Abstract
Objectives
This study aimed at comparing the microtensile bond strength (MTBS) and interfacial adaptation of a modern self-curing and a light-curing restorative bulk-fill composite to a conventional composite applied with the layering technique.
Methods
Forty-eight occlusal cavities were divided in three main groups (16/group) based on tested materials: (i) STELA, bulk-fill self-curing restorative (STELA, SDI Ltd.); (ii) 3 M-BULK, bulk-fill composite (Filtek One Bulk-Fill, 3 M Oral Care); and (iii) 3 M-CTR, a conventional composite (Filtek Supreme XTE, 3 M Oral Care). These were used in combination with their adhesives in self-etch (SE) or etch-and-rinse (ER) mode. Specimens stored in artificial saliva (24 h or 12 months) were evaluated for MTBS and fractography. The interfacial analysis was performed through confocal microscopy. ANOVA and Fisher’s LSD post hoc tests were performed with a level of significance of 5%.
Results
All the tested materials applied in ER mode presented (24 h) greater bond strength than in SE mode. Although all materials showed a significant drop in the bond strength after prolonged storage, STELA showed the highest bonding performance and interfaces with few gaps. 3 M-BULK had the lowest bond strength and an interface with several voids and gaps.
Conclusions
All materials were affected by interface degradation and bonding reduction over prolonged aging. However, their use in combination with adhesives applied in ER mode may offer greater immediate bonding performance.
Clinical relevance.
The use of restorative light-curing bulk-fill composites may generate gaps at the bonding interface and voids. STELA may represent a suitable alternative to avoid such issues.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dental resin composites consist of a combination of hydrophobic cross-linking resin monomers, silanated inorganic fillers, and photo-sensible activators, which trigger the polymerisation through free radical reaction when irradiated by visible light [1, 2]. It is well known that conventional composites are characterised by polymerisation shrinkage during light-curing procedures, which results in a volumetric reduction that may jeopardise the integrity of the bonding interface, as well as the longevity of restorations [3, 4]. Indeed, such a shrinkage during “rapid photo-polymerisation”, in combination with the high elastic modulus developed by the composite, generates internal stresses that can destroy the bonding interfaces between the composite and dental tissues (e.g. dentine and enamel); this leads to gap formation and decrease in bonding performance [3,4,5].
Several strategies have been advocated to minimise the deleterious effect of shrinkage stress on the marginal integrity of composite restorations [6]. For instance, techniques based on incremental composite application have been widely demonstrated to reduce cuspal deflection, incidence of micro-cracks/fractures in enamel, formation of premature gaps at the resin-dentine interface [7, 8], and dentine hypersensitivity after restorative procedures [6, 9]. The rationale behind the use of such an incremental layering technique is based on the application and light-curing of small layers of composite (1–2 mm), as shrinkage stress is minimised when there are fewer bonded walls involved during the polymerisation; this is due to a reduction of the C-factor while the composite is applied obliquely onto the cavity walls [10]. Moreover, the reduced thickness of such layers favours better light diffusion though the composite, which also increase the degree of conversion in such materials [11, 12].
However, there is currently a growing interest in “low shrinkage” bulk-fill composites (BFCs) as complementary restorative materials. Such products can be found as either flowable or high-viscosity “sculptable” composites, and they have been developed as a substitute of conventional composites to avoid the need for incremental layering, so providing simpler and faster clinical procedures [13]. According to manufacturers, such BFCs are intended to be applied in a single incremental application with thickness up to 4 or 5 mm. The success of such a simplified procedure can be assigned to the presence of more reactive photo-initiators within their composition, as well as to an increased translucency in modern BFCs, which permits deeper transmission of the light [14, 15]. Furthermore, such relatively new BFCs are claimed to have low shrinkage stress thanks to the presence of stress reliever molecules and polymerisation modulators incorporated by the manufacturers within their compositions [16]. Nevertheless, BFCs are still affected by polymerisation shrinkage at certain extent, so in order to reduce the detrimental effects of shrinkage on the bonding interfaces, the use of a flowable liner has been advocated before application of BFCs; it seems to be a clinical procedure that may have a pronounced stress-relieving effect [17].
The use of glass ionomer cements has been also demonstrated to attenuate the stress produced by composite shrinkage and maintain the bonding performance of simplified universal adhesives after load-cycling challenge (350,000 cycles; 3 Hz; 70 N) and after load-cycling aging followed by 8 months of immersion in artificial saliva (AS) [18]. It was also demonstrated that the use of a glass ionomer-based liner produced a significant reduction in gap formation volume when using some bulk-fill composites [19].
Moreover, the use of self-curing materials has been advocated to have a lower shrinkage stress at the bonded interfaces of restorations. Indeed, less risk of gap formation is encountered in such materials, since they are characterised by a “slower” polymerisation reaction that generates a polymeric structure with reduced cross-linking density compared to light-curing composites [20,21,22].
Recently, a self-cure, bulk-fill restorative material has been introduced in the market (STELA Automix, SDI Ltd., Australia), which comprises of an adhesive system that require no light-curing as it polymerises upon contact with the restorative material. This material contains different fillers such as strontium fluoroaluminosilicate glass, ytterbium trifluoride agglomerates, silica, and calcium aluminate. Regarding this latter aspect, although one may wonder whether this restorative material belongs to the compomer family, the manufacturer categorises STELA as a new-generation resin-based bulk-fill restorative material with unique chemico-physical characteristics.
Unfortunately, there is little information available on the physico-mechanical properties of STELA [23], and no information has been yet disclosed by the manufacturer about the amount of strontium fluoroaluminosilicate glass and calcium aluminate contained within the composition of this new class of bulk-fill restorative material, as well as whether these fillers were introduced in a pre-reacted form and/or pre-treated with any coupling agent (e.g. silane).
Thus, the aim of the present study was to compare the microtensile bond strength (MTBS) and interfacial adaptation through dye-assisted confocal microscopy after 24 h and 12 months of storage in artificial saliva (AS) of a self-curing resin-based bulk-fill system and a commercial “sculptable” light-curing bulk-fill composite to a conventional composite applied as manufacturer’s instructions. All the tested materials were used in combination with their respective adhesive systems, which were applied in self-etching (SE) or etch-and-rinse (ER) mode. The first hypothesis of this study was that the two tested bulk-fill materials would show a superior interfacial adaptation, along with less gap formation compared to conventional composite applied with the layering technique. The second hypothesis was that the bonding procedures performed in ER or SE mode would influence the bonding performance of the tested materials both at 24 h and 12 months of storage in AS.
Material and methods
Sample preparation
Human molars (n = 48) were extracted for periodontal or surgical reasons under a protocol reviewed and approved by the Ethical Committee of the University CEU Cardenal Herrera (ethical approval number: CEEI22/309). Prior to extraction, patients were informed about the use of their teeth for research purposes, which was confirmed with a written consent. The teeth were stored in distilled water at 4 °C and used within 6 months since extraction.
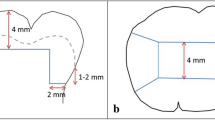
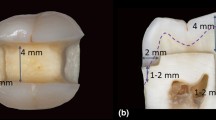
The crowns of the teeth were separated from their roots using a diamond blade (Isomet Diamond Wafering Blades, no. 11–4244, Buehler Ltd., Lake Buff, USA) under continuous water cooling. Standardised, box-shaped class I preparations (4 mm mesio-distal width × 3 mm buccolingual width × 5 mm deep), with margins located in the occlusal enamel and the cavity floor located in the dentine were prepared using a diamond bur (FG#3145, KG Sorensen, Cotia, Brazil) and finished with a fine diamond bur (FG#3145FF, KG Sorensen). The burs were used in a high-speed air turbine hand piece, and the preparations were performed under continuous water irrigation. The specimens were maintained in distilled water at 37 °C (pH 6.5) not longer than 1 h before bonding procedures, and restorations were performed.
Materials and experimental groups
Three main groups (n = 16 specimens/group) were created based on the restorative materials used in this study: (i) 3 M-CTR (Filtek™ Supreme XTE, 3 M Oral Care, GmbH, Seefeld, Germany) was used as the control conventional composite in combination with the adhesive system (SCH: Scotchbond Universal, 3 M Oral Care); (ii) 3 M-BULK (high-viscosity bulk-fill composite, Filtek™ One Bulk-Fill restorative, 3 M Oral Care) was used in combination with Scotchbond Universal (3 M Oral Care); (iii) STELA, a self-cure bulk-fill restorative system (STELA Automix, SDI Ltd, Bayswater, Australia) was used in combination with an adhesive system (STELA Primer, SDI Ltd.) that requires no light-curing, as it polymerises upon contact with the STELA restorative material.
The experimental design of this study (Fig. 1) required that the specimens were further divided into two subgroups (n = 8 specimens/group) based on the bonding procedures employed for the preparation of the specimens (self-etching (SE) or etch-and-rinse (ER) mode). In the ER groups, acid etching was performed in dentine using a 37% orthophosphoric acid gel for 15 s, which was subsequently rinsed with distilled water (15 s) and blotted, leaving the substrate moist. All the materials and adhesive systems were used according to the manufacturer’s instructions and light-cured (when necessary) using light-emitting diode curing unit (Radii Plus, SDI) with a mono-wavelength of 470 nm. The irradiance of this unit was 1200 mW/cm2, which was checked using a laboratory-grade spectral radiometer.
The specimens were finally restored with the tested restorative materials as previously aforementioned. The conventional 3 M-CTR was placed in two horizontal increments of 2 mm, and each increment was measured suing a periodontal probe and they were separately light-cured for 30 s. 3 M-BULK was placed in a single increment (4 mm thick) and light-cured as per manufacturer’s instructions. The self-cure bulk-fill restorative system (STELA) was placed in a single increment and allowed to self-cure at 37 °C for 4 min as per manufacturer’s instructions (Table 1).
Microtensile bond strength (MTBS) testing and fracture analysis (FIB-SEM)
The specimens were serially sectioned after 24 h of storage in AS at 37 °C (as per the experimental design) using a low-speed diamond blade (Isomet Diamond Wafering Blades, Buehler Ltd., Lake Buff, USA) operated under constant water cooling. This latest procedure created parallel-sided sticks (0.9 mm2) devoid of enamel on their sides. Half of the sticks from each group were submitted to microtensile bond strength (MTBS), and the half part of specimens were tested after 12 months of storage in AS at 37 °C. The composition of the artificial saliva was 0.103 g L−1 of CaCl2, 0.019 g L−1 of MgCl2·6H2O, 0.544 g L−1 of KH2PO4, 30 g L−1 of KCl, and 4.77 g L−1 HEPES (acid) buffer and pH 7.4 [24].
For MTBS testing, the sticks were glued to a jig and tested in a MTBS testing device (BISCO Corp., Schaumburg, IL, USA). This latest procedure was performed by applying a tensile force at a crosshead speed of 1 mm/min until a failure occurred at the bonding interface. The maximum tensile load of the recorded failure (N) was divided by the respective cross-sectioned area of each stick, and the bond strength values were converted into MPa. Fractographic analyses were performed with stereomicroscope to analyse the failure mode of each specimen (adhesive, mixed, or cohesive). Representative fractured specimens were selected from each group and mounted on stubs, gold-coated (MED 010, Balzers, Balzer, Liechtenstein), and finally observed through an ultra-high-resolution analytical focused ion beam scanning electron microscope (FIB-SEM, Thermo Scientific Scios 2 DualBeam, Waltham, MA, USA) in secondary electron mode.
Bond strength values in MPa were initially assessed for normality distribution and variance homogeneity using Kolmogorov–Smirnov and Levene’s tests, respectively. Analysis of variance (ANOVA factors: restorative material, adhesive bonding protocol, aging time) and Fisher’s LSD post hoc test were performed. A significance level of 0.05 was used throughout the analysis, which was carried out using Bioestat v.5.3 (Instituto Mamirauá, Manaus, AM, Brazil).
Confocal microscopy evaluation of the bonded-dentine interfaces
Before cutting the specimens in sticks as described in the “Microtensile bond strength (MTBS) testing and fracture analysis (FIB-SEM)” section, one composite-dentine slab was taken from the centre of each specimen in every experimental group. These were first polished for 30 s each side with 1200-grit SiC papers and subsequently ultrasonicated in distilled water for 5 min. The specimens were covered with a varnish, which was applied 1 mm away from the resin-dentine interface. Subsequently, the specimens were immersed in rhodamine B (Merck KGaA, Darmstadt, Germany) water solution (0.15 wt.%, pH 7) for 24 h. The specimens were then rinsed with distilled water and immersed in an ultrasonic bath for 5 min. These were finally polished for 30 s each side with a 1200-grit SiC paper and ultrasonicated again in distilled water for 5 min [24].
The slabs were analysed through a confocal microscope (Olympus FV1000, Olympus Corp., Tokyo, Japan) equipped with a 63X/1.4 NA oil-immersion lens, and a 543-nm LED illumination, reflection, and fluorescence images were obtained with a 1-µm z-step to optically section the specimens to a depth of up to 20 µm below the surface. The z-axis scan of the interface surface was pseudo-coloured arbitrarily for improved exposure and compiled into single projections using the CLSM image-processing software (Fluoview Viewer, Olympus Corp., Tokyo, Japan). The configuration of the system was throughout the entire experiment. Each dentine interface was investigated completely, and images were randomly obtained in five different zones of the bonding interface. These micrographs represented the most common morphological features observed along the resin-dentine interfaces.
Results
Microtensile bond strength (MTBS) and failure mode analysis
Mean bond strength was affected by the restorative material (F = 21.66; p < 0.001), adhesive bonding protocol (F = 49.11; p < 0.001), and aging time (F = 95.01; p < 0.001). Interactions were also significant (p < 0.05). The results obtained during the MTBS test are depicted in Table 2. During analysis of the results, it was observed that at 24 h of AS storage, the lowest bond strength (p < 0.05) was obtained with the 3 M-BULK composite applied on dentine using the adhesive SCH in SE mode (9.9 MPa); this group showed a great number of pre-fails, with most of the specimens failed in adhesive mode during the test. STELA applied both in SE (23.2 MPa) and ER (32.4 MPa) showed comparable (p > 0.05) results to those obtained with the conventional 3 M-CTR composite applied layer by layer on dentine with the SCH adhesive (SE 22.4 MPa; ER 38.8 MPa); in both cases, the highest results were obtained in ER mode compared to SE mode (p < 0.05) with 0% pre-fails. The application of 3 M-BULK composite applied on dentine using the adhesive SCH in ER mode gave comparable results to STELA and 3 M-CTR applied in dentine bonded in ER mode (p > 0.05). However, 3 M-BULK composite applied in combination with the adhesive SCH applied in ER failed prevalently in adhesive more (58%), while STELA and 3 M-CTR failed mainly in mixed mode. STELA applied in SE mode failed prevalently in adhesive mode (54%), and 3 M-CTR applied with SCH in SE mode failed prevalently in mixed mode (58%) (Table 1).
All the materials tested in this study applied on dentine bonded in SE or ER mode presented an important reduction of the bond strength after 12-month aging (p < 0.05) with a greater number of specimens failed in adhesive mode compared to those obtained at baseline (24 h). However, STELA applied in ER mode presented the highest bond strength (22.2 MPa) after 12-month storage; a clear higher bond strength (p < 0.05) was detected when compared to STELA applied in SE mode (12.4 MPa).
Confocal microscopy evaluation of the bonded-dentine interfaces
The results of confocal microscopy of the tested different materials analysed at baseline (24 h) and after 12 months of AS storage are presented in Fig. 2 and Fig. 3, respectively. The specimens created with the 3 M-CTR composite applied on dentine with the SCH adhesive applied in SE mode often presented gaps and at the dentine-composite interface. This latter structure appeared prevalently permeable to the diffusion of the fluorescent dye, which accumulated inside the dentinal tubules and particularly within the hybrid layer (Fig. 2A). The specimens created with the 3 M-CTR composite applied on dentine with the SCH adhesive applied in ER mode were characterised by a bonding interface relatively free from gaps, but with the presence of micro-cracks within the composite that were infiltrated by the fluorescent dye. This latter also highlighted the presence of a hybrid layer evidently porous (Fig. 2B). Conversely, a constant presence of gaps was observed in the specimens created with the 3 M-BULK composite applied on dentine with the SCH adhesive applied in SE mode. Also in this case, the bonding interface resulted highly permeable to the diffusion of the fluorescent dye, which also infiltrated some micro-cracks within the composite (Fig. 2C). Specimens created with the 3 M-BULK composite applied on dentine with the SCH adhesive applied in ER mode were often characterised by the presence of thin gaps and a dentine-composite interface permeable to the diffusion of the fluorescent dye, particularly within the hybrid layer (Fig. 3D). STELA applied both in SE (Fig. 2E) and ER mode (Fig. 2F) rarely showed the presence of gaps, although a bonding interface permeable to the diffusion of the fluorescent dye into the dentinal tubules and particularly within the hybrid layer.
Confocal microscopy images of the resin-dentine interfaces tested at 24 h (baseline). A CLSM projection image exemplifying the interfacial characteristics of the resin-dentine interface created by the 3 M-CTR in combination with the SCH applied in SE mode. It is possible to see the presence of thin gap (pointer) and a dentine-composite interface permeable to the diffusion of the fluorescent dye used in this study inside the dentinal tubules (d) and particularly within the hybrid layer (pointer). B CLSM projection image of the resin-dentine interface created by 3 M-CTR in combination with the SCH applied in ER mode. In this case, it is possible to observe a gap-free interface, but a dentine-composite interface permeable to the penetration of the fluorescent dye into dentinal tubules (d) and predominantly within the hybrid layer (hl) and the adhesive layer (ad). However, the fluorescent dye infiltrated some micro-cracks within the composite (pointers) created due to polymerisation shrinkage. C CLSM projection image of the resin-dentine interface created by application of 3 M-BULK in combination with SCH applied in SE mode. Please note the presence of thin gap (white pointer) and bonding interface permeable to the diffusion of the fluorescent dye used in this study. Also, in this case, the fluorescent dye infiltrated some micro-cracks within the composite (black pointer), which formed due to polymerisation shrinkage. D CLSM projection image of the resin-dentine interface created by application of 3 M-BULK in combination with SCH applied in ER mode. Also, in this case, it is possible to observe the presence of thin gap (pointer) and a dentine-composite interface permeable to the diffusion of the fluorescent dye, especially within the hybrid layer (hl). E CLSM projection image exemplifying the interfacial characteristics of the resin-dentine interface created by application of STELA in SE. There is no presence of gaps, but a bonding interface permeable to the diffusion of the fluorescent dye into the dentinal tubules (d) and within the hybrid layer and the primer (pointer). F CLSM projection image of the resin-dentine interface created by application of STELA in ER mode. Also, in this case, it is possible to observe no gap within a bonding interface permeable to the diffusion of the fluorescent dye deep into the dentinal tubules (d) and especially within the hybrid layer (hl) and the primer (pointer)
Confocal microscopy images of the resin-dentine interfaces tested at 12 months of storage in AS. A CLSM projection image representing the interfacial characteristics of the resin-dentine interface created by the 3 M-CTR in combination with the SCH applied in SE mode. Note the presence of a gap and clear signs of degradation (pointer) at the bonding interface. It is also possible to see a prominent dye diffusion and degradation within the adhesive layer (ad). The fluorescent dye also infiltrated some micro-cracks within the composite (c). B CLSM projection image of the resin-dentine interface created by the 3 M-CTR in combination with the SCH applied in ER mode. In this case, it is possible to observe the presence of a gap and clear signs of degradation in hybrid layer and adhesive layer (pointer). The fluorescent dye also infiltrated some micro-cracks within the composite (c). C CLSM projection image of the resin-dentine interface created by application of 3 M-BULK in combination with SCH applied in SE mode. Also in this case, it is possible to observe the presence of an evident gap (pointer) and a bonding interface totally affected by severe dye uptake as a sign of polymer degradation of the adhesive (ad). The fluorescent dye also highlighted the presence of voids within the composite (*). D CLSM projection image of the resin-dentine interface created by application of 3 M-BULK in combination with SCH applied in ER mode. The presence of an important gap (pointer) and a bonding interface totally degraded is clearly observed. E The resin-dentine interface created by application of STELA in SE mode is characterised by the presence of no gap, but it is possible to see that fluorescent dye infiltrated the hybrid layer and the adjacent composite, which appears characterised by the presence of several porosities (pointer). F CLSM projection image of the resin-dentine interface created by application of STELA in ER mode. Also in this case, it is possible to observe no gap at the bonding interface, but there is a compact layer (pointer) free from dye uptake at the interface between the dentine (d) and the composite
After 12 months of storage in AS, the specimens created with the 3 M-CTR composite applied on dentine with the SCH adhesive applied in SE mode were always characterised by the presence but with a prominent dye diffusion at the resin-dentine interface and evident signs of degradation within the adhesive layer. The presence of micro-cracks within the composite was more often observed in these specimens (Fig. 3A). Also, in the specimens created with the 3 M-CTR composite applied on dentine with the SCH adhesive applied in ER mode, the presence of a gap and clear signs of degradation in hybrid layer and adhesive layer were always detected. Moreover, the presence of micro-cracks within the composite was frequently detected and observed also in these specimens (Fig. 3B).
The resin-dentine interface of the specimens created with the SCH adhesive applied in SE mode and followed by the 3 M-BULK composite build up was often characterised by the presence of gaps with severe dye diffusion and signs of polymer degradation of the adhesive and hybrid layer. Most of the specimens created with the 3 M-BULK composite presented several voids within the composite, predominantly in proximity of the bonding interface (Fig. 3C). The situation was quite similar in the specimens created with 3 M-BULK in combination with SCH adhesive applied in ER mode. Indeed, the presence of large gaps was always detected along with a bonding interface completely affected by degradation process of the adhesive and hybrid layer (Fig. 3D). The situation was rather different in the specimens created with STELA as the presence of gaps was rarely detected. However, it was always detected a clear fluorescent dye infiltration within a bonding interface characterised by several porosities in the specimens created with the SE bonding protocol (Fig. 3E). Conversely, the resin-dentine interface created by application of STELA Primer in ER mode was characterised by a compact layer between dentine and composite, which was often free from dye uptake (Fig. 3F).
FIB-SEM fractographic analysis
The results of the ultra-morphological analysis performed using the FIB-SEM on the tested materials that fractured during microtensile bond strength at baseline (24 h) and after 12 months of AS storage are presented in Fig. 4 and Fig. 5, respectively. The specimens created with the 3 M-CTR composite applied on dentine in combination with the SCH adhesive applied in ER mode that failed in mixed mode (Fig. 4A) often showed a fracture underneath the hybrid layer with no clear presence of exposed acid-etched collagen fibrils, but there were several dentine tubules occluded by resin tags (Fig. 4 A1). The specimens created with the same composite used in combination with SCH applied in SE mode that failed in mixed or adhesive mode (Fig. 4B) were often characterised by the presence of residual smear layer on dentine surface and inside the dentinal tubules, along with the presence of a porous residual adhesive still bonded to the dentine (Fig. 4 B1). The specimens created with the 3 M-BULK composite applied on dentine in combination with the SCH adhesive applied in ER mode that failed in mixed and adhesive mode (Fig. 4C) showed fractures occurring prevalently within the hybrid layer and with an evident presence of exposed acid-etched collagen fibrils, along with fractured resin tags inside dentinal tubules (Fig. 4 C1). When the specimens created with the 3 M-BULK in combination with the SCH applied in SE mode failed in mixed or adhesive mode (Fig. 4D), it was possible to observe on the dentine surface and on the dentinal tubule residual smear layer and parts of adhesive still partially bonded to the dentine (Fig. 4 D1). The specimens created with the STELA composite with STELA Primer applied in ER mode that failed in mixed or adhesive mode (Fig. 4E) were characterised by fractures that occurred underneath the hybrid layer. In this case, the presence of exposed acid-etched collagen fibrils was rarely detected, but it was often observed dentine tubules rarely occluded by resin tags (Fig. 4 E1). Conversely, the specimens of STELA applied in SE mode that failed in mixed or adhesive mode (Fig. 4F) often showed fractures occurred within the hybrid layer, but with the dentine covered by residual adhesive, as well as smear layer (Fig. 4 F1).
SEM fractographic analysis of the specimens tested at 24-h storage (baseline). A Representative SEM fractography of a specimen created by application of 3 M-CTR in combination with the SCH applied in ER mode that failed in mixed mode. Analysing the exposed dentine (black circle) at higher magnification, it is possible to see (A1) that the fracture occurred underneath the hybrid layer with no clear presence of exposed acid-etched collagen fibrils, but dentine tubules occluded by resin tags (rt). B The specimens created by application of 3 M-CTR in combination with the SCH applied in SE mode, when analysed at higher magnification (black circle), showed (B1) little presence of residual smear layer on the dentine surface and inside the dentinal tubules (dt). Moreover, it is possible to see the presence of a porous residual adhesive still bonded to the dentine (ad). C Representative SEM fractography of a specimen created by application of 3 M-BULK in combination with SCH applied in ER mode that failed in mixed mode. Analysing the exposed dentine (black circle) at higher magnification, it is possible to note (C1) that the fracture occurred within the hybrid layer with a clear presence of exposed acid-etched collagen fibrils and fractured resin tags (rt) inside dentinal tubules. D The specimens created by application of 3 M-BULK in combination with SCH applied in SE mode that failed in mixed, at higher magnification (black circle), showed (D1) the presence of residual smear layer (sm) on the dentine surface with no exposed dentinal tubules (dt). Also in this case, please note the presence of a porous residual adhesive still partially bonded to the dentine (ad). E Representative SEM fractography of a specimen created by application of STELA in ER mode that failed in mixed mode. At higher magnification (black circle), it is possible to see (E1) that the fracture occurred underneath the hybrid layer with no clear presence of exposed acid-etched collagen fibrils, but with exposed dentine tubules (dt) occluded rarely by resin tags. F Representative SEM fractography of a specimen created by application of STELA in SE mode that failed in mixed mode or adhesive mode. Examining the exposed dentine (black circle) at higher magnification, it is possible to see (F1) that the fracture occurred within the hybrid layer and that the dentine is covered by a layer of residual primer
SEM fractographic analysis of the specimens tested at 12 months of storage in AS. A Representative SEM fractography of a specimen created by application of 3 M-CTR in combination with the SCH applied in ER mode that failed in mixed mode. Analysing the exposed dentine (black circle) at higher magnification, it is possible to see (A1) that the fracture occurred within a degraded hybrid layer characterised by little presence of residual exposed collagen fibrils and few dentine tubules (dt) still occluded by resin tags (rt). B The specimens created by application of 3 M-CTR in combination with the SCH applied in SE mode that failed in mixed or adhesive mode, when analysed at higher magnification (black circle), showed (B1) that the fracture occurred also in this case due to degradation of hybrid layer. It is possible to note little presence of residual exposed collagen fibrils, with large dentine tubules (dt) still occluded by resin tags or smear layer. C Representative SEM fractography of a specimen created by application of 3 M-BULK in combination with SCH applied in ER mode that failed in mixed mode or adhesive mode. Investigating the exposed dentine (black circle) at higher magnification, it is possible to note (C1) that the fracture occurred due to severe degradation of the hybrid layer. It is also possible to note the presence of fractured resin tags (rt) and residual presence of partially denatured collagen fibrils. D The specimens created by application of 3 M-BULK in combination with SCH applied in SE mode that failed in mixed, when analysed at higher magnification (black circle), showed (D1) the presence of a degraded hybrid layer which left the underneath dentine exposed, but with dentinal tubules still occluded by smear layer. E Representative SEM fractography of a specimen created by application of STELA in ER mode that failed in mixed or adhesive mode. At higher magnification (black circle), it is possible to see (E1) that in the fracture occurred underneath the hybrid layer, very few exposed demineralised collagen fibrils, but with occluded dentine tubules (dt). F Representative SEM fractography of a specimen created by application of STELA in SE mode that failed in mixed mode. Examining the exposed dentine (black circle) at higher magnification, it is possible to see (F1) that the fracture occurred within the bonding interface, with the presence of no exposed collagen fibrils but with dentinal tubules still occluded by smear layer
After 12 months of storage in AS, the specimens created with the 3 M-CTR composite applied on dentine with the SCH adhesive applied in ER mode that failed in adhesive and mixed mode (Fig. 5A) presented most of the time fractures within a degraded hybrid layer. These dentine surfaces were characterised by little presence of collagen fibrils, and with only few dentine tubules still occluded by resin tags (Fig. 5 A1).
The specimens created by application of SCH in SE mode followed by the CTR composite build up that failed in mixed and adhesive mode (Fig. 5B) were prevalently characterised by fractures occurred at the bonding interface with clear signs of degradation of hybrid layer. It was often observed little presence of residual collagen fibrils and the presence of obliterated dentine tubules (Fig. 5 B1).
The specimens created with the 3 M-BULK composite applied on dentine with the SCH adhesive applied in ER mode that failed in adhesive and mixed mode (Fig. 5C) presented fractures occurred at the bonding interface with severe degradation of the hybrid layer. The exposed dentine surface was often characterised by the presence of fractured resin tags and residual occurrence of partially denatured collagen fibrils (Fig. 5 C1). Also, the specimens created with the 3 M-BULK composite applied on dentine in combination with the SCH adhesive applied in SE mode that failed in mixed and adhesive mode (Fig. 4D) showed the presence of a degraded hybrid layer which left the underneath dentine exposed. However, the dentine surface frequently presented dentinal tubules still occluded by smear layer.
The specimens created with the STELA composite and STELA Primer applied in ER mode that failed in mixed or adhesive mode (Fig. 5E) were characterised by fractures that the fracture occurred underneath the hybrid layer. Few exposed demineralised collagen fibrils and occluded dentine tubules were detected, but with no clear sign of hybrid layer degradation (Fig. 5 E1). Conversely, the specimens of STELA applied in SE mode that failed in mixed or adhesive mode (Fig. 5F) often showed a dentine surface characterised by degradation of the bonding interface, but with no presence of exposed collagen fibrils and with dentinal tubules still occluded by smear layer (Fig. 5 F1).
Discussion
In view of the results obtained in this study, the first hypothesis that the two tested restorative bulk-fill materials (STELA and 3 M-BULK) would show in confocal microscopy analysis superior interfacial adaptation and less gap formation compared to conventional composite applied layer by layer must be only partially accepted. Indeed, 3 M-BULK was characterised by the presence of gaps and voids in all tested cases, while STELA applied in class I cavities, both in SE and ER mode, demonstrated at 24 h greater adaptation and no presence of gaps at the resin-dentine interface compared to the conventional resin composite. Moreover, after 12 months of storage, STELA was the only material tested in this study that showed no presence of gaps at the resin-dentine interface.”
It was advocated that gaps can originate from various factors, such as inadequate adhesion at the tooth-restoration interface, in combination with polymerisation shrinkage, adhesive resin degradation, insufficient light-curing, fatigue resulting from the aging procedure, differences in the coefficients of thermal expansion of the tooth substrate and the restorative material, the finishing and polishing procedures, and voids as a consequence of the lack of restorative material placement in the cavity [25].
We consider that the absence of gaps at the bonding interface of STELA-treated specimens after 24 h (Fig. 2E, F), pre-fails (0%), and a mode of failure occurring prevalently in mixed and cohesive mode (Table 2) must be principally attributed to a possible low shrinkage effect that such an innovative restorative bulk-fill composite may have had during its self-polymerisation reaction. Indeed, it has been previously reported that the self-curing polymerisation induced by chemical activators is characterised by a slower initiation rate, which may lead to less shrinkage stress on the bonding interfaces compared to light-cured resin-based composite [20]. Self-curing materials are known to have delayed gel point, which may allow the polymers to flow from the unbonded surface due to their extended viscous phase; this results in lower shrinkage stress on the bonded surfaces, with consequent reduction of gap formation [21, 22].
The rate and time to achieve maximum rate of polymerisation (shrinkage kinetics) is an important issue for the chemical–mechanical properties [23] and clinical performance of a resin-based restorative material [26]. Hence, since the polymeric structure produced by such a “slower” polymerisation reaction may be affected by inferior cross-linking density compared to light-curing composites, the effect of self-cure polymerisation in bulk-fill composites on mechanical properties should be further examined for STELA.
However, scrutinising the results of the current study, we believe that there must have been also a synergic bonding factor that further contributed to avoid excessive stress at the interface related to the use of the STELA Primer, as it self-polymerised together with the STELA restorative. This latter issue should be further investigated in the future to better understand the mechanism behind such results.
It was also observed that STELA applied in ER mode presented the greatest MTBS values after 12 months of AS storage (p < 0.05) and a bonding interface prevalently free form gaps and voids, both when it was applied in ER and SE mode (Fig. 3E, F). Also in this case, when examining the fractographic results of STELA in details, it was possible to see that the dentine tubules remained often occluded after MTBS testing. Moreover, when there was the presence of exposed collagen fibrils, these were often protected by minerals, which probably precipitated consequently to the ions released from STELA during prolonged storage in AS (Fig. 5E, E1). Indeed, this innovative material contains within its composition strontium fluoroaluminosilicate glass (SFASg), which is a typical component of GIC-based materials and calcium aluminate (Table 1). It is well known that SFASg in GIC-based materials can undertake a dynamic ionic exchange (e.g. fluoride, strontium) with the surrounding environment for a relatively long period of time [27,28,29]. Furthermore, recent studies have demonstrated that materials incorporating calcium aluminate agglomerates might be considered bioactive, as they may be able to remineralise tooth-material interface and create and a natural and durable seal [30].
Although the potential bioactivity of STELA must be further investigated, the confocal microscopy showed a sort of mineral-enriched layer between the material and dentine; this layer appeared porous in the specimens created with the SCH adhesive applied in SE mode (Fig. 3E) and a more compact and thicker layer in acid-etched dentine (Fig. 3F). This latter outcome was possibly a consequent of the fact that such interfaces received more minerals from opened acid-etched tubules over time in AS storage, which increased the maturation of that particular layer at the bonding interface. This latter observation may also justify the reason why, although the absence of gaps at the interfaces created by STELA both applied in SE and ER mode, there was a significant drop of the MTBS values after prolonged AS storage (12 months); a remineralised bonding interface is often more brittle than a conventional resin-dentine interface and it can fracture very easily during MTBS testing [31, 32].
However, we need to emphasise on the results at 24 h that all the tested materials used with their adhesives applied on dentine in ER mode gave higher MTBS results compared to obtained when they were used in combination with adhesives used in SE mode (Table 2). Conversely, after 12 months of AS storage, there was a significant drop (p < 0.05) in all tested groups, except for the 3 M-BULK used in combination with the SCH adhesive applied in SE mode, which showed nearly total failure in bonding already at 24 h. Thus, also the second hypothesis of this study that the bonding procedures performed in ER or SE mode would influence the bonding performance of the tested materials both at 24 h and 12 months of storage AS must be partially accepted.
As previously stated, 3 M-BULK used in combination with the SCH adhesive applied in dentine in SE mode showed the lowest bonding results at 24 h, as well as the greatest number of specimen pre-fails (75%) and debonding in adhesive mode during MTBS testing. It was also observed that the resin-dentine interface of the specimens created with 3 M-BULK applied with the SCH in SE mode was often characterised by a typical thin hybrid layer, voids, and large gaps (Fig. 2C); these latter defects were very likely due to an excessive shrinkage effect of the composite that the adhesive applied in SE mode could not cope with [33]. Conversely, the voids at the interface were mainly due to lack of adequate adaptation of such a high-viscosity restorative material in the “class I” cavity prepared as per experimental design [15, 16].
In addition to the gap formation, clear signs of aging at the resin-dentine interface and within the adhesive and hybrid layer were often observed after 12 months of storage in AS. This result may be a consequence of severe water sorption from the one-bottle “simplified” SCH adhesive, which trigged severe hydrolytic degradation processes at the resin-dentine bonding interface [24, 34]. A further support to this latter hypothesis comes from the fractographic analysis, which confirmed that fractures occurred often within a degraded hybrid layer, leaving behind an exposed dentine surface with dentine tubules partially only occluded and no presence of intact collagen fibrils (Fig. 5D, D1).
The results of the current study showed an interesting aspect of the 3 M-BULK applied on dentine in combination with the SCH adhesive used in ER mode. It was the only group of specimens created with the tested restorative materials in combination with the SCH adhesive applied in ER bonding mode that presented a high pre-fail rate (10%) and a high number of specimens that debonded in adhesive mode during MTBS testing at 24 h. Although this group of specimens presented no significant difference in MTBS at 24 h compared to the other tested materials, the resin-dentine interface was always characterised by the presence of gaps and by a hybrid layer totally infiltrated by the fluorescent dye (Fig. 2D). Moreover, the fractographic analysis showed that the fracture occurred within the hybrid layer with a clear presence of acid-etched collagen fibrils not well infiltrated by the resin adhesive (Fig. 4C, C1). In view of these outcomes, we theorise that conventional bulk-fill restorative materials should be used, especially if they are used in “class I” cavities in combination with simplified adhesive applied in SE mode, with the layering technique as the conventional composites or applied on a liner of resin-modified GIC or flowable resin composites. Nevertheless, most of the manufacturers claim that bulk-fill composites have lower polymerisation shrinkage than conventional composites; there is no substantial information in the literature regarding the gap formation of restorative bulk-fill composites used with or without intermediate liners [35, 36]. However, the results of this study showed that both the conventional 3 M-CTR applied using the layering technique and the 3 M-BULK showed a reduced bonding performance after 12-month AS storage; a significant (p < 0.05) drop of the MTBS was observed in both composites compared to their baseline results obtained at 24 h of AS storage (Table 2). Moreover, in both composites, a predominant presence of a large gap within the interface (Fig. 3A–D) and clear signs of hybrid layer degradation were frequently observed after prolonged storage. The issue about the longevity of such adhesive restorations can be related to two main mechanisms of degradation that occur simultaneously at the resin-dentine interface during aging in water-based fluids: (i) intrinsic or degradation of the hybrid layer due to intrinsic or proteolytic degradation of the organic matrix and (ii) extrinsic or hydrolytic degradation of the resin matrix [24, 37]. Furthermore, there is a common consent that collagen degradation within the hybrid layer created by simplified adhesive applied ER mode is still a current issue in modern adhesive dentistry. This is due to the use of phosphoric acid etchants that have a strong demineralisation effect in dentine, leaving collagen fibrils completely exposed and susceptible to proteolytic degradation by the endogenous dentinal enzymes (e.g. MMPs and cysteine cathepsins) [38, 39]. Regarding the hydrolytic degradation of the resin matrix, it has been widely demonstrated that water can be trapped within the acid-etched dentine collagen, which may jeopardise the penetration of hydrophilic resin monomers during bonding procedures [40, 41]. Such a remaining water, along with the unevaporated solvents of the adhesives, can cause phase separation [42, 43], as well as water-tree formation between the polymerised hydrophilic and hydrophobic resin phases. Such issues can favour water uptake over time at the bonding interface, jeopardising the mechanical properties of the resin adhesive, as well as the hybrid layer [44,45,46]. Hydrolysis of resinous polymers due to water sorption is more evident in modern “simplified all-in-one” adhesives as they contain a higher amount of solvents and hydrophilic components (e.g. HEMA and/or functional carboxylic and phosphate monomers such as 4-META and 10-MPD) compared to multi-step adhesive systems, which allow excessive water sorption that lead to hydrolytic degradation of the resin matrix poorly polymerised [47, 48].
However, it is important to highlight that the layering technique is one of the most appropriate methods to reduce the shrinkage stress on the bonding interface created with both conventional composites and high-viscosity bulk-fill composite [14]. Indeed, the results of the current results demonstrated that the conventional composite layered at 2 mm on a dentine bonded with the SCH applied in SE or ER mode had good results in terms of immediate bond strength (24 h) compared to the bulk-fill composite used alone (Fig. 2). Indeed, the conventional composite 3 M-CTR applied layer by layer on a dentine bonded using the universal adhesive in ER, although it performed equally (p < 0.05) in terms of MTBS to 3 M-BULK applied in ER mode, often generated a gap-free interface, but with the diffusion of the fluorescent dye into micro-cracks within the composite probably created due to polymerisation shrinkage (Fig. 2B). However, this group showed 0% of pre-test fails, while the specimens submitted to microtensile failed prevalently in mixed mode and this was the only group to show a higher number of cohesive failures (28%) compared to the other groups at 24 h (Table 2).
It was interesting to observe at 24 h of AS storage that although the 3 M-CTR composite applied on dentine bonded using the SCH adhesive in SE mode showed a MTBS comparable to that obtained by STELA and debonded prevalently in adhesive and mixed mode during bond strength testing, its interface was often characterised by the presence of gaps and great dye permeability between the dentine and composite (Fig. 2A). This was probably due to the inability of the interface created with the SE bonding technique to cope with shrinkage forces (12–15 MPa) of the composite during polymerisation [49], which caused such gaps at the cavity margins; this issue may be responsible for marginal bacterial leakage and higher risks for secondary caries [50, 51].
We believe that such an issue may not directly be related to the bonding ability of the SCH, but instead to the mechanical properties of the resin-dentine interface and the hybrid layer created by applying SE adhesives in dentine. Indeed, a previous study reported that such a layer type of resin dentine interface is particularly stiff and less viscoelastic than that created in acid-etched dentine; this layer exhibits a “smooth” gradient of stiffness that precludes localised stress concentrations and prevent debonding at the interface due to mechanical stress [52].
Conclusion
In conclusion, the use of light-curing bulk-fill restorative composites might create resin-dentine interfaces characterised by the presence of gaps and voids. These interfaces may also have poor immediate bonding performance, especially when used in combination with simplified universal adhesives applied in dentine in SE mode. However, the ER bonding technique may prevent formation of gaps at the resin-dentine interface and produce greater immediate bonding performance (24 h), although the resin-dentine bonds created using all the tested materials both in SE and ER mode were affected by a drop of bond strength after prolonged AS storage. Conversely, self-curing bulk-fill restorative systems such as STELA may represent a suitable alternative to conventional and bulk-fill composites to create a resin-dentine interface less affected by gaps and voids also after prolonged AS storage. Moreover, the possible “bioactivity” of STELA is being tested to evaluate if such material can induce remineralisation through mineral precipitation of bonding interfaces created in caries-affected dentine. It is important to consider that this is an in vitro study, so its limitation is that the results presented in this article may not reflect a real clinical scenario; hence, in vivo studies and clinical studies are required to confirm or reject the conclusions presented above.
References
Sideridou I, Tserki V, Papanastasiou G (2002) Effect of chemical structure on degree of conversion in light-cured dimethacrylate-based dental resins. Biomater 23:1819–1829. https://doi.org/10.1016/s0142-9612(01)00308-8
Ferracane JL (2020) Resin composite-state of the art. Dent Mater 27:29–38. https://doi.org/10.1016/j.dental.2010.10.020
Schneider LF, Cavalcante LM, Silikas N (2010) Shrinkage stresses generated during resin-composite applications: a review. J Dent Biomech 2010:131630. https://doi.org/10.4061/2010/131630
Ferracane JL (2005) Developing a more complete understanding of stresses produced in dental composites during polymerisation. Dent Mater 21:36–42. https://doi.org/10.1016/j.dental.2004.10.004
Cho E, Sadr A, Inai N, Tagami J (2011) Evaluation of resin composite polymerisation by three dimensional micro-CT imaging and nanoindentation. Dent Mater 27:1070–1078. https://doi.org/10.1016/j.dental.2011.07.008
Carvalho RM, Pereira JC, Yoshiyama M, Pashley DH (1996) A review of polymerisation contraction: the influence of stress development versus stress relief. Oper Dent 21:17–24
Versluis A, Douglas WH, Cross M, Sakaguchi RL (1996) Does an incremental filling technique reduce polymerization shrinkage stresses? J Dent Res 75:871–8. https://doi.org/10.1177/00220345960750030301. (Mar)
Rosatto CM, Bicalho AA, Veríssimo C, Bragança GF, Rodrigues MP, Tantbirojn D, Versluis A, Soares CJ (2015) Mechanical properties, shrinkage stress, cuspal strain and fracture resistance of molars restored with bulk-fill composites and incremental filling technique. J Dent 43:1519–1528. https://doi.org/10.1016/j.jdent.2015.09.007
Umer F, Khan FR (2011) Postoperative sensitivity in class V composite restorations: comparing soft start vs. constant curing modes of LED. J Conserv Dent 14:76–79. https://doi.org/10.4103/0972-0707.80738
Yu P, Xu YX, Liu YS (2022) Polymerization shrinkage and shrinkage stress of bulk-fill and non-bulk-fill resin-based composites. J Dent Sci 17:1212–1216. https://doi.org/10.1016/j.jds.2021.12.004
Hansen EK (1986) Effect of cavity depth and application technique on marginal adaptation of resins in dentin cavities. J Dent Res 65:1319–1321. https://doi.org/10.1177/00220345860650110701
Hilton TJ, Schwartz RS, Ferracane JL (1997) Microleakage of four class II resin composite insertion techniques at intraoral temperature. Quintessence Int 28:135–144
Sengupta A, Naka O, Mehta SB, Banerji S (2023) The clinical performance of bulk-fill versus the incremental layered application of direct resin composite restorations: a systematic review. Evid Based Dent 24:143. https://doi.org/10.1038/s41432-023-00905
Fronza BM, Ayres A, Pacheco RR, Rueggeberg FA, Dias C, Giannini M (2017) Characterization of inorganic filler content, mechanical properties, and light transmission of bulk-fill resin composites. Oper Dent 42:445–455. https://doi.org/10.2341/16-024-L
Son SA, Park JK, Seo DG, Ko CC, Kwon YH (2017) How light attenuation and filler content affect the microhardness and polymerisation shrinkage and translucency of bulk-fill composites? Clin Oral Investig 21:559–565. https://doi.org/10.1007/s00784-016-1920-2
Moszner N, Fischer UK, Ganster B, Liska R, Rheinberger V (2008) Benzoyl germanium derivatives as novel visible light photoinitiators for dental materials. Dent Mater 24:901–907. https://doi.org/10.1016/j.dental.2007.11.004
Kaisarly D, Meierhofer D, El Gezawi M, Rösch P, Kunzelmann KH (2021) Effects of flowable liners on the shrinkage vectors of bulk-fill composites. Clin Oral Investig 25:4927–4940. https://doi.org/10.1007/s00784-021-03801-2
Sauro S, Makeeva I, Faus-Matoses V, Foschi F, Giovarruscio M, Maciel Pires P, Martins Moura ME, Almeida Neves A, Faus-Llácer V (2019) Effects of ions-releasing restorative materials on the dentine bonding longevity of modern universal adhesives after load-cycle and prolonged artificial saliva aging. Materials (Basel) 12:722. https://doi.org/10.3390/ma12050722
Oglakci B, Kazak M, Donmez N, Dalkilic EE, Koymen SS (2019) The use of a liner under different bulk-fill resin composites: 3D GAP formation analysis by x-ray microcomputed tomography. J Appl Oral Sci 25(28):e20190042. https://doi.org/10.1590/1678-7757-2019-0042
Burrer P, Par M, Fürer L, Stübi M, Marovic D, Tarle Z, Attin T, Tauböck TT (2023) Effect of polymerization mode on shrinkage kinetics and degree of conversion of dual-curing bulk-fill resin composites. Clin Oral Investig 27:3169–3180. https://doi.org/10.1007/s00784-023-04928-0
Ilie N (2022) Fracture and viscoelastic behavior of novel self-adhesive materials for simplified restoration concepts. J Mech Behav Biomed Mater 125:104970
Braga RR, Ferracane JL (2002) Contraction stress related to degree of conversion and reaction kinetics. J Dent Res 81:114–118
Thadathil Varghese J, Raju R, Farrar P, Prentice L, Prusty BG (2023) Comparative analysis of self-cure and dual cure-dental composites on their physico-mechanical behaviour. Aust Dent J 22. https://doi.org/10.1111/adj.13004
Sauro S, Pashley DH (2016) Strategies to stabilise dentine-bonded interfaces through remineralising operative approaches: state of the art. Int J Adhes Adhes 69:39–57. https://doi.org/10.1016/j.ijadhadh.2016.03.014
Al-Zain AO, Baeesa L, Jassoma E, Alghilan MA, Hariri M, Ismail EH, Münchow EA (2023) Assessment of internal porosities for different placement techniques of bulk-fill resin-based composites: a micro-computed tomography study. Clin Oral Invest. https://doi.org/10.1007/s00784-023-05337-z
Ferracane JL, Hilton TJ (2016) Polymerization stress-is it clinically meaningful? Dent Mater 32:1–10. https://doi.org/10.1016/j.dental.2015.06.020
Maciel Pires P, Ionescu AC, Pérez-Gracia MT, Vezzoli E, Soares IPM, Brambilla E, de Almeida NA, Sauro S (2022) Assessment of the remineralisation induced by contemporary ion-releasing materials in mineral-depleted dentine. Clin Oral Investig 26:6195–6207. https://doi.org/10.1007/s00784-022-04569-9
Pires PM, Neves AA, Makeeva IM, Schwendicke F, Faus-Matoses V, Yoshihara K, Banerjee A, Sauro S (2020) Contemporary restorative ion-releasing materials: current status, interfacial properties and operative approaches. Br Dent J 229:450–458. https://doi.org/10.1038/s41415-020-2169-3
Watson TF, Bartlett DW (1994) Adhesive systems: composites, dentine bonding agents and glass ionomers. Br Dent J 19(176):227–231. https://doi.org/10.1038/sj.bdj.4808410
Lööf J, Svahn F, Jarmar T, Engqvist H, Pameijer CH (2008) A comparative study of the bioactivity of three materials for dental applications. Dent Mater 24(5):653–659. https://doi.org/10.1016/j.dental.2007.06.028
Sauro S, Osorio R, Watson TF, Toledano M (2015) Influence of phosphoproteins’ biomimetic analogs on remineralization of mineral-depleted resin-dentin interfaces created with ion-releasing resin-based systems. Dent Mater 31:759–777. https://doi.org/10.1016/j.dental.2015.03.013
Abuna G, Feitosa VP, Correr AB, Cama G, Giannini M, Sinhoreti MA, Pashley DH, Sauro S (2016) Bonding performance of experimental bioactive/biomimetic self-etch adhesives doped with calcium-phosphate fillers and biomimetic analogs of phosphoproteins. J Dent 52:79–86. https://doi.org/10.1016/j.jdent.2016.07.016
Sofan E, Sofan A, Palaia G, Tenore G, Romeo U, Migliau G (2017) Classification review of dental adhesive systems: from the IV generation to the universal type. Ann Stomatol (Roma) 8:1–17. https://doi.org/10.11138/ads/2017.8.1.001
Maciel Pires P, Dávila-Sánchez A, Faus-Matoses V, Nuñez Martí JM, Lo Muzio L, Sauro S (2022) Bonding performance and ultramorphology of the resin-dentine interface of contemporary universal adhesives. Clin Oral Investig 26(6):4391–4405. https://doi.org/10.1007/s00784-022-04402-3
Hayashi J, Espigares J, Takagaki T, Shimada Y, Tagami J, Numata T, Chan D, Sadr A (2019) Real-time in-depth imaging of gap formation in bulk-fill resin composites. Dent Mater 35(4):585–596. https://doi.org/10.1016/j.dental.2019.01.020
Nie J, Yap AU, Wang XY (2018) Influence of shrinkage and viscosity of flowable composite liners on cervical microleakage of class II restorations: a micro-CT analysis. Oper Dent 43(6):656–664. https://doi.org/10.2341/17-091-L
Hashimoto M (2010) A review–micromorphological evidence of degradation in resin-dentin bonds and potential preventional solutions. J Biomed Mater Res B Appl Biomater 92(1):268–280. https://doi.org/10.1002/jbm.b.31535
Sabatini C, Pashley DH (2014) Mechanisms regulating the degradation of dentin matrices by endogenous dentin proteases and their role in dental adhesion. A Rev Am J Dent 27(4):203–214
Nishitani Y, Yoshiyama M, Wadgaonkar B, Breschi L, Mannello F, Mazzoni A, Carvalho RM, Tjäderhane L, Tay FR, Pashley DH (2006) Activation of gelatinolytic/collagenolytic activity in dentin by self-etching adhesives. Eur J Oral Sci 114(2):160–166. https://doi.org/10.1111/j.1600-0722.2006.00342.x
Tay FR, Pashley DH (2003) Water treeing–a potential mechanism for degradation of dentin adhesives. Am J Dent 16:6–12
Sauro S, Watson TF, Mannocci F, Miyake K, Huffman BP, Tay FR, Pashley DH (2009) Two-photon laser confocal microscopy of micropermeability of resin-dentin bonds made with water or ethanol wet bonding. J Biomed Mater Res B Appl Biomater 90(1):327–337. https://doi.org/10.1002/jbm.b.31290
Spencer P, Wang Y (2002) Adhesive phase separation at the dentin interface under wet bonding conditions. J Biomed Mater Res 62(3):447–456. https://doi.org/10.1002/jbm.10364
Ye Q, Park JG, Topp E, Wang Y, Misra A, Spencer P (2008) In vitro performance of nano-heterogeneous dentin adhesive. J Dent Res 87(9):829–833. https://doi.org/10.1177/154405910808700911
Sauro S, Mannocci F, Toledano M, Osorio R, Thompson I, Watson TF (2009) Influence of the hydrostatic pulpal pressure on droplets formation in current etch-and-rinse and self-etch adhesives: a video rate/TSM microscopy and fluid filtration study. Dent Mater 25(11):1392–1402. https://doi.org/10.1016/j.dental.2009.06.010
De Munck J, Van Landuyt K, Peumans M, Poitevin A, Lambrechts P, Braem M, Van Meerbeek B (2005) A critical review of the durability of adhesion to tooth tissue: methods and results. J Dent Res 84(2):118–132. https://doi.org/10.1177/154405910508400204
Anchieta RB, Machado LS, Martini AP, Santos PH, Giannini M, Janal M, Tovar N, Sundfeld RH, Rocha EP, Coelho PG (2015) Effect of long-term storage on nanomechanical and morphological properties of dentin-adhesive interfaces. Dent Mater 31(2):141–153. https://doi.org/10.1016/j.dental.2014.11.010
Sauro S, Pashley DH, Montanari M, Chersoni S, Carvalho RM, Toledano M, Osorio R, Tay FR, Prati C (2007) Effect of simulated pulpal pressure on dentin permeability and adhesion of self-etch adhesives. Dent Mater 23(6):705–713. https://doi.org/10.1016/j.dental.2006.06.010
Tay FR, Frankenberger R, Krejci I, Bouillaguet S, Pashley DH, Carvalho RM, Lai CN (2004) Single-bottle adhesives behave as permeable membranes after polymerization I In vivo evidence. J Dent 32(8):611–621. https://doi.org/10.1016/j.jdent.2004.04.006
Davidson CL, de Gee AJ, Feilzer A (1984) The competition between the composite-dentin bond strength and the polymerization contraction stress. J Dent Res 63(12):1396–1399. https://doi.org/10.1177/00220345840630121101
Pashley DH (1991) Dentin bonding: overview of the substrate with respect to adhesive material. J Esthet Dent 3(2):46–50. https://doi.org/10.1111/j.1708-8240.1991.tb00808.x
Perdigão J, Reis A, Loguercio AD (2013) Dentin adhesion and MMPs: a comprehensive review. J Esthet Restor Dent 25(4):219–241. https://doi.org/10.1111/jerd.12016
Sauro S, Osorio R, Watson TF, Toledano M (2012) Assessment of the quality of resin-dentin bonded interfaces: an AFM nano-indentation, μTBS and confocal ultramorphology study. Dent Mater 28(6):622–631. https://doi.org/10.1016/j.dental.2012.02.005
Acknowledgements
All the materials used in this study were regularly purchased from local distributors. The authors also gratefully acknowledge SDI Ltd. (Bayswater, Australia), for a generous donation of STELA Automix restorative and STELA Primer used in this study.
Funding
Paula Maciel Pires was undertaking a post-doctorate exchange program at Cardenal Herrera University during a part of the experimental assay and was supported by a FAPERJ grant from Brazil (E-26/205.718/2022). This study was also supported in part by a grant funded by Generalitat Valenciana (CIAICO/2022/198).
Author information
Authors and Affiliations
Contributions
S.S. and P.M.P. wrote the main manuscript, performed the experimental tests and analysis and interpretation of the results; P.F. contributed to the experimental design; A. A.N. performed the statistical analysis and reviewed the final version of the manuscript; M.L.S. contributed to the experimental design and reviewed the final version of the manuscript; A.F.C. performed part of the experimental tests and reviewed the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, human molars used in this study were collected according to the guidelines of the local Ethics Committee (CEEI22/309).
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pires, P.M., de Almeida Neves, A., Lukomska-Szymanska, M. et al. Bonding performance and interfacial adaptation of modern bulk-fill restorative composites after aging in artificial saliva: an in vitro study. Clin Oral Invest 28, 132 (2024). https://doi.org/10.1007/s00784-024-05525-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00784-024-05525-5