Abstract
Key message
The aluminium tolerance of durum wheat was markedly enhanced by introgression of TaALMT1 and TaMATE1B from bread wheat. In contrast to bread wheat, TaMATE1B conferred greater aluminium tolerance than TaALMT1.
Abstract
Durum wheat (tetraploid AABB, Triticum turgidum) is a species that grows poorly on acid soils due to its sensitivity of Al3+. By contrast, bread wheat (hexaploid AABBDD, T. aestivum) shows a large variation in Al3+ tolerance which can be attributed to a major gene (TaALMT1) located on chromosome 4D as well as to other genes of minor effect such as TaMATE1B. Genotypic variation for Al3+ tolerance in durum germplasm is small and the introgression of genes from bread wheat is one option for enhancing the ability of durum wheat to grow on acid soils. Introgression of a large fragment of the 4D chromosome previously increased the Al3+ tolerance of durum wheat demonstrating the viability of transferring the TaALMT1 gene to durum wheat to increase its Al3+ tolerance. Here, we used a ph1 (pairing homoeologous) mutant of durum wheat to introgress a small fragment of the 4D chromosome harboring the TaALMT1 gene. The size of the 4D chromosomal fragment introgressed into durum wheat was estimated by markers, fluorescence in situ hybridisation and real-time quantitative PCR. In a parallel strategy, we introgressed TaMATE1B from bread wheat into durum wheat using conventional crosses. Both genes separately increased the Al3+ tolerance of durum wheat in both hydroponics and soil cultures. In contrast to bread wheat, the TaMATE1B gene was more effective than TaALMT1 in increasing the Al3+ tolerance of durum wheat grown on acid soil.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Durum wheat (Triticum turgidum var durum) has a genetic makeup comprising the A and B genomes (2n = 4x = 28; AABB), but lacks the D-genome of bread wheat (T. aestivum) where major genes for aluminium and salt tolerances are located. Durum wheat is grown in many regions of the world for the production of pasta and bread (Sissons 2008). Drought and temperature extremes have been identified as limiting factors for durum production in many regions of the world (Habash et al. 2010). Extensive root systems are clearly attributes that enable plants to take up water from depth, improving a plant’s ability to tolerate drought. Durum as a species is particularly sensitive of acid soils which can be attributed to its sensitivity of Al3+. Acid soils typically liberate poorly soluble forms of Al into toxic Al3+ that inhibits growth by causing roots to become stunted and thickened (Delhaize and Ryan 1995). Acid soils are widespread globally (von Uexküll and Mutert 1995) and can reduce yields or limit the area that an Al3+-sensitive species such as durum wheat is grown. Shortened roots reduce the ability of plants to take up water and nutrients resulting in reduced grain yields. Screens of durum wheat genotypes showed that this species is Al3+-sensitive (Cosic et al. 1994; Moustakas et al. 1992) and an extensive screen of over 600 genotypes failed to identify any useful levels of tolerance (Ryan et al. 2010). Reports of Al3+-tolerant durum genotypes have either proven to be due to contaminating Al3+-tolerant varieties of hexaploid wheat (Han et al. 2014) or are yet to be confirmed.
In contrast to durum wheat, hexaploid wheat (2n = 6x = 42; AABBDD, bread wheat) shows a large variation in Al3+ tolerance largely due to allelic variation in the major Al3+ tolerance gene TaALMT1 located on chromosome 4D (Delhaize et al. 2012). TaALMT1 encodes a transport protein located on the plasma membrane that has been characterized as a malate-permeable anion channel that is activated by Al3+. The malate secreted by Al3+ tolerant wheat is thought to protect roots by chelating the Al3+ in the apoplast and rendering it non-toxic.
When limited genetic variation for a trait exists within durum germplasm, the use of related species to donate genes is an option for crop improvement. For instance, the introgression of genes encoding gluten proteins from the D-genome of hexaploid wheat is a strategy for improving durum for bread making, while maintaining pasta quality (Sissons et al. 2014). Similarly, the Al3+ and Na+ tolerances of durum wheat have been improved by introgression of fragments of chromosome 4D (Dubcovsky et al. 1996; Dvorak and Gorham 1992; Dvorak et al. 1994; Han et al. 2014; Luo et al. 1996). The starting germplasm for the introgression of 4D-chromosomal fragments was a substitution line of durum where the 4D chromosome of hexaploid wheat replaced the 4B chromosome of durum wheat. Subsequently, the use of the ph1c mutant enabled the transfer of chromosomal fragments with the desired gene into the durum genetic background (Dvorak and Gorham 1992; Luo et al. 1996). In the absence of the ph1 locus, a spontaneous translocation of a fragment from chromosome 4D to chromosome 4B was identified and used to develop durum lines that were both Al3+- and Na+-tolerant while maintaining the semi-dwarf habit of an elite durum cultivar (Han et al. 2014). The translocation allowed both TaALMT1 and KNa1 on chromosome arm 4DL to be transferred to durum wheat while maintaining the semi-dwarf allele of Rht-B1 on chromosome arm 4BS of durum.
A mutant ph1 locus has enabled efficient transfer of relatively small chromosomal regions from alien chromosomes to homeologous chromosomes of wheat. The ph1 mutants have been used by breeders to not only allow the transfer of desirable genes into wheat germplasm but also to avoid detrimental phenotypes due to linkage drag of undesirable genes (Able and Atienza 2014; Ayala-Navarrete et al. 2013; Marais et al. 2010; Niu et al. 2014; Qi et al. 2007). The introgression of a 4D chromosomal fragment into durum wheat did not show any obvious detrimental phenotypes apart from reduced grain size, which was presumably caused by genes other than TaALMT1 located on the introgressed fragment (Han et al. 2014). To avoid other subtle effects on grain quality or yield, the ph1c mutation of durum wheat could be used to reduce the size of 4D chromosomal fragment introgressed into durum while maintaining Al3+ tolerance. Alternatively, if suitable genes are located on either the A or B genomes of hexaploid wheat or related diploid species, they can be introgressed into durum wheat without the need of the ph1c mutation. For example, the Nax loci from T. monococcum (2n = 2x = 14; AA) were introgressed into durum germplasm to enhance its ability to exclude and tolerate Na+ (James et al. 2012). Bread wheat and durum wheat can be crossed to generate pentaploid progeny and these progeny backcrossed to durum wheat to quickly eliminate the D-genome and to introgress the desired genes into elite cultivars (Ceoloni et al. 1996).
While TaALMT1 is the major gene for Al3+ tolerance in hexaploid wheat, other genes of minor effect have been identified on the A and B genomes (Cai et al. 2008; Ma et al. 2006; Navakode et al. 2014; Navakode et al. 2009; Raman et al. 2010; Ryan et al. 2009; Zhou et al. 2007). The only Al3+ tolerance gene other than TaALMT1 that has been isolated from hexaploid wheat and its function characterized is TaMATE1B located on chromosome 4B (Ryan et al. 2009; Tovkach et al. 2013). TaMATE1B also encodes a transport protein but the protein belongs to a family unrelated to TaALMT1. In contrast to the Al3+-activated efflux of malate conferred by TaALMT1, TaMATE1B confers constitutive citrate efflux from root apices and like malate, citrate is thought to bind Al3+ to form a non-toxic complex. Although TaALMT1 generates a stronger Al3+ tolerance phenotype than TaMATE1B in bread wheat, the location of TaMATE1B on the B-genome indicates that it should be relatively simple to introgress this gene into durum wheat since the B-genome is shared by both species. Here, we describe the introgression and characterization of a small fragment of chromosome 4D containing TaALMT1 into a durum genetic background as well as the development of durum lines where TaMATE1B has been introgressed from bread wheat. We describe crosses that avoid hybrid necrosis when durum wheat was crossed to bread wheat and compare the effectiveness of the two genes to confer Al3+ tolerance in a durum genetic background in both hydroponic and soil cultures.
Materials and methods
Germplasm
A durum line with the Langdon genetic background where the 4B chromosome is substituted with the 4D chromosome of hexaploid wheat (Joppa and Williams 1988) was crossed with a homozygous ph1c mutant (cv Cappelli) as the female parent. The 4D(4B) substitution line possesses an Al3+-tolerant allele of TaALMT1 derived from Chinese Spring (Sasaki et al. 2004). The TaALMT1 allele of Chinese Spring has a large duplication in its promoter region associated with Al3+ tolerance. The progeny were assessed for Al3+ tolerance by hydroponics using previously described methods (Han et al. 2014) to confirm that the crosses were successful. The F1 plants as pollen donors were backcrossed to the homozygous ph1c mutant to generate 40 grains which were germinated and screened for Al3+ tolerance. Two plants were Al3+ tolerant with the remainder found to be Al3+ sensitive. The Al3+-tolerant plants were screened for the ph1c mutation by PCR (Wang et al. 2002). We established that one Al3+-tolerant plant was homozygous for the ph1c mutation. Since the plant was Al3+ tolerant and homozygous for ph1c, it would have been heterozygous for the 4D chromosome and progeny suitable to screen for recombinations between chromosomes 4D and 4B. To generate sufficient grains to screen by PCR for identifying recombinant chromosomes, Al3+-tolerant progeny of the single plant was grown to maturity and the grain collected. This not only generated additional grain for screening but also enhanced the likelihood that further recombinations could occur. Collecting progeny from heterozygous Al3+-tolerant plants that were homozygous for ph1c was repeated a number of times. At each generation, progeny seedlings were screened for Al3+ tolerance and only those progeny batches that were segregating were analyzed by PCR. If a batch of progeny was segregating for Al3+ tolerance, then the parental plant must have been heterozygous for the 4D-chromosomal fragment. Therefore, recombinations between the 4D and 4B chromosomes could have occurred. If a batch of progeny was all Al3+ tolerant, then the parental plant would have been homozygous for the 4D chromosomal fragment and would not have enabled recombination with the 4B chromosome so these populations were discarded. After screening the batches of progeny, DNA from Al3+-tolerant seedlings was extracted only from populations that were segregating for Al3+ tolerance. DNA samples were analyzed by PCR and seedlings at each generation that possessed the smallest recombination based on markers proximal and distal to TaALMT1 were identified. These seedlings were allowed to self-fertilize to generate further potential recombinations in subsequent populations. After four rounds of selfing, the seedling with TaALMT1 and with the smallest amount of recombined 4D chromosome as identified with markers was crossed to the elite Australian durum cv Jandaroi. The progeny were backcrossed twice more to cv Jandaroi to generate lines that possessed the small 4D introgression (SF TaALMT1 line) and sister lines that lacked the introgression.
An allele of TaMATE1B conferring Al3+ tolerance to bread wheat was introgressed into durum wheat. The TaMATE1B gene is located on chromosome 4B and in theory could already be present in some durum genotypes. However, to-date no Al3+-tolerant allele of TaMATE1B has been identified in durum germplasm. We crossed durum wheat with hexaploid wheat lines including a line with the cv Westonia background that possessed the Al3+-tolerant allele of TaMATE1B donated by cv Carazinho. When either Carazinho or Westonia was crossed to several Australian durum cultivars (Jandaroi, Tamaroi and Bellaroi), all F1 progeny showed symptoms of hybrid necrosis and subsequently died before setting seed. Hybrid necrosis is a syndrome that is commonly encountered in crosses between bread and tetraploid wheats. It is controlled by interaction of complementary genes Ne1 and Ne2 (Tsunewaki 1992). To develop successful crosses between cv Carazinho or cv Westonia and durum germplasm, we crossed both hexaploid genotypes to each of 10 durum cultivars selected at random and originating from diverse countries to establish if any could act as a “bridge” between hexaploid wheat and elite Australian durum cultivars. The durum cultivars used in the crosses to hexaploid wheat included Bouffarick, Forex, Durex, Icaro, Greece 14, Langdon, Kalka, Arrivato, Leeds Dwarf and Castelporziano. The progeny were grown and of the crosses, the cultivars Greece 14 and Leeds Dwarf were the only durum parents to produce F1 progeny that did not develop hybrid necrosis. The F1 progeny of the cross between Leeds Dwarf and the cv Westonia line that possessed TaMATE1B were crossed to cv Jandaroi. The resulting progeny was crossed again to cv Jandaroi to develop homozygous TaMATE1B lines that had been effectively crossed three times to durum wheat including a backcross to cv Jandaroi. The availability of a co-dominant molecular marker for TaMATE1B (Tovkach et al. 2013) facilitated the screening of progeny and allowed the rapid development of lines homozygous for the Al3+-tolerant allele of TaMATE1B in a durum background. The availability of a dominant marker for TaALMT1 (Sasaki et al. 2004) allowed us to verify that the lines only possessed the TaMATE1B gene and that TaALMT1 had not been introgressed by a spontaneous translocation.
Methods previously described for short-term hydroponic and soil cultures were used to assess the Al3+ tolerance of the durum germplasm (Han et al. 2014). For soil experiments, we used soil that had been amended with various amounts of lime to generate a range of Al3+ toxicities with 4 g of lime per kg soil totally detoxifying the Al3+ toxicity. Pots (1.3 kg) were prepared and plants grown as described previously (Han et al. 2014). Plants were harvested after six (SF TaALMT1 lines) or seven (TaMATE1B lines) days, roots washed and lengths measured by scanning and WinRhizo Pro V software (2002). For hydroponic experiments, seedlings were grown in separate 11 L containers for each Al3+ treatment. Within a container, individual seedlings from each line were randomized with 7–10 replicate seedlings planted for each line. For experiments that used soil, pots were set up in a randomized block arrangement with 5 or 6 replicate blocks used in each experiment. Data were analyzed by one-way ANOVA, two-way ANOVA or Student’s t test as specified in figure legends using SigmaPlot v 12.3. Where required to ensure normal distributions, data were log10 transformed prior to statistical analysis.
Markers
Various markers located on chromosome 4D of hexaploid wheat were sourced from the Graingenes database (http://wheat.pw.usda.gov/cgi-bin/graingenes). Markers for TaALMT1 and TaMATE1B were implemented as described previously (Sasaki et al. 2004; Tovkach et al. 2013). A deletion series of chromosome 4D (Mickelson-Young et al. 1995) was used to verify the location of markers.
Fluorescence in situ hybridisations (FISH)
FISH analyses were undertaken using previously described methods (Zhang et al. 2004).
Bacterial artificial chromosomes 676D4 and 9M13 contain dispersed repetitive sequences specifically derived from the A- and D-genomes, respectively (Zhang et al. 2004). One microgram each of 676D4 and 9M13 DNA was labeled with tetramethyl-rhodamine-5-dUTP (Roche Diagnostics Australia P/L, NSW) using nick-translation and biotin-14-dATP (BioNick Labeling System, Invitrogen Australia P/L, Vic.), respectively. The hybridisation and post-hybridisation washes were conducted as described in Zhang et al. (2004). The biotin-labeled probe was detected with fluorescein-avidin DN (Vector Laboratories, Burlingame, CA, USA). Chromosome preparations were analyzed with an epifluorescence Zeiss Axio Imager microscope (Carl Zeiss Microimaging Gmbh, Göttingen, Germany). Images were captured with a Retiga EXi CCD (charge-coupled device) camera (QImaging, Surry, BC, Canada) operated with Image-Pro Plus 7.0 software (Media Cybernetics Inc., Bethesda, MD, USA) and processed with Photoshop version 8.0 software (Adobe Systems, San Jose, CA, USA).
Real-time PCR
To rapidly estimate the amount of D-genome recombined into durum lines, we used real-time quantified PCR (RT-qPCR). The Dgas44 sequence is a member of a family of repeated elements (Dgas) specific to the D-genome (McNeil et al. 1994) and primers were generated as described previously (Han et al. 2014). Primers (CTGATCTTCTGTGAAGGGT forward primer; TGATAGAACTCGTAATGGGC reverse primer) that amplified 28S ribosomal RNA genes were used as reference genes for RT-qPCR undertaken as described previously (Delhaize et al. 2004). Ribosomal RNA genes are repeated within genomes and are sequences common to all the A, B and D-genomes. Expressing the amount of Dgas DNA as a ratio of the genes encoding 28S ribosomal RNA would provide a measure for the relative abundance of Dgas between different lines and hence could be used to estimate the relative amount of the D-genome introgressed into the durum background. To test the method, we used DNA (approximately 10 ng) from lines that possessed differing amounts of D-genome ranging from hexaploid wheat (cv Chinese Spring; full complement of the D-genome), a 4D(4B) durum substitution line (possesses only the 4D chromosome), a durum line where a large fragment of chromosome 4D (LF line) had been introgressed (Han et al. 2014) and a durum line that lacked the D-genome altogether.
Results
Introgression of TaALMT1 into durum wheat
We verified the published locations of markers on chromosome 4D (http://wheat.pw.usda.gov/cgi-bin/graingenes) using a 4D-chromosomal deletion series and mapped a homolog of TaMATE1B to a particular bin on the long arm of chromosome 4D (Fig. 1a). The TaALMT1 gene was previously mapped to a bin delineated by break-points 4DL-12 and 4DL-14 in hexaploid wheat with marker wmc331 identified as closely linked. Use of the ph1c mutation resulted in progressively smaller introgressions of chromosome 4D that possessed TaALMT1 and a seedling was identified where the only marker from chromosome 4D that remained linked to TaALMT1 was wmc331 (Fig. 1b). Marker wmc331 is located in the same bin as TaALMT1 and is in a position distal to TaALMT1 on chromosome 4D. The closest marker proximal to TaALMT1 had been recombined. Chromosome 4B of this line that had the smallest fragment of D-genome introgressed (SF line) is shown in Fig. 1b.
Introgression of a fragment of chromosome 4D containing TaALMT1 into chromosome 4B of durum wheat. a Markers on chromosome 4D used to analyze DNA samples by PCR. The markers were used on deletion lines of chromosome 4D to verify to which “deletion bin” they belonged. The designations on the left of chomosome 4D denote the chomosomal break points and the name of deletion lines as described previously (Mickelson-Young et al. 1995). The designations on the right of chromosome 4D denote markers most of which were dominant for the 4D chromosome although a few were co-dominant since a fragment amplified from chromosome 4B could be distinguished from the fragment(s) amplified from chromosome 4D. A homolog of TaMATE1B (on chromosome 4B) was mapped to the interval between break points 4DL-11 and 4DL-2 (DMATE). b The size of the 4D chromosomal fragment introgressed into chromosome 4B as estimated from marker analysis. The D-genome is represented in black and the B-genome in white
To assess the relative size of 4D chromosomal introgressions into a durum genetic background, we used a method based on RT-qPCR. This allowed rapid estimation of the amount of D-genome introgressed and was tested using DNA derived from wheat lines with varying amounts of D-genome. The cultivar Chinese Spring showed the largest relative amount of D-genome consistent with it having a full complement of the D-genome chromosomes (Fig. 2). The line identified with markers (Fig. 1) as having the smallest amount of 4D chromosome introgressed into a durum background (SF line) had the smallest amount of DNA estimated by RT-qPCR and could not be distinguished from the durum cv Jandaroi (Fig. 2).
Quantification of D-genome introgressed into a durum background using RT-q PCR. The amount of Dgas sequence is expressed as a ratio of the rRNA sequence with the data normalized to show the 4D(4B) substitution line having a value of 1. Lines Chinese Spring with the full complement of D chromosomes, 4D(4B) is a durum line with chromosome 4B substituted with chromosome 4D, 4DL is a durum line that has a large fragment of chromosome 4D recombined with chromosome 4B (Han et al. 2014), SF TaALMT1 is the durum line which incorporates a small fragment of the 4D chromosome described in this paper and Jandaroi is a durum cultivar with no D-genome chromosome introgressed. Error bars show the SE (standard error) for two to four independent DNA extractions of each line. Data were log10 transformed before a one-way ANOVA. Bars with different letters signifying statistically significant differences at P < 0.05
The amount of D chromosome was assessed visually by FISH using genome-specific probes. Figure 3a shows a pair of complete chromosomes in substitution line 4D(4B) as being derived from the D-genome, while a chromosome arm of the LF line hybridized with the probe for the D-genome (Fig. 3b). Only a relatively small signal of D-genome was present in the arm of a chromosome for the SF line (Fig. 3c) and this line had the smallest fragment of chromosome 4D introgressed as identified by markers (Fig. 1) and RT-qPCR (Fig. 2).
FISH analysis identifies an introgression of a small fragment of D-genome into durum wheat. The green signals denote the presence of D-genome, whereas pink and blue denote the A- and B-genomes. a The 4D(4B) substitution line where the whole 4D chromosome has replaced the 4B chromosome of durum wheat. b A spontaneous translocation of a relatively large fragment from chromosome 4D into durum wheat (Han et al. 2014) consistent with a whole-arm translocation. c The small 4D fragment recombined into chromosome 4B using the ph1c mutant as described in this paper. d The durum cv Jandaroi that lacks any D-genome. The white arrows indicate signals for D-genome in each panel
Introgression of TaMATE1B into durum wheat
TaMATE1B is located on chromosome 4B and durum wheat possesses the Al3+ sensitive allele (lacks citrate efflux). A co-dominant marker was used to track the introgression of TaMATE1B into durum. Because the hexaploid donor also possessed a tolerant allele of TaALMT1, we needed to verify that any Al3+ tolerance was caused solely by TaMATE1B and not due to a spontaneous translocation of TaALMT1 from chromosome 4D to either the A- or B-genomes of durum wheat. The marker for TaALMT1 indicated that this gene had not been introgressed into the durum lines and the RT-qPCR assay also showed little or no D-genome had been introgressed into the durum line (Supplementary Fig. S1). Based on these findings, we were confident that TaMATE1B was responsible for the observed increases in Al3+ tolerance.
Al3+ tolerance of durum introgression lines
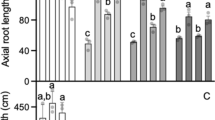
The Al3+ tolerance of SF TaALMT1 and TaMATE1B durum lines was assessed by hydroponics with varying Al3+ concentrations (Fig. 4). Both TaALMT1 and TaMATE1B enhanced the Al3+ tolerance of durum with TaALMT1 conferring the stronger phenotype. The level of Al3+ tolerance conferred by the SF line in hydroponics was comparable to that conferred by the full 4D chromosome (Fig. 4a).
Introgression of TaALMT1 or TaMATE1B into durum wheat enhances Al3+ tolerance as assessed by hydroponics. Effects of a SF TaALMT1 and b TaMATE1B on Al3+ tolerance of durum wheat. Seed was pre-germinated prior to planting and seedlings were grown in solutions that contained various concentrations of AlCl3. The data show root length after 5 days of the longest seminal root with the black bar denoting the introgression line and the white bar the cultivar Jandaroi (n = 7; error bars show the SE). For a, the gray bar is a line where chromosome 4B is substituted with chromosome 4D and for b the gray bar is cv Leeds Dwarf which was the line used as a “bridge” to transfer TaMATE1B from bread wheat to durum wheat before the resulting line was crossed to cv Jandaroi. Note that the maximum AlCl3 concentration used was 10 µM for the TaMATE1B line and 20 µM for the SF TaALMT1 line
When assessed in an acid soil, TaALMT1 enhanced root growth of cv Jandaroi, but this was only apparent in the length of thick roots in the most acidic soils (Fig. 5a). By contrast, the growth of fine roots was not enhanced on any of the soils (Fig. 5b). When total roots were measured, the TaALMT1 line had improved root growth but this could be attributed entirely to enhanced growth of thick roots (Fig. 5). The 4D(4B) line had better growth of both thick and fine roots in soil than the SF TaALMT1 line (Fig. 5a).
The SF TaALMT1 fragment improves growth of thick (seminal) but not fine (lateral) roots of durum grown in acid soil. Lengths of a thick roots, b fine roots and c total roots. Pre-germinated seed were planted into 1.3 kg pots of soil and seedlings grown for 6 days before roots were washed out and scanned. After scanning, data were analyzed in groups as thick roots composed mainly of seminal roots, fine roots composed mainly of lateral roots and the total composed of combined data for thick and fine roots. Data show the mean of five replicates and error bars denote the SE. For a there was a significant interaction identified from a two-way ANOVA between genotype and treatment and the LSD for P < 0.05 is shown. For b and c data needed to be log10 transformed before two-way ANOVA. For each lime rate, the different letters indicate significant differences between genotypes at P < 0.05
The TaMATE1B line of durum had a markedly improved growth of both thick and fine roots on acid soils (Fig. 6). Consequently, the enhanced growth of total root length was a result of increased growth of both classes of roots. Both thick and fine roots of cv Jandaroi behaved similarly in the two soil experiments (SF TaALMT1 and TaMATE1B lines), indicating that a similar range of Al3+ toxicity was encompassed in both experiments. In contrast to hydroponic culture, the TaMATE1B line showed greater Al3+ tolerance than the SF TaALMT1 line when assessed on this acid soil (Figs. 5, 6).
TaMATE1B improves growth of both thick (seminal) and fine (lateral) roots in acid soil. Lengths of a thick roots, b fine roots and c total roots. Pre-germinated seeds were planted into 1.3 kg pots of soil and seedlings grown for 7 days before roots were washed out and scanned. Data show the mean of six replicates and error bars denote the SE. The asterisks denote significant differences (P < 0.001) between genotypes at each lime rate as determined by a t test
Al3+ tolerance of near-isogenic hexaploid wheat lines possessing different TaMATE1B alleles
The greater Al3+ tolerance on soil conferred by TaMATE1B than TaALMT1 in a durum genetic background was intriguing and prompted us to determine whether hexaploid wheat behaved in a similar fashion. Previously, it was shown that hexaploid wheat lines that possessed the Al3+-tolerant allele of TaMATE1B had improved Al3+ tolerance in hydroponic culture, but this tolerance was considerably less than observed for lines that possessed TaALMT1 on its own (Ryan et al. 2009). The lines used in that study were F3 families derived from single crosses between cvs Carazinho and Egret so were not necessarily genetically similar to one another. Here, we used backcrossed germplasm where TaMATE1B was introgressed into cultivars that were either homozygous for a tolerant (cv EGA-Burke) or sensitive (cv Egret) TaALMT1 allele. In the presence of the sensitive TaALMT1 allele, TaMATE1B conferred improved Al3+ tolerance in hydroponic culture (Fig. 7a). When TaMATE1B was present in combination with a tolerant TaALMT1 allele, it conferred a small increase in Al3+ tolerance (Fig. 7b). The effectiveness of TaALMT1 could be seen in the considerably higher AlCl3 concentration used to screen EGA-Burke (60 µM) compared to Egret (10 µM). In contrast to durum wheat, the effectiveness of the lines on a range of amended acid soils showed a similar pattern to the hydroponics experiments with TaMATE1B improving Al3+ tolerance in the Egret background but not improving Al3+ tolerance in the EGA-Burke background above the high background of Al3+ tolerance conferred by TaALMT1 (Fig. 8).
TaMATE1B improves the Al3+ tolerance of hexaploid wheat cultivars grown in hydroponic culture. a Cultivar Carazinho was backcrossed to cv Egret six times using Egret as the recurrent parent to generate near isogenic lines (NILs) that differ in TaMATE1B alleles. The line that has the TaMATE1B allele that confers citrate efflux is named Egret TaMATE1B. Egret has an Al3+-sensitive allele of TaALMT1 and lacks Al3+-activated malate efflux. b Cultivar Carazinho was backcrossed to cv EGA-Burke nine times using EGA-Burke as the recurrent parent to generate NILs that differ in TaMATE1B alleles. The line that has the TaMATE1B allele that confers citrate efflux is named EGA-Burke TaMATE1B. EGA-Burke has an Al3+-tolerant allele of TaALMT1 that confers Al3+-activated malate efflux. Data show the means of 10 replicates and error bars denote the SE. For a data were normally distributed but for b data were log10 transformed prior to a two-way ANOVA. For a there was a significant interaction between genotype and treatment (P < 0.001). For b there were significant genotype and treatment effects (P < 0.001), but they did not interact. The asterisks denote significant differences between genotypes at each Al treatment
TaMATE1B improves the tolerance of an Al3+-sensitive wheat cultivar grown in acid soil. a The cv Carazinho was backcrossed to cv Egret six times using Egret as the recurrent parent to generate NILs that differ in TaMATE1B alleles. The line that has the TaMATE1B allele that confers citrate efflux is named Egret TaMATE1B. Egret has an Al3+-sensitive allele of TaALMT1 that lacks Al3+-activated malate efflux. b The cv Carazinho was backcrossed to cv EGA-Burke nine times using EGA-Burke as the recurrent parent to generate NILs that differ in TaMATE1B alleles. The line that has the TaMATE1B allele that confers citrate efflux is named EGA-Burke TaMATE1B. EGA-Burke has an Al3+-tolerant allele of TaALMT1 that possesses Al3+-activated malate efflux. Data show the mean of six replicates and error bars denote the SE. The asterisks denote significant differences (P < 0.05) between genotypes at each lime rate as determined by a t test
Discussion
When TaALMT1 and TaMATE1B were introgressed separately into a durum wheat background, they each enhanced the Al3+ tolerance of roots in hydroponic culture and improved root growth on acid soil. Previously, when a large fragment of chromosome 4D was introgressed into durum wheat, the Al3+ tolerance of roots was increased in hydroponic culture and only growth of thick (seminal) roots was improved in acid soil without improving the growth of fine (lateral) roots. Here, we show that a similar phenotype was obtained when a much smaller fragment of chromosome 4D that possessed the TaALMT1 gene was introgressed into durum. By contrast, the TaMATE1B gene enhanced the Al3+ tolerance of both thick and fine roots of durum when grown in acid soil. However, when assessed in hydroponic culture TaMATE1B was not as effective as TaALMT1 in conferring Al3+ tolerance (Fig. 4). Although both genes enhanced Al3+ tolerance of hexaploid wheat, TaALMT1 conferred the stronger phenotype in both hydroponic and soil cultures (Figs. 7, 8).
It is not clear why TaMATE1B conferred a stronger phenotype than TaALMT1 in durum wheat, but not bread wheat and specifically in soil. This illustrates that while hydroponic culture has proven to be useful to screen plants for Al3+ tolerance, the findings are not always reflected in experiments using acid soils. Similar to our findings with durum wheat, the Al3+ tolerance rankings of barley in hydroponic culture did not necessarily reflect rankings when the same lines were assessed on acid soil (Moroni et al. 2010). It is probable that the root systems of bread and durum wheat grown in acid soil differ sufficiently from one another such that the efflux of citrate was more effective for durum wheat roots whereas malate efflux was more effective for hexaploid wheat roots.
A relatively small fragment of chromosome 4D was introgressed into durum wheat and this was verified using three methods. Molecular markers located on chromosome 4D provided a genetic estimate of the size of fragments introgressed (Fig. 1), whereas FISH provided a direct image of the size of the fragment (Fig. 3). The use of a PCR-based method to quantify the relative amount of D-genome in the lines was in general agreement with both the marker and FISH analyses (Fig. 2). It provided a rapid method to estimate the relative sizes of fragments and another tool for selecting the recombinants with the smallest introgressions. A background signal was found in the absence of D-genome (cv Jandaroi: Fig. 2; Supplementary Fig. S1) which can be attributed to the primers annealing to related sequences in the A- and B-genomes albeit at lower efficiency relative to the D-genome. Here, we also show that the LF TaALMT1 durum line generated previously (Han et al. 2014) by a spontaneous translocation had transferred the long arm of chromosome 4D to the short arm of chromosome 4B (Fig. 3). While the RT-qPCR method is useful to estimate the amount of D-genome introgressed, in some cases the method could provide distorted estimates if the distributions of the two classes of genes vary throughout the genome. Nevertheless, the technique proved useful in establishing that the D-genome in the TaMATE1B lines had been quickly eliminated by backcrossing the bread wheat/durum wheat hybrids to durum wheat (Supplementary Fig. S1).
The TaALMT1 and TaMATE1B genes each independently improved the Al3+ tolerance of durum wheat. It should be possible to combine the genes to determine if their effects are additive and to further increase the acid soil tolerance of durum wheat. Figure 7b shows that TaMATE1B increased the Al3+ tolerance in hydroponics of a bread wheat cultivar that possesses a tolerant TaALMT1 allele although the effect was small in the hexaploid wheat background. Combining the 4D chromosomal fragment containing TaALMT1 with TaMATE1B into a durum wheat background will require that the durum lines with each gene introgressed be crossed and a suitable recombination event identified. If TaMATE1B in durum is located in a homeologous region to where TaMATE1B is located in bread wheat, then it will be closely linked to the fragment containing TaALMT1 (Fig. 1a). This could make identifying a suitable recombination event difficult. However, once generated it would simplify the introgression into other cultivars as the closely linked genes would generally be transferred together in crosses.
The development of a durum line that possesses a small fragment from the D-genome showed a similar Al3+ tolerance phenotype to lines that possessed the whole 4DL arm. The introgression of the small fragment containing TaALMT1 appears to have avoided the smaller grain phenotype that was observed when the full 4DL arm was introgressed (Han et al. 2014) but the Kna1 salt tolerance locus was absent from this fragment. To ensure that lines are also salt tolerant, a small fragment possessing the Kna1 in a durum wheat background (Luo et al. 1996) could be recombined with the Al3+ tolerance genes on chromosome 4B. Alternatively, the Nax genes that confer salt tolerance to durum wheat could be other sources of genes that are located on chromosomes 2A (Nax1) and 5A (Nax2) (Byrt et al. 2007; Lindsay et al. 2004). Nevertheless, the small fragment harboring TaALMT1 should enable the TaMATE1B gene to be combined with TaALMT1 as discussed above, whereas the full 4DL chromosome arm would not allow straightforward recombination of both genes.
Author contribution statement
ED directed the work, undertook some of the experimental work and wrote the manuscript. CH undertook the bulk of the experimental work to identify recombinants and cross genotypes to develop germplasm. CH also revised the manuscript. PZ undertook the FISH experimental work and revised the manuscript, PRR revised the manuscript, TR undertook real-time PCR aspects of the work and revised the manuscript, Z-H Y revised the manuscript.
References
Able J, Atienza S (2014) Special issue: durum wheat for the future: challenges, research and prospects in the 21st century. Crop Pasture Sci 65:1–124
Ayala-Navarrete LI, Mechanicos AA, Gibson JM, Singh D, Bariana HS, Fletcher J, Shorter S, Larkin PJ (2013) The Pontin series of recombinant alien translocations in bread wheat: single translocations integrating combinations of Bdv2, Lr19 and Sr25 disease-resistance genes from Thinopyrum intermedium and Th. ponticum. Theor Appl Genet 126:2467–2475
Byrt CS, Platten JD, Spielmeyer W, James RA, Lagudah ES, Dennis ES, Tester M, Munns R (2007) HKT1;5-like cation transporters linked to Na+ exclusion loci in wheat, Nax2 and Kna1. Plant Physiol 143:1918–1928
Cai S, Bai G-H, Zhang D (2008) Quantitative trait loci for aluminum resistance in Chinese wheat landrace FSW. Theor Appl Genet 117:49–56
Ceoloni C, Biagetti M, Ciaffi M, Forte P, Pasquini M (1996) Wheat chromosome engineering at the 4x level: the potential of different alien gene transfers into durum wheat. Euphytica 89:87–97
Cosic T, Poljak M, Custic M, Rengel Z (1994) Aluminum tolerance of durum-wheat germplasm. Euphytica 78:239–243
Delhaize E, Ryan PR (1995) Aluminum toxicity and tolerance in plants. Plant Physiol 107:315–321
Delhaize E, Ryan PR, Hebb DM, Yamamoto Y, Sasaki T, Matsumoto H (2004) Engineering high-level aluminum tolerance in barley with the ALMT1 gene. Proc Natl Acad Sci 101:15249–15254
Delhaize E, Ma JF, Ryan PR (2012) Transcriptional regulation of aluminium tolerance genes. Trends Plant Sci 17:341–348
Dubcovsky J, Maria GS, Epstein E, Luo MC, Dvorak J (1996) Mapping of the K+/Na+ discrimination locus Kna1 in wheat. Theor Appl Genet 92:448–454
Dvorak J, Gorham J (1992) Methodology of gene-transfer by homoeologous recombination into Triticum turgidum—transfer of K+/Na+ discrimination from Triticum aestivum. Genome 35:639–646
Dvorak J, Noaman MM, Goyal S, Gorham J (1994) Enhancement of the salt tolerance of Triticum turgidum by the Kna1 locus transferred from the Triticum aestivum chromosome 4D by homoeologous recombination. Theor Appl Genet 87:872–877
Habash DZ, Kehel Z, Nachit M (2010) Genomic approaches for designing durum wheat ready for climate change with a focus on drought. J Exp Bot 61:1249
Han C, Ryan PR, Yan Z, Delhaize E (2014) Introgression of a 4D chromosomal fragment into durum wheat confers aluminium tolerance. Ann Bot 114:135–144
James RA, Blake C, Zwart AB, Hare RA, Rathjen AJ, Munns R (2012) Impact of ancestral wheat sodium exclusion genes Nax1 and Nax2 on grain yield of durum wheat on saline soils. Funct Plant Biol 39:609–618
Joppa LR, Williams ND (1988) Langdon durum disomic substitution lines and aneuploid analysis in tetraploid wheat. Genome 30:222–228
Lindsay MP, Lagudah ES, Hare RA, Munns R (2004) A locus for sodium exclusion (Nax1), a trait for salt tolerance, mapped in durum wheat. Funct Plant Biol 31:1105–1114
Luo MC, Dubcovsky J, Goyal S, Dvorak J (1996) Engineering of interstitial foreign chromosome segments containing the K+/Na+ selectivity gene Kna1 by sequential homoeologous recombination in durum wheat. Theor Appl Genet 93:1180–1184
Ma H-X, Bai G-H, Lu W-Z (2006) Quantitative trait loci for aluminum resistance in wheat cultivar Chinese Spring. Plant Soil 283:239–249
Marais GF, Kotze L, Eksteen A (2010) Allosyndetic recombinants of the Aegilops peregrina-derived Lr59 translocation in common wheat. Plant Breed. 129:356–361
McNeil D, Lagudah ES, Hohmann U, Appels R (1994) Amplification of DNA-sequences in wheat and its relatives—the Dgas44 and R350 families of repetitive sequences. Genome 37:320–327
Mickelson-Young L, Endo TR, Gill BS (1995) A cytogenetic ladder-map of the wheat homoeologous group-4 chromosomes. Theor Appl Genet 90:1007–1011
Moroni JS, Sato K, Scott BJ, Conyers M, Read BJ, Fisher R, Poile G (2010) Novel barley (Hordeum vulgare L.) germplasm resistant to acidic soil. Crop Pasture Sci 61:540–553
Moustakas M, Yupsanis T, Symeonidis L, Karataglis S (1992) Aluminum toxicity effects on durum-wheat cultivars. J Plant Nutr 15:627–638
Navakode S, Weidner A, Lohwasser U, Roder MS, Borner A (2009) Molecular mapping of quantitative trait loci (QTLs) controlling aluminium tolerance in bread wheat. Euphytica 166:283–290
Navakode S, Neumann K, Kobiljski B, Lohwasser U, Boerner A (2014) Genome wide association mapping to identify aluminium tolerance loci in bread wheat. Euphytica 198:401–411
Niu Z, Klindworth DL, Yu G, Friesen TL, Chao S, Jin Y, Cai X, Ohm JB, Rasmussen JB, Xu SS (2014) Development and characterization of wheat lines carrying stem rust resistance gene Sr43 derived from Thinopyrum ponticum. Theor Appl Genet 127:969–980
Qi L, Friebe B, Zhang P, Gill BS (2007) Homoeologous recombination, chromosome engineering and crop improvement. Chromosome Res 15:3–19
Raman H, Stodart B, Ryan PR, Delhaize E, Emebiri L, Raman R, Coombes N, Milgate A (2010) Genome-wide association analyses of common wheat (Triticum aestivum L.) germplasm identifies multiple loci for aluminium resistance. Genome 53:957–966
Ryan PR, Raman H, Gupta S, Horst WJ, Delhaize E (2009) A second mechanism for aluminum resistance in wheat relies on the constitutive efflux of citrate from roots. Plant Physiol 149:340–351
Ryan PR, Raman H, Gupta S, Sasaki T, Yamamoto Y, Delhaize E (2010) The multiple origins of aluminium resistance in hexaploid wheat include Aegilops tauschii and more recent cis mutations to TaALMT1. Plant J 64:446–455
Sasaki T, Yamamoto Y, Ezaki B, Katsuhara M, Ahn SJ, Ryan PR, Delhaize E, Matsumoto H (2004) A wheat gene encoding an aluminum-activated malate transporter. Plant J 37:645–653
Sissons M (2008) Role of durum wheat composition on the quality of pasta and bread. Food. 2:75–90
Sissons M, Pleming D, Margiotta B, D’Egidio MG, Lafiandra D (2014) Effect of the introduction of D-genome related gluten proteins on durum wheat pasta and bread making quality. Crop Pasture Sci 65:27–37
Tovkach A, Ryan PR, Richardson AE, Lewis DC, Rathjen TM, Ramesh S, Tyerman SD, Delhaize E (2013) Transposon-mediated alteration of TaMATE1B expression in wheat confers constitutive citrate efflux from root apices. Plant Physiol 161:880–892
Tsunewaki K (1992) Aneuploid analyses of hybrid necrosis and hybrid chlorosis in tetraploid wheats using the D-genome chromosome substitution lines of durum-wheat. Genome 35:594–601
von Uexküll HR, Mutert E (1995) Global extent, development and economic impact of acid soils. Plant Soil 171:1–15
Wang XW, Lai JR, Liu GT, Chen F (2002) Development of a scar marker for the Ph1 locus in common wheat and its application. Crop Sci 42:1365–1368
Zhang P, Li WL, Friebe B, Gill BS (2004) Simultaneous painting of three genomes in hexaploid wheat by BAC-FISH. Genome 47:979–987
Zhou L-L, Bai G-H, Ma H-X, Carver BF (2007) Quantitative trait loci for aluminum resistance in wheat. Mol Breed 19:153–161
Acknowledgments
C.H. thanks the China Scholarship Council for financial support of a studentship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Communicated by X. Xia.
Electronic supplementary material
Below is the link to the electronic supplementary material.
122_2015_2661_MOESM1_ESM.docx
Supplementary material 1 (DOCX 12 kb) Supplementary Fig. S1. Relative quantity of D-genome in the TaMATE1B line as estimated by a RT-qPCR assay of the Dgas family of sequences. The Dgas sequence is expressed as a ratio of the 28S ribosomal RNA sequences with the data normalized to show the value of a durum line that has the long arm of chromosome 4D (LF line) set at 1.0. The durum wheat cv Jandaroi was used as a negative control for the D-genome. Error bars show the SE with n = 3. Bars with different letters denote statistically different values at P < 0.05 as determined with a one-way ANOVA
Rights and permissions
About this article
Cite this article
Han, C., Zhang, P., Ryan, P.R. et al. Introgression of genes from bread wheat enhances the aluminium tolerance of durum wheat. Theor Appl Genet 129, 729–739 (2016). https://doi.org/10.1007/s00122-015-2661-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-015-2661-3