Abstract
The use of composites in dentistry has evolved with the integration of chemical and micromechanical retention to tooth structure. Modern adhesives allow for a sound bond between tooth structure and filling material which is clinically significant for the success of restorations and long-term prognosis of the tooth. Bonding agents aim to tackle the challenges of using composite materials when restoring tooth form and function. The main areas of concern are related to the inherent properties of resin polymerization such as shrinkage stress, long-term stability, bond strength, and gap formation. As clinicians begin to adopt new procedures and technology of the bulk fill technique, ensuring good bond is essential.
Bulk-fill materials are appealing to clinicians with the possibility of 4–5 mm restorations in an efficient time frame and limited steps to prevent errors. Successful bulk-fill would be greatly advantageous for both practitioner and patients, but concerns persist about the ability of such materials to provide long-term positive results. One of the main areas of focus with this approach is the interface between tooth structure and composite. Gaps between the tooth and restoration material can lead to unfavorable developments like secondary caries or post-surgical sensitivity.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
3.1 The Evolution of Adhesives
Historically both composite polymers and metal restorations relied on mechanical retention to tooth structure. This technique required the practitioner to remove large amounts of affected and even sound tooth structure. Retention forms were more invasive, and yielded poor results in terms of biocompatibility, aesthetics, and efficacy. These non-adhesive restorations also created a potential gap at the interface of composite and hard tissue, which was susceptible to leakage, demineralization, and secondary caries. Issues derived from the mechanical retention practice ultimately led to restoration failure and filling dislodgement. The advent of adhesive dentistry addressed these barriers and simultaneously adopted a minimally invasive approach.
Adhesive dentistry is the conservative practice of using resin-based materials bonded directly to tooth structure. This preserves uncompromised hard tissues, eliminates mechanical retention, and improves the marginal seal of composite fillings.
3.1.1 The Acid Etch Technique and Early Dentin Bond
Enamel bonding was initially achieved in the 1950s with penetration of resin monomer to acid-etched enamel [1]. The etching approach only was sufficient for bonding to enamel but bonding to dentin was significantly more challenging due to its organic composition and inhomogeneous deposition. Recall that enamel is approximately 90% hydroxyapatite crystals (mineral content) in the form of parallel enamel prisms and interrod enamel. Dentin on the other hand is a complex organic matrix of odontoblasts, type I collagen, mineral and fluid filled microchannel dentinal tubules. The water and hydrophilic composition of dentin repelled the hydrophobic resins from penetrating deep into the dentin. This meant that with acid-etch technique resin was not impregnating deep enough for the micromechanical retention.
Bonding to dentin was eventually achieved by Fusayama, who pioneered the “total-etch” (also called “etch-and-rinse”) technique [2]. This process was most noteworthy for removing the smear layer created by instrumentation. This was achieved through chelating excess metal and mineral from the preparation surface. Though it may seem trivial, the smear layer occluded the dentinal tubules and directly inhibited primer or bond from impregnating fully into the dentin. Fusayama found that demineralizing the dentin with 30–40% acid also exposed the collagen fibrils which could then be used to integrate and copolymerize a hydrophilic monomer. Demineralization of dentin to 3–5 μm created ideal pores or voids that are filled by primer/resin for micromechanical retention.
Traditionally, the term of adhesion to dentin referred to the three-step process of etching, priming, and bonding adhesive resin into a tooth surface. Adhesive bonds use micromechanical retention, which is achieved through acid etching and priming underlying tooth structure. These steps allow adhesive resin to integrate into the porous surface of prepared tooth structure, rather than polymerizing on the surface of the preparation. Etching is the application of phosphoric acid (H3PO4) or a similar strong acid to demineralize the bonding surface. Exposed projections of hydroxyapatite increase the surface area to bond to and are more receptive to diffusion of hydrophobic resin. When polymerized, monomers of the adhesive resin interlock with these extensions. Priming is the step that removes excess water and expands the collagen network to allow for better wettability by the adhesive. Preparing the bonding surface for bond in this way is not as vital for an etched and dried enamel, but is very important for bonding to dentin. Finally, the bond is worked into the extensions of the prep and light cured to lock into the prepared microenvironment. It is this final layer that links the composite filling material to the tooth surface. Without adhesive bonds, composite restorations are just retentive resin fillings and have failures associated with an incomplete marginal seal and gap formation. OptiBond FL (Kerr, Brea, CA) has shown a record of accomplishment of success over decades after its introduction [1].
The mechanism for this loss of integrity was hydrolytic dissolution of both the polymer and the demineralized tissue (collagen fibrils). The organic component of dentin was left unprotected at sites of incomplete infiltration of resin, a process known as nanoleakage. Dentinal collagen is susceptible to hydrolytic cleavage in water, as well as attack from host-derived enzymes such as matrix metalloproteases (MMPs). To remedy this, researchers sought resins that promoted chemical interaction and provided an impermeable interface with the tooth substrate.
The advent of etch-and-rinse technique solved the issues associated with penetrating the smear layer for dentinal bonding. However, there was still hydrophilic and organic substances within the microcosm of the preparation surface that would not mix well with hydrophobic resin. Hydrophobicity is important for bonding agents to inhibit degradation in the oral environment. Thus, an adhesive needed to integrate the hydrophilic natural dentin structure with the hydrophobic adhesive monomers. It is the role of the primer to accomplish this necessary component of dental bonding. The primer acts to: (1) remove water and (2) chemically interact with collagen and hydroxyapatite. Removing excess water is accomplished through evaporation, which the primers can promote with polar solvents, mainly acetone. Primers also contain water, ethanol or acetone to decrease viscosity during the initial application, which helps the primer flow into all areas of the preparation. In addition, a successful primer contains substrates that can chemically attract both the hydrophobic resin and the hydrophilic tooth. Lastly, the literature supports mechanical stimulation of the primer by the practitioner at the time of application. In conjunction with etch-and-rinse, primers allow for adhesive polymerization to interlock the resin and the tooth structure intimately. The hydrophobic resin is the final component (and layer) to the three-step procedure of bonding. Monomer entanglement with collagen fibrils creates a mixed structure at the resin-dentin interface is known as the “hybrid layer,” as coined by Nakabayashi. After curing, this final mixture of primer, resin, and etched tooth extensions at the margin of a preparation creates an impermeable seal.
3.1.2 The Single-Bottle Adhesive and Wet Bonding
As technology sought to increase efficiency, manufacturers tried to conserve the number of steps required for proper bonding technique. In the fourth-generation system, the first operator coats H3PO4 on all prepared surfaces; this is the “total etch” or “etch and rinse” technique. After rinsing, a primer is used that allows the collagen fibers in the tooth to take on a more suitable spatial organization. Drying is important to allow volatile solvents to evaporate off excess water at this time. A third and final step is the application of bond, which integrates into the prepped collagen fibers. The attempt to simplify adhesive steps resulted in fifth generation of adhesives, which is a two-step system; etchant is the first step as before, and the second step includes a primer and bond in the same solution. The challenge was to keep the dentin wet enough to prevent the collapse of the collagen after phosphoric acid etching but not to leave too much moisture (visible water droplets) that would hamper effective polymerization. The term “wet-bonding” technique described this challenge for the fifth generation adhesives. Research showed that penetration of these single-bottle adhesives “two-step etch-and-rinse” into dentin was more challenging than originally thought, with areas of incompletely impregnated collagen at the base of hybrid layer, increasing the chance for long-term degradation of the bond and leakage.
3.1.3 The Self-Etch Approach
Development of the functional acidic monomer played a pivotal role in the introduction of clinically effective self-etching adhesives. One of the most successful acidic monomers in the composition of self-etch systems is 10-methacryloxydecyl dihydrogen phosphate (MDP) originally developed by Kuraray Noritake Dental, Tokyo, Japan. This monomer has a C=C bond on one end for polymerization and a reactive acidic moiety on the other end. The concept of chemical bonding to apatite gained strength by the observations of Van Meerbeek et al. on the self-orientation of the MDP monomer when reacting with the apatite, termed nanolayering. Electron microscopic observation of the interface between an MDP-containing two-step self-etch system, Clearfil SE Bond (Kuraray) and dentin after acid-base challenge showed that the self-etch adhesive system demineralized dentin mildly and partially, leaving hydroxyapatite crystals in the base of the hybrid layer. The phosphoric acid moiety in MDP could form an insoluble salt with the calcium-rich apatite, thereby forming a stable bond. The sixth generation adhesive, two-step self-etch, uses a self-etching primer in the first step, and a pure resin bond applied in the last [3].
Seventh generation relies on an all-in-one solution where all three components: etchant, primer, and bond are within one product. The all-in-one adhesives developed further to include additional components such as silane coupling agents for ceramic bonding and hydrophilic components facilitating penetration of the adhesive into etched dentin. This allowed more versatility, and the clinicians could choose a total-etching technique followed by application of the universal adhesive. The etching of dentin is an option open to the clinicians for these universal self-etch adhesives; however, they generally have a higher pH to improve their shelf-life stability, which means they are less effectiveness in enamel etching. Therefore, a selective enamel etching step is highly recommended for these generation systems by etching the enamel alone [4]. This step is particularly important, since the debonding at external margins has been typically considered more important than the internal dentin interface gaps, due to the increased risk of discoloration, bacterial leakage, and formation of caries around open margins [5]. While the dentin bond strength values are similar between the phosphoric acid-etching and self-etching of dentin with universal adhesives across studies [6, 7] Some have advised against etching of the dentin and particularly the dentin-enamel junction (DEJ) zone, due to the increased risk of hydrolytic degradation of hybrid layer and undermined cohesive strength of the DEJ [8, 9].
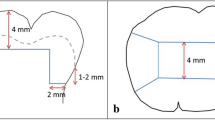
Self-adhesive bulk-fill restorative are the latest introduction in this category. These materials are mainly developed based on a combination of functional adhesive monomer technologies and bulk-fill composites through polymerizable acid polymers [10]. While promising, at the time, there is no evidence on the long-term clinical success of these materials. Historically, the bond strengths obtained with self-adhesive composites and glass-ionomer family of products have been inferior to those of multi-step adhesives, simply due to the fact that sufficient penetration of monomers in a liquid form is considered crucial for successful bonding (Fig. 3.1).
3.2 Bonding Implications for Bulk Fill Composites
Creating a sound bond to tooth structure important for any composite restoration, and clinicians should be reminded of the implications of adhesives for the bulk-fill technique. The most notable subject is shrinkage stress, accounting for a number of issues when attempting the bulk fill technique.
Polymerization shrinkage is a decrease in volume as methacrylate composite monomer is converted into polymer. This decrease in entropy creates a force that disrupts the adhesive hybrid layer that the clinician worked so hard to establish. Shrinkage stress may pull adhesive from the margin to ruin micromechanical retention. The shrinkage force causes fractures within the composite, adhesive and even the remaining sound tooth structure, known as a cohesive fault. Fractures allow for bacterial leakage, which can lead to demineralization and secondary caries. Additionally, when the composite restoration is placed under masticatory forces and thermal stress, a compromised margin can lead to even further damage (A). Polymerization can also lead to measurable gap formation at the pulpal floor of cavity preparations, contributing to a loss of marginal seal. These occurrences are observed as a direct result of shrinkage, but the bulk-fill technique alone is not to blame, obtaining proper adhesion is key.
Gap formation and loss of marginal seal is linked to the direction of polymerization shrinkage, and not only the size of the restoration. That is, polymerization from the outer most or occlusal/coronal surfaces toward the pulpal extensions of the prep will lead to the composite pulling inward and off the pulpal floor or away from the preparation walls. This is seen in vitro as measurable gaps and loss of margins. In contrast, polymerization shrinkage that originates at the margins of the restoration will result in shrinkage at the outermost layer, pulling composite into the preparation. The latter direction is the beneficial result of shrinkage that eliminates the issues associated with marginal seal and gap formation. In trying to standardize shrinkage to achieve this desirable direction, it was thought that the origin of the light source (occlusal versus marginal) was key. However, Versluis determined that the direction of shrinkage was mostly determined by the quality of the bond to the tooth and presence of unbonded surfaces [11]. Thus, it is advised that clinicians who are trying to create clinically acceptable results should appreciate the importance of adhesion and manage the polymerization shrinkage stress when placing large restorations.
3.3 What Adhesive Strategy Should Work for Bulk Fill Composites?
With the challenges of the bulk fill strategy, selection of the bonding system is an important step for a successful restoration. It is important to create adequate and timely copolymerization between the adhesive layer and the bulk fill composite to resist the competition between shrinkage stress development and maturing dentin bond.
From a clinical chair time management point of view, it makes sense to combine a bulk filling approach with a single-step all-in-one or universal adhesive. This offers a simplified application procedure resulting in reduced chair time compared with the two-step self-etching adhesives that required at least two separate application steps. While most of these bonding systems have reached comparable bond strength values to the tooth structure under laboratory conditions, the performance of the simplified adhesives has been questioned due to concerns regarding inherent limitations of their chemical mix of hydrophobic and hydrophilic components [12]. The water-free hydrophobic bonding agent of the two-step approach could slow down the hydrolytic degradation of the hybrid layer and contribute to tight sealing [13].
The real-time imaging of one-step and two-step self-etch adhesives when a flowable composite was bulk-filled showed that in the former, the separation mainly occurred only 7–14 s after the light curing started [14]. It is noteworthy that under that study, composite polymerization developed slower in deeper areas. Therefore, the dentin gap formation at the deeper cavity interface seemed to have occurred when the shallower composite had reached the postgel phase. Those findings also demonstrated that new polymerization shrinkage-related gaps were unlikely to initiate after the 20-s light-curing period; however, the propagation of existing defects evidently continued in a logarithmic pattern in the few minutes following completion of the light irradiation, as the postgel shrinkage continued in the composite despite the expected relaxation of residual stress [14] (Fig. 3.2).
Maximum intensity projection images from optical coherence tomography (OCT) three-dimensional (3D) data after light curing (a–d, a′–d′) at each time. At the enamel margins, Bond Force (seventh gen, BF), Scotchbond Universal Adhesive (universal, SBU), and OptiBond XTR (sixth gen, XTR) showed high signal intensity that progressed along the enamel interface after 10 min (a1–a5, b1–b5, c1–c5). On the other hand, Clearfil SE Bond 2 (sixth gen, SE2) did not show a high signal intensity at the enamel interface during the observation (d1–d5). At the dentin floors, BF and SBU showed bright areas that expanded at the floor after light curing for 10 min (a′1–a′5, b′1–b′5). On the other hand, XTR and SE2 showed almost no gap at the cavity floor during the observation (c′1–c′5, d′1–d′5)
3.4 Curing and Polymerization of the Adhesive
Light curing is the process to initiate and accelerate the free radical based polymerization reaction of the adhesive. As technology advanced, so did the light source evolve along with it and today it is possible to find LED light sources that emit multiple wavelengths, usually within the visible blue light spectrum. There are several factors that play a role in the equation that attempts to yield a high conversion ratio, minimize temperature increase, high power output [15, 16], light density [17], and time required [18]. Generally, the higher the output and flow of photons from the source, the quicker the reaction would be. It is possible in theory to create a light source that is strong enough to minimize curing time. The challenges facing that would be a high sharp increase in temperature that could have adverse effects on the pulp in vital teeth. Degree of conversion is another concern when using high intensity light curing devices [17, 19] Physical properties of the polymer also affect the ability of light to penetrate the deeper portions of the restoration [20], this is a result of how light passes through a non-clear composite material, but also a result of the propagation of the reaction unevenly across the restoration.
To ensure a restoration margin that has been well adapted with adhesive resin, the composite must copolymerize well with the bond in the extensions of the preparation. In the traditional incremental approach, this was successfully achieved with shallow composite layers, ensuring for repeated and excessive light exposure for polymerizing reactions. However, the bulk-fill technique blocks light from reaching the deepest extensions of the preparation. In other words, in the traditional incremental placement of composite, there are several separate light curing steps with each small layer of composite places. This gradually allows for maturation of the bond to dentin, and increased polymerization performance of the adhesive layer, due to increased irradiance. Therefore, a notable difference between traditional incremental placement of composite and bulk fill is several separate light curing with each layer of composite versus one large layer. Lack of sufficient light irradiation may inhibit complete curing of the composite and copolymerization of composite with the bond. In measuring the difference in polymerization completion at various depths, it was found that across various types and shades of traditional composites, incomplete polymerization occurred at greater depths using the bulk-fill technique, while no significant difference in hardness was observed at various depths using the incremental method [21].
In deep preparations with single increment fillings, the intensity of light reaching the adhesive is reduced due to the distance from the occlusal surface to the deep area such as the proximal gingival margin. In these cases, bulk filling will be a challenge since with a partially polymerized adhesive, the amount of light reaching the interface of the thick layer of bulk-fill composite and adhesive could be insufficient to establish a good seal. This is the reason why some researchers have advocated for application of a composite coating or intermediate layer in these areas prior bulk placement of the subsequent layer, to allow for improved polymerization of the adhesive-composite complex [22, 23]. With the challenges of bonding to deep dentin due to increased tubules density, orientation, and water content, it does appear that the bulk-fill strategy in this dentin region may be challenging without an additional resin-coating technique. Optical coherence tomography (OCT) experiments showed that compared to other conventional flowable materials, Surefill SDR Flow (Dentsply Sirona, Milford, DE) did have lower polymerization stress; in fact when applied in a bulk 2-mm increment to restore a dentin cavity, it showed perfect adaptation to the walls and floor. In contrast, the regular flowable composite, showed formation of gaps at the 2-mm deep cavity with the same type of one-step self-etch adhesive as was used for the bulk fill composite. However, when applied in one increment a 4-mm deep setup, both SDR and regular flowable showed interfacial defects [24].
It has been shown that the pattern of shrinkage stress for light-cured materials is from the bottom up in direction from the floor of the cavity to the top surface, placing a stretch on the adhesive layer with the largest vectors of polymerization stress at the deepest area of the preparation [25]. This is a critical issue for light-cured bulk-fill resin composites, particularly given the possibility of insufficient irradiation of the adhesive prior to and after placement of the composite. As mentioned earlier, in order to reach the post-gel phase, a sufficient intensity of light need to irradiate the composite and penetrate throughout the material to ensure curing of the deeper areas to achieve the cross-linking of the composite, as well as copolymerization with the adhesive layer and overall development of mechanical properties. One strategy in development of bulk-fill composite was increasing the overall translucency of the material to enable better light penetration, distribution and internal reflections [26].
A newly developed bulk-fill system incorporates high irradiance LED light curing unit (3 s PowerCure, Ivocalt-Vivadent AG, Schaan, Liechtenstein). The light curing process in this system takes only 3 s of irradiance using the light curing unit with high output (3000 mW/cm2). When the high intensity light delivery was compared to the regular LED curing (1200 mW/cm2), it was apparent that at the initial stage of composite polymerization, the high intensity system resulted in copolymerization between the bulk-fill composite and the adhesive at the cavity floor interface, which resisted against separation during polymerization. Overall, this light curing strategy together with increased translucency of the composite and improved phot-initiator chemistry contributed to the maintenance of the composite bond to the deepest areas in the preparation [27].
Additionally, insufficient solvent evaporation from simplified adhesives may contribute to overall lower polymerization performance and quality of the adhesive layer. Therefore, application of an additional hydrophobic resin as a coating (such as a bonding agent) seem beneficial for these adhesives [28]. It was shown that mechanical properties of the polymerized bonding agent of a two-step self-etch adhesive was superior to that of an all-in-one adhesive from the same manufacturer with a similar composition [29].
Depending on the size and geometry of the preparation, homogeneity of the adhesive thickness may be another issue with single step adhesives. While the adhesive may pool in the preparation corner and create thick bond layer (up to 100 μm), it may be too thin at other areas (<5 μm) increasing the risk of a weak bond layer due to formation of an oxygen inhibition layer through most of the adhesive thickness [23]. The oxygen-inhibited layer is always produced as a thin soft and sticky superficial layer when a bonding resin is polymerized via free-radical initiation in air. In case of a super thin adhesive, the majority of the thickness will be oxygen-inhibited [30].
3.5 Adhesion of Bulk-Fill Dual-Cure Composites
Ducal cure composite systems have a combination of chemical initiators and light initiators materials. The goal of having dual cure materials is having the advantages in using light curing systems of speed for initial set, while combining the advantage of chemical cure that can provide a higher degree of conversion (DC) in deeper portions of the composite [31]. The slower initial chemical polymerization reaction preceding light activation has other advantages for the dual-cure bulk-fill resin composite; it permits viscoelastic flow within the material and stress relaxation as copolymerization occurs between the resin composite and adhesive at the bottom of the cavity. In other words, under such situation, there is more time for the composite-adhesive bond to mature due to the slower setting reaction of the composite. The depth of cure in an efficient self-cure system is not a function of light irradiance; however, a lower degree of cross-linking (i.e., more linear polymer) has been reported for self-cured materials. Even though the final DC in the self-cured mode may not be necessarily lower than the dual-cured mode, additional light-curing would improve the polymer cross-linking. This final light curing step may also contribute to the completion of copolymerization of composite and adhesive.
However, a concern that needs to be taken into account is the compatibility between the adhesive and bulk-fill composite. While light cured adhesive and composite systems are considered generally compatible, dual-cured bulk-fill composite may not be compatible with certain adhesives, particularly the all-in-one adhesives that use an acidic functional monomer. An adverse acid-base reaction with the basic tertiary amine of the dual-cure composite can prevent polymerization of the dual-cured composite [32]. This is why some adhesive manufacturers recommend the use of a dual-cure activator that mixes with the adhesive to reduce these adverse reactions with amine-based dual-cure composites. For example, Scotchbond Universal Adhesive must be mixed with its dual-cure activator (3 M ESPE, St Paul, MN, USA) containing sodium p-toluenesulfinate and ethanol, when an amine-based resin composite is used [32].
In order to address the adhesive incompatibilities, amine-free low-viscosity dual-cured bulk-fill resin composites have been introduced (such as BulkEZ, Danville Materials, Carlsbad, CA, USA). This material contains a proprietary hydroperoxide oxidizing agent and a thiourea reducing agent as the catalyst redox system that allows polymerization reaction within 2 min after mixing. The comparison of gap formation at the cavity floor among the dual-cure and several light-cured bulk-fill composites confirmed the advantage of the dual-cure approach in terms of bonding to a deep preparation.
3.6 The Issue of a Too Strong Bond
When the bonding to the tooth structure is strong enough to resist an uncontrolled polymerization shrinkage stress and there is no debonding or stress relaxation (through viscoelastic flow of the composite), the shrinkage stress could result in cuspal deflection, reduced bond strength, or worse, crack formation, and propagation. Another important finding in OCT real-time imaging study was the development of postcuring enamel cracks along the enamel walls, which was explained by the residual stress in the bulk-filled composite according to the postgel concept [33]. It is therefore evident that for a bulk-fill strategy to work, besides a good bonding ability, polymerization shrinkage management of the composite must be considered for the success of the restoration.
If a strong bond to dentin is successfully achieved, protecting the tooth and the bonded restoration from harmful stresses is the next challenge to tackle. Bulk-fill composites are not zero-shrinkage composites; therefore, the application of a stress-absorbing layer such as a resin-coating with flowable composite [23] or continuous fiber-reinforced composite layer [34] prior to bulk filling is recommended. Since shrinkage is an intrinsic resin property, reducing resin volume by adding non-monomer components such as organic or non-organic fillers has been considered as an effective way to reduce the magnitude of shrinkage. Incorporation of plasma-treated leno-weaved ultra-high molecular-weight polyethylene fiber (Ribbond, Seattle, WA) at the base of a deep cavity carried such an effect on polymerization shrinkage, while enhancing physical properties of the composite and potentially acting as a crack stopping mechanism (Fig. 3.3).
Cross-sectional microscopic images of composite adaptation at 4-mm deep cavity bottom. (a) confocal laser scanning microscopy (CLSM) of the cavity floor in bulk-filled composite shows debonding of composite (Surefil) at the line angle. (b, b′, b″, and b‴) SEM images of the cross section in (a), it appears that polymerization shrinkage has pulled away the composite from the bonding layer (bold white arrow). (c) CLSM image of the cavity floor shows that fiber-reinforced increment reached a thickness of approximately 0.3 mm. A gap can be observed between the bulk placed composite and fiber-reinforced layer in c (blank arrow). (d, d′, d″, and d‴) SEM images of the cavity floor cross section presented in C, good adaptation of with fiber-reinforced increment at all interfaces can be observed (arrowheads)
3.7 Conclusion
Based on our current knowledge bonding of a bulk-fill composite to deep dentin is a possibility as long as the challenges of polymerization efficiency in depth and polymerization shrinkage stress have been addressed with the state-of-the-art technologies such as the enhanced light-cure or dual-cure mechanisms described in this chapter. Addition of continuous fiber would add value to stress distribution and protection of the bond to dentin. Clinicians are advised to select the most suitable bonding strategy for their bulk-fill technique, which appear to the authors of this chapter to be multi-step adhesives that have a separate hydrophobic bonding agent, namely the three-step etch-and-rinse (fourth gen) or the two-step self-etch (sixth gen) adhesive.
References
Van Meerbeek B, Yoshihara K, Van Landuyt K, Yoshida Y, Peumans M. From Buonocore’s pioneering acid-etch technique to self-adhering restoratives. A status perspective of rapidly advancing dental adhesive technology. J Adhes Dent. 2020;22(1):7–34.
Sadr A, Nikaido T, Takagaki T, Hariri I, Nazari A, Tagami J. Ultra-morphological and nanomechanical characterization of reinforced enamel and dentin by self-etch adhesives: the super tooth. J Nano Res. 2012;16:131–40.
Matsui N, Takagaki T, Sadr A, Ikeda M, Ichinose S, Nikaido T, et al. The role of MDP in a bonding resin of a two-step self-etching adhesive system. Dent Mater J. 2015;34(2):227–33.
Nazari A, Shimada Y, Sadr A, Tagami J. Pre-etching vs. grinding in promotion of adhesion to intact enamel using self-etch adhesives. Dent Mater J. 2012;31(3):394–400.
Turkistani A, Nakashima S, Shimada Y, Tagami J, Sadr A. Microgaps and demineralization progress around composite restorations. J Dent Res. 2015;94(8):1070–7.
Hu X, Luong MN, Zhang H, Zhu H, Chan DC, Sadr A. Influence of phosphoric acid etching on the dentin bond durability of universal adhesives. J Adhes Sci Technol. 2019;33(21):2356–68.
de Oliveira da Rosa WL, Piva E, da Silva AF. Bond strength of universal adhesives: a systematic review and meta-analysis. J Dent. 2015 Jul;43(7):765–76.
Tabata T, Shimada Y, Sadr A, Tagami J, Sumi Y. Assessment of enamel cracks at adhesive cavosurface margin using three-dimensional swept-source optical coherence tomography. J Dent. 2017;61:28–32.
Alshahni RZ, Shimada Y, Zhou Y, Yoshiyama M, Sadr A, Sumi Y, et al. Cavity adaptation of composite restorations prepared at crown and root: optical assessment using SS-OCT. Dent Mater J. 2019;38(5):779–89.
Klee JE, Renn C, Elsner O. Development of novel polymer technology for a new class of restorative dental materials. J Adhes Dent. 2020;22(1):35–45.
Versluis A, Tantbirojn D, Douglas WH. Do dental composites always shrink toward the light? J Dent Res. 1998;77(6):1435–45.
Sadr A, Ghasemi A, Shimada Y, Tagami J. Effects of storage time and temperature on the properties of two self-etching systems. J Dent. 2007;35(3):218–25.
Van Landuyt KL, Peumans M, De Munck J, Lambrechts P, Van Meerbeek B. Extension of a one-step self-etch adhesive into a multi-step adhesive. Dent Mater. 2006;22(6):533–44.
Hayashi J, Shimada Y, Tagami J, Sumi Y, Sadr A. Real-time imaging of gap progress during and after composite polymerization. J Dent Res. 2017;96(9):992–8.
Bouschlicher MR, Rueggeberg FA. Effect of ramped light intensity on polymerization force and conversion in a photoactivated composite. J Esthet Restor Dent. 2000;12(6):328–39.
Lovell LG, Newman SM, Donaldson MM, Bowman CN. The effect of light intensity on double bond conversion and flexural strength of a model, unfilled dental resin. Dent Mater. 2003;19(6):458–65.
Sakaguchi RL, Berge HX. Reduced light energy density decreases post-gel contraction while maintaining degree of conversion in composites. J Dent. 1998;26(8):695–700.
Lovell LG, Lu H, Elliott JE, Stansbury JW, Bowman CN. The effect of cure rate on the mechanical properties of dental resins. Dent Mater. 2001;17(6):504–11.
Silikas N, Eliades G, Watts DC. Light intensity effects on resin-composite degree of conversion and shrinkage strain. Dent Mater. 2000;16(4):292–6.
Emami N, Söderholm K-JM, Berglund LA. Effect of light power density variations on bulk curing properties of dental composites. J Dent. 2003;31(3):189–96.
Lazarchik DA, Hammond BD, Sikes CL, Looney SW, Rueggeberg FA. Hardness comparison of bulk-filled/transtooth and incremental-filled/occlusally irradiated composite resins. J Prosthet Dent. 2007;98(2):129–40.
Han S-H, Sadr A, Shimada Y, Tagami J, Park S-H. Internal adaptation of composite restorations with or without an intermediate layer: effect of polymerization shrinkage parameters of the layer material. J Dent. 2019;80:41–8.
Yahagi C, Takagaki T, Sadr A, Ikeda M, Nikaido T, Tagami J. Effect of lining with a flowable composite on internal adaptation of direct composite restorations using all-in-one adhesive systems. Dent Mater J. 2012;31(3):481–8.
Nazari A, Sadr A, Shimada Y, Tagami J, Sumi Y. 3D assessment of void and gap formation in flowable resin composites using optical coherence tomography. J Adhes Dent. 2013;15(3):237–43.
Cho E, Sadr A, Inai N, Tagami J. Evaluation of resin composite polymerization by three dimensional micro-CT imaging and nanoindentation. Dent Mater. 2011;27(11):1070–8.
Ferracane JL. Placing dental composites—a stressful experience. Oper Dent. 2008;33(3):247–57.
Hayashi J, Tagami J, Chan D, Sadr A. New bulk-fill composite system with high irradiance light polymerization: integrity and degree of conversion. Dent Mater. 2020;36(12):1615–23.
Sadr A, Shimada Y, Tagami J. Effects of solvent drying time on micro-shear bond strength and mechanical properties of two self-etching adhesive systems. Dent Mater. 2007;23(9):1114–9.
Sadr A, Shimada Y, Lu H, Tagami J. The viscoelastic behavior of dental adhesives: a nanoindentation study. Dent Mater. 2009;25(1):13–9.
Yamaji A, Koga K, Tsujimoto A, Shimizu Y, Tsubota K, Takamizawa T, et al. Influence of oxygen-inhibited layer on dentin bond strength of chemical-cured resin composite. Eur J Oral Sci. 2013;121(5):497–503.
Feilzer AJ, de Gee AJ, Davidson CL. Setting stresses in composites for two different curing modes. Dent Mater. 1993;9(1):2–5.
Meda EM, Rached RN, Ignácio SA, Fornazari IA, Souza EM. Effect of different adhesive strategies and time on microtensile bond strength of a CAD/CAM composite to dentin. Oper Dent. 2019;44(3):262–72.
Versluis A, Tantbirojn D, Douglas WH. Distribution of transient properties during polymerization of a light-initiated restorative composite. Dent Mater. 2004;20(6):543–53.
Sadr A, Bakhtiari B, Hayashi J, Luong MN, Chen Y-W, Chyz G, et al. Effects of fiber reinforcement on adaptation and bond strength of a bulk-fill composite in deep preparations. Dent Mater. 2020;36(4):527–34.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Sadr, A., Margalit, O., Palander, A., Tagami, J. (2023). Bulk Fill Composites: Adhesion and Interfacial Adaptation. In: Sabbagh, J., McConnell, R. (eds) Bulk Fill Resin Composites in Dentistry. Springer, Cham. https://doi.org/10.1007/978-3-031-16388-3_3
Download citation
DOI: https://doi.org/10.1007/978-3-031-16388-3_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-16387-6
Online ISBN: 978-3-031-16388-3
eBook Packages: MedicineMedicine (R0)