Abstract
Scrapie belongs to a group of diseases known as the transmissible spongiform encephalopathies or prion diseases. Two different categories of naturally occurring scrapie have been identified: classical scrapie, which was first recorded around 1750, and atypical scrapie or ‘Nor-98’, which was first identified in Norway in 1998. The molecular characteristics of atypical scrapie have been well defined, but detailed descriptions of the neuropathological phenotype are rare since the majority of cases have been detected through active surveillance programmes where only brainstem and cerebellum are collected for statutory diagnosis. In order to characterise the neuropathology of naturally occurring atypical scrapie in sheep, we examined multiple brain levels from 15 whole brains from field cases of atypical scrapie, both clinical suspects and fallen stock, collected in Great Britain between 2004 and 2006. We found that the distribution of disease-associated prion protein (PrPSc) and vacuolation in atypical scrapie cases are very different to both classical scrapie and experimental bovine spongiform encephalopathy in sheep. Immunolabelling for PrPSc is mild and restricted at the obex and more intense and widespread rostrally, particularly in the cerebellum, substantia nigra, thalamus and basal nuclei. Intracellular immunolabelling types are not seen, but distinctive white matter immunolabelling is widespread. Vacuolation associated with PrPSc deposits was not observed in the brainstem neuroanatomical areas commonly affected in classical scrapie and bovine spongiform encephalopathy, but was instead most prominent in the cerebellar cortex and neocortex. This is the largest comprehensive descriptive study of atypical scrapie pathology to date, and provides baseline data against which other natural or experimental cases can be compared. It also reinforces the current recommendation to collect cerebellum in addition to brainstem to enable confident confirmation of this distinct disease phenotype within surveillance programmes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Scrapie belongs to a group of diseases known as the transmissible spongiform encephalopathies (TSEs) or prion diseases, which also includes bovine spongiform encephalopathy (BSE) in cattle, chronic wasting disease in deer, and (variant) Creutzfeldt–Jakob disease, Gerstmann–Sträussler–Scheinker disease, and fatal familial insomnia in humans. It is now known that sheep (and goats) may be affected by at least two forms of naturally occurring scrapie which have different molecular and neuropathogical phenotypes. Classical scrapie has been known for at least 250 years, but was only added to the list of OIE notifiable diseases in 1993 [41]. Cases of TSE may be detected through ‘passive’ surveillance, where clinical suspects are subject to a full neurological examination, or ‘active’ surveillance, where brain samples are taken from apparently healthy animals at the abattoir or animals found dead on-farm (fallen stock). Atypical scrapie, also known as Nor-98, was first reported in Norway [4] and has since been identified in Great Britain [11, 15], Germany and France [8], Sweden [17], Portugal [36], Ireland [35], Belgium [12], the Falkland Islands [14], Switzerland [34] and the United States of America [5]. The first case of atypical scrapie to be detected in Great Britain was in 2002, but one case has been retrospectively identified from 1989 [7].
According to the protein-only hypothesis, the infectious agent in prion diseases is an abnormal isoform (PrPSc) of the host-encoded prion protein (PrPc) [37]. One of the biochemical properties which allows PrPSc to be distinguished from PrPc is the relative resistance of PrPSc to degradation by proteinase K (PK): under defined conditions PrPc is completely degraded, but degradation of PrPSc is incomplete so that a resistant core (PrPres) remains. In the EU, TSE surveillance is based on the use of molecular ‘rapid’ tests which detect PrPSc. The electrophoretic mobility of PrPres and relative amounts of un-, mono-, and di-glycosylated fragments were originally used to differentiate human TSE strains using Western blot [10]. More recently the addition of parallel antibody testing with monoclonal antibodies raised to different amino acid sequences of the PrP molecule has given further discriminatory potential [45]. Western blot of brain material taken from the medulla at the level of the obex from the first cases of atypical scrapie showed molecular weights and glycoform ratios of PrPres fragments which were different to both classical scrapie and BSE [4]. Subsequent histological examination of obex and cerebellum samples from active surveillance cases [4, 5], and whole brains from Swiss atypical scrapie cases [34] has allowed the identification of differences in vacuolation profiles and patterns of PrPSc deposition between classical and atypical scrapie cases. However, the low apparent prevalence of atypical scrapie [31, 46] combined with its identification mainly through active surveillance means that whole brains are not often available for examination.
Previous studies have shown that the medulla at the level of the obex is the first area of the brain to become affected in pre-clinical BSE and scrapie cases [2, 25, 38, 47]. Therefore, when active surveillance for small ruminant TSEs was introduced in 2002, testing was performed on obex samples. However, following the identification of atypical scrapie in 1998 [4] and bovine amyloidotic spongiform encephalopathy in 2004 [9], both of which have more extensive pathology rostral to the obex, there was concern that there may have been additional forms of TSE that were not being detected. In Great Britain, it was decided that it would not be feasible to collect whole brains from apparently healthy animals slaughtered at abattoirs, but that whole brains could be collected from fallen stock and clinical suspects. During the period June 2004–June 2006 whole brains were collected and multiple brain levels were examined for any novel TSE-related pathology.
We have produced a detailed description of the immunohistopathology of whole brains from natural atypical scrapie cases, providing a baseline against which to assess the stability of atypical scrapie field strains and experimental disease. In addition, we have investigated whether genotype and/or incubation period affect the pathological phenotype of atypical scrapie, as has been demonstrated for classical scrapie [18, 19, 44].
Materials and methods
Case selection
Whole brains from clinical suspects were sampled either at one of the VLA’s regional laboratories, or following ante-mortem clinical examination at Weybridge [27]. During the period June 2004–June 2006, heads from fallen stock were submitted to VLA Regional Laboratories and the whole brain was removed. Cases were screened using an EU approved rapid test (Bio-Rad ELISA). Cases with a positive rapid test result were further tested using immunohistochemistry, a ‘stringent’ Western blot (VLA Discriminatory WB) and ‘Mild-PK’ Western blot (Bio-Rad TeSeE WB) as described in the EFSA Opinion on the classification of atypical Transmissible Spongiform Encephalopathy (TSE) cases in Small Ruminants [13].
A total of 15 whole brains (eight from clinical suspects and seven from fallen stock, Table 1) which were Bio-Rad ELISA positive, VLA Discriminatory WB negative, and Bio-Rad TeSeE WB positive were subject to a full neuropathological investigation.
Fixation and section preparation
The obex region of the brainstem was removed and placed in 10% formal saline for a minimum of 3 days, for statutory diagnostic purposes. The remainder of each brain was hemi-sected sagitally, and one half fixed in 10% formal saline for a minimum of 2 weeks. The other half was retained frozen. In three cases in which atypical scrapie was not suspected pre-mortem, brains were initially collected to an alternative protocol in which the obex was fixed and the remaining brain frozen. In these cases frozen tissue blocks were sampled and placed into frozen fixative, placed on a shaker, and allowed to return to room temperature gradually. Fixation continued for a further 3 days prior to processing.
Up to nine coronal brain levels were examined from each animal: medulla at level of the obex, rostral medulla, cerebellum, caudal midbrain, rostral midbrain, occipital cortex (with hippocampus), caudal thalamus, parietal thalamus and basal ganglia with frontal cortex. Not all levels were available for all animals due to differences in sampling technique and degree of tissue autolysis. Tissues were processed routinely, and embedded in paraffin wax.
Histopathology
For examination of vacuolar changes serial 5-µm sections were cut and stained with haematoxylin and eosin (H&E).
Immunohistochemistry
Immunohistochemical detection of PrPSc was performed using mouse MAb 2G11 (Institute Pourquier), raised against ovine PrP peptide sequence 146-R154R171-182. R145 is our routine Ab for statutory purposes and was used in the initial confirmation of all selected cases. However, 2G11 was shown to give slightly superior immunolabelling without affecting the morphology of the deposits in either atypical scrapie or classical scrapie controls (data not shown), and was selected for this study to ensure optimal definition of minimal lesions. Sections of the formalin-fixed, paraffin embedded tissues were cut at 4µm onto charged slides and incubated at 60°C for 30 min to improve adhesion. Sections were thoroughly de-waxed in xylene, and rehydrated. Epitope demasking was performed by immersion of sections for 30 min in undiluted formic acid, then washing in running tap water for 15 min, followed by autoclaving at 121°C in citrate buffer pH 6.1 (8.8 mM tri-sodium citrate dihydrate, 1.3 mM citric acid in 2 l purified water). Endogenous peroxidase was blocked using 3% hydrogen peroxide (100w/vol) in methanol, and washing buffer used throughout the procedure was tris buffered saline, supplemented with 0.2% tween20 (TBST). Primary antibody was applied at dilution of 1/400 for one hour at room temperature, with immunodetection performed using biotinylated goat anti mouse and avidin–biotin–peroxidase-complex (Vector Elite) technique using diaminobenzidine chromogen prepared in McIlvane’s citrate buffer. Sections were counterstained using Mayers haematoxylin, then routinely dehydrated, cleared and mounted in dibutylphthalate in xylene (DPX), before examination by light microscopy.
Examination of H&E stained sections
The interpretation of H&E stained sections was difficult or impossible in most cases due to autolysis and/or tissue treatment prior to fixation. Therefore, full vacuolation lesion profiling was not performed. Rather, neuroanatomical areas with vacuolation were recorded and a note was made of any areas in which the vacuolation was associated with PrPSc deposition.
Examination of immunolabelled sections
At each brain level, readily identifiable neuroanatomical areas (n = 262) were examined and the PrPSc immunolabelling types present in each area was recorded. PrPSc immunolabelling types observed in the grey matter were described using the nomenclature of Spiropoulos et al. [44] with two modifications: (1) like Benestad et al. [4], we have used the term ‘fine granular’ instead of ‘fine punctate’ to describe the powdery, diffuse PrPSc deposits observed in the neuropil, (2) we have used the term ‘aggregates’ instead of ‘coalescing’ to describe the large, amyloid-like deposits seen in the grey and white matter. This reflects the fact that there are subtle differences in the morphology of such deposits between classical and atypical scrapie cases.
Atypical scrapie aggregate deposits may be further classified into ‘plaque-like’ deposits, indicating that the deposit has a lucent core (in contrast to ‘large aggregates’, which do not), and ‘multi-centric aggregates’ which are composed of two or more large aggregates and/or plaque-like deposits (Fig. 1b, arrow).
Immunohistochemistry for PrPSc using mouse MAb 2G11. a Thalamus, fine granular immunolabelling, aggregates and plaque-like deposits of various sizes in the ventral thalamic nucleus. Scale bar 500 μm. b Thalamus, higher powered view of a showing aggregates of various sizes and a multi-centric-aggregate with a plaque-like deposit at its centre (arrow). Scale bar 50 μm. c Midbrain, linear immunolabelling and small aggregates in the substantia nigra. Scale bar 200 μm. d Neocortex, fine granular immunolabelling occurs in three distinct bands (laminar pattern). Scale bar 500 μm. e Globular white matter immunolabelling in the internal capsule including deposits in the shape of crescents (arrows) and rings (arrow heads). Scale bar 50 μm. f Punctate white matter immunolabelling in the fasciculus subcallosus. Scale bar 50 μm. g Cerebellum, fine granular immunolabelling in the granular and molecular layers. This is the most common pattern seen in the cerebellum. Scale bar 500 μm. h Cerebellum, mixed pattern including linear (left of sulci), aggregates (right of sulci) and fine granular immunolabelling (top right). Scale bar 500 μm
In our experience, the PrPSc signal strength of immunolabelling in the grey matter can be affected by many factors, including fresh tissue quality, method of fixation, storage, processing and immunolabelling run. Semi-quantitative scoring of the severity of grey matter immunolabelling was not performed in this study since, (1) fresh tissue quality was suboptimal in a number of cases, particularly brains from fallen stock, (2) protocols for fixation and storage were different, for example, three brains were frozen before fixation, and (3), not all slides were stained in the same IHC run. The PrPSc signal strength of white matter immunolabelling does not appear to be affected by any of the factors mentioned above. Therefore, white matter immunolabeling was scored semi-quantitatively on a scale from 0 (negative) to 3 (very prominent).
‘PrPd profiles’ for the corpus striatum (basal nuclei and frontal cortex) were generated according to the method of Gonzalez et al. [20], modified slightly to include plaque-like, globular and punctuate immunolabelling types. Briefly, in 11 neuroanatomical areas the severity of 15 immunolabelling types was semi-quantatively scored. The scores for each immunolabelling type were averaged across all areas and these values plotted on a graph for comparison with available data for other ovine TSEs.
Western blotting
Fresh brain samples (frontal cortex) were available from 12 of the 15 animals and were subjected to the Bio-rad TeSeE Western blot (Bio-Rad Cat No: 355 1169). Tissue (0.35 g) from each sample was ribolysed, purified, PK treated and PrPres concentrated following the manufacturer’s instructions and reagents supplied. Samples were heated for 4 min at 100°C prior to loading on gels. Molecular mass markers (Sigma) were included at either end of the gel. A single lane each of a UK classical ovine scrapie, UK bovine BSE and a Nor-98 ovine scrapie case were included for profile comparisons. Fifteen microlitres of each sample (tissue equivalent of 0.02 g) was loaded in duplicate lanes onto pre-cast 12% bis–tris gels (Criterion) and subjected to electrophoresis for 50 min at 200 V. The proteins were then transferred onto PVDF membranes (115 V for 60 min) and blocked (Bio-Rad blocking solution) for 40 min at room temperature. They were probed with the primary monoclonal antibody SHA31 (1:10 dilution of the stock solution supplied by the manufacturer) for 30 min at room temperature. The membranes were washed with PBS + 0.1% Tween (supplied by the manufacturer), incubated for 20 min in Bio-Rad secondary antibody at room temperature, washed again and incubated with ECL substrate (Amersham) for 45 s–1 min. The signal was detected with the Fluor-S MultiImager (Bio-Rad).
Data recording and statistical analyses
Up to 262 neuroanatomical areas were examined per case, depending on the number of coronal sections available. For each case the PrPSc immunolabelling types present and semi-quantitative white matter scores for each neuroanatomical area were recorded and tabulated in Excel. A grey matter score for each area was calculated by summing of the number of immunolabelling types (excluding globular and punctate immunolabelling) present in that area. To investigate the effect of surveillance stream on the severity of PrPSc immunolabelling, we calculated average grey and white matter scores for each neuroanatomical area by group (clinical suspects and fallen stock) and compared them using the Wilcoxon–Mann–Whitney nonparametric test with exact P values.
Results
The brains examined came from older sheep (4–8 years old) of both common (Mule, Welsh Mountain, Cheviot) and less common (Portland) breeds (Table 1). All cases had PrP genotypes which are more resistant to classical scrapie [5, 30, 32, 33, 40].
Immunohistochemistry
Only five PrPSc immunolabelling types (fine granular, aggregates, plaque-like, linear, perineuronal) were observed in the grey matter of these atypical scrapie cases, less than half the number routinely seen in classical scrapie [44, J. Spiropoulos, M.M. Simmons, S. J. Moore, unpublished] (Table 2). All cases had distinctive immunolabelling in the white matter which can be classified into two main types (Fig. 1e, f). The ‘globular’ type is characterised by complete or incomplete (crescent) ring- or oval-shaped deposits, which may have an irregular outline (Fig. 1e). This type is widely distributed and is prominent in the spinocerebellar tracts, reticular formation, cerebellar white matter, crus cerebri, internal capsule, optic tract, and cerebral white matter. The ‘punctate’ type is characterised by deposits which are smaller than those observed in the globular type, and which usually take the shape of a small circle or elongated tear-drop but may have an irregular outline (Fig. 1f). This type has a more restricted distribution and is prominent in the reticular formation, substantia nigra, fimbria hippocampi, amygdala, external capsule, fasciculus subcallosus, rostral commisure, and stratum multiforme of the neocortex (Fig. 2; Table 3). Punctate immunolabelling is also seen in some grey matter areas. Both globular and punctate immunolabelling may be present in the same area. This commonly occurs in the reticular formation, cerebellar white matter, crus cerebri, substantia nigra, internal capsule and cerebral white matter, but may also occur in other areas. The average white matter scores from the fallen stock and clinical suspects were not significantly different (Wilcoxon–Mann–Whitney test, P < 0.05).
PrPSc deposition pattern maps showing the types and distribution of immunolabelling observed in the brains of atypical scrapie cases at the level of the a obex, b rostral medulla (with cerebellum), c caudal midbrain, d rostral midbrain, e occipital cortex, f parietal cortex and g frontal cortex. See Table 3 for list of neuroanatomical areas
The neuroanatomical distribution of PrPSc immunolabelling types at each brain level is described in detail below using the following nomenclature: for immunolabelling in the grey matter, ‘prominent’ indicates that the average number of immunolabelling types observed in that area was at least 0.8, that is, most cases examined had at least one immunolabelling type and a few cases were negative. For immunolabelling in the white matter, ‘prominent’ indicates an average white matter score of at least 1.0. These cut-off values were chosen to reflect the observer’s subjective impression of severity of immunolabelling at low magnification.
Obex
The obex was present in 12 cases. Fine granular immunolabelling was most prominent in the spinal tract of the trigeminal nerve and ventro-lateral solitary tract. White matter immunolabelling was most prominent in the spinocerebellar tracts (dorsal and ventral), nucleus raphe magnus, reticular formation and spinothalamic tract.
Rostral medulla
The rostral medulla was present in five cases. Fine granular immunolabelling was most prominent in the spinal tract of the trigeminal nerve. White matter immunolabelling was most prominent in the nucleus dorsalis corporis trapezoidei, genu of facial nerve, nucleus raphe magnus, pyramidal tract, caudal cerebellar peduncle, cochlear nucleus, reticular formation and nucleus parasympatheticus n. intermedii.
Cerebellum
The deep cerebellar nuclei (nucleus dentatus, nuclei interpositi cerebelli, nucleus fastigii), were positive in five cases, negative in seven cases, and not represented in three cases. In positive cases, fine granular immunolabelling was most common, with aggregates observed in two cases. White matter immunolabelling of varying intensity was present in most (13/15) cases, and was more intense around the deep cerebellar nuclei and less intense in the cerebellar folia.
Immunolabelling was present in the granular layer of all cases, and was generally moderate to marked. The molecular layer was positive in all cases and a range of PrPSc deposition patterns were seen (Fig. 1g, h). The Purkinje cells were always negative.
Caudal midbrain
The caudal midbrain was present in ten cases. Grey matter immunolabelling was most prominent in the substantia nigra and rubrospinal tract. In the substantia nigra, fine granular immunolabelling was seen in 8/10 cases, and four of these cases also had aggregates and linear immunolabelling (Fig. 1c). In the rubrospinal tract, only fine granular immunolabelling was seen (7/10 cases). White matter immunolabelling was most prominent in the crus cerebri and substantia nigra.
Rostral midbrain
The rostral midbrain was present in 12 cases. Grey matter immunolabelling was most prominent in the substantia nigra and the medial geniculate nucleus. In the substantia nigra, the immunolabelling types observed were identical in the caudal midbrain (fine granular, aggregates, linear) (Fig. 1c), but the overall strength of the immunolabelling was greater. White matter immunolabelling was most prominent in the crus cerebri and substantia nigra.
Occipital cortex (with hippocampus)
The hippocampus was present in 14 cases. In nine cases both the dorsal and ventral horns were present, but three and two cases had just the dorsal or ventral horn, respectively. Fine granular immunolabelling was seen in all layers except the stratum granulosum and alveus in the majority of cases. The intensity of this immunolabelling varied between layers, and in some cases the patterns observed in the dorsal and ventral horns were different.
Caudal thalamus
The caudal thalamus was present in 12 cases. Grey matter immunolabelling was most prominent in the substantia nigra, lateral thalamic nuclei (nucleus lateralis caudalis, nucleus ventralis caudalis, nucleus suprageniculatus), external medullary lamina, medial geniculate nucleus and lateral geniculate nucleus. The immunolabelling types and intensity of the immunolabelling in the substantia nigra were similar to those at the level of the rostral midbrain (fine granular, aggregates, linear) (Fig. 1c). The lateral thalamic nuclei had fine granular immunolabelling and aggregates in the majority of cases, with large, plaque-like aggregates (Fig. 1b) seen in four (three active, one passive) cases. The external medullary lamina had fine granular immunolabelling in the majority of cases, with aggregates observed in three (active) cases. The geniculate nuclei had fine granular immunolabelling with aggregates in two cases. White matter immunolabelling was most prominent in the crus cerebri, external medullary lamina and substantia nigra.
Parietal thalamus
The parietal thalamus was present in all cases. Grey matter immunolabelling was most prominent in the ventral thalamic nuclei, intralaminar thalamic nuclei, rostral ventral nucleus, and caudate nucleus. The ventral thalamic nuclei, intralaminar thalamic nuclei and rostral ventral nucleus had fine granular immunolabelling, with aggregates in the intralaminar thalamic nuclei and ventral thalamic nuclei, and large, plaque-like aggregates in the ventral thalamic nuclei (Fig. 1a, b). White matter immunolabelling was prominent in most white matter areas examined, but was particularly strong in the capsula externa/extrema/interna, fasciculus subcallosus and crus cerebri.
Aggregates of various sizes (Fig. 1a, b) were seen in the thalamus of all cases. The AHQ allele seemed to be associated with smaller aggregates and fewer of them, while the ARR allele seemed to be associated with larger aggregates and more of them. The aggregates seen in cases carrying the ARQ allele were intermediate in size in number. Multi-centric aggregates were seen in the thalamus in nine cases, six of which (67%) were fallen stock. Five out of six (84%) of the cases without multi-centric aggregates were clinical suspects. Six out of nine cases (67%) had at least one plaque-like deposit with a lucent core but no relationship with surveillance stream or genotype was found.
Basal nuclei
The basal nuclei and frontal cortex were present in 14 cases. Grey matter immunolabelling was most prominent in the basal nuclei. Fine granular immunolabelling was observed in all basal nuclei, with aggregates seen in the globus pallidus in four cases. White matter immunolabelling was most prominent in the internal capsule, rostral commissure, septal nuclei and nucleus accumbens.
Neocortex
Fine granular immunolabelling was observed in a diffuse laminar pattern in up to three of the neocortical layers (Fig. 1d). The multiform layer was affected in all cases, and the molecular layer and internal granular layer were affected in 12/15 and 11/15 cases, respectively. White matter immunolabelling was most prominent in the multiform layer, but was also seen in the internal pyramidal and internal granular layers.
In the cortical white matter, multi-centric aggregates were seen in two fallen stock (genotypes ARQ/ARQ and ARR/ARR) and one passive surveillance case (genotype ARQ/ARQ). These cases represent three out of only four cases (75%) that were not carrying an AHQ allele (see Table 1 for genotype distribution).
PrPSc deposition pattern (PDP)
Although the written descriptions above provide detailed information about the type and distribution of immunolabelling throughout the brain, we thought that a pictorial representation (Fig. 2) may be more meaningful to those who are less familiar with the neuroanatomy of the brain. Therefore, out of the 262 areas examined we selected 37 areas (Table 3) which were present in the majority (>50%) of cases and in which the immunolabelling was ‘prominent’ according to the definition given above. Each area is coloured/patterned to indicate the most common (present in >50% of cases) immunolabelling types observed in that area.
Fine granular immunolabelling was also present but not prominent in the following neuroanatomical areas: amygdala, nucleus reticulates thalami, accessory/external cuneate nuclei, stratum griseum profundum, nucleus dorsomedialis thalami, capsula externa, nucleus pretectalis caudalis, capsula extrema and claustrum. Neuropil immunolabelling was also observed in an additional 40 areas, many of which were present in only a minority of cases and are therefore not numbered in Fig. 2.
PrPd profiles
Profiling of the location and severity of PrPSc deposition in the basal nuclei and frontal cortex has previously been used to characterise natural and experimental scrapie strains and BSE in sheep [20]. When we applied this method to the atypical scrapie cases we found that the profiles for clinical suspects and fallen stock were similar (data not shown), and were distinctly different from experimental scrapie strains and ovine BSE described previously (Fig. 3).
PrPd profiles for the brain at the level of the basal nuclei for atypical scrapie (purple), experimental classical scrapie strains (blue) and BSE in sheep of different genotypes (green). Experimental classical scrapie and ovine BSE data from Gonzalez et al. [20], with the published data for ‘FG/A/PL’ deposits subdivided according to our current nomenclature (L. Gonzalez, personal communication). Immunolabelling types: ITNR intraneuronal, ITAS intra-astrocytic, ITMG intramicroglial, STEL stellate, SBPL subpial, SBEP subependymal, PRVS perivascular, PVAC perivacuolar, FG/A fine granular and aggregates, PL plaque-like aggregates, LIN linear, PN perineuronal, VASC vascular plaques, GL/PT globular and punctuate
Vacuolation
Neuropil vacuolation, where it could be confidently distinguished from artefact, was very prominent in the molecular layer of cerebellar cortex, neocortex, hippocampus, basal nuclei and nucleus accumbens. The severity of neuropil vacuolation was not always similar to the severity of IHC immunolabelling. For example, in the cerebellar cortex the severity of vacuolation and immunolabelling was comparable. In contrast, in the neocortex vacuolation affected all six neocortical layers, while immunolabelling was restricted to up to three layers. Intraneuronal vacuolation was not seen. Vacuolation of the white matter was seen in some cases but was generally difficult to interpret due to suboptimal tissue quality.
Western blot
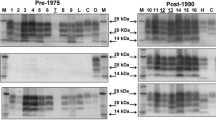
Variability in the biochemical characteristics of atypical scrapie has been reported and this may be related to PK cleavage sites and the method and antibodies used for detection [5]. In all the cases reported here, whether clinical suspects or fallen stock, a banding profile similar to that previously described for atypical scrapie/Nor-98 [4, 8, 15] was seen, the distinct profile showing a downward shift of all PrPSc fragments including one below 15 kD (Fig. 4). In one case (1403/04) there appears to be a subtle change in the banding pattern, but this may be due to lower protein levels in this sample. Due to limited sample availability it was not possible to standardise the loading fully.
Bio-Rad TeSeE Western blot. All cases have a banding profile similar to that previously described for atypical scrapie/Nor-98 [3, 4, 7, 12] with a downward shift of all PrPSc fragments including one below 15 kD. In lane 12 there is a subtle difference in the banding pattern which could be due to lower protein levels in this sample. Lane key: 1 biotinylated marker, 2 case 0076/06, 3 0092/06, 4 0603/06, 5 0667/05, 6 0781/04, 7 0832/06, 8 1239/05, 9 1270/05, 10 1347/04, 11 1367/05, 12 1403/04, 13 1572/05, 14 atypical scrapie positive control, 15 bovine BSE positive control, 16 classical scrapie positive control, 17 biotinylated marker
Discussion
All of the atypical scrapie cases examined in this report had similar PDP throughout the brain, in contrast to Nentwig et al. [34] who reported prominent differences in the distribution of abnormal prion protein in the brains of their ovine atypical scrapie cases. This may be because we examined whole brains from 15 cases, while Nentwig et al. had three cases with whole brains, one case with the obex, cerebellum and medulla, one case with the obex and cerebellum, and one case with the obex only. With only a few whole brains available, it may have been difficult for Nentwig et al. to interpret the significance of differences observed in individual cases. In addition, although different antibodies were used for detection of PrPSc in these two studies and it has been shown that this can affect the PDP in classical scrapie and ovine BSE [19, 23], further investigation would be required to ensure that the type and severity of immunolabelling detected in atypical scrapie cases is never affected by the antibody used for detection of PrPSc.
However, we are in agreement with Nentwig et al. [34] that the PDP of atypical scrapie cases are distinctly different from classical scrapie. In classical scrapie, at clinical end-point, PrPSc deposition occurs throughout the neuraxis at a moderate to marked severity. In atypical scrapie the distribution of PrPSc deposition is restricted, with the cerebellum, substantia nigra, thalamus and basal nuclei most affected, and the overall severity of immunolabelling is generally mild to moderate. Therefore, although ‘prominent’ has been used in the descriptions above, the intensity of immunolabelling in atypical scrapie is significantly less than in classical scrapie, particularly with regard to fine granular and perineuronal immunolabelling types. We found that the sum of the number of immunolabelling types in a particular area correlated well with our subjective impression of severity.
According to the protein only hypothesis, PrPSc is the sole component of the infectious agent [37]. Therefore, different strain phenotypes, including morphological PrPSc immunolabelling types and distribution, must be determined by molecular differences in PrPSc which affect the ability of a TSE agent strain to target particular cell types and influence the rates of production, accumulation and clearance of prion protein in cells of the brain and other tissues [6, 39, 43]. It is well established that the susceptibility of sheep to scrapie is associated with polymorphisms at codons 136, 141, 154 and 171 of the PrP gene [3, 33], and that atypical scrapie tends to occur in genotypes which are more resistant to classical scrapie [5, 30, 32, 33, 40]. However, this genotype–susceptibility relationship does not hold in all cases and multiple pathological phenotypes have been observed in sheep of the same genotype [19, 20, 44], so other molecular/cellular factors must also be involved in phenotype expression. Some suggested molecular differences include the size and/or conformation of the protease resistant fragment, the degree of asparagine-liked glycosylation, the sub-cellular compartmentalisation of the available pool of PrPc, and/or other cell- or tissue-specific factors. These hypotheses have been the focus of numerous in vitro and in vivo studies, but the exact mechanisms by which they influence PrPSc structure remain unclear (reviewed in Lawson et al. [28]).
Like Benestad et al. [4] and Nentwig et al. [34], we observed that the PDP in the cerebellum show much more variation than any other brain area. Although there are differences between individuals, the overall pattern is instantly recognisable as different from classical scrapie, both with regard to the relative severity of immunolabelling in the granular, Purkinje cell and molecular layers, and the PrPSc immunolabelling types present. In all three studies, in the majority of cases examined the severity of immunolabelling in the granular and molecular layers was approximately equal (Fig. 1g), while a smaller proportion of cases had immunolabelling which was most severe in the molecular (Fig. 1h) or granular layers. The Purkinje cells are always negative. Since the Western blots from the animals in all three studies are similar, it is unlikely that the observed differences in PDPs in the cerebellum of atypical scrapie cases are due to differences in PrPSc fragment size or glycosylation, but may instead be attributed to as yet unknown cell-specific factors which affect susceptibility to infection and/or rate of PrPSc production and accumulation.
Throughout the brain, atypical scrapie cases have fewer morphological PrPSc immunolabelling types than classical scrapie cases. Fine granular, aggregates, linear and white matter immunolabelling types are commonly seen in atypical scrapie, while intracellular and stellate types, which are prominent in classical scrapie, are not seen in atypical scrapie. Other immunolabelling types which have previously been recorded in classical scrapie but which are not seen in atypical scrapie are subpial, perivascular, perivacuolar, ependymal, subependymal and vascular plaques (Table 2). Why are the PrPSc immunolabelling types seen in classical and atypical scrapie so different?
Most PrPSc immunolabelling types are associated with PrPSc accumulation in particular cell types, so variations in the cellular tropism of the classical and atypical scrapie strains may explain the differences in PrPSc immunolabelling types seen. The large, round granules of varying sizes which appear in the perikaryon of large neurones and the cytoplasm of glial cells in classical scrapie are thought to be associated with the accumulation of partially digested PrPSc within lysosomes [24]. Intracellular immunolabelling is not seen in the central or peripheral nervous tissue or lymphoreticular tissues in natural [16, 34] or experimental atypical scrapie cases [42, S.J. Moore, unpublished]. Perineuronal and linear immunolabelling is characterised by PrPSc deposition around the soma, dendrites and/or axons of large neurones [18]. These labelling types are seen in both classical and atypical scrapie but are much more common and prominent in classical atypical scrapie. Glial cell associated PrPSc immunolabelling types (stellate, intraglial, perivascular, subpial, subependymal, perivacuolar) are commonly seen in classical scrapie but are not seen in atypical scrapie. The most commonly observed PrPSc immunolabelling types (fine granular and aggregates) in atypical scrapie are not associated with particular cell types, but represent PrPSc which aggregates and may form fibrils on distal neuronal processes or near the synapse [1, 22].
Overall, the atypical scrapie agent appears to have no, or very low, tropism for glial cells and large neurones, but instead tends to accumulate on the distal neuronal processes in the neuropil. The fact that intracellular immunolabelling is prominent in all classical scrapie strains but is never seen in atypical scrapie cases suggests that the cellular processing of classical and atypical scrapie prions is different. Perhaps since atypical scrapie PrPSc is more PK sensitive than classical scrapie PrPSc [5, 26], it may more readily undergo enzymatic digestion [42] and so does not accumulate in lysosomes and form intracellular deposits.
Globular and punctuate white matter immunolabelling (Fig. 1e, f) was observed throughout the neuraxis and is thought to be associated with oligodendrocytes [42]. To the authors’ knowledge, these immunolabelling types have not been previously reported in other TSE strains. Review of 115 active surveillance cases revealed that white matter immunolabelling is present in most, but not all, cases and may be the only immunolabelling type present at the obex (S. J. Moore, unpublished).
Incubation period and genotype have previously been shown to affect PDP in naturally occurring classical scrapie [19, 44]. We found that surveillance stream as well as genotype had some effect on the morphology and frequency of aggregates and the severity of immunolabelling in the neocortex, although our interpretation was limited by the small sample size and the fact that the exact incubation periods were not known. However, the clinical stage of disease could be inferred from the surveillance stream, that is, clinical suspects are possibly (and counter-intuitively) at an earlier point post-infection than fallen stock, which may have had clinical signs that were not recognised by the stockperson.
Aggregates of various sizes (Fig. 1a, b) were seen in the thalamus of all cases and the cortical white matter in four cases. In the thalamus, the size and number of aggregate appears to be influenced by both surveillance stream and genotype. Fallen stock are more likely to have multi-centric deposits than clinical suspects. Cases carrying multiple copies of the AHQ allele are more likely to have smaller and fewer aggregates than cases carrying ARQ or ARR alleles. No relationship was found between the presence of plaque-like deposits and surveillance stream or genotype. In the cortical white matter the occurrence of aggregates may be influenced by genotype, since hetero- or homozygosity for histidine at codon 154 appears to have an inhibitory effect on PrPSc aggregation. However, this interpretation is limited by the fact that aggregates in the cortical white matter were only present in three cases.
Amyloid PrPSc deposits in human prion diseases are intimately associated with astrocytes and microglia. Amyloid plaques are surrounded by astrocytic processes and invaded by microglial cells, and amyloid bundles may be attached to microglial cell membranes [21, 29]. In contrast to the compact structures typical of human amyloid deposits, plaques in mice and sheep contain haphazardly orientated fibrils which are heavily decorated with PrPSc [29], while aggregates may have no visible fibrils [22]. As discussed above, atypical scrapie prions appear to have low or no tropism for glial cells, so it seems unlikely that the aggregates and plaque-like deposits seen in atypical scrapie are associated with these cell types. Further studies are needed to define the ultra-structure of aggregates and plaque-like deposits in atypical scrapie.
Fine granular immunolabelling in the neocortex was present in up to three horizontal bands, roughly corresponding to the stratum moleculare, stratum granulare internum, and stratum multiforme (Fig. 1d). Severity of immunolabelling in the neocortex, as indicated by the number of layers affected, appeared to be affected by genotype. The presence of glutamine at codon 171 was associated with more severe immunolabelling, while sheep with arginine at codon 154 and/or 171 had less severe immunolabelling. Severity of immunolabelling in the neocortex was not influenced by surveillance stream.
This series of atypical scrapie cases showed a consistent pathological presentation which is distinct from any PDP previously reported in classical scrapie, and which is maintained on experimental transmission [42]. This supports previous observations that in atypical scrapie the amount of PrPSc immunolabelling in the cerebellum is consistently greater than in the brainstem [4], reinforcing the current recommendation [41] to collect cerebellum in addition to brainstem to enable confident confirmation all forms of TSE within surveillance programmes. Further work is needed to determine the full spectrum of PDP in both classical and atypical scrapie, and to develop a comprehensive understanding of the factors which influence it.
References
Andréoletti O, Berthon P, Levasseur E et al (2002) Phenotyping of protein-prion (PrPSc)-accumulating cells in lymphoid and neural tissues of naturally scrapie-affected sheep by double-labeling immunohistochemistry. J Histochem Cytochem 50(10):1357–1370
Arnold ME, Ryan JBM, Konold T et al (2007) Estimating the temporal relationship between PrPSc detection and incubation period in experimental bovine spongiform encephalopathy (BSE) of cattle. J Gen Virol 88(11):3198–3208
Baylis M, Chihota C, Stevenson E et al (2004) Risk of scrapie in British sheep of different prion protein genotype. J Gen Virol 85(9):2735–2740
Benestad SL, Sarradin P, Thu B, Schonheit J, Tranulis A, Bratberg B (2003) Cases of scrapie with unusual features in Norway and designation of a new type, Nor98. Vet Rec 153(7):202–208
Benestad SL, Arsac JN, Goldmann W, Noremark M (2008) Atypical/Nor-98 scrapie: properties of the agent, genetics, and epidemiology (review). Vet Res 39:19
Birkett CR, Hennion RM, Bembridge DA et al (2001) Scrapie strains maintain biological phenotypes on propagation in a cell line in culture. EMBO J 20(13):3351–3358
Bruce ME, Nonno R, Foster J et al (2007) Nor98-like sheep scrapie in the United Kingdom in (1989). Vet Rec 160(19):665–666
Buschmann A, Biacabe AG, Ziegler U et al (2004) Atypical scrapie cases in Germany and France are identified by discrepant reaction patterns in BSE rapid tests. J Virol Methods 117(1):27–36
Casalone C, Zanusso G, Acutis P et al (2004) Identification of a second bovine amyloidotic spongiform encephalopathy: molecular similarities with sporadic Creutzfeldt-Jakob disease. Proc Natl Acad Sci 101(9):3065–3070
Collinge J, Sidle KCL, Meads J, Ironside J, Hill AF (1996) Molecular analysis of prion strain variation and the aetiology of “new Variant” CJD. Nature 383:685–690
Defra National Scrapie Plan: atypical cases of Scrapie. Available at http://www.defra.gov.uk/animalH/bse/othertses/scrapie/nsp/atypicalcases/index.htm
De Bosschere H, Roels S, Benestad SL, Vanopdenbosch E (2004) Scrapie case similar to Nor98 diagnosed in Belgium via active surveillance. Vet Rec 155(22):707–708
SA EF (2005) Opinion of the scientific panel on biological hazards on classification of atypical spongiform encephalopathy (TSE) cases in small ruminants. J EFSA 276:1–30
Epstein V, Pointing S, Halfacre S (2005) Atypical scrapie in the Falkland Islands. Vet Rec 157(21):667–668
Everest SJ, Thorne L, Barnicle DA et al (2006) Atypical prion protein in sheep brain collected during the British scrapie-surveillance programme. J Gen Virol 87(2):471–477
Foster J, Toovey L, McKenzie C et al (2008) Atypical scrapie in a closed UK flock with endemic natural classical scrapie. Vet Rec 162(22):723–725
Gavier-Widén D, Nöremark M, Benestad S et al (2004) Recognition of the Nor98 variant of scrapie in the Swedish sheep population. J Vet Diagn Invest 16(6):562–567
González L, Martin S, Begara-McGorum I et al (2002) Effects of agent strain and host genotype on PrP accumulation in the brain of sheep naturally and experimentally affected with scrapie. J Comp Pathol 126(1):17–29
González L, Martin S, Jeffrey M (2003) Distinct profiles of PrP(d) immunoreactivity in the brain of scrapie- and BSE-infected sheep: implications for differential cell targeting and PrP processing. J Gen Virol 84(5):1339–1350
González L, Martin S, Houston FE et al (2005) Phenotype of disease-associated PrP accumulation in the brain of bovine spongiform encephalopathy experimentally infected sheep. J Gen Virol 86(3):827–838
Guiroy DC, Wakayama I, Liberski PP, Gajdusek DC (1994) Relationship of microglia and scrapie amyloid-immunoreactive plaques in kuru, Creutzfeldt-Jakob disease and Gerstmann–Sträussler syndrome. Acta Neuropathol 87(5):526–530
Jeffery M, Goodsir CM, Bruce ME, McBride PA, Fowler N, Scott JR (1994) Murine scrapie-infected neurones in vivo release excess prion protein into the extracellular space. Neurosci Lett 174:39–42
Jeffrey M, Martin S, Gonzalez L, Ryder SJ, Bellworthy SJ, Jackman R (2001) Differential diagnosis of infections with bovine spongiform encephalopathy (BSE) and scrapie agents in sheep. J Comp Path 125:271–284
Jeffrey M, Martin S, González L (2003) Cell-associated variants of disease-specific prion protein immunoimmunolabelling are found in different sources of sheep transmissible spongiform encephalopathy. J Gen Virol 84(4):1033–1045
van Keulen LJ, Bossers A, van Zijderveld F (2008) TSE pathogenesis in cattle and sheep. Vet Res 39(4):24
Klingeborn M, Wik L, Simonsson M, Renström LH, Ottinger T, Linné T (2006) Characterization of proteinase K-resistant N- and C-terminally truncated PrP in Nor98 atypical scrapie. J Gen Virol 87(6):1751–1760
Konold T, Davis A, Bone G, Bracegirdle J, Chaplin M, Simmons MM (2007) Clinical findings in two cases of atypical scrapie in sheep: a case report. BMC Vet Res 3:2
Lawson VA, Collins SJ, Masters CL, Hill AF (2005) Prion protein glycosylation (review). J Neurochem 93(4):793–801
Liberski PP, Bratosiewicz J, Waliś A, Kordek R, Jeffrey M, Brown P (2001) A special report I. Prion protein (PrP)-amyloid plaques in the transmissible spongiform encephalopathies, or prion diseases revisited (review). Folia Neuropathol 39(4):217–235
Lühken G, Buschmann A, Brandt H, Eiden M, Groschup MH, Erhardt G (2007) Epidemiological and genetical differences between classical and atypical scrapie cases. Vet Res 38(1):65–80
McIntyre KM, Del Rio Vilas VJ, Gubbins S (2008) No temporal trends in the prevalence of atypical scrapie in British sheep, 2002–2006. BMC Vet Res 4:13
Moreno CR, Moazami-Goudarzi K, Laurent P et al (2007) Which PrP haplotypes in a French sheep population are the most susceptible to atypical scrapie? Arch Virol 152(6):1229–1232
Moum T, Olsaker I, Hopp P et al (2005) Polymorphisms at codons 141 and 154 in the ovine prion protein gene are associated with scrapie Nor98 cases. J Gen Virol 86(1):231–235
Nentwig A, Oevermann A, Heim D et al (2007) Diversity in neuroanatomical distribution of abnormal prion protein in atypical scrapie. PLoS Pathog 3(6):e82
Onnasch H, Gunn HM, Bradshaw BJ, Benestad ST, Bassett HF (2004) Two Irish cases of scrapie resembling Nor98. Vet Rec 155(20):636–637
Orge L, Galo A, Machado C et al (2004) Identification of putative atypical scrapie in sheep in Portugal. J Gen Virol 85(11):3487–3491
Prusiner SB (1982) Novel proteinaceous infectious particles cause scrapie. Science 216:136–144
Ryder SJ, Spencer YI, Bellerby PJ, March SA (2001) Immunohistochemical detection of PrP in the medulla oblongata of sheep: the spectrum of staining in normal and scrapie-affected sheep. Vet Rec 148(1):7–13
Safar J, Wille H, Itri V et al (1998) Eight prion strains have PrPSc molecules with different conformations. Nature Med 4:1157–1165
Saunders GC, Cawthraw S, Mountjoy SJ, Hope J, Windl O (2006) PrP genotypes of atypical scrapie cases in Great Britain. J Gen Virol 87(11):3141–3149
Scrapie, Chapter 2.4.8 in OIE manual of diagnostic tests and vaccines for terrestrial animals. Available at http://www.oie.int/eng/normes/mmanual/2008/pdf/2.07.13_SCRAPIE.pdf
Simmons MM, Konold T, Simmons HA et al (2007) Experimental transmission of atypical scrapie to sheep. BMC Vet Res 3:20
Somerville RA (1999) Host and transmissible spongiform encephalopathy agent strain control glycosylation of PrP. J Gen Virol 80(7):1865–1872
Spiropoulos J, Casalone C, Caramelli M, Simmons MM (2007) Immunohistochemistry for PrPSc in natural scrapie reveals patterns which are associated with the PrP genotype. Neuropathol Appl Neurobiol 33(4):398–409
Stack MJ, Chaplin MJ, Clark J (2002) Differentiation of prion protein glycoforms from naturally occurring sheep scrapie, sheep passaged scrapie strains (CH1641 and SSBP1), bovine spongiform encephalopathy (BSE) cases and Romney and Cheviot breed sheep experimentally inoculated with BSE using two monoclonal antibodies. Acta Neuropathol 104:279–286
Tongue SC (2006) The diagnosis and relevance of ‘Atypical’ scrapie in Great Britain. Proc Sheep Vet Soc 30:71–75. Available at http://www.sheepvetsoc.org.uk/docs/Proc06/Tongue.pdf
Wells GA, Hawkins SA, Green RB et al (1998) Preliminary observations on the pathogenesis of experimental bovine spongiform encephalopathy (BSE): an update. Vet Rec 142(5):103–106
Acknowledgments
We would like to thank the VLA regional laboratories for collection of samples, the staff of the Neuropathology and Histopathology groups (VLA Weybridge) for processing of tissues, staff of the Molecular Pathogenesis and Genetics department (VLA Weybridge) for performing the Western blotting, Lorenzo Gonzalez (VLA Lasswade) for training and advice on PrPd profiling, and Alberto Vidal-Diez (VLA Weybridge) for assistance with statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moore, S.J., Simmons, M., Chaplin, M. et al. Neuroanatomical distribution of abnormal prion protein in naturally occurring atypical scrapie cases in Great Britain. Acta Neuropathol 116, 547–559 (2008). https://doi.org/10.1007/s00401-008-0433-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-008-0433-8