Abstract
Here, I present a review of the global pre-Pleistocene fresh- to brackish-water fossil record of the gastropod family Lymnaeidae based on a thorough literature survey of over 450 scientific works. I discuss the putative origin of the family, assess diversity development through geological time (based on the fossil records of Europe and North America), and illustrate the family’s geographic distribution over the past 200 Myr using paleogeographic maps. The following section deals with potential dispersal mechanisms to explain the family’s disjunct fossil distribution. A final part is devoted to peculiar cases of morphological evolution toward limpet-like and/or strongly sculptured shells.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
6.1 Introduction
Today, the Lymnaeidae are a diverse family in the world’s fresh water (e.g., Pyron and Brown 2015; Aksenova et al. 2018; Vinarski et al. 2019; and other articles in this volume). The family is near globally distributed, whereas the northern hemisphere maintains a much higher diversity (Vinarski et al. 2019). Also morphologically, they are highly diverse and comprise a great variety of shell shapes (e.g., Baker 1911; Hubendick 1951; Correa et al. 2011; Campbell et al. 2017; Aksenova et al. 2018; Vinarski et al. 2019; Alda et al. 2023). Moreover, Lymnaeidae play a vital role in the transmission of liver fluke and are thus of medical importance (e.g., Correa et al. 2010; Mahulu et al. 2019).
Since lymnaeids occur in a great range of habitats, they are a common component of fossil freshwater communities and are often used as paleoecological indicators (e.g., Good 1987; Carobene et al. 2018; Salvador et al. 2018a; Esu and Girotti 2020; Kadolsky 2020; Rasser et al. 2020). Numerous species have been described from fossil deposits all around the globe (e.g., Wenz 1923). Yet, in spite of their high diversity and relevance to paleoecology, a thorough review of the family’s diversity and distribution through geological time is absent.
Here, I review the literature to provide an overview over the global fossil record of lymnaeids and their geographic and stratigraphic distribution. Given the number of publications documenting lymnaeids in fossil deposits, the overview focuses on the pre-Pleistocene record. Although not including every single record in the literature, I sought to cover the complete geographic range of the family through time. I review the oldest records of fossil species attributed to the Lymnaeidae and discuss the family’s potential origin. Based on the available data for Europe and North America, which contain particularly rich records, I reconstruct continental diversity through time. Lymnaeid distribution through time and space is illustrated via paleogeographic maps. Two further chapters deal with potential dispersal mechanisms and morphological evolution.
6.2 Material and Methods
Information for European and North American faunas was retrieved in the frame of a project dealing with diversification dynamics of freshwater gastropods over the past 200 Myr. This project aligns with a continuous effort to gather and complete an inventory of all fossil freshwater gastropods worldwide. Most of the taxonomic information has already been made available via MolluscaBase (MolluscaBase eds 2021). Data were mostly obtained directly from the literature. Additional information were derived from various online sources, but in all cases these data were checked for accuracy and correctness against the primary literature. In total, the data were derived from 454 scientific works (Supplementary Table S1).
Systematically, I include in this review all species currently classified in the Lymnaeidae. Since fossil (as well as recent) lymnaeids have been regularly mixed up with members of the terrestrial families Succineidae (e.g., Marshall 1926), Bulimulidae (e.g., Salvador et al. 2018b), and Oleacinidae (e.g., Wenz 1923; Harzhauser et al. 2014b), I follow the latest revisions to account for systematic changes. This concerns also some genera formerly placed in the family Clivunellidae (Delminiella, Neoclivunella, and Neodelminiella), now a monotypic family restricted to the Miocene of the Balkans (Harzhauser et al. 2016). Neoclivunella and Neodelminiella comprise limpet-shaped shells and are classified tentatively in the lymnaeid subfamily Valencienniinae (Harzhauser et al. 2016). That taxon is still poorly defined and its genus- and species-level taxonomy is not well resolved and depends on the author (e.g., Gorjanović-Kramberger 1923; Moos 1944; Taktakishvili 1967; Marinescu 1970). Because of their descent from a species belonging to the genus Radix, Bouchet et al. (2017) treated the Valencienniinae as a synonym of Amphipepleinae. Here, I follow the traditional concept and treat the subfamily as distinct. In addition to the two abovementioned genera, I include here Velutinopsis, Undulotheca, Hiscerus, Velutinellus, Provalenciennesia, and Valenciennius. Despite the morphological similarity to Delminiella and the Valencienniinae, the family Clivunellidae is believed to be closely related to Planorbidae (Harzhauser et al. 2016) and is thus not treated herein. The Mesozoic genus Proauricula Huckriede, 1967, which was considered a basal Basommatophora and probably predecessor of the Lymnaeidae by Bandel (1991), was lately reclassified in the Ellobiidae by Yu et al. (2021).
Stratigraphically, the review is limited to the pre-Pleistocene fossil record. With few exceptions (e.g., the Caspian Sea; Andrusov 1923), the Early Pleistocene faunas resemble in species composition the modern fauna (e.g., Taylor 1960, 1966; La Rocque 1963; Esu and Girotti 1975; Schlickum and Puisségur 1978; Dehm 1979). Only the overview of the number of fossil species discovered per year and decade is based on all fossil species. Data were derived from a MolluscaBase query for all fossil-only species (including nomina dubia, taxa inquirenda and temporary names, e.g., junior homonyms without replacement name; queried 7 November 2021; Supplementary Table S2).
Age classifications of the localities are based on the most recent assessments of the fossil-bearing strata. For most European sites, age data are available from Neubauer et al. (2015a, b, c, 2021). Stratigraphic data on formations in China were derived mostly from Zhang (2009). Based on the ages, paleocoordinates were estimated using the GPlates Portal under the PALEOMAP model (http://portal.gplates.org/service/d3_demo/?view=points).
The statistical analyses were performed using R v. 4.0.3 (R Core Team 2020). Diversity reconstructions are made only for Europe (including Turkey) and North America because of the paucity of data for the other continents as well as the uncertain stratigraphy or taxonomy of many of those records. Subspecies, doubtful records or identifications (“cf.”, “aff.”), nomina nuda and nomina dubia were not considered. Taxa inquirenda and temporary names were included. Range-through species richness binned over stratigraphic stages was calculated using the package divDyn v. 0.8.0 (Kocsis et al. 2019). In order to avoid a biased decrease of range-through richness toward the Pleistocene by assuming that all taxa went extinct at the end of the Pliocene, I included species occurring today in a separate Holocene time bin for the calculation. Data for the modern species was taken from MolluscaBase. Stage boundaries follow the International Chronostratigraphic Chart (July 2021; https://stratigraphy.org/chart).
Since all fossil time series are inevitably skewed by uneven preservation (Alroy 2010), I applied a subsampling approach to assess whether any observed diversity pattern is the result of sampling. For this purpose, I used the shareholder quorum subsampling (SQS) method of Alroy (2010) as implemented and advanced in the package divDyn using 1000 iterations and a quorum level varying between 0.3 and 0.7 at increments of 0.1. The SQS approach was only applied to the European dataset, since not enough data are available for other continents.
Genus-level diversity is not discussed herein due to the problems associated with genus classification in lymnaeids. While modern lymnaeid systematics rely on anatomical characters and results from molecular phylogenetics (e.g., Vinarski 2013; Aksenova et al. 2018), any fossil species classification is inevitably based on shell characters, which are often of limited value (see also Vinarski 2013 for an in-depth discussion of the subject). Most fossil species are placed in “standard” genera like Lymnaea or Galba, whereas probably very few of them actually belong in these genera.
Institutional abbreviations. MBFSZ – Mining and Geological Survey of Hungary, Budapest; NHMW – Natural History Museum Vienna; ZMBH – Zemaljski Muzej Bosne i Hercegovine, Sarajevo.
6.3 Diversity Through Time
The first fossil lymnaeids were described by Alexandre Brongniart in 1810 (Brongniart 1810). He introduced nine new species from Eocene and Oligocene deposits of the Paris Basin in France; all of them are still considered as valid. Since then, the number of species descriptions per year has almost linearly increased (Fig. 6.1a). Noteworthy publications advancing the number of species above average were contributed by Deshayes (1863), who described 15 species also from the Paleogene of the Paris Basin species, and Gorjanović-Kramberger (1901), who introduced eleven species for the Valencienniinae of Lake Pannon. Overall, with 18 described species each, these two authors introduced the most (still valid) fossil lymnaeid species (Fig. 6.1b).
(a) Number of lymnaeid species described per year (cumulative, green) and decade (orange). Indicated are additionally the three publications that have contributed the most to the number of species. (b) Authors that described more than five fossil lymnaeid species. Invalid or unavailable names (e.g., synonyms, nomina nuda) were not considered in any of the two plots
The curve of new species descriptions is not saturated yet. Only since the year 2000, twenty new species of Lymnaeidae have been described from fossil freshwater deposits (Macaleț 2000; Pierce and Constenius 2001; Kovalenko 2004, 2017; Prysjazhnjuk et al. 2006; Pan and Zhu 2007; Harzhauser et al. 2012, 2014a, 2016; Neubauer et al. 2014, 2015d; Vinarski and Frolov 2017; Yu et al. 2021).
Altogether, 379 valid accepted species have been described over the 210 year-long history of fossil lymnaeid taxonomy (Supplementary Table S2). Of these, pre-Pleistocene distribution data are available for 376 species in 32 genera (of which 15 are only known from fossil species) and four subfamilies (Lymnaeinae, Amphipepleinae, Lancinae, and Valencienniinae) (Supplementary Table S1).
The species richness reconstructions showed similar tendencies for Europe and North America, yet in quite different magnitudes (Fig. 6.2). While the pre-Pleistocene European fossil record comprised altogether 262 species, only 56 species were recorded for North America. Species richness remained low in both continents during the Mesozoic and earliest Paleogene. No notable diversity decline was observed at the Cretaceous–Paleogene boundary, which contrasts a massive breakdown of the overall freshwater gastropod diversity in Europe (Neubauer et al. 2021).
Diversification speeded up about coevally in both continents in the Lutetian (Middle Eocene). In Europe, a diversity peak was reached with 45 species in the Rupelian. Diversity dropped in both continents toward the Oligocene–Miocene boundary and increased again during the Miocene. The all-time species richness peak was reached in both continents during the Tortonian. In Europe, this peak is partly owed to major diversification events in Lake Pannon and the Dacian Basin. Hence, not all of the 77 species recorded for the Tortonian lived simultaneously.
The diversity trend for Europe derived from the raw data was confirmed by the subsampling (SQS) approach (Fig. 6.2), suggesting that the observed pattern is not biased by uneven sampling. There is unfortunately not enough data for North America to use subsampling to assess the reliability of the measured trend.
6.4 Geographic Distribution Through Time and the Origins of Lymnaeidae
In this chapter, I give an overview of the geographic distribution of Lymnaeidae from their first occurrence in the fossil record to the Pliocene, illustrated by a series of paleographic maps (Figs. 6.3, 6.4, 6.5, 6.6). Given the high number of records in some stratigraphic intervals, these maps are not exhaustive and do not contain all records as single points but are rather intended to cover the entire geographic range of lymnaeids in each interval. Also, the lymnaeid fossil record is clearly geographically biased toward Europe and North America (Supplementary Fig. S1). The spotty occurrences on other continents, such as Asia, where lymnaeids are common today (Vinarski et al. 2019), suggest a strong preservation, sampling, and/or research bias.
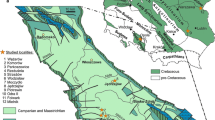
Geographic distribution of Lymnaeidae in the Jurassic. Close-by localities are represented by single points. Question marks indicate species of uncertain lymnaeid affinity. Base map from Cao et al. (2017) used under Creative Commons Attribution 3.0 (CC BY 3.0) license. Yellow areas mark land masses, orange indicates mountain ranges
Geographic distribution of Lymnaeidae in the Cretaceous. Close-by localities are represented by single points. Base map from Cao et al. (2017)
Geographic distribution of Lymnaeidae in the Paleogene. Close-by localities are represented by single points. Asterisks mark localities with doubtful stratigraphic age. Base map from Cao et al. (2017)
Geographic distribution of Lymnaeidae in the Neogene. Close-by localities are represented by single points. Asterisks mark localities with doubtful stratigraphic age. Base map from Cao et al. (2017)
6.4.1 Mesozoic Roots
The ancestry of the Lymnaeidae is unresolved. Inaba (1969) hypothesized a late “pro-lymnaeid” ancestor, from which modern lineages arose starting already in the late Paleozoic, but without any evidence from fossils. Kruglov (2005) suggested an origin and initial diversification in the Triassic and hypothesized that the ancestor of Lymnaeidae is related to Chilinidae. This assumption would at least match the basal position of Chilinidae within the Hygrophila as indicated by phylogenies based on morphology and molecular data (Hubendick 1978; Nordsieck 1992; Jörger et al. 2010), although their fossil record is comparably sparse and young (Dayrat et al. 2011). However, the latest molecular phylogeny by Saadi et al. (2020) suggests that the ancestor of Lymnaeidae is more closely related with Physidae. Yet, the fossil record of that family only starts in the Late Jurassic (Huckriede 1967).
A Triassic origin of Lymnaeidae as supposed by Kruglov (2005) predates the fossil record of the family. The earliest putative mention of lymnaeids in the fossil record derives from the United States (Fig. 6.3). Robinson (1915) described the two species Valvata gregorii and Limnaea hopii from “Painted Desert beds” in Arizona, which he attributed to the Morrison Formation, suggesting a Late Jurassic age for the fossils. Yen (1951) later attributed these findings erroneously to the Upper Triassic Chinle Formation (Harshbarger et al. 1957). The stratigraphic mystery was finally solved by Harshbarger et al. (1957), who correlated the fossil-bearing strata with the silty facies of the Kayenta Formation, pointing toward an Early Jurassic (Pliensbachian) age (Marsh and Rowe 2018). However, the type material has apparently not been studied since the first description (see, e.g., Yen 1952, p. 27), and the illustrations and description provided by Robinson (1915) do not unequivocally ascertain placement in the Lymnaeidae.
The next oldest records of supposed Lymnaeidae come from the Middle Jurassic of China (Fig. 6.3). Pan (1977) described Galba lufengensis and Galba yunnanensis from the Zhanghe Formation and Hepingxiang Formation of Yunnan, and Yü and Pan (1980) recorded Galba tongshangensis from the Tongshan Group of Zhejiang. All these stratigraphic units are presently attributed to the Middle Jurassic (Zhang 2009). The preservation of these fossil species is poor (Yü and Pan 1980) and, as for Robinson’s material above, the attribution to Lymnaeidae cannot be confirmed with certainty. In fact, Galba lufengensis resembles terrestrial succineids.
A careful revision of these earliest fossils is required to assess the origin of the Lymnaeidae. At least from the Late Jurassic onward, the number of lymnaeid records increases and they come from various continents (Fig. 6.3). This includes species from the Morrison Formation in the USA (White 1886; Yen 1952; Solem and Yochelson 1979; Gray 1988; Evanoff et al. 1998), the Tithonian of Shandong in China (Pan 1983) and, possibly, the Kimmeridgian of Lower Saxony in Germany (Huckriede 1967), involving several species undoubtedly belonging in the family Lymnaeidae.
Records from the Jurassic–Cretaceous boundary were derived from the Tithonian–Valanginian Purbeck Formation in southern England (Arkell 1941) and its stratigraphic equivalents (mostly of Berriasian age) in Switzerland (De Loriol and Jaccard 1865) and Germany (Dunker 1846) (Fig. 6.4). Early Cretaceous lymnaeids were documented from the Valanginian–Hauterivian and the Aptian–Albian of Siberia (Reis 1910; Martinson 1956, 1961), the Guantou, Yehe, Banlashan, Yixian and Xiazhuang formations of Zhejiang, Jilin, Liaoning and Beijing in China (Yü and Pan 1980; Zhu 1980; Yü 1987; Pan and Zhu 2007) and the Aptian–Albian Cloverly Formation (Yen 1946c, 1951) and the Albian Bear River Formation (White 1876; Yen 1954) in the USA. This also involves findings of Yen and Reeside (1946) and Yen (1952), who formerly attributed their fossils to the Morrison Formation, but Evanoff et al. (1998) identified the strata as belonging to the Cloverly Formation.
The Late Cretaceous (potentially) saw the first appearance of Lymnaeidae on the southern hemisphere (Fig. 6.4). Beu et al. (2014) reported an unidentified species from the Cenomanian of New Zealand, which they tentatively referred to the genus Austropeplea. Additional early Late Cretaceous records come from the Cenomanian of France (Repelin 1902; age after Fabre-Taxy 1948) and Myanmar (Yu et al. 2021). After a long stratigraphic gap, end-Cretaceous taxa appear in the Campanian–Maastrichtian St. Mary River Formation (Whiteaves 1885; Tozer 1956), the Campanian–Danian Edmonton Group (Tozer 1956) and the Maastrichtian lower Willow Creek Formation (Tozer 1956) of Canada, the Maastrichtian Laramie Formation in the USA (Meek 1873), the Maastrichtian Nanxiong Formation of Guangdong, China (Yü 1977), the Maastrichtian of India (Sowerby 1840; Hislop 1860; Hartman et al. 2008), the Campanian–Maastrichtian (“Rognacien”) of France (Matheron 1843; Fabre-Taxy 1959), and the Maastrichtian of Hațeg in Romania (Pană et al. 2002). (Note that Pană et al. (2002) introduced a new genus and three new species names for Lymnaeidae but without describing them or designating type material, rendering all these names unavailable.)
6.4.2 The Paleogene Diversification
Rich Paleocene faunas containing Lymnaeidae have been documented from the USA and Canada, including the Green River Formation (White 1880; La Rocque 1960), Paskapoo Formation (Whiteaves 1885; Russell 1926; Tozer 1956), Devils Basin Formation (Love 1989) and Fort Union Formation (Meek and Hayden 1856; Yen 1948b; Bickel 1977) (Fig. 6.5). European records were derived from the “Liburnian” (Danian) of Croatia (Sandberger 1870–1875; Stache 1889) and the Thanetian of France, i.e., the “Sables de Bracheux” (Cossmann 1889; Glibert 1962) and the “Calcaire de Rilly” in the Paris Basin (Bayan 1870) and the “Calcaire à Physa prisca” in southern France (Noulet 1854; Rey and Villatte 1971). Only a single Paleocene record comes from Asia, namely from the Shanghu Formation of Guangdong, China (Yü 1977).
The Eocene diversification is represented by numerous localities in Europe and North America (Fig. 6.5). The majority of records come from the Paris Basin in France (e.g., Brongniart 1810; Deshayes 1863; Leriche 1899; Cossmann 1902; Jodot and Feugueur 1953; Glibert 1962) and the Hampshire Basin in southern England (e.g., Sowerby 1826–1829; Edwards 1852; Glibert 1962; Paul 1989; Munt 2014), as well as from the Upper Rhine Graben in Germany and France (e.g., Sandberger 1870–1875; Rollier 1910), the “Calcaires de Mas-Saintes-Puelles”, “Calcaire de Castres” and “Molasse de Castelnaudary” in southern France (Serres 1844; Noulet 1854, 1857; Jodot and Rey 1956), and the Anjou (Glibert 1962), Matelles (Rey 1962b) and Aix-en-Provence (Matheron 1843; Roule 1886; Rey 1962a) basins, amongst several others. In North America, several species have been reported from the Middle–Late Eocene of the Kishenehn Basin (Russell 1952, 1955; Pierce and Constenius 2001, 2014). Additional records come from the Upper Eocene Florissant Formation (Cockerell 1906, 1908), the Upper Eocene to Lower Oligocene White River Formation (Evans and Shumard 1856; Meek 1876) and several more localities in the mid-west (e.g., Yen 1946b, 1948a; Good 1987; Hartman and Roth 1998). Only three Asian regions have yielded Eocene lymnaeids. They are located in China (Guangdong and Hebei provinces; Yü and Pan 1982; Yü and Zhang 1982) and Korea (Suzuki 1949). Finally, Lymnaeidae first appeared in the fossil record of Africa during the Eocene, with two species from the Lutetian of Namibia (Pickford et al. 2008).
During the Oligocene, Lymnaeidae maintain a diverse fossil record in France, specifically in the Paris Basin (e.g., Brongniart 1810; Deshayes 1863; Dollfus 1906, 1920; Glibert 1962; Lozouet and Maestrati 1981), the “Calcaires d’Albi” in southern France (e.g., Fontannes 1884), and the Limagne Basin (e.g., Rey 1965) (Fig. 6.5). Numerous findings have been reported from the Chattian “Ramondi Beds” and “Formation du Gypse d’Aix” in France, Switzerland, and Germany (e.g., Fontannes 1881, 1884; Douxami 1904; Jodot 1954; Rey 1965). Additional important records come from the Upper Rhine Graben in France (e.g., Sandberger 1870–1875; Gillet 1953; Jodot 1954), the Mainz Basin (e.g., Thomä 1845; Mödden et al. 2000), and the Hessian Depression in Germany (e.g., Dunker 1853) and Limburg in Belgium (Glibert and Heinzelin de Braucourt 1954; Janssen 1980; Marquet et al. 2008). Only few records come from outside central Europe; these include localities in Portugal (Roman 1907; Pais 2012), Spain (Almera 1894; Royo Gómez 1922; Esu 1984), and Italy (Sacco 1886; Esu and Girotti 2010). Comparably little is known about North American Oligocene Lymnaeidae. Only three species have been documented from the Renova Formation (Pierce 1993) and the Antero Formation (Russell 1938) in the USA. Similarly, only few Asian localities have yielded lymnaeids. Popova (1964, 1981) described a few taxa from the Upper Oligocene–Lower Miocene (?) Bayanday Formation of Siberia, and Yen (1969) documented one species from Shandong Province, China. Finally, an unidentified lymnaeid was found in the Upper Oligocene Tremembé Formation of São Paulo State, Brazil (Salvador et al. 2018b), being the oldest fossil Lymnaeidae of South America.
6.4.3 The Neogene Diversity Peak
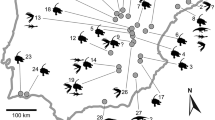
The Miocene record of Lymnaeidae is by far the richest (Figs. 6.2 and 6.6), and the sheer number of records and works mentioning lymnaeid fossils makes a detailed listing impractical (Supplementary Table S1). The European Miocene fossil record includes 158 species from over 1340 records from 26 countries, spanning from Portugal in the West to the Caspian Sea in the East (Fig. 6.6). The majority of records come from Germany and France, particularly from the Aquitaine Basin (e.g., Grateloup 1828; d’Orbigny 1852; Degrange-Touzin 1892; Peyrot 1933; Wenz 1936), the Upper Freshwater Molasse (e.g., Klein 1853; Sandberger 1870–1875; Clessin 1885; Jooss 1913; Wenz 1935; Schlickum 1976; Salvador and Rasser 2016; Salvador et al. 2016), and the Mainz Basin (e.g., Thomä 1845; Braun 1851; Kadolsky 1989). Paleogeographically, most records were derived from the Late Miocene Lake Pannon. This huge long-lived brackish-water lake alone yielded over 50 species of Lymnaeidae, most of which are endemic to the lake (see also chap. 6.1). Important contributions were made by Brusina (1874, 1902), Gorjanović-Kramberger (1901, 1923), Pavlović (1927), Strausz (1942), Moos (1944), Stevanović (1951, 1955, 1990b, c), Bartha (1955, 1971), Papp (1956), Kochansky-Devidé and Pikija (1976), Sremac (1981), Marinescu (1973), Lennert et al. (1999), Vrsaljko (1999), and Botka et al. (2019), amongst many others. Further rich faunas were reported from the Dacian Basin in Romania (Krejci-Graf and Wenz 1932; Wenz 1942; Marinescu 1970; Hanganu 1972; Huică 1977; Marinescu and Papaianopol 1990; Macaleț 2000) and the Bakony Mountains in Hungary (Kókay 2006).
Important North American faunas yielding Miocene lymnaeids were described by Hanna (1923), Yen (1946a, 1947), Taylor (1954, 1957, 1966, 1983), Carr and Trimble (1963), Firby (1966), and Pierce (1993). A South American record comes from the Middle Miocene San José Formation of Argentina (Morton and Herbst 2003; Salvador et al. 2018b). Asian taxa were recorded by Suzuki (1949) from Korea, Willis (1967) from Iraq, and Yen (1969) from China. Additional reports of undefined Neogene age were made by Martinson (1954), Popova (1981), and Yü (1982) from Russia, Mongolia, and Tibet. Pickford (2008) reported two species from the Early Miocene of Namibia.
The Pliocene distribution reflects more or less the Miocene one, with many records in Europe and North America, a few in Asia and a single one in South America (Fig. 6.6). The center of European diversity is slightly shifted to the east compared to the Miocene, with the Dacian Basin holding the most diverse fauna (e.g., Macarovici 1940; Hanganu 1972; Macaleț 2000; Papaianopol et al. 2003; Van Baak et al. 2015). Additionally, the Transylvanian Basin in Romania (Jekelius 1932) and the Lower Rhône Basin in France (Serres 1853; Michaud 1855; Fontannes 1879, 1883) have yielded faunas rich in lymnaeids. The North American faunas mostly come from the Blancan deposits and were summarized by Taylor (1966). Additional records were listed by Henderson and Rodeck (1934), Nations (1974), Taylor and Smith (1981), and Love (1989). In Asia, taxa were documented from the Kyzylgir Formation in Siberia (Popova et al. 1970; Popova 1981), the Late Pliocene of Java (van Benthem Jutting 1937) and several localities in China (Ping 1931; Suzuki 1949; Zhu 1985; note that some of these records may range into the Early Pleistocene). Finally, an unidentified lymnaeid species was documented from the Pliocene Meyer Desert Formation of Antarctica by Ashworth and Preece (2003), representing the sole fossil record of that continent as yet.
6.5 Dispersal in the Fossil Record
When viewing the distribution patterns of Lymnaeidae in the fossil record and their spread from the presumed origin in the Early Jurassic of North America to nearly all over the world, the most obvious question is: how did they get there? Already during the Jurassic, the early Atlantic Ocean separated North America and Eurasia (Cao et al. 2017; Fig. 6.3). In fact, during most of the Mesozoic and Cenozoic, broad seaways isolated the continents, and only short intermittent phases of terrestrial pathways are known (Brikiatis 2014, 2016; Cao et al. 2017). However, these land bridges existed only between selected continents and it is unlikely that any of them offered hydrological connections allowing direct dispersal. A prime example of dispersal with an obvious lack of land connection is posed by the Pliocene occurrence of a lymnaeid on Antarctica (Ashworth and Preece 2003).
Dispersal in freshwater snails today knows active and passive pathways. Snails move actively along streams or are passively dispersed via ingestion and defecation by fish or waterbirds or via attachment to birds, mammals, amphibians, or even larger insects (e.g., Walther et al. 2008; Kappes and Haase 2012; van Leeuwen et al. 2012, 2013; Kolenda et al. 2017). Dispersal by both fish and waterfowl has already been witnessed for Lymnaeidae previously (Baker 1911; Kappes and Haase 2012; van Leeuwen and van der Velde 2012). Especially long-distance aerial dispersal is invoked to explain the pan-continental distribution of the family and disjunct occurrences on islands or in distant water bodies (Aksenova et al. 2018; Vinarski et al. 2021). Only Lancinae may form an exception here. In contrast to most lymnaeids, lancines have a poorly developed lung and probably do not survive transport outside of the water for long, constraining the possibility for dispersal by birds or mammals over long distances (Campbell et al. 2017).
As concerns fossil representatives, aerial dispersal has been proposed to explain the disjunct occurrence of Delminiella in Austria, separated from the remaining records in the Dinaride Lake System by a marine connection between the Mediterranean and Paratethys seas (Harzhauser et al. 2016). Similarly, Esu and Girotti (2018) suggested transport by waterfowl as likely mechanism to explain the occurrence of Corymbina, otherwise restricted to the Aegean region, in Lower Pliocene deposits of Italy. The Antarctic Pliocene record of a lymnaeid is likely a result of bird-mediated dispersal as well (Ashworth and Preece 2003) and the same might be true for many a disjunct occurrence in the Late Cretaceous or Cenozoic (Taylor 1988). However, waterfowl did not originate before the Late Cretaceous (Claramunt and Cracraft 2015). The mechanisms for dispersal prior to their evolution remain enigmatic.
Migrating land vertebrates, amphibians, or insects may have constituted viable options to reach water bodies not hydrologically connected, but it is unclear how lymnaeids could disperse across large distances and seaways. Early Cretaceous birds show similar anatomical features as modern migratory birds, but it is uncertain if they also underwent long-distance migration (Falk 2011). A possible yet so far entirely unsupported hypothesis involves pterosaurs (Yu et al. 2021). Some species of pterosaurs were likely capable of long-distance travels (Witton and Habib 2010), and some of them probably contributed to angiosperm seed dispersal during the Cretaceous (Fleming and Lips 1991). A few species probably fed on mollusks (Bestwick et al. 2018). Perhaps, freshwater mollusks could have been dispersed via ingestion and defecation or via attachment to the feet, just like in modern birds. However, to support this hypothesis a joint fossil record of gastropod and pterosaur species would be necessary.
Another aerial dispersal alternative may be offered by strong winds and storms. Several examples are known of terrestrial and freshwater mollusks and even fishes to be transported via storms (Rees 1965; Ożgo et al. 2016). Although the number of records is still anecdotal, Ożgo et al. (2016) considered storms a possible means of long-distance dispersal, at least for terrestrial gastropods. However, the chance that storms pick up lymnaeids and deliver them safely to new freshwater environments might be comparatively low.
6.6 Exploring the Limits of Morphological Disparity
The typical lymnaeid shell, both today and in the fossil record, is smooth and turbiniform. However, a number of “special cases” of morphological evolution happened over the long evolutionary history of the Lymnaeidae. Four of these are detailed in this chapter.
6.6.1 Valencienniinae
Probably the most outstanding among those cases is the evolution of the Valencienniinae. This subfamily originated during the Early Tortonian (Late Miocene) in early Lake Pannon from a small ancestor species currently attributed to the genus Radix (Gorjanović-Kramberger 1923; Moos 1944; Taktakishvili 1967; Marinescu 1970; Neubauer et al. 2016). During the c. 7-Myr-lifetime of the long-lived Lake Pannon, several evolutionary lineages evolved from that ancestor exhibiting widely expanded apertures and/or limpet-shaped shells (Fig. 6.7a–c, f, i). This also involved a massive size increase over time: starting from about 20 mm in height (Gorjanović-Kramberger 1890), some species developed shell sizes of up to 132 mm (Taktakishvili 1967). As such, they are among the largest non-marine aquatic snails ever recorded. Some of the species also developed a peculiar siphonal fold (Fig. 6.7c) and/or sculpture in the form of strong concentric ribs (Fig. 6.7a–c, f), both of which are rare features among lymnaeids in general.
Examples of lymnaeid species with extraordinary morphological adaptations. (a) Undulotheca halavatsi (Gorjanović-Kramberger, 1901), Gușterița, Romania, Late Miocene, coll. Botka (no number) (from Botka et al. 2019). (b) Provalenciennesia arthaberi (Gorjanović-Kramberger, 1901), Beočin, Serbia, Late Miocene, MBFSZ Pl. 2545. (c) Valenciennius reussi Neumayr in Neumayr and Paul, 1875, Tirol, Romania, Late Miocene, NHMW 1900/IX/5 (from Vermeij 2017). (d) Delminiella cf. soklici, Podhum, Bosnia, and Herzegovina, Early Miocene, ZMBH Mg3657. (e) Velutinopsis velutina (Deshayes, 1838), Mihalț, Romania, Late Miocene, coll. Botka. (f) Valenciennius reussi, Okrugljak near Zagreb, Croatia, Late Miocene, NHMW 1888/XIV/2. (i) Radix kobelti (Brusina, 1884), Okrugljak near Zagreb, Croatia, Late Miocene, NHMW 1888/XIV/9. (g) Delminiella soklici Kochansky-Devidé and Slišković, 1972, Vučipolje near Tomislavgrad, Bosnia, and Herzegovina, Early Miocene, holotype, ZMBH Mg3631 (from Harzhauser et al. 2016). (h) Delminiella excentrica Kochansky-Devidé and Slišković, 1972, Eminovo selo near Tomislavgrad, Bosnia and Herzegovina, Early Miocene, holotype, ZMBH Mg3623 (from Harzhauser et al. 2016). (j) Corymbina bicarinata (Fuchs, 1877), Livanates, Greece, Early Pleistocene, holotype, NHMW 1878/XX/3. (k, l) Corymbina elegans (Cantraine, 1841), Livanates, Greece, Early Pleistocene, NHMW 1878/XX/1 (label reads “Lymnaeus Adelinae”, a junior objective synonym of C. elegans). (m, n) Lanx kirbyi Hanna and Gester, 1963, Dorris, California, Early Pleistocene, paratype, CASG 34807.02 (formerly CASG 12451). Photos by D. Botka (a, b), CAS (m, n), O. Mandic (d, g, h), T.A. Neubauer (j, l), A. Schumacher (c), M. Vinarski (f, i, k). Scale bars = 10 mm (a–c, f), 5 mm (d, e, g–n)
While starting in Lake Pannon, these lineages were not confined to it. Several species have been reported from the Upper Miocene and Pliocene of the Dacian Basin (Krejci-Graf and Wenz 1932; Marinescu 1970; Hanganu 1972; Huică 1977; Marinescu and Papaianopol 1990; Papaianopol and Marinescu 1995) and Black Sea Basin (Rousseau 1842; Sinzov 1875; Davitashvili 1930; Taktakishvili 1967; Özsayar 1977). During the Pontian (latest Miocene), the subfamily expanded as far as the Kura Basin in Azerbaijan in the East (Davitashvili 1931). The last known occurrence is Valenciennius kujalnicus Taktakishvili, 1962 from the lower Kuyalnikian (latest Pliocene to earliest Pleistocene) of the Gurian region in Georgia (Taktakishvili 1967). (“?Valenciennesia metochiana” Pavlović, 1933 from the Middle Miocene Peć Series in Kosovo is probably not related with the Valencienniinae; a revision is required.)
Because of the sequential appearance of (morpho-)species of these evolutionary lineages, the individual species have been used as biostratigraphic markers (Moos 1944; Taktakishvili 1967; Botka et al. 2019). In the Black Sea Basin, stratigraphers even used to distinguish lower and upper “Valenciennius clays” in Pontian strata (Seninski 1905; Stevanović 1990a; Popov et al. 2016).
Little is known about the causes for that peculiar morphological diversification. In Lake Pannon, the Valencienniinae seem to be constricted to deep-water (sublittoral and profundal) environments (Harzhauser and Mandic 2008; Cziczer et al. 2009). One hypothesis is that the broad, flattened shell is an adaptation to life on mudgrounds in deeper settings (Marinescu 1970; Müller et al. 1999). Cziczer et al. (2009) proposed, in turn, that the limpet-like shape serves floating or swimming in the water column.
6.6.2 Delminiella
A similar case of morphological evolution toward a limpet-like shell is found in the Early Miocene genus Delminiella (Fig. 6.7d, g, h). Unlike for Valencienniinae, no ancestor–descendant relationship is known for that genus, but a lymnaeid ancestor has been proposed (Harzhauser et al. 2016). The genus is known by three species only; two occur in the Dinaride Lake System in Croatia and Bosnia and Herzegovina (Kochansky-Devidé and Slišković 1972), the third one is endemic to the paleo-lake Lavant in southern Austria (Harzhauser et al. 2016). They are all much smaller than Valencienniinae and range around 15 mm in maximum diameter. All species have widely expanded apertures and concentric growth lines or riblets, and all retain a small, coiled apex (Kochansky-Devidé and Slišković 1972; Harzhauser et al. 2016; Fig. 6.7d, g, h).
In the Dinaride Lake System, their appearance in the fossil record parallels the evolution of the similarly limpet-shaped but presumably unrelated family Clivunellidae. In the Livno and Tomislavgrad basins in Bosnia and Herzegovina, Clivunella and Delminiella even co-occur in the same strata (Kochansky-Devidé and Slišković 1972). In contrast to the alleged deep-water Valencienniinae, Delminiella (as well as Clivunellidae) are suggested to be more generalist species that lived in a greater variety of paleohabitats (Harzhauser et al. 2016). They occurred in muddy lake bottoms in deeper water with intermittent low oxygen levels (De Leeuw et al. 2011) and in littoral settings alike (Bulić and Jurišić-Polšak 2009).
6.6.3 Lancinae
Similar to Valencienniinae and Delminiella, the subfamily Lancinae comprises limpet-like shells (Vermeij 2017). Species of that group have completely lost coiling and are the only true patelliform lymnaeids (Campbell et al. 2017; Fig. 6.7m, n). Shells are smooth aside from concentric growth lines. Because of their close resemblance of “true” freshwater limpets, species of Lancinae have been previously placed in the planorbid subfamily Ancylinae (Campbell et al. 2017).
Lancinae are restricted to North America and include the three genera Lanx, Fisherola, and Idaholanx (Campbell et al. 2017), of which only Lanx is known to have a fossil record. Four fossil species are recognized and their appearance in the fossil record is patchy, which might indicate that not all of them belong to the same genus. This is especially true for the oldest record of the genus and subfamily, Lanx nevadensis MacNeil, 1939 from the Lower Cretaceous of Nevada (MacNeil 1939). Given that molecular data indicate a close relationship between Lancinae and Lymnaeinae (Saadi et al. 2020), it is unlikely that the subfamily originated already in the Cretaceous. Probably, Lanx nevadensis does not belong in Lancinae; its systematic position still needs to be assessed.
The stratigraphically next occurrence of (probably true) Lancinae derives from Middle Miocene strata of California, over 100 Myryounger (Firby 1966). Further occurrences have been documented from the Late Miocene, Pliocene, and Pleistocene of the western USA (Hannibal 1912; Hanna 1922; Yen 1944, 1947; Hanna and Gester 1963; Taylor 1966, 1981; Fig. 6.7m, n). Today, Lancinae dwell primarily in cool, flowing, well-oxygenated water, often in rivers, springs or spring-influenced areas of the Pacific drainage region, where they are commonly found under stones or attached to them (Taylor 1981; Burch 1982; Campbell et al. 2017).
6.6.4 Corymbina
A different type of morphospace expansion is exhibited by the fossil genus Corymbina (syn. Adelina Cantraine, 1841, non Dejean, 1835; Adelinella Wenz, 1922). The genus includes five species that are characterized by the presence of strong axial ribs, sometimes accompanied by a spiral keel (Fig. 6.7j–l). Most species have a classical lymnaeid-type shell, but some forms developed a detached aperture or an even uncoiled body whorl (Willmann 1981). The genus is mostly confined to the Aegean region (Greece and Turkey), where it occurs in Middle Miocene to Middle Pleistocene strata (Oppenheim 1919; Willmann 1981; Schütt and Kavuşan 1984; Esu and Girotti 2020). Two exceptions of disjunct occurrences were found in the Sarmatian (late Middle Miocene) of Romania (Marinescu 1992) and the early Pliocene of Italy (Esu and Girotti 2018). Because of the limited occurrence in space and time, the genus has been used for biostratigraphic correlations between Greek and Turkish Neogene strata (Willmann 1981, 1982; Böger 1983).
6.7 Synthesis
The fossil record of Lymnaeidae is patchy but indicates a widespread distribution over long periods of time of the family’s evolutionary history. Given the paucity of freshwater deposits over large parts of the globe, especially in the Mesozoic, we know little about the family’s origin, early history, and distribution. The earliest supposed member of the family occurred during the Early Jurassic in North America. Already in the course of the Jurassic the Lymnaeidae spread to Europe and Asia. Diversity remained low until the early Paleogene until the family started to diversify during the Middle Eocene—approximately 150 Myr after its (presumed) origin. The all-time peak of lymnaeid diversity was reached in the Late Miocene and coincided with major morphological diversification of the subfamily Valencienniinae. The resulting species developed partly huge and strongly ribbed, limpet-like shells and were probably adapted to dysoxic deep-water settings. The associated evolutionary lineages evolved in long-lived lakes, which are widely considered natural laboratories of evolution (e.g., Michel et al. 1992; Harzhauser et al. 2013; Van Damme and Gautier 2013; Jenny et al. 2020). A similar yet smaller event of morphological diversification happened during the Early Miocene in central to southeastern Europe (genus Delminiella). The earliest supposed member of the North American Lancinae, a small subfamily of truly patelliform species that still exists today, dates back to the Early Cretaceous.
The disjunct distribution of Lymnaeidae through geological time is strongly shaped by long-distance dispersal. During the Late Cretaceous to Cenozoic, waterfowl probably constituted the main vector, suggested by numerous examples of isolated occurrences. In fact, the diversification of waterfowl and development of migratory routes may have contributed markedly to the diversification of Lymnaeidae. Conversely, the mechanisms for long-distance dispersal prior to waterfowl evolution remain doubtful and potentially involve Mesozoic ornithurine birds, pterosaurs, or storms. However, the patchy Mesozoic fossil record and the uncertainties regarding the systematic classification of early alleged lymnaeids further complicate the reconstruction of dispersal pathways.
References
Aksenova OV, Bolotov IN, Gofarov MY et al (2018) Species richness, molecular taxonomy and biogeography of the radicine pond snails (Gastropoda: Lymnaeidae) in the Old World. Sci Rep 8:11199. https://doi.org/10.1038/s41598-018-29451-1
Alda P, Bonel N, Alba A et al (2023, this volume) Molecular techniques for the study of ecological and evolutionary processes in lymnaeids. In: Vinarski MV, VÃzquez A (eds) The Lymnaeidae. A handbook on their natural history and parasitological significance. Springer, Cham, pp 121–146
Almera J (1894) Descripción de los depósitos pliocénicos de la cuenca del Bajo Llobregat y llano de Barcelona. Mem R Acad Cien Art Barcelona 3:1–355
Alroy J (2010) Geographical, environmental and intrinsic biotic controls on Phanerozoic marine diversification. Palaeontology 53(6):1211–1235. https://doi.org/10.1111/j.1475-4983.2010.01011.x
Andrusov N (1923) Apsheronskiy yarus. Trudy Geologicheskoy Komissii novaya seriya 110:1–294. [in Russian]
Arkell WJ (1941) The gastropods of the Purbeck beds. Quart Journ Geol Soc London 97:79–128. https://doi.org/10.1144/GSL.JGS.1941.097.01-04.04
Ashworth AC, Preece RC (2003) The first freshwater molluscs from Antarctica. J Molluscan Stud 69(1):89–92. https://doi.org/10.1093/mollus/69.1.89
Baker FC (1911) The Lymnaeidae of North and Middle America, recent and fossil. The Chicago Academy of Sciences, Chicago, IL. https://doi.org/10.5962/bhl.title.20500
Bandel K (1991) Gastropods from brackish and fresh water of the Jurassic – Cretaceous transition (a systematic reevaluation). Berliner geowiss Abh A 134:9–55
Bartha F (1955) A várpalotai pliocén puhatestü fauna biosztratigrafiai vizsgálata. Magyar All Földt Intezet Evk 43(2):275–359
Bartha F (1971) A magyarországi pannon biosztratigráfiai vizsgálata. In: Góczán F, Benkö J (eds) A Magyarországi pannonkori képzödmények kutatásai. Akadémiai Kiadó, Budapest, pp 9–172
Bayan F (1870) Études faites dans la collection de l’École des Mines sur des fossiles nouveaux ou mal connus. Premier fascicule. Mollusques tertiaires. F. Savy, Paris
Bestwick J, Unwin DM, Butler RJ et al (2018) Pterosaur dietary hypotheses: a review of ideas and approaches. Biol Rev 93(4):2021–2048. https://doi.org/10.1111/brv.12431
Beu AG, Marshall BA, Reay MB (2014) Mid-Cretaceous (Albian–Cenomanian) freshwater Mollusca from the Clarence Valley, Marlborough, New Zealand, and their biogeographical significance. Cretac Res 49:134–151. https://doi.org/10.1016/j.cretres.2014.02.011
Bickel D (1977) A new genus and species of freshwater gastropod from the Paleocene Tongue River formation of North Dakota. J Paleontol 54(1):123–130
Böger H (1983) Stratigraphische und tektonische Verknüpfungen kontinentaler Sedimente des Neogens im Ägäis-Raum. Geol Rundsch 72(3):771–814
Botka D, Magyar I, Csoma V et al (2019) Integrated stratigraphy of the Guşterița clay pit: a key section for the early Pannonian (late Miocene) of the Transylvanian Basin (Romania). Austrian J Earth Sci 112(2):221–247. https://doi.org/10.17738/ajes.2019.0013
Bouchet P, Rocroi J-P, Hausdorf B et al (2017) Revised classification, nomenclator and typification of gastropod and monoplacophoran families. Malacologia 61(1–2):1–526. https://doi.org/10.4002/040.061.0201
Braun A (1851) Darstellung der geognostischen Verhältnisse des Mainzer Beckens und seiner fossilen Fauna und Flora. In: Walchner FA (ed) Handbuch der Geognosie zum Gebrauche bei seinen Vorlesungen und zum Selbststudium mit besonderer Berücksichtigung der geognostischen Verhältnisse des Grossherzogthums Baden. Christian Theodor Gross, Karlsruhe, pp 1121–1169
Brikiatis L (2014) The De Geer, Thulean and Beringia routes: key concepts for understanding early Cenozoic biogeography. J Biogeogr 41(6):1036–1054. https://doi.org/10.1111/jbi.12310
Brikiatis L (2016) Late Mesozoic North Atlantic land bridges. Earth-Sci Rev 159:47–57. https://doi.org/10.1016/j.earscirev.2016.05.002
Brongniart A (1810) Sur des Terrains qui paroissent avoir été formés sous l’eau douce. Ann Mus Natl Hist Nat 15:357–405
Brusina S (1874) Fossile Binnen-Mollusken aus Dalmatien. Kroatien und Slavonien nebst einem Anhange, Actienbuchdruckerei, Agram
Brusina S (1902) Iconographia Molluscorum Fossilium in tellure tertiaria Hungariae, Croatiae, Slavoniae, Dalmatiae, Bosniae, Herzegovinae, Serbiae and Bulgariae inventorum. Officina Soc, Typographicae, Agram
Bulić J, Jurišić-Polšak Z (2009) Macropalaeontology and stratigraphy of lacustrine Miocene deposits at Crnika Beach on the Island of Pag (Croatia). Geol Croat 62(3):135–156. https://doi.org/10.4154/gc.2009.16
Burch JB (1982) Freshwater snails (Mollusca: Gastropoda) of North America. In: U.S. environmental protection agency, office of research and development, environmental monitoring and support laboratory, Cincinnati, OH
Campbell DC, Clark SA, Lydeard C (2017) Phylogenetic analysis of the Lancinae (Gastropoda, Lymnaeidae) with a description of the U.S. federally endangered Banbury Springs lanx. Zookeys 663:107–132. https://doi.org/10.3897/zookeys.663.11320
Cantraine F (1841) Malacologie méditerranéenne et littorale, ou description des mollusques qui vivent dans la Méditerranée ou sur le continent de l’Italie, ainsi que des coquilles qui se trouvent dans les terrains tertiaires italiens, avec des observations sur leur anatomie, leurs moeurs, leur analogie et leur gisement. Ouvrage servant de faune malacologique italienne et de complément à la Conchiologia fossile subapennina de Brocchi. Nouv Mem Acad R Sci Bruxelles 13:1–173
Cao W, Zahirovic S, Flament N et al (2017) Improving global paleogeography since the late Paleozoic using paleobiology. Biogeosciences 14(23):5425–5439. https://doi.org/10.5194/bg-14-5425-2017
Carobene D, Harzhauser M, Mandic O et al (2018) Taxonomy and palaeoecology of continental Gastropoda (Mollusca) from the Late Pleistocene mammoth-bearing site of Bullendorf in NE Austria. Riv Ital Paleontol Stratigr 124(3):509–534. https://doi.org/10.13130/2039-4942/10616
Carr WJ, Trimble DE (1963) Geology of the American Falls quadrangle, Idaho. U.S. Geol Surv Bull 1121-G:1–44
Claramunt S, Cracraft J (2015) A new time tree reveals Earth history’s imprint on the evolution of modern birds. Sci Adv 1(11):e1501005. https://doi.org/10.1126/sciadv.1501005
Clessin S (1885) Die Conchylien der Obermiocaenen Ablagerungen von Undorf. Malakozool Bl Neue Folge 7:71–95
Cockerell TDA (1906) The fossil Mollusca of Florissant, Colorado. Bull Am Mus Nat Hist 22:459–462
Cockerell TDA (1908) The Miocene species of Lymnaea. The Nautilus 22(6):69–70
Correa AC, Escobar JC, Durand P et al (2010) Bridging gaps in the molecular phylogeny of the Lymnaeidae (Gastropoda: Pulmonata), vectors of fascioliasis. BMC Evol Biol 10:381. https://doi.org/10.1186/1471-2148-10-381
Correa AC, Escobar JS, Noya O (2011) Morphological and molecular characterization of Neotropic Lymnaeidae (Gastropoda: Lymnaeoidea), vectors of fasciolosis. Infect Genet Evol 11:1978–1988. https://doi.org/10.1016/j.meegid.2011.09.003
Cossmann M (1889) Catalogue illustré des coquilles fossils de l’Éocène des environs de Paris. Ann Soc R zool malacolog Belg 24:3–376
Cossmann M (1902) Appendice No. 3 au Catalogue illustré des coquilles fossils de l’Éocène des environs de Paris. Ann Soc R zool malacolog Belg 36:9–110
Cziczer I, Magyar I, Pipík R et al (2009) Life in the sublittoral zone of long-lived Lake Pannon: paleontological analysis of the Upper Miocene Szák Formation, Hungary. Int J Earth Sci 98:1741–1766. https://doi.org/10.1007/s00531-008-0322-3
d’Orbigny A (1852) Prodrome de Paléontologie. Stratigraphique universelle des animaux mollusques et rayonnés faisant suitre au cours élémentaire de paléontologie et de géologie stratigraphique. Troisième volume, Victor Masson, Paris. https://www.biodiversitylibrary.org/page/31730040
Davitashvili LS (1930) Kimmeriyskiy yarus. In: Archangelsky AD, Davitashvili LS (eds) Rukovodyashchiye iskopayemyye neftyenosnykh rayonov Krymsko-Kavkazskoy oblasti, VIII, vol 6. Trudy Gosudarstvennogo Issledovatel’skogo Neftyanogo Instituta, pp 1–44. [in Russian]
Davitashvili LS (1931) Ponticheskiy yarus. In: Archangelsky AD, Davitashvili LS (eds) Rukovodyashchiye iskopayemyye neftyenosnykh rayonov Krymsko-Kavkazskoy oblasti, VII. Trudy Gosudarstvennogo Issledovatel’skogo Neftyanogo Instituta, pp 1–56. [in Russian]
Dayrat B, Conrad M, Balayan S et al (2011) Phylogenetic relationships and evolution of pulmonate gastropods (Mollusca): new insights from increased taxon sampling. Mol Phylogenet Evol 59(2):425–437. https://doi.org/10.1016/j.ympev.2011.02.014
De Leeuw A, Mandic O, Krijgsman W et al (2011) A chronostratigraphy for the Dinaride Lake System deposits of the Livno-Tomislavgrad Basin: the rise and fall of a long-lived lacustrine environment. Stratigraphy 8(1):29–43
De Loriol P, Jaccard A (1865) Étude géologique et paléontologique de la formation d’eau douce infracrétacée du Jura et en particulier de Villers-le-Lac. Mem Soc phys hist nat Genève 18(1):63–128
Degrange-Touzin A (1892) Étude sur la faune terrestre, lacustre et fluviatile de l’Oligocène supérieur et du Miocène dans le Sud-Ouest de la France et principalement dans la Gironde. Actes Soc Linn Bordeaux 45:125–230
Dehm R (1979) Artenliste der altpleistozänen Molluskenfauna vom Uhlenberg bei Dinkelscherben. Geol Bavarica 80:123–125
Dejean PFMA (1833–1836) Catalogue des coléoptères de la collection de M. le comte Dejean. Méquignon-Marvis Père et Fils, Paris
Deshayes GP (1861–1864) Description des animaux sans vertèbres découverts dans le bassin de Paris pour servir de supplément a la description des coquilles fossiles des environs de Paris comprenant une revue générale de toutes les espèces actuellement connues. Tome deuxième. – Texte. Mollusques Acéphalés Monomyairew et Brachiopodes. Mollusques céphalés. Première Partie. J.-B. Baillière et Fils, Paris. https://www.biodiversitylibrary.org/page/35623270
Dollfus GF (1906) Bassin de Paris. Feuille de Bourges au 320.000e (révision des faunes continentales). In: Anonymous (ed.) Comptes rendus des collaborateurs pour la campagne de 1905. Bull Serv Carte Géol Fr 16(110):284–304
Dollfus GF (1920) Bassin de Paris. Feuille de Paris au 80.000e. In: Anonymous (Ed.) Comptes rendus des collaborateurs pour la campagne de 1919. Bull Serv Carte Géol Fr 24(140):1–13
Douxami H (1904) Étude sur la molasse rouge. Ann Soc Linn Lyon nouv ser 51:1–30. https://www.biodiversitylibrary.org/page/54584266
Dunker W (1846) Monographie der Norddeutschen Wealdenbildung. Ein Beitrag zur Geognosie und Naturgeschichte der Vorwelt, Oehme und Müller, Braunschweig
Dunker W (1853) Ueber die in der Braunkohlenformation von Großalmerode in neuerer Zeit entdeckten Süßwasser-Mollusken. Programm der höheren Gewerbschule in Cassel zu Michaelis 1853:3–18
Edwards FE (1852) Part II. Pulmonata. In: Edwards FE, Wood SV (eds) A monograph of the Eocene Mollusca, or descriptions of shells from the older Tertiaries of England. Paleontographical Society, London, pp 57–122. https://www.biodiversitylibrary.org/page/12894018
Esu D (1984) Gasteropodi dei bacini continentali terziari eocenico-oligocenici dell’isola di Maiorca (Baleari). Thalassia Salent 14:85–99. https://doi.org/10.1285/i15910725v14p85
Esu D, Girotti O (1975) La malacofauna continentale del Plio-Pleistocene dell’Italia centrale. I Paleontologia Geol Rom 13:203–294
Esu D, Girotti O (2010) The Late Oligocene molluscan fauna from Otranto (Apulia, southern Italy): an example of alternating freshwater, lagoonal and emerged environments. Palaeontology 53(1):137–174. https://doi.org/10.1111/j.1475-4983.2009.00923.x
Esu D, Girotti O (2018) Valvata mathiasi n. sp. (Gastropoda: Heterobranchia: Valvatidae) from the lower Pliocene of the Val di Pesa (Tuscany, Central Italy). Arch Molluskenkd 147(1):49–54. https://doi.org/10.1127/arch.moll/147/049-054
Esu D, Girotti O (2020) Updating a late Early – Middle Pleistocene non-marine molluscan fauna from Achaia (Greece). Systematics and palaeoecological remarks. Boll Malacol 56:59–83
Evanoff E, Good SC, Hanley JH (1998) An overview of the freshwater mollusks from the Morrison Formation (Upper Jurassic, Western Interior, USA). Mod Geol 22:423–450
Evans J, Shumard BF (1856) Descriptions of new fossil species from the fresh water Tertiary formation of Nebraska, collected by the North Pacific railroad expedition, under Gov. J. J Stevens. Proc Acad Nat Sci Philad 7:164–165. https://www.biodiversitylibrary.org/page/26299385
Fabre-Taxy S (1948) Faunes lagunaires et continentales du Crétacé Supérieur de Provence. I – Le Turonien saumâtre. Ann Paléont 34:63–95
Fabre-Taxy S (1959) Faunes lagunaires et continentales du Crétacé Supérieur de Provence. III – Le Maestrichtien et le Danien. Ann Paléont 45:55–124
Falk AR (2011) Tracking Mesozoic birds across the world. J Syst Palaeontol 9(1):85–90. https://doi.org/10.1080/14772019.2010.512616
Firby JR (1966) New non-marine Mollusca from the Esmeralda formation, Nevada. Proc Calif Acad Sci 4th ser 33(14):453–480. https://www.biodiversitylibrary.org/page/26236670
Fleming TH, Lips KR (1991) Angiosperm endozoochory: were pterosaurs Cretaceous seed dispersers? Am Nat 138(4):1058–1065. https://doi.org/10.1086/285269
Fontannes F (1879) Note sur la découverte d’un gisement de marne a Limnées a Celleneuve, près Montpellier. Revue Sci Nat 8(64–75):159
Fontannes F (1881) Les terrains tertiaires du bassin de Crest. Ann Soc Agr Hist Nat Arts Utiles Lyon 5th ser 3:827–1046. http://gallica.bnf.fr/ark:/12148/bpt6k6154617d/f856.image
Fontannes F (1883) Diagnoses d’espèces et de variétés nouvelles des terrains tertiaires du bassin du Rhône. Mougin-Rusand, Lyon
Fontannes F (1884) Description sommaire de la faune malacologique des formations saumâtres et d’eau douce du groupe d’Aix (Bartonien-Aquitanien) dans le Bas-Languedoc, la Provence et le Dauphiné. Georg, F. Savy, Lyon, Paris. http://gallica.bnf.fr/ark:/12148/bpt6k55007367
Gillet S (1953) Les marnes à Cyrènes de l’Oligocène d’Alsace. Rev Inst Francais Pétrole 8(8):395–422
Glibert M (1962) Euthyneura et Pulmonata fossiles du Cénozoïque étranger des collections de l’Institut Royal des Sciences Naturelles de Belgique. Mem Inst R Sci nat Belgique 2nd ser 70:1–140. www.vliz.be/imisdocs/publications/252233.pdf
Glibert M, Heinzelin de Braucourt J (1954) Le gîte des vertébrés tongriens de Hoeleden. Bull Inst R Sci nat Belgique 30(1):1–14
Good SC (1987) Mollusc-based interpretations of lacustrine paleoenvironments of the Sheep Pass Formation (latest Cretaceous to Eocene) of east central Nevada. PALAIOS 2:467–478. https://doi.org/10.2307/3514618
Gorjanović-Kramberger C (1890) Die praepontischen Bildungen des Agramer Gebirges. Glasnik Hrvatsk Nar Društva 5:151–163. https://www.biodiversitylibrary.org/page/11105025
Gorjanović-Kramberger K (1901) Über die Gattung Valenciennesia und einige unterpontische Limnaeen. Ein Beitrag zur Entwicklungsgeschichte der Gattung Valenciennesia und ihr Verhältnis zur Gattung Limnaea. Beitr Paläont Österr-Ung Orient 13(3):121–140. https://www.biodiversitylibrary.org/page/14512804
Gorjanović-Kramberger C (1923) Die Valenciennesiden und einige anderen Limnaeiden der pontischen Stufe des unteren Pliocaens in ihrer stratigraphischen und genetischen Bedeutung. Glasnik Hrvatsk Prir Društva 35:87–114
Grateloup J-PS (1828) Tableau des coquilles Fossiles qu’on rencontre dans des terrains calcaires tertiaires (faluns) des environs de Dax, dans le département des Landes (Articles 1–3). Bull Hist Nat Soc linn Bordeaux 2:72–109. 123–158, 192–204. https://www.biodiversitylibrary.org/page/35729945
Gray J (1988) Evolution of the freshwater ecosystem: the fossil record. Palaeogeogr Palaeoclimatol Palaeoecol 62:1–214. https://doi.org/10.1016/0031-0182(88)90054-5
Hanganu E (1972) Des espèces de Valenciennius au Pontien du Bassin dacique. Revue Roum Geol Geophys Geogr 16(1):21–39
Hanna GD (1922) Fossil freshwater mollusks from Oregon, contained in the Condon Museum of the University of Oregon. Univ Oregon Publ 1(12):1–22. https://www.biodiversitylibrary.org/page/20825593
Hanna GD (1923) Upper Miocene lacustrine mollusks from Sonoma County, California. Proc Calif Acad Sci 4th ser 12(3):31–41. https://www.biodiversitylibrary.org/page/15974899
Hanna GD, Gester GC (1963) Pliocene lake beds near Dorris, California. Occas Pap Calif Acad Sci 43:1–17
Hannibal H (1912) A synopsis of the recent and tertiary freshwater Mollusca of the Californian province, based upon an ontogenetic classification. Proc Malacol Soc London 10(2–3):112–165. 167–211. https://www.biodiversitylibrary.org/page/3158751
Harshbarger JW, Repenning CA, Irwin JH (1957) Stratigraphy of the uppermost Triassic and the Jurassic rocks of the Navajo country. US Geol Surv Prof Paper 291:1–74. https://pubs.usgs.gov/pp/0291/report.pdf
Hartman JH, Roth B (1998) Late Paleocene and Early Eocene nonmarine molluscan faunal change in the Bighorn Basin, northwestern Wyoming and south-central Montana. In: Aubrey MP, Lucas SG, Berggren WA (eds) Late Paleocene–Early Eocene biotic and climatic events in the marine and terrestrial records. Columbia University Press, pp 323–379
Hartman JH, Erickson DN, Bakken A (2008) Stephen Hislop and his 1860 Cretaceous continental molluscan new species descriptions in Latin from the Deccan Plateau. India. Palaeontology 51(6):1225–1252. https://doi.org/10.1111/j.1475-4983.2008.00807.x
Harzhauser M, Mandic O (2008) Neogene lake systems of Central and South-Eastern Europe: faunal diversity, gradients and interrelations. Palaeogeogr Palaeoclimatol Palaeoecol 260(3–4):417–434. https://doi.org/10.1016/j.palaeo.2007.12.013
Harzhauser M, Neubauer TA, Mandic O et al (2012) A Middle Miocene endemic freshwater mollusc assemblage from an intramontane Alpine lake (Aflenz Basin, Eastern Alps, Austria). Palaeontol Z 86(1):23–41. https://doi.org/10.1007/s12542-011-0117-x
Harzhauser M, Mandic O, Kern AK et al (2013) Explosive demographic expansion by dreissenid bivalves as a possible result of astronomical forcing. Biogeosciences 10:8423–8431. https://doi.org/10.5194/bg-10-8423-2013
Harzhauser M, Neubauer TA, Gross M et al (2014a) The early Middle Miocene mollusc fauna of Lake Rein (Eastern Alps, Austria). Palaeontogr Abt A 302(1–6):1–71
Harzhauser M, Neubauer TA, Georgopoulou E et al (2014b) The Early Miocene (Burdigalian) mollusc fauna of the North Bohemian Lake (Most Basin). Bull Geosci 89(4):819–908. https://doi.org/10.3140/bull.geosci.1503
Harzhauser M, Mandic O, Neubauer TA et al (2016) Disjunct distribution of the Miocene limpet-like freshwater gastropod genus Delminiella. J Molluscan Stud 82(1):129–136. https://doi.org/10.1093/mollus/eyv040
Henderson J, Rodeck HG (1934) New species of Pliocene Mollusca from Eastern Oregon. J Paleontol 8(3):264–269
Hislop S (1860) On the Tertiary deposits, associated with trap-rock, in the East Indies. Quart Journ Geol Soc London 16:154–182. http://archive.org/stream/quarterlyjournal161860ge#page/154/mode/2up
Hubendick B (1951) Recent Lymnaeidae. Their variation, morphology, taxonomy, nomenclature and distribution. Kungliga Svenska Vetenskapsakademiens Handlingar Fjärde Serien 3(1):1–223
Hubendick B (1978) Systematics and comparative morphology of the Basommatophora. In: Fretter V, Peake J (eds) Pulmonates. Volume 2A – systematics, evolution and ecology. Academic Press, London, pp 1–47
Huckriede R (1967) Molluskenfaunen mit limnischen und brackischen Elementen aus Jura, Serpulit und Wealden NW-Deutschlands und ihre paläogeographische Bedeutung. Geol Jb Beih 67:1–263
Huică IV (1977) Studiul geologic al depozitelor miocene și pliocene dintre Valea Sohodol și Valea Blahnița, județul Gorj (depresiunea getica). Anuarul Institutului de Geologie și Geofizică 51:5–68
Inaba A (1969) Cytotaxonomic studies of lymnaeid snails. Malacologia 7(2–3):143–168. https://www.biodiversitylibrary.org/page/13122332
Janssen AW (1980) A mollusc-fauna with ‘Pseudamnicola’ helicella (Braun) from the Atuatuca formation (Oligocene) at St.-Truiden (Belgium, province of Limburg). Meded Werkgr Tert Kwart Geol 17(2):43–55
Jekelius E (1932) Fauna Neogenă a României. Die Molluskenfauna der dazischen Stufe des Beckens von Braşov. Memoriile Institutului geologic al României 2:1–118
Jenny J-P, Anneville O, Arnaud F et al (2020) Scientists’ warning to humanity: rapid degradation of the world’s large lakes. J Gt Lakes Res 46(4):686–702. https://doi.org/10.1016/j.jglr.2020.05.006
Jodot P (1954) Mollusques de petites dimensions des formations continentales ludienne, sannoisienne et stampienne du Jura méridional et de la Haute-Savoie. Bull Soc Geol Fr 6th ser 4(7–9):537–556
Jodot P, Feugueur L (1953) Le passage du Lutétien au Bartonien a Montagny-en-Vexin (Oise). Présence d’un calcaire lacustre a faune bartonienne subordonnée aux couches à Potamides lapidum. Bull Soc Geol Fr 6th ser 3(9):933–940
Jodot P, Rey R (1956) Observations stratigraphiques et malacologiques sur les bassins lacustres de Saint-Alban-sur-Limagnole (Lozère) et de Massiac (Cantal). Bull Soc Geol Fr 6th ser 6(7–9):937–968
Jooss CH (1913) Ueber Limnaea (Limnaea s.str.) turrita Klein emend. Jooss. Centralbl Min Geol Paläont 1913(2):58–64. https://www.biodiversitylibrary.org/page/49054066
Jörger KM, Stöger I, Kano Y et al (2010) On the origin of Acochlidia and other enigmatic euthyneuran gastropods, with implications for the systematics of Heterobranchia. BMC Evol Biol 8:323. https://doi.org/10.1186/1471-2148-10-323
Kadolsky D (1989) Stratigraphie und Molluskenfaunen von “Landschneckenkalk” und “Cerithienschichten” im Mainzer Becken (Oberoligozän bis Untermiozän?). Stratigraphische, paläogeographische und paläoökologische Ergebnisse. Geol Jb A 110:69–133
Kadolsky D (2020) A remarkable non-marine mollusc fauna of early Eocene age from a fissure infill in Karsdorf quarry (Sachsen-Anhalt, Germany). Geol Saxonica 65:31–76. https://doi.org/10.26049/GEOLSAX65-66-2019-2020-04
Kappes H, Haase P (2012) Slow, but steady: dispersal of freshwater molluscs. Aquat Sci 74(1):1–14. https://doi.org/10.1007/s00027-011-0187-6
Klein A (1853) Conchylien der Süsswasserkalkformation Württembergs. Jahresh Ver vaterl Naturk Württemberg 9:203–223. https://www.biodiversitylibrary.org/page/7983357
Kochansky-Devidé V, Pikija M (1976) Panonske Clivunellidae (Gastropoda) sjeverne Hrvatske. Geol Vjesn 29:397–407
Kochansky-Devidé V, Slišković T (1972) Revizija roda Clivunella Katzer, 1918 i Delminiella n.gen. (Gastropoda). Geol glasn 16:47–70
Kocsis AT, Alroy J, Reddin CJ et al (2019) divDyn: diversity dynamics using fossil sampling data. R package version 0.8.0. http://cran.r-project.org/package=divDyn. Accessed 1 November 2020
Kókay J (2006) Nonmarine mollusc fauna from the Lower and Middle Miocene, Bakony Mts., W Hungary. Geol Hung Ser Palaeont 56:1–196
Kolenda K, Najbar A, Kusmierek N et al (2017) A possible phoretic relationship between snails and amphibians. Folia Malacol 25(4):281–285. https://doi.org/10.12657/folmal.025.019
Kovalenko V (2004) Lymnaeidae iz mestonahodždeni Trijebine i Vračević, Serbia. Bull Acad Serbe Sci Arts 42:327–339
Kovalenko VA (2017) Presnovodnyye mollyuski (Gastropoda, Pulmonata, Lymnaeidae) v sarmatskikh otlozheniyakh yuga Ukrainy. Heoloho-mineralohichnyy visnyk Kryvoriz’koho natsional’noho universytetu 1(37):17–31. [in Russian]
Krejci-Graf K, Wenz W (1932) Stratigraphie und Paläontologie des Obermiozäns und Pliozäns der Muntenia (Rumänien). Z Dtsch Geol Ges 83:65–163
Kruglov ND (2005) Lymnaeid snails of Europe and Northern Asia. Smolensk State Pedagogical University Press, Smolensk. [in Russian]
La Rocque A (1960) The molluscan faunas of the Flagstaff Formation of central Utah. Geol Soc Am Mem 78:1–100. https://doi.org/10.1130/MEM78-p1
La Rocque A (1963) Late Cenozoic non-marine molluscan associations in eastern North America. Sterkiana 11:1–50
Lennert J, Szónoky M, Szuromi-Korecz A et al (1999) The Lake Pannon fossils of the Bįtaszék brickyard. Acta Geol Hung 42(1):67–88
Leriche M (1899) Description de la Faune d’eau douce sparnacienne de Cuvilly (Oise). Ann Soc geol Nord 28:95–104. http://iris.univ-lille1.fr/bitstream/handle/1908/1774/DP11_28.pdf?sequence=2
Love JD (1989) Names and descriptions of new and reclassified formations in northwestern Wyoming (geology of the Teton-Jackson Hole region, northwestern Wyoming). US Geol Surv Prof Paper 932-C:1–45. https://pubs.usgs.gov/pp/0932c/report.pdf
Lozouet P, Maestrati P (1981) Sables de Vauroux-St.-Antoine. Afzett Werkgr Tert Kwart Geol 3(2):61–71
Macaleț R (2000) New Radix species identified in the Neogene deposits of the Dacic Basin. Acta Palaeont Romaniae 2:251–259
Macarovici N (1940) Recherches géologiques et paléontologiques dans la Bessarabie méridionale (Roumanie). Ann sci Univ Jassy 36(1):177–422
MacNeil FS (1939) Fresh-water invertebrates and land plants of cretaceous age from Eureka, Nevada. J Paleontol 13:355–360
Mahulu A, Clewing C, Stelbrink B et al (2019) Cryptic intermediate snail host of the liver fluke Fasciola hepatica in Africa. Parasit Vectors 12:573. https://doi.org/10.1186/s13071-019-3825-9
Marinescu F (1970) Velutinellus, nouveau genre fossile de la famille des Lymnaeidae, et ses relations avec Velutinopsis et Valenciennius. Malacologia 9(2):313–325
Marinescu F (1973) Les mollusques pontiens de Tirol (Banat Roumain). Mem Inst Geol Geophys 18:7–56
Marinescu F (1992) Radix (Adelinella) coronatus n. sp. (Mollusca, Gastropoda) dans le Sarmatien du Bassin de Borod. Rom J Palaeont 75:9–10
Marinescu F, Papaianopol I (1990) Faciostratotypes et stratotypes de limite del’avantpays Carpatho-Balkanique et du Bassin Dacique (Roumanie). In: Stevanović P, Nevesskaya LA, Marinescu F et al (eds) Chronostratigraphie und Neostratotypen. Neogen der Westlichen (“Zentrale”) Paratethys, Bd. VIII, Pl1. Pontien. Verlag der Jugoslawischen Akademie der Wissenschaften und Künste und der Serbischen Akademie der Wissenschaften und Künste, Zagreb, Beograd, pp 398–410
Marquet R, Lenaerts J, Karnekamp C et al (2008) The molluscan fauna of the Borgloon formation in Belgium (Rupelian, Early Oligocene). Palaeontos 12:1–100
Marsh AD, Rowe TB (2018) Anatomy and systematics of the sauropodomorph Sarahsaurus aurifontanalis from the early Jurassic Kayenta formation. PLoS One 13(10):e0204007. https://doi.org/10.1371/journal.pone.0204007
Marshall WB (1926) New fossil fresh-water mollusks from Florida. Proc U S Nat Mus 68(2612):1–4. https://www.biodiversitylibrary.org/page/7610769
Martinson GG (1954) Nekotoryye presnovodnyye bryukhonogiye mollyuski iz neogenovykh otlozheniy Irkutskogo amfiteatra. Trudy Baikal’skoy Limnologicheskoy Stantzii Akademii Nauk SSSR 13:108–121. [in Russian]
Martinson GG (1956) Opredelitel’ mezozoyskikh i kaynozoyskikh presnovodnykh mollyuskov Vostochnoy Sibiri. Izdatel’stvo Akademii Nauk SSSR, Moskva, Leningrad. [in Russian]
Martinson GG (1961) Mezozoiskie i Kainozoiskie Molliuski kontinentalnykh otlozhenii Sibirskoi Platformy Zabaikalia i Mongolii. Trudy Baikal’skoy Limnologicheskoy Stantzii Akademii Nauk SSSR 19:1–332. [in Russian]
Matheron P (1843) Catalogue méthodique et descriptif des corps organisés fossiles du départment des Bouches-du-Rhône et lieux circonvoisins; précédé D’un Mémoire sur les terrains supérieurs au Grès Bigarré du S. E. de la France. Rep Trav Soc stat Marseille 6:1–269
Meek FB (1873) Preliminary paleontological report, consisting of lists and descriptions of fossils, with remarks on the ages of the rocks in which they were found, etc., etc. US Geol Geogr Surv Terr Ann rep 6:431–541. https://biodiversitylibrary.org/page/15677973
Meek FB (1876) A report on the invertebrate Cretaceous and Tertiary fossils of the upper Missouri country. Rep US Geol Surv Terr 9:1–629. https://www.biodiversitylibrary.org/page/2024294
Meek FB, Hayden FV (1856) Descriptions of new species of Acephala and Gastropoda, from the Tertiary formations of Nebraska territory, with some general remarks on the geology of the country about the sources of the Missouri River. Proc Acad Nat Sci Philad 8:111–126. https://www.biodiversitylibrary.org/page/1935165
Michaud G (1855) Descriptions de coquilles fossiles découvertes dans les environs de Hauterive (Drôme). Ann Soc linn Lyon nouv ser 2:33–64. https://www.biodiversitylibrary.org/page/3487714
Michel AE, Cohen AS, West K et al (1992) Large African Lakes as natural laboratories for evolution: examples from the endemic gastropod fauna of Lake Tanganyika. Mitt Intern Ver Theor Angew Limnol 23:85–99
Mödden C, Schäfer P, Reichenbacher B et al (2000) Säugetiere, Fisch-Otolithen, Ostracoden, Mollusken und Charophyten aus den Süßwasser-Schichten (Oligozän) von Wolfsheim im Mainzer Becken. Palaeontol Z 74(3):343–361. https://doi.org/10.1007/BF02988106
MolluscaBase (eds) (2021) MolluscaBase. https://www.molluscabase.org. Accessed 7 Nov 2021
Moos A (1944) Neue Funde von Lymnaeiden, insbesondere von Valenciennesiiden im Pannon Kroatiens. Vjestnik Hrvatskog državnog geološkog zavoda i Hrvatskog državnog geološkog muzeja 2(3):341–390
Morton LS, Herbst R (2003) Moluscos dulceacuícolas de las Formaciones San José y Chiquimil (Mioceno) del Valle de Santa María (Catamarca y Tucumán). Argentina Ameghiniana 40(2):205–216
Müller P, Geary DH, Magyar I (1999) The endemic molluscs of the Late Miocene Lake Pannon: their origin, evolution, and family-level taxonomy. Lethaia 32:47–60
Munt MC (2014) Mollusca from the Insect Limestone (Bembridge Marls Member: Bouldnor Formation: Solent Group), Palaeogene, Isle of Wight, southern England. Earth Env Sci Trans Roy Soc Edinb 104:263–274. https://doi.org/10.1017/S1755691014000048
Nations JD (1974) Paleontology, biostratigraphy, and paleoecology of the Verde Formation of late Cenozoic age, north-central Arizona. In: TNV K, Swann GA, Eastwood RL (eds) Geology of northern Arizona with notes on archaeology and paleoclimate. Part II – Area studies and field guides. Geological Society of America, Rocky Mountain Section Meeting, Flagstaff, Arizona. Geological Society of America, Flagstaff, AZ, pp 611–629
Neubauer TA, Harzhauser M, Kroh A et al (2014) Replacement names and nomenclatural comments for problematic species-group names in Europe’s Neogene freshwater Gastropoda. Part 2. ZooKeys 429:13–46. https://doi.org/10.3897/zookeys.429.7420
Neubauer TA, Harzhauser M, Kroh A et al (2015a) A gastropod-based biogeographic scheme for the European Neogene freshwater systems. Earth-Sci Rev 143:98–116. https://doi.org/10.1016/j.earscirev.2015.01.010
Neubauer TA, Harzhauser M, Georgopoulou E et al (2015b) Tectonics, climate, and the rise and demise of continental aquatic species richness hotspots. Proc Natl Acad Sci U S A 112(37):11478–11483. https://doi.org/10.1073/pnas.1503992112
Neubauer TA, Georgopoulou E, Kroh A et al (2015c) Synopsis of European Neogene freshwater gastropod localities: updated stratigraphy and geography. Palaeontol Electron 18(1):3T. https://doi.org/10.26879/478
Neubauer TA, Harzhauser M, Pipík R (2015d) Upper Miocene endemic lacustrine gastropod fauna of the Turiec Basin: addressing taxonomic, paleobiogeographic and stratigraphic issues. Geol Carpathica 66(2):139–156. https://doi.org/10.1515/geoca-2015-0016
Neubauer TA, Georgopoulou E, Harzhauser M et al (2016) Predictors of shell size in long-lived lake gastropods. J Biogeogr 43(10):2062–2074. https://doi.org/10.1111/jbi.12777
Neubauer TA, Hauffe T, Silvestro D et al (2021) Current extinction rate in European freshwater gastropods greatly exceeds that of the late Cretaceous mass extinction. Commun Earth Environ 2:97. https://doi.org/10.1038/s43247-021-00167-x
Nordsieck H (1992) Phylogeny and system of the Pulmonata (Gastropoda). Arch Molluskenkd 121:31–52
Noulet J-B (1854) Mémoires sur les coquilles fossiles des terrains d’eau douce du Sud-Ouest de la France. Victor Masson, Paris
Noulet J-B (1857) Coquilles fossiles nouvelles des terrains d’eau douce du Sud-Ouest de la France. Editions Masson, Paris
Oppenheim P (1919) Das Neogen in Kleinasien. Z Dtsch Geol Ges 70:1–210. https://www.biodiversitylibrary.org/page/43778447
Ożgo M, Örstan A, Kirschenstein M et al (2016) Dispersal of land snails by sea storms. J Molluscan Stud 82(2):341–343. https://doi.org/10.1093/mollus/eyv060
Özsayar TY (1977) Karadeniz kiyi bölgesindeki Neojen formasyonlari ve bunlarin mollusk faunasinin incelenmesi. Karadeniz Teknik Üniversitesi Yayin 79:1–80
Pais J (ed) (2012) The Paleogene and Neogene of Western Iberia (Portugal): a Cenozoic record in the European Atlantic domain. Springer, Berlin
Pan HZ (1977) Mesozoic and Cenozoic fossil Gastropoda from Yunnan. In: Nanjing Institute of Geology and Palaeontology (ed) Mesozoic Fossils from Yunnan. 2. Science Press, Beijing, pp 83–152
Pan HZ (1983) Jurassic-cretaceous non-marine gastropod from Shandong Province. Acta Palaeontol Sin 22(2):210–218
Pan HZ, Zhu XG (2007) Early Cretaceous non-marine gastropods from the Xiazhuang Formation in North China. Cretac Res 27:215–224. https://doi.org/10.1016/j.cretres.2006.12.001
Pană I, Grigorescu D, Csiki Z et al (2002) Paleo-ecological significance of the continental gastropod assemblages from the Maastrichtian dinosaur beds of the Hațeg Basin. Acta Palaeont Romaniae 3:337–343
Papaianopol I, Marinescu F (1995) Faune de mollusques daciens du Bassin Dacique. In: Marinescu F, Papaianopol I (eds) Chronostratigraphie und Neostratotypen. Neogen der Zentrale Paratethys, Bd. IX, Pl1. Dacien. Editura Academiei Române, Bucuresti, pp 130–267
Papaianopol I, Marinescu F, Macaleț R (2003) Les coupes représentatives (stratotypes, faciostratotypes, stratotypes de limite). In: Papaianopol I, Marinescu F, Krstić N et al (eds) Chronostratigraphie und Neostratotypen. Neogen der Zentrale Paratethys, Bd. X, Pl2. Romanien. Editura Academiei Române, Bucuresti, pp 133–173
Papp A (1956) Paläontologische Beobachtungen im Pannon von Podsused bei Zagreb (Kroatien). Geol Vjesn 8–9:67–79
Paul CRC (1989) The molluscan faunal succession in the Hatherwood Limestone Member (Upper Eocene), Isle of Wight, England. Tert Res 10(4):147–162
Pavlović PS (1927) Donjopontiski mekušci iz okoline Beograda (s narocitim obzirom na fosilnu faunu okoline sela Vrcina). Sprska Akademija nauka, posebna izdanja 66(Prirodnjački i matematički spisi 17):1–121
Pavlović PS (1933) O fosilnoj fauni mekušaca iz okoline Peci. Glas Srpske Kraljevske Akademije 158(78):75–91
Peyrot A (1933) Conchologie néogènique de l’Aquitaine. Gastropodes Actes Soc Linn Bordeaux 84(2):129–288. 11–18. https://www.biodiversitylibrary.org/page/47871688
Pickford M (2008) Freshwater and terrestrial Mollusca from the Early Miocene deposits of the northern Sperrgebiet, Namibia. Mem Geol Surv Namibia 20:65–74
Pickford M, Senut B, Morales J et al (2008) Fossiliferous Cainozoic carbonates of the northern Sperrgebiet. Mem Geol Surv Namibia 20:25–42
Pierce HG (1993) The nonmarine mollusks of the Late Oligocene-Early Miocene Cabbage Patch fauna of western Montana III Aquatic mollusks and conclusions. J Paleont 67(6):980–993
Pierce HG, Constenius KN (2001) Late Eocene-Oligocene nonmarine mollusks of the northern Kishenehn Basin, Montana and British Columbia. Ann Carnegie Mus 70(1):1–112. https://www.biodiversitylibrary.org/page/52462132
Pierce HG, Constenius KN (2014) Terrestrial and aquatic mollusks of the Eocene Kishenehn Formation, Middle Fork Flathead River, Montana. Ann Carnegie Mus 82(4):305–329. https://doi.org/10.2992/007.082.0401
Ping C (1931) Tertiary and Quaternary non-marine gastropods of North China. Palaeont Sin Ser B 6(6):1–39
Popov SV, Rostovtseva YV, Fillippova NY et al (2016) Paleontology and stratigraphy of the Middle–Upper Miocene of the Taman Peninsula: Part 1. Description of key sections and benthic fossil groups. Paleont J 50(10):1039–1206. https://doi.org/10.1134/S0031030116100014
Popova SM (1964) K poznaniyu paleogenovykh i neogenovykh presnovodnykh mollyuskov Pribaykal’ya i yuga Sovetskogo Dal’nego Vostoka. In: Popova SM, Martinson GG (eds) Stratigrafiya i paleontologiya mezozoyskikh i kaynozoyskikh otlozheniy Vostochnoy Sibiri i Dal’nego Vostoka. Trudy Limnologicheskogo instituta 4(24):151–271. [in Russian]
Popova SM (1981) Kaynozoyskaya kontinental’naya malakofauna yuga Sibiri i sopredel’nykh territoriy. Nauka, Moskva. [in Russian]
Popova SM, Devyatkin YV, Starobogatov YI (1970) Mollyuski kyzylgirskoy svity Gornogo Altaya. Nauka, Moskva. [in Russian]
Prysjazhnjuk V, Kovalenko V, Górka M et al (2006) The fresh-water gastropods from the Sarmatian (Lymnaeidea, Bulinidae, Planorbiidae) of Zwierzyniec (Central Poland). In: Gozhik PF (ed) Problemy paleontologii ta biostratigrafii proterozoyu i fanerozoyu Ukraini. Natsional’naya Akademiya Nauk Ukraini, Kiev, pp 256–264
Pyron M, Brown KM (2015) Introduction to Mollusca and the class Gastropoda. In: Thorp JH, Rogers DC (eds) Thorp and Covich’s freshwater invertebrates: ecology and general biology, vol 1. Elsevier, London, pp 383–421
R Core Team (2020) R: A language and environment for statistical computing. Version 4.0.3. R Foundation for Statistical Computing, Vienna. http://www.R-project.org. Accessed 1 November 2020
Rasser MW, Höltke O, Salvador RB (2020) Gastropod palaeohabitats of Miocene Lake Randeck Maar and its hinterland defined by an actualistic genus-level approach. Lethaia 53(2):229–241. https://doi.org/10.1111/let.12353
Rees WJ (1965) The aerial dispersal of Mollusca. J Molluscan Stud 36(5):269–282. https://doi.org/10.1093/oxfordjournals.mollus.a064955
Reis O (1910) Fauna rybnykh slantsev Zabaykal’skoy oblasti. Geologicheskiye issledovaniya i razvedyvatel’nyye raboty po linii Sibirskoy zheleznoy dorogi 29:117–126. [in Russian]
Repelin J (1902) Description des faunes et des gisements du Cénomanien saumâtre ou d’eau douce du Midi de la France. Ann Mus hist nat Marseille Sect Geol 7:1–133. https://www.biodiversitylibrary.org/page/11806897
Rey R (1962a) Remarques sur les faunes malacologiques de l’Éocène inférieur de Provence. C r somm seanc Soc geol Fr 1962a(8):235–236
Rey R (1962b) Sur l’importance paléontologique de faunes malacologiques continentales de très petites dimensions, comme celles de la région de Montpellier. C r somm seanc Soc geol Fr 1962b(10):312–313
Rey R (1965) L’Oligocène et le Miocène inférieur de la Limagne bourbonnaise (Notes de paléontologie malacologique). Rev sci Bourbonnais Cent 1964:56–81
Rey R, Villatte J (1971) Gastéropodes des calcaires lacustres du Thanétien sous-pyrénéen entre les vallées du Douctouyre et de l’Aude. Bull Soc Hist nat Toulouse 107(3):414–422. http://gallica.bnf.fr/ark:/12148/bpt6k65564713/f66.item
Robinson WI (1915) Two new fresh-water gastropods from the Mesozoic of Arizona. Am J Sci 4th ser 40(240):649–651. https://doi.org/10.2475/ajs.s4-40.240.649
Rollier L (1910) Troisième supplément à la description géologique de la partie jurassienne de la feuille VII de la carte géologique de la Suisse au 1:100,000. Mat Carte Geol Suisse Nouv ser 25:i–vii:1–230
Roman F (1907) 1re partie – Paléontologie. In: Roman F, Torres A (eds) Le Néogène continental dans la basse vallée du Tage (rive droite). Imprimerie de l’Académie Royale des Sciences, Lisbonne, pp 1–88
Roule L (1886) Nouvelle recherches sur les mollusques du terrain lacustre inférieur de Provence. Ann Malacolog 2:195–228. https://www.biodiversitylibrary.org/page/16301303
Rousseau L (1842) Description des principaux fossiles de la Crimée. In: Demidov A (ed) Voyage dans la Russie méridionale et la Crimée, par la Hongrie, la Valachie et la Moldavie, exécuté en 1837, sous la direction de M. Anatole de Démidoff, par MM. de Sainson, Le Play, Huot, Léveillé, Raffet, Rousseau, de Nordmann et Du Ponceau. Tome 2. Ernest Bourdin et Ce., Paris, pp 781–823
Royo Gómez J (1922) El Mioceno continental ibérico y su fauna malacológica. Com invest paleontol prehist 30:1–230
Russell LS (1926) Mollusca of the Paskapoo Formation in Alberta. Trans R Soc Can 3rd ser Geol Sci 20:207–220
Russell LS (1938) New species of Gastropoda from the Oligocene of Colorado. J Paleontol 12(5):505–507
Russell LS (1952) Molluscan fauna of the Kishenehn Formation, Southeastern British Columbia. In: Anonymous (Ed.) Annual report of the National Museum of Canada for the fiscal year 1950–51. Natl Mus Can Bull 126:120–141
Russell LS (1955) Additions to the molluscan fauna of the Kishenehn formation, Southeastern British Columbia and adjacent Montana. In: Anonymous (Ed.) Annual report of the National Museum of Canada for the fiscal year 1953–54. Natl Mus Can Bull 136:102–119
Saadi AJ, Davison A, Wade CM (2020) Molecular phylogeny of freshwater snails and limpets (Panpulmonata: Hygrophila). Zool J Linnean Soc 190(2):518–531. https://doi.org/10.1093/zoolinnean/zlz177
Sacco F (1886) Nuove species terziarie di molluschi terrestri e d’aqua dolce e salmastra del Piemonte. Atti Soc Ital Sci Nat 29:427–476. https://www.biodiversitylibrary.org/page/16237730
Salvador RB, Rasser MW (2016) Fossil land and freshwater gastropods from the Middle Miocene of Bechingen and Daugendorf, southwestern Germany. Arch Molluskenkd 145(1):111–124. https://doi.org/10.1127/arch.moll/1869-0963/145/111-124
Salvador RB, Höltke O, Rasser MW et al (2016) Annotated type catalogue of the continental fossil gastropods in the Staatliches Museum für Naturkunde Stuttgart, Germany. Palaeodiversity 9:15–70. https://doi.org/10.18476/pale.v9.a3
Salvador RB, Tütken T, Tomotani BM et al (2018a) Paleoecological and isotopic analysis of fossil continental mollusks of Sandelzhausen (Miocene, Germany). Palaeontol Z 92:395–409. https://doi.org/10.1007/s12542-017-0400-6
Salvador RB, Cabrera F, Martínez S et al (2018b) Annotated catalogue of the fossil Hygrophila and Eupulmonata (Mollusca: Gastropoda) from South America (cretaceous – Neogene). N Jb Geol Paläont Abh 289(3):249–280. https://doi.org/10.1127/njgpa/2018/0760
Sandberger CLF (1870–1875) Die Land- und Süßwasser-Conchylien der Vorwelt. C. W. Kreidel, Wiesbaden. https://www.biodiversitylibrary.org/bibliography/112296
Schlickum WR (1976) Die in der pleistozänen Gemeindekiesgrube von Zwiefaltendorf a. d. Donau abgelagerte Molluskenfauna der Silvanaschichten. Arch Molluskenkd 107(1–3):1–31
Schlickum WR, Puisségur J-J (1978) Die Molluskenfauna der Schichten mit Viviparus burgundinus und Pyrgula nodotiana von Montagny-les-Beaune (Dép. Côte-d’Or). Arch Molluskenkd 109(1/3):1–26
Schütt H, Kavuşan G (1984) Mollusken der miozänen Süßwasserablagerungen in der Umgebung von Harmancık bei Kütahya-Bursa in Nordwestanatolien. Arch Molluskenkd 114(4/6):217–229
Seninski K (1905) Novyya Dannyya o neogenovych’ plastach’ Yugozapadnogo Zakavkaz’ya. Trudy Obshchestva Estestvoispytateley pri Imperatorskom Yuryevskom Universitete 16:1–80. [in Russian]
Serres M (1844) Notice sur les terrains d’eau douce du Bassin émergé de Castelnaudary (Aude). Ann Sci Nat Zool 3rd ser 2:168–190. https://www.biodiversitylibrary.org/page/2272726
Serres M (1853) Note sur les dépôts diluviens, les sables et les marnes teriaires d’eau douce mis à découvert lors des fondations du Palais-de-Justice de Montpellier, où l’on a rencontré des débris de Singes fossiles. Revue Mag Zool 2nd ser 5:446–461. 557–565. https://www.biodiversitylibrary.org/page/13711970
Sinzov I (1875) Opisaniye novykh i maloissledovannykh form rakovin iz tretichnykh obrazovaniy Novorossii. Zapiski Novorossiiskogo obshchestva estestvoispytatelei 3:1–59. [in Russian]
Solem A, Yochelson EL (1979) North American Paleozoic land snails, with a summary of other Paleozoic nonmarine snails. US Geol Surv Prof Paper 1072:1–42. https://pubs.usgs.gov/pp/1072/report.pdf
Sowerby JdC (1826–1829) The mineral conchology of Great Britain; or, Coloured figures and descriptions of those remains of testaceous animals or shells, which have been preserved at various times and depths in the earth. Vol. VI. Privately published by author, London. https://www.biodiversitylibrary.org/page/14386101
Sowerby JC (1840) Organic remains collected by Mr. Malcolmson and described by Mr. James de Carle Sowerby. In: Malcolmson JG. On the fossils of the eastern portion of the Great Basaltic District of India. Trans Geol Soc London Series 2 5(3):550–551. https://www.biodiversitylibrary.org/page/36236116
Sremac J (1981) Neke nove i manje poznate vrste mekušaca Croatica-naslaga i Banatica-naslaga sjeverne Hrvatske. Geol Vjesn 33:107–121
Stache G (1889) Die Liburnische Stufe und deren Grenzhorizonte. Eine Studie über die Schichtenfolgen der cretacisch-eocänen oder protocänen Landbildungsperiode im Bereiche der Küstenländer von Österreich-Ungarn mit einer einleitenden Uebersicht der geologischen Verhältnisse dieses Gebietes. Erste Abtheilung: Geologische Uebersicht und Beschreibung der Faunen und Floren-Reste. Abh k k Geol R-A 13:1–170
Stevanović PM (1951) Pontische Stufe im engeren Sinne – obere Congerienschichten Serbiens und der angrenzenden Gebiete. Sprska Akademija nauka, posebna izdanja 187(Geološki institut 2):1–361
Stevanović PM (1955) Neue Beiträge zur Kenntnis der kaspibrackischen Fazies des Portaferrian (O.-Pont s. str.) in Serbien. Ann Geol Penins Balk 23:45–65
Stevanović P (1990a) Discussion on the Pontian in the Pannonian Basin of the Western (“Central”) Paratethys. In: Stevanović P, Nevesskaya LA, Marinescu F et al (eds) Chronostratigraphie und Neostratotypen. Neogen der Westlichen (“Zentrale”) Paratethys, Bd. VIII, Pl1. Pontien. Verlag der Jugoslawischen Akademie der Wissenschaften und Künste und der Serbischen Akademie der Wissenschaften und Künste, Zagreb, Beograd, pp 31–38
Stevanović P (1990b) Pontien südlich von der Sava und Donau in Serbien und Bosnien. In: Stevanović P, Nevesskaya LA, Marinescu F et al (eds) Chronostratigraphie und Neostratotypen. Neogen der Westlichen (“Zentrale”) Paratethys, Bd. VIII, Pl1. Pontien. Verlag der Jugoslawischen Akademie der Wissenschaften und Künste und der Serbischen Akademie der Wissenschaften und Künste, Zagreb, Beograd, pp 119–153
Stevanović P (1990c) Pontien nördlich von der Sava und Donau, in Syrmien, Bačka und Banat. In: Stevanović P, Nevesskaya LA, Marinescu F et al (eds) Chronostratigraphie und Neostratotypen. Neogen der Westlichen (“Zentrale”) Paratethys, Bd. VIII, Pl1. Pontien. Verlag der Jugoslawischen Akademie der Wissenschaften und Künste und der Serbischen Akademie der Wissenschaften und Künste, Zagreb, Beograd, pp 195–211
Strausz L (1942) Das Pannon des mittleren Westungarns. Ann hist nat Mus Natl Hung Pars Min Geolg Palaeont 35:1–102
Suzuki K (1949) Development of the fossil non-marine molluscan faunas in Eastern Asia. Japan. J Geol Geogr Trans 21(1–4):91–133
Taktakishvili IG (1962) Novyye dannyye o stratigraficheskom rasprostranenii roda Valenciennius Rousseau. Soobshcheniya Akademii nauk Gruzinskoy SSR 29(3):301–306. [in Russian]
Taktakishvili IG (1967) Istoricheskoye razvitiye semeystva valensiyenniid. Metsniyereba, Tbilisi. [in Russian]
Taylor DW (1954) Nonmarine mollusks from Barstow formation of southern California. US Geol Surv Prof Paper 254-C:67–80. https://doi.org/10.3133/pp254C
Taylor DW (1957) Pliocene fresh-water mollusks from Navajo County, Arizona. J Paleont 31(1):654–661
Taylor DW (1960) Late Cenozoic molluscan faunas from the High Plains. US Geol Surv Prof Paper 337:1–94
Taylor DW (1966) Summary of North American Blancan nonmarine mollusks. Malacologia 4(1):1–172. https://www.biodiversitylibrary.org/page/13147234
Taylor DW (1981) Freshwater mollusks of California: a distributional checklist. Calif Fish Game 67(3):140–163
Taylor DW (1983) Late Tertiary mollusks from the lower Colorado River valley. Contr Mus Paleont Univ Michigan 26(13):289–298
Taylor DW (1988) Aspects of freshwater mollusc ecological biogeography. Palaeogeogr Palaeoclimatol Palaeoecol 62(1–4):511–576. https://doi.org/10.1016/0031-0182(88)90071-5
Taylor DW, Smith GR (1981) Pliocene molluscs and fishes from northeastern California and northwestern Nevada. Contr Mus Paleont Univ Michigan 25(18):339–413
Thomä C (1845) Fossile Conchylien aus den Tertiärschichten bei Hochheim und Wiesbaden gesammelt und im naturhistorischen Museum zu Wiesbaden ausgestellt. Jb Ver Naturk Nassau 2:125–162
Tozer ET (1956) Uppermost Cretaceous and Paleocene nonmarine molluscan faunas of western Alberta. Geol Surv Can Mem 280:1–125. https://doi.org/10.4095/101507
Van Baak CGC, Mandic O, Lazăr I et al (2015) The Slanicul de Buzau section, a unit stratotype for the Romanian stage of the Dacian Basin (Plio-Pleistocene, Eastern Paratethys). Palaeogeogr Palaeoclimatol Palaeoecol 440:594–613. https://doi.org/10.1016/j.palaeo.2015.09.022
van Benthem Jutting T (1937) Non marine Mollusca from fossil horizons in Java with special reference to the Trinil fauna. Zool Meded 20(11):83–180. https://www.repository.naturalis.nl/document/149951
Van Damme D, Gautier A (2013) Lacustrine mollusc radiations in the Lake Malawi Basin: experiments in a natural laboratory for evolution. Biogeosciences 10:5767–5778. https://doi.org/10.5194/bg-10-5767-2013
van Leeuwen CHA, van der Velde G (2012) Prerequisites for flying snails: external transport potential of aquatic snails by waterbirds. Freshw Sci 31(3):963–972. https://doi.org/10.1899/12-023.1
van Leeuwen CHA, van der Velde G, van Lith B et al (2012) Experimental quantification of long distance dispersal potential of aquatic snails in the gut of migratory birds. PLoS One 7(3):e32292. https://doi.org/10.1371/journal.pone.0032292
van Leeuwen CHA, Huig N, van der Velde G et al (2013) How did this snail get here? Several dispersal vectors inferred for an aquatic invasive species. Freshw Biol 58:88–99. https://doi.org/10.1111/fwb.12041
Vermeij GJ (2017) The limpet form in gastropods: evolution, distribution, and implications for the comparative study of history. Biol J Linn Soc 120(1):22–37. https://doi.org/10.1111/bij.12883
Vinarski MV (2013) One, two, or several? How many lymnaeid genera are there? Ruthenica 23(1):41–58
Vinarski MV, Frolov PD (2017) A new late Miocene Lymnaea with aberrant suture structure unique for the family (Gastropoda: Pulmonata: Lymnaeidae). Hist Biol 29(4):480–487. https://doi.org/10.1080/08912963.2016.1192618
Vinarski MV, Clewing C, Albrecht C (2019) Lymnaeidae Rafinesque, 1815. In: Lydeard C, Cummings KS (eds) Freshwater mollusks of the world. A distribution atlas. Johns Hopkins University Press, Baltimore, MD, pp 158–162
Vinarski MV, Bolotov IN, Aksenova OV et al (2021) Freshwater Mollusca of the circumpolar Arctic: a review on their taxonomy, diversity and biogeography. Hydrobiologia 848:2891–2918. https://doi.org/10.1007/s10750-020-04270-6
Vrsaljko D (1999) The Pannonian palaeoecology and biostratigraphy of molluscs from Kostanjek-Medvednica Mt., Croatia. Geol Croat 52:9–27
Walther AC, Benard MF, Boris LP et al (2008) Attachment of the freshwater limpet Laevapex fuscus to the hemelytra of the water bug Belostoma flumineum. J Freshw Ecol 23(2):337–339. https://doi.org/10.1080/02705060.2008.9664207
Wenz W (1922) Zur Nomenklatur tertiärer Land- und Süßwassergastropoden. IV. Senckenbergiana 4:5–7. https://www.biodiversitylibrary.org/page/30068315
Wenz W (1923) Fossilium Catalogus I: Animalia. Gastropoda extramarina tertiaria. Vol. IV. W. Junk, Berlin. https://www.biodiversitylibrary.org/page/40593222
Wenz W (1935) Weitere Beiträge zur Land- und Süßwasser-Molluskenfauna der subalpinen Molasse des Pfändergebietes. Senckenbergiana 17:223–225
Wenz W (1936) Die Molluskenfauna der Mergel von Paulhiac (Lot-et-Garonne). Arch Molluskenkd 68(6):228–238
Wenz W (1942) Die Mollusken des Pliozäns der rumänischen Erdöl-Gebiete als Leitversteinerungen für die Aufschluß-Arbeiten. Senckenbergiana 24:1–293
White CA (1876) Invertebrate paleontology of the plateau province, together with notice of a few species from localities beyond its limits in Colorado. In: Powell JW (ed) Report on the geology of the eastern portion of the Uinta Mountains and a region of country adjacent thereto. With atlas. U. S. Geological and Geographic Survey of the Territories, Washington, pp 74–135. https://www.biodiversitylibrary.org/page/19391191
White CA (1880) Descriptions of new invertebrate fossils from the Mesozoic and Cenozoic rocks of Arkansas, Wyoming, Colorado, and Utah. Proc US Nat Mus 3:157–162. https://www.biodiversitylibrary.org/page/14917257
White CA (1886) On the fresh-water invertebrates of the North American Jurassic. US Geol Surv Bull 29:691–725. https://doi.org/10.3133/b29
Whiteaves JF (1885) Report on the Invertebrata of the Laramie and Cretaceous rocks of the vicinity of the Bow and Belly Rivers and adjacent localities in the North-west Territory. Contrib Can Palaeont 1(1):1–89. https://www.biodiversitylibrary.org/page/38502645
Willis RP (1967) Geology of the Arabian peninsula. Bahrain. US Geol Surv Prof Paper 560-E:1–54. https://pubs.usgs.gov/pp/0560e/report.pdf
Willmann R (1981) Evolution, Systematik und stratigraphische Bedeutung der neogenen Süßwassergastropoden von Rhodos und Kos/Ägäis. Palaeontogr Abt A 174:10–235
Willmann R (1982) Biostratigraphisch wichtige Süßwassergastropoden (Prosobranchia, Hydrobiidae) aus dem Neogen des Ägäis-Raumes. N Jb Geol Paläont Abh 162(3):304–331
Witton MP, Habib MB (2010) On the size and flight diversity of giant pterosaurs, the use of birds as pterosaur analogues and comments on pterosaur flightlessness. PLoS One 5(11):e13982. https://doi.org/10.1371/journal.pone.0013982
Yen TC (1944) Notes on fresh-water mollusks of Idaho formation at Hammett, Idaho. J Paleont 18(1):101–108
Yen TC (1946a) Late Tertiary fresh-water mollusks from southeastern Idaho. J Paleontol 20(5):485–494
Yen TC (1946b) Eocene nonmarine gastropods from Hot Spring County, Wyoming. J Paleont 20(5):495–500
Yen TC (1946c) On Lower Cretaceous fresh-water mollusks of Sage Creek, Wyoming. Not Nat Acad Nat Sci 166:1–13
Yen TC (1947) Pliocene fresh-water mollusks from northern Utah. J Paleontol 21(3):268–277
Yen TC (1948a) Eocene fresh-water Mollusca from Wyoming. J Paleontol 22(5):634–640
Yen TC (1948b) Paleocene fresh-water mollusks from southern Montana. US Geol Surv Prof Paper 214-C:35–50
Yen TC (1951) Fresh-water mollusks of Cretaceous age from Montana and Wyoming. Part 1: a fluviatile fauna from the Kootenai formation near Harlowton, Montana. US Geol Surv Prof Paper 233-A:1–9. https://pubs.usgs.gov/pp/0233a/report.pdf
Yen TC (1952) Molluscan fauna of the Morrison formation. US Geol Surv Prof Paper 233-B:21–51. https://doi.org/10.3133/pp233B
Yen TC (1954) Nonmarine mollusks of Late Cretaceous age from Wyoming, Utah and Colorado. Part 1: a fauna from western Wyoming. US Geol Surv Prof Paper 254-B:45–59. https://pubs.usgs.gov/pp/0254b/report.pdf
Yen JTC (1969) Fossile nicht-marine Mollusken-Faunen aus Nordchina. Sitzber Österr Akad Wiss 177(1/3):21–64. http://www.zobodat.at/stable/pdf/SBAWW_177_0021-0064.pdf
Yen TC, Reeside JB Jr (1946) Fresh-water mollusks from the Morrison formation (Jurassic) of Sublette County, Wyoming. J Paleont 20(1):52–58
Yü W (1977) Cretaceous and early Tertiary non-marine gastropods from South China with their stratigraphical significance. Acta Palaeontol Sin 16(2):191–213
Yü W (1982) Some fossil gastropods from Xizang. In: Anonymous (ed) The series of the comprehensive scientific expedition to the Qinghai-Xizang plateau, Palaeontology of Xizang (Tibetan) plateau, vol 4. Science Press, Beijing, pp 255–281
Yü XH (1987) Late Jurassic and Early Cretaceous fresh water gastropods (Mollusca) from western Liaoning Province, China. In: Yu X, Wang W, Liu X et al (eds) Mesozoic stratigraphy and palaeontology of Western Liaoning, vol 3. Geological Publishing House, Beijing, pp 29–116
Yü W, Pan HZ (1980) Mesozoic non-marine gastropods from Zhejiang and South Anhui. In: Anonymous (ed) Divisions and correlation of the Mesozoic volcano-sedimentary rocks in Zhejiang and Anhui provinces, China. Academia Sinica, Nanking Institute of Geology and Paleontology, Nanking, pp 135–172
Yü W, Pan HZ (1982) Eocene non-marine Gastropoda from Zhuo Xian, Hebei. Bull Nanjing Inst Geol Palaeont 4:189–212
Yü W, Zhang X-Q (1982) Late Cretaceous and early Tertiary non-marine gastropods from Sanshui Basin, Guangdong. Mem Nanjing Inst Geol Palaeont Acad Sin 17:37–84
Yu T, Neubauer TA, Jochum A (2021) First freshwater gastropod preserved in amber suggests long-distance dispersal during the Cretaceous. Geol Mag 158(7):1327–1334. https://doi.org/10.1017/S0016756821000285
Zhang SX (2009) Geological formation names of China (1866–2000), vol 1. Springer, Dordrecht
Zhu GX (1980) Mollusca. In: Shenyang Institute of Geology and Mineral Resources (ed) Paleontological Atlas of Northeast China II. Mesozoic and Cenozoic volume. Geological Publishing House, Beijing, pp 8–59
Zhu XG (1985) Late Cenozoic non-marine gastropods from Xili Basin in Gansu. Acta Palaeontol Sin 24(6):672–679
Acknowledgments
I am grateful to Maxim Vinarski for the invitation to contribute to this book. Dániel Botka, Christine Garcia, Oleg Mandic, Alice Schumacher, and Maxim Vinarski kindly provided photographs. Heinz Kollmann, Simon Schneider, and Maxim Vinarski helped retrieving rare pieces of literature. Maxim Vinarski and Mathias Harzhauser provided helpful comments on an early draft of the manuscript. The study contributes to the project “Unraveling drivers of species diversification—an integrative deep-time approach on continental aquatic biota” funded by the German Research Foundation (DFG grant no. NE 2268/2-1).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
6.1 Electronic Supplementary Material
Supplementary Fig. 6.1
Number of species, species records, and literature sources of pre-Pleistocene Lymnaeidae compared across continents. The pie charts indicate the relative size of each continent. The uneven distribution of lymnaeids across continents as recorded in the literature suggests a major preservation, sampling, and/or research bias (ZIP 143 kb)
Supplementary Table 6.1
Dataset of all fossil Lymnaeidae used in this study, with information on taxonomy, geography, stratigraphy, sources (of original and revised record if available), and indication which records were used in diversity reconstructions. The geographic precision index of GPS data follows Neubauer et al. (2015c) (XLSX 466 kb)
Supplementary Table 6.2
List of all fossil-only Lymnaeidae species queried from MolluscaBase on 11 November 2020. The list includes accepted species, nomina dubia, taxa inquirenda as well as temporary names (XLSX 38 kb)
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Neubauer, T.A. (2023). The Fossil Record of the Lymnaeidae: Revisiting a 200-Myr-Long Story of Success. In: Vinarski, M.V., Vázquez, A.A. (eds) The Lymnaeidae. Zoological Monographs, vol 7. Springer, Cham. https://doi.org/10.1007/978-3-031-30292-3_6
Download citation
DOI: https://doi.org/10.1007/978-3-031-30292-3_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-30291-6
Online ISBN: 978-3-031-30292-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)