Abstract
It is estimated by the World Health Organization that around 5% of the world’s population has some kind of disabling hearing loss that requires rehabilitation (WHO. 2021, https://www.who.int/news-room/fact-sheets/detail/deafness-and-hearing-loss). Cochlear implantation is widely regarded as the most effective treatment for patients with severe-to-profound sensorineural hearing loss (Mertens et al. B-ENT, 17(3):155–163; 2021). Since its inception, much research has been devoted to improving the technology of cochlear implants, and the effectiveness and reliability of the devices have improved markedly. However, there has been little research into less invasive ways of placing them. Only since the advent of robotic surgery in general during the past 20 years has robotically assisted cochlear implantation surgery developed. With this technique, unlike the conventional method, it is not necessary to drill away a significant part of the mastoid; one can reach the round window directly with a 1.8-mm and then a 1-mm drill bit, where the electrode can also be placed. The conventional method has the disadvantage that placing the electrode is seldom non-traumatic because of the angle at which the surgeon operates. The advantage of this technique is that it requires little drilling next to the facial nerve, which reduces the risk of facial paralysis. In robotically assisted cochlear implantation surgery there is less space between the drill and the facial nerve, so electrode placement can be more accurate and less traumatic. Here, pre-operative imaging, intra-operative imaging and facial nerve monitoring are of crucial importance. Using pre-operative imaging, one can plan the safest and most efficient drilling trajectory and angles, ensuring atraumatic intra-cochlear electrode array placement. This in turn ensures that the anatomy and physiology of the inner ear are preserved as far as possible, thus reducing the risk of losing the rest of the patient’s natural hearing levels. This could lead to a broadening of the indications for cochlear implant placement for patients who do not wish to take this risk (Topsakal et al. Front Neurol. 13, 2022).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Cochlear implantation
- Robotically assisted cochlear implantation surgery
- Sensorineural hearing loss
- HEARO procedure
- Image-guided surgery
6.1 The Evolution of Cochlear Implantation Surgery
Before discussing the developments of cochlear implantation surgery, it is necessary to describe the evolution of cochlear implant devices themselves. During the 1800s, Alessandro Volta showed that electrical stimulation of metal rods placed in a person’s ear can produce an auditory sensation. The first single-channel cochlear implant device was introduced in 1972, and 12 years later the first multichannel cochlear implant device was devised, stimulating different parts of the cochlea at different frequencies [1]. With each stage in the development of cochlear implant devices, steps have been taken to improve sound and speech processing, thus achieving better speech recognition in patients [2].
One of the first cochlear implantation surgical techniques to be described is the posterior tympanoplasty or facial recess approach. This is considered one of the safest techniques, with a low risk of injuring the facial nerve. The first major steps in the operation are opening a retro-auricular skin flap and drilling the mastoid until the surgeon has approached the round window safely. After the round window is opened, the electrode array is inserted into the scala tympani of the cochlea [3]. Other techniques have been described in the past, differing in facial nerve injury risk, the risk of perforating the tympanic membrane, and electrode array insertion angles [4].
Following the advent of robotic surgery in general, robotically assisted cochlear implantation surgery (RACIS) has been developed in recent years. Implementing robotics into cochlear implantation surgery has made a minimally invasive round window approach without mastoidectomy a real possibility. More than 10 years have passed since RACIS was first described. RACIS could not have been developed without the progress afforded by image-guided technology. Pre- and intra-operative imaging makes it possible for the surgeon to plan a safe and efficient trajectory. A direct approach to the round window with a drill is only possible if the drill can pass safely through the space of the facial recess. This is the space between the facial nerve and the chorda tympani. It measures approximately 2.5–3.5 mm. Pre-clinical studies have shown that the facial recess space has to measure a minimum of 2.5 mm, and the spaces between the drill and the facial nerve/chorda tympani have to measure 0.4 mm and 0.3 mm, respectively, to avoid injury. It is understandable that the trajectory has to be planned carefully and accurately for a 1.8-mm drill to avoid these structures [5]. Because of small imaging inaccuracies, it is still necessary to ensure that the real trajectory does not deviate from the planned trajectory. Intra-operatively, the trajectory is double-checked using imaging and facial nerve monitoring to verify a safe distance from the facial nerve [4].
The first clinical application of RACIS is the drill passing through the space of the facial recess, approaching the round window. After having passed the facial recess robotically, the surgeon still had to open the round window manually and insert the electrode array under the microscope [6]. Recently, the HEARO- procedure has provided a step toward full automation of the surgery. With this procedure, the round window is opened autonomously because the drill opens its bony overhang (canonostomy), providing autonomous inner ear access [4].
6.2 The HEARO-Procedure
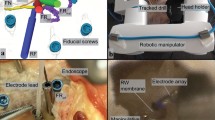
The HEARO-procedure has three main phases. The first phase is scanning and planning. After the patient’s head is immobilized and five screws are placed in the mastoid cortex, pre-operative imaging is performed. The images are reconstructed into three dimensions using software. Using the 3D image, an ideal trajectory to the round window is planned (Fig. 6.1) [4, 7]. The second phase is accessing the middle ear. This phase requires careful intra-operative monitoring using imaging with a mobile cone-beam CT, and facial nerve monitoring. The HEARO-robotic system performs the drilling (Fig. 6.2) in three stages. The first stage is drilling from the cortex of the mastoid part of the temporal bone to 3 mm before the facial recess. A rod is then placed in the drilling hole followed by imaging. This stage is necessary to assess the safety margins and the executed trajectory. When a safe trajectory has been guaranteed, the second stage follows: further drilling through the facial recess in smaller steps of 0.5 mm, with facial nerve monitoring between successive steps. The third stage is the fastest part of the drilling process and achieves complete middle ear access (Fig. 6.3) [4]. The third and final phase of the HEARO-procedure is to attain inner ear access or milling of the canonus (Fig. 6.4), the bony overhang of the round window [8] (Fig. 6.5). During canonostomy, the drilling depth is calculated from both imaging data and an intra-operative force-torque sensor (Fig. 6.6). After canonostomy, complete access to the inner ear has been achieved.
The surgeon then takes over manually. The electrode array of the cochlear implant device has to be placed correctly for the surgery to be deemed successful. From a transmeatal view, the insertion of the electrode array can be visualized by microscope or endoscope. After careful insertion through the drilling hole in the scala tympani of the cochlea, the surgeon fixates the implant and closes the wound as in conventional cochlear implantation surgery. After surgery, post-operative imaging and electrophysiological tests are used to determine whether the placement of the electrode is correct [4, 9].
6.3 Conclusion and Future Perspectives
Robotically assisted cochlear implantation surgery is a minimally invasive way of performing autonomous inner ear access. Careful pre- and intra-operative planning makes atraumatic insertion of the electrode array possible, potentially providing better results than conventional surgery and also reducing operative and post-operative recovery times. The HEARO-procedure is still not a fully autonomous method of cochlear implantation surgery because autonomous electrode array insertion is not yet possible. Further studies are expected to make the procedure more efficient and safer.
References
Mertens G, Brandt A, Boudewyns A, Cochet E, Govaerts P, Lammers M, Topsakal V, Heyning P, Vanderveken O, Rompaey V. More than a quarter century of cochlear implantations: a retrospective study on 1161 implantations at the Antwerp University Hospital. B-ENT. 2021;17(3):155–63.
Topsakal V, Heuninck E, Matulic M, Tekin A, Mertens G, Van Rompaey V, Galeazzi P, Zoka-Assadi M, van de Heyning P. First study in men evaluating a surgical robotic tool providing autonomous inner ear access for Cochlear implantation. Front Neurol. 2022;13
Brown, C., Geers, A., Herrmann, B., Kirk, K., Tomblin, B. and Waltzman, S., 2004. Cochlear implants.
House W. Preface. Ann Otol Rhinol Laryngol. 1976;85(3_suppl):i–i.
Majdani O, Rau T, Baron S, Eilers H, Baier C, Heimann B, Ortmaier T, Bartling S, Lenarz T, Leinung M. A robot-guided minimally invasive approach for cochlear implant surgery: preliminary results of a temporal bone study. Int J Comput Assist Radiol Surg. 2009;4(5):475–86.
Caversaccio M, Gavaghan K, Wimmer W, Williamson T, Ansò J, Mantokoudis G, Gerber N, Rathgeb C, Feldmann A, Wagner F, Scheidegger O, Kompis M, Weisstanner C, Zoka-Assadi M, Roesler K, Anschuetz L, Huth M, Weber S. Robotic cochlear implantation: surgical procedure and first clinical experience. Acta Otolaryngol. 2017;137(4):447–54.
Topsakal V, Matulic M, Assadi M, Mertens G, Rompaey V, de Heyning P. Comparison of the surgical techniques and robotic techniques for cochlear implantation in terms of the trajectories toward the inner ear. J Int Adv Otol. 2020;16(1):3–7.
tenDonkelaar H, Elliott K, Fritzsch B, Kachlik D, Carlson M, Isaacson B, Topsakal V, Broman J, Tubbs S, Baud R. An updated terminology for the internal ear with combined anatomical and clinical terms. J Phonetics Audiol. 2022;6(2)
Tekin AM, Matulic M, Wuyts W, Assadi MZ, Mertens G, Rompaey VV, Li Y, Heyning PV, Topsakal V. A new pathogenic variant in POU3F4 causing deafness due to an incomplete partition of the cochlea paved the way for innovative surgery. Genes. 2021;12(5):613.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Tekin, A.M., Abari, J., Topsakal, V. (2023). Robotics for Approaches to the Mastoid/Mastoidectomy. In: Al-Salihi, M.M., Ayyad, A., Tubbs, R.S., Oertel, J. (eds) Robotics in Skull-Base Surgery. Springer, Cham. https://doi.org/10.1007/978-3-031-38376-2_6
Download citation
DOI: https://doi.org/10.1007/978-3-031-38376-2_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-38375-5
Online ISBN: 978-3-031-38376-2
eBook Packages: MedicineMedicine (R0)