNA complexes shaped through shorter and near monodisperse chitosan oligomers (24-mer) have extra applicable properties than ultrapure chitosan and are therefore extra attractive as gene shipping systems than the traditional high molecular weight chitosans (Koping-Hoggard et al. 2003). Besides this, the degree of chitosan deacetylation additionally acts as an important aspect in chitosan–DNA nanoparticle method because it impacts DNA binding, launch, and gene transfer performance in vitro and in vivo assays (Kiang et al. 2004).
In particular, the ability of use of CHT NPs for genetic transformation is proposed by using its capability to shape, via electrostatic interactions, a complicated where DNA is included from nuclease degradation (Mao et al. 2010; PichyaIriti and Varoni 2015). Interesting effects were obtained by Wang et al. (2013), who devolped QD-labeled CHT-DNA complexes to display nanoparticle-mediated genetic transformation of cultured cells of Jatropha curcas. This approach gave an upward push to solid transformants with higher performance than other conventional strategies of gene transfer. Recent data confirmed that primarily CHT-based nanoparticles with highly positive surface coatings can passively penetrate throughout the chloroplast membrane.
Once in the chloroplast, these nanoparticles showcase both restricted diffusion and convection before attaining an irreversibly trapped state (Wong et al. 2016). These results propose that CHT nanoparticles may be used as viable molecular transporters into plastids, just like the chloroplast. Fungal resistance of transgenic potato flowers expressing thionin genes isolated from Brassicaceae species (Arabidopsis thaliana) was evaluated against the phytopathogenic fungi. Thionins inhibit the increase in vitro of approximately 20 one-of-a-kind fungal plant pathogens that include Botrytis cinerea, Fusarium spp., Phytophthora infestans, and Rhizoctonia solani (Cammue et al. 1992). It is obvious that protein extract of five replicates of transgenic potato that incorporate thionin proteins showed antifungal activity against some pathogenic fungi with reduction in mycelial growth, illustrate the inhibitory effect of protein extract of the transgenic potato cultivars on radial growth of the PDA plates of four different pathogenic fungi after incubation for 7 days at 28 °C compared with control treatment (Abdel-Razik et al. 2017).
Two thionin resistance genes have been transferred into potato cultivars using chitosan nanoparticle, and then examined the resistance of transgenic species with the fungal pathogens Alternaria alternata and Rhizoctonia solani (Abdel-Razik et al. 2017). Recently, chitosan has attracted sizeable attention for use in formulations with small interfering RNA (siRNA). Because of the cationic nature, chitosan could make complex with siRNA in nanoparticles profile. Some studies suggest the utility of chitosan nanoparticle-entrapped siRNA as a service for siRNA delivery (Ragelle et al. 2013). Zhang et al. (2010) have proven that chitosan nanoparticles successfully added dsRNA (against chitin synthase genes) in stabilized shape to mosquito larvae through feeding. Chitosan nanoparticles must verify to be effective in dsRNA delivery due to their efficient binding with RNA, protection, and the ability to penetrate through the cellular membrane. These effects really suggest that chitosan nanoparticles primarily based siRNA formulations may additionally make contributions to plant pathogen and pest control while warding off the lengthy method of conventional plant transformations. There are a few mixed consequences with reference to gene transport through chitosan nanoparticles, but given the potential benefits of chitosan nanoparticles to assist within the transfer of genetic material to design new and enhanced plant genotypes (Kashyap et al. 2015).
13.5 Future Prospects
Therefore the incorporation of natural antimicrobial compounds in based-chitosan release matrices as micro- and nanoparticles is a topic of interest for future research. This may represent a promising alternative for use in the control of bacteria, fungi and insects in pre- and put up-harvest foods, contributing to the eradication of the troubles related to the usage of chemicals on food crops (Cota-Arriola et al. 2013). However, nanotechnology can both enhance crop productivity and reduce nutrient losses. As demonstrated in the course of this chapter, the nano-chitosan have been widely used in research in order to develop new formulations with active compounds of interest in plant protection. These formulations not only release the active compound in a slow manner but also after degradation increase the crop output besides proving the water-holding capacity of the soil. Cu and Zn have traditionally been used in agrochemicals. Cu and Zn, blended with chitosan may additionally reduce the threat of hazardous agrochemicals for crop improvement and protection. Chitosan nanofertilizer assessments should be done using mixtures of nanoscale nutrients to mimic conventional fertilizer application regimens typically involving multiple nutrients applied simultaneously (e.g., NPK).
13.6 Conclusions
The role of chitosan NPs in the agricultural sector is highlighted as insecticides and growth promoters for plants, and additionally as preservatives in the course of post-harvest and packaging of agriproducts. Studies of those nanomaterials are specifically focused on components of in vitro control, so it is important to perform in situ control assessments to offer answers and alternatives to the problems that the agricultural field faces. Additionally, the numerous makes use of nanochitosan inside the plant protection turned into mentioned, beginning with the utility of it as a pesticide remediation and gene delivery for the plants and controlling post-harvest diseases. The shape of chitosan NPs and their impact is determined by their type, concentration, mixture (monomer or combined with other compounds), and response (temperature and time). However, this biopolymer requires investigation and further exploration to better understand its multifunctionality, properties, and mechanisms. Use of nanochitosan for transport of agrochemicals (insecticides, micronutrients, fertilizers, and plant growth hormones) would be the most promising discipline in the coming years for nanotechnology utility in agriculture. In conclusion, chitosan formulations may be used for more secure use of nano-agrochemicals.
References
Abdel-Aziz HMM, Hasaneen MNA, Omar AM (2018) Effect of foliar application of nano chitosan NPK fertilizer on the chemical composition of wheat grains. Egypt J Bot. https://doi.org/10.21608/EJBO.2018.1907.1137
Abdel-Razik AB, Hammad IA, Tawfik E (2017) Transformation of Thionin genes using chitosan nanoparticle into potato plant to be resistant to fungal infection. IOSR J Biotechnol Biochem 3(3):1–13
Abd–Elsalam KA, Vasil’kov AY, Said–Galiev EE, Rubina MS, Khokhlov AR, Naumkin AV, Shtykova EV, Alghuthaymi MA (2017) Bimetallic and chitosan nanocomposites hybrid with trichoderma: novel antifungal agent against cotton soil–borne fungi. Eur J Plant Pathol. https://doi.org/10.1007/s10658–017–1349–8
Agnihotri AA, Mallikarjuna NN, Aminabhavi TM (2004) Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J Control Release 100(1):5–28
Alves NM, Mano JF (2008) Chitosan derivatives obtained by chemical modifications for biomedical and environmental applications. Int J Biol Macromol 43:401–414
Ambrosio L (2009) Biomedical composites. Woodhead Publishing, Cambridge
Aziz N, Faraz M, Pandey R, Sakir M, Fatma T, Varma A, Barman I, Prasad R (2015) Facile algae-derived route to biogenic silver nanoparticles: synthesis, antibacterial and photocatalytic properties. Langmuir 31:11605–11612. https://doi.org/10.1021/acs.langmuir.5b03081
Aziz N, Pandey R, Barman I, Prasad R (2016) Leveraging the attributes of Mucor hiemalis-derived silver nanoparticles for a synergistic broad-spectrum antimicrobial platform. Front Microbiol 7:1984. https://doi.org/10.3389/fmicb.2016.01984
Badawy MEI, Rabea EI, Rogge TM, Stevens CV, Steurbaut W, Höfte M, Smagghe G (2005) Fungicidal and insecticidal activity of O-acyl chitosan derivatives. Polym Bull 54:279–289
Behboudi F, Tahmasebi SZ, Kassaee MZ, Modares Sanavi SAM, Sorooshzadeh A (2017) Phytotoxicity of chitosan and SiO2 nanoparticles to seed germination of wheat (Triticum aestivum L.) and barley (Hordeum vulgare L.) plants. Not Sci Biol 9(2):242–249
Berscht PC, Nies B, Liebendorfer A, Kreuter J (1994) Incorporation of basic ibroblast growth factor into methylpyrrolidinone chitosan leeces and determination of the in vitro release characteristics. Biomaterials 15:593–600
Beyki M, Zhaveh S, Tahere S, Rahmani-Cherati T, Abollahi A, Mansour B, Bayat Tabatabaei M, Mohsenifarc A (2014) Encapsulation of Mentha piperita essential oils in chitosan–cinnamic acid nanogel with enhanced antimicrobial activity against Aspergillus flavus. Ind Crop Prod 54:310–319
Bharani RA, Namasivayam SKR, Shankar SS (2014) Biocompatible chitosan nanoparticles incorporated pesticidal protein beauvericin (CSNp-BV) preparation for the improved pesticidal activity against major groundnut defoliator Spodoptera litura (Fab.) (Lepidoptera; Noctuidae). Int J Chem Tech Res 6:5007–5012
Bhattacharyya A, Bhaumik A, Usha Rani P, Suvra Mandal S, Epidi TT (2010) Nano- particles - a recent approach to insect pest control. Afr J Biotechnol 9(24):3489–3493
Bin Hussein MZ, Hashim N, Yahaya AH, Zainal Z (2009) Controlled release formulation of agrochemical pesticide based on 4-(2,4-dichlorophenoxy)butyrate nanohybrid. J Nanosci Nanotechnol 9:2140–2147
Boehm AL, Martinon I, Zerrouk R, Rump E, Fessi H (2003) Nanoprecipitation technique for the encapsulation of agrochemical active ingredients. J Microencapsul 20:433–441
Borges J, Mano JF (2014) Molecular interactions driving the layer-by-layer assembly of multilayers. Chem Rev 114:8883–8942
Borges J, Rodrigues LC, Reis RL et al (2014) Layer-by-layer assembly of light-responsive polymeric multilayer systems. Adv Funct Mater 24:5624–5648
Brunel F, El Gueddari NE, Moerschbacher BM (2013) Complexation of copper(II) with chitosan nanogels: Toward control of microbial growth. Carbohydr Polym 92(2):1348–1356
Bueter CL, Specht CA, Levitz SM (2013) Innate sensing of chitin and chitosan. PLoS Pathog 9(1):e1003080
Cabrera JC, Wégria G, Onderwater RCA, González G, Nápoles MC, Falcón-Rodríguez AB, Costales D, Rogers HJ, Diosdado E, González S, Cabrera G, González L, Wattiez R (2013) In: Saa Silva S et al (eds) Proc. 1st world Congresson the use of biostimulants in agriculture, Acta horticultural 1009 ISHS
Cammue BPA, De Bolle MFC, Terras FRG, Proost P, Van Damme J, Rees SB, Vanderleydenand J, Broekaert WF (1992) Isolation and characterization of a novel class of plant antimicrobial peptides from Mirabilis jalapa L. seeds. J Biol Chem 267:2228–2233
Campos EVR, Proença PLF, Oliveira JL, Melville CC, Della Vechia JF, de Andrade DJ, Fraceto LF (2018) Chitosan nanoparticles functionalized with β-cyclodextrin: a promising carrier for botanical pesticides. Sci Rep 8:2067. https://doi.org/10.1038/s41598-018-20602-y
Caridade SG, Merino EG, Alves NM, Bermudez VZ, Boccaccini AR, Manoa JF (2013) Chitosan membranes containing micro or nano-size bioactive glass particles: evolution of biomineralization followed by in situ dynamic mechanical analysis. J Mech Behav Biomed Mater 20:173–183
Cea M, Cartes P, Palma G, Mora ML (2010) Atrazine efficiency in an andisol as affected by clays and nanoclays in ethylcellulose controlled release formulations. R C Suelo Nutr Veg 10:62–77
Celis R, Adelino MA, Hermosín MC, Cornejo J (2012) Montmorillonite–chitosan bionanocomposites as adsorbents of the herbicide clopyralid in aqueous solution and soil/water suspensions. J Hazard Mater 209:21067–21076
Chandra JH, Raj LFAA, Namasivayam SKR, Bharani RSA (2013) Improved pesticidal activity of fungal metabolite from nomureae rileyi with chitosan nanoparticles. Proceedings of the international conference on advanced nanomaterials and emerging engineering technologies. Chennai. pp 387–390
Chandra S, Chakarborty N, Dasgupt A, Sarkar J, Panda K, Acharya K (2015) Chitosan nanoparticle: a positive modulator of innate immune responses in plants. Sci Rep 5:1–13
Chaudhry Q, Scotter M, Blackburn J, Ross B, Boxall A, Castle L, Aitken R, Watkins R (2008) Applications and implications of nanotechnologies for the food sector. Food Addit Contam Part A 25:241–258
Chen J, Zou X, Liu Q, Wang F, Feng W, Wan W (2014) Combination effect of chitosan and methyl jasmonate on controlling Alternaria alternata and enhancing activity of cherry tomato fruit defense mechanisms. Crop Prot 56:31–36
Chirkov SN (2002) The antiviral activity of chitosan (review). Appl Biochem Microbiol 38:1–8
Chookhongkha N, Sopondilok T, Photchanachai S (2012) Effect of chitosan and chitosan nanoparticles on fungal growth and chilliseed quality. International conference on postharvest pest and diseas e management in exporting horticultural crops-PPDM2012 973:231–237
Chookhongkha N, Sopondilok T, Photchanachai S (2013) Effect of chitosan and chitosan nanoparticles on fungal growth and chilli seed quality. Acta Hortic 973:231–237
Choudhary MK (2017) Development and evaluation of Cu chitosan nanocomposite for its antifungal activity against post flowering stalk rot (PFSR) disease of maize caused by Fusarium verticillioids (Sheldon). Ph.D. Thesis, Maharana Pratap University of Agriculture and Technology, Udaipur, India. 79 pages
Chowdappa P, Shivakumar C, Chethana S, Madhura S (2014) Antifungal activity of chitosan-silver nanoparticles composite against Colletotrichum gloeosporioides associated with mango anthracnose. Afr J Microbiol Res 81:1803–1812
Cindi MD, Shittu T, Sivakumar D, Bautista-Baños S (2015) Chitosan boehmite alumina nanocomposite films and thyme oil vapour control brown rot in peaches (Prunus persica L.) during postharvest storage. Crop Prot 72:127–131
Corradini E, De Moura M, Mattoso L (2010) A preliminary study of the incorporation of NPK fertilizer into chitosan nanoparticles. Express Polym Lett 4(8):509–515
Costa RR, Mano JF (2014) Polyelectrolyte multilayered assemblies in biomedical technologies. Chem Soc Rev 43:3453–3479
Cota-Arriola O, Cortez-Rocha MO, Ezquerra-Brauer JM, Lizardi-Mendoza J, Burgos-Hernández A, Robles-Sánchez RM (2013) Ultrastructural, morphological, and antifungal properties of micro and nanoparticles of chitosan crosslinked with sodium tripolyphosphate. J Polym Environ 21:971–980
Decher G (1997) Fuzzy nanoassemblies: toward layered polymeric multicomposites. Science 277:1232–1237
Dehaghi SM, Rahmanifar B, Moradi AM, Azar PA (2014) Removal of permethrin pesticide from water by chitosan–zinc oxide nanoparticles composite as an adsorbent. J Saudi Chem Soc 18:348–355
Di Carlo G, Curulli A, Toro RG, Bianchini C, De Caro T, Padeletti G, Zane D, Ingo GM (2012) Green synthesis of gold? Chitosan nanocomposites for caffeic acid sensing. Langmuir 28:5471–5479
Duncan TV (2011) Applications of nanotechnology in food packaging and food safety: barrier materials, antimicrobials and sensors. J Colloid Interface Sci 363(1):1–24
Dzung NA, Khanh VTP, Dung TT (2011) Research on impact of chitosan oligomer on biophysical characteristics, growth, development and drought resistance of coffee. Carbohydr Polym 84:751–755
El Hadrami A, El Hadrami I, Daayf F (2009) Suppression of induced plant defense responses by fungal pathogens. In: Bouarab K, Brisson N, Daayf F (eds) Molecular-plant microbe interactions. CABI, Wallingford Chapter 10, pp 231–268
El Hassni M, El Hadrami A, Daayf F, Chérif M, Ait Barka E, El Hadrami I (2004) Chitosan, antifungal product against fusarium oxysporum f. Sp. albedinis and elicitor of defence reactions in date palm roots. Phytopathol Mediterr 43:195–204
El-Sawy NM, Abd El-Rehim HA, Elbarbary AM, Hegazy E-SA (2010) Radiation-induced degradation of chitosan for possible use as a growth promoter in agricultural purposes. Carbohydr Polym 79:555–562
Fang H, Huang J, Ding L, Li M, Chen Z (2009) Preparation of magnetic chitosan nanoparticles and immobilization of laccase. J Wuhan Univ Technol Mater Sci Ed 24:42–47. https://doi.org/10.1007/s11595–009–1042–7
Faoro F, Sant S, Iriti M, Appiano A (2001) Chitosan-elicited resitance to plant viruses: a histochemical and cytochemical study. In: Muzzarelli RAA (ed) Chitin enzymology. Atec, Grottammare, pp 57–62
Feng J, He J, Ma Z, Wang Z, Zhang X (2009) Plant source fruit and vegetable fresh-keeping agent and its preparation method. Patent number: CN101305747–A
Fernández-Saiz P, Lagaron JM (2011) Chitosan for film and coating applications. In: Plackett D (ed) Biopolymers: new materials for sustainable films and coatings. Wiley, West Sussex, pp 87–105
Freire TM, Dutra LMU, Queiroz DC, Ricardo NMPS, Barreto K, Denardin JC, Wurm FR, Sousa CP, Correia AN, de Lima-Neto P, Fechine PBA (2016) Fast ultrasound assisted synthesis of chitosan-based magnetite nanocomposites as a modified electrode sensor. Carbohydr Polym 151:760–769
Furbank RT, White RJ, Palta A, Turner NC (2004) Internal recycling of respiratory CO2 in pods of chickpea (Cicerarietinum L.): the role of pod wall, seed coat, and embryo. J Exp Bot 55:1687–1696
Geng B, Jin Z, Li T, Qi X (2009) Preparation of chitosan-stabilized Fe(0) nanoparticles for removal of hexavalent chromium in water. Sci Total Environ 407:4994–5000
Ghormade V, Deshpande MV, Paknikar KM (2011) Perspectives for nano-biotechnology enabled protection and nutrition of plants. Biotechnol Adv 29:792–803
Gornik K, Grzesik M, Duda BR (2008) The effect of chitosan on rooting of gravevine cuttings and on subsequent plant growth under drought and temperature stress. J Fruit Ornamental Plant Resour 16:333–343
Grillo R, Pereira AES, Nishisaka CS, de Lima R, Oehlke K, Greiner R, Fraceto LF (2014) Chitosan/tripolyphosphate nanoparticles loaded with paraquat herbicide: an environmentally safer alternative for weed control. J Hazard Mater 278:163–171
Guan H, Chi D, Yu J, Li X (2008) A novel photodegradable insecticide: preparation, characterization and properties evaluation of nano-Imidacloprid. Pestic Biochem Physiol 92:83–91
Guan YJ, Hu J, Wang XJ, Shao CX (2009) Seed priming with chitosan improves maize germination and seedling growth in relation to physiological changes under low temperature stress. J Zhejiang Univ Sci B 10:427–433
Hammerschmidt R (1999) Phytoalexins: what have we learned after 60 years? Annu Rev Phytopathol 37:285–306
Hasaneen MNA, Abdel-Aziz HMM, El-Bialy DMA, Omer AM (2014) Preparation of chitosan nanoparticles for loading with NPK. Afr J Biotech 13:3158–3164
He L, Liu Y, Mustapha A, Lin M (2011) Antifungal activity of zinc oxide nanoparticles against Botrytis cinerea and Penicillium expansum. Microbiol Res 166:207–215
Higueras L, López-Carballo G, Cerisuelo JP et al (2013) Preparation and characterization of chitosan/HP-β-cyclodextrins composites with high sorption capacity for carvacrol. Carbohydr Polym 97:262–268
Hussain MR, Devi RR, Maji TK (2012) Controlled release of urea from chitosan microspheres prepared by emulsification and cross-linking method. Iran Polym J 21:473–479
Hwang IC, Kim TH, Bang SH, Kim KS, Kwon HR, Seo MJ, Youn YN, Park HJ, Yasunaga-Aoki C, Yu YM (2011) Insecticidal effect of controlled release formulations of etofenprox based on nano-bio technique. J Fac Agric Kyushu Univ 56:33–40
Ibrahima EA, Ramadan WA (2015) Effect of zinc foliar spray alone and combined with humic acid or/and chitosan on growth, nutrient elements content and yield of dry bean (Phaseolus vulgaris L.) plants sown at different dates. Sci Hortic 184:101–105
Ichikawa S, Iwamoto S, Watanabe J (2005) Formation of biocopmpatible nanoparticles by selfassembly of enzymatic hydrolysates of chitosan and carboxymethyl cellulose. Biosci Biotechnol Biochem 69:1637–1642 PMID: 16195579
Iler RK (1966) Multilayers of colloidal particles. Colloid Interf Sci J 21:569–594
Ing LY, Zin NM, Sarwar A, Katas H (2012) Antifungal activity of chitosan nanoparticles and correlation with their physical properties. Int J Biomater 2012:632698. https://doi.org/10.1155/2012/632698
Jaiswal M, Chauhan D, Sankararamakrishnan N (2012) Copper chitosan nanocomposites: synthesis, characterization, and application in removal of organophosphorous pesticide from agricultural runoff. Environ Sci Pollut Res 19:2005–2062
Jayaseelan C, Ramkumar R, Rahuman AA, Perumal P (2013) Green synthesis of gold nanoparticles using seed aqueous extract of Abelmo schusesculentus and its antifungal activity. Ind Crop Prod 45:423–429
Jo YK, Kim BH, Jung G (2009) Antifungal activity of silver ions and nanoparticles on phytopathogenic fungi. Plant Dis 93:1037–1043
Juárez-Maldonado A, Ortega-Ortiz, H, Pérez-Labrada F, Cadenas-Pliego G, Benavidez-Mendoza A (2016) Cu nanoparticle absorbed on chitosan hydrogels positively alter morphological production and quality characteristics of tomato. J Appl Bot Food Qual 89:183–189
Kah M, Hofmann T (2014) Nanopesticide research: current trends and future priorities. Environ Int 63:224–235
Kashyap PL, Kumar S, Srivastava AK, Sharma AK (2013) Myconanotechnology in agriculture: a perspective. World J Microbiol Biotechnol 29(2):191–207
Kashyap PL, Xiang X, Heiden P (2015) Chitosan nanoparticle based delivery systems for sustainable agriculture. Int J Biol Macromol 77:36–51
Katiyar D, Hemantarajan A, Sing B (2015) Chitosan as a promising natural compound to enhance potential physiological responses in plant: a review. Indian J Plant Physiol 20(1):1–9
Kaur P, Thakur R, Choudhary A (2012) An in vitro study of the antifungal activity of silver/chitosan nanoformulations against important seed borne pathogens. Int J Sci Technol Res 1:83–86
Kendra DF, Hadwiger LA (1984) Characterization of the smallest chitosan oligomer that is maximally antifungal to Fusarium solani and elicits pisatin formation by Pisum sativum. Exp Mycol 8:276–281
Khalili ST, Mohsenifar A, Beyki M, Zhaveh S, Rahmani-Cherati T, Bayat M et al (2015) Encapsulation of thyme essential oils in chitosan–benzoic acid nanogel with enhanced antimicrobial activity against Aspergillus flavus. LWT Food Sci Technol 60:502–508
Kheiri A, Moosawi Jorf SA, Malihipour A, Saremi H, Nikkhah M (2016) Application of chitosan and chitosan nanoparticles for the control of fusarium head blight of wheat (Fusarium graminearum) in vitro and greenhouse. Int J Biol Macromol 93:1261–1272
Kiang T, Wen J, Lim HW, Leong KW (2004) The effect of the degree of chitosan deacetylation on the efficiency of gene transfection. Biomaterials 25(22):5293–5301
Kim SW, Jung JH, Lamsal K, Kim YS, Min JS, Lee YS (2012) Antifungal effects of silver nanoparticles (AgNPs) against various plant pathogenic fungi. Mycobiology 40:53–58
Koping-Hoggard M, Mel’nikova YS, Varum KM, Lindman B, Artursson B (2003) Relationship between the physical shape and the efficiency of oligomeric chitosan as a gene delivery system in vitro and in vivo. J Gene Med 5:30–141
Kowalski B, Terry FJ, Herrera L, Peñalver DA (2006) Application of soluble chitosan in vitro and in the greenhouse to increase yield and seed quality of potato minitubers. Potato Res 49:167–176
Kulikov SN, Chirkov SN, Il’ina AV, Lopatin SA, Varlamov VP (2006) Effect of the molecular weight of chitosan on its antiviral activity in plants. Prikl Biokhim Mikrobiol 42(2):224–228
Lamsal K, Kim SW, Jung JH, Kim YS, Kim KS, Lee YS (2011) Inhibition effects of silver nanoparticles against powdery mildews on cucumber and pumpkin. Mycobiology 39:26–32
Lao S-B, Zhang Z-X, Xu H-H, Jiang G-B (2010) Novel amphiphilic chitosan derivatives: synthesis, characterization and micellar solubilization of rotenone. Carbohydr Polym 82:1136–1142
Lee P-W, Peng S-F, Su C-J, Mi F-L, Chen H-L, Wei M-C, Lin H-J, Sung H-W (2008) The use of biodegradable polymeric nanoparticles in combination with a low-pressure gene gun for transdermal DNA delivery. Biomaterials 29:742–751
Li SJ, Zhu TH (2013) Biochemical response and induced resistance against anthracnose (Colletotrichum camelliae) of camellia (Camellia pitardii) by chitosan oligosaccharide application. For Pathol 43:67–76. https://doi.org/10.1111/j.1439-0329.2012.00797
Li B, Wang GL, Wu ZY, Qiu W, Tang QM, Xie GL (2009a) First report of bacterial head rot of broccoli caused by Pseudomonas fluorescens in China. Plant Dis 93:12–19
Li B, Yu RR, Yu SH, Qiu W, Fang Y, Xie GL (2009b) First report on bacterial heart rot of garlic caused by Pseudomonas fluorescens in China. Plant Pathol J 25:91–94
Li B, Fang Y, Zhang GQ, Yu RR, Lou MM, Xie GL, Wang YL, Sun GC (2010) Molecular characterization of Burkholderia cepacia complex isolates causing bacterial fruit rot of apricot. Plant Pathol J 26:223–230
Li C, Guo T, Zhou D, Hu Y, Zhou H, Wang S, Chen J, Zhang Z (2011) A novel glutathione modified chitosan conjugate for efficient gene delivery. J Control Release 154:177–188
Liu Y, Yan L, Heiden P, Laks P (2001) Use of nanoparticles for controlled release of biocides in solid wood. J Appl Polym Sci 79:458–465
Liu H, Cai X, Wang Y, Chen J (2011) Adsorption mechanism-based screening of cyclodextrin polymers for adsorption and separation of pesticides from water. Water Res 45:3499–3511
Liu H, Tian WX, Li B, Wu GX, Ibrahim M, Tao ZY, Wang YL, Xie GL, Li HY, Sun GC (2012) Antifungal effect and mechanism of chitosan against the rice sheath blight pathogen, Rhizoctonia solani. Biotechnol Lett 34:2291–2298
Llorens A, Lloret E, Picouet PA, Trbojevich R, Fernandez A (2012) Metallic-based micro and nanocomposites in food contact materials and active food packaging. Trends Food Sci Technol 24:19–29
Lopez-Leon T, Carvalho ELS, Seijo B, OrtegaVinuesa JL, Bastos-Gonzalez D (2005) Physicochemical characterization of chitosan nanoparticles: Electrokinetic and stability behavior. Colloid Interf Sci J 283:344–351. https://doi.org/10.1016/j.jcis.2004.08.186
Lou MM, Zhu B, Muhammad I, Li B, Xie GL, Wang YL, Li HY, Sun GC (2011) Antibacterial activity and mechanism of action of chitosan solutions against apricot fruit rot pathogen Burkholderia seminalis. Carbohydr Res 346:1294–1301. https://doi.org/10.1016/j.carres.2011.04.042
Ma L, Li J, Yy YCM, Wang Y, Xm L, Li N (2014) Germination and physiological response of wheat (Triticum aestivum) to pre-soaking with oligochitosan. Int J Agric Biol 16:766–770
Malerba M, Cerana R (2016) Chitosan effects on plant systems. Int J Mol Sci 17:996. https://doi.org/10.3390/ijms17070996
Malerba M, Crosti P, Cerana R (2012) Defense/stress responses activated bychitosan in sycamore cultured cells. Protoplasma 249:89–98. https://doi.org/10.1007/s00709–011–0264–7
Manikandan A, Sathiyabama M (2016) Preparation of chitosan nanoparticles and its effect on detached rice leaves infected with Pyricularia grisea. Int J Biol Macromol 84:58–61
Mano JF (2008) Stimuli-responsive polymeric systems for biomedical applications. Adv Eng Mater 10:515–527
Mao S, Sun W, Kissel T (2010) Chitosan-based formulations for delivery of DNA and siRNA. Adv Drug Deliv Rev 62:12–27
Martelli MR, Barros TT, de Moura MR, Mattoso LH, Assis OB (2013) Effect of chitosan nanoparticles and pectin content on mechanical properties and water vapor permeability of banana puree films. J Food Sci 78:N98–N104
Martins A, Reis RL, Neves NM (2008) Electrospinning: processing technique for tissue engineering scaffolding. Int Mater Rev 53:257–274
Maruyama CR, Guilger M, Pascoli M et al (2016) Nanoparticles based on chitosan as carriers for the combined herbicides Imazapic and Imazapyr. Sci Rep 6:19768. https://doi.org/10.1038/srep19768
Mathew T, Kuriakose S (2013) Photochemical and antimicrobial properties of silver nanoparticle-encapsulated chitosan functionalized with photoactive groups. Mater Sci Eng C 33:4409–4415
Meng XH, Yang LY, Kennedy JF, Tian SP (2010) Effects of chitosan and oligochitosan on growth of two fungal pathogens and physiological properties in pear fruit. Carbohydr Polym 81:70–75
Mihindukulasuriya SDF, Lim LT (2014) Nanotechnology development in food packaging: a review. Trends Food Sci Technol 40:149–167
Mohammadi A, Hashemi M, Hosseini SM (2015) Chitosan nanoparticles loaded with Cinnamomum zeylanicum essential oil enhance the shelf life of cucumber during cold storage. Postharvest Biol Technol 110:203–213
Molina EB, Mejía LZ (2016) New bioactive biomaterials based on chitosan A2 – Baños B, Silvia. In: Chitosan in the preservation of agricultural commodities. Chapter 2, pp 33–64, Academic Press, Elsevier, USA
Moura D, Mano JF, Paiva MC, Alves NM (2016) Chitosan nanocomposites based on distinct inorganic fillers for biomedical applications. Sci Technol Adv Mater 17(1):626–643
Nair R, Varghese SH, Nair BG, Maekawa T, Yoshida Y, Sakthi Kumar D (2010) Nanoparticulate material delivery to plants. Plant Sci 179:154–163
Namasivayam SKR, Aruna A, Gokila G (2014) Evaluation of silver nanoparticles-chitosan encapsulated synthetic herbicide paraquat (AgNp-CS-PQ) preparation for the controlled release and improved herbicidal activity against Eichhornia crassipes. Res J Biotechnol 9:19–27
Nguyen VS, Dinh MH, Nguyen AD (2013) Study on chitosan nanoparticles on biophysical characteristics and growth of Robusta coffee in green house. Biocatal Agric Biotechnol 2:289–294
Palerice DRG, Gatehouse JA (2008) RNAi-mediated crop protection against insects. Trends Biotechnol 26:393–309
Palma-Guerrero J, López-Jiménez JA, Pérez-Berná AJ, Huang IC, Jansson HB, Salinas J, Villalaín J, Read ND, Lopez-Llorca LV (2010) Membrane fluidity determines sensitivity of fiamentous fungi to chitosan. Mol Microbiol 75:1021–1032
Park HJ, Kim SH, Kim HJ, Choi SH (2006) A new composition of nanosized silica–silver for control of various plant diseases. Plant Pathol J 22:295–302
Park Y, Kim MH, Park SC, Cheong H, Jang MK, Nah JW, Hahm KS (2008) Investigation of the antifungal activity and mechanism of action of LMWS-chitosan. J Microbiol Biotechnol 18:1729–1734
Perez-de-Luque A, Cifuentes Z, Beckstead JA, Sillero JC, Anila C, Rubio J, Ryan RO (2012) Effect of amphotericin B nanodisks on plant fungal disease. Pest Manag Sci 68:67–74
PichyaIriti M, Varoni EM (2015) Chitosan-induced antiviral activity and innate immunity in plants. Environ Sci Pollut Res 22:2935–2944
Pospieszny H, Chirkov S, Atabekov J (1991) Induction of antiviral resistance in plants by chitosan. Plant Sci 79:63–68
Prasad R (2014) Synthesis of silver nanoparticles in photosynthetic plants. J Nanopart:963961. https://doi.org/10.1155/2014/963961
Prasad R, Kumar V, Prasad KS (2014) Nanotechnology in sustainable agriculture: present concerns and future aspects. Afr J Biotechnol 13(6):705–713
Prasad R, Bhattacharyya A, Nguyen QD (2017a) Nanotechnology in sustainable agriculture: recent developments, challenges, and perspectives. Front Microbiol 8:1014. https://doi.org/10.3389/fmicb.2017.01014
Prasad R, Gupta N, Kumar M, Kumar V, Abd-Elsalam KA (2017b) Nanomaterials acts as plant defense mechanism. In: Prasad R, Kumar V, Kumar M (eds) Nanotechnology: food and environmental paradigm. Springer Pvt Ltd, Cham, pp 253–269
Qi L, Xu Z, Jiang X, Hu C, Zou X (2004) Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr Res 339:2693–2700
Qiu M, Wu C, Ren G, Liang X, Wang X, Huang X (2014) Effect of chitosan and its derivatives as antifungal and preservative agents on postharvest green asparagus. Food Chem155:105–111
Rabea EI, Badawy MEI, Rogge TM, Stevens CV, Hofte M, Steurbaut W, Smagghe G (2005) Insecticidal and fungicidal activity of new synthesized chitosan derivatives. Pest Manag Sci 61:951–960
Racovita S, Vasiliu S, Popa M, Luca C (2008) Polysaccharides based on micro-and nanoparticles obtained by ionic gelation and their applications as drug delivery systems. Rev Roum Chim 54:709–718
Raftery R, O’Brien FJ, Cryan SA (2013) Chitosan for gene delivery and orthopedic tissue engineering applications. Molecules 18:5611–5647
Ragelle H, Vandermeulen G, Préa V (2013) Chitosan-based siRNA delivery systems. J Control Release 172:207–218
Raho N, Ramirez L, Lanteri ML, Gonorazky G, Lamattina L, ten Have A, Laxalt AM (2011) Phosphatidic acid production in chitosan-elicited tomato cells, via both phospholipase D and phospholipase C/diacylglycerol kinase, requires nitric oxide. J Plant Physiol 168:534–539. https://doi.org/10.1016/j.jplph.2010.09.004
Reddy MV, Arul J, Angers P, Couture L (1999) Chitosan treatment of wheat seeds induces resistance to Fusarium graminearun and improves seed quality. J Agric Food Chem 47:1208–1216
Reglinski T, Elmer PAG, Taylor JT, Wood PN, Hoyte SM (2010) Inhibition of Botrytis cinerea growth and suppression of botrytis bunch rot in grapes using chitosan. Plant Pathol 59:882–890
Rhim JW, Hong SI, Park HM, Ng PKW (2006) Preparation and characterization of chitosan-based nanocomposite films with antimicrobialactivity. J Agric Food Chem 54:5814–5822
Rhoades J, Roller S (2000) Antimicrobial actions of degraded and native chitosan against spoilage organisms in laboratory media and foods. Appl Environ Microbiol 66:80–86
Richardson JJ, Bjornmalm M, Caruso F (2015) Multilayer assembly. Technology-driven layer-by-layer assembly of nanofilms. Science (New York, NY) 348:2348–2491
Rinaudo M (2006) Chitin and chitosan: properties and applications. Prog Polym Sci 31:603–632
Rubina RS, Vasil’kov AY, Naumkin AV, Shtykova EV, Abramchuk SS, Alghuthaymi MA, Abd–Elsalam KA (2017) Synthesis and characterization of chitosan–copper nanocomposites and their fungicidal activity against two sclerotia–forming plant pathogenic fungi. J Nanostruct Chem. https://doi.org/10.1007/s40097–017–0235–4
Sabbour MM (2016) Observations of the effect of Nano chitosan against the locust Schistocerca gregaria (Orthoptera: Acrididae). J Nanosci Nanoengin 2:28–33
Sahab AF, Waly AI, Sabbour MM, Lubna SN (2015) Synthesis, antifungal and insecticidal potential of chitosan (CS)-g-poly (acrylic acid) (PAA) nanoparticles against some seed borne fungi and insects of soybean. Int J ChemTech Res 8(2):589–598
Saharan V, Mehrotra A, Khatik R, Rawal P, Sharma SS, Pal A (2013) Synthesis of chitosan based nanoparticles and their in vitro evaluation against phytopathogenic fungi. Int J Biol Macromol 62:677–683
Saharan V, Khatik R, Choudhary MK, Mehrotra A, Jakhar S, Raliya R, Nallamuthu I, Pal A (2014) Nano-materials for plant protection with special reference to Nano chitosan. In: Proceedings of 4th annual international conference on advances in biotechnology. GSTF, Dubai, pp 23–25
Saharan V, Sharma G, Yadav M, Choudhary MK, Sharma SS, Pal A, Biswas P (2015) Synthesis and in vitro antifungal efficacy of cu–chitosan nanoparticles against pathogenic fungi of tomato. Int J Biol Macromol 75:346–353
Saharan V, Kumaraswamy RV, Choudhary RC, Kumari S, Pal A, Raliya R, Biswas P (2016) Cu-chitosan nanoparticle mediated sustainable approach to enhance seedling growth in maize by mobilizing reserved food. J Agric Food Chem 64(31):6148–6155
Saifuddin N, Nian CY, Zhan LW, Ning KX (2011) Chitosan-silver nanoparticles composite as point of-use drinking water filtration system for household to remove pesticides in water. Asian J Biochem 6:142–159
Sailaja AK, Amareshwar P, Chakravarty P (2013) Chitosan nanoparticles as a drug delivery system. Res J Pharm Biol Chem Sci 1(3):474–484
Salaberria AM, Diaz RH, Andrés MA, Fernandes SCM, Labidi J (2017) The antifungal activity of functionalized chitin nanocrystals in poly (Lactid acid) films. Anzai J, ed. Materials 10(5):546
Sánchez EA, Tiznado HME, Ojeda CAJ, Valenzuela-Quintanar AI, Troncoso-Rojas R (2009) Induction of enzymes and phenolic compounds related to the natural defence response of netted melon fruit by a bio-elicitor. J Phytopathol 157:24–32
Sangeetha J, Thangadurai D, Hospet R, Harish ER, Purushotham P, Mujeeb MA, Shrinivas J, David M, Mundaragi AC, Thimmappa AC, Arakera SB, Prasad R (2017) Nanoagrotechnology for soil quality, crop performance and environmental management. In: Prasad R, Kumar M, Kumar V (eds) Nanotechnology. pp 73–97, Springer Nature, Singapore Pte Ltd.
Sanuja S, Agalya A, Umapathy MJ (2015) Synthesis and characterization of zinc oxide–neem oil–chitosan bionanocomposite for food packaging application. Int J Biol Macromol 74:76–84
Saharan V, Mehrotra A, Khatik R, Rawal P, Sharma SS, Pal A (2013). Synthesis of chitosan based nanoparticles and their in vitro evaluation against phytopathogenic fungi. Int J Biol Macromol 62:677–683
Sarmento B, Neves J (2012) Chitosan-based systems for biopharmaceuticals: deliver, targeting and polymer therapeutics. John Wiley & Sons, Ltd.
Shantha Siri JG, Fernando CAN, De Silva N (2017) Eco-friendly chitosan nanoparticles cross linked with genipin: basis to develop control release nanofertilizer. J SciTech Res 7:26–31
Sharon M, Choudhary A, Kumar R (2010) Nanotechnology in agricultural diseases and food safety. J Phytology 2:83–92
Sharp RG (2013) A review of the applications of chitin and its derivatives in agriculture to modify plant-microbial interactions and improve crop yields. Agronomy 3:757–793
Shi LE, Fang XJ, Xing LY, Chen M, Zhu DS et al (2011) Chitosan nanoparticles as drug delivery carriers for biomedical engineering. J Chem So Pak 33:929–934
Shikata F, Tokumitsu H, Ichikawa H, Fukumori Y (2002) In vitro cellular accumulation of gadolinium incorporated into chitosan nanoparticles designed for neutron-capture therapy of cancer. Eur J Pharm Biopharm 53:57–63 PMID: 11777753
Shukla SK, Mishra AK, Arotiba OA, Mamba BB (2013) Chitosan-based nanomaterials: a state-of-the-art review. Int J Biol Macromol 59:46–58
Siddaiah CN, Prasanth KVH, Satyanarayana NR, Mudili V, Gupta VK, Kalagatur NK, Satyavati T, Dai XF, Chen JY, Mocan A, Singh BP, Srivastava RK (2018) Chitosan nanoparticles having higher degree of acetylation induce resistance against pearl millet downy mildew through nitric oxide generation. Sci Rep 8(1):2485. https://doi.org/10.1038/s41598-017-19016-z
Siddiqui MH, Al-Whaibi MH, Firoz M, Al-Khaishany MY (2015) Role of nanoparticles in plants. Nanotech Plant Sci:19–35. https://doi.org/10.1007/978-3-319-14502-0-2
Silva MS, Cocenza DS, Grillo R, de Melo NFS, Tonello POS, deOliveira LC, Cassimiro DL, Rosa AH, Fraceto LF (2011) Paraquat-loaded alginate/chitosan nanoparticles: preparation, characterization and soil sorption studies. J Hazard Mater 190:366–374
Sivamani E, DeLong RK, Qu R (2009) Protamine-mediated DNA coating remarkably improves bombardment transformation efficiency in plant cells. Plant Cell Rep 28:213–221
Sotelo-Boyás ME, Bautista-Baños S, Correa-Pacheco ZN, Jiménez-Aparicio A, Sivakumar D (2016) Biological activity of chitosan nanoparticles against pathogenic fungi and bacteria. Chapter 13. In: Bautista-Banos S, Romanazzi G, Jiménez-Aparicio A (eds) Chitosan in the preservation of agricultural commodities, pp 339–349, Academic Press, Elsevier, USA
Sun B, Zhang L, Yang L, Zhang F, Norse D, Zhu Z (2012) Agricultural non-point source pollution in China: causes and mitigation measures. Ambio 41:370–337
Swati, Choudhary MK, Joshi A, Saharan V (2017) Assessment of cu-chitosan nanoparticles for its antibacterial activity against Pseudomonas syringae pv. glycinea. Int J Curr Microbiol App Sci 6(11):1335–1350
Tan H, Ma R, Lin C, Liu Z, Tang T (2013) Quaternized chitosan as an antimicrobial agent: antimicrobial activity, mechanism of action and biomedical applications in orthopedics. Int J Mol Sci 14:1854–1869
Tao S, Pang R, Chen C et al (2012) Synthesis, characterization and slow release properties of O-naphthylacetyl chitosan. Carbohydr Polym 88:1189–1194
Teo WE, Ramakrishna S (2006) A review on electrospinning design and nanofibre assemblies. Nanotechnolgy 17:R89–R106
Tiyaboonchai W (2003) Chitosan nanoparticles: a promising system for drug delivery. Naresuan Univ J 11:51–66
Vander P, Vaêrum KM, Domard A, El Gueddari NE, Moerschbacher BM (1998) Comparison of the ability of partially N-acetylated chitosans and chitooligosaccharides to elicit resistance reactions in wheat leaves. Plant Physiol 118:1353–1359
Vasyukova NI, Zinovèva SV, Ilìnskaya LI, Perekhod EA, Chalenko GI, Gerasimova NG, Il’ina AV, Varlamov VP, Ozeretskovskaya OL (2001) Modulation of plant resistance to diseases by water-soluble chitosan. App Biochem Microbiol 37:103–109
Wang X, El Hadrami A, Adam LR, Daayf F (2008) Differential activation and suppression of potato defence responses by Phytophthora infestans isolates representing US-1 and US-8 genotypes. Plant Pathol 57:1026–1037
Wang Q, Chen JN, Zhan P, Zhang L, Kong QQ (2013) Establishment of a suspension cell system for transformation of Jatropha curcas using nanoparticles. Adv Mater Res 608–609:314–319
Wang P, Lombi E, Zhao FJ, Kopittke PM (2016) Nanotechnology: a new opportunity in plant sciences. Trends Plant Sci 21:699–712
Wani IA, Ahmad T (2013) Size and shape dependant antifungal activity of gold nanoparticles: a case study of Candida. Colloids Surf B101:162–170
Wazed AS, Joshi M, Rajendran S (2011) Novel, selfassembled antimicrobial textile coating containing chitosan nanoparticles. AATCC Rev 11:49–55
Wen Y, Yuan Y, Chen H et al (2010) Effect of chitosan on the enantioselective bioavailability of the herbicide dichlorprop to Chlorella pyrenoidosa. Environ Sci Technol 44:4981–4949
Wen Y, Chen H, Yuan Y et al (2011) Enantioselective ecotoxicity of the herbicide dichlorprop and complexes formed with chitosan in two fresh water green algae. J Environ Monitor JEM 13:879–88587
Wong MH, Misra RP, Giraldo JP, Kwak SY, Son Y, Landry MP, Swan JW, Blankschtein D, Strano MS (2016) Lipid exchange envelope penetration (LEEP) of nanoparticles for plant engineering: a universal localization mechanism. Nano Lett 16:1161–1172
Wu L, Liu M (2008) Preparation and properties of chitosan-coated NPK compound fertilizer with controlled-release and water retention. Carbohydr Polym 72:240–247
Wu L, Liu M, Liang R (2008) Preparation and properties of a double-coated slow-release NPK compound fertilizer with superabsorbent and water-retention. Bioresour Technol 99:547–554
Xing K, Zhu X, Peng X, Qin S (2014) Chitosan antimicrobial and eliciting properties for pest control in agricultural: a review. Agron Sustain Dev 35(2):569–588
Xing K, Zhu X, Peng X, Qin S (2015) Chitosan antimicrobial and eliciting prop erties for pest control in agriculture: a review. Agronomy for sustainable development. Springer Verlag/EDP Sci/INRA 35(2):569–588
Xing K, Shen X, Zhu X, Ju X, Miao X, Tian J, Feng Z, Peng X, Jiang J, Qin S (2016) Synthesis and in vitro antifungal efficacy of oleoyl-chitosan nanoparticles against plant pathogenic fungi. Int J Biol Macromol 82:830–836
Xu L, Cao LD, Li FM, Wang XJ, Huang QLJ (2014) Utilization of chitosan-lactide copolymer nanoparticles as controlled release pesticide carrier for pyraclostrobin against Colletotrichum gossypii Southw. Dispers Sci Technol 35:544–550
Yu-qin F, Lu-hua L, Pi-wu W, Jing Q, Yong-ping F, Hui W, Jing-ran S, Chang-li L (2012) Delivering DNA into plant cell by gene carriers of ZnS nanoparticles. Chem Res Chin Univ 28(4):672–676
Zahid N, Alderson P, Ali A, Maqbool M, Manickam S (2013) In vitro control of Colletotrichum gloeosporioides by using chitosan loaded nanoemulsions. Acta Hortic 1012:769–774
Zeng D, Luo X, Tu R (2012) Application of bioactive coatings based on chitosan for soybean seed protection. Int J Carbohydr Chem 1:1–5
Zhang X, Zhang J, Zhu KY (2010) Chitosan/double-stranded RNA nanoparticle-mediated RNA interference to silence chitin synthase genes through larval feeding in the African malaria mosquito (Anopheles gambiae). Insect Mol Biol 19(5):683–693
Zhao X, She X, Du Y, Liang X (2007) Induction of antiviral resistance and stimulary effect by oligochitosan in tobacco. Pestic Biochem Phys 87:78–84
Zheng L, Hong F, Lu S, Liu C (2005) Effect of nano-TiO2 on strength of naturally aged seeds and growth of spinach. Biol Trace Elem Res 104:83–91
Zheng YY, Monty J, Linhardt RJ (2015) Polysaccharide-based nanocomposites and their applications. Carbohydr Res 405:23–32
Acknowledgment
This research was supported by the Science and Technology Development Fund (STDF), Joint Egypt (STDF)–South Africa (NRF) Scientific Cooperation, Grant ID. 27837 to Kamel Abd-Elsalam.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Al-Dhabaan, F.A., Mostafa, M., Almoammar, H., Abd-Elsalam, K.A. (2018). Chitosan-Based Nanostructures in Plant Protection Applications. In: Abd-Elsalam, K., Prasad, R. (eds) Nanobiotechnology Applications in Plant Protection. Nanotechnology in the Life Sciences. Springer, Cham. https://doi.org/10.1007/978-3-319-91161-8_13
Download citation
DOI: https://doi.org/10.1007/978-3-319-91161-8_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-91160-1
Online ISBN: 978-3-319-91161-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)