Abstract
Robotic surgery in the last few decades has become more and more popular in all surgical specialties and during the last few years has also created interest in otology with the objective of improving the surgical accuracy by overcoming the surgeon’ hand limitations such as tremor, drift, and accurate force control feedback. Cochlear implantation may take advantage from robotic assistance in all the steps of the surgery: the approach to the middle ear through a tunnel from the postauricular skin to the inner ear (i.e., direct cochlea access); the minimally invasive cochleostomy by robot-assisted drilling tool; the alignment of the correct insertion axis on the cochlear basal turn, and the insertion of the electrode array via an automated insertion tool. The development of bone-attached parallel robots and image-guided surgical robot system allowed in the last few years the successful first cochlear implantation procedures in patients via a single hole drilled tunnel. Other robotic systems allowed successful implantation with reduced trauma cochleostomy and slow speed accurate insertion in the scala tympani.
Despite these promising results and the increasing number of procedures, the use of robotics in cochlear implantation does not represent yet the standard care. Further laboratory research and clinical studies are necessary to prove the superiority of robotic procedures with respect to standard procedure with the aim of making the intracochlear implant insertion an atraumatic and reversible gesture for a total preservation of the inner ear structure anatomy and physiology.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
17.1 Introduction
Robotic surgery in the last few years has gained popularity in ear surgery as an effective tool to improve the accuracy of surgical gesture by reducing involuntary movements of the surgeon such as tremor, drift, undershoot and overshoot, and the jerk, a sudden reflex or spasmodic muscular movement related to lack of experience or to tiredness. Other advantage reported by the use of robotic devices is the improvement of the force control feedback. Recent studies report that cochlear implantation may take advantage from robotic assistance in all the steps of the surgery: (i) the approach to the middle ear by automated mastoidectomy and posterior tympanotomy through a tunnel also known as direct cochlea access (DCA); (ii) a minimally invasive cochleostomy by means of robot-assisted drilling tool; (iii) the alignment of the correct insertion axis on the cochlear basal turn; and (iv) the insertion of the electrode array via motorized insertion tools [1].
The reduction of the intracochlear trauma during cochlear implant (CI) insertion is currently the standard care in cochlear implantation surgery with the objective of maintaining the integrity of inner ear structures for all cochlear implant recipients, even for those candidates with severe-to-profound hearing loss where there is no residual hearing to preserve. The reduction of the trauma during implantation offers several advantages indeed. The preservation of the residual low-frequency hearing is necessary for the electro-acoustic stimulation, but has also been demonstrated to contribute to better speech perception scores in patients with only classic electric stimulation [2]. Moreover, limiting the scalar translocation and thus the intracochlear damage has been proved to be a positive prognostic factor of hearing performances also for those patients without preoperative residual hearing [3, 4]. In addition, the reduced trauma limits the intracochlear fibrosis and/or ossification facilitating the possible revision surgery, especially for children who will probably require one or more reimplantation during their lifetime. Other advantages of limiting intracochlear injury include the potential application of future technologies, such as cellular regeneration or other novel cochlear nerve stimulation modalities [5]. In fact, providing a reversible atraumatic gesture may leave the possibility to remove the intracochlear device and eventually perform a reimplantation without compromising the inner ear structures.
The use of robotic devices in CI surgery has been demonstrated to be reliable in experimental models, to improve structure and hearing preservation in animals [6] or temporal bone cadaveric model [7], and more recently to have excellent postoperative results in implanted patients [8]. Different groups have studied and developed CI robotic devices, during the last two decades. In this chapter, a review of the different robots and automated devices capable of improving the surgeon gesture in the different steps of the cochlear implant surgery, from the inner ear access to the electrode array insertion, is presented.
17.2 Direct Access to the Cochlea
The standard and mostly used access to the cochlea for CI surgery requires a mastoidectomy and the passage of the electrode array through the posterior tympanotomy, although alternative and less invasive approaches have been described (trans-canal or supra-meatal) [9, 10]. The conventional approach needs significant drilling of the mastoid cells to identify the anatomical landmarks and to obtain a sufficient field of vision before opening the facial recess in safe conditions. A direct percutaneous tunnel from the retro-auricular mastoid region to the cochlear basal turn would reduce the invasiveness of the surgical procedure and, probably, the surgical time. During the last few decades, the improvement of imaging resolution and the development of surgical navigation systems, also known as image-guided systems, provided a solution to compensate the loss of visual control over the tunnel to be drilled to reach the inner ear. In fact, the development and the recent improvements of this technology allowed the landmark recognition and real-time positioning of the surgical instruments in the temporal bone.
Several laboratory experimental studies have been performed in the past few years to test the different variables for obtaining a reliable and safe direct cochlear access (DCA) for CI insertion [10,11,12,13,15] (Fig. 17.1).
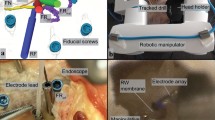
Direct cochlear access. Postoperative CT scan. Note the intact mastoid compartment and the direct linear access to the inner ear. The tunnel drilled by the Hearo® system allowed the implantation of a MED-EL electrode array in the scala tympani. Modified from Caversaccio, et al. (2019) Robotic middle ear access for cochlear implantation: First in man. PLoS One 14(8): e0220543. https://doi.org/10.1371/journal.pone.0220543
Currently, several systems are able to create a straight trajectory from the mastoid surface to the scala tympani at the ideal approach angle for entering the cochlea through the round window in temporal bone; two of them have been used in clinical practice (see section: clinical applications).
17.2.1 HEARO® System
The group from Bern, Switzerland, developed a robot, named HEARO®, which was designed for drilling a preoperatively planned tunnel based on CT images, passing through the facial recess with close proximity and sufficient margin to the facial nerve (>0.4 mm) [16]. The device is controlled by either a computer or manually using a 3D mouse. The robotic arm attaches directly to the operating table and controls the position of the surgical tool using active optical tool tracking relative to the patient via a bone-anchored active dynamic reference base (DRB) marker. All robot movements remain under the control of the user via an activation hand switch [17].
In the preoperative phase, four surgical screws are positioned into the mastoid under local anesthesia. A CT scan of the temporal bone is then performed and the images are transferred to a custom-developed planning software system to create a drilling plan based on the individual patient’s anatomy. The software enables automatic location of the implanted fiducial screws and the segmentation of anatomical structures to define a safe drilling trajectory from the mastoid surface to a target selected on the cochlea (round window or cochleostomy). Distances to surrounding anatomy are automatically calculated and displayed by the software, to assist in optimizing the trajectory for a scala tympani placement of the electrode array.
In the operating room, the patient’s head is secured in a head-holder and the robot is fixed onto the surgical table (Fig. 17.2). The surgical plan is subsequently transferred to the robotic system. The process starts with recording the fiducial screw positions by an optically tracked system. Once all fiducial screws locations have been digitized by the camera, the tracked tool is displayed in the surgical plan on the graphical user interface for navigation. The DRB is then attached to the temporal bone, and a second patient-to-image registration is performed relative to the DRB. The DRB allows the robot system to compensate for small changes in patient position during drilling. Robotic drilling of the tunnel is controlled by the surgeon using a hand-held switch and is performed using a custom heat-reducing drill to reduce the thermal damage to the facial nerve. Electromyographic facial nerve monitoring performed at preoperatively planned positions controls the facial functioning during the procedure. When the last portion of the tunnel is completed and the middle ear is reached, a tympano-meatal flap is performed to allow microscopic supervision of electrode array insertion in the cochlea.
The Hearo® robotic system during a cochlear implantation procedure. Modified from Caversaccio, et al. (2019) Robotic middle ear access for cochlear implantation: First in man. PLoS One 14(8): e0220543. https://doi.org/10.1371/journal.pone.0220543
17.2.2 Vanderbilt Robotic System
Bone-attached parallel robot requires neither head fixation nor intraoperative registration. The systems are based on a patient’s customized stereotaxic frame directly fixed on the head during both imaging and surgical intervention [18]. The group of Labadie and colleagues from Vanderbilt University (Nashville, USA) designed and developed a robotic system for DCA by using a micro-stereotactic frame attached to the patient skull via three rigid bone anchors [18]. In the preoperative CT scan, the anatomy of interest is automatically segmented and a safe linear trajectory avoiding vital anatomical structures and targeting the scala tympani is defined. Segmentation is accomplished via atlas-based segmentation for the external ear canal and ossicles and active shape modeling for the labyrinth and its subcomponents for facial nerve and chorda tympani. Trajectory generation specifies avoidance of the facial nerve and external ear canal but does allow violation of the chorda tympani if no other solutions are possible. In the operating room, three titanium self-tapping anchors attached to extenders with spherical tip are screwed into the skull. The spherical tips act both as fiducials for registering the patient’s anatomy to the CT scan and as attachment points for a miniature stereotactic frame. An intraoperative cone-beam CT scan is performed, and the segmented anatomy and drill trajectory from the preoperative CT scan are mapped to the intraoperative CT scan. A miniature tabletop that mounts onto the spherical fiducial markers via legs of specified lengths is then fabricated “on-the-fly” using a computer-numeric-control machine [19]; a specifically designed drill is attached to the micro-stereotactic frame, and then mounted on the fiducial markers. A cutaneous incision is made and the temporal bone is exposed. The tunnel is wider when lateral to the facial nerve (3.797 mm diameter) and then smaller at the level of the facial recess (1.59 mm diameter). A second intraoperative CT scan is obtained for additional safety to confirm the correct position of the drill and then confirmed under endoscopic vision. A tympano-meatal flap is lastly raised to allow access to the middle ear and perform the cochleostomy. Finally, the electrode array is passed into the drilled tunnel, and inserted in the cochlea under direct visualization. Confirmation of correct insertion and assessment of intracochlear positioning is made with a final intraoperative CT scan.
17.2.3 Hannover Robotic Systems
The group from Hannover, Germany, developed during the last decade different robot-based systems with the principal aim of reaching the inner ear via a minimally invasive approach, which can be divided into three technologies:
-
(i)
System based on an industrial robot adapted to the cochlear implant surgery: The KR3 (KUKA GmbH, Augsburg, Germany) is a six-joint light robot, with 6 degrees of freedom, a maximum operating distance of 635 mm in x/y-axis, and maximum loading capacity of 3 kg [13].
-
(ii)
Custom-made parallel kinematics with optimized structural elements [20, 21]: These devices have passive legs made of micrometer gauges and mounted via magnetic bearings directly on the skull. The more recent one is based on a spherical platform, using three non-rigid bone anchors letting the possibility to change the anchors location, adapting to each patient, so that the device provided a straight-line trajectory guidance. The device was tested on an artificial temporal and the target accuracy was 0.4 ± 0.12 mm, a result potentially suitable for minimally invasive cochlear implant surgery.
-
(iii)
Customized targeting platforms, consisting of a reusable base frame and a disposable patient-specific instrument: The disposable template was customized in the operating room to set the correct drill position. The plates are positioned with a mechatronics system in the desired position for the correct drilling of the tunnel, and the fixation methods for the micro-stereotactic frame were based on bone cement-filled struts [22].
17.3 Robot-Based Cochleostomy
The access to the scala tympani can be achieved by either a cochleostomy or direct round window insertion. Several studies compared the two techniques with some advantages reported in favor of the round window membrane insertion. Wanna et al. [23] showed the cochleostomy to be more traumatic and prone to scalar translocation than pure round window insertion. Nevertheless, considering the trajectory of the electrode array during the insertion, the first point of contact of lateral wall arrays is the 180° region in case of cochleostomy insertion, while in pure round window membrane insertion the array tip would hit the modiolar region before continuing its trajectory in the basal turn [24]. The choice of the entry point in the cochlea should be done on the basis of the anatomical variation of the hook region of cochlea [25].
Cochleostomy is a delicate step in the cochlear implantation surgery and consists in drilling the promontory to access the scala tympani to insert the electrode array. When drilling through the bone tissue of the cochlea, the inadvertent protrusion of the drill through the endosteal membrane can lead to its perforation in more than 60% of cases and inner ear damages [26]. Recent studies showed that manual opening of the endosteal membrane was a predictive factor of residual hearing preservation. On the contrary, a perforation of such a thin membrane (0.1–0.2 mm thick) by a rotating drill can lead to (i) increase of pressure into the endolymphatic fluids; (ii) direct trauma to the basilar membrane; and (iii) contamination of the fluids with bone dust [27]. To facilitate such a gesture, a smart hand-guided robotic microdrill system, coupled to a force sensor, was developed by Brett and colleagues [26]. The device was able to sense the changes in force transients and to stop on the interface of bone and soft tissue, preserving the endosteal membrane. Set at 700 RPM at a progression speed of 0.1 mm/s [28], and compared to manual drilling, robotic drilling reduced to 1% of the velocity induced on the endosteal membrane [29] with a mean force level of 1°N (0.6°N–1.3°N).
Klenzer and colleagues developed a force-controlled robot system with a conventional industrial robot (RX90CR, Staeubli AG, PfäYkon, Switzerland) using articulated arm kinematic structures coupled to a registration software to program a semi-automated cochleostomy. Coupled to 2D images and virtual endoscopic vision, the ideal localization of the cochleostomy was chosen and the cochleostomy performed with a target error of 0.25 mm [0.13–0.37 mm] [12].
17.4 Motorized Electrode Array Insertion Tool
Standard insertion of the electrode array is in general manually performed with limited visual and tactile feedback with the use of forceps possibly aided with micro-forks. In order to further control this high accuracy step, several insertion tools have been designed and commercialized. Indeed, the insertion technique is influenced by many factors, and some of them such as tremor, fits and starts, and insertion speed can be controlled with the use of such devices.
The motorized insertion tools have been designed to assist robotic implantation. Motorization of the insertion procedure could minimize intracochlear trauma as it allows for (i) standardization of the insertion, independently by the electrode array characteristics or surgical expertise; (ii) smoothing the insertion forces profile, and elimination of human tremor; and (iii) a very low speed and continuous insertion beyond those which are manually feasible.
Experimental insertion tools were developed by several groups (Hannover, Nashville, New York, Paris) [29,30,31,32,33,34,36]. The different automated motorized insertion tools allowed the insertion of straight electrodes, pre-curved electrodes, or both, enabling even a controlled stylet removal.
JT Roland (NYU, New York) and colleagues designed and developed an insertion device for a robotic advance-off-stylet (AOS) pre-curved electrode insertion. The device was intended for the bench evaluation of cochlear implant electrode insertion dynamics by fluoroscopic analysis and intracochlear hydraulic and mechanical force and lastly to evaluate the histological intracochlear trauma, and not for clinical application [30].
The group from Hannover, Germany, developed a first automated insertion tool in 2008 for AOS insertion and was tested and validated in synthetic scala tympani and cadaveric temporal bone models [31, 32]. Successively in cooperation with the Department of Mechanical Engineering, Vanderbilt University, Nashville, USA, they developed a force sensing unit which can be attached to the automated insertion tool (force resolution: 1000 μN) [33]. The tool was secondarily developed with another integrated force sensor (force resolution: 30 μN) and was designed to insert a straight electrode array, but could also pull the stylet during AOS insertion [34]. Recently, the same group proposed a new concept of a simple tool designed to automate the intracochlear cochlear insertion of electrode array with an easy adaptation to electrode arrays from different manufacturers, and meeting all sterility needs and regulations for intraoperative use [35]. The prototype facilitates automated forward motion using a syringe connected to an infusion pump. The design of the device purposes a commercially available, sterile, disposable syringe as hydraulic cylinder that provides automated hydraulic actuation. The plunger of the syringe serves as a piston and transforms the pressure inside the barrel into a continuous and linear movement at constant speed that pushes the electrode array into the scala tympani. The prototype is then connected to a standard surgical retractor with a flexible arm for manually adjusting the correct positioning under the direct control of the surgeon. Insertion velocity can be controlled via the infusion pump by setting a corresponding flow rate according to the desired insertion speed.
The group from Paris, France (Sorbonne University/Inserm), developed their first version of a motorized insertion tool a decade ago [36]. The tool was composed of a rotary electrical motor mounted on a micromanipulator. A threaded screw on the micromanipulator converted the rotary movement into a linear actuation. Motor speed was controlled via the input voltage current using an analog-to-digital interface card. The linear movement of the micromanipulator pushed the array via a blunt pin inside an insertion tube in order to eject the array. With this device, smooth slow insertion could be obtained, to achieve a reproducible insertion quality, necessary to evaluate force profiles in artificial cochleae and trauma in temporal bones. In the experimental insertion model, a 6-axis force was placed under the temporal bone. Force profiles for normal insertions, complete fold-over, or incomplete insertion could be measured with this test bench [37]. The current version of the tool took into consideration the visual field impairment and the possibility to be connected to a robotic arm (RobOtol®, Collin, Bagneux, France). The actuator speed was controlled via laboratory power supply and set at 0.8 mm/s. This tool was validated on plastic scala tympani model, in temporal bones, and in an animal model [36, 37].
17.5 Robot-Assisted Electrode Array Insertion
The most important phase of cochlear implantation surgery is the insertion of the electrode array. The optimal array insertion includes both a full insertion into the scala tympani and a preservation of the inner ear structures. In addition to array specifications (size, shape, stiffness, etc.), different predictive factors have been correlated to an atraumatic insertion of the electrode array, mainly depending on the human hand accuracy and therefore on the surgeon’s experience. The insertion axis, ideally aligned to the basal turn of the cochlea [38], a low speed of insertion (0.25 mm/s) [39], and low forces applied during insertion were correlated to a high number of electrodes correctly inserted in the scala tympani, and reduced intracochlear trauma [40].
17.5.1 RobOtol ® System, Paris, France
A robot-based arm developed to be able to operate in restrained areas was first designed to be dedicated to middle ear procedures, for the assistance of precise gestures such as otosclerosis surgery [41]. The current version of the RobOtol® is a 6 degrees-of-freedom (DOF) teleoperated robot arm [41,42,44] (Fig. 17.3). It is provided by two adjustable arms that can be used separately: one is a dedicated micro-instrument holder and the other is an endoscope holder. Three linear actuators were integrated into a XYZ cross-table. The table was built with 2 orthogonal (X–Y) precision linear stages with 70 mm travel, and a Z precision linear stage with 95 mm travel. Three DC micromotors with a magnetic incremental encoder were used for all rotary actuators. These were connected to a harmonic drive gearhead with a 100:1 reduction ratio. Two command modes are available to teleoperate the RobOtol® system: (i) The Phantom Omni® (SensAble Technologies, Inc., Woburn, MA) a pen-like interface with 6 DOF. It works with a position-to-position command from the master to the slave arms. The command is based on a registered correspondence between the local stylus frame and the robot tool frame. (ii) The Space-Mouse (3Dconnexion, Waltham, MA), with a position-to-velocity command. The registration mode allows master arm configuration to be uncoupled from the robot, and allows indirect visual feedback (e.g., angled endoscope). In the velocity command mode, stylus motion codes for robot speed (as with a joystick). In this mode, the user has to release the dead man’s foot switch (DMFS) to stop the robot. In both modes, the DMFS allows the surgeon to confirm movement commands. In both command modes, a down-scale ratio between the master and slave arms can be implemented to enhance control for a more accurate movement. The use of RobOtol® system in cochlear implantation is with micro-instrument holder arm, the robot to align the motorized insertion tool, or the other dedicated electrode array’s holder instrument for the insertion in the scala tympani. After the round window membrane opening, the array is pushed in the scala tympani by the motorized insertion tool or, in the clinical setting, directly by the forward movement of the robotic arm. In the latter scenario, all the axes of freedom of the robot are blocked (X-,Y-, and all rotation axes), with the exception of the Z-axis that allowed the robot arm to move only linearly along the chosen insertion trajectory toward the scala tympani. Using a constant speed insertion of 0.25 mm/s and compared to a manual insertion on temporal bones, robot-assisted insertion was found to be more accurate if the optimal insertion axis was respected [7] and less traumatizing to the inner ear structures [45].
Left: Overall view of the RobOtol®. The system comprises a cart carrying a controller, a human–machine interface and a slave robot-based arm. Right: operating room setting where both the microscope and the robot with the instrument holder arm are present. Modified from Vittoria S., et al. Robot-based assistance in middle ear surgery and cochlear implantation: first clinical report. Eur Arch Otorhinolaryngol. 2021;278:77–85. https://doi.org/10.1007/s00405-020-06070-z
17.6 Curvature-Controlled and Steerable Electrode Arrays
Two different groups [46, 47] worked on the development of prototype arrays able to bend or curve in order to facilitate the surgical insertion by providing additional degrees of freedom to achieve more precise control of the array trajectory during insertion. The aim of these prototypes is to guide the trajectory of the insertion following the cochlear coiling and thus to minimize the contact with the lateral wall or modiolar region so that the occurrence of an insertion trauma would be limited.
Zhang and colleagues developed a robot capable of achieving a cochlear implantation with a controlled steerable electrode array. In their actively electrodes, a strand is embedded off the centerline of an elastomeric electrode array and attached internally to the tip of the electrode inside. The rest of the strand can move inside the electrode. When pulling the strand while holding the base of the electrode array, the electrode array bends to predetermined shapes. The last prototype developed by the researchers was a parallel robot with force sensing capability, 6 actuators that manipulate its moving platform through 6 independently controlled linear actuators (cylinders) [46]. The base of this robot is held stationary during surgery through a mechanical lock. The steerable electrode is held on the moving platform. The electrode steering includes 2 levels of motions. First, the electrode itself can be bent to a certain shape by the embedded strand. Different amounts of pull on the strand correspond to different bent shapes of the steerable electrode. Second, the bent electrode as a whole unit can be rotated by the robot, which further increases its steerability. The rotation from the robot can adjust the electrode approach angle toward the scala tympani. The robot was controlled through a haptic joystick that allows surgeons to move the robot, following the hand motions of surgeons. The computer shows and records the surgery information, such as insertion depth, insertion speed, and sensed force.
Unfortunately, steerable electrode array technology, despite their promising results, has not yet been developed by cochlear implant manufacturers.
17.7 Clinical Applications
At present, three robots are used for cochlear implantations in patients. Two of them (Vanderbilt system and HEARO®) were designed for the DCA, the subsequent electrode array insertion being manually performed; the last one (RobOtol®) is not intended for a mastoid tunnel drilling but for the robotic alignment of the electrode array through a mastoidectomy and its subsequent insertion in the scala tympani.
17.7.1 Vanderbilt System
The first preliminary report showed 9 patients who underwent a CI surgery. Eight of them had a successful intracochlear insertion of the array, although one procedure has to be converted to standard open technique. Mean surgical time was 182 ± 36 min. Styleted CI electrode arrays were used in 6 patients, and the other 3 patients received lateral wall electrode array. As major complication, one patient developed a mild postoperative permanent mild facial nerve palsy due to overheating [48]. No further clinical studies have been performed pending improvement of safety mechanisms to protect against possible mechanical or thermal damage [49].
17.7.2 HEARO® System
Nine patients were enrolled for the first cochlear implantation trial with the robotic system [17], the mean total surgical time was 4 h, and the drilling accuracy was very high (0.2 ± 0.10 mm). In 3 cases, the procedure was converted to standard open technique due to critical distance of the tunnel to vital structures.
17.7.3 RobOtol® System
The RobOtol® was available from February 2018 at Pitié-Salpetrière Hospital (Paris, France) where the first clinical trial took part [50] and successively was adopted in other centers in Europe and China (Video 17.1). Cochlear insertions were possible using specific tools available for different devices (straight electrode array of Advanced Bionics® (Valencia, CA, USA), Cochlear® (Sydney, Australia), MED-EL® (Innsbruck, Austria), Nurotron® (Hangzhou, China), and Oticon Medical® (Vallauris, France) [8, 48,49,52]. The AOS insertion of a pre-curved electrode array (Advanced Bionics, Mid-Scala®) was also possible, but was partially robot-assisted. The stylet was positioned and maintained by the robot arm, and then the electrode array was pushed manually into the cochlea [52]. At present, more than one-hundred CIs were implanted with the aid of RobOtol® system and the numbers are rapidly increasing. Published data reported that the robot-assisted insertion of straight electrode array is less traumatizing for the inner ear, compared to the manual one, with 7% versus 16% of translocated electrodes, respectively (p < 0.001) [52]. The average total time of surgery (including mastoidectomy and posterior tympanotomy) was 138 ± 7.1 min, an acceptable performance with approximately 30 min more than the manual technique (average of 109 ± 4.0 min). The group from Liege, Belgium, reported the preliminary results of their first five MED-EL® Flex 24 electrodes implanted with the robotic system. The insertion speed was 0.88 ± 0.12 mm/s, the mean postoperative PTA (500–4000 Hz) hearing loss was 13.60 ± 7.70 dB, and the postoperative radiological analysis did not show any electrodes translocation or damage to the inner ear structures [8].
17.8 Future Perspectives
The ideal robot for cochlear implantation should encompass all the best characteristics from the available devices, from both the direct cochlear access systems and the robotic arm and insertion tools.
To date, among the 3 robotic systems that have been used for cochlear implantation in patients, the RobOtol® is the more widespread used. Considering the intracochlear trauma, the RobOtol®, compared to standard manual cochlear implantation surgery, has been demonstrated to reduce the intracochlear forces in temporal bones and to correctly position the array in the scala tympani with both low translocation and high hearing preservation rates in patients; partial hearing preservation rate after the HEARO® system procedure was reported in 4 out of 9 patients, while the Vanderbilt robot mainly designed for a mini-invasive inner ear access does not have yet published data concerning hearing preservation results.
17.8.1 Robotic Arm Alignment for Insertion Axis Control
The optimal insertion axis of the electrode array is parallel to the basal turn of the cochlea. Torres et al. [38] showed that the mental representation of the basal-turn anatomy was experience-dependent but that even experts had non-optimal representation to some extent (<7 degrees error). In successive studies, the same authors reported that the robotic alignment of the insertion tool with the aid of a navigation system reduced the angle error with an optimal insertion vector below 2 degrees in temporal bone models [7]. Successive temporal bone studies indicate that the angle of approach to the scala tympani centerline is a critical factor in intracochlear trauma [45]. Unpublished data show that the automated insertion system (RobOtol® + navigation system) could accurately align the tip of the pre-curved Advanced Bionics Mid-scala® electrode array to the axis of the basal turn and then the subsequent coiling of the scala tympani, and dramatically reduce intracochlear trauma and translocation. Considering these results, we can assume that the application of the image guidance systems to the RobOtol® for cochlear implantation on patients should improve the hearing preservation rate and therefore ameliorate the postoperative auditory outcomes for these patients.
17.8.2 Insertion Force and Electrocochleography (EcochG) Feedback Control During CI Insertion
Preserving the amplitude of EcochG responses has shown to predict the extent of hearing preservation [53]. Moreover, the electrode insertion forces were demonstrated to be correlated with structural damage in human temporal bone [40] and by extension with the level of post-implantation hearing loss. In a guinea pig model study, by Lo and colleagues from Melbourne, Australia, modified cochlear implants were inserted while monitoring the ECochG responses and insertion forces. Results showed that intraoperative compound action potential (CAP) amplitude during implantation was predictive of an atraumatic insertion and reduced post-implantation hearing loss [54]. A rise in force usually preceded the reporting of resistance, although by less than 1 s. These results suggest that intraoperative CAPs may offer a robust feedback mechanism for improving hearing preservation rates than electrode insertion force recordings, especially considering the rapid changes in insertion force and relatively slow human reaction times. However, a recent systematic review concluded that ECochG recordings were achieved in over 90% of patients, but accuracy to predict postoperative hearing loss remained limited [55].
New robotic insertion tools should be provided with loop feedback systems capable of modifying the insertion parameters on the basis of both insertion forces and electrocochleographic responses.
17.8.3 Electrode Insertion Path Planning
In a recent study, Wang et al. [56] proposed a method to study the optimal insertion path into the scala tympani of a perimodiolar electrode array and plan a strategy for a robotic insertion following the designed path (centerline of scala tympani). The coordinate information of the optimal path was combined with the stylet extraction state to conduct the path planning for robotic cochlear implant, and the result was sent to the robot for kinematic inverse solution to obtain the robot motion trajectory. The results of the study showed a reduction of the insertion forces using the path planning insertion method. The path planning methodology seems to be a promising tool to reduce intracochlear trauma and could be in further studies modified for the application to other electrode array (e.g., steerable or straight electrodes).
17.9 Conclusion
First clinical trials in patients implanted with robotic devices began a few years ago, and since then, three different systems have been successfully used. Robotic cochlear implantation is currently used in few centers, but its application and the number of procedures is rapidly increasing. Moreover, a great acceptance of patients and parents toward task-autonomous robotic cochlear implantation was recently reported in a survey in patients scheduled for manual cochlear implantation [57]. Considering the promising results in terms of mini-invasiveness, reduced trauma, and better hearing preservation, further laboratory research and clinical studies should continue with the aim of improving the results of robotic cochlear implantation making the implant insertion an atraumatic and reversible gesture for a total preservation of the inner ear structure and physiology.
References
Daniele, De Seta Hannah, Daoudi Renato, Torres Evelyne, Ferrary Olivier, Sterkers Yann, Nguyen. Robotics automation active electrode arrays and new devices for cochlear implantation: A contemporary review. Hear Res. 2022. 414108425-10.1016/j.heares.2021.108425.
Schaefer S, Sahwan M, Metryka A, et al. The benefits of preserving residual hearing following cochlear implantation: a systematic review. Int J Audiol. 2021;60:1–17. https://doi.org/10.1080/14992027.2020.1863484.
Aschendorff A, Kromeier J, Klenzner T, et al. Quality control after insertion of the nucleus contour and contour advance electrode in adults. Ear Hear. 2007;28:75S–9S. https://doi.org/10.1097/AUD.0b013e318031542e.
Finley CC, Skinner MW. Role of electrode placement as a contributor to variability in cochlear implant outcomes. Otol Neurotol. 2008;29:920–8. https://doi.org/10.1097/MAO.0b013e318184f492.
Devare J, Gubbels S, Raphael Y. Outlook and future of inner ear therapy. Hear Res. 2018;368:127–35. https://doi.org/10.1016/j.heares.2018.05.009.
Mamelle E, Kechai NE, Granger B, et al. Effect of a liposomal hyaluronic acid gel loaded with dexamethasone in a Guinea pig model after manual or motorized cochlear implantation. Eur Arch Otorhinolaryngol. 2017;274:729–36. https://doi.org/10.1007/s00405-016-4331-8.
Torres R, Kazmitcheff G, De Seta D, et al. Improvement of the insertion axis for cochlear implantation with a robot-based system. Eur Arch Otorhinolaryngol. 2017;274:715–21. https://doi.org/10.1007/s00405-016-4329-2.
Barriat S, Peigneux N, Duran U, et al. The use of a robot to insert an electrode array of Cochlear implants in the cochlea: a feasibility study and preliminary results. Audiol Neurotol. 2021;26(5):361–7.
Kiratzidis T. “Veria operation”: cochlear implantation without a mastoidectomy and a posterior tympanotomy. A new surgical technique. Adv Otorhinolaryngol. 2000;57:127–30. https://doi.org/10.1159/000059218.
Kronenberg J, Migirov L, Dagan T. Suprameatal approach: new surgical approach for cochlear implantation. J Laryngol Otol. 2001;115:283–5. https://doi.org/10.1258/0022215011907451.
Caversaccio M, Stieger C, Weber S, et al. Navigation and robotics of the lateral skull base. HNO. 2009;57:975–82. https://doi.org/10.1007/s00106-009-1985-1.
Klenzner T, Ngan CC, Knapp FB, et al. New strategies for high precision surgery of the temporal bone using a robotic approach for cochlear implantation. Eur Arch Otorhinolaryngol. 2009;266:955–60. https://doi.org/10.1007/s00405-008-0825-3.
Majdani O, Rau TS, Baron S, et al. A robot-guided minimally invasive approach for cochlear implant surgery: preliminary results of a temporal bone study. Int J Comput Assist Radiol Surg. 2009;4:475–86. https://doi.org/10.1007/s11548-009-0360-8.
Stieger C, Caversaccio M, Arnold A, et al. Development of an auditory implant manipulator for minimally invasive surgical insertion of implantable hearing devices. J Laryngol Otol. 2011;125:262–70. https://doi.org/10.1017/S0022215110002185.
Nguyen Y, Miroir M, Vellin J-F, et al. Minimally invasive computer-assisted approach for Cochlear implantation: a human temporal bone study. Surg Innov. 2011;18:259–67. https://doi.org/10.1177/1553350611405220.
Anso J, Balmer TW, Jegge Y, et al. Electrical impedance to assess facial nerve proximity during robotic Cochlear implantation. IEEE Trans Biomed Eng. 2019;66:237–45. https://doi.org/10.1109/TBME.2018.2830303.
Caversaccio M, Gavaghan K, Wimmer W, et al. Robotic cochlear implantation: surgical procedure and first clinical experience. Acta Otolaryngol (Stockh). 2017;137:447–54. https://doi.org/10.1080/00016489.2017.1278573.
Kratchman LB, Blachon GS, Withrow TJ, et al. Design of a bone-attached parallel robot for percutaneous cochlear implantation. IEEE Trans Biomed Eng. 2011;58:2904–10. https://doi.org/10.1109/TBME.2011.2162512.
Labadie RF, Mitchell J, Balachandran R, et al. Customized, rapid-production microstereotactic table for surgical targeting: description of concept and in vitro validation. Int J Comput Assist Radiol Surg. 2009;4:273–80. https://doi.org/10.1007/s11548-009-0292-3.
Kobler J-P, Kotlarski J, Oltjen J, et al. Design and analysis of a head-mounted parallel kinematic device for skull surgery. Int J Comput Assist Radiol Surg. 2012;7:137–49. https://doi.org/10.1007/s11548-011-0619-8.
Kobler J-P, Nuelle K, Lexow GJ, et al. Configuration optimization and experimental accuracy evaluation of a bone-attached, parallel robot for skull surgery. Int J Comput Assist Radiol Surg. 2016;11:421–36. https://doi.org/10.1007/s11548-015-1300-4.
Vollmann B, Müller S, Kundrat D, et al. Methods for intraoperative, sterile pose-setting of patient-specific microstereotactic frames. Proc SPIE. 2015;9415(2015):94150M.
Wanna GB, Noble JH, Carlson ML, et al. Impact of electrode design and surgical approach on scalar location and cochlear implant outcomes. Laryngoscope. 2014;124(Suppl 6):S1–7. https://doi.org/10.1002/lary.24728.
Zhou L, Friedmann DR, Treaba C, et al. Does cochleostomy location influence electrode trajectory and intracochlear trauma? Laryngoscope. 2015;125:966–71. https://doi.org/10.1002/lary.24986.
Atturo F, Barbara M, Rask-Andersen H. On the anatomy of the “hook” region of the human cochlea and how it relates to cochlear implantation. Audiol Neurootol. 2014;19:378–85. https://doi.org/10.1159/000365585.
Brett PN, Taylor RP, Proops D, et al. A surgical robot for cochleostomy. Annu Int Conf IEEE Eng Med Biol Soc. 2007;2007:1229–32. https://doi.org/10.1109/IEMBS.2007.4352519.
Coulson CJ, Reid AP, Proops DW. Robotics can lead to a reproducibly high-quality operative result for ear, nose, and throat patients. Proc Inst Mech Eng. 2010;[H] 224:735–42. https://doi.org/10.1243/09544119JEIM714.
Coulson CJ, Assadi MZ, Taylor RP, et al. A smart micro-drill for cochleostomy formation: a comparison of cochlear disturbances with manual drilling and a human trial. Cochlear Implants Int. 2013;14:98–106. https://doi.org/10.1179/1754762811Y.0000000018.
Assadi MZ, Du X, Dalton J, et al. Comparison on intracochlear disturbances between drilling a manual and robotic cochleostomy. Proc Inst Mech Eng. 2013;227:1002–8. https://doi.org/10.1177/0954411913488507.
Roland JT. A model for Cochlear implant electrode insertion and force evaluation: results with a new electrode design and insertion technique. Laryngoscope. 2005;115:1325–39. https://doi.org/10.1097/01.mlg.0000167993.05007.35.
Hussong A, Rau T, Eilers H, et al. Conception and design of an automated insertion tool for cochlear implants. Annu Int Conf IEEE Eng Med Biol Soc. 2008;2008:5593–6. https://doi.org/10.1109/IEMBS.2008.4650482.
Hussong A, Rau TS, Ortmaier T, et al. An automated insertion tool for cochlear implants: another step towards atraumatic cochlear implant surgery. Int J Comput Assist Radiol Surg. 2010;5:163–71. https://doi.org/10.1007/s11548-009-0368-0.
Schurzig D, Webster RJ, Dietrich MS, et al. Force of cochlear implant electrode insertion performed by a robotic insertion tool: comparison of traditional versus advance off-stylet techniques. Otol Neurotol. 2010;31:1207–10. https://doi.org/10.1097/MAO.0b013e3181f2ebc3.
Majdani O, Schurzig D, Hussong A, et al. Force measurement of insertion of cochlear implant electrode arrays in-vitro: comparison of surgeon to automated insertion tool. Acta Otolaryngol (Stockh). 2010;130:31–6. https://doi.org/10.3109/00016480902998281.
Rau TS, Zuniga MG, Salcher R, et al. A simple tool to automate the insertion process in cochlear implant surgery. Int J Comput Assist Radiol Surg. 2020;15:1931–9. https://doi.org/10.1007/s11548-020-02243-7.
Miroir M, Nguyen Y, Kazmitcheff G, et al. Friction force measurement during cochlear implant insertion: application to a force-controlled insertion tool design. Otol Neurotol. 2012a;33:1092–100. https://doi.org/10.1097/MAO.0b013e31825f24de.
Nguyen Y, Miroir M, Kazmitcheff G, et al. Cochlear implant insertion forces in microdissected human cochlea to evaluate a prototype array. Audiol Neurootol. 2012;17:290–8. https://doi.org/10.1159/000338406.
Torres R, Kazmitcheff G, Bernardeschi D, et al. Variability of the mental representation of the cochlear anatomy during cochlear implantation. Eur Arch Otorhinolaryngol. 2016;273:2009–18. https://doi.org/10.1007/s00405-015-3763-x.
Rajan GP, Kontorinis G, Kuthubutheen J. The effects of insertion speed on inner ear function during cochlear implantation: a comparison study. Audiol Neurootol. 2013;18:17–22. https://doi.org/10.1159/000342821.
De Seta D, Torres R, Russo FY, et al. Damage to inner ear structure during cochlear implantation: correlation between insertion force and radio-histological findings in temporal bone specimens. Hear Res. 2017;344:90–7. https://doi.org/10.1016/j.heares.2016.11.002.
Nguyen Y, Bernardeschi D, Sterkers O. Potential of robot-based surgery for otosclerosis surgery. Otolaryngol Clin N Am. 2018;51:475–85. https://doi.org/10.1016/j.otc.2017.11.016.
Kazmitcheff G, Miroir M, Nguyen Y, et al. Evaluation of command modes of an assistance robot for middle ear surgery. In: Presented at the 2011 IEEE/RSJ International Conference on Intelligent Robots and System, International Conference on Intelligent Robots and Systems. San Francisco, CA, USA: IEEE; 2011. p. 2532–8. https://doi.org/10.1109/IROS.2011.6094634.
Miroir M, Nguyen Y, Szewczyk J, et al. Design, kinematic optimization, and evaluation of a teleoperated system for middle ear microsurgery. Sci World J. 2012;2012:e907372. https://doi.org/10.1100/2012/907372.
Miroir M, Nguyen Y, Szewczyk J, et al. RobOtol: from design to evaluation of a robot for middle ear surgery. In: Presented at the 2010 IEEE/RSJ International Conference on Intelligent Robots and Systems. San Francisco, CA, USA: IEEE; 2010. p. 850–6. https://doi.org/10.1109/IROS.2010.5650390.
Torres R, Jia H, Drouillard M, et al. An optimized robot-based technique for Cochlear implantation to reduce array insertion trauma. Otolaryngol Head Neck Surg. 2018;159:900–7. https://doi.org/10.1177/0194599818792232.
Zhang J, Wei W, Ding J, et al. Inroads toward robot-assisted cochlear implant surgery using steerable electrode arrays. Otol Neurotol. 2010;31:1199–206. https://doi.org/10.1097/MAO.0b013e3181e7117e.
Wu J, Yan L, Xu H, et al. A curvature-controlled 3D micro-electrode array for cochlear implants. In: The 13th International Conference on Solid-State Sensors, Actuators and Microsystems, 2005. Seoul, Korea: Digest of Technical Papers; 2005. p. 1636–9. https://doi.org/10.1109/SENSOR.2005.1497402.
Labadie RF, Balachandran R, Noble JH, et al. Minimally-invasive, image-guided Cochlear implantation surgery: first report of clinical implementation. Laryngoscope. 2014;124:1915–22. https://doi.org/10.1002/lary.24520.
Robert F, Labadie Katherine, Riojas Kathleen, Von Wahlde Jason, Mitchell Trevor, Bruns Robert, Webster Benoit, Dawant J. Michael, Fitzpatrick Jack, Noble. Clinical Implementation of Second-generation Minimally Invasive Image-guided Cochlear Implantation Surgery. Otol Neurotol. 2021. Publish Ahead of Print https://doi.org/10.1097/MAO.0000000000003025
Sykopetrites V, Lahlou G, Torres R, et al. Robot-based assistance in middle ear surgery and cochlear implantation: first clinical report. Eur Arch Otorhinolaryngol. 2020;278(1):77–85. https://doi.org/10.1007/s00405-020-06070-z.
Jia H, Pan JX, Li Y, et al. Preliminary application of robot-assisted electrode insertion in cochlear implantation. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2020;55:952–6. https://doi.org/10.3760/cma.j.cn115330-20200228-00141.
Daoudi H, Lahlou G, Torres R, et al. Robot-assisted Cochlear implant electrode array insertion in adults: A comparative study with manual insertion. Otol Neurotol. 2021;42(4):e438–44. https://doi.org/10.1097/MAO.0000000000003002.
O’Connell BP, Holder JT, Dwyer RT, et al. Intra- and postoperative electrocochleography may be predictive of final electrode position and postoperative hearing preservation. Front Neurosci. 2017;11:291. https://doi.org/10.3389/fnins.2017.00291.
Lo J, Bester C, Collins A, et al. Intraoperative force and electrocochleography measurements in an animal model of cochlear implantation. Hear Res. 2018;358:50–8. https://doi.org/10.1016/j.heares.2017.11.001.
Yin LX, Barnes JH, Saoji AA, Carlson ML. Clinical utility of electrocochleography (ECochG) during cochlear implantation: a systematic review and quantitative analysis. Otol Neurotol. 2021;42:363–71. https://doi.org/10.1097/MAO.0000000000002996.
Labadie RF, Riojas K, Von Wahlde K, Mitchell J, Bruns T, Webster III R, Dawant B, Fitzpatrick JM, Noble J. Clinical implementation of second-generation minimally invasive image-guided cochlear implantation surgery. Otol Neurotol. 2021;42(5):702–5. https://doi.org/10.1097/MAO.0000000000003025.
De Seta D, Daoudi H, Torres R, Ferrary E, Sterkers O, Nguyen Y. Robotics automation active electrode arrays and new devices for cochlear implantation: A contemporary review. Hear Res. 2021;414:108425. https://doi.org/10.1016/j.heares.2021.108425
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
17.1 Electronic Supplementary Material
Robot based Cochlear Implant insertion (MP4 821201 kb)
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
De Seta, D., Nguyen, Y., Torres, R., Mosnier, I., Sterkers, O. (2022). Robot-Assisted Cochlear Implantation. In: DeSaSouza, S. (eds) Cochlear Implants. Springer, Singapore. https://doi.org/10.1007/978-981-19-0452-3_17
Download citation
DOI: https://doi.org/10.1007/978-981-19-0452-3_17
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-0451-6
Online ISBN: 978-981-19-0452-3
eBook Packages: MedicineMedicine (R0)