Abstract
Background
Ionizing radiation use for medical diagnostic purposes has substantially increased over the last three decades. Moderate to high doses of radiation are well established causes of cancer, especially for exposure at young ages. However, cancer risk from low-dose medical imaging is debated.
Objective
To review the literature on cancer risks associated with prenatal and postnatal medical diagnostic ionizing radiation exposure among children and to assess this risk through a meta-analysis.
Materials and methods
A literature search of five electronic databases supplemented by a hand search was performed to retrieve relevant epidemiological studies published from 2000 to 2019, including patients younger than 22 years of age exposed to medical imaging ionizing radiation. Pooled odds ratio (ORpooled) and pooled excess relative risk (ERRpooled) representing the excess of risk per unit of organ dose were estimated with a random effect model.
Results
Twenty-four studies were included. For prenatal exposure (radiographs or CT), no significant increased risk was reported for all cancers, leukemia and brain tumors. For postnatal exposure, increased risk was observed only for CT, mostly for leukemia (ERRpooled=26.9 Gy−1; 95% confidence interval [CI]: 2.7–57.1) and brain tumors (ERRpooled=9.1 Gy−1; 95% CI: 5.2–13.1).
Conclusion
CT exposure in childhood appears to be associated with increased risk of cancer while no significant association was observed with diagnostic radiographs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Medical diagnostic imaging using ionizing radiation is a very important tool in patients’ care and substantial benefits arise from its use. Recent decades have been marked by an increased use of medical radiation imaging [1] representing an annual growth of about 5% to 8% per capita [2], mostly in developed countries.
Although single doses delivered per examination have decreased over the years, thanks to advances in technologies, protocol improvements, awareness and the reactivity of radiologists to improve their daily practice in accordance with scientific and medical recommendations, overall collective doses continue to increase [3,4,5], resulting from the growing number of tests performed and the use of procedures, such as computed tomography (CT), that are known to deliver much higher doses than conventional radiology procedures.
Several epidemiological studies of populations exposed to high to moderate doses of ionizing radiation have shown an increased risk of cancer [6,7,8,9]. Increased risk of cancer with decreasing age at exposure has been described [2, 10], hence fetuses and children are more radiosensitive [2].
Studies in the 1950s and 1960s linked prenatal and postnatal diagnostic X-ray exposure to an increased risk of childhood cancer [11,12,13,14,15]. However, with the decrease of doses observed over the years, the association became weak, especially for postnatal exposure [16].
Since former reviews of literature on children exposed to medical diagnostic radiation [9, 16,17,18,19] did not include recent cohorts on CT and interventional procedures, or quantitative summaries, we aimed to assess cancer risk subsequent to prenatal and postnatal medical diagnostic radiation exposure through a systematic review, and to provide a quantitative summary on the overall risk estimate.
Materials and methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [20] adapted to observational studies.
Online searches
An online-based literature search was conducted in July 2019 in PubMed, Scopus, Web of Science, Global Health and EMBASE. Specific keywords: (neoplasms OR cancer) AND risk AND medical AND (diagnosis OR diagnostic) AND (“radiation exposure” OR (radiation AND exposure)) AND (child OR children). An additional search was carried out by hand through references from relevant publications and international reports such as BEIR VII [21] and UNSCEAR 2006 & 2013 [2, 22].
All relevant articles fulfilling the selection criteria based (see below) on their title and abstract were selected and reviewed by two different authors with experience in the health effects of ionizing radiation (E.R., with 12 years of experience, and K.D.A., with 2 years of experience), with a review by a third author (M.- O.B., with 26 years of experience) in case of discrepancy. Duplicate studies from the different databases were removed and studies providing completed quantitative information and risk estimate were then included in the meta-analysis.
Selection criteria
Eligible studies were cohort and case-control studies, published in English from Jan. 1, 2000, to July 31, 2019, involving children younger than 22 years at exposure. The exposure period was restricted to 1970 onward to ensure comparability with more recent practices since doses tend to decrease over time. Abstracts of congresses, meta-analyses, letters and authors’ comments were ineligible but were checked to find any relevant reference. In case of publications on overlapping populations or updated publications [23,24,25,26,27,28,29,30], only data from the most complete study were considered [25, 27, 28, 30].
Methodological quality assessment of individual studies
To assess the risk of bias for individual studies, the Newcastle-Ottawa Scale (NOS) for quality assessment of non-randomized studies [31] and Agency for Healthcare Research and Quality (AHRQ) standards [32] for observational study were applied by two investigators (E.R. and K.D.A.). NOS quality tools uses eight items, grouped into three domains of potential bias such as selection (representation of the sample, sample size, nonrespondents, ascertainment of the exposure), comparability (the subjects in different outcome groups are comparable, based on the study design or analysis, and confounding factors are controlled) and outcome/exposure (assessment of outcome or exposure and statistical test). A maximum of one star can be given for each item within the selection and outcome categories and a maximum of two stars can be given for comparability. To convert the NOS into AHRQ standards (good, fair and poor quality), thresholds are as follows:
-
Good quality: 3 or 4 stars in selection domain AND 1 or 2 stars in comparability domain AND 2 or 3 stars in outcome/exposure domain.
-
Fair quality: 2 stars in selection domain AND 1 or 2 stars in comparability domain AND 2 or 3 stars in outcome/exposure domain.
-
Poor quality: 0 or 1 star in selection domain OR 0 stars in comparability domain OR 0 or 1 star in outcome/exposure domain.
Statistical analysis
Studies providing a comprehensive risk estimate were set together to generate a summarized risk of cancer following medical diagnostic radiation exposure. In radiation epidemiology, however, the association between cancer risk and exposure is most often described by a risk difference or excess risk rather than a risk ratio or relative risk. In the case of an excess relative risk (ERR) model, a linear multiplicative relationship between risk and exposure is assumed rather than an exponential relationship [21]. The ERR is the proportional increase in risk over the background rate of cancer (in the absence of exposure) per unit of dose, as follows: RR = 1 + βD, where RR is the relative risk, β is the ERR and D is the dose received. For example, RR of 1.2 equals an ERR of 0.2 per unit of dose, which corresponds to an increase in risk of 20% per unit of dose.
We estimated a pooled ERR to assess the strength of the association when available from the individual studies, otherwise a pooled RR or odds ratio (OR) was computed.
An analysis was performed by period of exposure (pre- or postnatal) and type of cancer. The DerSimonian and Laird random-effect model was used to estimate the overall effect size [33] to account for within- and between-study heterogeneities. The confidence interval (CI) bounds of ERRs commonly reported from epidemiological studies may be nonsymmetrical when estimated under different hypotheses with different methods (Wald test, maximum likelihood, profile likelihood). Inference of standard deviation from the ERR’s CIs in such circumstances could lead to biased results. An alternative DerSimonian and Laird-based model proposed by Richardson et al. [34] was used to estimate the pooled effect of ERRs.
We assessed a study’s small size effect and quantified the contribution of heterogeneity to the summarized estimate with the I2 statistic, calculated as follows:
where Q is the Cochran’s statistic of heterogeneity, which follows a standard χ2 distribution with df = k − 1 degree of freedom (k is the number of individual studies).
I2 is interpreted as the proportion of the total variation of the estimated effect due to heterogeneity between studies [33]. Publication and selection bias were assessed and tested using the Egger test [35, 36]. Statistical significance was defined by P<0.05.
All statistical analyses were conducted using Stata statistical software, STATA/MP 15.1 (Stata Corp, College Station, Texas) and R 3.5.1 software (R Foundation for Statistical Computing, Vienna, Austria).
Results
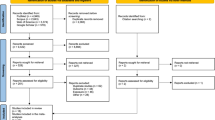
The systematic search yielded 1,674 articles. Figure 1 displays the flow diagram of selection of the relevant studies. After excluding duplicated studies (n=181), 1,493 articles were screened and 254 eligible articles were reviewed, with 24 included in the review according to prenatal (Table 1) [37,38,39,40,41,42,43,44] and postnatal radiation exposure (Tables 2 and 3) [25, 26, 29, 30, 37, 38, 41, 43,44,45,46,47,48,49,50,51,52,53,54,55]. There were 13 case-control studies [26, 37,38,39,40,41, 43, 44, 52,53,54,55,56] and 11 cohort studies [25, 29, 30, 42, 45,46,47,48,49,50,51] (Fig. 1).
Prenatal diagnostic radiation exposure
Cancer risks related to prenatal radiation exposure have been investigated in seven case-control studies [37,38,39,40,41, 43, 44] and in one cohort study [42] (Table 1). Medical examinations investigated were X-ray in five out of eight studies [37,38,39,40, 43], and X-ray coupled with CT in two studies [41, 44]. Intravenous pyelograms and radionuclide tests were evaluated in one study [41].
In the case-control studies, cancer cases were identified from cancer registries while controls were randomly selected from population registries and matched to cases on gender and age at cancer diagnosis. Additional matching criteria such as geographic region and residence were applied in several studies [37, 39,40,41, 44]. Age at cancer diagnosis ranged from 0 to 16 years except in one study in which the diagnosis age ranged from 7 to 19 years [44]. Maternal exposure to radiation was ascertained by questionnaires or interviews reporting the type of examination, the trimester of pregnancy at the time of the examination and the body part examined. In three out of seven studies, questionnaires were completed by obstetrical records [39, 42, 43].
No statistically significant increased risk of all cancer, leukemia or brain tumors, neither for X-ray nor CT exposure, were reported in the eight studies considered. Since doses to the fetus were not estimated, no study was able to derive dose-response analyses.
Risk summaries were estimated for leukemia and brain tumors based on four [38, 39, 41, 43] and three studies [37, 43, 44], respectively. The pooled analyses included 6,274 cases and 12,426 controls for the leukemia subgroup and 3,461 cases and 7,924 controls for the brain tumors subgroup. Methodological quality scores of included studies were all satisfied, with NOS scores ranging from 6 to 9 (good quality according to AHRQ scores).
No increased risk for leukemia following prenatal exposure (any exposure versus no exposure) could be observed (ORpooled=1.08, 95% CI: 0.90–1.28) (Fig. 2), with no reported heterogeneity between studies (I2=23.2%, P=0.27).
No increased risk of brain tumors was reported (ORpooled=0.93, 95% CI: 0.68–1.28) (Fig. 3) and no heterogeneity between studies was observed (I2=0.0, P=0.72).
No publication bias was identified by the Egger tests for leukemia (P=0.52) and brain tumors (P=0.49).
Postnatal diagnostic radiation exposures
There were 21 studies [25, 26, 29, 30, 37, 38, 41, 43,44,45,46,47,48,49,50,51,52,53,54,55,56] on childhood radiation medical exposure (Tables 2 and 3). Beside X-ray and CT, which were the most frequently studied types of procedures, some specific examinations such as cardiac catheterization or cystography were also considered. CT exposure was mainly investigated in cohort studies whereas case-control studies predominantly explored X-ray exposure.
Subjects’ exposures were identified from hospital records or from health insurance databases while cancer cases were retrieved from cancer registries [25, 27, 30, 41, 44,45,46,47, 49, 56]. In some CT studies with medical information available, children with cancer-predisposing factors [25, 28, 46] and children subjected to CT because of suspected cancer [30, 56] were excluded from the analyses. To deal with reverse causality (cancers that were caused by the underlying medical conditions prompting the CT rather than by the dose delivered during the examination), various latency periods were applied, ranging from 3 to 24 months for lymphohematopoietic malignancies, and from 12 to 60 months for solid cancers. Age at inclusion, i.e. at first exposure, varied from 0 to 22 years, with some studies focusing only on children first exposed before the age of 10 years [28] or 15 years [25, 49]. Mean follow-up extended from 4 years [49] to 8.5 years [50].
Organ doses were estimated only for CT studies, based on patient characteristics (age, gender), type of examination and machine-specific settings retrieved from radiology protocols [28], published radiologic survey data [30, 45, 49], or from the Picture Archiving and Communication System (PACS) [57]. The cumulative estimated doses ranged from 5.9 mGy to 10.1 mGy to the red bone marrow and from 18.3 mGy to 49.0 mGy to the brain (Table 2).
X-ray exposure was not associated with increased risks of all cancers [45, 49, 50], lymphohematopoietic malignancies or brain tumors [37, 43, 44, 52, 54].
Exposure to cystourethrography procedures was statistically associated with increased risks of genital and urinary system cancers as well as hematological system malignancies [47]. Standardized incidence ratio (SIR) of all cancers (SIR 3.01, 95% CI 2.09–4.19) and lymphoma (SIR 9.15, 95% CI 5.66–13.97) were increased and significantly associated with childhood cardiac catheterization procedures [51] but were no more increased after censoring transplant recipients (SIR 0.90, 95% CI 0.49–1.49 for all cancers with 0 cases for lymphoma).
CT studies reported significant increased risks for lymphohematopoeitic malignancies [30, 45, 50] and for leukemia [56] while others found nonsignificant increased risks for leukemia [41, 49] by comparing children undergoing one or more CTs versus none. An almost two-fold increase in risk of brain tumor has been reported [45, 46, 50] (one or more CTs versus none) while some studies have not shown any increased risk [44, 54] regardless of the region exposed.
Among the six CT studies providing organ doses [28, 30, 45, 49, 50, 56], pooled ERRs per Gy were calculated for leukemia and brain tumors. Overall, the pooled analysis included 11,398,728 and 11,393,070 subjects for leukemia and brain tumor risk analyses, respectively. Among them, 437 leukemia and 478 brain tumor cases were observed. The studies were comparable, and the methodological quality of the included studies was good, according to AHRQ, with NOS scores ranging from 7 to 9. We observed a significant increased risk for leukemia (ERRpooled=26.9 Gy−1, 95% CI: 2.7–57.1) based on 6 studies [28, 30, 45, 49, 50, 56] (Fig. 4), which represents an increase of 2.69% per mGy of dose over the background risk of leukemia. There was moderate heterogeneity between studies (I2=60.3%, P=0.03). Sensitivity analyses in which the pooled ERR was calculated excluding each study one at a time revealed no substantial alteration of the aggregate ERR except when excluding the Dutch study [50], which accounted for a large weight of the pooled analysis, leading to a higher pooled ERR after the exclusion of this study. Publication bias was suspected (P=0.03) suggesting that small studies with negative results were less often published.
The pooled ERR for brain tumors was significantly increased (ERRpooled=9.1 Gy−1, 95% CI: 5.2–13.1) based on 5 studies [28, 30, 45, 49, 50] (Fig. 5), which represents an increase of 0.91% per mGy of dose over the background risk of brain tumors. Small heterogeneity between study was found I2=32% and no publication or selection bias was suspected (P=0.16).
Discussion
Cancer risks after prenatal or postnatal medical diagnostic radiation exposure were analyzed based on 24 studies. Our review did not find any statistically increased risks of all cancers, leukemia and brain tumors after prenatal X-ray or CT exposures. For postnatal exposure, increased risks were observed for leukemia as well as brain tumors after CT exposure while no evidence of an increased risk of all cancers was observed after X-ray exposure.
Early published data in the 1950s, mainly the Oxford Survey of Childhood Cancers studies [11, 13, 58] and other epidemiological studies [59, 60], reported an increased cancer risk related to prenatal X-ray exposure [11, 13]. However, studies carried out a few years later did not show such a statistically significant association [16, 61, 62]. Nevertheless, the positive association, albeit non-statistically significant, between prenatal X-ray and leukemia (ORpooled 1.08, 95% CI: 0.90–1.28) is consistent with earlier much more statistically informative analyses that found results rejecting the null [13, 60].
A possible explanation for the difference between the cancer risk associated with prenatal irradiation estimated in this meta-analysis and estimated from previous studies may be linked to the decline in X-ray frequency during pregnancy by shifting to nonionizing procedures such as sonography or magnetic resonance imaging [15, 63] and the decrease in X-ray doses due to the setting and improvement of radiation protection rules for patients. Current X-ray systems deliver fetal radiation doses of about 1.7 μGy for spine measurement and 2.7 μGy for femur measurement during the first trimester [64].
Little is known about the potential harm of CT exposure to the fetus. Because of higher doses delivered by CT compared to conventional radiology [63, 65], the increased use of CT during pregnancy [64, 66] for non-obstetrical conditions might be an issue. Only one study [42] focused specifically on the link between maternal CTs and a subsequent malignancy in the child and did not observe any cancer risk in children (hazard ratio [HR] 0.68, 95% CI: 0.25–1.80) [42]. But a limit of this study is the lack of dosimetry assessment of the fetus exposure [42].
Postnatal diagnostic X-ray exposure has been the focus of numerous studies in the last half century. Early studies [11, 67, 68] reported increased risks of leukemia in patients exposed to diagnostic X-ray compared to controls while more recent studies [69,70,71] did not report increased risks.
Because of the large increase in CT use over the years, several recent epidemiological studies have assessed the risk of cancer following CT exposure in childhood [28, 30, 45]. Among these studies, most reported increased risks of leukemia [28, 30, 45] and brain tumors [30, 45, 46], some of them without reaching significance [48]. In the present analysis, we reported a summarized ERR of ERRpooled=26.9 Gy−1 (95% CI: 2.7–57.1) for leukemia and ERRpooled=9.1 Gy−1 (95% CI: 5.2–13.1) for brain tumors, indicating an increase in the risks of leukemia and brain tumors over the background risks of 2.69% and 0.91%, respectively, per unit of mGy due to postnatal CT exposure based on linear dose-response models. That means, for a given CT delivering 10 mGy to the red bone marrow (or to the brain), the leukemia (or brain tumor) risk increases by about 27% (or 9% for brain tumor risk) over the respective background risks, holding all other factors constant. The major limits encountered in the CT studies are indication and reverse causation bias, uncertainties in dose reconstruction and insufficient statistical power. Indication and reverse causation bias can be suspected when cancer-predisposing factors or early symptoms of undetected cancer are the indication of the CT [72]. Thus, the apparent excess incidence of cancer is not linked to the CTs performed but to the underlying conditions or undetected cancer that motivate the indication of the CT. In most of the studies on CT [30, 45, 46], no information on the indication of the CT examination was available. Then, the association between CT and cancer risk might likely be overestimated in case of bias. Authors challenged reverse causation bias by applying several increasing lag periods (minimal latent time between the exposure and the cancer diagnosis) to exclude as much as possible CTs that could be performed after the cancer initiation but before the diagnosis of cancer. Because leukemia genesis is rather short and diagnosis is not assessed by CT examination, reverse causation bias is unlikely to obscure the leukemia dose response analysis. The similar values of ERR for leukemia (ERR=0.045 per mGy, 95% CI: 0.016–0.188) reported in the Life Span Study supports this hypothesis [6, 30, 45]. It is much more difficult to exclude such a bias for brain tumors as their development might take years, when several exams, especially CTs, could be performed to investigate the undetected condition.
Confounding bias linked to underlying conditions predisposing to cancer has been scarcely investigated [23, 28]. Reanalysis of the previous published data [30] according to medical information available for 40% of the UK CT cohort showed a decrease of previously estimated ERRs of 15% for leukemia and 30% for brain tumors, albeit still significantly increased. In the French cohort [28], analysis restricted to the patients without predisposing factors to cancer (97% of the studied population) reported risk estimates in the same range as those obtained in the whole cohort, ruling out a potential bias linked to predisposing conditions to cancer. However, the rather small number of cases and the short duration of follow-up prevent definitive conclusions.
Aside from CT, studies of other procedures such as fluoroscopy and cardiac catheterization were scarce. A study of adults with congenital heart defects reported significantly higher cancer risks associated with increasing numbers of diagnostic and treatment cardiac procedures [73]. Cancer risk associated with cardiac catheterization during childhood has been analyzed in three studies with divergent results [51, 74, 75]. The most recent study from the UK with individual dose reconstruction [51] reported no increase in all cancers after disregarding transplant patients, whose conditions might predispose to cancer. The study had relatively low statistical power to detect an association as only 11,270 children were included.
One of the major limits in the reviewed studies is the lack of precise dose assessment, especially in earlier studies. Although the doses might be quite easily estimated based on machine-specific parameters, this information was scarcely documented until recently. The assessment of X-ray exposure based on interviews or questionnaires in case-control studies may lead to a recall bias, unless confirmed by a review of medical records [39, 42, 43].
One strength of our study is the ability to estimate pooled ERR thanks to the alternative random-effect method of Richardson et al. [34], which allows the derivation of estimates of variance of published ERRs in case of nonsymmetrical confidence intervals.
Assessing the quality of individual studies included in a systematic review is fundamental to interpreting the review. Quality assessment is challenging due to the methodological intricacies and its subjective nature. We used two recognized tools, NOS for quality assessment of non-randomized studies [31] and AHRQ standards [32] to assess quality and methodological limits of included studies in a standardized manner, and we applied the PRISMA recommendations [20] for the reporting of the study’s results. However, we did not weigh the pooled estimates on quality criteria, as quality scores were in the same range of values (NOS scores 6 to 9, and good quality for AHRQ scores) for the studies considered in each analysis. We used a random effect model to calculate the pooled estimates, which allows for potential between- and within-study heterogeneities, even if the hypothesis of heterogeneity was rejected in most of our analyses. Publication bias linked to the absence of studies of small size with negative results seems not to be a major limitation of our analysis as demonstrated by statistical tests. Restricting the study period to published articles from 2000 to 2019 ruled out studies with exposure before the 1970s, for the purpose of insuring a certain homogeneity in the exposure scenario since a downturn in doses per X-ray exam has been reported and CT machines had been introduced after that period [15]. This prevented the inclusion of old studies with outdated exposure conditions and medical practices.
An important common limitation is the lack of statistical power linked to the small expected risk, the rather low frequency of these procedures during pregnancy and childhood, and the short follow-up of recent studies. Hopefully, ongoing international studies will be able to assess, with greater statistical power, the risks associated with radiation-induced malignancies in medical exposures and to address some limits of previous published data.
In that way, the ongoing European collaborative project EPI-CT (epidemiological study to quantify risks for pediatric computed tomography and to optimize doses) pools nine national cohorts of children exposed to CT and provides individual organ doses taking into account uncertainties in dose assessment. With the inclusion of about 1 million patients, EPI-CT will provide statistically powerful estimates of cancer risk associated with CT exposure [76]. An extension of the follow-up of the main cohorts of EPI-CT is also planned in the international MEDIRAD project (Implications of Medical Low Dose Radiation Exposure) [77], which aims to enhance the scientific bases and clinical practice of radiation protection in the medical field. Another ongoing study, HARMONIC (Health Effects of Cardiac Fluoroscopy and Modern Radiotherapy in Paediatrics) [78], is partly devoted to assessing the risk of radiation-related malignancies in children undergoing cardiac catheterizations.
Conclusion
Although prenatal medical radiation during the last 50 years appeared unrelated to a subsequent later life risk of cancer, pooled results from studies on CT exposure during childhood showed greater risks for leukemia and brain tumors. Published studies present some methodological limitations. Although the benefits of prenatal and postnatal diagnostic radiation examinations outweigh the risks associated with the doses delivered by these procedures, the results of this analysis justify continued efforts to optimize doses to patients.
Change history
22 October 2020
The original version of Table 2 included two incorrect values.
References
Hall EJ, Brenner DJ (2008) Cancer risks from diagnostic radiology. Br J Radiol 81:362–378
United Nations Scientific Committee on the Effects of Atomic Radiation (2008) Effects of ionizing radiation: United Nations Scientific Committee on the Effects of Atomic Radiation—UNSCEAR 2006 report, volume 1—report to the general assembly, with scientific annexes A and B. United Nations Office at Vienna, United Nations
Linet MS, Slovis TL, Miller DL et al (2012) Cancer risks associated with external radiation from diagnostic imaging procedures. CA Cancer J Clin 62:75–100
Institut de Radioprotection et de Sûreté Nucléaire (IRSN) (2015) [Exposure of the French population to ionising radiation.] Fontenay-aux-Roses. https://www.irsn.fr/FR/expertise/rapports_expertise/Documents/radioprotection/IRSN-Exposition-Population-Rayonnements-Ionisants_2015-00001.pdf. Accessed 20 Oct 2018
Watson SJ, Jones AL, Oatway WB, Hughes SJ (2005) Ionising radiation exposure of the UK population: UK review. HPA-RPD-001. Health Protection Agency, Centre for Radiation, Chemical and Environmental Hazards. Chilton, Oxfordshire
Preston DL, Kusumi S, Tomonaga M et al (1994) Cancer incidence in atomic bomb survivors. Part III: leukemia, lymphoma and multiple myeloma, 1950-1987. Radiat Res 137:S68–S97
Folley JH, Borges W, Yamawaki T (1952) Incidence of leukemia in survivors of the atomic bomb in Hiroshima and Nagasaki, Japan. Am J Med 13:311–321
Ozasa K, Shimizu Y, Suyama A et al (2011) Studies of the mortality of atomic bomb survivors, report 14, 1950–2003: an overview of cancer and noncancer diseases. Radiat Res 177:229–243
Wakeford R (2013) The risk of childhood leukaemia following exposure to ionising radiation—a review. J Radiol Prot 33:1–25
Preston DL, Cullings H, Suyama A et al (2008) Solid cancer incidence in atomic bomb survivors exposed in utero or as young children. J Natl Cancer Inst 100:428–436
Stewart A, Webb J, Hewitt D (1958) A survey of childhood malignancies. Br Med J 1:1495–1508
Wakeford R (2008) Childhood leukaemia following medical diagnostic exposure to ionizing radiation in utero or after birth. Radiat Prot Dosim 132:166–174
Giles D, Hewitt D, Stewart A, Webb J (1956) Malignant disease in childhood and diagnostic irradiation in utero. Lancet 271:447
MacMahon B (1962) Prenatal x-ray exposure and childhood cancer. J Natl Cancer Inst 28:1173–1191
Linet MS, Kim KP, Rajaraman P (2009) Children’s exposure to diagnostic medical radiation and cancer risk: epidemiologic and dosimetric considerations. Pediatr Radiol 39:S4–S26
Schulze-Rath R, Hammer GP, Blettner M (2008) Are pre-or postnatal diagnostic X-rays a risk factor for childhood cancer? A systematic review. Radiat Environ Biophys 47:301–312
Baysson H, Etard C, Brisse HJ, Bernier M-O (2012) Diagnostic radiation exposure in children and cancer risk: current knowledge and perspectives. Arch Pediatr 19:64–73
Baysson H, Journy N, Roué T et al (2016) Exposure to CT scans in childhood and long-term cancer risk: a review of epidemiological studies. Bull du Cancer 103:190–198
Bernier M-O, Journy N, Baysson H et al (2015) Potential cancer risk associated with CT scans: review of epidemiological studies and ongoing studies. Prog Nucl Energy 84:116–119
Liberati A, Altman DG, Tetzlaff J et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 6:e1000100
National Research Council (2006) Health risks from exposure to low levels of ionizing radiation: BEIR VII phase 2. National Academies Press
United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) (2013) Sources, effects and risks of ionizing radiation: Volume II. Scientific annex B: Effects of radiation exposure of Children. New York
de Gonzalez AB, Salotti JA, McHugh K et al (2016) Relationship between paediatric CT scans and subsequent risk of leukaemia and brain tumours: assessment of the impact of underlying conditions. Br J Cancer 114:388–394
Hammer GP, Seidenbusch MC, Schneider K et al (2009) A cohort study of childhood cancer incidence after postnatal diagnostic X-ray exposure. Radiat Res 171:504–512
Hammer GP, Seidenbusch MC, Regulla DF et al (2011) Childhood cancer risk from conventional radiographic examinations for selected referral criteria: results from a large cohort study. AJR Am J Roentgenol 197:217–223
Infante-Rivard C (2003) Diagnostic x rays, DNA repair genes and childhood acute lymphoblastic leukemia. Health Phys 85:60–64
Infante-Rivard C, Mathonnet G, Sinnett D (2000) Risk of childhood leukemia associated with diagnostic irradiation and polymorphisms in DNA repair genes. Environ Health Perspect 108:495–498
Journy N, Rehel J-L, Le Pointe HD et al (2015) Are the studies on cancer risk from CT scans biased by indication? Elements of answer from a large-scale cohort study in France. Br J Cancer 112:185–193
Journy N, Roué T, Cardis E et al (2016) Childhood CT scans and cancer risk: impact of predisposing factors for cancer on the risk estimates. J Radiol Prot 36:N1–N7
Pearce MS, Salotti JA, Little MP et al (2012) Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet 380:499–505
Wells GA, Shea B, O’Connell D et al (2015) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 24 Nov 2018
Viswanathan M, Ansari MT, Berkman ND et al (2012) Assessing the risk of bias of individual studies in systematic reviews of health care interventions. In: Methods guide for effectiveness and comparative effectiveness reviews. https://effectivehealthcare.ahrq.gov/sites/default/files/pdf/methods-guidance-bias-individual-studies_methods.pdf. Accessed 24 Nov 2018
Sterne JA (2009) Meta-analysis in Stata: an updated collection from the Stata journal. StataCorp LP, College Station, Texas
Richardson DB, Abalo K, Bernier M-O et al (2020) Meta-analysis of published excess relative rate estimates. Radiat Environ Biophys. https://doi.org/10.1007/s00411-020-00863-w
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Steichen T (1998) Tests for publication bias in meta-analysis. Stata Tech Bull 7:14
Schüz J, Kaletsch U, Kaatsch P et al (2001) Risk factors for pediatric tumors of the central nervous system: results from a German population-based case-control study. Med Pediatr Oncol 36:274–282
Shu XO, Potter JD, Linet MS et al (2002) Diagnostic X-rays and ultrasound exposure and risk of childhood acute lymphoblastic leukemia by immunophenotype. Cancer Epidemiol Biomark Prev 11:177–185
Roman E, Simpson J, Ansell P et al (2005) Perinatal and reproductive factors: a report on haematological malignancies from the UKCCS. Eur J Cancer 41:749–759
Goel R, Olshan AF, Ross JA et al (2009) Maternal exposure to medical radiation and Wilms tumor in the offspring: a report from the Children’s Oncology Group. Cancer Causes Control 20:957–963
Bailey HD, Armstrong BK, de Klerk NH et al (2010) Exposure to diagnostic radiological procedures and the risk of childhood acute lymphoblastic leukemia. Cancer Epidemiol Biomark Prev 19:2897–2909
Ray JG, Schull MJ, Urquia ML et al (2010) Major radiodiagnostic imaging in pregnancy and the risk of childhood malignancy: a population-based cohort study in Ontario. PLoS Med 7:e1000337
Rajaraman P, Simpson J, Neta G et al (2011) Early life exposure to diagnostic radiation and ultrasound scans and risk of childhood cancer: case-control study. BMJ 342:d472
Tettamanti G, Shu X, Adel Fahmideh M et al (2017) Prenatal and postnatal medical conditions and the risk of brain tumors in children and adolescents: an international multicenter case-control study. Cancer Epidemiol Biomark Prev 26:110–115
Mathews JD, Forsythe AV, Brady Z et al (2013) Cancer risk in 680 000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. BMJ 346:f2360
Huang W-Y, Muo C-H, Lin C-Y et al (2014) Paediatric head CT scan and subsequent risk of malignancy and benign brain tumour: a nation-wide population-based cohort study. Br J Cancer 110:2354–2360
Liao Y-H, Lin C-L, Wei C-C et al (2014) Subsequent cancer risk of children receiving post voiding cystourethrography: a nationwide population-based retrospective cohort study. Pediatric Nephrol 29:885–891
White IK, Shaikh KA, Moore RJ et al (2014) Risk of radiation-induced malignancies from CT scanning in children who underwent shunt treatment before 6 years of age: a retrospective cohort study with a minimum 10-year follow-up. J Neurosurg Pediatri 13:514–519
Krille L, Dreger S, Schindel R et al (2015) Risk of cancer incidence before the age of 15 years after exposure to ionising radiation from computed tomography: results from a German cohort study. Radiat Environ Biophys 54:1–12
Meulepas JM, Ronckers CM, Smets AMJB et al (2018) Radiation exposure from pediatric CT scans and subsequent cancer risk in the Netherlands. J Nal Cancer Inst 111:256–263
Harbron RW, Chapple C-L, O’Sullivan JJ et al (2018) Cancer incidence among children and young adults who have undergone x-ray guided cardiac catheterization procedures. Eur J Epidemiol 33:393–401
Mellemkjær L, Hasle H, Gridley G et al (2006) Risk of cancer in children with the diagnosis immaturity at birth. Paediatr Perinat Epidemiol 20:231–237
Khan S, Evans AA, Rorke-Adams L et al (2010) Head injury, diagnostic X-rays, and risk of medulloblastoma and primitive neuroectodermal tumor: a Children’s Oncology Group study. Cancer Causes Control 21:1017–1023
Milne E, Greenop KR, Fritschi L et al (2014) Childhood and parental diagnostic radiological procedures and risk of childhood brain tumors. Cancer Causes Control 25:375–383
Shih T-Y, Wu J, Muo C-S, Kao C-H (2014) Association between leukaemia and X-ray in children: a nationwide study. J Paediatr Child Health 50:615–618
Nikkilä A, Raitanen J, Lohi O, Auvinen A (2018) Radiation exposure from computerized tomography and risk of childhood leukemia: Finnish register-based case-control study of childhood leukemia (FRECCLE). Haematologica 103:1873–1880
Lee C, Kim KP, Bolch WE et al (2015) NCICT: a computational solution to estimate organ doses for pediatric and adult patients undergoing CT scans. J Radiol Prot 35:891–909
Bithell JF, Stewart AM (1975) Pre-natal irradiation and childhood malignancy: a review of British data from the Oxford survey. Br J Cancer 31:271–287
Bithell JF, Stiller CA (1988) A new calculation of the carcinogenic risk of obstetric X-raying. Stat Med 7:857–864
Doll R, Wakeford R (1997) Risk of childhood cancer from fetal irradiation. Br J Radiol 70:130–139
Rodvall Y, Pershagen G, Hrubec Z et al (1990) Prenatal X-ray exposure and childhood cancer in Swedish twins. Int J Cancer 46:362–365
Inskip PD, Harvey EB, Boice JD Jr et al (1991) Incidence of childhood cancer in twins. Cancer Causes Control 2:315–324
Børretzen I, Lysdahl KB, Olerud HM (2007) Diagnostic radiology in Norway—trends in examination frequency and collective effective dose. Radiat Prot Dosim 124:339–347
Chen MM, Coakley FV, Kaimal A, Laros RK Jr (2008) Guidelines for computed tomography and magnetic resonance imaging use during pregnancy and lactation. Obstet Gynecol 112:333–340
Mettler FA Jr, Bhargavan M, Faulkner K et al (2009) Radiologic and nuclear medicine studies in the United States and worldwide: frequency, radiation dose, and comparison with other radiation sources—1950-2007. Radiology 253:520–531
Righini M, Robert-Ebadi H, Elias A et al (2018) Diagnosis of pulmonary embolism during pregnancy: a multicenter prospective management outcome study. Ann Intern Med 169:766–773
Graham S, Levin ML, Lilienfeld AM et al (1966) Preconception, intrauterine, and postnatal irradiation as related to leukemia. Natl Cancer Inst Monogr 19:347–371
Polhemus DW, Koch R (1959) Leukemia and medical radiation. Pediatrics 23:453–461
Ager EA, Schuman LM, Wallace HM et al (1965) An epidemiological study of childhood leukemia. J Chronic Dis 18:113–132
Shu XO, Gao YT, Brinton LA et al (1988) A population-based case-control study of childhood leukemia in Shanghai. Cancer 62:635–644
Hartley AL, Birch JM, McKinney PA et al (1988) The Interregional Epidemiological Study of Childhood Cancer (IRESCC): past medical history in children with cancer. J Epidemiol Community Health 42:235–242
Boice JD Jr (2015) Radiation epidemiology and recent paediatric computed tomography studies. Ann ICRP 44:236–248
Cohen S, Liu A, Gurvitz M et al (2018) Exposure to low-dose ionizing radiation from cardiac procedures and malignancy risk in adults with congenital heart disease. Circulation 137:1334–1345
McLaughlin JR, Kreiger N, Sloan MP et al (1993) An historical cohort study of cardiac catheterization during childhood and the risk of cancer. Int J Epidemiol 22:584–591
Modan B, Keinan L, Blumstein T, Sadetzki S (2000) Cancer following cardiac catheterization in childhood. Int J Epidemiol 29:424–428
Bernier M-O, Baysson H, Pearce MS et al (2018) Cohort profile: the EPI-CT study: a European pooled epidemiological study to quantify the risk of radiation-induced cancer from paediatric CT. Int J Epidemiol 48:379–381g
MEDIRAD. Home. http://www.mediradproject.eu/. Accessed 11 Mar 2020
HARMONIC. ISGlobal website. https://www.isglobal.org/en/-/harmonic. Accessed 11 Mar 2020
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abalo, K.D., Rage, E., Leuraud, K. et al. Early life ionizing radiation exposure and cancer risks: systematic review and meta-analysis. Pediatr Radiol 51, 45–56 (2021). https://doi.org/10.1007/s00247-020-04803-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-020-04803-0