Abstract
The excessive and irrational use of synthetic fungicides has perturbed us with irrevocable soil-water-air contaminations, development of resistance in microbes, and disturbing biosphere. Thus, search for biodegradable/ecofriendly materials has emerged as the main goal to replace/reduce the synthetic fungicides in agriculture for crop protection. Under this scenario, nanobiotechnology seems to be a boon for the synthesis of ecofriendly, biocompatible, and safe fungicides which will not only improve the soil health and the defense system of plants but also help in obtaining healthy food for the continuously growing population. Among the available biomaterials/biopolymers, chitosan is being explored as new generation smart material to be used in agriculture especially for plant protection. This chapter describes various chitosan-based nanomaterials (NMs) which have been used from laboratory to field for control of fungal disease in crops.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Past few years have witnessed a tremendous growth in world’s total population which is expected to reach up to 8.6 billion by 2030. This sets a great difficulty for the scientists in achieving sustainable agriculture production in view of global warming. To ensure the adequate food supply for growing population, application of synthetic agrochemicals has, therefore, increased many folds. Global application of agrochemicals is ~4.6 million tons, 90% of which gets runs-off into the environment and seep to agricultural products. Pesticides are among these agrochemicals which are being used since long to provide protection against damage caused by severe phytopathogens. Plant pathogens cause significant damage to almost all crops worldwide and this loss compels the farmers to use more and more pesticides to get maximum crop production (Zhang et al. 2011a, b). It is estimated that pesticides are used for one-third of total agricultural production; due to which crop loss declined by 35 to 42% (Pimentel 1997; Liu et al. 2002; Zhang et al. 2011a, b). 32%, 78%, and 54% loss in cereals, fruits, and vegetables, respectively, may be caused if pesticides are not used (Cai 2008).
Global consumption of these pesticides is increasing day by day. The average annual usage of fungicides and bactericides (kg/ha) from 2010 to 2014 in Japan is the greatest (7.934) followed by Mexico (3.275), France (2.162), UK (1.332), Germany (1.194), and Brazil (0.814), which are higher than global average (0.32). The last two countries in the list are USA (0.229) and India (0.058) (Zhang 2018). Although these agrochemicals have significantly contributed to agriculture production, their reckless and non-judicious use has been causing an irreversible damage to the ecosystem due to their nondegradable and toxic nature (Kumaraswamy et al. 2018). Further, most of these agrochemicals are not fully absorbed by plants and seep into the soil/groundwater and eventually get accumulated in living organisms too (Alister and Kogan 2006; Dietz and Herth 2011; Kah 2015; Marutescu et al. 2017). Global pesticide use has also resulted in the loss of biodiversity (Zhang et al. 2011b; Kumar et al. 2013). In addition, pesticide use has led to various human/animal diseases and injured human fecundity and intelligence quotient in past few years (Chen et al. 2004; Zhang 2018). Moreover, the increment of resistance in plant pathogens against these agrochemicals has become a serious issue (Hahn 2014; Xing et al. 2017). Due to this, either new kinds of agrochemicals have been developed or higher doses of the existing ones have been used which in turn has increased the cost and further expedites the resurgence of new plant pathogens.
With the emergence of nanoscience, application of nanotechnological tools has raised hope to deliver new generation agrochemicals which are safe to environment and effective at low doses. New generation pesticides could be comprised of nanostructured materials which act on target in slow/controlled release manner when need arises. Unexplored various bioactive compounds (inorganic and organic) can be used alone or in composite forms through nanotechnology to deliver novel nano-based products for use in agriculture for crop protection especially against fungal disease. Therefore, various inorganic and organic materials for synthesis of nanomaterials (NMs) having biocompatibility, biodegradability, wide biological activities, and ecological safety characteristics are in the forefront list of scientists (Shukla et al. 2013; Kah and Hofmann 2014; Kashyap et al. 2015).
In pursuit of this, chitosan, β-(1,4)-2-amino-2-deoxy-d-glucose, a hetero-amino-polysaccharide which can easily be obtained from the waste produce of shrimp, crab shells, and cell wall of fungi (Katiyar et al. 2015; Malerba and Cerana 2016), has been in high demand. Chitosan NMs can competently perform many biological applications due to their small size, higher surface area, and cationic nature. Furthermore, they are excellent blending materials for different organic and inorganic molecules due to the availability of functional groups in their structures (Choudhary et al. 2019a, b). Utility of chitosan has been acknowledged in developing chitosan nanoparticles (NPs) either alone or in combination with inorganic and organic substances. The developed chitosan-based nanocomposites could ensure slow, systemic, targeted, and protected release of active ingredients to improve their efficacy and avoid toxicity to environment (Saharan et al. 2015; Saharan and Pal 2016a, b; Choudhary et al. 2017a, b). Chitosan functionalized with various inorganic and organic inputs might ultimately lead to precision farming in a cost-effective manner and can deliver a smart chitosan-based nano-agri-input.
Herein, this chapter highlights various chitosan-based NMs, in-depth, which have potential to protect the plants from fungal diseases (Table 1).
2 Chitosan-Based NMs
Chitosan, being an excellent antimicrobial, plant growth regulator and plant elicitor, has been explored in sole as well as functionalized NM forms with other bioactive compounds of inorganic and organic nature. Herein, we have classified chitosan-based NMs in three categories (a) sole chitosan NMs, (b) inorganic based chitosan NMs, and (c) organic based chitosan NMs.
2.1 Sole Chitosan NMs
Since last few years, chitosan NMs have been explored for their diverse biological activities. They have been tested against many plant pathogenic fungi and found to be effective in significantly controlling fungal growth.
Chitosan NPs, in in vitro experiments, at a concentration of 0.6% (w/v), significantly delayed mycelia growth of Rhizopus sp. Colletotrichum capsici, Colletotrichum gloeosporioides, and Aspergillus niger. NPs exhibited better tendency as compared with bulk chitosan towards reduction of mycelia growth. In addition, chitosan coated/treated chickpea (Cicer arietinum) seeds had higher vigor and very good antifungal activity which could be explained by two facts: (1) chitosan directly inhibits mycelia growth and (2) seeds treated with chitosan produce more phenolic compounds and lignin (Chookhongkha et al. 2012).
Chitosan NPs, synthesized using ionic gelation method, were investigated against phytopathogenic fungi (Alternaria alternata, Macrophomina phaseolina, and Rhizoctonia solani) at various concentrations ranging from 0.001 to 0.1% under in vitro conditions. The maximum growth inhibitory effects (87.6%) were found against Macrophomina phaseolina at 0.1% concentration. The radial growth of Rhizoctonia solani was reduced by all concentrations of chitosan NPs in a dose-dependent manner (Saharan et al. 2013).
In another study, a biological method was used to prepare chitosan NMs using anionic proteins isolated from Penicillium oxalicum culture. These biologically synthesized chitosan NMs were significantly found to inhibit the growth of Pyricularia. grisea, Alternaria solani, Fusarium oxysporum (Sathiyabama and Parthasarathy 2016). The inhibition rate for Pyricularia grisea, Fusarium oxysporum ciceri, and Alternaria solani was found to be 92%, 87%, and 72%, respectively. Seed treatment with these NPs exhibited positive morphological effect including enhanced percent germination, vegetative biomass, and seed vigor index of chickpea (Cicer arietinum) seedlings. The efficacy of NMs could be attributed to their size as well as highly permeable nature towards biological membranes (Shukla et al. 2013; Saharan et al. 2015). Their small size, lower PDI value, and higher zeta-potential make these NMs more stable and effective against tested phytopathogens.
A 100% suppression of rice (Oryza sativa) blast disease symptoms was observed, in vivo, under detached leaf condition, when treated with chitosan NMs prepared using ionic gelation method (Manikandan and Sathiyabama 2016). Chitosans of different molecular weights have also been used to prepare chitosan NMs and check their antifungal property against Fusarium head blight (FHB) in wheat (Triticum aestivum) caused by Fusarium graminearum. The dynamic light scattering (DLS) study indicated variable z-average size of NMs (180.9, 339.4225.7, and 595.7 nm). Different concentrations of these NMs were tested to evaluate the inhibitory effect on this pathogen, and the maximum growth reduction (77.5%) was found at 5000 ppm. In greenhouse trials, the area under disease progress curve (AUDPC) decreased in plants treated with NMs (Kheiri et al. 2017).
2.2 Inorganic Based Chitosan NMs
Metals such as copper (Cu), zinc (Zn), and silver (Ag) have been explored in developing chitosan-based NMs as chitosan can easily chelate the metals (Choudhary et al. 2017a). Functionalized chitosan with metals has enabled chitosan NMs more suitable for controlling fungal diseases in plant.
2.2.1 Cu–Chitosan NMs
Copper (Cu) is a constituent of many enzymes like ascorbic acid oxidase, laccase, phenolase, cytochrome oxidase, etc., and is therefore vital for photosynthesis, respiration, and carbon-nitrogen balance. Traditionally, it has been used as antifungal agent in many commercially available pesticides (Saharan et al. 2015). Cu, therefore, has been tested for synthesis of smart chitosan-based NMs for controlling fungal disease in plants.
In in vitro model, Saharan et al. (2013) observed 89.5, 63.0, and 60.1% growth inhibition of Alternaria alternata, Macrophomina phaseolina, and Rhizoctonia solani, respectively, at various concentrations of Cu–chitosan NMs. The higher zeta-potential of chitosan NMs bestowed them a greater binding affinity for negatively charged fungal membrane. In fungi, Cu (II) reduces to Cu (I) which produces toxic H2O2, resulting in destruction of fungal cell viability. Pure chitosan nanogels were produced to adsorb Cu (II) and assess their antimicrobial activities against Fusarium graminarium. Antifungal activity was observed due to the strong synergistic effect between chitosan and Cu. The MIC (Minimum Inhibitory Concentration) of Cu (II) was observed as 250 μg/mL which decreased exponentially upon addition of low amounts of chitosan either in solution or dispersion. Therefore, Cu (II) and chitosan not only seem to be biocompatible and bioactive, but also display a strong synergistic effect in antifungal activities (Brunel et al. 2013).
Porous Cu–chitosan NMs were also examined for their antifungal efficacy in tomato (Solanum lycopersicum Mill). DLS, TEM, FTIR, SEM-EDS, and AAS were used for physico-chemical characterization of NMs. In in vitro model, 0.12% concentration caused 70.5 and 73.5% inhibition of mycelia growth and 61.5 and 83.0% inhibition of spore germination in Alternaria solani and Fusarium oxysporum, respectively. In pots, tomato plants exhibited 87.7% percent efficacy of disease control (PEDC) in early blight, while 61.1% in Fusarium wilt. Cu–chitosan NMs markedly exhibited higher antifungal activity along with only 1–2 mm small black or brown lesions as compared with control plants. At 0.10 and 0.12% concentrations, Cu–chitosan NMs were equally effective on early blight disease as was the commercial fungicide (Saharan et al. 2015).
These NMs were further tested to boost defense responses in Zea mays maize crop against Curvularia leaf spot (CLS) disease under in vitro as well as field conditions (Choudhary et al. 2017b). Plants showed significant defense response through higher activities of antioxidant (superoxide dismutase, SOD and peroxidase, POD) and defense enzymes (polyphenol oxidase, PPO and phenylalanine ammonia-lyase, PAL) . In NMs treated plants, disease symptoms in the form of small lesions without chlorosis were visualized after 7–8 days of fungal inoculation in pot experiment. PAL activity increased from 46.15 to 66.66%, while PPO activity increased from 3.05 to 16.39%. Application of these NMs increased the activities of POD, PAL, and PPO in plant which further enhanced the production of suberin, melanin, and lignin for cell wall strengthening acting as a mechanical barrier to invading plant pathogen (Kuźniak and Urbanek 2000; Fugate et al. 2016). In pot experiments, at 0.04 to 0.16% concentrations, Cu–chitosan NPs significantly controlled CLS disease while the same effect was observed at 0.12 to 0.16% concentrations of Cu–chitosan NPs in field condition. Study further revealed that these NMs are pH responsive as the Cu release rate increases as pH decreases in plant cell due to fungal infection. The released Cu, therefore, acts smartly on invading fungi (Rubina et al. 2017). Cu–chitosan NMs were prepared using metal vapor synthesis method and their in vitro antifungal effects were checked on hyphal morphology and sclerotia formation in Sclerotium rolfsii and Rhizoctonia solani AG-4. These NMs were found effective against both the tested fungi in a dose dependent manner (Rubina et al. 2017).
2.2.2 Zn-Chitosan NMs
Zinc (Zn) is an essential micronutrient which helps the plants in maintaining their cellular homeostasis. It plays a crucial role during plant’s reproductive and grain filling stage and therefore its deficiency or unavailability can result into poor growth and lower grain yield. Zn helps to carry out several biological processes such as electron transport, gene expression, protein and auxin metabolism, structural and functional integrity of biomembranes. It has been found that Zn deficiency in crop also leads to disease suitability.
Zn-chitosan NMs were synthesized and evaluated for their antifungal activity via seed priming and foliar application in maize plants (Fig. 1). These NMs (0.01–0.16%) showed strong in vitro antifungal activity as evident by inhibition of fungal spore germination. The plant immunity was further improved due to enhanced antioxidant and defense enzymes activity, balanced reactive oxygen species (ROS) levels, and more lignin accumulation caused by these NMs. In the field, 0.01–0.16% concentrations were used for seed treatment and foliar application which significantly controlled CLS disease and enriched the grain with Zn micronutrient from 41.27 to 62.21 μg/g DW.
Zn-chitosan NMs displayed high encapsulation efficiency (82%) and exhibited slow release of Zn ions . At acidic pH (from 3 to 1), 20.84–42.80% Zn ions were released rapidly due to protonation of chitosan (Choudhary et al. 2017a, b; Kumaraswamy et al. 2018). It is important as these NMs act strongly when plants are infected with fungi since sudden exposure of Zn (at low pH caused by fungi) creates ions toxicity which averts the growth of fungal cells. Zn-chitosan NMs controlled CLS disease up to 39.5% with significantly higher grain yield. Hence, these NPs could be an effective growth promoting, fungal disease controlling, and micronutrient fortifying agent in maize crop (Choudhary et al. 2019a, b).
2.2.3 Ag-Chitosan NPs
Silver (Ag)displays multiple modes of inhibitory action against microorganisms (Park et al. 2006). Although metallic Ag is relatively nonreactive, Ag nanoparticles are exceedingly reactive because of their high ability to generate Ag+ ions, which are well known to induce ROS production. ROS are highly detrimental to microbial cells as they can damage surface and interior proteins, lipids, and nucleic acids (Storz and Imlayt 1999; Hwang et al. 2008). Therefore, Ag may be used to prepare NMs as an antifungal treatment for various seed borne plant pathogens. Ag-chitosan NMs exhibited the highest inhibition against Aspergillus followed by Alternaria and Rhizoctoniaspecies . The observed zone of inhibition was 19.66 ± 0.28, 16.33 ± 0.29, 12.66 ± 0.76 against Aspergillus, Alternaria, and Rhizoctonia, respectively. Thus, Ag-chitosan NMs may be used as an alternative to fungicides for controlling seed borne phytopathogens (Kaur et al. 2012).
Nano Ag with irradiated chitosan NMs were investigated along with native chitosan for their ability to hamper the growth of Botrytis cinerea Pers, the gray mold of strawberry (Fragaria ananassa), that causes great losses in other agricultural crops too. Ag-irradiated chitosan (IrCTS), as compared with its native fungal chitosan , was found more effective and showed highest antifungal activity at a minimal inhibitory concentration of 125 μg/mL (comprised of 20% Ag and 80% IrCTS). Botrytis cinerea treated with the NMs had an obvious alteration in mycelial shape as well as moderate lysis in fungal hyphae. Coating with these NMs led to 90% control of gray mold infection after 7 days of storage and treated fruits still gave fresh-like appearance at the end of storage. Hence, coating with nano Ag-IrCTS solution could be highly recommended regarding its efficiency in prohibiting Botrytis cinerea growth , preventing gray mold decay and enhancing the overall quality of coated strawberry fruits (Moussa et al. 2013).
Chitosan was functionalized with 4-((E)-2-(3-hydroxynaphthalen-2-yl) diazen-1-yl) benzoic acid by coupling of hydroxyl functional groups of chitosan with carboxylic acid group of dye by DCC coupling method. The Ag NPs were prepared by sol-gel method while Ag NPs-encapsulated functionalized chitosan was prepared by phase transfer method. The products were characterized by FTIR, UV-VIS, fluorescence, and Nuclear Magnetic Resonance (NMR) spectroscopic methods and by SEM and TEM analysis . The light-fastening properties of the chromophoric system were enhanced when attached to chitosan and they were further improved by the encapsulation of Ag NMs. Their antibacterial analysis was carried out against Aspergillus flavus and Aspergillus terreus by diffusion plate method and found inhibition zone (20.2 ± 0.15 and 27.0 ± 0.38 mm), showing that NPs can be used for antifungal applications (Mathew and Kuriakose 2013).
2.2.4 AgNPs, Chitosan, and Fungicide Zineb (Zi) NMs
Ngoc and Nguyen (2018) examined the synergistic effect of AgNPs, chitosan (CS), and fungicide zineb (Zi) as antifungal materials against Neoscytalidium dimidiatum in (Hylocereus undatus) dragon fruit. The researchers synthesized Ag@CS by encapsulating AgNPs in CS polymer and then combined with Zi. 4.11 ± 0.37 nm was recorded as diameter of spherical nanoparticles as confirmed by TEM. Ag@CS showed better antifungal ability as compared with each component alone against N. dimidiatum. At 5 ppm of Ag@CS, the zone of inhibition was found to be 15.00 ± 0.00 mm which was better than that of Ag alone (13.33 ± 0.58 mm) at 10 ppm . When pure Zi at 500 and 1000 ppm (inhibition zone, 5.00 ± 0.00 mm) was incapable of removing the fungi, the zone of inhibition of Ag@CS-Zi increased to 12.00 ± 0.00 mm, which was nearly equivalent to 5 ppm Ag (12.33 ± 0.58 mm) and much higher than 5000 ppm of Zi (9.00 ± 0.00 mm). Ag@CS-Zi at 2500 ppm of Zi gave inhibition zone of 20.67 ± 0.58 which showed its high antifungal activity as compared with each of individual component.
2.3 Organic Based Chitosan NMs
Essential oils (EOs) which obtained from plants are aromatic and volatile. They are present in stems, bark, leaves, fruits, etc. (Oussalah et al. 2006). Compounds such as terpenoids and phenolic acids are some of the EOs which are extracted from plants. The food industry used EOs as natural antimicrobials because of their antifungal and antimicrobial properties. (Tassou et al. 1995; Burt 2004; du Plooy et al. 2009).
Many reports have shown that NMs functionalized with essential oils have significant antimicrobial activity because of their chemical stability and solubility, decreased fast evaporation and degradation of EO active components. The controlled and sustained released nature of encapsulated EOs which enhance their bioavailability and efficacy against multidrug-resistant pathogens (Chouhan et al. 2017). As EOs have the property of hydrophobicity, it helps in the partition of lipid present in the cell membrane of the pathogen resulting in the leakage of molecules and ions leading to its death. The activity of essential oils depends on its composition, functional groups present in active components, and their synergistic interactions. Nanoencapsulation of bioactive compounds can be used as an efficient approach to enhance the physical stability of the active ingredient. It can also prevent their interactions with the food components, thus enhancing their bioactivity due to their subcellular size (Donsi et al. 2011). Chitosan, having the properties of biocompatibility, low toxicity , and biodegradability, its encapsulating with EOs is of much interest. (Muzzarelli 2010; Donsi et al. 2011; Harris et al. 2011; Luo et al. 2011).
2.3.1 Zataria multiflora Essential Oils in Chitosan
Chemical fungicides have been used as a preventive measure of fungal attack during post-harvest storage. However, use of these synthetic fungicides has raised health related questions. So, application of plant EOs at post-harvest stage has been considered as an alternative management to prevent post-harvest decay (Aloui et al. 2014). Zataria multiflora Boiss EOs (ZEO) is one of the EOs which appear as potential natural compounds for controlling post-harvest loss in fruits. Quantitatively, the most abundant components in hydro-distilled ZEO are oxygenated monoterpenes (~70%) followed by monoterpene, sesquiterpenes, and oxygenated sesquiterpenes (Sajed et al. 2013). The volatile compounds of EOs are used to maintain fruit quality and decrease fungal decay, but they are easily degraded by high temperature, pressure, light, and oxygen. Furthermore, they are insoluble in water and, for certain applications, a controlled release is required (Martin et al. 2010). Therefore, sustained and controlled released is crucial to obtain maximum benefits of using EOs as antimicrobial agents.
Nano-/microencapsulation technology of these compounds can be a practical and efficient approach to solve some of these problems such as the physical instability. Mohammadi et al. (2015) investigated the nanoencapsulation of ZEO in chitosan nanoparticles (CSNPs) to enhance antifungal activity and stability of the oils against Botrytis cinerea, the causal agent of gray mold disease in strawberry. Ionic gelation method was used for encapsulation of ZEO with CSNPs and found an average size of 125–175 nm, as observed by TEM. In vitro release studies also demonstrated a controlled and sustained release of ZEO for 40 days. There was a superior activity of ZEO when encapsulated by CSNPs under both in vitro and in vivo conditions in comparison with unmodified ZEO against Botrytis cinerea. At 1500 ppm of encapsulated oils, both disease severity and incidence of Botrytis-inoculated strawberries significantly decreased during 7 days of storage at 4 °C followed by 2–3 more days at 20 °C. These findings showed the potential role of CSNPs as a controlled release system for EOs in order to enhance antifungal activities.
2.3.2 Chitosan-Thymol Nanoparticles
Thymol (2-isopropyl-5-methylphenol) is the major antimicrobial agent of the aromatic plant thyme (Thymus vulgaris). It has a strong antimicrobial property because of its capability of binding bacterial proteins and giving rise to disintegration and permeability of the cell membrane (Juven et al. 1994). Thymol affects energy-generating processes, which makes the cell unable to recover (Ahmad et al. 2011). It, therefore, may be incorporated as a natural antifungal agent in an active packaging to increase shelf-life of foods (Mirdehghan and Valerob 2017).
Medina et al. (2019) conducted the experiment to improve the performance of quinoa protein/chitosan edible films on the extension of post-harvest life of blueberries (Cyanococcus) and tomato cherries by addition of chitosan-thymol nanoparticles prepared by ionic gelation method. They obtained NPs with a hydrodynamic diameter (175 ± 21 nm) similar to the diameter measured by TEM (153 ± 42 nm). The PDI and zeta-potential values were 0.4 ± 0.1 and 37 ± 2.7 mV, respectively. Inhibition of radial mycelia growth by chitosan-thymol nanoparticles (CTNPs), chitosan nanoparticles (CNPs), and chitosan/thymol (CT) blend was evaluated in different dilutions added to the potato dextrose agar having the same concentrations of active compounds. CTNPs formulation recorded 100% inhibition for all dilutions (10, 25, and 50%, v/v), whereas CT blend showed total inhibition only at a higher concentration (50% v/v). CNP showed lowest inhibition of mycelia growth (74%) at higher concentration (50% v/v). Therefore, CTNPs was the only treatment that showed inhibitory effect at the lowest dose (10%).
2.3.3 Chitosan-Thyme-Oregano, Thyme-Tea Tree and Thyme-Pepper Mint Essential Oils
Bio-nanocomposite based packaging containing plant-derived EOs are presently playing an important role in controlling fungal contamination and proliferation in processed food (Hossain et al. 2017). EOs are more efficiently used in foods when encapsulated in proper delivery systems to overcome dosage limitations and increase the biological stability of active compounds (Van Long et al. 2016). Bioactivities of EOs get enhanced when encapsulated at the nanosize. They pass the cell membranes through passive mechanism or tissue infusion, thereby enabling the reduction of the EOs doses required to ensure antimicrobial activity (Bilia et al. 2014). Flavor, natural aroma, and taste of food maintain the same because of low doses of the bioactive compound applied (Lu et al. 2016).
Hossaina et al. (2019) prepared cellulose nanocrystals (CNCs) reinforced chitosan-based antifungal films by encapsulating EOs nanoemulsion. Chitosan-based nanocomposite films carried with thyme-oregano, thyme-tea tree, and thyme-peppermint EOs mixtures showed reduction of fungal growth by 51–77% against Aspergillus niger, Aspergillus flavus, Aspergillus parasiticus, and Penicillium chrysogenum in inoculated rice during 8 weeks of storage at 28 °C. Nanoemulsion prepared with thyme-oregano, thyme-tea tree, and thyme- peppermint have z-averages 76.58, 69.9, 57.9 nm, PDI 0.25, 0.21, 0.32, and zeta-potential −51, −50, −53 mV, respectively. They showed 83.73 ± 2.55, 75.60 ± 1.27, and 87.95 ± 6.81%, respectively, inhibition against A. niger after 24 hrs of inoculation. There was a slow release of volatile compounds (26%) and the rice samples packed with bioactive film showed no different change in color, taste, and odor over 12 weeks of storage. CNCs incorporated with chitosan matrix played an important function in stabilizing the physico-chemical and release properties of the nanocomposite films.
2.3.4 Chitosan with Cymbopogon martinii Essential Oil
Cymbopogon martinii, also known as Indian geranium/motia/rosha, is a tropical herbaceous grass belonging to family Poaceae (Duke 1993). Bioactive compounds such as geraniol caryophyllene, humulene, geranyl acetate, linalool, selinenes, limonene, etc. are some of the chemical constituents of its EOs (Rao et al. 2005; Cannon et al. 2013; Verma et al. 2013; Kakaraparthi et al. 2015).
The antifungal activity of Cymbopogon martini EOs (CMEOs) was investigated against Fusarium head blight disease in maize, caused by the post-harvest pathogen Fusarium graminearum (Kalagatur et al. 2018). They found that the minimum inhibitory concentration and minimum fungicidal concentration of CMEOs were 421.7 ± 27.14 and 618.3 ± 79.35 ppm, respectively. There was a morphological change in vesicles, craters, protuberance, and rough surfaces in macroconidia when exposed with CMEOs as compared with control. ROS content and lipid peroxidation were increased, which induced the death of fungi. Chitosan encapsulated CMEOs nanoparticles (Ce-CMEO-NPs) were synthesized with spherical morphology of size 455–480 nm and zeta-potential of 39.3–37.2 mV. FTIR analysis confirmed that bioactive constituents of CMEOs were well stabilized due to chitosan conjugation and successfully formed Ce-CMEO-NPs. A stabilized complex structure formed between chitosan and CMEOs increased the lifetime antifungal activity of CMEOs by gradual release of antifungal constituents of Ce-CMEO-NPs. Maize grains were used as sample material to check the antifungal and antimycotoxin activities of CMEOs and Ce-CMEO-NPs against F. graminearum under laboratory conditions over a storage period of 28 days. Ce-CMEO-NPs and CMEOs reduced fungal growth at 700 ppm and 900 ppm, respectively. Ce-CMEO-NPs offered competent and enhanced antifungal and antimycotoxin activities as compared with CMEO, and it could be due to persistence of antifungal activity by controlled release of antifungal constituents from Ce-CMEO-NPs.
2.3.5 Chitosan with Pepper Tree (Schinus molle) Essential Oil
Schinus molle (Anacardiaceae), also known as pepper tree, has EOs with antimicrobial properties (Lopez et al. 2014). The chemical constituents of EOs, such as ǖFC;-pinene, ǖFC;-phellandrene, 𝛽-phellandrene, limonene, monoterpenes, and myrcene, are found in pepper tree. Efficacy of its EOs against the filamentous fungi of Fusarium solani has been proved (Rhouma et al. 2009). At 500 ppm of pepper tree EOs, the mycelium inhibition of up to 53.5% was found against Aspergillus flavus (Dikshit et al. 1986). It also exhibited substantial antifungal activity against A. japonicus, A. niger, and A. oryzae (Martins et al. 2014). A minimum inhibitory concentration of >1000 mg/mL of the oil was found against A. fumigates (Alanis-Garza et al. 2007).
Luque-Alcaraz et al. (2016) synthesized chitosan nanoparticles, encapsulating pepper tree EOs having the size distribution of 754 ± 7.5 nm and zeta-potential of +9.1 ± 1.74 mV. They tested the effect of different concentrations of chitosan nanoparticles encapsulated pepper tree EOs on the viability of A. parasiticus spores. It was found out that all treatments reduced the viability of fungal spores compared with control. These results indicated that the addition of pepper tree EOs in chitosan bionanocomposites is an alternative that preserves the antifungal properties of both components, decreasing the tendency to volatilization of EOs and consequent loss of activity.
2.3.6 Mentha piperita Essential Oils in Chitosan–Cinnamic Acid Nanogel
Beykia et al. (2014) investigated the encapsulation of Mentha piperitaEOs in chitosan–cinnamic acid nanogel to increase stability of oils and antimicrobial activity against Aspergillus flavus. They found out that because of encapsulation, the extract possessed remarkable antifungal properties against A. flavus. The minimum inhibitory concentrations of encapsulated and free EOs against A. flavus under sealed condition were 500 and 2100 ppm, respectively. However, when experimented under non-sealed condition, the encapsulated oils performed better result (800 ppm) compared with the free oils which failed to caused complete inhibition within the concentration range tested (up to 3000 ppm). These findings revealed the promising role of chitosan–cinnamic acid nanogel as a carrier for EOs to enhance their antimicrobial properties.
2.3.7 Thyme Oil with Chitosan/Boehmite
Cindi et al. (2015) had done their investigation on polyethylene terephthalate (PET) punnets which contained thyme oil (TO sachets) and also packed with chitosan/boehmite nanocomposite lidding films. They found out that, in artificially inoculated peach fruits (cv. Kakawa) (Prunus persica) by Monilinia laxa, the incidence and severity of brown rot were reduced when stored at 25 °C for 5 days. Moreover, in naturally infected fruits, the brown rot incidence was reduced to 10% when stored at 0.5 °C, 90% RH for 7 days. Active compounds such as thymol (56.43%), β-linalool (37.6%), and caryophyllen (9.47%) were maintained within the punnet. The appearance, taste, and natural peach flavor were remains as such so people preferred fruits packed from commercial punnet containing thyme oil (sachets) and sealed with chitosan/boehmite nanocomposite lidding films.
2.3.8 Thiadiazole-Functionalized Chitosan Derivatives
Li et al. (2013) revealed that a group of novel water-soluble chitosan derivatives, such as 1,3,4-thiadiazole (TPCTS), 2-methyl-1,3,4-thiadiazole (MTPCTS), and 2-phenyl-1,3,4-thiadiazole (PTPCTS), had antifungal activities against plant-threatening fungi such as Colletotrichum lagenarium, Phomopsi asparagi, and Monilinia fructicola. The inhibitory index was recorded as 31.6% at 1.0 mg/mL against the growth of C. lagenarium. The antifungal activities of chitosan derivatives were given better result as compared with chitosan. Among the chitosan derivatives tested, MTPCTS gave the best result with the inhibitory indices of 75.3, 82.5, and 65.8% against C. lagenarium, P. asparagi, and M. fructicola, respectively, at 1.0 mg/mL . The length of alkyl substituent in thiadiazole and the hydrophobic moiety tend to affect the antifungal activity of chitosan derivatives.
2.3.9 Salicylic Acid-Chitosan NMs
Salicylic acid (SA) is a naturally occurring vital phenolic compound involved in plant’s signal transduction pathway for the onset of systemic acquired resistance (SAR) (Raskin et al. 1990; Malamy et al. 1992; Vlot et al. 2009). It is a key element for photosynthesis, vegetative growth, respiration, flower formation, up-regulation of seed germination, senescence, thermogenesis, and cellular redox homeostasis (Khan et al. 2015).
Exogenous application of SA as seed treatment and foliar application induced many metabolic processes in plants and could be an alternative approach for controlling disease, enhancing plant growth and yield . Therefore, SA-chitosan nanoparticles (SA-CS NPs) have been investigated as a biostimulant for promoting plant defense and growth in maize. SA-CS NPs induced significant physiological-biochemical responses under in vitro and in vivo conditions (Fig. 2), as elevated antioxidant-defense enzyme activities (SOD, catalase, peroxidase, etc.), balanced ROS, cell wall reinforcement by lignin deposition, disease control, and plant growth in maize. In field 59.4% and in pots 37.3–49.5% (at 0.01–0.16% concentration) control of post-flowering stalk rot (PFSR) disease and 57.8% yield enhancement was evident in SA-CS NPs application. NPs at the concentrations of 0.08 and 0.16% significantly evaded in vitro mycelia growth from 62.2 to 100% and spore germination from 48.3 to 60.5%. In NPs treatments (0.01–0.16%), plants endowed reasonably reduced disease severity (25.2 to 33.0%) and higher disease control (PEDC values from 40.5 to 59.4%).
Salicylic acid-chitosan NMs (Kumaraswamy et al. 2019, copyright permission from Elsevier)
With +34.1 mV zeta-potential, SA-CS NPs were stable in aqueous due to electrostatic repulsion between NPs which averts aggregation and agglomeration of NPs. FTIR study revealed the interaction of –COOH group of SA to primary amide of chitosan. Slow release of SA from SA-CS NPs significantly amended physiological and biochemical responses in maize plant for commendable disease control, plant growth and yield as compared with sole SA application. Fusarium verticillioides is an intercellular endophytic pathogen where symptoms appear at flowering stage, so most of the approaches of disease control may not be effective. Thus, application of SA-CS NPs as seed treatment and foliar application before flowering stage can be an effective and preventive approach through boosting plant innate immunity even before the onset of pathogen infection (Kumaraswamy et al. 2019).
2.3.10 Silica-Chitosan NMs
Since the discovery of Mobil Crystalline Material 41 (MCM-41), research and development of mesoporous silica nanoparticles (MSNs) has gained worldwide interest due to MSNs’ unique properties. These include biocompatibility, low cost, large surface area, tunable pore size for high loading capacity, and ability for targeted and controlled release with surface functionalization and polymer coatings (Wu et al. 2013; Sun et al. 2015).
Bernardos et al. (2015) reported that EOs loaded into MSNs had sustained antifungal activity against A. niger. MSNs were synthesized by liquid crystal templating mechanism. A water-soluble chitosan derivative (N-(2-hydroxyl) propyl-3-trimethyl ammonium chitosan chloride, HTCC) was used to encapsulate pyraclostrobin (a fungicide)-loaded MSNs. Through physico-chemical and structural analyses, it was proved that electrostatic interactions and hydrogen bonding were responsible for the formation of HTCC-capped MSNs. The loading efficiency of NPs increased to 40.3% by HTCC coating as compared with using bare MSNs as a single encapsulating material (26.7%). Initially, a rapid release of pyraclostrobin-loaded NPs was observed but later it showed a slow and sustained release. Almost same fungicidal activity was expressed by pyraclostrobin-loaded HTCC-capped MSNs with half doses of pyraclostrobin against Phomopsis asparagi (Sacc.), which resulted into lower application of pesticide and improved utilization efficiency. Therefore, HTCC-decorated MSNs demonstrated great potential as nanocarriers in agrochemical applications (Cao et al. 2016).
2.3.11 Chitosan–Saponin NMs
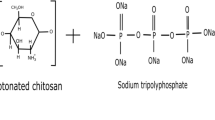
Saponins are complex glycosidic compounds known for their fungistatic activities (Chapagain et al. 2007). Their self-assembly property in aqueous media has been successfully exploited in chitosan–saponin nanoformulation against cancer cells (Rejinold et al. 2011). But its ability to suppress plant fungal growth was first studied by Saharan et al. (2013), when they synthesized chitosan–saponin NPs to test their synergistic activity against phytopathogenic fungi (A. alternata, M. phaseolina, and R. solani). These NPs were prepared using ionic gelation method by interaction of chitosan, sodium tripolyphosphate, and saponin. Their particle size, polydispersity index, zeta-potential, and structures were confirmed by DLS, FTIR, TEM, and SEM. These NPs inhibited 80.9% of mycelia growth at 0.1% w/v concentration and also showed a dose dependent effect on mycelia growth.
2.3.12 Oleoyl-Chitosan NMs
Many scientists have reported about the hydrophobic modifications of chitosan and NP formation by self-aggregation in water. These modifications can introduce hydrophobic groups into chitosan and produce chitosan amphiphilic polymers. Some of these chitosan amphiphilic derivatives can form nanosized self-aggregates in aqueous solution. Derivatives of chitosan having long chain fatty acyl are novel hydrophobic modifications that can form nanoparticles (Xing et al. 2016).
Therefore, oleoyl-chitosan NPs were synthesized using oil-water emulsification method based on O-chitosan, which involved grafting a monounsaturated fatty acid residue, C18 oleoyl group, onto the NH2 at C-2 in the chitosan structure (Xing et al. 2016). These NPs were examined for their antifungal activity against Verticillium dahlia which causes wilting in woody and herbaceous plants, a problem for which no effective controls have been devised yet. Oleoyl-chitosan NPs dramatically decreased the mycelium growth showing the highest antifungal indexes of 86.81% at 2 mg/mL, and also affected the spore germination and hyphae morphology as crumpled hyphae and spores, thickened cell walls, disappearance of membranous organelles, massive vacuolation of the cytoplasm, and cell wall-plasmalemma separation as observed in SEM and TEM studies. O-chitosan NPs showed inhibitory effect at all tested concentrations which was reversibly concentration-dependent. The dry weight of mycelia was much lower than the control group at pH 4.5 and 5.0. The inactivation of spores by NPs occurred via one of the following mechanisms. Specifically, O-chitosan NPs at lower concentrations could mainly induce an inhibition effect, while at higher concentrations, they primarily led to flocculation. Therefore, the antifungal capability of O-chitosan NPs could restrain the germination and tube growth of conidia. Moreover, these NPs having the characteristics of both coagulants and flocculants could disrupt the dispersion state of spores (Dong et al. 2014; Xing et al. 2017).
3 Conclusion
Review of literature confirms that chitosan is a versatile biomaterial that exhibits remarkable fungicidal activity. It can be easily maneuvered through various physical and chemical techniques. Functional groups of chitosan (–NH2 and –OH) enable this biopolymer to provide unique platform to make smart fungicides by functionalizing it with inorganic/organic substances to expend its application horizon. In this notation, new generation agrochemicals (like fungicides) can be synthesized which can act smart and timely at lower dose. Chitosan biopolymer has flexible physico-chemical properties to convert into smart nano-chitosan product with the help of other bioactive compounds. Therefore, we expect to achieve the following characteristics in new generation fungicides: (a) multi-targeted/multi-mode action to arouse plant immune responses, (b) show direct antifungal activity, (c) slow/controlled release of active component for timely and long lasting effects in crop (Fig. 3). Therefore, chitosan-based NMs have great scope for creation of new generation fungicides which may be economical and ecofriendly, and give minimum chemical load to the biosphere.
References
Ahmad A, Khan A, Akhtar F, Yousuf S, Xess I, Khan L, Manzoor N (2011) Fungicidal activity of thymol and carvacrol by disrupting ergosterol biosynthesis and membrane integrity against Candida. Eur J Clin Microbiol Infect Dis 30:41–50
Alanis-Garza BA, Gonzalez-Gonzalez GM, Salazar-Aranda R, Waksman de Torres N, Rivas-Galindo VM (2007) Screening of antifungal activity of plants from the northeast of Mexico. J Ethnopharmacol 114(3):468–471
Alister C, Kogan M (2006) ERI: environmental risk index. A simple proposal to select agrochemicals for agricultural use. Crop Prot 25(3):202–211
Aloui H, Khwaldia K, Licciardello F, Mazzaglia A, Muratore G, Hamdi M, Restuccia C (2014) Efficacy of the combined application of chitosan and locust bean gum with different citrus essential oils to control postharvest spoilage caused by Aspergillus flavus in dates. Int J Food Microbiol 170:21–28
Bernardos A, Marina T, Žáček P, Pérez-Esteve É, Martínez-Mañez R, Lhotka M, Klouček P (2015) Antifungal effect of essential oil components against Aspergillus niger when loaded into silica mesoporous supports. J Sci Food Agric 95(14):2824–2831
Beykia M, Zhaveha S, Khalilib ST, Rahmani-Cheratic T, Abollahic A, Bayatd M, Tabatabaeie M, Mohsenifar A (2014) Encapsulation of Mentha piperita essential oils in chitosan–cinnamic acid nanogel with enhanced antimicrobial activity against Aspergillus flavus. Ind Crop Prod 54:310–319
Bilia AR, Guccione C, Isacchi B, Righeschi C, Firenzuoli F, Bergonzi MC (2014) Essential oils loaded in nanosystems: a developing strategy for a successful therapeutic approach. In: Evidence-based complement alternative medicine. Oxford University Press, Oxford
Brunel F, Gueddari NEE, Moerschbacher BM (2013) Complexation of copper (II) with chitosan nanogels: toward control of microbial growth. Carbohydr Polym 92:1348–1356
Burt S (2004) Essential oils: their antibacterial properties and potential applications in foods—a review. Int J Food Microbiol 94:223–253
Cai DW (2008) Understand the role of chemical pesticides and prevent misuses of pesticides. Bull Agric Sci Technol 1(6):36–38
Cannon JB, Cantrell CL, Astatkie T, Zheljazkov VD (2013) Modification of yield and composition of essential oils by distillation time. Ind Crops Prod 41:214–220
Cao L, Zhang H, Cao C, Zhang J, Li F, Huang Q (2016) Quaternized chitosan-capped mesoporous silica nanoparticles as nanocarriers for controlled pesticide release. NMs 6(7):126
Chapagain BP, Wiesman Z, Tsror L (2007) In vitro study of the antifungal activity of saponin-rich extracts against prevalent phytopathogenic fungi. Ind Crops Prod 26(2):109–115
Chen JP, Lin G, Zhou BS (2004) Correlation between pesticides exposure and morbidity and mortality of breast cancer. Chin J Public Health-Shenyang 20:289–290
Chookhongkha N, Sopondilok T, Photchanachai S (2012) Effect of chitosan and chitosan nanoparticles on fungal growth and chilli seed quality. In: International conference on postharvest Pest and disease Management in Exporting Horticultural Crops-PPDM2012 973. Acta Horticulturae, Bangkok, pp 231–237
Choudhary RC, Kumaraswamy RV, Kumari S, Sharma SS, Pal A, Raliya R, Biswas P, Saharan V (2017a) Synthesis, characterization, and application of chitosan NMs loaded with zinc and copper for plant growth and protection. In: Prasad R et al (eds) Nanotechnology. Springer Nature Singapore Pte Ltd., Singapore
Choudhary RC, Kumaraswamy RV, Kumari S, Sharma SS, Pal A, Raliya R, Biswas P, Saharan V (2017b) Cu-chitosan nanoparticle boost defense responses and plant growth in maize (Zea mays L.). Sci Rep 7:9754
Choudhary RC, Kumaraswamy RV, Kumari S, Sharma SS, Pal A, Raliya R, Saharan V (2019a) Zinc encapsulated chitosan nanoparticle to promote maize crop yield. Int J Biol Macromol 127:126–135
Choudhary RC, Kumari S, Kumaraswamy RV, Pal A, Raliya R, Biswas P, Saharan V (2019b) Characterization methods for chitosan-based NMs. Plant Nanobionics. Springer, Cham, pp 103–116
Chouhan S, Sharma K, Guleria S (2017) Antimicrobial activity of some essential oils-present status and future perspectives. Medicines 4(3):58
Cindi MD, Shittu T, Sivakumar D, Bautista-Banos S (2015) Chitosan boehmite-alumina nanocomposite films and thyme oil vapour control brown rot in peaches (Prunus persica L.) during postharvest storage. Crop Prot 72:127–131
Dietz KJ, Herth S (2011) Plant nanotoxicology. Trends Plant Sci 16(11):582–589
Dikshit A, Nanqvi AA, Husain A (1986) Schinus molle: a new source of natural fungitoxicant. Appl Environ Microbiol 51(5):1085–1088
Dong C, Chen W, Liu C (2014) Flocculation of algal cells by amphoteric chitosan-based flocculant. Bioresour Technol 170:239–247
Donsi F, Annunziata M, Sessa M, Ferrari G (2011) Nanoencapsulation of essential oils to enhance their antimicrobial activity in foods. LWT- Food Sci Technol 44:1908–1914
du Plooy W, Regnier T, Combrinck S (2009) Essential oil amended coatings as alternative to synthetic fungicides in citrus postharvest management. Postharvest Biol Technol 53:117–122
Duke JA (1993) CRC handbook of alternative cash crops. CRC Press, Boca Raton
Fugate KK, Ribeiro WS, Lulai EC, Deckard EL, Finger FL (2016) Cold temperature delays wound healing in postharvest sugarbeet roots. Front Plant Sci 7:499
Hahn M (2014) The rising threat of fungicide resistance in plant pathogenic fungi: Botrytis as a case study. J Chem Biol 7(4):133–141
Harris R, Lecumberri E, Mateos-Aparicio I, Mengíbar M, Heras A (2011) Chitosan nanoparticles and microspheres for the encapsulation of natural antioxidants extracted from Ilex paraguariensis. Carbohydr Polym 84:803–806
Hossain F, Follett P, Salmieri S, Vu KD, Jamshidian M, Lacroix M (2017) Perspectives on essential oil loaded nanodelivery packaging technology for controlling stored cereal and grain pests. In: Nollet LML, Rathore HS (eds) Green pesticides handbook essential oils for Pest control. Routledge, London, pp 487–508
Hossaina F, Follettb P, Salmieria S, Vua KD, Fraschinic C, Lacroixa M (2019) Antifungal activities of combined treatments of irradiation and essential oils (EOs) encapsulated chitosan nanocomposite films in in vitro and in situ conditions. Int J Food Microbiol 295:33–40
Hwang ET, Lee JH, Chae YJ, Kim YS, Kim BC, Sang BI, Gu MB (2008) Analysis of the toxic mode of action of silver nanoparticles using stress-specific bioluminescent bacteria. Small 4(6):746–750
Juven BJ, Kanner J, Schved F, Weisslowicz H (1994) Factors that interact with the antibacterial action of thyme essential oil and its active constituents. J Appl Bacteriol 76:626–631
Kah M (2015) Nanopesticides and nanofertilizers: emerging contaminants or opportunities for risk mitigation? Front Chem 3:64. 13
Kah M, Hofmann T (2014) Nanopesticide research: current trends and future priorities. Environ Int 63:224–235
Kakaraparthi PS, Srinivas KVNS, Kumar JK, Kumar AN, Rajput DK, Anubala S (2015) Changes in the essential oil content and composition of palmarosa (Cymbopogon martini) harvested at different stages and short intervals in two different seasons. Ind Crops Prod 69:348–354
Kalagatur NK, Nirmal Ghosh OS, Sundararaj N, Mudili V (2018) Antifungal activity of chitosan nanoparticles encapsulated with Cymbopogon martinii essential oil on plant pathogenic Fungi Fusarium graminearum. Front Pharmacol 9:610
Kashyap PL, Xiang X, Heiden P (2015) Chitosan nanoparticle based delivery systems for sustainable agriculture. Int J Biol Macromol 77:36–51
Katiyar D, Hemantaranjan A, Singh B (2015) Chitosan as a promising natural compound to enhance potential physiological responses in plant: a review. Indian J Plant Physiol 20(1):1–9
Kaur P, Thakur R, Choudhary A (2012) An in vitro study of the antifungal activity of silver/chitosan nanoformulations against important seed borne pathogens. Int J Sci Technol Res 1(7):83–86
Khan MIR, Fatma M, Per TS, Anjum NA, Khan NA (2015) Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front Plant Sci 6:462
Kheiri A, Jorf SM, Malihipour A, Saremi H, Nikkhah M (2017) Synthesis and characterization of chitosan nanoparticles and their effect on Fusarium head blight and oxidative activity in wheat. Int J Biol Macromol 102:526–538
Kumar JN, Bora A, Kumar RN, Amb MK, Khan S (2013) Toxicity analysis of pesticides on cyanobacterial species by 16S rDNA molecular characterization. Proc Int Acad Ecol Environ Sci 3(2):101
Kumaraswamy RV, Kumari S, Choudhary RC, Pal A, Raliya R, Biswas P, Saharan V (2018) Engineered chitosan based NMs: bioactivities, mechanisms and perspectives in plant protection and growth. Int J Biol Macromol 113:494–506
Kumaraswamy RV, Kumari S, Choudhary RC, Sharma SS, Pal A, Raliya R, Saharan V (2019) Salicylic acid functionalized chitosan nanoparticle: a sustainable biostimulant for plant. Int J Biol Macromol 123:59–69
Kuźniak E, Urbanek H (2000) The involvement of hydrogen peroxide in plant responses to stresses. Acta Physiol Plant 22(2):195–203
Li Q, Ren J, Dong F, Feng Y, Gu G, Guo Z (2013) Synthesis and antifungal activity of thiadiazole-functionalized chitosan derivatives. Carbohydr Res 373:103–107
Liu CJ, Men WJ, Liu YJ, Zhang H (2002) The pollution of pesticides in soils and its bioremediation. Syst Sci Compr Stud Agric 18(4):295–297
Lopez A, Castro S, Andina MJ (2014) Insecticidal activity of microencapsulated Schinus molle essential oil. Ind Crop Prod 53:209–216
Lu W, Kelly AL, Miao S (2016) Emulsion-based encapsulation and delivery systems for polyphenols. Trends Food Sci Technol 47:1–9
Luo Y, Zhang B, Whent M, Yu L, Wang Q (2011) Preparation and characterization of zein/chitosan complex for encapsulation of tocopherol and its in vitro controlled release study. Colloids Surf B: Biointerfaces 85:145–152
Luque-Alcaraz AG, Cortez-Rocha MO, Velázquez-Contreras CA, Acosta-Silva AL, Santacruz-Ortega HDC, Burgos-Hernández A, Argüelles-Monal WM, Plascencia-Jatomea M (2016) Enhanced Antifungal Effect of Chitosan/Pepper Tree (Schinus molle) Essential Oil Bionanocomposites on the Viability of Asppergillus parasiticus Spores. Journal of Nanomaterials 2016:1–10
Malamy J, Hennig J, Klessig DF (1992) Temperature-dependent induction of salicylic acid and its conjugates during the resistance response to tobacco mosaic virus infection. Plant Cell 4(3):359–366
Malerba M, Cerana R (2016) Chitosan effects on plant systems. Int J Mol Sci 17(7):996
Manikandan A, Sathiyabama M (2016) Preparation of chitosan nanoparticles and its effect on detached rice leaves infected with Pyricularia grisea. Int J Biol Macromol 84:58–61
Martin A, Varona S, Navarrete A, Cocero MJ (2010) Encapsulation and co-precipitation processes with supercritical fluids: applications with essential oils. Open Chem Eng J 4(1)
Martins MDR, Arantes S, Candeias F, Tinoco MT, Cruz-Morais J (2014) Antioxidant, antimicrobial and toxicological properties of Schinus molle L. essential oils. J Ethnopharmacol 151(1):485–492
Marutescu L, Popa M, Saviuc C, Lazar V, Chifiriuc MC (2017) Botanical pesticides with virucidal, bactericidal, and fungicidal activity. In: New pesticides and soil sensors. Academic Press, London, pp 311–335
Mathew TV, Kuriakose S (2013) Photochemical and antimicrobial properties of silver nanoparticle-encapsulated chitosan functionalized with photoactive groups. Mater Sci Eng C 33(7):4409–4415
Medina E, Caro N, Abugoch L, Gamboa A, Diaz-Dosque M, Tapia C (2019) Chitosan thymol nanoparticles improve the antimicrobial effect and the water vapour barrier of chitosan-quinoa protein films. J Food Eng 240:191–198
Mirdehghan HS, Valerob D (2017) Bioactive compounds in tomato fruit and its antioxidant activity as affected by incorporation of Aloe, eugenol, and thymol in fruit package during storage. Int J Food Prop 20:798–806
Mohammadi A, Hashemi M, Hosseini SM (2015) Nanoencapsulation of Zataria multiflora essential oil preparation and characterization with enhanced antifungal activity for controlling Botrytis cinerea, the causal agent of gray mould disease. Innov Food Sci Emerg Technol 28:73–80
Moussa SH, Tayel AA, Alsohim AS, Abdallah RR (2013) Botryticidal activity of nanosized silver-chitosan composite and its application for the control of gray mold in strawberry. J Food Sci 78(10):M1589–M1594
Muzzarelli RAA (2010) Chitins and chitosans as immunoadjuvants and nonallergenic drug carriers. Mar Drugs 8:292–312
Ngoc UTP, Nguyen DH (2018) Synergistic antifungal effect of fungicide and chitosan-silver nanoparticles on Neoscytalidium dimidiatum. Green Proc Synth 7:132–138
Oussalah M, Caillet S, Saucier L, Lacroix M (2006) Inhibitory effects of selected plant essential oils on the growth of four pathogenic bacteria: E. coli O157:H7, Salmonella Typhimurium, Staphylococcus aureus and Listeria monocytogenes. Food Control 18:414–420
Park HJ, Kim SH, Kim HJ, Choi SH (2006) A new composition of nanosized silica-silver for control of various plant diseases. Plant Pathol J 22(3):295–302
Pimentel D (1997) Pest management in agriculture: techniques for reducing pesticide use: environmental and economic benefits. Wiley, New York, p 456
Rao BR, Kaul PN, Syamasundar KV, Ramesh S (2005) Chemical profiles of primary and secondary essential oils of palmarosa (Cymbopogon martinii (Roxb.) Wats var. motia Burk.). Ind Crops Prod 21:121–127
Raskin I, Skubatz H, Tang W, Meeuse BJ (1990) Salicylic acid levels in thermogenic and non-thermogenic plants. Ann Bot 66(4):369–373
Rejinold NS, Muthunarayanan M, Muthuchelian K, Chennazhi KP, Nair SV, Jayakumar R (2011) Saponin-loaded chitosan nanoparticles and their cytotoxicity to cancer cell lines in vitro. Carbohydr Polym 84(1):407–416
Rhouma A, Daoud HB, Ghanmi S, Salah HB, Romdhane M, Demak M (2009) Antimicrobial activities of leaf extracts of Pistacia and Schinus species against some plant pathogenic fungi and bacteria. J Plant Pathol 91(2):339–345
Rubina MS, Vasil’kov AY, Naumkin AV, Shtykova EV, Abramchuk SS, Alghuthaymi MA, Abd-Elsalam KA (2017) Synthesis and characterization of chitosan–copper nanocomposites and their fungicidal activity against two sclerotia-forming plant pathogenic fungi. J Nanostruct Chem 7(3):249–258
Saharan V, Pal A (2016a) Chitosan based NMs in plant growth and protection. Springer, Berlin
Saharan V, Pal A (2016b) Current and future prospects of chitosan-based NMs in plant protection and growth. In: Chitosan based NMs in plant growth and protection. Springer, New Delhi, pp 43–48
Saharan V, Mehrotra A, Khatik R, Rawal P, Sharma SS, Pal A (2013) Synthesis of chitosan based nanoparticles and their in vitro evaluation against phytopathogenic fungi. Int J Biol Macromol 62:677–683
Saharan V, Sharma G, Yadav M, Choudhary MK, Sharma SS, Pal A, Raliya R, Biswas P (2015) Synthesis and in vitro antifungal efficacy of cu-chitosan nanoparticles against pathogenic fungi of tomato. Int J Biol Macromol 75:346–353
Sajed H, Sahebkar A, Iranshahi M (2013) Zataria multiflora Boiss.(Shirazi thyme)-an ancient condiment with modern pharmaceutical uses. J Ethnopharmacol 145(3):686–698
Sathiyabama M, Parthasarathy R (2016) Biological preparation of chitosan nanoparticles and its in vitro antifungal efficacy against some phytopathogenic fungi. Carbohydr Polym 151:321–325
Shukla SK, Mishra AK, Arotiba OA, Mamba BB (2013) Chitosan-based NMs: a state-of-the-art review. Int J Biol Macromol 59:46–58
Storz G, Imlayt JA (1999) Oxidative stress. Curr Opin Microbiol 2(2):188–194
Sun R, Wang W, Wen Y, Zhang X (2015) Recent advance on mesoporous silica nanoparticles-based controlled release system: intelligent switches open up new horizon. NMs 5(4):2019–2053
Tassou C, Drosinos EH, Nychas GJE (1995) Effects of essential oil of mint (Mentha piperita) on Salmonella enteritidis and Listeria monocytogenes in model food systems at 4°C and 10°C. J Appl Bacteriol 78:593–600
Van Long NN, Joly C, Dantigny P (2016) Active packaging with antifungal activities. Int J Food Microbiol 220:73–90
Verma RS, Padalia RC, Chauhan A (2013) Introduction of Cymbopogon distans (Nees ex Steud.) Wats to the sub-tropical India: evaluation of essential oil yield and chemical composition during annual growth. Ind Crops Prod 49:858–863
Vlot AC, Dempsey DMA, Klessig DF (2009) Salicylic acid, a multifaceted hormone to combat disease. Annu Rev Phytopathol 47:177–206
Vokhidova NR, Sattarov ME, Kareva ND, Rashidova SS (2014) Fungicide features of the nanosystems of silkworm (Bombyx mori) chitosan with copper ions. Microbiology 83(6):751–753
Wu SH, Mou CY, Lin HP (2013) Synthesis of mesoporous silica nanoparticles. Chem Soc Rev 42(9):3862–3875
Xing K, Shen X, Zhu X, Ju X, Miao X, Tian J, Qin S (2016) Synthesis and in vitro antifungal efficacy of oleoyl-chitosan nanoparticles against plant pathogenic fungi. Int J Biol Macromol 82:830–836
Xing K, Liu Y, Shen X, Zhu X, Li X, Miao X, Qin S (2017) Effect of O-chitosan nanoparticles on the development and membrane permeability of Verticillium dahliae. Carbohydr Polym 165:334–343
Zhang W (2018) A long-term trend of cancer-induced deaths in European countries. Network 3(1–2):1–9
Zhang W, Jiang F, Ou J (2011a) Global pesticide consumption and pollution: with China as a focus. Proc Int Acad Ecol and Environ Sci 1(2):125
Zhang WJ, van der Werf W, Pang Y (2011b) A simulation model for vegetable-insect pest-insect nucleopolyhedrovirus epidemic system. J Environ Entomol 33(3):283–301
Acknowledgments
Financial support from the DBT-RA Program in Biotechnology and Life Sciences is gratefully acknowledged (Khaidem Aruna Devi). Financial support to Ashok Kumar from ICMR-JRF (ICMR-JRF/2018/30716) and DBT-JRF to Damyanti Prajapati (DBT/2018/MPUAT/1123) also acknowledged.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Devi, K.A. et al. (2020). Smart Nano-Chitosan for Fungal Disease Control. In: Fraceto, L.F., S.S. de Castro, V.L., Grillo, R., Ávila, D., Caixeta Oliveira, H., Lima, R. (eds) Nanopesticides. Springer, Cham. https://doi.org/10.1007/978-3-030-44873-8_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-44873-8_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-44872-1
Online ISBN: 978-3-030-44873-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)