Abstract
Patients with urinary stones are exposed to substantial amounts of radiation during diagnosis, treatment, and follow-up. Percutaneous nephrolithotomy (PCNL) is commonly performed for the management of large and complex renal stones and requires image-guided percutaneous access. This access is commonly done using fluoroscopy, exposing both patients and surgeons to radiation. The understanding of potential risks associated with radiation exposure has recently expanded from malignancy to include ischemic heart disease, cataracts, arthritis, and inflammation. This chapter will first review the hazards associated with radiation exposure, with emphasis on ionizing radiation used for medical imaging. Then, options for evaluation of patients that minimize radiation exposure will be discussed, including ultrasound and low and ultra-low dose CT scans. Next, intraoperative techniques designed to reduce radiation exposure during PCNL will be presented including ultrasound, pulsed fluoroscopy, shielding, distance, and other low dose techniques. In addition, follow-up strategies that minimize radiation exposure will be presented. Finally, after reading this chapter, the surgeon will be well-versed in the tenants of the ALARA principle (as low as reasonably achievable), and able to employ this principle in their practice to keep their patients and themselves safe from the harmful effects of excessive radiation exposure.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The world around us is filled with potential dangers that intrude into our consciousness. These dangers include many visible and tangible threats, including poisonous spiders, venomous snakes, sharks, and lightning strikes. Although these tangible threats may arouse fear within us, the actual risks associated with many of these are quite low. In the United States, spider bites cause six deaths, rattlesnakes kill five people, shark attacks end the life of one, and lightning strikes kill eleven people on average per year.
In contrast, ionizing radiation cannot be felt, heard, or touched, and is completely invisible. For this reason, the risks associated with its use are easy to overlook. Despite being invisible, silent, and intangible, the effects of radiation pose a clear and present danger to the health and safety of kidney stone patients, urologic surgeons, and operating room staff. For patients with urinary stone disease specifically, radiation is used for diagnosis, during treatment, and for follow-up. Exposure to medical radiation from computed tomography (CT) scans, is projected to cause approximately 15,000 deaths per year in the USA alone (Berrington de Gonzalez et al. 2007).
Similarly, fluoroscopy employs ionizing radiation. The fluoroscopy machine was invented by Thomas Edison and much of the early work was done by his assistant Clarence Dally. Tragically, Mr. Dally developed severe non-healing skin burns on his hands requiring amputation, and ultimately became one of the first known casualties of medical radiation exposure when he died at the age of 39 from metastatic cancer. This had a profound effect upon Thomas Edison, who in 1903 said “Don’t talk to me about x-rays; I am afraid of them” (Anon 1903). If the inventor of the fluoroscopy machine was afraid of its risks, perhaps we also should be afraid. Afraid enough that we do not use fluoroscopy indiscriminately and use it only when necessary and in accordance with the principles of ALARA (as low as reasonably achievable).
The aim of this chapter is to outline and review the health hazards associated with the ionizing radiation used in CT scans for the diagnosis and follow-up of stone disease, and fluoroscopy used to treat kidney stones. In addition, percutaneous nephrolithotomy (PCNL) techniques will be presented to facilitate diagnosis, treatment and follow-up of stones using the lowest possible dose of radiation.
2 Basic Principles of Medical Ionizing Radiation
Ionizing radiation has high frequency and energy, and is able to remove electrons from atoms, whether in living tissue or the surrounding environment. X-rays are ionizing radiation that have widespread use in medical imaging. In fact, diagnostic x-ray exposure is considered the largest source of all radiation exposure in the USA, accounting for over 40% of lifetime exposure (Donya et al. 2014). All radiographic studies including conventional plain films, CT scans, fluoroscopy, and nuclear medicine studies expose patients to ionizing radiation.
During medical imaging, sources of radiation include primary beam radiation, which travels directly from the source to the patient and constitutes the largest component of patient exposure, and scatter radiation, which are dispersed waves that are reflected when primary waves encounter the patient or some other object. Scatter radiation doses are lower than primary beam, but constitute the majority of radiation exposure to healthcare workers present during imaging or when performing radiation-guided procedures.
Different dose measurements can be used to quantify radiation exposure, which can create confusion among healthcare professionals. The three most commonly used doses are: absorbed dose, equivalent dose, and effective dose (Mitchell and Furey 2011).
Absorbed dose refers to the ionizing radiation deposited on and absorbed by tissue and is quantified by the unit Gray (Gy). Equivalent dose takes into account the properties of different types of ionizing radiation in causing harm and is calculated from the absorbed dose using radiation weighting factors. For x-rays, the radiation weighting factor is 1; therefore, both absorbed and equivalent doses are equal for medical radiation exposure. Effective dose is a calculated dose that further accounts for the susceptibility of different organs to radiation effects and is calculated from the equivalent dose using tissue weighting factors for a standard reference. Both equivalent dose and effective dose are reported in the unit Sievert (Sv). Effective dose is the radiation measure used in radiation protection practices, including setting annual exposure limits and measuring occupational exposures.
3 Health Risks of Ionizing Radiation
3.1 Radiation Effects—Defining the Terms
The harmful effects of radiation on cells and tissues can be classified into deterministic and stochastic effects. Deterministic effects refer to harms conferred to sensitive tissues as a result of radiation exposure that depends on the duration and dose. After a threshold is reached, any radiation exposure causes harm, and the severity is directly proportional to the dose. Common deterministic effects include dermatitis/burns, alopecia, infertility, cataracts, radiation sickness, and death (in cases of extensive exposure such as nuclear accidents).
Stochastic effects, on the other hand, occur by chance. No radiation threshold is needed for stochastic effects to occur and the severity of the effect is not related to the exposure dose. However, the risk of developing these stochastic effects is proportional to the radiation exposure, the greater the exposure, the more likely stochastic effects will take place. Stochastic effects include malignancy and genetic alterations.
3.2 Radiation Hazards
-
(A)
Cancer
Evidence on the increased risk of malignancy associated with ionizing radiation exposure originates from epidemiological studies conducted on the atomic bomb survivors in Japan (Ozasa 2016). Shortly after the bombings, a high incidence of leukemia was noticed among survivors. For those exposed at a younger age (10 years), the risk of developing leukemia was 70 times higher. A decade after the bombings, an increased incidence of solid malignancies was also recorded and the risk of malignancy was dependent upon the individual dose exposure, such that the risk of developing a solid malignancy increased by 40–50% for each 1 Gy exposure (Ozasa 2016). These studies provided the evidence for the no threshold and linear dose–response relationship between radiation exposure and stochastic effects.
An increased risk of malignancy has also been reported after medical radiation exposure. This is largely based on cohort and case–control studies conducted on pediatric populations who underwent medical imaging. A meta-analysis conducted on these studies estimated the risk of malignancy associated with CT scan imaging in children and reported pooled excess relative risks of 26.9 per Gy for leukemia and 9.1 per Gy for brain tumors (Abalo et al. 2021). In fact, an increasing number of CT scans are being performed in both adults and children, such that up to 2% of malignancies in the United States may be attributable to the use of CT scans (Brenner and Hall 2007).
Similarly, physicians exposed to radiation and those performing radiation-guided interventions are at a higher risk of developing malignancies. In the United States, radiologists were found to have 38% higher all-cancer deaths compared to other specialists, and when analyzing leukemia specifically, the observed deaths were twice as many as expected (Yoshinaga et al. 2004). Radiation technologists were also found to have a higher incidence of leukemia, which was significantly associated with holding 50 or more patients during x-ray exams (relative risk 2.6) and working for five or more years before 1950 (relative risk 6.6) (Linet et al. 2005). The relationship between occupational radiation exposure among health professionals and the ensuing malignancy risk is further supported by the finding of a disproportionately high incidence of left-sided brain tumors among interventional radiologists. These right-handed interventionalists stand with the left side of the brain close to the source and subsequently receive much higher doses on that side (Roguin et al. 2013).
-
(B)
DNA damage
Ionizing radiation can lead to DNA damage, including direct induction of double-strand DNA breaks. In fact, radiation-induced DNA damage leading to cell death forms the basis of radiation therapy for certain malignant neoplasms. However, not all radiation-induced DNA damage leads to cell death and if abnormal DNA accumulates or is inappropriately repaired, this may lead to consequences such as chromosomal abnormalities or malignancies.
One study measured the effects of acute radiation exposure on DNA among vascular surgeons and interventional radiologists after performing endovascular aortic repair (El-Sayed et al. 2017). In this study, all operators wore standard lead aprons and thyroid shields. After the procedure, blood samples from surgeons were collected. White blood cell markers of DNA damage and DNA repair were measured and were significantly elevated compared to baseline. A repeat test 24 hours later, revealed the markers had returned to baseline. During the same study, dosimeters placed on the surgeons’ legs recorded significantly higher radiation exposures. A subset of surgeons was later asked to wear lower leg shields, and when doing so, there was no increase in DNA damage and repair markers after the procedure, suggesting that these leukocytes acquired DNA damage as they circulated in unshielded regions.
-
(C)
Cardiovascular disease
Astronauts must be completely healthy with no medical comorbidities. Subsequently, it was not surprising that astronauts who have never flown or who flew low Earth orbital missions were significantly less likely to die of cardiovascular disease (CVD) compared to the age-matched general population. Conversely, the Apollo Lunar astronauts who flew into deep space had significantly higher CVD mortality compared to the other groups of astronauts (Delp et al. 2016). This may be attributed to the large amounts of cosmic radiation astronauts were exposed to when travelling beyond Earth’s atmosphere.
Radiation can induce oxidative stress, increase inflammation, promote a profibrogenic state, and cause direct endothelial dysfunction (Meerman et al. 2021). This in turn may lead to accelerated atherosclerosis, ischemic heart disease, arrythmias, conduction defects, myocardial remodeling, cardiomyopathy, and heart failure.
Similarly, exposure to medical radiation can also increase the risk of CVD. Although medical imaging utilizes relatively less radiation than deep space travel, there is evidence of increased CVD with these smaller exposure levels, as demonstrated by a recent study on a large cohort of patients with tuberculosis who underwent repetitive fluoroscopy screenings (Tran et al. 2017). They reported approximately 25% higher excess relative risks per Gy for the development of all circulatory disease (p = 0.021) and for ischemic heart disease specifically (p = 0.048) among those exposed to cumulative radiation doses less than 0.5 Gy.
-
(D)
Thyroid disease
Ionizing radiation is a known dose and age-related risk factor for developing thyroid cancer. Adults with low occupational radiation exposure have higher rates of thyroid cancer and a dose-related higher incidence of subclinical hypothyroidism (Luna-Sanchez et al. 2019).
-
(E)
Cataracts
Occupational radiation exposure and its relationship to cataracts has been well documented. In one study, 52% of interventional cardiologists were found to have posterior lens opacities compared to 9% of controls, giving a significant relative risk of 5.7 (Ciraj-Bjelac et al. 2012). A similar high prevalence of 45% was also found among nurses working in interventional cardiology. A recent meta-analysis of 15 studies (n > 5,600), reported a 4.96 times greater risk of cataracts in the group with occupational radiation exposure (p < 0.00001) (Alhasan and Aalam 2022).
-
(F)
Skin Changes
Patients receiving radiation therapy often experience radiation dermatitis, a common adverse effect due to the high amounts of exposure. Acute changes include desquamation, erythema, and hair loss, while chronic changes include atrophy, fibrosis, and pigmentation abnormalities (Hegedus et al. 2017). These skin changes are deterministic effects, which depend on the radiation dose received.
The exposures from medical imaging are unlikely to reach the threshold to cause radiation dermatitis similar to that seen in radiation therapy. However, recently more than 200 patients undergoing CT brain scans for the evaluation of stroke developed alopecia and skin burns in a single academic institution (Kuehn 2010). A subsequent FDA review found that the doses were increased to improve the image quality of the studies, with patient radiation exposure reaching up to 8 times greater than expected. Alarmingly, a review of regional hospitals found similar exposures in two other institutions. This led the US FDA to issue a white paper in 2010 specifically calling for a reduction in radiation exposure during CT scans, fluoroscopy, and nuclear medicine studies (United States Food and Drug Administration 2010).
In addition, surgeons who perform image-guided procedures with their hands under the direct radiation beam are exposed to relatively high doses for prolonged periods, leading to significant cumulative exposures. In fact, one study estimated that cumulative dose exposures to the dominant hand of urologists can reach levels up to 75 times higher than other regions (Park et al. 2021). In another study on orthopedic surgeons, high amounts of direct radiation exposure to the hands was associated with skin and nail pigmentation abnormalities (Fig. 1) (Asari et al. 2022).
Surgeon’s hands demonstrating skin changes from occupational radiation exposure. These include nail discoloration, depigmentation, hyperkeratosis, and papules. Figure reproduced from Asari et al. (2022). With permission from Elsevier
-
(G)
Other Effects
Ionizing radiation can affect any organ, however different organs have different susceptibilities and the effects depend on the dose and site of exposure. Radiation cystitis, radiation proctitis, and infertility are some effects of pelvic radiotherapy. Stomatitis, dysphagia, and alopecia are some effects of head and neck radiotherapy. Exposure to ionizing radiation may also trigger autoimmune diseases (Yahyapour et al. 2018), and chronic occupational exposure may lead to male infertility by causing damage to sperm DNA (Zhou et al. 2016). Surgeons with their hands in the direct radiation beam may experience hand joint pain and osteoarthritic changes (Willey et al. 2013).
3.3 Exposure Limits
To control radiation exposure and minimize harms, the International Commission on Radiological Protection (ICRP) has set occupational limits to an effective dose of 20 mSv per year, averaged over 5 years (no more than 100 mSv over 5 years) and also states the effective dose should not exceed 50 mSv annually (International Commission on Radiological Protection (ICRP) Guidance for Occupational Exposure 2023). The United States Nuclear Regulatory Commission (NRC) also sets whole body effective dose to 50 mSv per year (United States Nuclear Regulatory Commision Occupational Dose Limits 2023). These exposure limits are occupational upper limits. Furthermore, there are no guidelines regarding patient medical radiation exposure limits, but the goal should be to keep exposure as low as reasonably achievable (ALARA).
4 Radiation in Stone Disease—Diagnosis and Pre-operative Use
Patients with nephrolithiasis often undergo imaging to establish the diagnosis and plan for surgical intervention. The three most commonly used modalities are CT scan, kidney ureter bladder (KUB), and ultrasound (US).
The estimated radiation exposure to a patient from a single KUB is 0.7 mSv (Brisbane et al. 2016). However, a KUB film has low reported sensitivity (57%) in diagnosing nephrolithiasis and a specificity of 76% (Brisbane et al. 2016). A KUB is limited in that it only detects stones from a single angle and cannot visualize all stone types, including uric acid, cystine, and struvite stones.
US does not utilize ionizing radiation and is relatively inexpensive. There is marked variation in the reported sensitivity (45–84%) and specificity (53–94%) of US as a diagnostic modality (Brisbane et al. 2016). This may be attributed to the user-dependent nature of US imaging, as well as many factors which might affect image production including stone size, echogenicity, and patient body habitus.
CT scans have become widely available and are the most commonly used imaging modality for investigating urinary stones (Smith-Bindman et al. 2014). Up to three-quarters of patients presenting to the emergency department with flank pain or hematuria may receive CT imaging (Broder et al. 2007). CT has the highest sensitivity (95%) and specificity (98%) for detecting renal stones (Brisbane et al. 2016). It is able to detect almost all stone types, can provide multi-dimensional information regarding stone burden, and information regarding stone density. It also provides important anatomic information regarding the location of surrounding organs and the lungs, and detailed anatomic positions of the stones in three dimensions that can assist the surgeon in selecting the site of access prior to PCNL. Conversely, it is 10 times more expensive than a KUB and exposes patients to almost 15 times more radiation, at an average effective dose of 10–20 mSv per CT scan (Brisbane et al. 2016; Smith-Bindman et al. 2009). It is estimated that a single conventional non-contrast CT will lead to 1 in 1000 patients developing a fatal malignancy (Jellison et al. 2009).
Given the relative affordability of US and lack of radiation, the European Association of Urology (EAU) recommends US as the first diagnostic imaging modality for patients presenting with a picture suggestive of nephrolithiasis and recommends CT scan for confirmation of nephrolithiasis after the initial ultrasound (Skolarikos et al. 2022). The American Urological Association (AUA) guidelines for surgical management of stones recommend clinicians order a non-contrast CT scan prior to performing PCNL (Assimos et al. 2016). They also suggest clinicians order CT scans in stone patients to help determine the best management option when deciding between shock-wave lithotripsy (SWL) and ureteroscopy (URS).
Reducing radiation during evaluation of stone patients:
Safe radiation practices start from the time of patient presentation with a clinical picture suggestive of a urinary stone. Emergency care and primary care providers should be judicious in their selection of the appropriate diagnostic tests and imaging modality based on their clinical evaluation of the patient. In a multicenter prospective study (n = 2759), patients presenting to the emergency department with flank or abdominal pain were randomized to undergo either CT scan, formal US, or point-of-care US (Smith-Bindman et al. 2014). All three groups had similar adverse events and emergency department returns, but the two US groups had 40–46% lower radiation exposure compared to the CT group (p < 0.001).
If a CT scan is desired or indicated based on clinical judgement, the radiation exposure can be reduced by lowering the mAs or kVp. Low dose CT scans utilize settings to deliver < 3–4 mSv to the patient while maintaining diagnostic accuracy for urinary stones, with a meta-analysis including seven studies reporting pooled sensitivity and specificity of 97% and 95% respectively (Niemann et al. 2008). Ultra-low dose CT scans deliver doses less than 1–2 mSv and have also been reported to have satisfactory diagnostic ability for stones > 4 mm in size (Pooler et al. 2014). A cadaveric study, with blinded radiologists showed that reducing the mAs (140 to 7.5) resulted in no change in sensitivity and specificity in detecting calcium oxalate stones and resulted in a 95% reduction in radiation exposure (Fig. 2) (Jellison et al. 2009).
Comparison of imaging between conventional 140 mAs CT with low and ultra-low dose CT settings. Figure reproduced from Jellison et al. (2009). With permission from Wolters Kluwer
The main drawback of lowering the CT scan dose is a decline in the sensitivity and specificity for stone detection in patients who are overweight or underweight. The diagnostic accuracy of ultra-low dose CT scan (≤ 7.5 mAs) was found to be significantly lower with high and low body weights compared to average weight, however when the mAs was increased to 15, the diagnostic accuracy was similar (Heldt et al. 2012). Reconstruction algorithms can also affect the image produced and alter the diagnostic ability of low dose CT scans. It has been reported that iterative image reconstruction is preferable for diagnosing stones in overweight patients using low dose CT scans (Chang et al. 2019). One exception to the performance of ultra-low dose CT would be a suspected infected obstructed stone, where the 20% mortality of urosepsis trumps the 0.1% risk of mortality from the conventional CT scan (Borofsky et al. 2013). Also, patients with metal implants or hardware provide suboptimal picture quality with ultra-low dose CT due to the hardware-induced noise.
5 Radiation in Stone Disease—Intra-Operative Use
After diagnosing nephrolithiasis, surgical management may be indicated including SWL, URS, or PCNL. All of these approaches require image guidance, and fluoroscopy has commonly been used. Fluoroscopy is used to localize stones for SWL, and is used for stone localization and to guide insertion of wires and instruments in retrograde intrarenal surgery. For PCNL, image guidance is particularly crucial for establishing percutaneous access into the renal collecting system, which is also commonly performed under fluoroscopic guidance. During PCNL, fluoroscopy is also used to insert guidewires, for tract dilation, sheath insertion, identifying renal and surrounding anatomy, evaluating for residual stones, and stent or nephrostomy tube placement. One study reported a mean effective dose of 8.66 mSv from fluoroscopic radiation exposure during PCNL (Mancini et al. 2010). It is at this stage that surgeons may be exposed to both direct and scatter radiation. More recently, use of intraoperative CT scan as a surgical adjunct to confirm stone-free status has been reported (Patel et al. 2022). Use of conventional CT intraoperatively could substantially increase radiation exposure.
5.1 Fluoroscopy Settings and Doses
The radiation dose from fluoroscopy depends on: (1) the amount of radiation produced by the machine, measured in milliamperes (mA); and (2) the energy of the x-ray produced, reflecting its ability to penetrate objects, which is measured as the kilovoltage peak (kVp). The standard fluoroscopy machine setting is automatic exposure control (AEC), which automatically adjusts mA and kVp to optimize image quality.
5.2 Factors Affecting Radiation Dose During PCNL
Several studies have investigated factors which affect radiation doses during urologic procedures. Mancini et al. reported significantly higher effective dose during PCNL with increased BMI (p < 0.0001), greater stone burden (p = 0.04), and more access tracts (p = 0.024) (Mancini et al. 2010). Balaji et al. examined surgeon radiation exposure during PCNL and also reported significantly higher exposures with larger stone burden, multiple tracts, larger sheath size, and lower stone Hounsfield units (Balaji et al. 2019).
Although many of the above factors are beyond the control of the treating surgeon, it is important to consider their potential contribution to higher radiation exposure. In contrast, there are many factors which are directly under the control of the surgeon, including machine settings, shielding, collimation, positioning, and surgical technique.
5.3 Reducing Radiation During PCNL
-
(A)
Fluoroscopy settings and use
The standard fluoroscopy mode is AEC, which uses continuous fluoroscopy at 30 pulses per second (pps), and continuously adjusts the mA and kVp to optimize image quality. However, many tasks during PCNL do not require optimal image quality, including confirming the position of guidewires, endoscopes, nephrostomies, and stents. For these tasks, where optimal image quality is not required, the surgeon can manually adjust the mA, kVp, pulse rate, and employ the “low dose” modality. Depression of the “low dose” button (Fig. 3) which is present on most modern c-arms has been shown to cut radiation exposure by more than half (Yecies et al. 2018).
Canales et al. compared radiation exposures and outcomes of PCNL between standard AEC (30 pps) and a protocol where the pulse rate was cut to 12 pps and the dose output was cut in half. They reduced the mean radiation exposure from 35.5 to 23.9 mGy (p < 0.001) (Canales et al. 2016). In their study, no patient required conversion from the reduced radiation protocol back to AEC, and the outcomes were similar.
In addition to altering the output dose and pulse rate, other settings on the fluoroscopy machine can also be employed to reduce radiation. Using a second screen with last image hold allows the surgeon to review the image without the use of live fluoroscopy (Mitchell and Furey 2011). Surgeon, instead of technician, fluoroscopy activation can also reduce radiation dose by eliminating unnecessary pedal activation. Another technique which significantly reduces radiation exposure is the use of collimation. In collimation, the x-ray beam leaving the source is narrowed to limit the field of exposure to only the areas of interest. Collimation is associated with improved image quality and reduced scatter radiation (Mitchell and Furey 2011).
-
(B)
Positioning and Set-Up
C-arm positioning is crucial and can affect radiation dose (Mitchell and Furey 2011). Keeping the source underneath the patient reduces the scatter to staff members in the operating room (Harris 2018). When the x-ray source is close to the patient, the entrance dose will be higher. Subsequently, it is important to keep the x-ray source as far away from the patient as possible. In other words, the image intensifier or flat panel detector should be as close to the patient as possible. In addition, all staff should stand as far from the source as possible while completing their tasks. According to the inverse square law (dose ∝ 1/distance2), if the distance from the source is doubled, the radiation dose is cut to a fourth (Le Heron et al. 2010). Non-essential personnel can step out of the room when fluoroscopy is in use.
Some c-arms come equipped with a laser pointer which will allow positioning of the c-arm without fluoroscopy activation. Markings on the drape can help with instrument location and depth, minimizing the need to use imaging to confirm placement. Markings on the drape or the floor can also be used to guide the technician in positioning the c-arm appropriately, minimizing “trial-and-error” in obtaining the desired image.
-
(C)
Surgical technique
Modification of surgical techniques can further reduce the need for fluoroscopy. For example, in many steps of the procedure, relying on tactile feedback can decrease the need for imaging. Guidewires and the ureteroscope can be positioned using tactile feedback, reducing the need for fluoroscopy (Blair et al. 2013). Positioning of the surgeons’ hands and equipment out of the primary beam can also reduce radiation exposure (Hajiha et al. 2019).
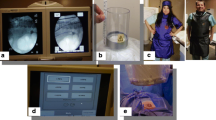
During PCNL, ancillary techniques have been developed that can reduce the requirement for fluoroscopy while obtaining percutaneous renal access. These modifications include use of ultrasound, endoscopic, laser, and electromagnetic guidance. US is extremely effective in locating the lung, pleura, and surrounding organs. US can also assess depth to the calyx of interest and aid in needle selection. Many authors have reported use of US to facilitate renal access and some have even performed PCNL completely under US guidance (Fig. 4A) (Emiliani et al. 2020).
Use of ureteroscopy in an endoscopic combined intrarenal approach (ECIRS) for access, can further reduce radiation as it will allow direct visual confirmation of needle position (Fig. 4B). Small corrections in needle position can be made under direct visualization. Similarly, balloon dilation and sheath insertion can be performed under direct vision to allow precise positioning that is not possible using fluoroscopy. A study comparing conventional fluoroscopic access to combined US and endoscopic access for PCNL reported 99% lower total fluoroscopy times, with similar outcomes in terms of complication and stone-free rates (Alsyouf et al. 2016).
The laser direct alignment radiation reduction technique (DARRT) is a hybrid technique which employs US and laser guidance. US is used to identify surrounding structures and the laser beam on the image intensifier is used to guide and maintain needle orientation during insertion (Fig. 4C), without the need for continuous fluoroscopic guidance. This has also been reported to significantly reduce fluoroscopy time (Khater et al. 2016).
Finally, a new robotic platform has recently become available which uses electromagnetic-guided percutaneous access. This device has just been approved by the FDA and may show promise for reducing radiation exposure to both patients and surgeons.
-
(D)
Shielding
Use of lead shielding by medical personnel can significantly reduce their radiation exposure by more than 90% (Ong et al. 2021). These include aprons, vests and kilts, thyroid shields, and goggles. Given the availability of these personal protective equipment, it is expected that healthcare workers will take all necessary precautions to minimize harms to themselves, however a recent European study reported that although more than 99% wear aprons, only 52% wear thyroid shields, and only 7% wear lead glasses (Ong et al. 2021). In addition, maintaining the condition of these protective garments, with proper storage and periodic checks on their efficacy is also important to ensure maximal radiation protection. Other protective shields may also include under-table shields, table skirts, suspended shields, and portable rolling shields.
-
(E)
Awareness
Awareness of radiation safety and reduction techniques has been demonstrated to cut fluoroscopy time in half (Weld et al. 2014). In one study, almost 44% of operating room staff working with radiation, reported receiving no training on radiation safety (Ong et al. 2021). It is imperative that staff utilizing fluoroscopy are aware of its potential risks and strategies for reducing these risks. Surgeons and other staff, especially those who work in high volume centers, should also wear dosimeters to keep track of their cumulative exposures. Use of dedicated radiology technicians, who know how to calibrate, orient, and maneuver the c-arm, provides optimal images at the lowest radiation possible.
Keeping track of radiation during the procedure can help focus the surgeon’s attention on reducing radiation exposure. This includes providing real time feedback to the surgeon on fluoroscopy time used, verbal warning by the surgeon to other personnel when fluoroscopy will be activated, and developing institutional protocols and standards to limit radiation. Figure 5 summarizes many different strategies that can be employed to reduce radiation during PCNL.
6 Radiation in Stone Disease—Follow-Up
After PCNL and other stone procedures, it is important to ensure there are no residual fragments which can subsequently lead to complications such as infection, dislodgement, obstruction, or growth and recurrence of stones. Post-operative imaging is also important to make sure there is no obstruction or hydronephrosis. There is no unanimous consensus on the appropriate imaging after PCNL, however similar to its use for diagnosis, CT scan has the highest sensitivity in detecting residual fragments, with a reported sensitivity of 100% compared to direct flexible nephroscopy (Pearle et al. 1999).
The AUA guidelines do not make explicit recommendations regarding follow-up of patients who underwent PCNL or other stone surgeries (Assimos et al. 2016). They do, however, recommend follow-up for stone patients managed by observation, but do not recommend a specific imaging modality or frequency. The EAU do make recommendations on follow-up after surgical treatment and give recommended durations and frequencies for imaging, depending on the presence of residual fragments post-operatively, the size of the residual fragments, and patient risk stratification (Skolarikos et al. 2022). The EAU also proposes KUB, US, or a combination of both as the imaging modality for follow-up, unless a patient becomes symptomatic, for which a CT scan should be ordered.
Reducing radiation for follow-up:
Ordering a low dose or ultra-low dose CT scan after PCNL to check for residual fragments or obstruction is an appropriate alternative to full dose CT scan. Similar to pre-operative and diagnostic use, deciding on the appropriate follow-up imaging should consider all patient factors and risks, while keeping radiation safety principles in mind. One should only order imaging studies with ionizing radiation that will affect management.
7 Special Populations
7.1 Children
Although the general approach to managing stone disease in children while maintaining radiation safety precautions is fairly similar to adults, clinicians should remember that because of their younger age and smaller body habitus, children are at a significantly higher risk for malignancy.
One recent study quantified the radiation dose for pediatric PCNL and reported a median fluoroscopy time of 11.7 minutes, with an estimated effective dose of 16.8 mSv (Ristau et al. 2015). One unique concern in children is the technical challenge in inserting the sheath, which may require additional fluoroscopy. This can be minimized by using retrograde ureteroscopic guidance for tract dilation and sheath insertion. Another study reported that the mean effective dose of an abdomen-pelvic CT scan in a child ranged from 10.6 to 14.8 mSv, with up to 25% of children receiving doses greater that 20 mSv (Miglioretti et al. 2013). When adding these numbers up, the exposure to a child undergoing preoperative CT scan, PCNL, and postoperative CT scan may surpass the annual occupational exposure limit for an adult.
With regards to guidelines on managing stones in the pediatric population, the AUA recommends obtaining a low dose CT scan if PCNL is planned (Assimos et al. 2016), while the EAU recommends US as the initial diagnostic test, followed by a KUB or a low dose CT if needed (Skolarikos et al. 2022).
7.2 Pregnancy
Developing fetuses are particularly susceptible to ionizing radiation. For diagnostic purposes, the EAU recommends US, followed by magnetic resonance imaging (MRI) if needed, with low dose CT scan being a last resort after careful consideration (Assimos et al. 2016). First-line management should be observation, with URS being a possible alternative should observation fail (Skolarikos et al. 2022). For pregnant women with stones, PCNL and SWL are contraindicated.
Pregnant surgeons and healthcare workers should be vigilant regarding the cumulative radiation dose they are exposed to and ensure all safety measures are in place, as the occupational exposure limit to the fetus during the entire pregnancy is set by the IRCP at 1 mGy (International Commission on Radiological Protection (ICRP) 2000).
8 Clinical Scenarios
Case A: Mr. Smith is a 55-year-old man who presented to the urgent care with right flank pain and hematuria. A conventional CT scan of the abdomen and pelvis was ordered and revealed a 3 cm right renal pelvic stone. Mr. Smith was referred to a urologist and PCNL was performed under fluoroscopy guidance with no radiation reduction protocols. The total fluoroscopy time was 9 minutes. A follow-up conventional CT scan was ordered on postoperative day 1 and revealed no residual fragments and a successful procedure. The total radiation doses received by Mr. Smith during the management of his stone were calculated and yielded: 16 mSv from preoperative CT scan, 6.3 mSv from intraoperative fluoroscopy, and 12 mSv from postoperative CT scan. Total radiation exposure was 34.3 mSv.
Case B: Mr. Jones is a 62-year-old man who presented to the urgent care with left flank pain. A renal ultrasound was ordered and revealed a large stone in the left kidney. Upon referral to a urologist trained in radiation safety, a low-dose CT scan was ordered for planning before PCNL. PCNL was then performed using a low-radiation protocol and combined access with ultrasound, laser, and endoscopic guidance, giving a total fluoroscopy time of 10 seconds. To ensure complete stone removal and no obstruction, an ultra-low dose CT scan confirmed the stone-free status. Mr. Jones received no radiation during ultrasound, 3.3 mSv from preoperative CT, 0.17 mSv from intraoperative fluoroscopy, and 1.1 mSv from postoperative CT scan. Total radiation exposure was 4.57 mSv.
When calculating the total radiation received during the stone episode, Mr. Smith received 7.5 times more ionizing radiation than Mr. Jones, yet both underwent similar procedures that resulted in similar favorable outcomes. Implementing radiation safety practices is essential, particularly in stone patients who may have recurrent episodes and may require repeat imaging and additional image-guided procedures. In one study, the radiation exposure received by stone patients over one year was quantified and was reported to be a median annual total effective dose of 29.7 mSv per patient, with one in five patients receiving radiation doses greater than the annual occupational limit (> 50 mSv) (Ferrandino et al. 2009). What is even more striking, is that these large values were calculated only from imaging studies and did not include radiation exposures from any operative procedures.
9 Summary of Key Points
-
Radiation is important for diagnostic imaging and procedural guidance in endourology but can lead to high exposure levels to both patients and surgeons.
-
High radiation exposure has been associated with an increased risk of malignancy, DNA damage, cardiovascular disease, thyroid disorders, cataracts, skin disease, and other health hazards.
-
Use of low dose or ultra-low dose non-contrast CT scan is a suitable alternative to standard CT in most patients and minimizes radiation exposure without compromising sensitivity and specificity for diagnosis and follow-up of urolithiasis.
-
Fluoroscopy settings and positioning that can lower radiation include pulsed mode, low dose settings, collimation, last image hold, surgeon foot pedal activation, x-ray source as far as possible from the patient, source below patient, and use of lasers and markings to guide c-arm positioning.
-
Use of ultrasound, ECIRS, laser, or electromagnetic guidance during percutaneous renal access can significantly reduce the fluoroscopy needed, while ensuring safety and efficacy.
-
Use of lead shielding, tracking radiation times, staff awareness and education are important to reduce radiation exposure.
-
The concept of ALARA, as low as reasonably achievable, should be followed in everyday practice.
References
Abalo KD, Rage E, Leuraud K, Richardson DB, Le Pointe HD, Laurier D, et al. Early life ionizing radiation exposure and cancer risks: systematic review and meta-analysis. Pediatr Radiol. 2021;51(1):45–56. https://doi.org/10.1007/s00247-020-04803-0.
Alhasan AS, Aalam WA. Eye lens opacities and cataracts among physicians and healthcare workers occupationally exposed to radiation: a systematic review and meta-analysis. Saudi Med J. 2022;43(7):665–77. https://doi.org/10.15537/smj.2022.43.7.20220022.
Alsyouf M, Arenas JL, Smith JC, Myklak K, Faaborg D, Jang M, et al. Direct endoscopic visualization combined with ultrasound guided access during percutaneous nephrolithotomy: a feasibility study and comparison to a conventional cohort. J Urol. 2016;196(1):227–33. https://doi.org/10.1016/j.juro.2016.01.118.
Anon. Edison fears hidden perils of the X-Rays. New York World; 1903.
Asari T, Rokunohe D, Sasaki E, Kaneko T, Kumagai G, Wada K, et al. Occupational ionizing radiation-induced skin injury among orthopedic surgeons: A clinical survey. J Orthop Sci. 2022;27(1):266–71. https://doi.org/10.1016/j.jos.2020.11.008.
Assimos D, Krambeck A, Miller NL, Monga M, Murad MH, Nelson CP, et al. Surgical management of stones: American urological association/endourological society guideline. PART II J Urol. 2016;196(4):1161–9. https://doi.org/10.1016/j.juro.2016.05.091.
Balaji SS, Vijayakumar M, Singh AG, Ganpule AP, Sabnis RB, Desai MR. Analysis of factors affecting radiation exposure during percutaneous nephrolithotomy procedures. BJU Int. 2019;124(3):514–21. https://doi.org/10.1111/bju.14833.
Berrington de Gonzalez A, Mahesh M, Kim KP, Bhargavan M, Lewis R, Mettler F, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071–7. https://doi.org/10.1001/archinternmed.2009.440.
Blair B, Huang G, Arnold D, Li R, Schlaifer A, Anderson K, et al. Reduced fluoroscopy protocol for percutaneous nephrostolithotomy: feasibility, outcomes and effects on fluoroscopy time. J Urol. 2013;190(6):2112–6. https://doi.org/10.1016/j.juro.2013.05.114.
Borofsky MS, Walter D, Shah O, Goldfarb DS, Mues AC, Makarov DV. Surgical decompression is associated with decreased mortality in patients with sepsis and ureteral calculi. J Urol. 2013;189(3):946–51. https://doi.org/10.1016/j.juro.2012.09.088.
Brenner DJ, Hall EJ. Computed tomography–an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277–84. https://doi.org/10.1056/NEJMra072149.
Brisbane W, Bailey MR, Sorensen MD. An overview of kidney stone imaging techniques. Nat Rev Urol. 2016;13(11):654–62. https://doi.org/10.1038/nrurol.2016.154.
Broder J, Bowen J, Lohr J, Babcock A, Yoon J. Cumulative CT exposures in emergency department patients evaluated for suspected renal colic. J Emerg Med. 2007;33(2):161–8. https://doi.org/10.1016/j.jemermed.2006.12.035.
Canales BK, Sinclair L, Kang D, Mench AM, Arreola M, Bird VG. Changing default fluoroscopy equipment settings decreases entrance skin dose in patients. J Urol. 2016;195(4 Pt 1):992–7. https://doi.org/10.1016/j.juro.2015.10.088.
Chang DH, Slebocki K, Khristenko E, Herden J, Salem J, Grosse Hokamp N, et al. Low-dose computed tomography of urolithiasis in obese patients: a feasibility study to evaluate image reconstruction algorithms. Diabetes Metab Syndr Obes. 2019;12:439–45. https://doi.org/10.2147/DMSO.S198641.
Ciraj-Bjelac O, Rehani M, Minamoto A, Sim KH, Liew HB, Vano E. Radiation-induced eye lens changes and risk for cataract in interventional cardiology. Cardiology. 2012;123(3):168–71. https://doi.org/10.1159/000342458.
Delp MD, Charvat JM, Limoli CL, Globus RK, Ghosh P. Apollo lunar astronauts show higher cardiovascular disease mortality: possible deep space radiation effects on the vascular endothelium. Sci Rep. 2016;6:29901. https://doi.org/10.1038/srep29901.
Donya M, Radford M, ElGuindy A, Firmin D, Yacoub MH. Radiation in medicine: origins, risks and aspirations. Glob Cardiol Sci Pract. 2014;2014(4):437–48. https://doi.org/10.5339/gcsp.2014.57.
El-Sayed T, Patel AS, Cho JS, Kelly JA, Ludwinski FE, Saha P, et al. Radiation-induced DNA damage in operators performing endovascular aortic repair. Circulation. 2017;136(25):2406–16. https://doi.org/10.1161/CIRCULATIONAHA.117.029550.
Emiliani E, Kanashiro A, Chi T, Perez-Fentes DA, Manzo BO, Angerri O, et al. Fluoroless endourological surgery for stone disease: a review of the literature-tips and tricks. Curr Urol Rep. 2020;21(7):27. https://doi.org/10.1007/s11934-020-00979-y.
Ferrandino MN, Bagrodia A, Pierre SA, Scales CD, Jr., Rampersaud E, Pearle MS, et al. Radiation exposure in the acute and short-term management of urolithiasis at 2 academic centers. J Urol. 2009;181(2):668–72; discussion 73. https://doi.org/10.1016/j.juro.2008.10.012.
Hajiha M, Smith J, Amasyali AS, Groegler J, Shah M, Alsyouf M, et al. The effect of operative field instrument clutter during intraoperative fluoroscopy on radiation exposure. J Endourol. 2019;33(8):626–33. https://doi.org/10.1089/end.2019.0285.
Harris AM. Radiation exposure to the urologist using an overcouch radiation source compared with an undercouch radiation source in contemporary urology practice. Urology. 2018;114:45–8. https://doi.org/10.1016/j.urology.2017.12.011.
Hegedus F, Mathew LM, Schwartz RA. Radiation dermatitis: an overview. Int J Dermatol. 2017;56(9):909–14. https://doi.org/10.1111/ijd.13371.
Heldt JP, Smith JC, Anderson KM, Richards GD, Agarwal G, Smith DL, et al. Ureteral calculi detection using low dose computerized tomography protocols is compromised in overweight and underweight patients. J Urol. 2012;188(1):124–9. https://doi.org/10.1016/j.juro.2012.02.2568.
International Commission on Radiological Protection (ICRP) Guidance for Occupational Exposure. https://remm.hhs.gov/ICRP_guidelines.htm Accessed 15 Feb 2023.
International Commission on Radiological Protection (ICRP). Pregnancy and Medical Radiation; 2000.
Jellison FC, Smith JC, Heldt JP, Spengler NM, Nicolay LI, Ruckle HC, et al. Effect of low dose radiation computerized tomography protocols on distal ureteral calculus detection. J Urol. 2009;182(6):2762–7. https://doi.org/10.1016/j.juro.2009.08.042.
Khater N, Shen J, Arenas J, Keheila M, Alsyouf M, Martin JA, et al. Bench-top feasibility testing of a novel percutaneous renal access technique: the laser direct alignment radiation reduction technique (DARRT). J Endourol. 2016;30(11):1155–60. https://doi.org/10.1089/end.2016.0170.
Kuehn BM. FDA warning: CT scans exceeded proper doses. JAMA. 2010;303(2):124. https://doi.org/10.1001/jama.2009.1906.
Le Heron J, Padovani R, Smith I, Czarwinski R. Radiation protection of medical staff. Eur J Radiol. 2010;76(1):20–3. https://doi.org/10.1016/j.ejrad.2010.06.034.
Linet MS, Freedman DM, Mohan AK, Doody MM, Ron E, Mabuchi K, et al. Incidence of haematopoietic malignancies in US radiologic technologists. Occup Environ Med. 2005;62(12):861–7. https://doi.org/10.1136/oem.2005.020826.
Luna-Sanchez S, Del Campo MT, Moran JV, Fernandez IM, Checa FJS, de la Hoz RE. Thyroid function in health care workers exposed to ionizing radiation. Health Phys. 2019;117(4):403–7. https://doi.org/10.1097/HP.0000000000001071.
Mancini JG, Raymundo EM, Lipkin M, Zilberman D, Yong D, Banez LL, et al. Factors affecting patient radiation exposure during percutaneous nephrolithotomy. J Urol. 2010;184(6):2373–7. https://doi.org/10.1016/j.juro.2010.08.033.
Meerman M, Bracco Gartner TCL, Buikema JW, Wu SM, Siddiqi S, Bouten CVC, et al. Myocardial disease and long-distance space travel: solving the radiation problem. Front Cardiovasc Med. 2021;8: 631985. https://doi.org/10.3389/fcvm.2021.631985.
Miglioretti DL, Johnson E, Williams A, Greenlee RT, Weinmann S, Solberg LI, et al. The use of computed tomography in pediatrics and the associated radiation exposure and estimated cancer risk. JAMA Pediatr. 2013;167(8):700–7. https://doi.org/10.1001/jamapediatrics.2013.311.
Mitchell EL, Furey P. Prevention of radiation injury from medical imaging. J Vasc Surg. 2011;53(1 Suppl):22S-7Shttps://doi.org/10.1016/j.jvs.2010.05.139
Niemann T, Kollmann T, Bongartz G. Diagnostic performance of low-dose CT for the detection of urolithiasis: a meta-analysis. AJR Am J Roentgenol. 2008;191(2):396–401. https://doi.org/10.2214/AJR.07.3414.
Ong K, Warren H, Nalagatla S, Kmiotek E, Obasi C, Shanmugathas N, et al. Radiation safety knowledge and practice in urology theaters: a collaborative multicenter survey. J Endourol. 2021;35(7):1084–9. https://doi.org/10.1089/end.2020.0955.
Ozasa K. Epidemiological research on radiation-induced cancer in atomic bomb survivors. J Radiat Res. 2016;57 Suppl 1:i112-i7. https://doi.org/10.1093/jrr/rrw005
Park IW, Kim SJ, Shin D, Shim SR, Chang HK, Kim CH. Radiation exposure to the urology surgeon during retrograde intrarenal surgery. PLoS ONE. 2021;16(3): e0247833. https://doi.org/10.1371/journal.pone.0247833.
Patel PM, Kandabarow AM, Chuang E, McKenzie K, Druck A, Seffren C, et al. Using Intraoperative Portable CT Scan to Minimize Reintervention Rates in Percutaneous Nephrolithotomy: a Prospective Trial. J Endourol. 2022;36(10):1382–7. https://doi.org/10.1089/end.2022.0049.
Pearle MS, Watamull LM, Mullican MA. Sensitivity of noncontrast helical computerized tomography and plain film radiography compared to flexible nephroscopy for detecting residual fragments after percutaneous nephrostolithotomy. J Urol. 1999;162(1):23–6. https://doi.org/10.1097/00005392-199907000-00006.
Pooler BD, Lubner MG, Kim DH, Ryckman EM, Sivalingam S, Tang J, et al. Prospective trial of the detection of urolithiasis on ultralow dose (sub mSv) noncontrast computerized tomography: direct comparison against routine low dose reference standard. J Urol. 2014;192(5):1433–9. https://doi.org/10.1016/j.juro.2014.05.089.
Ristau BT, Dudley AG, Casella DP, Dwyer ME, Fox JA, Cannon GM, et al. Tracking of radiation exposure in pediatric stone patients: The time is now. J Pediatr Urol. 2015;11(6):339 e1–5. https://doi.org/10.1016/j.jpurol.2015.08.008.
Roguin A, Goldstein J, Bar O, Goldstein JA. Brain and neck tumors among physicians performing interventional procedures. Am J Cardiol. 2013;111(9):1368–72. https://doi.org/10.1016/j.amjcard.2012.12.060.
Skolarikos A, Neisius A, Petřík A, Somani B, Thomas K, Gambaro G. EAU guidelines on urolithiasis. EAU Guidelines Edn Presented at the EAU Annual Congress Amsterdam; 2022.
Smith-Bindman R, Lipson J, Marcus R, Kim KP, Mahesh M, Gould R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med. 2009;169(22):2078–86. https://doi.org/10.1001/archinternmed.2009.427.
Smith-Bindman R, Aubin C, Bailitz J, Bengiamin RN, Camargo CA Jr, Corbo J, et al. Ultrasonography versus computed tomography for suspected nephrolithiasis. N Engl J Med. 2014;371(12):1100–10. https://doi.org/10.1056/NEJMoa1404446.
Tran V, Zablotska LB, Brenner AV, Little MP. Radiation-associated circulatory disease mortality in a pooled analysis of 77,275 patients from the Massachusetts and Canadian tuberculosis fluoroscopy cohorts. Sci Rep. 2017;7:44147. https://doi.org/10.1038/srep44147.
United States Food and Drug Administration. Initiative to Reduce Unnecessary Radiation Exposure from Medical Imaging. 2010.
United States Nuclear Regulatory Commision Occupational Dose Limits. https://www.nrc.gov/reading-rm/doc-collections/cfr/part020/part020-1201.html Accessed 15 Feb 2023.
Weld LR, Nwoye UO, Knight RB, Baumgartner TS, Ebertowski JS, Stringer MT, et al. Safety, minimization, and awareness radiation training reduces fluoroscopy time during unilateral ureteroscopy. Urology. 2014;84(3):520–5. https://doi.org/10.1016/j.urology.2014.03.035.
Willey JS, Long DL, Vanderman KS, Loeser RF. Ionizing radiation causes active degradation and reduces matrix synthesis in articular cartilage. Int J Radiat Biol. 2013;89(4):268–77. https://doi.org/10.3109/09553002.2013.747015.
Yahyapour R, Amini P, Rezapour S, Cheki M, Rezaeyan A, Farhood B, et al. Radiation-induced inflammation and autoimmune diseases. Mil Med Res. 2018;5(1):9. https://doi.org/10.1186/s40779-018-0156-7.
Yecies T, Averch TD, Semins MJ. Identifying and managing the risks of medical ionizing radiation in endourology. Can J Urol. 2018;25(1):9154–60.
Yoshinaga S, Mabuchi K, Sigurdson AJ, Doody MM, Ron E. Cancer risks among radiologists and radiologic technologists: review of epidemiologic studies. Radiology. 2004;233(2):313–21. https://doi.org/10.1148/radiol.2332031119.
Zhou DD, Hao JL, Guo KM, Lu CW, Liu XD. Sperm quality and DNA damage in men from Jilin Province, China, who are occupationally exposed to ionizing radiation. Genet Mol Res. 2016;15(1). https://doi.org/10.4238/gmr.15018078.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Farkouh, A., Baldwin, D.D. (2023). Radiation Hazards in Endourology. In: Denstedt, J.D., Liatsikos, E.N. (eds) Percutaneous Renal Surgery. Springer, Cham. https://doi.org/10.1007/978-3-031-40542-6_8
Download citation
DOI: https://doi.org/10.1007/978-3-031-40542-6_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-40541-9
Online ISBN: 978-3-031-40542-6
eBook Packages: MedicineMedicine (R0)