Abstract
Scrapie is a naturally occurring transmissible spongiform encephalopathy (TSE) in sheep, goats and moufflons almost worldwide and is known for about 270 years. It is characterised by the accumulation of an abnormal isoform (PrPSc) of host-encoded prion protein (PrPC) in the central nervous system which leads to progressive neurodegeneration and death. Scrapie represents the prototype of the so-called prion diseases. It is observed to date as two types, classical and atypical scrapie. The susceptibility to both types is modulated by polymorphisms of the prion protein gene. Whereas classical scrapie is clearly a naturally occurring contagious disease, atypical scrapie is most probably non-contagious and caused by an age-related spontaneous misfolding of the prion protein. This review gives an overview on the current knowledge of classical and atypical scrapie in sheep and goats with special emphasis on epidemiology, clinical and pathological signs, genetic susceptibilities, diagnosis and scrapie prion strains.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Overview
Scrapie is the most common name for transmissible spongiform encephalopathy (TSE), which affects sheep, goats and moufflons almost worldwide. Like all other prion diseases, scrapie is a neurodegenerative progressive and eventually fatal disease. Scrapie is associated with a number of clinical signs ranging from subtle behavioural abnormalities to more obvious neurological signs. The clinical diagnosis needs to be confirmed by the demonstration of pathognomonic spongiform lesions which are associated with an immunodetection of pathological prion protein (PrPSc) depositions in the central nervous system (CNS) primarily (OIE 2009). PrPSc depositions can be revealed by immunohistochemical and biochemical methods (see Chap. 33). To date, two distinct scrapie types are known: classical and atypical scrapie.
2 History
Scrapie is not only the prototype of TSEs but also the prion disease with the longest history of publication. The first authentic report on scrapie was written in Germany and dates back to the year 1750 (Leopoldt 1750). However, a later publication (Comber 1772) even mentions cases in England that occurred already in 1732. Several authors at later times even referred to much earlier time periods, spanning from Roman times up to the seventeenth century, but without giving corresponding references (for a detailed review, see Schneider et al. 2008). Moreover in former times, many sheep diseases were confused with scrapie. Other difficulties were the various names that were used to describe this disease throughout Europe: “Goggles”, “Ricketts”, “Rubbing Disease” and “Trotting Disease” in England, “Scratchie” and “Yeukie pine” in Scotland, “Basqvilla Disease” in Spain, “La maladie convulsive”, “La Tremblante” and “Prurigo lumbaire” in France, “Rida” in Iceland, “Gnave-og travesjuke” in Norway and “Gnubberkrankheit”, “Petermännchen”, “Traber” or “Reiberkrankheit” in Germany. Altogether, at least 42 different names were used in Europe and India (Schneider et al. 2008) for this disease in small ruminants.
The infectious nature of scrapie was already reckoned in the eighteenth century (Leopoldt 1750). In the following decades and centuries, different transmission routes were discussed in which sexual intercourse was the most suspected modus. However, among other causes like atmospheric disturbances, a few authors proposed a mere coexistence of infected and non-infected animals or a spontaneous origin of the diseases (Schneider et al. 2008). In addition, broad consent existed already in the nineteenth century concerning the role of hereditary factors for scrapie. Initially, a hereditary predisposition and the transmission by asymptomatic animals were assumed (Thaer 1821; von Richthofen 1821) and even the existence of hereditary and non-hereditary scrapie forms was postulated (von Richthofen 1826).
A number of experimental transmission studies were subsequently carried out in order to clarify the origin and transmission routes of scrapie. These experiments included contact studies with infected and non-infected sheep and subcutaneous and intravenous inoculation studies using different tissues and bodily fluids from infected animals. However, most of these studies were terminated prematurely and therefore failed due to the long incubation period of scrapie (for a detailed review, see Schneider et al. 2008). However, in 1936, the transmissibility of scrapie was first time proven by experimental inoculation of healthy animals with the brain and spinal cord of diseased sheep. In this experiment, the inoculated animals were kept for longer periods of time, and sheep could develop scrapie after incubation periods of up to 2 years (Cuille and Chelle 1936, 1938a, b).
Since the 1930s, scrapie research was intensified when substantial financial losses to the sheep industry were caused by increasing numbers of cases. These losses prompted also studies on the true nature of the infectious agent. Besides parasites (M’Gowan 1914) and bacteria (Bastian 1979) as causative agents, virus infection was the most commonly proposed theory, already formulated in 1938 (Cuille and Chelle 1938a, b). In 1954, the term “slow virus infection” was first introduced (Sigurdsson 1954). However, already in 1966, an alternative to the virus origin was postulated as the causative agent, that is, polysaccharides (Alper et al. 1966, 1967; Field 1966) or lipids (Alper et al. 1978). In 1967, for the first time, a protein was assumed as infectious agent (Pattison and Jones 1967) and the first “protein-only-hypothesis” was enunciated (Griffith 1967) followed in the 1970s by the “virino” theory (Dickinson and Outram 1979). Finally, based on the resistance of the pathogen, in 1982, the term “proteinaceous infectious particle” (acronym: prion) was introduced (Prusiner 1982) and the conversion of a normal cellular protein (PrPc) into a pathological isoform (PrPSc) as a key event of TSE pathogenesis was postulated shortly after (Oesch et al. 1985). PrPSc is currently considered to be the biochemical marker and the causative agent of TSEs. However, the prion theory is still debated since PrPSc is not always infectious and the phenomenon of strains is still an enigma (Lasmezas et al. 1997; Piccardo et al. 2007).
In 1998, the atypical form of scrapie, termed Nor98, was first time discovered in Norwegian sheep (Benestad et al. 2003). However, retrospective studies revealed atypical scrapie cases in the UK already in the late 1980s. Therefore, this disease is not considered as a new emerging form of TSE (Bruce et al. 2007). Atypical scrapie is distinguished from classical scrapie by the genotypes affected, the clinical and epidemiological characteristics as well as by the molecular pattern and the (neuro)anatomical spread of PrPSc (for review see Cassman and Greenlee 2019; EFSA 2021a). The disease can be found in sheep and goats and shows clear characteristics of a rare disease with a homogenous prevalence across countries, surveillance streams and year of examination (Fediaevsky et al. 2008). It is not rare compared to classical scrapie in most countries. Based on current knowledge a recent Scientific Opinion from EFSA (2021a) concluded that atypical scrapie is more likely a non-contagious rather than a contagious disease. Scrapie in goats was initially described after an experimental exposure in 1939 (Cuille and Chelle 1939) and the first natural case was reported a few years later (Chelle 1942). The first experimental challenge of goats with sheep scrapie showed 100% susceptibility suggesting that goats are highly susceptible (Pattison et al. 1959; Cuille and Chelle 1939). Like classical scrapie, atypical scrapie cases were reported also in goats (Fediaevsky et al. 2008, for a detailed review, see Vaccari et al. 2009) but showed a lower prevalence as compared to sheep (EFSA 2010).
In moufflons, only classical scrapie was reported at least in six natural cases so far (Wood et al. 1992a, b).
3 Geographical Distribution and Surveillance
At the turn of the millennium, classical scrapie cases were still widespread in small ruminants in Europe, and thousands of animals died because of the disease every year. As a result of surveillance, eradication and resistance breeding programmes, however, the number of cases has since fallen significantly. As a result, in 2020, classical scrapie cases were recorded only in Spain (273), Greece (203), Italy (115), Iceland (53), Romania (57), Bulgaria (13) and the United Kingdom (2). East Timor, Israel, Ivory Coast, Japan, Palestinian Autonomous Territories and the USA reported also scrapie cases (atypical and/or classical) in the last 5 years. Only individual atypical scrapie cases were documented on the Falkland Islands and New Zealand (World Animal Health Data Base (OIE-WAHIS)).
An introduction of classical scrapie via imported sheep from the UK was suspected in countries like Australia and New Zealand (1952–1954), South Africa (1964–1972), Colombia (1968–1971) and Kenya (1970). After thorough eradication by slaughtering the imported sheep and their flock mates, Australia and New Zealand remained free of classical scrapie to date (OIE-WAHIS).
However, the true scrapie status of many countries remains unknown because there is usually only an inadequate passive surveillance system in place to detect infected animals. It is nearly impossible to establish freedom from infection without establishing an active surveillance system, which includes the examination of fallen stock and emergency slaughter (Detwiler and Baylis 2003, OIE 2009). This is exemplified by the introduction of a harmonised active surveillance programme for scrapie in sheep and goats throughout the EU in 2003. In the context of this programme, defined numbers of sheep and goats over 18 months of age (fallen stock, emergency slaughter, as well as healthy slaughtered animals) were examined for TSE.
4 Prion Protein Gene and Susceptibility
It has been shown in several epidemiological studies that the successful transmission of classical scrapie requires genetically susceptible sheep. In the year 1968, the effect of a so-called Sinc-gene (scrapie incubation gene) on the length of the incubation period of experimentally infected mice and a synonymously so-called Sip-gene (scrapie incubation period gene) in sheep were proposed (Dickinson et al. 1968a, b). Eventually, different polymorphisms of the prion protein gene (Prnp) were matched in the 1980s and 1990s with the Sip-/Sinc-genes (Oesch et al. 1985; Westaway et al. 1987; Goldmann et al. 1991; Moore et al. 1998; Hunter et al. 1996).
The murine Prnp consists of two alleles, s7 and p7, which differ in their PrP amino acid sequence at codons 108 and 189 and are associated with short or prolonged incubation times after infection with particular (i.e., ME-7) experimental strains. However, infections with other strains (i.e., 22A) showed reversed results (Dickinson et al. 1968a). Thus, the susceptibility and incubation period is determined at least by two factors: the genotype of the host and the agent strain. Similar results were obtained in sheep.
The ovine Prnp is located on chromosome 13 (Iannuzzi et al. 1998) and the functional length of the PrP gene is approximately 21 kb and is composed of three exons, from which exon III contains the complete uninterrupted open reading frame (ORF). The length of the unprocessed precursor protein is 256 amino acids. After post-translational modifications, about 210 amino acids remain in the mature protein (for a detailed review see Goldmann 2008).
Ovine PrP polymorphisms influence not only the susceptibility to the disease but also modulate the progression including the incubation period and clinical signs. The vast majority of polymorphisms are due to single nucleotide polymorphisms (SNP) in the DNA, which often cause single amino acid changes. Of particular interest are polymorphisms at codons 136, 154 and 171 within the ORF, which are clearly linked to scrapie susceptibility in sheep (Goldmann 2008). Standard abbreviations describe the alleles in reference to the three codons:
-
A136V in which alanine (A) is associated with resistance and valine (V) is associated with susceptibility (Goldmann et al. 1991; Hunter et al. 1994).
-
Q171R in which arginine (R) is associated with resistance and glutamine (Q) is associated with susceptibility (Westaway et al. 1994; Clouscard et al. 1995; O’Rourke et al. 1997).
-
R154H in which histidine (H) is associated with resistance (Goldmann et al. 1991; Laplanche et al. 1993).
The polymorphisms mentioned above result in five different alleles (ARQ, VRQ, AHQ, ARR and ARH), leading to 15 different genotypes, which are the only alleles with significant distribution worldwide (Goldmann 2008). Some further genotypes, ARK and TRQ among others, are known (Gombojav et al. 2003; Guo et al. 2003; Billinis et al. 2004), but due to their low frequencies, they are not included in a TSE genotype classification system (Dawson et al. 1998). This five-group risk classification (Table 26.1) is the basis for breeding and scrapie eradication programmes applied in the EU. The highest risk to develop scrapie carry VRQ/VRQ animals, the highest genetic resistance is associated with ARR/ARR sheep (Belt et al. 1995; Hunter et al. 1996; Hunter 1997). However, this classification is subject to restrictions as, for example, at least two ARR/ARR sheep from different flocks in France and Germany have been shown to be subclinical carriers of classical scrapie (Groschup et al. 2007). Additionally, ARQ/ARQ animals, classified in R3, can be at the highest risk in flocks where the VRQ allele is absent for example due to breed (Goldmann 2008).
Furthermore, several polymorphisms are described at other positions, for example 25% of all ARQ alleles revealed additional polymorphisms (Goldmann 2008). However, it is unclear whether such polymorphisms have a profound effect on the disease. Some studies refer to resistance and/or prolonged incubation times in sheep carrying for example AC151RQ, AT137RQ or ARQK176 (Vaccari et al. 2009; Acin et al. 2004; Thorgeirsdottir et al. 1999).
The classification system described above and in Table 26.1 does not work for atypical scrapie. In contrast to classical scrapie in most of the atypical cases, animals of PrP genotype risk groups R1-3 (Benestad et al. 2008) are affected. Most frequently found in such cases are haplotypes such as AHQ/ARQ, AHQ/ARR and ARR/ARR, respectively (for review see EFSA 2021a). It has been shown that polymorphisms at codons 141 (L/F) and 154 (R/H) are linked to susceptibility. Genotype AF141RQ for example encoded for a higher susceptibility than the AL141RQ allele or even the AHQ genotype (Goldmann 2008).
Although the wild-type amino acid sequence of goat and sheep PrP are similar, the PrP genetics in goats is much more variable, yet without polymorphisms at codons 136 and 171 surprisingly. In goats, more than 70 other polymorphisms of the caprine Prnp, resulting in amino acid changes, have been found in different countries and breeds (Vaccari et al. 2009; Goldmann et al. 2011, Fast unpublished results). At least some of them seem to be associated with a certain degree of TSE resistance (for a detailed review see Vaccari et al. 2009 and EFSA 2017):
-
I142M haplotypes show an incomplete resistance with a lengthened incubation period after experimental inoculation and an increased resistance to classical scrapie under natural conditions. However, some classical scrapie cases have been detected in such goats (Goldmann et al. 1996; Barillet et al. 2009; EFSA 2017).
-
G145D was detected in resistant goats in an Italian study (Maestrale et al. 2015), combining experimental and epidemiological approaches. However, the examined animal group was small and no further data exist.
-
R154H haplotypes are associated with some resistance to classical scrapie in different breeds and countries (Barillet et al. 2009; Billinis et al. 2002; Papasavva-Stylianou et al. 2007; Vaccari et al. 2006).
-
N146S/D is associated with profound resistance to classical scrapie in field and experimental studies; however, intracerebral inoculation results in high attack rates but with a significant increase in the incubation period (EFSA 2017). Unfortunately, N146D is confined to goats in Cyprus (Papasavva-Stylianou et al. 2007).
-
R211Q haplotypes have shown to convey an increased resistance to classical scrapie in French case–control studies (Barillet et al. 2009), but positive cases can be regularly found. There seems to be partial resistance and prolonged incubation periods, which might be strain dependent (EFSA 2017).
-
Q222K haplotype is associated with protection against classical scrapie in several breeds and countries, demonstrated in epidemiological and experimental studies. However, protection against intracerebral challenge is incomplete, and few heterozygous animals are reported to be naturally infected (Acutis et al. 2006; Barillet et al. 2009; Vaccari et al. 2006; EFSA 2017).
In summary, in the Scientific Opinion published by EFSA (2017) a ranking of alleles was given, which is based on “weight of evidence” and “strength of resistance”. This is, from high to weak scrapie resistance: K222 > D146 = S146 > Q211 = H154 = M142. As a consequence, both haplotypes 146S/D and 222 K can now be used for TSE resistance breeding and eradication programmes for goats. Most interestingly, Benestad et al. reported a single goat with a PrP-null allele in Norway (2012), which is resistant to scrapie (Salvesen et al. 2020).
Atypical scrapie in goats is most frequently associated with homozygous H154H and heterozygous R154H genotypes (Moum et al. 2005; Arsac et al. 2007; Seuberlich et al. 2007).
Taken together, the number of factors modulating the susceptibility to scrapie is high. The success of infection depends not only on the genotype of the host and the infectious agent strain but also on individual flocks, breeds and geographical location, not to forget the dose and route of inoculation effects.
5 Epidemiology of Scrapie
Summarising the prevalence of TSE infections in small ruminants worldwide is a difficult task in the face of the long incubation periods, the missing availability of a practical antemortem test (which prevents the detection of subclinical-infected animals), the variable clinical signs (which may result in unidentified animals), the potentially unknown host-encoded genetic components (which influence both the risk of infection and the incubation period) and the not yet fully understood routes of transmission (for detailed reviews concerning the epidemiology of classical and atypical scrapie see Hoinville 1996; Detwiler and Baylis 2003; Benestad et al. 2008; EFSA 2021a).
5.1 Prevalence in the EU
A comprehensive overview of the prevalence of classical and atypical scrapie in the European Union is given by Fediaevsky et al. as well as by EFSA (Fediaevsky et al. 2008, 2010; EFSA 2010, 2014, 2021a).
Following the introduction of active surveillance programmes for TSEs in sheep and goats in the EU in 2002, clearly defined epidemiological data were obtained for the first time. Most importantly, it has been shown that the prevalence of TSE in sheep/goats and the geographical distribution were much higher than originally assumed (EFSA 2014) and the number of cases in fallen stock was significantly higher as compared to healthy slaughter animals (Fediaevsky et al. 2008). Additionally, the prevalence of classical and atypical scrapie showed different patterns with more variation seen in classical scrapie (Fediaevsky et al. 2008). Moreover, the incidence rates of classical scrapie in geographical areas were non-uniform and clustered at the flock level (McIntyre et al. 2008). In some countries only a few, if any, cases were detected, whereas other EU member states experienced large epidemics (EFSA 2014). In 2002, a breeding and scrapie eradication programme was applied in the EU, and the results are heterogeneous (for a detailed review see EFSA 2014). From 2002 to 2012 only in six Member States a significantly decreasing trend was found, which was in all cases associated with an effective implementation of genetic and non-genetic measures for the control of the disease. Thus, a successful classical scrapie eradication policy cannot rely on postmortem testing and depopulation programmes solely, but should also include breeding programmes for resistance to classical scrapie. However, due to the long incubation time and particular pathogenesis of classical scrapie, an underestimation of the real prevalence may apply and substantial numbers of undetected cases (up to 17%) were reported (Jeffrey et al. 2002; Ligios et al. 2006; Reckzeh et al. 2007; González et al. 2009).
The distribution of atypical scrapie cases is remarkably homogenous in space and time as compared to classical scrapie and no infection clusters were observed in positive flocks. In most cases, individual animals are affected instead (Fediaevsky et al. 2008, 2010). The animals (sheep as well as goats) are significantly older usually as compared to animals affected by classical scrapie. In eight EU countries between 2007 and 2009, the incidence of atypical scrapie in healthy slaughtered sheep was similar to or higher than the incidence of classical scrapie. These data suggest that atypical scrapie represents a significant proportion of TSE-infected small ruminants (EFSA 2010). However, relying on the prevalence in the EU and on scientific data it can be concluded that atypical scrapie is most likely a non-contagious disease or has very low transmissibility under natural conditions (Fediaevsky et al. 2010; EFSA 2021a). The prevalence seems to be stable within the EU, with >100 cases on average per year for sheep and 10 cases for goats (EFSA 2020). Atypical scrapie is most frequently detected by active surveillance in fallen stock animals. Due to the generally used passive surveillance approach, atypical scrapie may also be underreported. Other problems in estimating the exact prevalence of atypical scrapie include the age-dependent variations and the inconsistent detection of atypical scrapie by using brainstem samples (Benestad et al. 2008), the low sensitivity of some rapid tests for atypical scrapie (EFSA 2005) and the absence of detectable pathological prion protein in the lymphoreticular tissues.
5.2 Transmission Routes in Scrapie
In the last centuries, a lengthy discussion about the mode of transmission of scrapie took place (Schneider et al. 2008), and even up to now the exact transmission routes are not resolved entirely. It is known that classical scrapie can transmit laterally between sheep under natural conditions. Such transmissions occur either via direct contact or through contamination of the environment. The oral route is most efficient (Jeffrey and Gonzalez 2007; van Keulen et al. 2008). Scrapie in goats is often found in mixed herds with sheep, but it has also been observed to spread from goat to goat (Wood et al. 1992b).
The main source of infection is the infectious placenta. Infectivity and PrPSc have been detected in the foetal parts of the placenta, depending on the genotype of the offspring (Pattison et al. 1972; Onodera et al. 1993; Race et al. 1998; Andreoletti et al. 2002; Alverson et al. 2006; Lacroux et al. 2007; O’Rourke et al. 2011). More recently, PrPSc and infectivity have also been detected in foetal tissue samples (Garza et al. 2011; Spiropoulos et al. 2014). The placenta and the amniotic fluid (Hoinville 1996) are shed into the environment during lambing and their ingestion by other sheep (and goats) is still assumed to be the most important infection mode within the flock (Pattison et al. 1972; Hoinville 1996). Moreover, it has been shown that scrapie agent remains infectious even after years in the environment (Brown and Gajdusek 1991; Seidel et al. 2007). Anecdotal data indicate even survival of infectivity for more than 16 years (Georgsson et al. 2006). Additional results indicate that released PrPSc may be sequestered near the soil surface and bound on soil minerals, which may then be ingested during grazing of farm animals (Johnson et al. 2006, for a detailed review, see Smith et al. 2011). Even more interestingly, amplifiable classical scrapie PrPSc has been detectable on surfaces of farm fomites and such fomites (i.e. water troughs) are sufficient to transmit the disease to naive sheep even after decontamination of barns, indicating that PrPSc in the dust was the source (Konold et al. 2015; Gough et al. 2015, 2018).
Besides the placenta, faeces (Terry et al. 2011) and milk (Konold et al. 2008; Lacroux et al. 2008; Maddison et al. 2009; Madsen-Bouterse et al. 2018) have been shown to contain PrPSc and/or infectivity. Recent results revealed PrPSc also in the oral cavity of scrapie-infected sheep (Maddison et al. 2010; Gough et al. 2011) and PrPSc and/or infectivity in urine was demonstrated in experimental scrapie models in hamsters and mice (Seeger et al. 2005; Gonzalez-Romero et al. 2008; Gregori et al. 2008). More artificial routes demonstrated in several experimental infections include transmissions via subcutaneous inoculation (Stamp et al. 1959; Kratzel et al. 2007), conjunctival exposure (Haralambiev et al. 1973), skin scarification (Taylor et al. 1996) and blood transfusions (Houston et al. 2008). Some scrapie infections were consequences of iatrogenic transmissions due to contaminated vaccines (Gordon 1946; Caramelli et al. 2001).
In most flocks, only a single case of atypical scrapie is found. The transmission mode of atypical scrapie under natural conditions is not understood at all and it is even questioned whether this disease is contagious under all circumstances. The intracerebral route of infection has been clearly established in both rodent and susceptible (AHQ/AHQ) sheep models (Le Dur et al. 2005; Simmons et al. 2007, 2010), but silent wild-type carriers (ARQ/ARQ) are also known (Okada et al. 2016). Under experimental conditions, an oral challenge of newborn lambs within 24-h post-partum was successful in AHQ homozygous sheep (Simmons et al. 2011). Epidemiological data obtained by active surveillance programmes indicate that the capacity of atypical scrapie to transmit disease within the herd under field conditions is quite low and most probably non-existent (Fediaevsky et al. 2009, 2010). This could be due to the fact that affected sheep shed little or no PrPSc infectivity, as atypical scrapie does not spread or only very little in peripheral tissues compared to classical scrapie (EFSA 2021a). However, also cohort cases of atypical scrapie are reported in flocks and also coinfections with classical scrapie in some herds (Konold et al. 2007a, b; Onnasch et al. 2004; Orge et al. 2010). Taken together, these data support the theory of a spontaneous origin of the disease, which might be associated with a very low or absent natural transmissibility (Benestad et al. 2003; Moum et al. 2005; Hopp et al. 2006; Green et al. 2007). Retrospective studies indicate that large flock sizes (>1000 sheep), over-average animal exchanges within flocks and vitamin and mineral feed supplements may be risk factors for atypical scrapie (Hopp et al. 2006; Green et al. 2007).
5.3 Incubation Period
The incubation time of scrapie depends on the infection route and the animal’s age at infection, its genotype, the involved agent strain and the infectious dose. Interestingly, certain strains proliferate easier in specific genotypes. Iatrogenic infections lead to slightly shorter incubation periods (Caramelli et al. 2001). In classical scrapie, sheep come down with clinical disease usually between 2 and 5 years of age, with an average age of 47.8 months in the time period 2002–2020 (EFSA 2020). Although both sexes appear to be equally affected, disease manifestations in rams occur often at a slightly younger age (Parry 1983; Wineland et al. 1998; Lühken et al. 2007; McIntyre et al. 2008). However, also shorter and longer incubation periods ranging between 1 and up to 11 years are reported (Parry 1983). Scrapie-diseased animals younger than 18 months are fairly rare (Dickinson and Stamp 1969). However, it is usually not possible to tell the time of infection in older scrapie-diseased sheep (Detwiler and Baylis 2003).
The frequency of atypical scrapie cases increases with the age of the animals, thus the average age of atypical scrapie cases in sheep and goats is significantly higher than that of classical scrapie (EFSA 2021b). Atypical cases in sheep are on average 82 months of age (EFSA 2021b). In a German (Lühken et al. 2007) and in a larger pan-European (20 countries) study, almost 60% or 70% of the atypical scrapie cases were 5 years or older, respectively (Fediaevsky et al. 2008).
As for sheep, the incubation period of goats is influenced by the genotype (Goldmann 2008). Data concerning the age distribution of TSE-infected goats are rare but indicate similarities to the distribution in sheep scrapie. The mean age of goats affected by classical scrapie from 2002 to 2020 was 51.6 months (EFSA 2021b); however, cases up to 10 years of age were also reported (Brotherston et al. 1968; Hourrigan et al. 1969; Harcourt and Anderson 1974; Wood et al. 1992b; Capucchio et al. 1998; Konold et al. 2007b; Papasavva-Stylianou et al. 2010; Niedermeyer et al. 2016). Atypical scrapie cases in goats from 2002 to 2020 have a mean age of 84 months (EFSA 2021b).
5.4 Pathogenesis and Tissue Distribution of PrPSc and/or Infectivity
The pathogenesis of TSEs is discussed separately in Chap. 27. Nevertheless, the most important facts are summarised here and in Fig. 26.1.
Schematic illustration of the most common theories concerning the pathogenesis of classical scrapie (Modified from van Keulen et al. 2002; Sisó et al. 2010). The time periods stated are from different studies showing PrPSc accumulation by immunohistochemistry (Andreoletti et al. 2000, 2002, 2004; van Keulen et al. 2000, 2002; Jeffrey et al. 2006; Everest et al. 2011), which mostly rely on VRQ/VRQ sheep. ARQ sheep and experimentally infected goats revealed (as far as known) similar distribution but delayed dynamics. In ARR sheep PrPSc is mainly confined to the CNS. Dotted arrows indicate possible but not yet clarified routes of dissemination. LN lymphonodus, GALT gut-associated lymphoid tissue, GIT gastrointestinal tract, PP Peyer’s patches, ENS enteric nervous system, ANS autonomous nervous system, PNS peripheral nervous system, CMGC celiac and mesenteric ganglion complex, IL Ileum, Duod duodenum
After oral uptake, it still remains an enigma how the infectious agent overcomes the mucosal barrier of the gut (for a detailed review see Mabbott and MacPherson 2006). The first results indicate that the genotype does not affect this process (Jeffrey et al. 2006). M cells within the follicle-associated epithelium of the gut and specialised for the transport of macromolecules are important sites of PrPSc uptake (Donaldson et al. 2012). Transport across the villous enterocytes (Jeffrey et al. 2006; Akesson et al. 2011) and a direct uptake by processes of dendritic cells extending into the gut lumen (Rescigno et al. 2001) are further options. In this regard, there is experimental evidence that dendritic cells play a crucial role in the transport of PrPSc towards follicular dendritic cells (FDC) within Peyer’s patches (PP) (Bradford et al. 2017). After crossing, the mucosal barrier PrPSc was found within 15 min after inoculation in the lacteals of the villi (Jeffrey et al. 2006; Akesson et al. 2011). The first accumulation of PrPSc was seen in the gut-associated lymphoid tissues (GALT) of the tonsil and PP in the intestines in lambs as early as 21-day post-partum (Andreoletti et al. 2000, 2002; van Keulen et al. 2002). Experimental infections indicate a rapid transport of inoculum into the GALT and corresponding lymph nodes, but replication and accumulation of de novo PrPSc were not seen before 1-month post-infection (Jeffrey et al. 2006). Experimental data demonstrate that FDC is the target cell for prion replication in lymphoid tissues (McCulloch et al. 2011). As shown in naturally infected lambs, the accumulation of PrPSc is restricted to the GALT and mesenteric lymph nodes for the first 2 months of age (Andreoletti et al. 2000, 2002; van Keulen et al. 2002). Subsequently in lambs older than 2 months, a spread to all lymph nodes of the lymphoreticular system (LRS) takes place and the amount of PrPSc in the LRS increases with age up to a plateau level around 6 months (Andreoletti et al. 2000). At this time, after one-third of the incubation period, infectivity is found first time in blood with increasing tendency until to the clinical stage (Houston et al. 2008). The enteric nervous system (ENS) of the duodenum and the ileum are the first parts of the peripheral nervous system, which become affected after 5 months (van Keulen et al. 2000). The exact route of infection is not understood completely yet, especially whether a prion replication in the GALT is necessary for further neuroinvasion. In this regard, sheep with more resistant genotypes fail to accumulate PrPSc in the LRS. For example, sheep of the VRQ/ARR genotype have no or only low amounts of PrPSc in the lymphoid tissues but develop scrapie albeit only after longer incubation periods (Bossers et al. 1996; van Keulen et al. 1996). Thus, a direct infection via subepithelial nerve endings or an indirect infection via infected Peyer’s patches and submucosal plexus of the ENS are conceivable (Jeffrey et al. 2006; van Keulen et al. 2008). With the progression of the disease starting at 14 months, PrPSc spreads within the ENS in all directions and other parts of the small intestine and at later stages (21–26 months) even the oesophagus, forestomach, large intestine and rectum become involved (van Keulen et al. 2000). Along parasympathetic and/or sympathetic nerve fibres, prions ascend after 10 months via the celiac and mesenteric ganglion complex to the spinal cord and/or brainstem (van Keulen et al. 2000). From these sites in the CNS, a further ascending and descending spread of PrPSc takes place (van Keulen et al. 2008).
There is also evidence for the hematogenous spread of the scrapie agent demonstrated by the transmission of the disease via blood and blood particles (Houston et al. 2008; Dassanayake et al. 2015) as well as by the early detection of PrPSc in the Circumventricular Organ of the CNS, an area without a blood–brain barrier (Siso et al. 2009).
Between 7 and 10 months of age, PrPSc can be demonstrated first time in the brainstem and spinal cord of young VRQ sheep (Andreoletti et al. 2000; Jeffrey et al. 2001; van Keulen et al. 2002). At 13 months of age, PrPSc is eventually identified in skeletal muscle (Andreoletti et al. 2004) and after 20–30 months in the liver of naturally infected sheep (Everest et al. 2011).
Most of the aforementioned data were obtained for VRQ/VRQ animals, which are considered to be most susceptible and having a comparatively fast dissemination dynamic. Only limited data are available for sheep of other genotypes (Jeffrey et al. 2001; Lacroux et al. 2008). However, these data indicate that the topology and the timing of the PrPSc dissemination in ARQ/ARQ and ARQ/VRQ sheep are quite similar, apart from a slightly delayed dynamic in ARQ carriers (EFSA 2010). There are only a few reports on classical scrapie in heterozygous ARR sheep, perhaps due to the lower susceptibility of animals carrying this genotype. In such cases, PrPSc is mainly confined to the CNS (van Keulen et al. 1996; Greenlee et al. 2014).
The dissemination dynamics of classical scrapie in goats is well documented but relies mostly on experimentally challenged wild-type goats. The spread of PrPSc during the prion ascension seems to be quite similar to classical scrapie in sheep (EFSA 2009; González et al. 2009, 2010a, b; Niedermeyer et al. 2016). However, a French study shows that the time course may be prolonged as compared to scrapie in sheep. In goat kids infected around birth, PrPSc was detectable in the GALT not before 4 months of age, peripheral lymphoid tissues turned PrPSc positive after 6 months of age and the CNS showed the first PrPSc accumulations at 18 months of age. In skeletal muscle, PrPSc was not detected before 21 months of age (EFSA 2010).
However, it should be noted that there is a high diversity of classical scrapie strains in sheep and goats. Their interaction with the particular host genotypes may result in different dissemination dynamics. Therefore, the tissue distribution described above cannot be considered as definitive (EFSA 2010). For example, several ARQ/VRQ and ARQ/ARQ sheep and some goats affected with classical scrapie were reported with few, if any detectable PrPSc in the LRS (Jeffrey et al. 2002; Ligios et al. 2006; Konold et al. 2007a, b; González et al. 2009; Niedermeyer et al. 2016). Additionally, results from experiments of scrapie-infected I142M goats revealed that the dissemination of the TSE agent in peripheral tissues is delayed as compared to wild-type goats (EFSA 2010).
The limited data concerning the tissue distribution of atypical scrapie indicate that detectable amounts of PrPSc seem to be confined to the CNS (Benestad et al. 2003, 2008; Simmons et al. 2007; Vidal et al. 2008). However, in mouse bioassays, infectivity was shown in the absence of any detectable PrPSc in peripheral tissues including the LRS (Andreoletti et al. 2011; Simmons et al. 2011).
6 Clinical Signs
Clinical signs are quite variable in different breeds, flocks, regions and countries and are influenced by genotype, agent strain and stage of the disease (for a detailed review see Parry 1983; Ulvund 2007, 2008).
The clinical phase mostly progresses slowly over several weeks and months, but acute onsets and durations up to 1 year with intermittent remission of the signs are also seen. Recumbent or sudden deaths of animals were recorded (Parry 1983; Clark et al. 1994; Capucchio et al. 2001; Healy et al. 2003; Humphrey et al. 2004).
Deficits in the disease recognition by shepherds/veterinarians, the subtle onset, the variability of signs as well as the slow clinical progression of the disease are reasons why the disease often remains unidentified. Isolation of animals from the flock is often the first clinical sign. More specific symptoms at the early stage are central nervous system deficits and loss of wool caused by pruritus. Affected animals may appear normal but stimulated by stress (i.e., sudden noise, excessive movement and handling) tremor becomes obvious. At later stages, the animal may even fall down into a convulsive state (Hörnlimann et al. 2007; Ulvund 2007). Clinical signs of scrapie fall into five different categories (Ulvund 2008):
-
General signs: Depression, wool loss, regurgitation and cardiac arrhythmia.
-
Changes in behaviour: Head tremor, altered mental status, nibble response (reflex), teeth grinding, altered head carriage, hyperresponsive, anxious, apprehensive, salivation, aggressiveness and reluctance to be milked.
-
Changes in sensitivity: Pruritus, “cannibalism”, allotriophagia and biting.
-
Changes in locomotion: Hind limb ataxia, dysmetria, abnormal posture, hind limb weakness and circling.
-
Other signs: Weight loss, labial oedema, visual impairment, brief epileptiform attacks and hypogalactia.
Not all symptoms are always present, but usually at least more than one is noticeable (Hörnlimann et al. 2007). Moreover, a nervous form may dominate in one flock, while the pruritic form prevails in another (Ulvund 2008). In general, head tremor, nibble response, hyperresponsiveness, salivation, pruritus and weight loss are the most often reported symptoms in different flocks and countries (Healy et al. 2003; Capucchio et al. 2001; Ulvund 2007; Vargas et al. 2005). The final stages are characterised by massive weight losses often despite of unchanged appetite and recumbency due to severe ataxia (Hörnlimann et al. 2007; Ulvund 2007).
Data on clinical signs in classical scrapie infected goats are rare and most authors refer to symptoms as described for sheep. Disease durations from 1 up to 3 months are described (Capucchio et al. 1998; Foster et al. 2001; Konold et al. 2007b). The most frequent signs described are weight loss despite of remaining appetite, ataxia and progression to recumbency and pruritus. Behavioural changes include apathy, nervousness or aggressiveness. Less frequently found symptoms are sometimes confined to single animals and include lateralisation of neurological signs such as circling, biting, ptyalism, hyperaesthesia, dribbling/regurgitation, visual impairment, difficulties with milk and tremor (Brotherston et al. 1968; Hourrigan et al. 1969; Harcourt and Anderson 1974; Wood et al. 1992a, b; Capucchio et al. 1998; Foster et al. 2001; Konold et al. 2007a, b).
Only a few reports are describing clinical signs of atypical scrapie in sheep and goats. This could be interpreted as if there was a less pronounced clinical phase. However, since normally only singleton animals are affected, they are recognised not quite well by veterinary professionals (Benestad et al. 2008). The overall clinical signs of atypical scrapie are ataxia and weight loss and behavioural changes such as nervousness and anxiety. Circling movements of the sheep may also occur. The tremor was hardly seen and—with the exception of two British cases—alopecia due to pruritus did not occur. Animals die unexpectedly or after a very acute progression phase. One goat was described as having blindness, stiff gait and apathy (Benestad et al. 2003; Gavier-Widen et al. 2004; Onnasch et al. 2004; Epstein et al. 2005; Nentwig et al. 2007; Simmons et al. 2007; Dagleish et al. 2008).
None of the clinical signs described above, in combination or alone, are pathognomonic for scrapie. Therefore, the clinical diagnosis must always be confirmed by laboratory investigations (Ulvund 2007, 2008).
7 Diagnosis of Scrapie
The diagnosis of TSEs is discussed separately in Chap. 35. Nevertheless, the most important facts are summarised here.
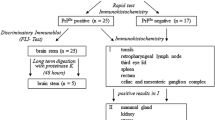
The TSE surveillance in small ruminants is based on rapid tests using brainstem material. To diagnose atypical scrapie, samples of the cerebellum have to be included as well (TSE EURL, a technical handbook for National Reference Laboratories in the EU). All samples of active surveillance with a reactive result in one of the approved rapid tests must be retested in the national reference laboratory using one of the OIE-approved confirmatory methods (Matthews et al. 2004). Clinical suspect animals (passive surveillance) may be directly examined by such methods. These are histopathology, immunohistochemistry (IHC), electron microscopy and scrapie-associated fibrils (SAF) immunoblot. For practical reasons, mainly the IHC and SAF immunoblot are of relevance today.
7.1 Discriminatory Immunoblot
According to the EU legislation (January 2005, EC regulation 36/2005), all confirmed classical scrapie cases in small ruminants should be examined by discriminatory testing to reveal BSE infections in sheep and goats. These include discriminatory immunoblots (following defined immunoblot protocols, see TSE EURL, a technical handbook for National Reference Laboratories in the EUs) and, in equivocal cases, mouse bioassays (for strain typing). Size differences of proteinase K (PK)-treated, non-glycosylated PrPSc can be shown by high-resolution sodium dodecyl sulphate polyacrylamide-gel electrophoresis (SDS-PAGE) followed by immunoblotting. Moreover, monoclonal antibodies binding to an epitope located on the ragged N-terminal end of PK-cleaved PrPSc are useful tools in discriminating classical scrapie from ovine BSE. One of these antibodies is, for example, mab P4, whose N-terminal epitope remains detectable after PK digestion of scrapie PrPSc, in contrast to BSE PrPSc, from which this epitope is trimmed off by this enzyme. Antibodies that recognise an epitope in the core region of PrPSc, mab L42 for example, detect scrapie as well as BSE PrPSc after PK digestion because this treatment has no influence on epitopes of the protein’s core region (Figs. 26.2 and 26.3).
Lack of detection of ovine and bovine BSE PrPSc by mab P4. As the PK cleavage sites vary between BSE and scrapie, mab P4 can be used to discriminate between these two TSE types. While BSE-related PrPSc is trimmed approximately to the amino acid 100 and the P4 epitope is therefore destroyed, the trimming of scrapie-related PrPSc stops 10–15 amino acid positions further N terminally. Therefore, the P4 epitope remains intact and the PK-digested PrPSc is easily detected by the antibody
In the last years, several biochemical strain typing techniques were developed, which utilise these differences in the PK cleavage site of PrPSc (Stack et al. 2002; Lezmi et al. 2004; Nonno et al. 2003; Thuring et al. 2004; Gretzschel et al. 2005, for details see TSE EURL, a technical handbook for National Reference Laboratories in the EU). In Germany, the so-called FLI test is applied (Gretzschel et al. 2005), which is a biochemical BSE/scrapie typing strategy that utilises the differences in the glycosylation and PK cleavage site of PK treated and immunoblotted ovine BSE and scrapie PrPSc. Detection antibodies are mabs L42 and P4.
According to the discriminatory testing PrPSc in a sample will be judged BSE-like, if the sample conforms to the following three biochemical attributes: (1) the diglycosylated band is the dominating moiety (FLI test: the glycoform ratio for the diglycosylated form is above 50%); (2) Certain N-terminal antibodies fail to detect PrPSc (FLI test: the antibody binding ratio P4/L42 has a lower value than 0.4) and (3) low molecular weight of the unglycosylated band (FLI test: the molecular mass is by >0.5 kDa lower than that of the internal scrapie standard). Deviations in one or more characteristics exclude BSE in the isolate concerned since only the complete characteristics define the BSE agent.
7.2 Histopathology
Gross lesions are not visible and the histomorphological alterations are confined to the central nervous system. The first description of typical scrapie lesions dates back to the nineteenth century (Besnoit and Morel 1898). Scrapie is a neurodegenerative disease with vacuolation of the grey matter as a hallmark, often accompanied by astrocytosis but without signs of inflammation. Neuronal loss is present, but significant cell losses are not evident on routine examination (Jeffrey and Gonzalez 2004; Wells et al. 2007). The development of clinical signs is not necessarily reflected by the severity of the pathology changes (Jeffrey and Gonzalez 2004, 2007).
Lesions are usually bilaterally symmetrical (Fraser 1993), especially at the brainstem at the level of the obex (Fig. 26.4), and the dorsal motor nucleus of the vagus nerve is the most commonly affected site (Wood et al. 1997). However, a considerable variation in the neuroanatomical distribution of the spongiform lesions is obvious, especially in more rostral areas of the brain. The formation of lesions depends not only on the prion strain but also on the genotype of the host, breed and presumably also other individual factors (Ligios et al. 2002; Begara-McGorum et al. 2002). Additionally, the magnitude of vacuolation is influenced by the age at the onset of clinical disease (Ligios et al. 2002).
In classical scrapie vacuolation is detectable in the neuronal perikarya and in the neuropil but can be rare in some naturally occurring and experimental scrapie cases (Zlotnik 1960; Dickinson 1976; Fraser 1976; Chaplin et al. 1998; Begara-McGorum et al. 2002). These membrane-bound vacuoles are found within the neuronal perikarya as single or multiple vacuoles distending the cell body and/or within processes leading to the typical spongiform appearance in the grey matter neuropil (Jeffrey et al. 1995; Jeffrey and Gonzalez 2004). The proportion of perikaryonal to neuropil vacuolation differs in respect of the disease and agent strain. In murine scrapie models, dendrites are most frequently affected, neuronal perikarya, axons and axon terminals to a lesser extent (for a detailed review see Jeffrey et al. 1995). Additional findings might be other signs of neuronal degeneration like chromatolysis, neuronophagia and dark shrunken neurons. Astrocytosis is also an inconsistent finding seen in some scrapie cases (Wood et al. 1997; Jeffrey and Gonzalez 2004; Wells et al. 2007).
In atypical scrapie, the vacuolation is most prominent in the molecular layer of the cerebellar cortex, neocortex hippocampus, basal nuclei and nucleus accumbens. The brainstem is, in contrast to classical scrapie, affected to a much lesser degree and no lesions are observed at the level of the obex (Benestad et al. 2003; Moore et al. 2008). Intraneuronal vacuolation is not (Moore et al. 2008) or only infrequently seen (Benestad et al. 2003).
7.3 Immunohistochemistry
The second hallmark of TSEs is the accumulation of PrPSc in the brain, which precedes morphological alterations (DeArmond 1993; Jeffrey et al. 2000). Previous studies (van Keulen et al. 2000) with classical scrapie demonstrated that the brainstem at the level of the obex, in particular the dorsal motor nucleus of the vagus nerve, is the first area in the CNS to become affected in advance of any morphological alterations. With the progression of the disease, the PrPSc accumulation becomes more widespread and spreads in ascending and descending directions to finally involve at clinical endpoint the entire neuraxis.
It is possible to differentiate several morphological types of PrPSc accumulation (Table 26.2). These PrPSc profiles provide strain and source-specific information on the cell types, which sustain the infection (cellular tropism) and the cellular processing of PrPSc. Not all these types and patterns are found in all scrapie cases. Furthermore, in immunohistochemistry (IHC), a differentiation between ovine/caprine BSE and scrapie is possible by using the immunoreactivity of antibodies recognising different epitopes of PrPSc (epitope mapping) (for a detailed review see Jeffrey and Gonzalez 2007). This method relies on the different protease cleavage sites for PrPSc in different cell types (the same principle as shown in Fig. 26.2).
Atypical scrapie cases are characterised by a distinctly different PrPSc distribution pattern as compared to classical scrapie. The brainstem at the level of the obex is only inconstantly involved. In contrast to classical scrapie, a PrPSc accumulation at the DMNV was never seen (Nentwig et al. 2007; Benestad et al. 2008). PrPSc accumulations found at the obex are mainly confined to the spinal tract nucleus of the trigeminal nerve with primary involvement of the white matter, formatio reticularis, ventrolateral solitary tract and ambiguous nucleus (for a detailed review see Benestad et al. 2008). The most pronounced immunostaining is usually detectable in the cerebellar (Fig. 26.5) and cerebral cortices (Benestad et al. 2008) as well as in the substantia nigra, thalamus and basal nuclei (Moore et al. 2008). However, cases without any cerebellar accumulation were also described (Nentwig et al. 2007). PrPSc accumulations are generally mild to moderate, and only a few morphological types (including fine granular, aggregates, plaque-like, linear and perineuronal) can be seen. An intraneuronal deposition staining has never been reported (Benestad et al. 2008; Moore et al. 2008).
8 Scrapie Agent Strains
It is important to recognise that classical scrapie can occur in different phenotypes in hosts of the same species or genotype, and this characteristic could be explained by the evolution of new prion strains (EFSA 2017; Nonno et al. 2019). The first reports on the existence of different prion strains date back to the 1960s (Fraser and Dickinson 1968, 1973) and three classes of scrapie strains were defined in the UK based on the phenotypes after passage in inbred wild-type mice (Bruce and Dickinson 1987; Westaway et al. 1987).
Prion strain properties depend on the specific conformation of PrPSc. Firstly, they are defined by their phenotypic characteristics in the natural host, including clinical signs, brain lesion profile, immunohistochemical and biochemical (e.g. protease sensitivity, molecular migration on Western blot, glycosylation patterns) pattern of PrPSc. Even more important are the disease properties in experimental rodent models, in particular attack rate and incubation period as well as lesion and PrPSc profiles in the mouse brain and biochemical characteristics of prions (EFSA 2015).
The process of prion strain evolution is still unknown, but the impact of selective pressure, that is, host genetic or environmental factors could induce an alteration of the propagation process, and only a “new” PrPSc is able to cross a transmission barrier, adapt and emerge. Another hypothesis states that selective pressure results in a selection of the most suitable PrPSc conformer already present in a given isolate, which is a mixture of different PrPSc conformers (Collinge and Clarke 2007; Li et al. 2010; Beringue et al. 2007). In any case, different replication environments most probably play an important role in determining the biological properties of scrapie isolates and their further evolution. Thus, the full characterisation of isolates today includes, besides bank voles, the use of transgenic mouse models, overexpressing the prion protein of animals and man. That way the influence of different hosts on the biological properties of the original isolate is determined (EFSA 2014, 2015).
Up to now, strain typing studies have identified several prion strains responsible for classical scrapie in sheep and goats (EFSA 2015, 2017; Nonno et al. 2019; Beringue et al. 2008; Marín-Moreno et al. 2021), but the exact number is unknown and most classical scrapie field isolates contain sub-strains in different proportions (Nonno et al. 2019). Evidently, some isolates are not completely stable and their biological properties can shift on transmission, even affecting the ability of certain strains to cross the species barrier (EFSA 2015), as shown for some classical scrapie isolates in humanised transgenic mice (Cassard et al. 2014). These divergences might be the reason for the high diversity of classical scrapie strains and could be the basis for the emergence of new prion strains.
References
Acin C, Martin-Burriel I, Goldmann W, Lyahyai J, Monzon M, Bolea R, Smith A, Rodellar C, Badiola JJ, Zaragoza P. Prion protein gene polymorphisms in healthy and scrapie-affected Spanish sheep. J Gen Virol. 2004;85:2103–10.
Acutis PL, Bossers A, Priem J, Riina MV, Peletto S, Mazza M, Casalone C, Forloni G, Ru G, Caramelli M. Identification of prion protein gene polymorphisms in goats from Italian scrapie outbreaks. J Gen Virol. 2006;87:1029–33.
Akesson CP, McGovern G, Dagleish MP, Espenes A, Press CM, Landsverk T, Jeffrey M. Exosome-producing follicle associated epithelium is not involved in uptake of PrPd from the gut of sheep (Ovis aries): an ultrastructural study. PLoS One. 2011;6:e22180.
Alper T, Haig DA, Clarke MC. The exceptionally small size of the scrapie agent. Biochem Biophys Res Commun. 1966;22:278–84.
Alper T, Cramp WA, Haig DA, Clarke MC. Does the agent of scrapie replicate without nucleic acids? Nature. 1967;214:764–6.
Alper T, Haig DA, Clarke MC. The scrapie agent: evidence against its dependence for replication on intrinsic nucleic acid. J Gen Virol. 1978;41:503–16.
Alverson J, O’Rourke KI, Baszler TV. PrPSc accumulation in fetal cotyledons of scrapie resistant lambs is influenced by fetus location in the uterus. J Gen Virol. 2006;87:1035–41.
Andreoletti O, Berthon P, Marc D, Sarradin P, Grosclaude J, van Keulen L, Schelcher F, Elsen JM, Lantier F. Early accumulation of PrP(Sc) in gut-associated lymphoid and nervous tissues of susceptible sheep from a Romanov flock with natural scrapie. J Gen Virol. 2000;81:3115–26.
Andreoletti O, Lacroux C, Chabert A, Monnereau L, Tabouret G, Lantier F, Berthon P, Eychenne F, Lafond-Benestad S, Elsen JM, Schelcher F. PrPSc accumulation in placentas of ewes exposed to natural scrapie: influence of foetal PrP genotype and effect on ewe-to-lamb transmission. J Gen Virol. 2002;83:2607–16.
Andreoletti O, Simon S, Lacroux C, Morel N, Tabouret G, Chabert A, Lugan S, Corbiere F, Ferre P, Foucras G, Laude H, Eychenne F, Grassi J, Schelcher F. PrPSc accumulation in myocytes from sheep incubating natural scrapie. Nat Med. 2004;10:591–3.
Andréoletti O, Orge L, Benestad SL, Beringue V, Litaise C, Simon S, Le Dur A, Laude H, Simmons H, Lugan S, Corbière F, Costes P, Morel N, Schelcher F, Lacroux C. Atypical/Nor98 scrapie infectivity in sheep peripheral tissues. PLoS Pathog. 2011;7:e1001285.
Arsac JN, Andreoletti O, Bilheude JM, Lacroux C, Benestad SL, Baron T. Similar biochemical signatures and prion protein genotypes in atypical scrapie and Nor98 cases, France and Norway. Emerg Infect Dis. 2007;13:58–65.
Barillet F, Mariat D, Amigues Y, Faugeras R, Caillat H, Moazami-Goudarzi K, Rupp R, Babilliot JM, Lacroux C, Lugan S, Schelcher F, Chartier C, Corbiere F, Andreoletti O, Perrin-Chauvineau C. Identification of seven haplotypes of the caprine PrP gene at codons 127, 142, 154, 211, 222 and 240 in French Alpine and Saanen breeds and their association with classical scrapie. J Gen Virol. 2009;90:769–76.
Bastian FO. Spiroplasma-like inclusions in Creutzfeldt-Jakob disease. Arch Pathol Lab Med. 1979;103:665–9.
Begara-McGorum I, Gonzalez L, Simmons M, Hunter N, Houston F, Jeffrey M. Vacuolar lesion profile in sheep scrapie: factors influencing its variation and relationship to disease-specific PrP accumulation. J Comp Pathol. 2002;127:59–68.
Belt PB, Muileman IH, Schreuder BE, Bos-De Ruijter J, Gielkens AL, Smits MA. Identification of five allelic variants of the sheep PrP gene and their association with natural scrapie. J Gen Virol. 1995;76:509–17.
Benestad SL, Sarradin P, Thu B, Schönheit J, Tranulis MA, Bratberg B. Cases of scrapie with unusual features in Norway and designation of a new type, Nor98. Vet Rec. 2003;153:202–8.
Benestad SL, Arsac JN, Goldmann W, Nöremark M. Atypical/Nor98 scrapie: properties of the agent, genetics, and epidemiology. Vet Res. 2008;39:19.
Benestad SL, Austbø L, Tranulis MA, Espenes A, Olsaker I. Healthy goats naturally devoid of prion protein. Vet Res. 2012;43:87.
Beringue V, Andréoletti O, Le Dur A, Essalmani R, Vilotte JL, Lacroux C, Reine F, Herzog L, Biacabé AG, Baron T, Caramelli M, Casalone C, Laude H. A bovine prion acquires an epidemic bovine spongiform encephalopathy strain-like phenotype on interspecies transmission. J Neurosci. 2007;27:6965–71.
Béringue V, Vilotte JL, Laude H. Prion agent diversity and species barrier. Vet Res. 2008;39:47.
Besnoit MM, Morel C. Note sur les l駸ions nerveuses de la tremblante du 883 mouton. Rev Veterinaire. 1898;23:397–400.
Billinis C, Panagiotidis CH, Psychas V, Argyroudis S, Nicolaou A, Leontides S, Papadopoulos O, Sklaviadis T. Prion protein gene polymorphisms in natural goat scrapie. J Gen Virol. 2002;83:713–21.
Billinis C, Psychas V, Leontides L, Spyrou V, Argyroudis S, Vlemmas I, Leontides S, Sklaviadis T, Papadopoulos O. Prion protein gene polymorphisms in healthy and scrapie-affected sheep in Greece. J Gen Virol. 2004;85:547–54.
Bossers A, Schreuder BE, Muileman IH, Belt PB, Smits MA. PrP genotype contributes to determining survival times of sheep with natural scrapie. J Gen Virol. 1996;77:2669–73.
Bradford BM, Reizis B, Mabbott NA. Oral prion disease pathogenesis is impeded in the specific absence of CXCR5-expressing dendritic cells. J Virol. 2017;91:e00124–e17.
Brotherston JG, Renwick CC, Stamp JT, Zlotnik I, Pattison IH. Spread of scrapie by contact to goats and sheep. J Comp Pathol. 1968;78:9–17.
Brown P, Gajdusek CD. Survival of scrapie virus after three years interment. Lancet. 1991;337:269–70.
Bruce ME, Dickinson AG. Biological evidence that scrapie agent has an independent genome. J Gen Virol. 1987;68:79–89.
Bruce ME, Nonno R, Foster J, Goldmann W, Di Bari M, Esposito E, Benestad SL, Hunter N, Agrimi U. Nor98-like sheep scrapie in the United Kingdom in 1989. Vet Rec. 2007;160:665–6.
Capucchio MT, Guarda F, Isaja MC, Caracappa S, Di Marco V. Natural occurrence of scrapie in goats in Italy. Vet Rec. 1998;143:452–3.
Capucchio MT, Guarda F, Pozzato N, Coppolino S, Caracappa S, Di Marco V. Clinical signs and diagnosis of scrapie in Italy: a comparative study in sheep and goats. J Vet Med A. 2001;48:23–31.
Caramelli M, Ru G, Casalone C, Bozzetta E, Acutis PL, Calella A, Forloni G. Evidence for the transmission of scrapie to sheep and goats from a vaccine against Mycoplasma agalactiae. Vet Rec. 2001;28:531–6.
Cassard H, Torres JM, Lacroux C, Douet JY, Benestad SL, Lantier F, Lugan S, Lantier I, Costes P, Aron N, Reine F, Herzog L, Espinosa JC, Beringue V, Andréoletti O. Evidence for zoonotic potential of ovine scrapie prions. Nat Commun. 2014;5:5821.
Cassman E, Greenlee J. Pathogenesis, detection and control of scrapie in sheep. Am J Vet Res. 2019;81:600–14.
Chaplin AD, Aldrich MJ, Stack M. Scrapie associated fibril detection from formaldehyde fixed brain tissue in natural cases of ovine scrapie. Res Vet Sci. 1998;64:41–4.
Chelle PL. Un cas de tremblante chez la chevre. Bull Acad Vet Fr. 1942;15:294–5.
Clark AM, Dawson M, Scott AC. Scrapie associated fibrils in found dead sheep. Vet Rec. 1994;134:650–1.
Clouscard C, Beaudry P, Elsen JM, Milan D, Dussaucy M, Bounneau C, Schelcher F, Chatelain J, Launay JM, Laplanche JL. Different allelic effects of the codons 136 and 171 of the prion protein gene in sheep with natural scrapie. J Gen Virol. 1995;76:2097–101.
Collinge J, Clarke AR. A general model of prion strains and their pathogenicity. Science. 2007;318:930–6.
Comber T. Real improvements in agriculture. 1st ed. London: W. Nicoll; 1772.
Cuille J, Chelle PL. Pathologie animale—La maladie dite tremblante du mouton est-elle inoculable? C R Hebd Seances Acad Sci. 1936;203:1552–4.
Cuille J, Chelle PL. La tremblante du mouton est-elle determinee par un virus filtrable? C R Hebd Seances Acad Sci. 1938a;206:1687–8.
Cuille J, Chelle PL. Le tremblante du mouton est bien inoculable. C R Hebd Seances Acad Sci. 1938b;206:78–9.
Cuille J, Chelle PL. Transmission experimentale de la tremblante a la chevre. C R Hebd Seances Acad Sci. 1939;208:1058–60.
Dagleish MP, Rodger SM, Simmons MM, Finlayson J, Buxton D, Chianini F. Atypical scrapie in a sheep in Scotland. Vet Rec. 2008;162:518–9.
Dassanayake RP, White SN, Madsen-Bouterse SA, Schneider DA, O’Rourke KI. Role of the PRNP S127 allele in experimental infection of goats with classical caprine scrapie. Anim Genet. 2015;46:341.
Dawson M, Hoinville LJ, Hosie BD, Hunter N. Guidance on the use of PrP genotyping as an aid to the control of clinical scrapie. Vet Rec. 1998;142:623–5.
DeArmond SJ. Overview of the transmissible spongiform encephalopathies: prion protein disorders. Br Med Bull. 1993;49:725–37.
Detwiler LA, Baylis M. The epidemiology of scrapie. Rev Sci Tech Off Int Épizoot. 2003;22:121–43.
Dickinson AG. Scrapie in sheep and goats. In: Kimberlin RH, editor. Slow virus diseases of animals and man. Amsterdam: North Holland; 1976. p. 209–41.
Dickinson AG, Outram GW. The scrapie replication-site hypothesis and its implication for pathogenesis. In: Prusiner SB, Hadlow WJ, editors. Slow transmissible disease of the nervous system: pathogenesis, immunology, virology and molecular biology of the spongiform encephalopathies, vol. 2. 1st ed. New York/London/Toronto/Sydney/San Francisco: Academic; 1979.
Dickinson AG, Stamp JT. Experimental scrapie in Cheviot and Suffolk sheep. J Comp Pathol. 1969;79:23–6.
Dickinson AG, Meikle VM, Fraser H. Identification of a gene which controls the incubation period of some strains of scrapie agent in mice. J Comp Pathol. 1968a;78:293–9.
Dickinson AG, Stamp JT, Renwick CC, Rennie JC. Some factors controlling the incidence of scrapie in cheviot sheep injected with a cheviot-passaged scrapie agent. J Comp Pathol. 1968b;78:313–21.
Donaldson DS, Kobayashi A, Ohno H, Yagita H, Williams IR, Mabbott NA. M cell-depletion blocks oral prion disease pathogenesis. Mucosal Immunol. 2012;5:216–25.
EFSA. Scientific report of the European Food Safety Authority on the evaluation of rapid post mortem TSE tests intended for small ruminants. EFSA J. 2005;31:1–17.
EFSA. Scientific Opinion on BSE/TSE infectivity in small ruminant tissues. EFSA J. 2010;8:1875.
EFSA. Scientific Opinion on the scrapie situation in the EU after 10 years of monitoring and control in sheep and goats. EFSA J. 2014;12:3781.
EFSA. Scientific Opinion on a request for a review of a scientific publication concerning the zoonotic potential of ovine scrapie prions. EFSA J. 2015;13:4197.
EFSA. Scientific Opinion on the Genetic resistance to transmissible spongiform encephalopathies (TSE) in goats. EFSA J. 2017;15:4962.
EFSA. The European Union summary report on surveillance for the presence of transmissible spongiform encephalopathies (TSE) in 2019. EFSA J. 2020;18:6303.
EFSA. Scientific Report on the analysis of the 2-year compulsory intensified monitoring of atypical scrapie. EFSA EFSA J. 2021a;19:6686.
EFSA. The European Union summary report on surveillance for the presence of transmissible spongiform encephalopathies (TSE) in 2020. EFSA J. 2021b;19:6934.
EFSA Panel on Biological Hazards (BIOHAZ). Scientific Opinion on genetic TSE resistance in goats in all European Union member states. EFSA J. 2009;7:1371–411.
Epstein V, Pointing S, Halfacre S. Atypical scrapie in the Falkland Islands. Vet Rec. 2005;19:667–8.
Everest SJ, Ramsay AM, Chaplin MJ, Everitt S, Stack MJ, Neale MH, Jeffrey M, Moore SJ, Bellworthy SJ, Terry LA. Detection and localisation of PrP(Sc) in the liver of sheep infected with scrapie and bovine spongiform encephalopathy. PLoS One. 2011;12:e19737.
Fediaevsky A, Tongue SC, Nöremark M, Calavas D, Ru G, Hopp P. A descriptive study of the prevalence of atypical and classical scrapie in sheep in 20 European countries. BMC Vet Res. 2008;4:19–43.
Fediaevsky A, Morignat E, Ducrot C, Calavas D. A case–control study on the origin of atypical scrapie in sheep France. Emerg Infect Dis. 2009;15:710–8.
Fediaevsky A, Maurella C, Nöremark M, Ingravalle F, Thorgeirsdottir S, Orge L, Poizat R, Hautaniemi M, Liam B, Calavas D, Ru G, Hopp P. The prevalence of atypical scrapie in sheep from positive flocks is not higher than in the general sheep population in 11 European countries. BMC Vet Res. 2010;6:9.
Field EJ. Transmission experiments with multiple sclerosis: an interim report. Br Med J. 1966;2:564–5.
Foster JD, Parnham D, Chong A, Goldmann W, Hunter N. Clinical signs, histopathology and genetics of experimental transmission of BSE and natural scrapie to sheep and goats. Vet Rec. 2001;148:165–71.
Fraser H. The pathology of natural and experimental scrapie. In: Kimberlin RH, editor. Slow virus diseases of animals and man. Amsterdam: North Amsterdam; 1976. p. 267–305.
Fraser H. Diversity in the neuropathology of scrapie-like diseases in animals. Br Med Bull. 1993;49:792–809.
Fraser H, Dickinson AG. The sequential development of the brain lesion of scrapie in three strains of mice. J Comp Pathol. 1968;78:301–11.
Fraser H, Dickinson AG. Scrapie in mice. Agent-strain differences in the distribution and intensity of grey matter vacuolation. J Comp Pathol. 1973;83:29–40.
Garza MC, Fernandez-Borges N, Bolea R, Badiola JJ, Castilla J, Monleon E. Detection of PrPres in genetically susceptible fetuses from sheep with natural scrapie. PLoS ONE. 2011;6:e27525.
Gavier-Widen D, Noremark M, Benestad S, Simmons M, Renstrøm L, Bratberg B, Elvander M, Segerstad CH. Recognition of the Nor98 variant of scrapie in the Sweedish sheep population. J Vet Diagn Investig. 2004;16:562–7.
Georgsson G, Sigurdarson S, Brown P. Infectious agent of sheep scrapie may persist in the environment for at least 16 years. J Gen Virol. 2006;87:3737–40.
Goldmann W. PrP genetics in ruminant transmissible spongiform encephalopathies. Vet Res. 2008;39:30.
Goldmann W, Hunter N, Benson G, Foster JD, Hope J. Different scrapie associated fibril proteins (PrP) are encoded by lines of sheep selected for different alleles of the Sip gene. J Gen Virol. 1991;72:2411–7.
Goldmann W, Martin T, Foster F, Hughes S, Smith G, Hughes K, Dawson M, Hunter N. Novel polymorphisms in the caprine PrP gene: a codon 142 mutation associated with scrapie incubation period. J Gen Virol. 1996;77:2885–91.
Goldmann W, Ryan K, Stewart P, Parnham D, Xicohtencatl R, Fernandez N, Saunders G, Windl O, González L, Bossers A, Foster J. Caprine prion gene polymorphisms are associated with decreased incidence of classical scrapie in goat herds in the United Kingdom. Vet Res. 2011;42:110.
Gombojav A, Ishiguro N, Horiuchi M, Shinagawa M. Unique amino acid polymorphisms of PrP genes in Mongolian sheep breeds. J Vet Med Sci. 2003;66:1293–5.
González L, Martin S, Sisó S, Konold T, Ortiz-Peláez A, Phelan L, Goldmann W, Stewart P, Saunders G, Windl O, Jeffrey M, Hawkins SA, Dawson M, Hope J. High prevalence of scrapie in a dairy goat herd: tissue distribution of disease-associated PrP and effect of PRNP genotype and age. Vet Res. 2009;40:65.
Gonzalez-Romero D, Barria MA, Leon P, Morales R, Soto C. Detection of infectious prions in urine. FEBS Lett. 2008;582:3161–6.
Gordon WS. Advances in scrapie research. Vet Rec. 1946;47:516–25.
Gough KC, Baker CA, Rees HC, Terry LA, Spiropoulos J, Thorne L, Maddison BC. The oral secretion of infectious scrapie prions occurs in pre-clinical sheep with a range of PRNP genotypes. J Virol. 2011;86(1):566–71.
Gough K, Baker C, Simmons HA, Hawkins SA, Maddison BC. Circulation of prions within dust on a scrapie affected farm. Vet Res. 2015;46:40.
Gough K, Baker CA, Hawkins S, Simmons H, Konold T, Maddison BC. Rapid recontamination of a farm building occurs after attempted prion removal. Vet Rec. 2018;184:97.
Gonzalez L, Siso S, Monleo E, Casalone C, van Keulen L, Balkema-Buschmann A, Ortiz-Pelaez A, Iulini B, Langeveld JPM, Hoffmann C, Badiola JJ, Jeffrey M, Acın C. Variability in disease phenotypes within a single PRNP genotype suggests the existence of multiple natural sheep scrapie strains within Europe. J Gen Virol. 2010a;91:2630–41.
Gonzalez L, Martin S, Hawkins SAC, Goldmann W, Jeffrey M, Siso S. Pathogenesis of natural goat scrapie: modulation by host PRNP genotype and effect of co-existent conditions. Vet Res. 2010b;41:48
Green DM, Del Rio Vilas VJ, Birch CP, Johnson J, Kiss IZ, McCarthy ND, Kao RR. Demographic risk factors for classical and atypical scrapie in Great Britain. J Gen Virol. 2007;88:3486–92.
Greenlee JJ, Kunkle RA, Richt JA, Nicholson EM, Hamir AN. Lack of prion accumulation in lymphoid tissues of PRNP ARQ/ARR sheep intracranially inoculated with the agent of scrapie. PLoS One. 2014;9:e108029.
Gregori L, Kovacs GG, Alexeeva I, Budka H, Rohwer RG. Excretion of transmissible spongiform encephalopathy infectivity in urine. Emerg Infect Dis. 2008;14:1406–12.
Gretzschel A, Buschmann A, Eiden M, Ziegler U, Lühken G, Erhardt G, Groschup MH. Strain typing of German transmissible spongiform encephalopathies field cases in small ruminants by biochemical methods. J Veterinary Med Ser B. 2005;52:55–63.
Griffith JS. Self-replication and scrapie. Nature. 1967;215:1043–4.
Groschup MH, Lacroux C, Buschmann A, Lühken G, Mathey J, Eiden M, Lugan S, Hoffmann C, Espinosa JC, Baron T, Torres JM, Erhardt G, Andreoletti O. Classic scrapie in sheep with the ARR/ARR prion genotype in Germany and France. Emerg Infect Dis. 2007;13:1201–7.
Guo X, Kupfer DM, Fitch GQ, Roe BA, DeSilva U. Identification of a novel lysine-171 allele in the ovine prion protein (PRNP) gene. Anim Genet. 2003;34:303–5.
Haralambiev H, Ivanov I, Vesselinova A, Mermerski K. An attempt to induce scrapie in local sheep in Bulgaria. Zbl Vet Med B. 1973;20:701–9.
Harcourt RA, Anderson KMA. Naturally-occurring scrapie in goats. Vet Rec. 1974;94:504.
Healy AM, Weavers E, McElroy M, Gomez-Parada M, Collins JD, O’Doherty E, Sweeney T, Doherty ML. The clinical neurology of scrapie in Irish sheep. J Vet Intern Med. 2003;17:908–16.
Hoinville LJ. A review of the epidemiology of scrapie in sheep. Rev Sci Tech Off Int Épizoot. 1996;15:827–52.
Hopp P, Omer MK, Heier BT. A case–control study of scrapie Nor98 in Norwegian sheep flocks. J Gen Virol. 2006;87:3729–36.
Hörnlimann B, van Keulen L, Ulvund M, Bradley R (2007) Portrait of scrapie in sheep and goats. In: Hörnlimann B, Riesner D, Kretzschmar H (eds) Prions in human and animals. Walter de Gruyter GmbH & CO KG, Berlin.
Hourrigan JL, Klingsporn AL, McDanie HA, Riemenschneider MN. Natural scrapie in a goat. J Am Vet Med Assoc. 1969;154:538–9.
Houston F, McCutcheon S, Goldmann W, Chong A, Foster J, Sisó S, González L, Jeffrey M, Hunter N. Prion diseases are efficiently transmitted by blood transfusion in sheep. Blood. 2008;112:4739–45.
Humphrey RW, Clark AM, Begara-McGorum I, Gunn GJ. Estimation of scrapie prevalence in cull and found-dead sheep on the Shetland Islands. Vet Rec. 2004;154:303–4.
Hunter N. PrP genetics in sheep and the implications for scrapie and BSE. Trends Microbiol. 1997;5:331–4.
Hunter N, Goldmann W, Smith G, Hope J. The association of a codon 136 PrP gene variant with the occurrence of natural scrapie. Arch Virol. 1994;137:171–7.
Hunter N, Foster JD, Goldmann W, Stear MJ, Hope J, Bostock C. Natural scrapie in a closed flock of Cheviot sheep occurs only in specific PrP genotypes. Arch Virol. 1996;141:809–24.
Iannuzzi L, Palomba R, Di Meo GP, Perucatti A, Ferrara L. Comparative FISHmapping of the prion protein gene (PRNP) on cattle, river buffalo, sheep and goat chromosomes. Cytogenet Cell Genet. 1998;81:202–4.
Jeffrey M, Gonzalez L. Pathology and pathogenesis of bovine spongiform encephalopathy and scrapie. Curr Top Microbiol Immunol. 2004;284:65–97.
Jeffrey M, Gonzalez L. Classical sheep transmissible spongiform encephalopathies: pathogenesis, pathological phenotypes and clinical disease. Neuropathol Appl Neurobiol. 2007;33:373–94.
Jeffrey M, Goodbrand IA, Goodsir C. Pathology of the transmissible spongiform encephalopathies with special emphasis on ultrastructure. Micron. 1995;26:277–98.
Jeffrey M, Halliday WG, Bell J, Johnston AR, Macleod NK, Ingham C, Sayers AR, Brown DA, Fraser JR. Synapse loss associated with abnormal PrP precedes neuronal degeneration in the scrapie infected murine hippocampus. Neuropathol Appl Neurobiol. 2000;26:41–54.
Jeffrey M, Martin S, Thomson JR, Dingwall WS, Begara-McGorum I, Gonzalez L. Onset and distribution of tissue prp accumulation in scrapie-affected Suffolk sheep as demonstrated by sequential necropsies and tonsillar biopsies. J Comp Pathol. 2001;125:48–57.
Jeffrey M, Begara-McGorum I, Clarky S, Martin S, Clarkz J, Chaplinz M, Gonzalez L. Occurrence and distribution of infection-specific PrP in tissues of clinical scrapie cases and cull sheep from scrapie-affected farms in Shetland. J Comp Pathol. 2002;127:264–73.
Jeffrey M, González L, Espenes A, Press CM, Martin S, Chaplin M, Davis L, Landsverk T, MacAldowie C, Eaton S, McGovern G. Transportation of prion protein across the intestinal mucosa of scrapie-susceptible and scrapie-resistant sheep. J Pathol. 2006;209:4–14.
Johnson CJ, Phillips KE, Schramm PT, McKenzie D, Aiken JM, Pedersen JA. Prions adhere to soil minerals and remain infectious. PLoS Pathog. 2006;2:e32.
Konold T, Davis A, Bone G, Bracegirdle J, Everitt S, Chaplin M, Saunders GC, Cawthraw S, Simmons MM. Clinical findings in two cases of atypical scrapie in sheep: a case report. BMC Vet Res. 2007a;3:2.
Konold T, Bone G, Simmons MM, Dexter G, Moore SJ, Pettitt RG. Scrapie in goats. Vet Rec. 2007b;161:395–6.
Konold T, Moore SJ, Bellworthy SJ, Simmons HA. Evidence of scrapie transmission via milk. BMC Vet Res. 2008;4:14.
Konold T, Hawkins SAC, Thurston L, Maddison B, Gough K, Duarte A, Simmons HA. Objects in contact with classical scrapie sheep act as a reservoir for scrapie transmission. Front Vet Sci. 2015;2:32.
Kratzel C, Mai J, Madela K, Beekes M, Krüger D. Propagation of scrapie in peripheral nerves after footpad infection in normal and neurotoxin exposed hamsters. Vet Res. 2007;38:127–39.
Lacroux C, Corbiere F, Tabouret G, Lugan S, Costes P, Mathey J, Delmas JM, Weisbecker JL, Foucras G, Cassard H, Elsen JM, Schelcher F, Andreoletti O. Dynamics and genetics of PrPSc placental accumulation in sheep. J Gen Virol. 2007;88:1056–61.
Lacroux C, Simon S, Benestad SL, Maillet S, Mathey J, Lugan S, Corbiere F, Cassard H, Costes P, Bergonier D, Weisbecker JL, Moldal T, Simmons H, Lantier F, Feraudet-Tarisse C, Morel N, Schelcher F, Grassi J, Andreoletti O. Prions in milk from ewes incubating natural scrapie. PLoS Pathog. 2008;4:e1000238.
Laplanche JL, Chatelain J, Westaway D, Thomas S, Dussaucy M, Brugere-Picoux J, Launay JM. PrP polymorphisms associated with natural scrapie discovered by denaturing gradient gel electrophoresis. Genomics. 1993;15:30–7.
Lasmezas CI, Deslys JP, Robain O, Jaegly A, Beringue V, Peyrin JM, Fournier JG, Hauw JJ, Rossier J, Dormont D. Transmission of the BSE agent to mice in the absence of detectable abnormal prion protein. Science. 1997;275:402–5.
Le Dur A, Beringue V, Andreoletti O, Reine F, Lai TL, Baron T, Bratberg B, Vilotte JL, Sarradin P, Benestad SL, Laude H. A newly identified type of scrapie agent can naturally infect sheep with resistant PrP genotypes. Proc Natl Acad Sci U S A. 2005;102:16031–6.
Leopoldt JG. Nützliche und auf die Erfahrung gegründete Einleitung zu der Land-Wirthschafft, vol. 5. Sorau: Johann Gottlieb Rothen; 1750.
Lezmi S, Martin S, Simon S, Comoy E, Bencsik A, Deslys JP, Grassi J, Jeffrey M, Baron T. Comparative molecular analysis of the abnormal prion protein in field scrapie cases and experimental bovine spongiform encephalopathy in sheep by use of Western blotting and immunohistochemical methods. J Virol. 2004;78:3654–62.
Li J, Browning S, Mahal SP, Oelschlegel AM, Weissmann C. Darwinian evolution of prions in cell culture. Science. 2010;327:869–72.
Ligios C, Jeffrey M, Ryder SJ, Bellworthy SJ, Simmons MM. Distinction of scrapie phenotypes in sheep by lesion profiling. J Comp Pathol. 2002;127:45–57.
Ligios V, Cancedda MG, Madau L, Santucciu C, Maestrale C, Agrimi U, Ru G, Di Guardo G. PrPSc deposition in nervous tissues without lymphoid tissue involvement is frequently found in ARQ/ARQ Sarda breed sheep preclinically affected with natural scrapie. Arch Virol. 2006;151:2007–20.
Lühken G, Buschmann A, Brandt H, Eiden M, Groschup MH, Erhardt G. Epidemiological and genetical differences between classical and atypical scrapie cases. Vet Res. 2007;38:65–80.
M’Gowan JP. Investigation into the disease of sheep called “scrapie”. 1st ed. Edinburgh: William Blackwood and Sons; 1914.
Mabbott NA, MacPherson GG. Prions and their lethal journey to the brain. Nat Rec Microbiol. 2006;4:201–11.
Maddison BC, Baker CA, Rees HC, Terry LA, Thorne L, Bellworthy SJ, Whitelam GC, Gough KC. Prions are secreted in milk from clinically normal scrapie-exposed sheep. J Virol. 2009;83:8293–6.
Maddison BC, Rees HC, Baker CA, Taema M, Bellworthy SJ, Thorne L, Terry LA, Gough KC. Prions are secreted into the oral cavity in sheep with preclinical scrapie. J Infect Dis. 2010;201:1672–6.
Madsen-Bouterse SA, Highland MA, Dassanayake RP, Zhuang D, Schneider DA. Low-volume goat milk transmission of classical scrapie to lambs and goat kids. PLoS One. 2018;13(9):e0204281.
Maestrale C, Cancedda MG, Pintus D, Masia M, Nonno R, Ru G, Carta A, Demontis F, Santucciu C, Ligios C. Genetic and pathological follow-up study of goats experimentally and naturally exposed to a sheep scrapie isolate. J Virol. 2015;89:10044–52.
Marín-Moreno A, Aguilar-Calvo P, Espinosa JC, Zamora-Ceballo M, Luis Pitarch J, González L, Fernández-Borges N, Orge L, Andreoletti O, Nonno R, Torres JM. Classical scrapie in small ruminants is caused by at least four different prion strains. Vet Res. 2021;52:57.
Matthews D, Jeffrey M, Simmons MM, Stack M, Wells GAH, Wilesmith JW. Bovine spongiform encephalopathy. In: OIE International Committee, editor. Manual of diagnostic tests and vaccines for terrestrial animals (mammals, birds and bees), vol. 2. World Organisation for Animal Health, OIE; 2004. p. 642–53.
McCulloch L, Brown KL, Bradford BM, Hopkins J, Bailey M, Rajewsky K, Manson JC, Mabbott NA. Follicular dendritic cell-specific prion protein (PrPc) expression alone is sufficient to sustain prion infection in the spleen. PLoS Pathog. 2011;7:e1002402.
McIntyre KM, Gubbins S, Goldmann W, Hunter N, Baylis M. Epidemiological characteristics of classical scrapie outbreaks in 30 sheep flocks in the United Kingdom. PLoS One. 2008;3:e3994.
Moore RC, Hope J, McBride PA, McConnell I, Selfridge J, Melton DW, Manson JC. Mice with gene targetted prion protein alterations show that Prnp, Sinc and Prni are congruent. Nat Genet. 1998;18:118–25.
Moore SJ, Simmons M, Chaplin M, Spiropoulos J. In naturally occurring atypical scrapie cases in Great Britain. Acta Neuropathol. 2008;116:547–59.
Moum T, Olsaker I, Hopp P, Moldal T, Valheim M, Moum T, Benestad SL. Polymorphisms at codons 141 and 154 in the ovine prion protein gene are associated with scrapie Nor98 cases. J Gen Virol. 2005;86:231–5.
Nentwig A, Oevermann A, Heim D, Botteron C, Zellweger K, Drögemüller C, Zurbriggen A, Seuberlich T. Diversity in neuroanatomical distribution of abnormal prion protein in atypical scrapie. PLoS Pathog. 2007;3:e82.
Niedermeyer S, Eiden M, Toumazos P, Papasavva-Stylianou P, Ioannou I, Sklaviadis T, Panagiotidis C, Langeveld J, Bossers A, Kuczius T, Kaatz M, Groschup MH, Fast C. Genetic, histochemical and biochemical studies on goat TSE cases from Cyprus. Vet Res. 2016;47:99.
Nonno R, Esposito E, Vaccari G, Conte M, Marcon S, Di Bari M, Ligios C, Di Guardo G, Agrimi U. Molecular analysis of cases of Italian sheep scrapie and comparison with cases of bovine spongiform encephalopathy (BSE) and experimental BSE in sheep. J Clin Microbiol. 2003;41:4127–33.
Nonno R, Marin-Moreno A, Carlos Espinosa J, Fast C, Van Keulen L, Spiropoulos J, Lantier I, Andreoletti O, Pirisinu L, Di Bari MA, Aguilar-Calvo P, Sklaviadis T, Papasavva-Stylianou P, Acutis PL, Acin C, Bossers A, Jacobs JG, Vaccari G, D'Agostino C, Chiappini B, Lantier F, Groschup MH, Agrimi U, Maria Torres J, Langeveld JPM. Characterization of goat prions demonstrates geographical variation of scrapie strains in Europe and reveals the composite nature of prion strains. Sci Rep. 2019;10:19.
O’Rourke KI, Holyoak GR, Clark WW, Mickelson JR, Wang S, Melco RP, Besser TE, Foote WC. PrP genotypes and experimental scrapie in orally inoculated Suffolk sheep in the United States. J Gen Virol. 1997;78:975–8.
O’Rourke KI, Zhuang D, Truscott TC, Yan H, Schneider DA. Sparse PrP Sc accumulation in the placentas of goats with naturally acquired scrapie. BMC Vet Res. 2011;7:7.
Oesch B, Westaway D, Wüchli M, McKinley MP, Kent SB, Aebersold R, Barry RA, Tempst P, Teplow DB, Hood LE, Prusiner SB, Weissmann C. A cellular gene encodes scrapie PrP 27–30. Protein Cell. 1985;40:735–46.
OIE. Manual of diagnostic tests and vaccines for terrestrial animals (mammals, birds and bees). Vol 2, Chapter 2.7.13—Scrapie. 2009. http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/2.07.13_SCRAPIE.pdf. Accessed 7 Nov 2011.
Okada H, Miyazawa K, Masujin K, Yokoyama T. Coexistence of two forms of disease-associated prion protein in extracerebral tissues of cattle infected with H-type bovine spongiform encephalopathy. J Vet Med Sci. 2016;78:1189–93.
Onnasch H, Gunn HM, Bradshaw BJ, Benestad SL, Bassett HF. Two Irish cases of scrapie resembling Nor98. Vet Rec. 2004;155:636–7.
Onodera T, Ikeda T, Muramatsu Y, Shinagawa M. Isolation of scrapie agent from the placenta of sheep with natural scrapie in Japan. Microbiol Immunol. 1993;37:311–6.
Orge L, Oliveira A, Machado C, Lima C, Ochoa C, Silva J, Carvalho R, Tavares P, Almeida P, Ramos M, Pinto MJ, Simas JP. Putative emergence of classical scrapie in a background of enzootic atypical scrapie. J Gen Virol. 2010;91:1646–50.
Papasavva-Stylianou P, Kleanthous M, Toumazos P, Mavrikiou P, Loucaides P. Novel polymorphisms at codons 146 and 151 in the prion protein gene of Cyprus goats, and their association with natural scrapie. Vet J. 2007;173:459–62.
Papasavva-Stylianou P, Windl O, Saunders G, Mavrikiou P, Toumazos P, Kakoyiannis C. PrP gene polymorphisms in Cyprus goats and their association with resistance or susceptibility to natural scrapie. Vet J. 2010;187:245–50.
Parry HB. Scrapie disease in sheep. London: Academic; 1983.
Pattison IH, Jones KM. The possible nature of the transmissible agent of scrapie. Vet Rec. 1967;80:2–9.
Pattison IH, Gordon WS, Millson GC. Experimental production of scrapie in goats. J Comp Pathol. 1959;69:200–312.
Pattison IH, Hoare MN, Watson WA. Spread of scrapie to sheep and goats by oral dosing with foetal membranes from scrapie-affected sheep. Vet Rec. 1972;90:465–8.
Piccardo P, Manson JC, King D, Ghetti B, Barron RM. Accumulation of prion protein in the brain that is not associated with transmissible disease. Proc Natl Acad Sci. 2007;104:4712–7.
Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216:136–44.
Race R, Jenny A, Sutton D. Scrapie infectivity and proteinase K-resistant prion protein in sheep placenta, brain, spleen, and lymph node: implications for transmission and antemortem diagnosis. J Infect Dis. 1998;178:949–53.
Reckzeh C, Hoffmann C, Buschmann A, Buda S, Budras KD, Reckling KF, Bellmann S, Knobloch H, Erhardt G, Fries R, Groschup MH. Rapid testing leads to the underestimation of the scrapie prevalence in an affected sheep and goat flock. Vet Microbiol. 2007;123:320–7.
Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Castagnoli RP. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–7.
Salvesen Ø, Espenes A, Reiten MR, Vuong TT, Malachin G, Tran L, Andréoletti O, Olsaker I, Benestad SL, Tranulis MA, Ersdal. Goats naturally devoid of PrPC are resistant to scrapie. Vet Res. 2020;51:1.
Schneider K, Fangerau H, Michaelsen B, Raab WHM. The early history of the transmissible spongiform encephalopathies exemplified by scrapie. Brain Res Bull. 2008;77:343–55.
Seeger H, Heikenwalder M, Zeller N, Kranich J, Schwarz P, Gaspert A, Seifert B, Miele G, Aguzzi A. Coincident scrapie infection and nephritis lead to urinary prion excretion. Science. 2005;310:324–6.
Seidel B, Thomzig A, Buschmann A, Groschup MH, Peters R, Beekes M, Terytze K. Scrapie agent (Strain 263 K) can transmit disease via the oral route after persistence in soil over years. PLoS One. 2007;2(5):e435.
Seuberlich T, Botteron C, Benestad SL, Brünisholz H, Wyss R, Kihm U, Schwermer H, Friess M, Nicolier A, Heim D, Zurbriggen A. Atypical scrapie in a Swiss goat and implications for transmissible spongiform encephalopathy surveillance. J Vet Diagn Investig. 2007;19:2–8.
Sigurdsson B. Rida, a chronic encephalitis of sheep. With general remarks on infections, which develop slowly, and some of their special characteristics. Br Vet J. 1954;110:341–54.
Simmons MM, Konold T, Simmons HA, Spencer YI, Lockey R, Spiropoulos J, Everitt S, Clifford D. Experimental transmission of atypical scrapie to sheep. BMC Vet Res. 2007;3:20.
Simmons MM, Konold T, Thurston L, Bellworthy SJ, Chaplin MJ, Moore SJ. The natural atypical scrapie phenotype is preserved on experimental transmission and sub-passage in PRNP homologous sheep. BMC Vet Res. 2010;6:14.
Simmons MM, Moore SJ, Konold T, Thurston L, Terry LA, Thorne L, Lockey R, Vickery C, Hawkins SA, Chaplin MJ, Spiropoulos J. Experimental oral transmission of atypical scrapie to sheep. Emerg Infect Dis. 2011;17:848–54.
Sisó S, Jeffrey M, González L. Neuroinvasion in sheep transmissible spongiform encephalopathies: the role of the haematogenous route. Neuropathol Appl Neurobiol. 2009;35:232–46.
Sisó S, González L, Jeffrey M. Neuroinvasion in prion diseases: the roles of ascending neural infection and blood dissemination. Interdiscip Perspect Infect Dis. 2010;2010:747892.
Smith CB, Booth CJ, Pedersen JA. Fate of prions in soil: a review. J Environ Qual. 2011;40:449–61.
Spiropoulos J, Hawkins SAC, Simmons MM, Bellworthy SJ. Evidence of In Utero Transmission of Classical Scrapie in Sheep. J Virol. 2014;88:4591–94.
Stack MJ, Chaplin MJ, Clark J. Differentiation of prion protein glycoforms from naturally occurring sheep scrapie, sheep-passaged scrapie strains (CH1641 and SSBP1), bovine spongiform encephalopathy (BSE) cases and Romney and Cheviot breed sheep experimentally inoculated with BSE using two monoclonal antibodies. Acta Neuropathol. 2002;104:279–86.
Stamp JT, Brotherston JG, Zlotnik I, Mackay JMK, Smith W. Further studies on scrapie. J Comp Pathol. 1959;69:268–80.
Taylor DM, McConnell I, Fraser H. Scrapie infection can be established readily through skin scarification in immunocompetent but not immunodeficient mice. J Gen Virol. 1996;77:1595–9.
Terry LA, Howells L, Bishop K, Baker CA, Everest S, Thorne L, Maddison BC, Gough KC. Detection of prions in the faeces of sheep naturally infected with classical scrapie. Vet Res. 2011;42:65.
Thaer S. Lässt sich irgend ein Grund der ersten Entstehung der Traber- oder Kreutzdreher-Krankheit annehmen? In: Königlich Preußische Akademie des Landbaues zu Möglin, vol. 7. 1st ed; 1821. p. 97–109.
Thorgeirsdottir S, Sigurdarson S, Thorisson HM, Georgsson G, Palsdottir A. PrP gene polymorphism and natural scrapie in Icelandic sheep. J Gen Virol. 1999;80:2527–34.
Thuring CM, Erkens JH, Jacobs JG, Bossers A, Van Keulen LJ, Garssen GJ, Van Zijderveld FG, Ryder SJ, Groschup MH, Sweeney T, Langeveld JP. Discrimination between scrapie and bovine spongiform encephalopathy in sheep by molecular size, immunoreactivity, and glycoprofile of prion protein. J Clin Microbiol. 2004;42:972–80.
TSE EURL. TSE strain characterisation in small ruminants. In: A technical handbook for National Reference Laboratories in the EU. Istituto Zooprofilattico Sperimentale del Piemonte, Liguria e Valle d’Aosta/Turin and Istituto Superiore di Sanità/Rome; 2020. http://www.izsto.it/images/stories/EURL_guidance.zip.
Ulvund M. Clinical findings in scrapie. In: Hörnlimann B, Riesner D, Kretzschmar H, editors. Prions in human and animals. Berlin: Walter de Gruyter GmbH & CO KG; 2007.
Ulvund MJ. Ovine scrapie disease: do we have to live with it? Small Rumin Res. 2008;76:131–40.
Vaccari G, Di Bari MA, Morelli L, Nonno R, Chiappini B, Antonucci G, Marcon S, Esposito E, Fazzi P, Palazzini N, Troiano P, Petrella A, Di Guardo G, Agrimi U. Identification of an allelic variant of the goat PrP gene associated with resistance to scrapie. J Gen Virol. 2006;87:1395–402.
Vaccari G, Panagiotidis CH, Acin C, Peletto S, Barillet F, Acutis P, Bossers A, Langeveld J, van Keulen L, Sklaviadis T, Badiola JJ, Andreoletti O, Groschup MH, Agrimi U, Foster J, Goldmann W. State-of-the-art review of goat TSE in the European Union, with special emphasis on PRNP genetics and epidemiology. Vet Res. 2009;40:48–66.
van Keulen LJ, Schreuder BE, Meloen RH, Mooij-Harkes G, Vromans ME, Langeveld JP. Immunohistochemical detection of prion protein in lymphoid tissues of sheep with natural scrapie. J Clin Microbiol. 1996;34:1228–31.
van Keulen LJ, Schreuder BE, Vromans ME, Langeveld JP, Smits MA. Pathogenesis of natural scrapie in sheep. Arch Virol Suppl. 2000;16:57–71.
van Keulen LJ, Vromans ME, van Zijderveld FG. Early and late pathogenesis of natural scrapie infection in sheep. APMIS. 2002;110:23–32.
van Keulen LJ, Vromans ME, Dolstra CH, Bossers A, van Zijderveld FG. TSE pathogenesis in cattle and sheep. Vet Res. 2008;39:24.
Vargas F, Bolea R, Monleón E, Acín C, Vargas A, De Blas I, Luján L, Badiola JJ. Clinical characterisation of natural scrapie in a native Spanish breed of sheep. Vet Rec. 2005;156:318–20.
Vidal E, Tortosa R, Costa C, Benavides J, Francino O, Sanchez-Robert E, Perez V, Pumarola M. Lack of PrP(sc) immunostaining in intracranial ectopic lymphoid follicles in a sheep with concomitant non-suppurative encephalitis and Nor98-like atypical scrapie: a case report. Vet J. 2008;177:283–8.
Von Richthofen F. Ueber die sogenannte Traberkrankheit der Schafe. Verhandlungen und Arbeiten der Ökonomisch-Patriotischen Sozietät der Fürstenthümer Schweidnitz und Jauer, Beilage G; 1821. p. 125–31.
von Richthofen F. Bemerkungen wegen der Bekanntmachung No. II. in den Mögl. Annalen der Landwirthschaft. In: 17ten Bandes 1stes Stück, unter der Aufschrift: Ueber die Traberkrankheit in Frankenfelde u.s.w., vom Königl. Landrathe Strigauschen Kreises und Direktor der ökonomisch-patriotischen Gesellschaft der Fürstenthümer Schweidnitz und Jauer etc., auf Barzdorf u.m.a. Güter Freiherrn, v. Richthofen. 1st ed. Vol 17 of Königlich Preußische Akademie des Landbaues zu Möglin; 1826. p. 501–39.
Wells GAH, Ryder SJ, Hadlow WJ (2007) The pathology of prion diseases in animals. In: Hörnlimann B, Riesner D, Kretzschmar H (eds) Prions in human and animals. Walter de Gruyter GmbH & CO, Berlin.
Westaway D, Goodman PA, Mirenda CA, McKinley MP, Carlson GA, Prusiner SB. Distinct prion proteins in short and long scrapie incubation period mice. Cell. 1987;51:651–62.
Westaway D, Zuliani V, Cooper CM, Da Costa M, Neumann S, Jenny AL, Detwiler L, Prusiner SB. Homozygosity for prion protein alleles encoding glutamine 171 renders sheep susceptible to natural scrapie. Genes Dev. 1994;8:959–69.
Wineland NE, Detwiler LA, Salman MD. Epidemiologic analysis of reported scrapie in sheep in the United States: 1,1 17 cases (1947–1992). J Am Vet Med Assoc. 1998;212:713–8.
Wood JL, Lund LJ, Done SH. The natural occurrence of scrapie in moufflon. Vet Rec. 1992a;130:25–7.
Wood JNL, Done SH, Pritchard GC, Wooldridge MJA. Natural scrapie in goats: case histories and clinical signs. Vet Rec. 1992b;131:66–8.
Wood JL, McGill IS, Done SH, Bradley R. Neuropathology of scrapie: a study of the distribution patterns of brain lesions in 222 cases of natural scrapie in sheep, 1982–1991. Vet Rec. 1997;140:167–74.
Zlotnik I. Cerebellar and midbrain lesions in scrapie. Nature. 1960;4715:785.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Fast, C., Groschup, M.H. (2023). Classical and Atypical Scrapie in Sheep and Goats. In: Zou, WQ., Gambetti, P. (eds) Prions and Diseases. Springer, Cham. https://doi.org/10.1007/978-3-031-20565-1_26
Download citation
DOI: https://doi.org/10.1007/978-3-031-20565-1_26
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-20564-4
Online ISBN: 978-3-031-20565-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)