Abstract
Embryo rescue is a tissue culture tool that is greatly used to facilitate breeding in plants. Embryo rescue provides an effective means for recovering hybrid embryos resulting from wide hybridizations, which often fail to develop in vivo into plants. Embryo rescue is mainly used to develop interspecific or intergeneric distant hybrids. This review elucidates the salient aspects of wide hybridizations toward plant improvement. The main causes of hybrid embryo failures in wide crosses that have been discussed are precocious seed germination, nutritional starvation of developing embryo, cytological aberrations in embryogenesis, endosperm balance number discrepancy, polar-nuclei activation hypothesis deviations, and post-zygotic barrier limitations in endosperm and embryo development. Wide hybridizations related to frequent embryo failures arising from pre-fertilization or postfertilization barriers are usually overcome through embryo rescue. In this chapter, various limitations of pre-fertilization and postfertilization barriers that are encountered in wide hybridizations have been reviewed. In addition, some significant factors that influence the success of embryo culture, such as embryo genotypic background, embryo developmental stage, nutrient media composition and growth temperature, and light conditions, have been elaborated. Furthermore, considerations such as the determination of appropriate embryo stage for rescue, nature of the embryo excision techniques, and media manipulations for efficient embryo culture are noteworthy for success in embryo rescuing. Discussed also are the following very useful embryo rescue techniques: embryo-nurse endosperm transplant method, in vitro ovary and ovule culture techniques, as well as the ovary and ovule slice or perforation procedure. Some important applications of the embryo rescue technique that have been mentioned include overcoming seed dormancy and embryo abortion, plants development in seedless varieties, germplasm conservation, and homozygous monoploid production. The most recent uses of embryo rescue in successful wide hybridizations and the achieved improved agronomic traits in various plant genera or species have been highlighted.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Wide hybridization
- Interspecific and intergeneric
- Pre- and postfertilization barriers
- Embryo rescue
- Hybrid plant development
1 Introduction
Crop improvement is successfully achieved by the introduction of wide genetic variability through various plant breeding techniques. In this regard, the enhancement in the quality and yield of virtually all major crops has been realized through interspecific and intergeneric hybridizations, followed by selection (Araujo et al., 2021; Hristova-Cherbadzhi, 2020; Mahoney & Brand, 2021). Interspecific and intergeneric hybridization enables the transfer of desirable genes or traits from wild relatives to the respective domesticated plants through wide crosses between or among species. Unfortunately, in wide sexual crosses, most often than not, the endosperm fails to properly develop and causes majority of the embryos to abort in vivo or switch into dormancy for prolonged periods (Sahijram et al., 2013). It has been established that low fertility or poor survival of endosperm or hybrid embryos is largely due to the effects of pre-fertilization (pre-zygotic) and postfertilization (post-zygotic) physiological barriers (Okamoto & Ureshino, 2015; Sun et al., 2018). Quite frequently, zygotic development barriers prevent distant hybridizations from going through the normal sexual reproductive physiological processes to yield viable plants. Notably, inherent physiological incompatibility in wide hybridizations causes abortion of embryos at virtually any developmental stages (Okamoto & Ureshino, 2015; Sun et al., 2018). The achievement of successful wide hybridizations in various species or genera is, therefore, arduous due to hindering physiological barriers.
The most useful approach and widely employed method for overcoming postfertilization barriers has been the application of the technology of embryo rescue or culture (Hristova-Cherbadzhi, 2020). This in vitro tissue culture approach involves the removal of immature, mature, or defective hybrid embryo from ovule and nurturing it in culture into a whole plant. This practice releases the hybrid embryo obtained through wide crosses from the hindering influence of hybridization barriers and enables the embryo that hitherto would have aborted or degenerated to instead develop into a viable plant. The essence of embryo rescue is, therefore, to enable the limitations imposed by post-zygotic or fertilization barriers to be circumvented. The embryo rescue approach facilitates the development of interspecific and intergeneric plant hybrids.
The rescue and culture of embryos are relatively quite an easy technique to carry out and requires basically a simple agar-based nutrient medium incorporated with sugar and minerals. Furthermore, using embryo rescue, embryonic development in relation to the physical and nutritional requirements has been elucidated through the study of the physiological processes involved in the growth of rescued young hybrid embryos. To a great extent, successful embryo rescue depends largely upon explant maturation, medium nutrient content, as well as the genotype (Okamoto & Ureshino, 2015; Sun et al., 2018). The embryo rescue technology aids effective rescue of hybrid embryos from wide hybridizations (Hu & Wang, 1986). The technique also enables haploid plant production and reduction in the duration of the breeding cycle, in cases of prolonged dormancy. Embryos from ripened seeds could be isolated and cultured to eliminate the influence of seed germination inhibitors in instances where dormancy is the identified constraint to hybrid embryo development. In addition, embryo culture presents a reliable means for testing seed viability and provides material for micro-propagation.
Using embryo rescue, young immature, mature, or weak defective embryos have been effectively rescued in a number of crops, forests, ornamentals, and wild plant species (Araujo et al., 2021; Buteme et al., 2021; Kuang et al., 2021; Mahoney & Brand, 2021). For example, embryo rescue and interspecific pollination techniques were combined in an attempt to produce doubled haploids in castor bean (Ricinus communis) (Baguma et al., 2019). In this instance, embryo rescue was used to save the resulting embryos that would have otherwise aborted. The procedure of embryo rescue has been extensively applied to achieve varied objectives for successful plant improvement. This review presents an update on the current knowledge and achievements made in the improvement of useful traits in plants through wide hybridizations mediated by embryo rescue.
2 Interspecific and Intergeneric Hybridization: Associated Constraints
Plant genetic hybridization is a breeding procedure in which parents derived from different species belonging to a genus (interspecific) or parents from different genera of a family (intergeneric) are crossed to combine their genomes through pollination, either naturally or by induction (Table 1). Hybridization in plant improvement also involves crosses between diploid and tetraploid species (Mwangangi et al., 2019). Furthermore, through backcrossing, successful gene transfer has been performed between two species of different genetic constitution leading to the development of cytoplasmic sterile male plants (Premjet et al., 2019). Wide hybridization is, therefore, a powerful approach used to facilitate gene transfer by overcoming the species barrier (Huylenbroeck et al., 2020). A summary of the main steps involved in hybrid plant generation through wide hybridizations and the validation processes commonly carried out for verifying the hybrid state is presented in Fig. 1a, b. The main target of such wide hybridization procedures is usually to successfully obtain interspecific or intergeneric gene transfer, with the aim of creating more variation in plants for desirable traits. Unfortunately, such crosses that involve distant genetic backgrounds more often than not fail and do not produce viable plants (Premjet et al., 2019). There are several physiological barriers that cause endosperm and embryo development to fail at the pre- and postfertilization phases and, thus, hinder the introgression of genes from wild relatives to crops (Mahoney & Brand, 2021).
Pre-fertilization barriers comprise impediments that prevent successful fertilization. For instance, ineffective fertilization is usually caused by the prevention of proper pollen germination, tube growth and guidance, due to low pollen quality or lack of stigma receptivity (Buteme et al., 2021). Other factors that have been implicated to cause pre-fertilization limitations in wide crosses include dissimilarities in flower morphology, failure of pollen capture, adhesion or hydration, impaired pollen-pistil or pollen-ovule interactions, and fertilization failure (Köhler et al., 2021). Table 2 contains some recently identified pre-fertilization barriers encountered in wide hybridizations in various plants. Postfertilization barriers on the other hand arise due to ploidy differences, chromosome elimination, and seed dormancy (Premjet et al., 2019). Postfertilization barriers also occur as mitotic mismatch of parental genomes, defective endosperm growth and embryo malnutrition, as well as hybrid weakness or sterility, arising from ploidy or parental incompatibilities (Köhler et al., 2021).
Post-zygotic barriers inhibit zygote growth after fertilization has occurred, leading to abnormal seed formation. Seed malformation often emanates from physiologically defective hybrid endosperm, which causes acute failure in nutrient supply to the hybrid embryo (Okamoto & Ureshino, 2015; Sun et al., 2018). Moreover, endosperm or embryo failure could also be due to embryo-endosperm physiological mismatch induced by the exudation of lethal toxins from the endosperm to poison the embryo (Okamoto & Ureshino, 2015; Sun et al., 2018). In principle, embryos of nonviable hybrids have an innate competence to transform into plantlets, but their development is hindered by failure to undergo normal differentiation.
2.1 Interspecific and Intergeneric Hybrid Failures: Main Causes
2.1.1 Effects of Precocious Seed Germination
Precocious seed germination is characterized by the germination of seeds on the parent plant before the crop is harvested. In this abnormal form of seed germination, embryos initiate germination prior to full normal embryo development and maturity (Cota-Sanchez, 2018). Generally, precocious germination arises due to the elimination of the influence of endogenous germination inhibitors through the removal of the seed testa. Precocious germination is also induced by higher negative osmotic potential in vivo. Usually, precocious seed germination produces weak developing plantlets. This phenomenon has been observed to be generally widespread in plants that lack seed dormancy (Cota-Sanchez, 2018). This seed dormancy trait is generally characteristic of many wild plant species. Similarly, some crops inherently undergo short-duration seed dormancy. In some crops, the wild ancestors possessed seed dormancy traits; however, such plants lost the dormancy trait through the period of adaptation from the wild (Nakamura et al., 2017; Subburaj et al., 2016).
The absence of dormancy sometimes causes precocious seed germination particularly, in the form of preharvest sprouting of grains on the maternal plants when conditions of high humidity prevail (Cota-Sanchez, 2018; Subburaj et al., 2016). The culture or rescue of embryos could be employed to resolve and guide proper growth of embryos. The rescue and nurture of embryos can be achieved under different established culture conditions to stimulate embryological transformation into healthy plants. Ramming (1985) reported that the precocious germination of seeds can be circumvented through ovule rescue and culture in appropriate media. In Prunus, Ramming (1985) successfully used ovule culture to overcome the limitations posed by the integuments and, thus, prevent precocious germination.
2.1.2 Influence of Nutritional Starvation on Embryo Development
In making a transition to become a mature plant, the embryo first forms multiple tissues that subsequently result into a whole plant. Initially, an asymmetric zygotic differentiation produces the embryo and a suspensor, with both localized in the endosperm (Hristova-Cherbadzhi, 2020). The embryo is nourished with nutrients through the suspensor at the early developmental stages. It is known that the embryo and endosperm undergo parallel as well as interconnected developmental processes. The hybrid embryo size, for example, is regulated by the endosperm enveloping it. Similarly, the embryo also influences the developmental faith of the endosperm. This interdependence is an indication that there might be an exchange or sharing of some metabolic factors between endosperm and embryo that tune their developmental processes. In instances of embryo development failure, therefore, nutritional starvation of the embryo has been implicated as the main cause of the failure, which then hinders the efficient development of both the endosperm and embryo in particularly interspecific hybrids (Dziasek et al., 2021). In Capsella, crosses of Capsella rubella and C. grandiflora produce unviable hybrids linked to chromatin abnormalities in the endosperm, which subsequently led to abortion (Dziasek et al., 2021). Hybrid seed collapse could also be due to nutrient starvation of the embryo arising from early retardation and disintegration of the endosperm as a result of overgrowth of the endothelium.
2.1.3 Implications of Cytological Aberrations in Embryogenesis
In eukaryotic organisms, it is essential that gene dosage exists in the right balance to enable normal physiological, biochemical, or gene function. Factors including ploidy level differences, chromosome structural changes that cause gene dosage disproportion in organisms, eventually lead to phenotypic abnormalities. Irregular cell divisions during mitosis are comparatively common and result in an increase or decrease in chromosomes during meiosis (Heslop-Harrison & Schwarzacher, 2011). Commonly, the phenomena of nondisjunction, aberrant spindles, lagging chromosomes, or chromosome breakages create mitotic abnormalities in in vitro culture that bring about the incidence of chromosomal variations and genome alterations. During in vitro culture, major genome changes produce karyotypic instability and cytogenetic irregularities such as ploidy level variation and chromosomal structure alterations due to chromosomal breakages that are often created in the course of in vitro multiplication (Neelakandan & Wang, 2012). Chromosome breakages cause rearrangements that induce direct mutational alterations in gene expression. In oat and maize, it was found that chromosome breakage occurred more frequently than ploidy variations (Kaeppler et al., 1998).
The introduction of polyploidy disparities during embryogenesis is more predominant compared to the occurrence of aneuploidy-chromosome karyotype deviation from the normal precise multiple of the haploid set (Kaeppler et al., 2000). Ploidy level variations in in vitro culture or embryogenesis are mostly associated with the phenomenon of endo-reduplication or nuclear fusion (Kaeppler et al., 2000). Lee et al. (2009) explained that endo-reduplication gives rise to polyploidy in instances where replication proceeds devoid of the successive cell division and eventually culminate in higher gene content in the nucleus. Aneuploidy manifests as monosomy or trisomy, which represents, respectively, the loss or gain of one or more specific chromosomes or, in some cases, large chromosomal fragments. Aneuploidy is generally observed during the initial stages of callus initiation and suspension cultures (Kaeppler et al., 1998, 2000). Aneuploidy is induced via nuclear fragmentation occurring prior to mitosis or by irregular chromosome actions in the course of mitosis. In embryogenesis, long-term callus cultures are more frequently prone to cytogenetic abnormalities in especially regenerated plants of a variety of species (Rodriguez-López et al., 2010). Some cytological aberrations are usually checked at the initial phases of embryogenesis via the transfer of growth-regulating substances from the endosperm to the embryo.
2.1.4 Biological Significance of the Endosperm Balance Number
The entirety of the factors that drive successful seed development is still not fully understood and is very much speculative. Many different postulates or hypotheses have been put forward by some plant biologists to explain some likely factors responsible for the successful development of seeds in plants. One such hypothesis is the Endosperm Balance Number (Johnston et al., 1996). The Endosperm Balance Number hypothesis has been used to generally interpret and predict the success or failure of interspecific and interploidy hybridizations in plants (Carputo et al., 1999). Also, this hypothesis emphasizes the significance of a balanced parental genome mix that enables formation of physiologically normal endosperm. Some opinions state that a ploidy ratio of 2:3:2 of maternal tissue: endosperm: Embryo is an important consideration for viable seed formation. Others emphasize that the endosperm: embryo ratio is most important (Johnston et al., 1996; Katsiotis et al., 1995). Another school of thought considers the maternal tissue: endosperm ratio as the most crucial cytological balance ideal for successful seed development. Some plant biologists, however, suggest that endosperm function is autonomous and has no significant dependence on the maternal tissue and the embryo (Shukla, 2016).
Furthermore, other views stress the effectiveness of the 2 maternal∶ 1 paternal ratio for maternal and paternal genomes in the endosperm (Lester & Kang, 1998; Johnston, et al. 1996; Katsiotis et al., 1995). It has been emphasized that the Endosperm Balance 2: 1 ratio of maternal: paternal is an important prerequisite for successful interspecific crossability (Katsiotis et al., 1995). Despite the fact that such inconsistencies exist in opinion regarding the Endosperm Balance Number, the hypothesis has served as a useful measure toward the achievement of complex interspecific hybridizations involving various ploidy levels irrespective of the criterion relied on for selection (Hawkes & Jackson, 1992). Normal seeds have been obtained by some researchers without consideration for the Endosperm Balance Number (Katsiotis et al., 1995). The role of the histology of the endosperm in normal development must, therefore, be further investigated and better established.
2.1.5 Importance of the Polar-Nuclei Activation Hypothesis
The polar-nuclei activation hypothesis establishes the linkage of endosperm development to the activation of the two polar nuclei by fusion with a compatible male nucleus. The hypothesis is, therefore, dependent on the intensity of the activating influence of the male nucleus and the reactive response of the female nucleus (Nishiyama et al., 1991; Nishiyama & Yabuno, 1978). The activation effect of the male nucleus is expressed as the activating value, and the reactive action of the female nucleus is expressed as the response value. Successful endosperm development or failure is closely linked to the difference in the Activation Index defined as the ratio of the activating value (AV) to the response value (RV). The physiological capability of the male nuclei to appropriately undergo mitotic divisions in the primary endosperm nucleus is dependent on a 2∶ 1 ratio between the AV and the RV (i.e., AV/2RV) (Nishiyama et al., 1991). The degree of endosperm development or failure is closely related to this ratio. Nishiyama and Yabuno (1978) recounted the achievement of crosses between various species of Avena, Triticum, and Aegilops based on the polar-nuclei activation hypothesis. Furthermore, in Brassica species, Nishiyama et al. (1991) estimated the relative activating value (AV) of diploid and tetraploid species to be, respectively, in the range 1.0–3.5 and 2.7–5.2. In addition, Nishiyama et al. (1991) found that hybridization based on the polar nuclei activation index of between 15% and 87% was effective, whereas crosses at activation index of less than 15% or more than 87% were incompatible.
2.1.6 Effects of Pre- or Post-Zygotic Barriers on Endosperm Development
Fertilization or zygotic barriers to endosperm development are broadly described as pre-zygotic (pre-fertilization) or post-zygotic (postfertilization). These barriers include factors such as pollen interactions with the pistil, hybrid zygote abnormality, and low hybrid fertility or sterility (Table 3). Lester and Kang (1998) described various postfertilization barriers to endosperm or seed development. Some of these barriers include embryo malformation or degeneration, endosperm and embryo death leading to abortion of ovules, early stage collapse of seeds during development, failure of hybridization between diploids, and their own autotetraploids as a result of embryo abortion. These barriers can be bypassed with the application of embryo rescue techniques (Okamoto & Ureshino, 2015; Sun et al., 2018). Appropriate pollinations or crosses within same species usually give rise to physiologically normal endosperm and embryo and, thus, result in viable seeds. Similarly, in some instances, no post-zygotic barriers arise in crosses between individuals of different species. In such cases also, the formation of normal zygotes or hybrids is achieved.
Nonetheless, instances arise where post hybrid zygote development and reproduction turn out unsuccessful. Gametes from different species can in some instances hybridize to produce hybrid zygotes, most of which turn out abnormal and never reach sexual maturity (Okamoto & Ureshino, 2015). Moreover, cases exist where reproduction is successful, and the resulting hybrids reach sexual maturity but are usually unable to reproduce. The reason is because an appreciable proportion of hybrid embryos turn out sterile and fail to produce viable gametes. In these examples, the reproduction of the different species fails because the offspring obtained are incapable of passing on their genes to the next generation. It is probable that the parents engaged in the hybridization have expended the energy involved in pollination and for producing hybrid offspring and yet end up with no transfer of their genetic materials to subsequent generations. The fitness of the hybrids is, therefore, considered to be zero due to the effect of zygotic barriers in reproduction.
3 Embryo Rescue Techniques and Essence of Application
An embryo in plants is a part of the seed that is formed after double fertilization and contains the preform of the plant organs. Embryo rescue and culture involve the nurturing of isolated defective hybrid embryos, under suitable sterile in vitro culture conditions in order to surmount the inhibiting effects of post-zygotic barriers on embryo initiation, growth, and development. Embryo rescue is, therefore, used to obtain fertile hybrid plants (Sahijram et al., 2013). Usually, the improper development of hybrid endosperm creates defective hybrid embryos from wide crosses. These failing embryos are saved from degenerating by isolating the embryo prior to its abortion and aseptically culturing the embryo under in vitro conditions. This strategy helps to circumvent the barriers that induce hybridization abortion (Konan et al., 2020; Okamoto & Ureshino, 2015; Sun et al., 2018).
In normal seed development, good physiological functioning of the embryo-nourishing tissue, the endosperm enables proper embryo development, which subsequently culminates into viable seeds. On the other hand, defective malfunctioning endosperm causes nutritional starvation which hinders proper embryo development and, consequently, gives rise to nonviable plants (Premjet et al., 2019; Sun et al., 2018; Okamotoan Ureshino, 2015). Frequently, endosperm resulting from crosses between two distant species or diploids and tetraploids hybridizations more often than not fail to properly develop and thus cause embryo abortion, degeneration, or nonviable embryos (Konan et al., 2020; Sun et al., 2018). Improper development of hybrid embryo usually causes flowers to abort and drop due to distinct physiological differences in the parental embryos (Konan et al., 2020; Sun et al., 2018; Okamotoan Ureshino, 2015). Furthermore, the release of toxic inhibitory metabolic substances from the hybrid endosperm is also known to hinder embryo growth (Okamoto & Ureshino, 2015; Sun et al., 2018). Physiologically, however, the otherwise defective hybrid embryos indeed have inherent ability to start growth and subsequently develop into viable seeds if aided. The embryo rescue and culture technique are, therefore, carried out to save and aid the recovery of defective hybrid embryos which hitherto would have aborted.
The embryo rescue and culture technique generally involves a careful aseptic isolation of immature or mature embryos without injuring the embryos. The isolated embryos are then cultured in an appropriate nutrient medium supplemented with suitable carbon and inorganic nitrogen sources. The embryo is subsequently nurtured under suitable in vitro temperature, light, humidity, and osmotic conditions to induce continued embryogenic growth and seedling development into viable plants (Fathi & Jahani, 2012) and, thereby, circumventing the hindering influence of hybridization barriers. The embryo culture approach has proven very useful in the rescue of embryos that would normally abort or fail to follow the normal progressive sequence of ontogeny. The most valuable use of the embryo rescue technology has been the success in the development of interspecific and intergeneric hybrid plants (Pratap et al., 2021; Kaminski et al., 2020; Yin et al., 2020).
Through embryo rescue, it has been practicable to obtain viable seeds by circumventing most of the physiological barriers arising through wide crosses. Embryo rescue is used to successfully recover crosses between diploids and tetraploid species (Fathi & Jahani, 2012; Pachakkil et al., 2019). Furthermore, embryo culture has also proven to be an effective technique for resynthesizing some plant hybrids. The technology has, therefore, been the most effective method of valuable gene transfer from wild species. In addition, zygotic or seed embryos from embryo rescue have often been used as explants to initiate, for instance, callus cultures for crop improvement (Debnath & Arigundam, 2020; Koltunow et al., 1996). Over the years, embryo rescue and culture have become an attractive and valuable in vitro tool for plant tissue culture and breeding. Embryo culture has enabled the rescue of embryos from interspecific and intergeneric wide crosses as well as the achievement of seedless triploid embryos and haploids production. Embryo culture has also made it possible to circumvent the effect of germination inhibitors and thereby overcome seed dormancy and shortening the breeding cycle in some plant species (Fathi & Jahani, 2012). Embryo rescue has aided in bypassing germination inhibitions, for example, dormancy or sterility characteristics that are linked to the first filial or F1 crossing generation in crop breeding (Pachakkil et al., 2019). The technique of embryo culture has also been effective in determining seed viability and development of plant variety from embryos that more often than not fail to fully develop naturally or the embryo aborts under the inhibiting influence of physiological factors.
3.1 Historical Notes on the Embryo Rescue and Culture Technology
Embryo rescue application began as early as the eighteenth century. This landmark in plant tissue culture is credited to Charles Bonnet who successfully regenerated whole plants from hybrid embryos obtained from crosses between Phaseolus and Fagopyrum. The procedure he carried out produced plants that were characteristically dwarf (Sharma et al., 1996). After that success by Charles Bonnet, many other plant scientists followed suit by culturing embryos in varied types of nutrient culture media. The period between 1890 and 1904 saw tremendous advancements in the efficiency of embryo rescue and culture techniques. Embryo culture became more systematic with the use of precise protocols of nutrient solutions supplemented with inorganic salts and carbon energy sources in the form of sugars and enhanced by aseptic manipulations (Amanate-Bordeos et al., 1992).
In 1904, Hanning became one of the first to succeed in obtaining viable plants in vitro from mature embryo culture (Hanning, 1904). He aseptically extracted mature embryos of two crucifers and cultured the embryos on a growth medium incorporated with minerals and sugar. Hanning (1904) described precocious germination tendencies in the embryos where he observed instantaneous initiation of growth in mature embryos, and thus, overcoming dormancy. The precocious germination characterized embryos developed into abnormal plantlets with small, weak architecture and nonviable (Mehetre & Aher, 2004). Subsequently, a successful culture of embryos of cherry by Tukey (1933) served as one of the very important advancements in the embryo rescue and culturing of fruit crops. Another authority in the applications of tissue culture whose work contributed greatly advanced the field was R. J. Gautheret. He was the first to obtain true plants from tissue cultures using cambial tissue of Acer pseudoplatanus (Gautheret, 1934, 1935). That feat at the time ushered in various different plant tissue culture procedures. Some of these culture methods include embryo culture, anther culture, pollen culture, shoot tip culture, root culture, and many others.
One of the early in vitro tissue culture methods that was effectively carried out to achieve efficient plant regeneration in crop improvement is embryo rescue. Yeung et al. (1981) indicated that embryo rescue is a useful tool for achieving a significant shortening in the breeding period by avoiding the delay that arises due to seed dormancy. In addition, embryo rescue is an appropriate approach to apply when the investigation of the endosperm and embryo germination involves a destructive analysis. Hu and Wang (1986) described several crosses that failed due to embryo abortion. Early embryo abortion is associated with failure of the endosperm to properly progress into physiological maturity or does not develop at all. The constraint of embryo failure or abortion is usually resolved by culturing the embryos in appropriate nutrient medium to aid the embryo bypass post-zygotic barriers within the parental plant. Many successful examples of embryo rescue assisted interspecific and intergeneric generated hybrid plants have been described (Kaminski et al., 2020; Bridgen, 1994). Li et al. (2014) presented an exceedingly efficient procedure for hybrid embryo rescue from wide crosses that gave rise to important enhancement in effective breeding for disease-resistant trait in seedless grapes. During the past decades, embryo rescue or culture has enabled better appreciation of the physiology of embryonic development.
Furthermore, embryo rescue has been a very useful tool for bypassing seed dormancy to significantly reduce the duration of breeding, assessing seed viability, enhancing micro propagation efficiency, and rescuing undeveloped hybrid embryos from mismatched hybridizations (Caruso et al., 2020; Uma et al., 2011). To date, embryo rescue is extensively applied routinely in several fruit crops, for varied objectives, for example, breeding for seedless fruits, triploid plants, and interspecific hybridization. Some of the fruit crops in which embryo rescue has been successfully applied include banana (Uma et al., 2011), citrus (Caruso et al., 2020), persimmon (Hu et al., 2013), and watermelon (Taskın et al., 2013).
3.2 Types of Embryo Culture Technique
Depending on the histological source from where the embryo explants were extracted for culture, two broad classes of embryo rescue techniques termed zygotic and somatic embryo culture are practiced. The embryo rescue technique is also classified as mature embryo culture and immature embryo culture based on the maturity level of the isolated explants. The mature embryo culture is used to circumvent seed dormancy in order to decrease duration of germination. Immature embryo culture on the other hand is carried out to achieve early embryo rescue. Practically, all the four broad types of embryo rescue or culture somewhat interconnect.
3.2.1 Mature Embryo Culture
Mature embryo rescue basically involves nurturing in vitro, the growth of mature embryos that are isolated out of ripe seeds. Mature embryo rescue or culture is carried out in instances where embryos fail to survive in vivo (Lentini et al., 2020). This in vitro procedure is also employed to remove restriction on seed germination, which causes seeds to stay dormant for protracted periods. In most plant species, seed dormancy is induced by chemical inhibitors in the embryonic tissue (Buteme et al., 2021). Another cause of seed dormancy is the effect of mechanical resistance created by structures casing the embryo. Some plant species also yield infertile seeds due to the defective physiological formation of embryos (Okamoto & Ureshino, 2015; Sun et al., 2018). Mature embryo rescue is done by surgically isolating the embryos that are autotrophic, out of the testa of the dormant seed that is at the stage of maturity. In seeds with hard coats, the seeds are first disinfected by sterilization and then soaked in sterile distilled water for an appropriate duration, which could be a few hours or even days. The soaked seeds are cut open and appropriately dissected to remove the embryos. The isolated embryos are then cultured using basal inorganic medium with sucrose incorporated as energy source in order to circumvent seed dormancy and enable germination (Lentini et al., 2020). Embryo rescue protocols may culminate in viable plants. Mature embryo culture is employed in instances where seed dormancy of the hybrids is protracted. In cases of poor survival of embryos in vivo, the mature embryo culture technique is a useful tool for deriving viable seedlings.
3.2.2 Immature Embryo Culture
Embryo culture involving immature explants is also referred to as embryo rescue. The embryo rescue approach is also carried out as pre- or post-germinal immature embryo culture. Pre-germinal embryo culture is usually performed to regenerate plantlets. On the other hand, post-germinal embryo culture is done to boost embryo growth and development after germination. The embryo rescue approach fundamentally involves in vitro nurturing of immature embryos in order to rescue hybrid embryos prone to failure that result from wide hybridizations (Ren et al., 2019). Immature embryo rescue also aids to regenerate plantlets, in instances where parents are seedless, or in cases of heavy premature fruit fall during the initial stages of embryo development. In addition, the method of immature embryo rescue is applied to save seedless triploid embryos. The technique has also proven very valuable in the creation of haploids, bypassing of seed dormancy, and estimating of seed viability (Lentini et al., 2020). Immature embryo rescue is frequently used to overcome embryo abortion in order to create viable hybrid plants. Improper functioning of the endosperm usually culminates in malnourishment of the failing embryo. Ren et al. (2019) established an efficient immature embryo rescue protocol for the improvement of the plant Ziziphus jujuba.
3.3 Factors That Influence the Success of Embryo Culture
3.3.1 Genotypic Background of Embryo
The genotype of the plant species involved in embryo rescue or culture is a key factor that determines the success or otherwise of the technique. In closely related or distant cultivars, embryos of some genotypes respond to in vitro culture conditions far more effectively and are easier to grow in culture than other genotypes whose culture in vitro is more daunting (Rangan, 1984). The ease of achievement of regeneration of whole physiologically normal plantlets through embryo culture, therefore, differs from genotype to genotype. Vidhanaarachchi et al. (2016) observed significant differences in in vitro culture germination response in embryos of different selected genotypes of coconut. Lu and Bridgen (1996) also reported significant effect of parental genotypes on embryo germination, callus, and shoot induction in interspecific hybridization of Alstroemeria.
3.3.2 Developmental Stage of the Isolated Embryo
The growth of immature embryos in culture is quite daunting in spite of the remarkable progress made and the successes attained in the application of embryo culture (Pen et al., 2018). In instances where the abortion or degeneration of the embryo sets in very early, embryo rescue turns out to be extremely difficult and most often unsuccessful. Practically, an important strategy used to achieve effective rescue of immature embryos has been to carry out a technique referred to as the embryo-nurse endosperm transplant (Sect. 15.5.2). In this procedure, the immature embryo of a species is isolated and placed in the endosperm of a different seed of the same species. For instance, a 30–40% survival rate was achieved with the implantation method in the hybridization of Hordeum x Secale, compared to one percent survival rate using the traditional approach of embryo rescue (Kruse, 1974). Invariably, the more mature the isolated embryo, the easier it is to culture in vitro and more likely to successfully achieve regeneration of physiologically normal plants.
3.3.3 Composition of the Nutrient Media
One of the essential prerequisites toward any successful embryo rescue undertaking is the choice of suitable in vitro culture medium to nurture orderly differentiation and development of cultured embryos. The formulation of the culture medium used is determined primarily by the nature of embryo culture which could be either pre-germinal or post-germinal (Kumari et al., 2018). Pre-germinal immature embryo culture is purposely for obtaining plantlets regeneration. In such instances, the embryos require a complex nutrient medium. On the other hand, the aim of post-germinal immature embryo culture is usually carried out to hasten the development of the embryo after germination. Comparatively, post-germinal embryo culture is attained with less complex medium which could be as simple as just sucrose or glucose solution. Nonetheless, for embryo rescue generally, the culture media composition of mineral salts, organic nutrients, or growth regulators may be an important consideration and useful for the efficient culture of embryos (Lentini et al., 2020; Li et al., 2014). The formulation of the culture medium must take cognizance of the developmental phase of the isolated embryo to be cultured. Embryos at a heterotrophic phase of development depend on the endosperm and the surrounding maternal tissues for nutrients, whereas autotrophic stage embryos metabolically produce growth substances required for development (Lentini et al., 2020; Li et al., 2014).
3.3.4 Growth Temperature and Light Conditions
Light and temperature are environmental factors that have been identified to be very important for the efficient culture of embryos (Narayanaswamy and Norstog, 1964). Embryo rescue protocols usually have integrated in them a step of incubating cultures the in dark for the first 1 to 2 weeks until embryos or calluses appear. The embryos are subsequently transferred to appropriate light and temperature conditions to enable the embryo to begin synthesizing chlorophyll (Kumari et al., 2018). Compared to intact seeds, it has been realized that isolated embryos frequently germinate, grow, and develop better in a wider temperature range. Narayanaswamy and Norstog (1964) explained that the plant species from which the embryo explant was taken influences the optimum temperature depending on the plant species from which the embryo was obtained. Nonetheless, normally a temperature range as high as 25–30 °C is used (Brits et al., 2015). Cultured embryos usually germinate in a wider temperature range than whole seeds. Meanwhile, embryos from plant species such as Allium require a lower temperature of around 17 °C, whereas in some other plant species, cold treatment of 4 °C is usually essential for the establishment and growth of embryos in in vitro culture. For example, the growth and development of embryos of sweet cherry were significantly enhanced when immature and mature embryos were subjected to 40 and 60 days of cold treatment, respectively (Hajmansoor et al., 2009).
3.4 Salient Considerations Involved in Embryo Rescue and Culture
3.4.1 Determination of Appropriate Embryo Stage for Rescue
Prior to carrying out embryo rescue, it is very useful to determine the physiological maturity stage of the embryos to be cultured. It is also helpful to know when abortion sets in for the particular plant species so that the dissection and isolation of the embryo can be carried out timely before the embryo degenerates or aborts. One of the recommended approaches for determining the stages of development of embryos is to make histological sections. Accurate establishment of the embryo development stage guides the appropriate medium formulation that is to be used (Haslam & Yeung, 2011). It is recommended that preliminary development of an efficient protocol for the selection of the right embryo development stage is very useful even though it could take appreciable time and some financial investment to achieve. However, such a tool, once in place, guides subsequent embryo isolation and collection at exactly the established most competent stage. This greatly enhances the success of the embryo rescue.
Another essential consideration in embryo culture is the condition under which the mother plant was growing prior to the isolation of the explant for culture initiation. The endosperm and the cotyledons have been found to develop more efficiently in cases where the mother plant was maintained under strictly controlled environments. Consequently, the embryo growth and development is well promoted. It has been established that the younger the embryo chosen for rescue, the more complex the nutritional formulation that is used to sustain its culture and growth. Maturing embryos are subsequently transferred to less complex inorganic salt culture media (Yeung et al., 2001). Yan et al. (2014), for instance, intimated that in cassava, the rescue of embryos should be initiated at immature stages before 38 DAP, the time when the highest proportion of embryos is at the cotyledonary stage with an already fully developed endosperm. At the cotyledonary stage, the embryos can be seen and are easier to dissect and isolate from pollinated ovules without any damage or injury to the embryos. After 38 DAP, the seeds become too hard, and high rate of injury is usually caused to the embryo during the excision and isolation procedure (Lentini et al., 2020; Yan et al., 2014).
3.4.2 Embryo Excision Techniques
More often than not embryos are found within the ovule which presents a sterile environment. For this reason, carrying out surface sterilization to make the isolated embryos aseptic is of no practical value. Instead, in most protocols, the more common practice is that the florets are removed and the ovules are isolated from the ovaries. This is followed by surface sterilization of whole ovules or ovaries. The embryo is normally effectively protected from the usually severe surface sterilization procedures, by the bordering tissues. In plant species such as corn (Zea mays L.), to assess the embryo, it is required that hard seed coats are broken. Furthermore, in some cases, endophytic pathogens may be present in the seed coats. For plants with such type of seeds, direct disinfection of embryos by sterilization is necessary in order to establish an aseptic culture. The surface sterilization procedure of the entire ovules or ovaries is followed by aseptic excision and isolation of embryos from the ovules or ovaries and surrounding tissues. The dissection and excision of large embryos pose little difficulty.
However, to isolate small embryos without injury, appropriate tools are used to carry out micro-excision and isolation procedure aided by a dissecting microscope. It is precautionary worthy to note that embryos are quite fragile when the seed coat is broken. In addition, another essential precaution is that isolated embryos must not be subjected to desiccation during in vitro culture (Rangan, 1984). The technique or protocol for efficient isolation of immature embryos is usually tailored specific for the particular plant species. However, quite often, incision of the immature ovule is targeted at the micropylar end, and with exertion of appropriate pressure at the other end, the embryo is released through the incision. In applying the pressure, caution must be exercised not to injure the delicate embryonic tissue. Hu and Wang (1986) indicated that in the isolation of heart-stage or immature embryos, it is essential that the suspensors are not damaged or impaired to boost the growth and development of the embryos in culture. For the rescue of mature embryo, usually isolated seeds that are physiologically mature are decontaminated or disinfected by surface sterilization prior to embryo isolation.
3.4.3 Media Manipulations for Efficient Embryo Culture
Raghavan (1966) described the two main stages of embryo development as heterotrophic and autotrophic. In the heterotrophic phase, the immature embryo relies primarily on the endosperm and the neighboring maternal tissues. During this phase, the young embryo requires a medium with more complicated media composition and osmotic potential that is higher than required for mature embryos. As the initially immature embryo further grows in culture, its efficient development is promoted by culturing it on complex media augmented with appropriate combinations of amino acids, growth hormones or regulators, and vitamins. In addition, the incorporation of plant extracts, for example, coconut milk, also helps embryo development. At the autotrophic phase, the embryo is physiologically more mature. During this stage, the embryo is metabolically able to synthesize most essential biomolecules needed to support its growth and development using available salts and sugar. The embryos at this stage are now capable of germination and development on basic inorganic medium augmented with as sucrose a carbon source.
The choice of the appropriate culture medium and growth conditions is some of the most essential considerations for a successful embryo rescue procedure. The application of suitable culture conditions enables effective physiological growth of the embryo, its maturation, and regeneration into whole plants. A key factor in a successful embryo rescue is the optimal composition of the culture medium used. The optimal medium nutrient composition depends on the embryo stage, and the medium constituents vary during the development process of the embryo. The two extensively used basal media are the Murashige and Skoog (1962) and Gamborg’s B5 medium (Gamborg et al., 1968), usually applied with some appropriate modifications to enhance efficient embryo culture growth. The required complexity or stringency of the media composition or growth conditions used is influenced by the level of maturity of the target embryo for rescue. In this regard, it has been successful to grow mature embryos using basal salt media with sucrose as an organic carbon source of energy. However, for immature embryos, in addition to the basal salt media and sucrose, different vitamins, amino acids, and growth regulators are incorporated in the culture medium. Varied compositions of mineral salts are incorporated in order to manipulate the growth of embryo cultures. In many protocols of embryo rescue and culture, amino acids and amino acid complexes such as casein hydrolysate as well as the vitamins, biotin, thiamine, pantothenic acid, and nicotinic have been widely used as additives in culture media to promote the development of the embryo. The various amino acids are also important components of culture media. Generally, the use of malic acid and trace amounts of organic nitrogen, for example, asparagine, glutamine, or casein hydrolysate, have often been observed to be useful. Pawar et al. (2015) reported that proline and glutamine improve in vitro callus induction and subsequent shooting in rice.
Sucrose is also a very important component in embryo culture media and serves two main purposes. The primary energy source in culture media is sucrose. Another important role of sucrose is that it stabilizes and maintains appropriate osmotic potential of the culture medium. Usually, mature embryos and immature embryos are cultured on media with 2–3% sucrose concentration. Immature embryos are commonly cultured on media with high sucrose concentration between 8% and 12%, which in principle mimics the high osmotic potential of the embryo sac’s intracellular environment and conditions. It has been found that generally, the more immature the isolated embryo, the higher the osmolarity condition required in the culture medium to promote growth and development of the embryo. Reinert et al. (1977) observed that high osmolarity inhibits precocious germination and prevents dividing cells from switching into a state of elongation. Moreover, the high osmotic environment together with the addition of hormones such as auxin and cytokinin in moderate amounts promotes the development of heart-stage embryos (Din et al., 2016). Mature embryos have been found to grow well on semisolid medium supplemented with only Knop’s mineral salts and 2–5% sucrose. Generally, the most effective sources of inorganic N in media for embryo cultures have been ammonium nitrate and potassium nitrate. Essentially, ammonium boosts appropriate growth and differentiation of immature embryos in culture (Umbeck & Norstog, 1979). Ammonium is often incorporated together with mainly malate or citrate anions as a source of organic acid. The use of natural plant extracts as media constituents has also been found useful in attaining greater recovery of growth and development of embryos. The commonly used natural extract from plants is coconut milk. Similarly, tomato juice and extracts of banana are also useful in culture media.
The incorporation of plant growth regulators in embryo culture media generally plays significant role in boosting embryo growth and development (Ming et al., 2019). However, it must be noted that high concentrations of exogenous auxins appear to induce inhibitory effect on plant embryo growth in vitro (Din et al., 2016; Manzur et al., 2014). Therefore, the induction of somatic embryo is better stimulated rather by low concentrations of exogenous auxin in the culture media. Exogenous use of growth regulators such as kinetin (Kin), benzyl-amino-purine (BAP), and naphthalene acetic acid (NAA) or thidiazuron (TDZ) improved regeneration frequency (Din et al., 2016). Similarly, high levels of cytokinins, for example, zeatin, have been found to only slightly promote young embryo growth, when used as the sole media hormone additive (Manzur et al., 2014). Auxins and cytokinins are, therefore, not generally used for embryo culture except in cases where the aim is to induce callus. However, some cytokinins and auxins combinations show better performance in boosting the growth and differentiation of embryos (Din et al., 2016). It has been found that hormones induce plant architectural abnormalities when included in an embryo culture media. For example, gibberellins in some cases stimulate precocious germination (White et al., 2000). Therefore, the use of hormones in media for embryo cultures is generally avoided or used with precaution.
Commonly, in the preparation of embryo rescue media, agar at 0.5–1.5% is used as the main solidifying agent (Hu & Wang, 1986). Higher concentrations of agar hold less water and likely presence of higher contaminating salts. The use of higher concentrations of agar is, therefore, not recommended because that could inhibit growth. Alternatively, Pinto et al. (1994) successfully used a vermiculite support system for small embryos obtained at fruit maturity. As earlier explained in Sect. 3.4, light and temperature conditions are very essential considerations in the technique of embryo culture. Light regulates cell division and rate of ethylene emission, a factor that affects caulogenesis—shoot initiation and rhizogenesis—root initiation. Timing and duration of exposure of explants to light play a vital role in the morphogenesis of embryos. In most species, the embryo stops growing between one and two weeks after inoculation. The embryos are then moved on to another medium with regular sucrose concentration as well as low levels of auxin and cytokinin in order to cause the embryo to regain growth and direct shoot initiation. In cases where the embryo fails to grow shoots directly, callus induction is performed followed by shoot induction and then eventually the development of plantlets in vitro. The plantlets are weaned on soil treated to become sterile and nurtured to develop under greenhouse conditions.
3.5 Major Embryo Rescue and Culture Procedures
3.5.1 Rescue and Culture of Embryos
Usually, hybrid embryos obtained from interspecific and intergeneric crosses turn out to be defective and more often abort at a point during development or yield nonviable seeds (Araujo et al., 2021; Hristova-Cherbadzhi, 2020). Embryo rescue is used to resolve this constraint of failure in embryo development or abortion following hybridization. Embryo culture serves as a tool of great value to plant breeding, particularly in interspecific hybridization (Hristova-Cherbadzhi, 2020). The technique of embryo culture serves as a very effective and useful means of creating normal hybrid plants and producing viable seeds. Usually, seeds of fruits obtained from controlled pollination of plants are collected at an appropriate stage in order to avoid the period embryo abortion is known to set in. The embryo rescue procedure is achieved by the excision of the embryos from the harvested seeds and placing the embryos directly onto an appropriate culture medium (Buteme et al., 2021; Kuang et al., 2021).
Embryo culture is undertaken in various forms to achieve different objectives. The seeds of some plant species, for example, orchids, lack nutritious tissues and are without plumule and radicle. In such irregular type of seeds, the strategy has been to culture whole seeds with intact undifferentiated embryos. In a similar technique, intact mature embryo culture and manipulations are carried out to enhance embryonic growth and to track the metabolic and biochemical interactions involved in overcoming seed dormancy and inducing germination. Moreover, instead of directly culturing the intact mature embryo, the embryo could be surgically excised into various segments and cultured in vitro on suitable media to enable the monitoring of the physiological processes that come to play as well as the growth of the different parts of the mature embryo (Buteme et al., 2021; Konan et al., 2020). In addition, the culture of immature embryos is also a common procedure that involves mainly in vitro culture of globular or heart-shaped embryo development phases in appropriate nutrient medium to enable the differentiation and progressive development of embryos (Buteme et al., 2021. Furthermore, in species such as lemons or oranges, embryos that result from nuclear tissue are more often defective and abortive. This challenge is overcome by culturing the embryos under artificial conditions and manipulated to attain clonal propagation. In such approach, adventitious embryos are in vitro cultured from polyembryonic seeds.
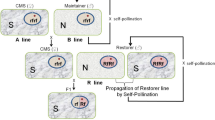
3.5.2 Embryo-Nurse Endosperm Transplant Method
It is often practically daunting to isolate defective immature or very tiny hybrid embryos that abort at the initial stages of growth. Besides, the initiation of such very small hybrid embryos for growth in vitro is quite challenging. Therefore, usually in handling very tiny or immature hybrid embryos, a specialized technique referred to as the embryo-nurse endosperm transplant method has often been used to improve success of the embryo culture (Shukla, 2016). In other instances, the hybrid embryo may be physiologically normal; however, the ovule may have a defective or immature endosperm which fails to serve as an important source of nutrition to the hybrid embryo. The embryo-nurse endosperm transplant technique involves a combination of a hybrid embryo resulting from a hybridization that is not compatible and a normal endosperm developed from a cross of related plant species that is compatible (Widiez et al., 2017). In this process, usually the small or very immature hybrid embryo is surgically inserted into an endosperm that has been extracted from a normal ovule of one of the parents crossed to produce the hybrid embryo, or alternatively, the normal endosperm is obtained from a different species (Shukla, 2016). The endosperm transplant technique is used mainly for rescuing immature embryos.
Basically, a hybrid embryo is excised from an ovule that is enclosed by an endosperm whose development has failed and degenerating (Fig. 2a). Next, from a normally developed endosperm, the ovule is dissected, and the normal embryo is taken out. This procedure creates a normal endosperm with an exit hole. The hybrid embryo is passed through the exit and placed into the endosperm (Fig. 2b). This procedure results in embryo-endosperm transplant that is subsequently transferred together and cultured on an appropriate in vitro medium (Williams et al., 1982). Typically, the embryo of an interspecific hybridization can be transplanted by inserting into an endosperm arising from an intraspecific hybridization that involves one of the parental species. Many interspecific and intergeneric plants have been obtained using the technique of embryo-endosperm transplant. Furthermore, modifications of the nurse endosperm technique, for example, embryo implantation or embryo transplantation, are employed in many different plant species. Embryo rescue via embryo-nurse endosperm transplants could enable about 30% recovery in wide hybridizations, compared to instances where the technique is not deployed (Shukla, 2016).
Steps involved in the embryo-nurse endosperm transplant technique. (a) Isolation of hybrid embryo from ovule with defective endosperm resulting from wide hybridization as well the isolation of normal embryo from normal ovule of foster plant species with physiologically functional endosperm. (b) Transplant of hybrid embryo into the normal ovule with physiologically normal endosperm and generation of hybrid plant through in vitro culture
3.5.3 In Vitro Ovary Culture
Ovary culture entails culturing the entire ovary in a culture medium. In an ovary culture procedure, ovaries are collected by isolating the ovaries followed by the removal of any remaining flower parts (Li et al., 2020). Pollinated ovaries are extracted by the removal of calyx, corolla, and stamens. The ovaries are then disinfested by surface sterilization to eliminate contaminants cautiously by avoiding damage to the ovaries. The ovaries are subsequently inoculated in culture medium and oriented such that the cut pedicle section is positioned direct contact with the culture nutrient medium (Ramming et al., 2003). The ovary culture is nurtured by varying in vitro conditions and monitoring the progress of culture growth toward eventually producing plants that will bear fully developed fruits with viable seeds. Typically, ovule culture involves surgically opening disinfected ovary to release the ovule. Comparatively, there is higher success rate of obtaining hybrid plants from the culture of ovary or ovule than from embryo culture (Lentini et al., 2020). The reason may likely be due to nutritional disparities and physical causes such as the protective influence of maternal or sporophytic tissues on the embryo.
3.5.4 Ovule Culture Technique
Ovule culture is an approach in which the entire ovule containing the ovaries is excised and placed onto an appropriate culture medium (Li et al., 2020). The advantage of this approach is that the likely damage to the embryo that may arise during the excision of the embryo is avoided (Lentini et al., 2020). Generally, ovule culture is either supported on filter paper and vermiculite support systems. In the filter paper technique, the ovule is cultured using filter papers positioned over liquid medium (Ramming et al., 2003). On the other hand, the vermiculite support method is carried out by placing in an orientation such that the micropylar section of the ovule is placed down making contact with a sterile vermiculite support.
3.5.5 Ovary and Ovule Slice or Perforation Procedure
In the ovary slice culture technique, transverse sections of ovaries are cut with a sterilized scalpel. The basal cut end of the sections is then placed in direct contact with the culture medium (Shukla, 2016). The ovary-slice culture technique is considered more efficient, less laborious, and takes less time than ovary culture or ovule culture. In the ovule perforation method, tiny holes are carefully created in the ovule using needles without damaging the embryos. The ovule perforation procedure is usually performed just before the ovule is placed on the culture medium. The tiny perforations enhance increased water and nutrient permeability and uptake by the ovule, thus stimulating embryo development (Pinto et al., 1994). It has been observed that surgically slicing the ovule also boosts embryo growth, probably because such a procedure enables better culture medium and embryo nutrient exchange.
3.6 Applications of the Embryo Rescue Technique
3.6.1 Overcoming Seed Dormancy
Embryo rescue and culture serves as a very important strategic approach for overcoming seed dormancy (Pramanik et al., 2021). Seeds of several plant types undergo conventional dormancy where seed germination is inhibited by some hormones in seeds containing the embryo. In some plant species, the seeds remain dormant for a very long period without initiating germination. Some seeds may, however, germinate extremely slowly, or in some cases, the seeds fail completely to germinate even in normal conditions. A number of factors have been implicated in diverse plant species to be responsible for inducing seed dormancy. Some of these seed dormancy-inducing factors include influence of endogenous inhibitors, specific temperature, humidity, or light requirements during seed storage and state of embryo maturity (Brits et al., 2015). Seed dormancy-causing factors particularly endogenous inhibitors of seed germination, for example, certain plant hormones, may be contained within the seed coat, the endosperm, or present in both locations.
The embryo rescue and culture strategy for bypassing dormancy, therefore, involves the exclusion of the dormant embryos from the effects of the germination inhibitors in order for the embryos to sprout and develop rapidly. The embryo rescue protocol for inducing germination of dormant embryos is usually formulated to provide the appropriate culture media composition, growth hormone combinations and culture temperature, and light or humidity environment to enable embryo germination and proper growth (Mohapatra & Rout, 2005). Burgos and Ledbetter (1993) employed embryo rescue and culture successfully in an apricot to obtain higher proportion of seedlings. Similar result was achieved by Balla and Brozik (1993) to circumvent seed dormancy in sweet cherry. Efficient protocols have been established for successful embryo rescue in several plant species.
3.6.2 Shortening of the Breeding Cycle in Plants
In some plant species, the embryo requires sufficient time to reach physiological maturity in order to break seed dormancy which in some species could be so long as to cause significant extension in the breeding cycle. Generally, seedlings fail to develop immediately after fruit ripening. Examples of crops in which the seeds do not germinate soon after fruit ripening include apples and oil palm. In such fruit crops, the embryo rescue approach has been used to reduce the breeding period by circumventing germination seed dormancy. Dormancy-induced delay in germination has been significantly reduced and shortened the breeding cycle from years to a few months by extracting the embryos out of the control of dormancy-inducing factors which are localized in the seed coat and endosperm, or both. Embryo rescue has been the most effective practical approach to freeing the embryos from dormancy-inducing factors in various horticultural crops. Removal and in vitro nurturing of immature embryos on appropriate culture medium enable germination in a short time and, therefore, reduce the breeding cycle (Fathi & Jahani, 2012). Shortening the breeding cycle enables the plant breeder to obtain many more generations of a crop per year.
Embryo rescue has been a very useful technique for shortening the breeding cycles in apple by achieving good germination via reduction in the duration of seed dormancy. The technology of rescuing embryos in culture has also aided a very effective and efficient increase in the germination rate of mature seeds in ripening fruits such as sweet cherry by between 30% and 60% (Fathi et al., 2002). Similarly, Tamaki et al. (2011) succeeded in shortening the duration of the breeding cycle of Carica papaya varieties by roughly three months, assisted by embryo rescue and culture breeding techniques.
3.6.3 Overcoming Embryo Abortion
Embryo abortion arises mainly through the malformation of the endosperm of the seed to properly develop into a physiologically normal nutritive tissue around the embryo (Berger et al., 2006). Embryo abortion is a major constraint that limits the effectiveness of conventional plant breeding and improvement in some plant species. This challenge to conventional breeding work exists because, more often than not, interspecific and intergeneric hybridization of diploids versus tetraploids results in endosperms that usually develop defectively or not at all. In this regard, embryo abortion is prevented by the application of the embryo rescue and culture approach to generate whole plants using in vitro culture protocols (Reed, 2005). The embryo rescue method has been very valuable in conventional breeding and crop improvement, in the effective rescue of young embryos resulting from intraspecific and intergeneric hybrids that usually yield seeds that are not viable (Table 4).
Generally, post-zygotic barriers such as developmental failure of defective endosperm are effectively circumvented by extracting the embryos out of the ovule and nursing them aseptically in culture on appropriate nutrient medium to develop and grow into whole physiologically normal plants. Yang et al. (2007) rescued triploid hybrid embryos from intraspecific hybridization in grape varieties using in vitro culture. Similarly, Guo et al. (2011) also used rescue of hybrid embryos in obtaining triploid grapes from the hybridization of diploid and tetraploid varieties. Furthermore, via embryo rescue procedures and manipulations, Zhiwu et al. (2009) obtained plants from young triploid hybrid embryos developed from intraspecific hybridization involving diploid and tetraploid crosses in daylily (Hemerocallis). In seedless mandarin oranges, embryo rescue and culture techniques were used to achieve the production of triploid plants (Aleza et al., 2012). Peach, cherry, apricot, and plum commonly yield nonviable seeds, and particularly, the early ripening varieties have been found to often fail to germinate even when exposed to natural or favorable conditions. Seed sterility which is usually caused by incomplete embryo development in the seeds of these crop varieties is often resolved by employing the technique of embryo rescue and culture to assist in germination and plant regeneration (Bohra et al., 2016).
3.6.4 Development of Plants in Seedless Varieties
In many seedless varieties, for example, grapes, the embryo ceases to develop postfertilization leading to failure of physiologically normal seed formation, a phenomenon known as stenospermocarpy (Picarella & Mazzucato, 2019). The constraints associated with stenospermocarpy render conventional breeding methods inefficient in the improvement of seedless varieties (Costantini et al., 2021; Picarella & Mazzucato, 2019). The technique of in ovulo embryo rescue was, therefore, developed and widely applied to rescue naturally immature, weak, or defective aborting embryos, with the ultimate aim of producing progeny from the hybridization of seedless parents. Typically, in ovulo embryo rescue is carried out by aseptically isolating the defective embryos surgically from ovules and manipulating the in vitro culture media and conditions until eventually plantlets are formed. However, in some cases, it has been practically very difficult to isolate the embryos out of the ovules. In such instances, the whole ovule containing the embryo is cultured (Sharma et al., 1996).
Seedless varieties in some crops are developed mainly via parthenocarpy and stenospermocarpy (Costantini et al., 2021; Picarella & Mazzucato, 2019; Pratt, 1971). In grapes, the large berried seedless ones are developed more often by manipulating stenospermocarpy and less from parthenocarpy (Costantini et al., 2021; Picarella & Mazzucato, 2019; Stout, 1936). Cain et al. (1983) were the first to employ embryo rescue in seedless grapes development. Later, the efficiency of the method was optimized for application in other crops (Kumari et al., 2018; Singh et al., 2011). It is now quite routine to grow rescued embryos into whole plants. Improvement in seedless lime has also been achieved with embryo rescue and culture techniques (Prasad et al., 1996). Embryo rescue has also enabled the generation of plants from triploid embryos obtained from diploids crossed with tetraploids of the same plant species.
3.6.5 In Vitro Vegetative Propagation of Plants
Embryo culture has also proven very valuable in vegetative propagation of plants. The embryos of some plant genera exhibit both juvenile and mature physiological characteristics. The embryos of such genera are usually used as initiation explants for vegetative propagation (Naing et al., 2019). The juvenile state of these embryos is more practically exploited because the embryos in this state are most responsive to manipulations for efficient vegetative propagation (Debnath & Arigundam, 2020). For instance, in the Poaceae, compared to mature callus tissue, juvenile callus gives rise to organogenesis easier. A similar observation was made in the propagation of conifers using immature calli produced via young embryos (Bornman, 2002). In this example also, comparatively, axillary shoot generation was found to be easier with juvenile calli. The major challenge associated with this approach, however, is that the resulting clones are more often not derived out of zygotic materials. Nonetheless, in cases where embryos form from nucellar tissue, as observed in citrus, zygotic embryos could serve as the basis for generated clones (Koltunow et al., 1996).
3.6.6 Germplasm Conservation: Preservation of Embryos and Regrowth
Somatic embryos are a very convenient form of tissues for medium- and long-term in vitro conservation, preservation, and micropropagation (Danso & Elegba, 2017; Danso & Ford-Lloyd, 2002). Generally, in vitro conservation of embryos is achieved by inducing growth reduction in the tissues in order for the embryo to enter and stay in a dormant state for a period. Growth reduction for medium-term conservation is carried out via manipulating the growth temperature, humidity, and in vitro culture medium conditions (Cruz-Cruz et al., 2013). However, in long-term embryo preservation and conservation, reduction in the rate of embryo growth is achieved at very low temperature by storing the embryos in liquid nitrogen at −196 °C, a technique termed cryopreservation (Danso & Ford-Lloyd, 2002; Jaisankar et al., 2018). The successive actions of the process of cryopreservation of embryos are carried out under precise conditions which are usually determined for each type of material to be preserved and conserved. For the practical use of preserved embryos, after the required medium- or long-term period of storage, the preserved embryos are regrown into whole plants (Shukla, 2016).
In direct regrowth of the stored embryos in a nursery or through direct planting, the culture medium is manipulated essentially to bypass any secondary callogenesis or embryogenesis or both. The ability of the preserved embryos to grow into physiologically normal plants is dependent on the embryo size and the maturity of the mother plants that donated the preserved embryos (Tessereau et al., 1994). Protocols and procedures of cryopreservation have been established for many crops of food, medicinal, and industrial value (Cruz-Cruz et al., 2013; Reed, 2011). One of the pioneering extensive uses of cryopreservation involved the conservation of somatic embryos of oil palm (Palanyandy et al., 2020). In coconuts, it is very cumbersome to transport whole nuts due to the weight and requirement for huge cargo space on flights or ships. It is, therefore, mandatory to internationally exchange germplasm of coconut in the form of embryo cultures or embryos containing endosperm plugs (Lédo et al., 2017). Besides, embryos serve as disease free and safest materials for cryogenic storage and preservation. Development of seedlings from in vitro embryo culture prevents pests and diseases introduction and spread (Tegen, 2016). It has been possible to directly regrow frozen-thawed embryos into carrot and coffee plantlets. Large-sized cryopreserved embryos of some plants usually do not survive cryopreservation; however, Tessereau et al. (1994) found that large carrot embryos could survive cryopreservation. Utami et al. (2017) reported an efficient embryo rescue or culture procedure for obtaining plantlets in the medicinal orchid (Dendrobium lasianthera) using mature seed culture.
3.6.7 Homozygous Monoploid Production
Embryo culture is a useful tool for creating haploids via chromosome elimination after wide hybridization has been carried out. Usually, in some instances, fertilization takes place, but the chromosomes of the pollen-donating parent are later in the process, removed by the seed parent (Dresselhaus et al., 2016). Such cases give rise largely to nonviable haploid embryos. Viable haploid embryos and haploid plants production are, therefore, achieved through the in vitro rescue of haploid maternal embryos that have the paternal chromosomes removed (Seguí-Simarro et al., 2021). Subsequently, the maternal chromosomes are doubled using colchicine treatment of the rescued embryos to create homozygous monoploid embryos which eventually develop into monoploid plants (Chase, 1969; Mehetre & Thombre, 1980). Monoploid embryo induction and plant regeneration serve as a very useful tool in plant breeding. Monoploids are more often than not obtained through the manipulation of mainly embryo and anther cultures (Chaikam et al., 2019). Monoploids are considered to present valuable advantages and serve as one of the best materials for breeding-related studies and crop improvement (Hooghvorst et al., 2020).
4 Conclusion
The embryo rescue technology presents a huge potential for the generation of interspecific and intergeneric hybrids with desired traits. Various salient aspects of embryo rescue technique in plant breeding and improvement through wide hybridizations have been elucidated. In addition, some of the current successful achievements in the improvement of agronomic traits using embryo rescue or culture emphasize the usefulness of the technique. This informative review will serve as a valuable resource that will enable a better understanding and more effective use of the embryo rescue technology to quicken the development of superior-performing plants and boost the sustenance of food and nutritional security.
References
Aleza, P., Juárez, J., Cuenca, J., et al. (2012). Extensive citrus triploid hybrid production by 2x × 4x sexual hybridizations and parent–effect on the length of the juvenile phase. Plant Cell Reports, 31(9), 1723–1735.
Amanate-Bordeos, A. D., Nelson, R. J., Oliva, N. P., Dalmacio, R. D., Leung, H., & Sitch, L. A. (1992). Transfer of blast and bacterial blight resistance from the tetraploid wild rice Oryza minuta to the cultivated rice, O. sativa. Theoretical and Applied Genetics, 84, 345–354.
Araujo, A. O. D., Peixoto, M., Souza, C. N. D., Gasparino, E. C., Faria, J. T., & Lombello, R. A. (2021). A natural intergeneric hybrid of Gesneriaceae from Brazil. Phytotaxa, 497(2), 20. https://doi.org/10.11646/phytotaxa.497.2.2
Baguma, J. K., Mukasa, S. B., Kawuki, R., Tugume, A. K., Buttibwa, M., et al. (2019). Fruit set and plant regeneration in cassava following interspecific pollination with castor bean. African Crop Science Journal, 27(1), 99–118.
Balla, I., & Brozik, S. (1993). Embryo culture of sweet cherry hybrids (pp. 385–386). In II International Cherry Symposium.
Ballesfin, M. L. E., Vinarao, R. B., Sapin, J., Kim, S. R., & Jena, K. K. (2018). Development of an intergeneric hybrid between Oryza sativa L. and Leersia perrieri (A. Camus) Launert. Breeding Science, 68(4), 474–480. https://doi.org/10.1270/jsbbs.18045. Epub. PMID: 30369822; PMCID: PMC6198897.
Berger, F., Grini, P. E., & Schnittger, A. (2006). Endosperm: An integrator of seed growth and development. Current Opinion in Plant Biology, 9(6), 664–670. https://doi.org/10.1016/j.pbi.2006.09.015
Bhatia, D., Joshi, S., Das, A., Vikal, Y., Sahi, G. K., Neelam, K., Kaur, K., & Singh, K. (2017). Introgression of yield component traits in rice (Oryza sativa ssp. indica) through interspecific hybridization. Crop Science. https://doi.org/10.2135/cropsci2015.11.0693
Bohra, A., Jha, U. C., Adhimoolam, P., Bisht, D., & Singh, N. P. (2016). Cytoplasmic male sterility (CMS) in hybrid breeding in field crops. Plant Cell Reports, 35(5), 967–993. https://doi.org/10.1007/s00299-016-1949-3
Bornman, C. H. (2002). Somatic seed in conifer biotechnology — A viable alternative to natural seed? South African Journal of Botany, 68, 119–126.
Bridgen, M. P. (1994). A review of plant embryo culture. Horticultural Science, 29, 1243–1246.
Brits, G. J., Brown, N. A. C., Calitz, F. J., & Van Staden, J. (2015). Effects of storage under low temperature, room temperature and in the soil on viability and vigour of Leucospermum cordifolium (Proteaceae) seeds. South African Journal of Botany, 97, 1–8. https://doi.org/10.1016/j.sajb.2014.11.003
Burgos, L., & Ledbetter, C. A. (1993). Improved efficiency in apricot breeding: Effects of embryo development and nutrient media on in vitro germination and seedling establishment. Plant Cell, Tissue and Organ Culture, 35(3), 217–222.
Buteme, R., Nakajiri, M., Kucel, N., Kabod, P. N., Sseremba, G., & Kizito, E. B. (2021). Intraspecific crossability and compatibility within Solanum aethiopicum. Heliyon, 7(7), e07645. https://doi.org/10.1016/j.heliyon.2021.e07645
Cabezas, D., de Bem, O. I., Acker, M., Lyrene, P., & Munoz, P. R. (2021). Evaluating wild germplasm introgression into autotetraploid blueberry. Agronomy, 11, 614. https://doi.org/10.3390/agronomy11040614
Cain, D. W., Emershad, R. L., & Tarailo, R. E. (1983). In-ovulo embryo culture and seedling development of seeded and seedless grapes (Vitis vinifera L.). Vitis, 22, 9–14.
Carputo, D., Monti, L., Werner, J. E., & Frusciante, L. (1999). Uses and usefulness of endosperm balance number. Theoretical and Applied Genetics, 98, 478–484.
Caruso, M., Smith, M. W., Froelicher, Y., Russo, G., & Gmitter, F. G., Jr. (2020). Traditional breeding. In M. Talon, M. Caruso, & F. G. Gmitter (Eds.), The genus citrus (1st ed., pp. 129–148). Elsevier.
Chaikam, V., Molenaar, W., Melchinger, A. E., et al. (2019). Doubled haploid technology for line development in maize: Technical advances and prospects. Theoretical and Applied Genetics, 132, 3227–3243. https://doi.org/10.1007/s00122-019-03433-x
Chase, S. S. (1969). Monoploids and monoploid-derivatives of maize (Zea mays L.). The Botanical Review, 35, 117–168. https://doi.org/10.1007/BF02858912
Chen, Q., Chen, B., Lu, H., Zhou, H., & Mei, Z. (2016). Innovation on distant hybridization of saline-tolerant mud flat Spartina and Rice germplasm. Asian Agricultural Research, 08(11), 87–94. https://doi.org/10.22004/ag.econ.253344
Costantini, L., Moreno-Sanz, P., Nwafor, C. C., et al. (2021). Somatic variants for seed and fruit set in grapevine. BMC Plant Biology, 21, 135. https://doi.org/10.1186/s12870-021-02865-2
Cruz-Cruz, C. A., González-Arnao, M. T., & Engelmann, F. (2013). Biotechnology and conservation of plant biodiversity. Resources, 2, 73–95. https://doi.org/10.3390/resources2020073
Danso, K., & Elegba, W. (2017). Optimization of somatic embryogenesis in cassava. Biotechnologies for Plant Mutation Breeding, 73–89. https://doi.org/10.1007/978-3-319-45021-6_5
Danso, K., & Ford-Lloyd, B. (2002). Induction of high-frequency somatic embryos in cassava for cryopreservation. Plant Cell Reports, 21, 226–232. https://doi.org/10.1007/s00299-002-0516-2
Debnath, S. C., & Arigundam, U. (2020). In vitro propagation strategies of medicinally important berry crop, lingonberry (Vaccinium vitis-idaea L.). Agronomy, 10, 744. https://doi.org/10.3390/agronomy10050744
Deng, Y., Sun, X., Gu, C., Jia, X., Liang, L., & Su, J. (2017). Identification of pre-fertilization reproductive barriers and the underlying cytological mechanism in crosses among three petal-types of Jasminum sambac and their relevance to phylogenetic relationships. PLoS One, 12(4), e0176026. https://doi.org/10.1371/journal.pone.0176026
Din, M. A. R. J., Ahmad, I. F., Wagiran, A., Samad, A. A., Rahmat, Z., & Sarmidi, M. R. (2016). Improvement of efficient in vitro regeneration potential of mature callus induced from Malaysian upland rice seed (Oryza sativa cv. Panderas). Saudi Journal of Biological Sciences, 23, S69–S77. https://doi.org/10.1016/j.sjbs.2015.10.022
Dresselhaus, T., Sprunck, S., & Wessel, G. M. (2016). Fertilization mechanisms in flowering plants. Current Biology, 26(3), 125–139. https://doi.org/10.1016/j.cub.2015.12.032
Dziasek, K., Simon, L., Lafon-Placette, C., Laenen, B., Wärdig, C., Santos-González, J., et al. (2021). Hybrid seed incompatibility in Capsella is connected to chromatin condensation defects in the endosperm. PLoS Genetics, 17(2), e1009370. https://doi.org/10.1371/journal.pgen.1009370
Fathi, H., & Jahani, U. (2012). Review of embryo culture in fruit trees. Annals of Biological Research, 3(9), 4276–4281.
Fathi, H., Arzani, K., Khalighi, A., & Ebadi, A. (2002). The application of embryo culture in sweet cherry (Prunus avium L.) breeding. Abstract Book of the Third Iranian Horticultural Congress. 31 August – 2 September, 355 pp.
Gamborg, O. L., Miller, R. A., & Ojima, K. (1968). Nutrient requirements of suspension cultures of soybean root cells. Experimental Cell Research, 50(1), 151–158. https://doi.org/10.1016/0014-4827(68)90403-5. PMID 5650857.
Gautheret, R. J. (1934). Culture de tissue cambial. C. R. Hebd. Seances Acad. Sc, 198, 2195–2196.
Gautheret, R. J. (1935). Recherches sur la culture des tissues végétaux. Ph. D. Thesis.
Guo, Y., Zhao, Y., Li, K., et al. (2011). Embryo rescue of crosses between diploid and tetraploid grape cultivars and production of triploid plants. African Journal of Biotechnology, 10(82), 19005–19010.
Hajmansoor, S., Bihamta, M. R., Nabipour, A., Mohammadi, A., Pirseyedi, S. M., & Nikkhah, H. R. (2009). Genetic diversity in barley genotypes: I. Seed storage proteins (hordeins) and agronomic traits. Seed and Plant Improvement Journal, 25(4), 51–64.
Han, C., Zhang, P., Ryan, P. R., Rathjen, T. M., Yan, Z., & Delhaize, E. (2016). Introgression of genes from bread wheat enhances the aluminium tolerance of durum wheat. Theoretical and Applied Genetics, 129, 729–739. https://doi.org/10.1007/s00122-015-2661-3
Hannig, E. (1904). Zur Physiologie Pflanzicher Embryonen I. Ueber die Cultur von Cruciferen-Embryonen ausserhalb des Embryosacks. Bot Ztg, 62, 45–80.
Haslam, T. M., & Yeung, E. C. (2011). Zygotic embryo culture: An overview. In T. A. Thorpe & E. C. Yeung (Eds.), Plant embryo culture: Methods and protocols (pp. 3–15). Springer. https://doi.org/10.1007/978-1-61737-988-8_1
Hawkes, J. G., & Jackson, M. T. (1992). Taxonomic and evolutionary implications of the endosperm balance number hypothesis in potatoes. Theoretical and Applied Genetics, 84, 180–185.
Heslop-Harrison, J. S., & Schwarzacher, T. (2011). Organization of the plant genome in chromosomes. The Plant Journal, 66, 18–33. https://doi.org/10.1111/j.1365-313X.2011.04544.x
Hooghvorst, I., Torrico, O., Hooghvorst, S., & Nogués, S. (2020). In situ parthenogenetic doubled haploid production in melon “Piel de Sapo” for breeding purposes. Frontiers in Plant Science, 11, 378. https://doi.org/10.3389/fpls.2020.00378
Hristova-Cherbadzhi, M. (2020). Intergeneric hybidization of sunflower (Helianthus annuus L.) with spiny plumeless thistle (Carduus acanthoides L.). Helia, 43(72), 83–98. https://doi.org/10.1515/helia-2020-0008
Hu, C., & Wang, P. (1986). Embryo culture: Technique and applications. In D. A. Evans, W. R. Sharp, & P. V. Ammirato (Eds.), Handbook of plant cell culture, Vol 4, techniques and applications (pp. 43–96). Macmillan Publishing.
Hu, D., Tang, X., Zhang, Q., & Luo, Z. (2013). Cross compatibilities of oriental persimmon ‘Mopanshi’ and ‘Luotian-tianshi’ with ‘Zenjimaru’. Acta Horticulturae, 996, 165–170.
Hugo Cota-Sánchez, J. (2018). Precocious germination (Vivipary) in tomato: A link to economic loss? Proceedings of the National Academy of Sciences, India Section B, 88, 1443–1451. https://doi.org/10.1007/s40011-017-0878-4
Huylenbroeck, V. J., Eeckhaut, T., Leus, L., Laere, V. K., & Dhooghe, E. (2020). Bridging the gap: Tools for interspecific and intergeneric hybridization in ornamentals. In P. Franken, C. Tränkner, & U. Drüge (Eds.), XXVI International Eucarpia Symposium Section Ornamentals: Editing Novelty (Vol. 1283, pp. 161–168). Acta Horticulturae. International Society for Horticultural Science. https://doi.org/10.17660/ActaHortic.2020.1283.22
Jaisankar, I., Velmurugan, A., & Sivaperuman, C. (2018). Biodiversity conservation: Issues and strategies for the tropical islands. Biodiversity and Climate Change Adaptation in Tropical Islands, 525–552. https://doi.org/10.1016/B978-0-12-813064-3.00019-3
Johnston, S. A., & Hanneman, R. E. (1996). Genetic control of Endosperm Balance Number (EBN) in the Solanaceae based on trisomic and mutation analysis. Genome, 39, 314–321.
Kaeppler, S. M., Phillips, R. L., & Olhoft, P. (1998). Molecular basis of heritable tissue culture-induced variation in plants. In Jain et al. (Eds.), Somaclonal variation and induced mutations in crop improvement. Current plant science and biotechnology in agriculture (Vol. 32, pp. 465–484). Kluwer Academic Publishers.
Kaeppler, S. M., Kaeppler, H. F., & Rhee, Y. (2000). Epigenetic aspects of somaclonal variation in plants. Plant Molecular Biology, 43, 179–188.
Kaminski, P., Marasek-Ciolakowska, A., Podwyszynska, M., Starzycki, M., Starzycka-Korbas, E., & Nowak, K. (2020). Development and characteristics of interspecific hybrids between Brassica oleracea L. and B. napus L. Agronomy, 10, 1339. https://doi.org/10.3390/agronomy10091339
Karim, M. M., Siddika, A., Tonu, N. N., Hossain, D. M., Meah, M. B., Kawanabe, T., Fujimoto, R., & Okazaki, K. (2014). Production of high yield short duration Brassica napus by interspecific hybridization between B. oleracea and B. rapa. Breeding Science, 63, 495–502.
Katsiotis, A., Hanneman, R. E., Jr., & Forsberg, R. A. (1995). Endosperm balance number and the polar-nuclei activation hypotheses for endosperm development in interspecific crosses of Solanaceae and Gramineae, respectively. Theoretical and Applied Genetics, 91, 848–855.
Köhler, C., Dziasek, K., & León, G. D. (2021). Postzygotic reproductive isolation established in the endosperm: Mechanisms, drivers and relevance. Philosophical Transactions of the Royal Society, B: Biological Sciences, 376, 20200118. https://doi.org/10.1098/rstb.2020.0118
Koltunow, A. M., Hidaka, T., & Robinson, S. P. (1996). Accumulation of seed storage proteins in seeds and in embryos cultured in vitro. Plant Physiology, 110, 599–609.
Konan, N., Jacquemin, J., Baudoin, J., & Mergeai, G. (2020). Phenotypic, cytological and molecular (AFLP) analyses of the cotton synthetic allohexaploid hybrid (G. hirsutum × G. longicalyx)2. Open Journal of Genetics, 10, 35–49. https://doi.org/10.4236/ojgen.2020.102004
Kruse, A. (1974). An in vivo/vitro embryo culture technique. Hereditas, 77(2), 219–224. https://doi.org/10.1111/j.1601-5223.1974.tb00935.x
Kuang, Y., Lu, C.-H., & Hsu, F.-C. (2021). Restoring fertility for novel interspecific hybrids between Kalanchoe garambiensis and K. nyikae using colchicine treatment. Plants, 10(2), 209. https://doi.org/10.3390/plants10020209
Kuligowska, K., Lütken, H., Christensen, B., et al. (2015). Evaluation of reproductive barriers contributes to the development of novel interspecific hybrids in the Kalanchoë genus. BMC Plant Biology, 15, 15. https://doi.org/10.1186/s12870-014-0394-0
Kumar, S., Choudhary, A. K., Rana, K. S., Sarker, A., & Singh, M. (2018). Bio-fortification potential of global wild annual lentil core collection. PLoS One, 13, e0191122. https://doi.org/10.1371/journal.pone.0191122
Kumari, P., Eshwari, T., & Hul, R. (2018). Embryo rescue in horticultural crops. International Journal of Current Microbiology and Applied Sciences, 7(6), 3350–3358. https://doi.org/10.20546/ijcmas.2018.706.393
Kumlehn, J., & Bradley, J. T. (2017). Biotechnologies for plant mutation breeding protocols (pp. 73–90). Springer Nature. https://doi.org/10.1007/978-3-319-45021-6
Lédo, A. S., Oliveira, L. A. A. R., Machado, C. A., Silva, A. V. C., & Ramos, S. R. R. (2017). Strategies for exchange of coconut germplasm in Brazil. Biology Ciencia Rural, 47, 3. https://doi.org/10.1590/0103-8478cr20160391
Lee, H. O., Davidson, J. M., & Duronio, R. J. (2009). Endoreplication: Polyploidy with purpose. Genes & Development, 23(21), 2461–2477. https://doi.org/10.1101/gad.1829209
Lentini, Z., Restrepo, G., Buitrago, M. E., & Tabares, E. (2020). Protocol for rescuing young cassava embryos. Frontiers in Plant Science, 11, 522. https://doi.org/10.3389/fpls.2020.00522
Lester, R. N., & Kang, J. H. (1998). Embryo and endosperm function and failure in Solanum species and hybrids. Annals of Botany, 82, 445–453. Article No. bo980695.
Li, G. R., Ji, W., Wang, G., Zhang, J. X., & Wang, Y. J. (2014). An improved embryo-rescue protocol for hybrid progeny from seedless Vitis vinifera grapes × wild Chinese Vitis species. In Vitro Cellular & Developmental Biology. Plant, 50, 110–120. https://doi.org/10.1007/s11627-013-9543-7
Li, J., Zhang, C., Guan, C., et al. (2017). Analysis of intergeneric sexual hybridization between transgenic Brassica oleracea and Sinapis alba. Euphytica, 213, 271. https://doi.org/10.1007/s10681-017-2063-5
Li, F., Cheng, Y., Zhao, X., et al. (2020). Haploid induction via un-pollinated ovule culture in Gerbera hybrida. Scientific Reports, 10, 1702. https://doi.org/10.1038/s41598-020-58552-z
Lu, C., & Bridgen, M. P. (1996). 1996. Effects of genotype, culture medium and embryo developmental stage of the in vitro responses from ovule culture of interspecific hybrids of Alstroemeria. Plant Science, 116, 205–212.
Mahoney, J. D., & Brand, M. H. (2021). Pre- and postzygotic barriers associated with intergeneric hybridization between Aronia melanocarpa (Michx.) Elliott x Pyrus communis L. and ×Sorbaronia dippelii (Zabel) CK Schneid. X Pyrus communis Hortscience, 56(2), 177–184. https://doi.org/10.21273/HORTSCI15412-20
Manzur, J. P., Calvache-Asensio, M. N., & Rodriguez-Burruezo, A. (2014). Growth regulators and darkness increase efficiency in in vitro culture of immature embryos from peppers. Genetics and Plant Breeding Sci agric (Piracicaba, Braz.), 71, 488. https://doi.org/10.1590/0103-9016-2013-0230
Manzur, J. P., Fita, A., Prohens, J., & Rodríguez-Burruezo, A. (2015). Successful wide hybridization and introgression breeding in a diverse set of common peppers (Capsicum annuum) using different cultivated ají (C. baccatum) accessions as donor parents. PLoS One, 10(12), e0144142. https://doi.org/10.1371/journal.pone.0144142
Martins, K. C., Pereira, T. N. S., Souza, S. A. M., Rodrigues, R., & Junior, A. T. A. (2015). Crossability and evaluation of incompatibility barriers in crosses between capsicum species crop breed. Applied Biotechnology, 15(03). https://doi.org/10.1590/1984-70332015v15n3a25
Mehetre, S. S., & Aher, A. R. (2004). Embryo rescue: A tool to overcome incompatible interspecific hybridization in Gossypium Linn. – A review. Indian Journal of Biotechnology, 3, 29–36.
Mehetre, S. S., & Thombre, M. V. (1980). Meiosis in monoploid asiatic cotton (Gossypium arboreum L.). Caryologia, 33(3), 393–400. https://doi.org/10.1080/00087114.1980.10796852
Ming, N., Mostafiz, B. S., Johon, N. S., Zulkifli, A. N. S., & Wagiran, A. (2019). Combination of plant growth regulators, maltose, and partial desiccation treatment enhance somatic embryogenesis in selected Malaysian rice cultivar. Plants (Basel), 8(6), 144. https://doi.org/10.3390/plants8060144
Mohapatra, A., & Rout, G. R. (2005). Study of embryo rescue in floribunda rose. Plant Cell Tiss Organ Cult, 81, 113–117. https://doi.org/10.1007/s11240-004-3128-4
Murashige, T., & Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum, 15(3), 473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Mwangangi, M., Muli, J. K., & Neondo, J. O. (2019). Plant hybridization as an alternative technique in plant breeding improvement immaculate. Asian Journal of Research in Crop Science, 4(1), 1–11. Article no. AJRCS.49096.
Naing, A. H., Kim, S. H., Chung, M. Y., et al. (2019). In vitro propagation method for production of morphologically and genetically stable plants of different strawberry cultivars. Plant Methods, 15, 36. https://doi.org/10.1186/s13007-019-0421-0
Nakamura, S., Pourkheirandish, M., Morishige, H., Sameri, M., Sato, K., & Komatsuda, T. (2017). Quantitative trait loci and maternal effects affecting the strong grain dormancy of wild barley (Hordeum vulgare ssp. spontaneum). Front. Plant Science, 8, 1840. https://doi.org/10.3389/fpls.2017.01840
Narayanaswami, S., & Norstog, K. (1964). Plant embryo culture. The Botanical Review, 30, 587–628.
Neelakandan, A. K., & Wang, K. (2012). Recent progress in the understanding of tissue culture-induced genome level changes in plants and potential applications. Plant Cell Reports, 31, 597–620. https://doi.org/10.1007/s00299-011-1202-z
Niemann, J., Olender, M., Wojciechowski, A., & Tomkowiak, A. (2015). Interspecific hybridization between Brassica napus and Brassica rapa ssp. chinensis genotypes through embryo rescue and their evaluation for crossability. Biotechnologia, 96, 184–191.
Nishiyama, I., & Yabuno, T. (1978). Causal relationships between the Polar nuclei in double fertilization and interspecific cross-incompatibility in Avena. Cytologia, 43(2), 453–466. https://doi.org/10.1508/cytologia.43.453
Nishiyama, I., Sarashima, M., & Matsuzawa, Y. (1991). Critical discussion on abortive interspecific crosses in Brassica. Plant Breeding, 107, 288. https://doi.org/10.1111/j.1439-0523.1991.tb00552.x
Okamoto, A., & Ureshino, K. (2015). Pre- and post-fertilization barriers in Iinterspecific hybridization between evergreen azalea species and Rhododendron uwaense H. Hara & T. Yamanaka. The Horticulture Journal, 84, 355. https://doi.org/10.2503/hortj.MI-036
Pachakkil, B., Terajima, Y., Ohmido, N., et al. (2019). Cytogenetic and agronomic characterization of intergeneric hybrids between Saccharum spp. hybrid and Erianthus arundinaceus. Scientific Reports, 9, 1748. https://doi.org/10.1038/s41598-018-38316-6
Palanyandy, S. R., Gantait, S., Subramaniam, S., & Sinniah, U. R. (2020). Cryopreservation of oil palm (Elaeis guineensis Jacq.) polyembryoids via encapsulation – Desiccation. Biotech, 10, 9. https://doi.org/10.1007/s13205-019-1997-9
Pant, U., Joshi, S., Lohani, P., & Dahiya, N. (2021). Pre-fertilization barrier, crossability and meiotic behavior of interspecific hybrids among Brassica species Euphytica. https://doi.org/10.21203/rs.3.rs-791559/v1
Patil, P., Malik, S. K., Negi, K. S., John, J., Yadav, S., Chaudhari, G., & Bhat, K. V. (2013). Pollen germination characteristics, pollen–pistil interaction and reproductive behaviour in interspecific crosses among Abelmoschus esculentus Moench and its wild relatives. Grana, 52(1), 1–14. https://doi.org/10.1080/00173134.2013.768699
Pawar, B., Kale, P., Bahurupe, J., Jadhav, A., Kale, A., & Pawar, S. (2015). Proline and glutamine improve in vitro callus induction and subsequent shooting in rice. Rice Science, 22, 283–289.
Pen, S., Nath, U. K., Song, S., Goswami, G., Lee, J.-H., Jung, H.-J., Kim, H.-T., Park, J.-I., & Nou, I. S. (2018). Developmental stage and shape of embryo determine the efficacy of embryo rescue in introgressing orange/yellow color and anthocyanin genes of Brassica species. Plants, 7, 99. https://doi.org/10.3390/plants7040099
Picarella, M. E., & Mazzucato, A. (2019). The occurrence of seedlessness in higher plants; insights on roles and mechanisms of parthenocarpy. Frontiers in Plant Science, 9, 1997.
Pinto, A. C., Rogers, S. M. D., & Byrne, D. H. (1994). Growth of immature peach embryos in response to media, ovule support method, and ovule perforation. Horticultural Science, 29(9), 1081–1083.
Pollegioni, P., Olimpieri, I., Woeste, K. E., Simoni, G. D., Gras, M., & Malvolti, M. E. (2013). Barriers to interspecific hybridization between Juglans nigra L. and J. regia L species. Tree Genetics & Genomes, 9, 291–305. https://doi.org/10.1007/s11295-012-0555-y
Pramanik, K., Sahoo, J. P., Mohapatra, P. P., Acharya, L. K., & Jena, C. (2021). Insights into the embryo rescue – a modern in vitro crop improvement approach in horticulture. Plant Cell Biotechnology and Molecular Biology, 22(15–16), 20–33. Retrieved from https://www.ikprress.org/index.php/PCBMB/article/view/6025
Prasad, M. B. N. V., Sahijram, L., & Rekha, A. (1996). Role of embryo culture techniques in the improvement of seedless lime fruit quality (pp. 28–30). Nat’l. Symp. Hortl. Biotech.
Pratap, A., Basu, P. S., Gupta, S., Malviya, N., Rajan, N., Tomar, R., et al. (2014). Identification and characterization of sources for photo- and thermo-insensitivity in Vigna species. Plant Breeding, 133, 756–764. https://doi.org/10.1111/pbr.12215
Pratap, A., Gupta, S., Basu, P. S., Tomar, R., Dubey, S., Rathore, M., et al. (2019). Towards development of climate smart mungbean: Challenges and opportunities. In C. Kole (Ed.), Genomic designing of climate-smart pulse crops (pp. 235–264). Springer. https://doi.org/10.1007/978-3-319-96932-9_5
Pratap, A., Das, A., Kumar, S., & Gupta, S. (2021). Current perspectives on introgression breeding in food legumes. Frontiers in Plant Science, 11. https://doi.org/10.3389/fpls.2020.589189
Pratt, C. (1971). Reproductive anatomy in cultivated grapes – A review. American Journal of Enology and Viticulture, 22, 92–109.
Premjet, D., Obeng, A. K., Kongbangkerd, A., & Premjet, S. (2019). Intergeneric hybrid from Jatropha curcas L. and Ricinus communis L.: Characterization and polyploid induction. Biology, 8(2), 50. https://doi.org/10.3390/biology8020050
Raghavan, V. (1966). Nutrition, growth and morphogenesis of plant embryos. Biological Reviews, 41, 1–58.
Ramming, D. W. (1985). In ovulo embryo culture of early maturing Prunus. Horticultural Science, 20, 419–420.
Ramming, D. W., Emershad, R. L., & Foster, C. (2003). In vitro factors during ovule culture affect development and conversion of immature peach and nectarine embryos. HortScience: A publication of the American Society for Horticultural Science, 38(3), 424. https://doi.org/10.21273/HORTSCI.38.3.424
Rangan, T. S. (1984). Culture of ovules. In I. K. Vasil (Ed.), Cell culture and somatic cell genetics of plants. vol. 1. Laboratory procedures and their applications (pp. 227–231). Academic.
Reed, S. M. (2005). Embryo rescue. In R. N. Trigiano & D. J. Gray (Eds.), Plant development and biotechnology (pp. 235–239). CRC Press.
Reed, B. M. (2011). Choosing and applying cryopreservation protocols to new plant species or tissues. Acta Horticulturae, 908(908), 363–372. https://doi.org/10.17660/ActaHortic.2011.908.48
Reinert, S., Bajaj, Y. P. S., & Zbell, B. (1977). Aspects of organization, organogenesis, embryogenesis, cytodifferentiation. In H. E. Street (Ed.), Plant tissue and cell culture (pp. 389–427). University of California Press.
Ren, H., Du, X., Li, D., Zhao, A., Wang, Y., Xue, X., Gong, G., & Du, J. (2019). An efficient method for immature embryo rescue and plant regeneration from the Calli of Ziziphus jujube ‘Lengbaiyu’. The Journal of Horticultural Science and Biotechnology, 94(1), 63–69. https://doi.org/10.1080/14620316.2018.1446767
Rodriguez-Lopez, C. M., Wetten, A. C., & Wilkinson, M. J. (2010). Progressive erosion of genetic and epigenetic variation in callus-derived cocoa (Theobroma cacao) plants. The New Phytologist, 186, 856–868. https://doi.org/10.1111/j.1469-8137.2010.03242.x
Sahijram, L., Soneji, J. R., Naren, A., & Madhusudhana, B. R. (2013). Hybrid embryo rescue: A non-conventional breeding strategy in horticultural crops. Journal of Horticultural Science, 8(1), 1–20.
Saxena, K. B., Sultana, R., Mallikarjuna, N., Saxena, R. K., Kumar, R. V., Sawargaonkar, S. L., et al. (2010). Male-sterility systems in pigeonpea and their role in enhancing yield. Plant Breeding, 129, 125–134. https://doi.org/10.1111/j.1439-0523.2009.01752.x
Seguí-Simarro, J., Jacquier, N. M. A., & Widiez, T. (2021). Overview of in vitro and in vivo doubled haploid technologies. In J. M. Segui-Simarro (Ed.), Doubled haploid technology (Vol. 2287). Methods in Molecular Biology, Humana. https://doi.org/10.1007/978-1-0716-1315-3_1
Sharma, D. R., Daur, R., & Kumar, K. (1996). Embryo rescue in plants – A review. Euphytica, 89, 325–337. https://doi.org/10.1007/BF00022289
Shukla, S. (2016). Embryo rescue technology: An approach for varietal development and in vitro germplasm conservation. International Journal of Tropical Agriculture, 34(3), 841–847.
Singh, N. V., Singh, S. K., & Singh, A. K. (2011). Standardization of embryo rescue technique and bio-hardening of grape hybrids (Vitis vinifera L.) using arbuscular mycorrhizal fungi (AMF) under sub-tropical conditions. Vitis, 50(3), 115–118.
Singh, M., Rani, S., Malhotra, N., Katna, G., & Sarker, A. (2018). Transgressive segregations for agronomic improvement using interspecific crosses between C. arietinum L. × C. reticulatum Ladiz and C. arietinum L. × C. echinospermum Davis species. PLoS One, 13(9), e0203082. https://doi.org/10.1371/journal.pone.0203082
Soto, S., Bullrich, L., Bologna, P., Facciuto, G., & Borja, M. (2012). Post-fertilization barriers in interspecific crosses between Nierembergia linariifolia and N. ericoides. Acta Horticulturae, 937, 947–952. https://doi.org/10.17660/ActaHortic.2012.937.117
Stout, A. B. (1936). Seedlessness in grapes. New York State Agricultural Experiment Station Technical Bulletin, 238, 68.
Subburaj, S., Cao, S., Xia, X., & He, Z. (2016). Phylogenetic analysis, lineage-specific expansion and functional divergence of seed dormancy 4-like genes in plants. PLoS One, 11, e0153717. https://doi.org/10.1371/journal.pone.0153717
Sun, C., Ma, Z., Zhang, Z., Sun, G., & Dai, Z. (2018). Factors influencing cross barriers in interspecific hybridizations of water lily. Journal of the American Society for Horticultural Science, 143(2), 130–135. https://doi.org/10.21273/JASHS04302-17
Tamaki, M., Urasaki, N., Nakamura, I., Motomura, K., & Adaniya, S. (2011). Shortening the breeding cycle of papaya (Carica papaya L.) by culturing embryos treated with ethrel. Plant Cell Tissue and Organ Culture, 106(2), 225–233. https://doi.org/10.1007/s11240-010-9910-6
Taskın, H., Yücel, N. K., Baktemur, G., Çömlekçioğlu, S., & Büyükalaca, S. (2013). Effects of different genotypes and gamma ray doses on haploidization with irradiated pollen technique in watermelon (Citrullus lanatus L.). Canadian Journal of Plant Science, 93, 1165–1168.
Tegen, H. (2016). The role of plant tissue culture to supply disease free planting materials of major horticultural crops in Ethiopia. Journal of Biology, Agriculture and Healthcare, 6(1), 1–122.
Tessereau, H., Florin, B., Meschine, M. C., Thierry, C., & Pétiard, V. (1994). Cryopreservation of somatic embryos: A tool for germplasm storage and commercial delivery of selected plants. Annals of Botany, 74(5), 547–555.
Tukey, H. B. (1933). Artificial culture of sweet cherry embryos. The Journal of Heredity, 24, 7–12.
Uma, S., Lakshmi, S., Saraswathi, M. S., Akbar, A., & Mustaffa, M. M. (2011). Embryo rescue and plant regeneration in banana (Musa spp.). Plant Cell, Tissue and Organ Culture, 105, 105–111. https://doi.org/10.1007/s11240-010-9847-9
Umbeck, P. F., & Norstog, K. (1979). Effects of abscisic acid and ammonium ion on morphogenesis of cultured barley embryos. Bulletin of the Torrey Botanical, 106, 110–116.
Utami, E. S. W., Sri, Y., & Manuhara, W. (2017). In vitro propagation of the endangered medicinal orchid, dendrobium lasianthera J. J. Sm through mature seed culture. Asian Pacific Journal of Tropical Biomedicine, 7(5), 385–504. https://doi.org/10.1016/j.apjtb.2017.01.011
Vidhanaarachchi, V. R. M., Suranjith, W. C., & Gunathilake, T. R. (2016). Effect of genotype, embryo maturity and culture medium on in vitro embryo germination of Sri Lankan coconut (Cocos nucifera L.) varieties. Journal of the National Science Foundation of Sri Lanka, 44(3), 273–278.
Wang, Y.-L., Guan, Z.-Y., Chen, F.-D., Fang, W.-M., & Teng, N.-J. (2012). Pollen viability, pistil receptivity, and embryo development in hybridization of Nelumbo nucifera Gaertn. The Scientific World Journal. Article ID 678706, 8 pages. https://doi.org/10.1100/2012/678706
White, C. N., Proebsting, W. M., Hedden, P., & Rivin, C. J. (2000). Gibberellins and seed development in maize. I. Evidence that gibberellin/abscisic acid balance governs germination versus maturation pathways. Plant Physiology, 122(4), 1081–1088. https://doi.org/10.1104/pp.122.4.1081
Widiez, T., Ingram, G. C., & Gutierrez-Marcos, J. (2017). Embryo-endosperm-sporophyte interaction in maize seeds. In Maize Kernel Development (pp. 95–107). CABI.
Williams, E. G., Verry, I. M., & Williams, W. M. (1982). Use of embryo culture in interspecific hybridization. In I. K. Vasil, W. R. Scowcroft, & K. J. Frey (Eds.), Plant improvement and somatic cell genetics (pp. 119–128). Academic.
Yan, H., Lu, L., Alzate, A., Ceballos, H., Hershey, C., Chen, S., et al. (2014). Fruit, seed and embryo development of different cassava (Manihot esculenta Crantz) genotypes and embryo rescue. African Journal of Biotechnology, 131, 1520–1524. https://doi.org/10.5897/AJB2013.13180
Yang, D., Li, W., Li, S., Yang, X., et al. (2007). In vitro embryo rescue culture of F1 progenies from crosses between diploid and tetraploid grape varieties. Plant Growth Regulation, 51(1), 63–71.
Yeung, E. C., Thorpe, T. A., & Jensen, C. J. (1981). In vitro fertilization and embryo culture. In T. A. Thorpe (Ed.), Plant tissue culture: Methods and applications in agriculture (pp. 253–271). Academic Press.
Yeung, E. C., Nickle, T. C., & Meinke, D. (2001). Embryology of flowering: An overview. Phytomorphology, 51, 289–304.
Yin, X., Zhan, R., He, Y., Song, S., Wang, L., Ge, Y., et al. (2020). Morphological description of a novel synthetic allotetraploid (A1A1G3G3) of Gossypium herbaceum L. and G. nelsonii Fryx. Suitable for disease-resistant breeding applications. PLoS One, 15(12), e0242620. https://doi.org/10.1371/journal.pone.0242620
Yoon, J. B., Yang, D. C., Do, J. W., & Park, H. G. (2006). Overcoming two post-fertilization genetic barriers in interspecific hybridization between Capsicum annuum and C. baccatum for introgression of anthracnose resistance. Breeding Science, 56(1), 31–38. https://doi.org/10.1270/jsbbs.56.31
Zhiwu, L., Pinkham, L., Campbell, N. F., et al. (2009). Development of triploid daylily (Hemerocallis) germplasm by embryo rescue. Euphytica, 169(3), 313–318.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Amiteye, S. (2023). In Vitro Embryo Rescue Techniques and Applications in Hybrid Plant Development. In: Raina, A., Wani, M.R., Laskar, R.A., Tomlekova, N., Khan, S. (eds) Advanced Crop Improvement, Volume 2. Springer, Cham. https://doi.org/10.1007/978-3-031-26669-0_15
Download citation
DOI: https://doi.org/10.1007/978-3-031-26669-0_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-26668-3
Online ISBN: 978-3-031-26669-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)