Abstract
Congenital anomalies of the kidneys and urinary tract, otherwise known as CAKUT, are disorders of development, the most common cause of kidney failure in children, and an antecedent cause of kidney dysfunction in adults. CAKUT consists of a polymorphic group of unilateral or bilateral disorders characterized by phenotypic and genetic heterogeneity with variable expression and severity. In this chapter, the clinical classification, epidemiology, clinical outcomes, molecular pathogenesis, genetic etiology, and clinical diagnosis and management are described based on studies in experimental animals and humans. Genetic pathways that control the embryonic development of the kidney and urinary tract are described as well as genetic mutations associated with CAKUT. Clinical management of specific types of CAKUT is discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Congenital anomalies of the kidneys and urinary tract, otherwise known as CAKUT, are classical disorders of development that are the most common cause of renal failure in children [1,2,3]. These disorders encompass a spectrum of entities including renal agenesis, renal hypodysplasia (RHD), multicystic kidney dysplasia, duplex renal collecting systems, ureteropelvic junction obstruction (UPJO), ureterovesical junction obstruction, megaureter, posterior urethral valves and vesicoureteral reflux (VUR). While congenital disorders like autosomal recessive and autosomal dominant polycystic kidney disease (PKD), nephronophthisis, and heritable nephrotic syndrome could also be considered as disorders of kidney formation, these generally occur later in kidney development as part of terminal cell differentiation events. However, some of the genes that cause nephronophthisis are also associated with CAKUT. In this chapter, we discuss disorders that arise during the early inductive events that lead to the formation of the kidneys and the urinary tracts. Both tissues arise from a common primordial tissue known as the mesonephric duct and thus, congenital kidney and urinary tract malformations commonly co-occur. In this chapter, we will focus on renal disorders encompassed within CAKUT and discuss their etiology, clinical manifestations and management. Other disorders within CAKUT with significant urinary tract pathology like UPJO, ureterovesical junction obstruction, megaureter, posterior urethral valves and VUR are discussed in other chapters.
Classification and Definition of Renal Malformations
Congenital malformations of the kidney can be defined at the macroscopic level by changes in size, shape, location, or number or microscopically by changes within specific lineages like the ureteric bud, the metanephric mesenchyme, or combinations of both [4]. In clinical practice, most congenital renal malformations are defined grossly using imaging methods like ultrasound and nuclear medicine scans. Sometimes renal tissue is obtained from biopsies or from nephrectomies, and in these cases, histological definitions of renal hypoplasia, renal dysplasia, and multicystic renal dysplasia can be utilized for classification. One can group congenital malformations of the kidney as follows:
Changes in size
-
Renal hypoplasia
-
Renal dysplasia
Changes in shape
-
Multicystic dysplastic kidney (MCDK)
-
Renal fusion
Changes in location
-
Renal ectopia
-
Renal fusion
Changes in number
-
Renal duplication
-
Renal agenesis
When induction events do not occur at the right time or location during embryogenesis, the kidneys may fail to form (agenesis, hypoplasia, dysplasia), the kidneys may form, but in the wrong location (ectopia ± hypoplasia/dysplasia), the kidneys may fail to migrate to the correct location (fusion ± hypoplasia/dysplasia) or there may be multiple induction events that arise (duplication). Malformations can be either unilateral or bilateral. Importantly, from animal models and human studies, disorders of renal formation are frequently observed with concurrent lower urinary tract malformations. In these cases, it is not clear if the impairment in induction of the kidney is primary or secondary to urinary tract obstruction. Renal agenesis refers to congenital absence of the kidney and ureter. Typically, renal malformations defined as renal hypoplasia or dysplasia are grossly small in size, defined as less than 2 SD below the mean for kidney length or weight [4,5,6]. Usually, renal hypoplasia or dysplasia is defined based on the presence of a small hyperechogenic kidney from ultrasound imaging [7]. Simple renal hypoplasia is defined as a small kidney with a reduced number of nephrons and normal architecture. Renal dysplasia is defined by the presence of malformed kidney tissue elements. Characteristic microscopic abnormalities include abnormal differentiation of mesenchymal and epithelial elements, a decreased number of nephrons, loss of corticomedullary differentiation and the presence of dysplastic elements including cartilage and bone (Fig. 8.1). As stated, dysplastic or hypoplastic kidneys are typically small, but can range in size and appear normal or even large due to the presence of multiple cysts or coincident urinary tract obstruction with hydronephrosis. The MCDK is an extreme form of renal dysplasia and is defined grossly as a non-reniform collection of cysts.
Anatomical features of human renal and lower urinary tract malformations. (a) MCDK characterized by numerous cysts (arrow) distorting the renal architecture. (b) Dysplastic renal tissue demonstrating lack of recognizable nephron elements, dilated tubules, large amounts of stromal tissue and primitive ducts (arrows) characterized by epithelial tubules with fibromuscular collars. (c) Ureteral duplication (right, white arrows) and dilated ureter (left, black arrow) associated with a ureterocele. All ureters are obstructed at the level of the bladder and are associated with hydronephrosis. (d) Crossed fused ectopia with fused orthotopic and heterotopic kidneys (arrow)
In addition to defects in renal formation that affect size, shape, location or number, and tissue patterning, congenital anomalies of the kidneys also encompass defects in the number of nephrons formed. Nephron number in the lower range of that normally observed, but not so low that it would result in renal insufficiency during childhood and/or adolescence, typically manifests in adulthood with hypertension and/or chronic kidney disease (CKD) [8,9,10].
Epidemiology and Longterm Outcomes of Renal Malformations
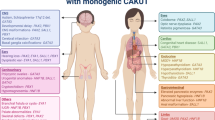
The prevalence of renal and urinary tract malformations is 0.3–17 per 1000 liveborn and stillborn infants [11, 12]. Due to their common embryonic origin from the mesonephric duct, lower urinary tract abnormalities are found in about 50% of patients with renal malformations and include VUR (25%), UPJO (11%), and ureterovesical junction obstruction (11%) [13, 14]. Renal malformations are commonly detected in the antenatal period and account for 20–30% of all anomalies detected [15]; upper urinary tract dilatation is the most frequent abnormality that is observed. All major organs are formed between the 4th and 8th week of gestation: the neural tube closes, the aortic arches undergo transformation, the cloacal membrane ruptures, and the kidneys begin to form. Renal malformations are therefore observed in association with non-renal malformations in about 30% of cases [12]. Indeed, there are over 100 multiorgan syndromes associated with renal and urinary tract malformations [16, 17] (Table 8.1).
Bilateral renal agenesis occurs in 1:3000–10,000 births and males are affected more often than females. Unilateral renal agenesis has been reported with a prevalence of 1:1000 autopsies. The incidence of unilateral hypoplasia/dysplasia is 1 in 3000–5000 births (1:3640 for the MCDK) compared to 1 in 10,000 for bilateral dysplasia [18]. The male to female ratio for bilateral and unilateral renal hypo/dysplasia is 1.32:1 and 1.92:1, respectively [19]. Nine percent of first degree relatives of patients with bilateral renal agenesis or bilateral renal hypoplasia/dysplasia have some type of renal malformation [20]. The incidence of renal ectopia is 1 in 1000 from autopsies, while from clinical studies it is estimated to be less frequent at 1 in 10,000 patients [21]. Males and females are equally affected. Renal ectopia is bilateral in 10% of cases; when unilateral, there is a slight predilection for the left side. The incidence of fusion anomalies is estimated to be about 1 in 600 infants [22].
While congenital renal malformations are relatively frequent birth defects, they become clinically evident at variable times during life and comprise a wide spectrum of outcomes ranging from no symptoms at all to CKD, which causes early mortality. The range in phenotypic severity makes it extremely difficult to counsel patients with certainty. Melo et al. reported a prevalence of CAKUT of 1.77 per 100 live births (524 cases of CAKUT in 29,653 newborns) in a tertiary care unit and a mortality rate of 24% in those affected (126/524) [11]. Amongst the 524 cases, risk factors for early mortality were the co-existence of non-renal and non-urinary tract organ disease, prematurity, low birth weight, oligohydramnios, and renal involvement (renal agenesis, RHD, multicystic renal dysplasia). Quirino et al. reported on the clinical course of 822 children with prenatally detected CAKUT that were followed for a median time of 43 months [23]. Their results demonstrate that most affected children do well: 29% of the children had urinary tract infection, 2.7% had hypertension, 6% had CKD, and 1.5% died during follow-up. Celedon et al. studied 176 children with chronic renal failure secondary to renal dysplasia, reflux nephropathy or urinary tract obstruction with a minimum of 5 years of follow-up [24]. They noted that patients with a urine albumin to creatinine ratio greater than 200 mg/mmol deteriorated faster compared to those with less than 50 mg/mmol (−6.5 mL/min/1.73 m2 year vs. −1.5 mL/min/1.73 m2 year change in estimate glomerular filtration rate [eGFR]). They also observed that those children with more than two febrile urinary tract infections deteriorated faster than those with fewer than two infections (median −3.5 mL/min/1.73 m2 vs. −2 mL/min/1.73 m2 year change in eGFR). Similar differences were noted for children with hypertension when compared to those without. Finally, they noted that the rate of decline in eGFR was greater during puberty (−4 mL/min/1.73 m2/year vs. −1.9 mL/min/1.73 m2/year change in eGFR). They noted no differences in deterioration of eGFR when comparing children with one or two functioning kidneys. In contrast, Sanna-Cherchi et al. examined the risk of progression to end-stage kidney disease (ESKD) in patients with CAKUT. They found that by 30 years of age, 58 out of 312 patients had initiated dialysis. They also noted that the risk for dialysis was significantly higher for patients with a solitary kidney [25]. The same group reported that patients with bilateral hypodysplasia, solitary kidney, or posterior urethral valves with RHD had a higher risk of dialysis requirement at 30 years when compared to patients with unilateral RHD or horseshoe kidney, and the risk was even higher if there was coexistence of VUR. Wuhl et al. compared patients with CAKUT to age-matched patients with other causes of renal failure who were receiving some form of renal replacement therapy (RRT) and registered within the European Dialysis and Transplant Association Registry [26]. Of 212,930 patients ranging in age from 0 to 75 years who commenced RRT, only 2.2% had renal failure secondary to CAKUT. Importantly, the median age for requirement of RRT was 31 years in the CAKUT cohort versus 61 years in the non-CAKUT cohort, suggesting that most children are likely to require dialysis and/or transplantation as adults. CAKUT was the most frequent cause of need for RRT in all pediatric age groups and peaked in incidence in the 15–19-year-old group.
Low birth weight and prematurity are associated with low nephron number and have therefore been studied as surrogate markers of low nephron number. The Helsinki Study followed approximately 20,000 people born between 1924 and 1944 until death or age 86 years and established that low birth weight was a risk factor for CKD in males, whereas prematurity (birth before 34 weeks of gestation) was a risk factor for CKD in females [9]. Similarly, Crump et al. demonstrated that preterm birth, defined as <37 weeks, and extreme preterm birth, defined as <28 weeks, were strongly associated with an increased risk of CKD in childhood and in adulthood [8]. Keller et al. demonstrated that low nephron number is a risk factor for hypertension in middle-aged adults compared with age-, sex-, and race-matched controls without hypertension [10].
Taken together, many questions remain in understanding the long-term outcome of CAKUT, but clearly most children are surviving into adulthood, and thus there is a need for adult nephrologists to understand these disorders.
Abnormal Molecular Signaling in the Malformed Kidney
Human renal development is complete by 34 weeks gestation [4]. Thus, by definition, renal malformation is a problem of disordered renal embryogenesis. The morphologic, cellular, and genetic events that underlie normal renal development are reviewed in Chap. 7. During human kidney development, two primordial tissues, the ureteric bud and the metanephric mesenchyme, undergo epithelial morphogenesis to form the final metanephric kidney [27]. The kidneys and the ureters arise from two epithelial tubes that extend along the length of the embryo, the mesonephric ducts. An epithelial swelling emerges from the mesonephric duct and is known as the ureteric bud. The ureteric bud invades the adjacent undifferentiated mesenchyme and induces the formation of the metanephric mesenchyme. Reciprocal signaling between the ureteric bud and the metanephric mesenchyme induces the ureteric bud to elongate and bifurcate in a process known as branching morphogenesis that ultimately gives rise to the collecting duct system of the adult kidney. The process of ureteric bud branching morphogenesis is critical for kidney development: each ureteric bud tip induces the adjacent ventrally located metanephric mesenchyme to undergo mesenchymal-to-epithelial transition and this determines the final number of nephrons formed in utero. Perturbations in ureteric bud outgrowth, branching morphogenesis and mesenchymal-to-epithelial transition are thought to underlie the majority of the malformations described in humans.
Failure of ureteric bud outgrowth and invasion of the metanephric blastema are events antecedent to renal agenesis or severe renal dysplasia. Studies in the mouse embryo, a model of human renal development, have identified genes that control ureteric bud outgrowth, ureteric bud branching morphogenesis, and mesenchymal-to-epithelial transition. Some of these genes are mutated in human renal malformations also characterized by agenesis or severe dysplasia (reviewed by [28]). If the ureteric bud fails to emerge, the ureter and the kidney do not develop, while if the ureteric bud emerges from an abnormal location, the ureter that forms will not connect to the bladder properly and potentially result in obstruction and/or VUR with a malformed kidney. Indeed, a pathogenic role for abnormal ureteric bud outgrowth from the mesonephric duct was first hypothesized based on the clinical-pathological observation that abnormal insertion of the ureter into the lower urinary tract is frequently associated with a duplex kidney. Moreover, the renal parenchyma associated with the ureter with ectopic insertion into the bladder is frequently dysplastic [29]. The local environment of transcription factors and signalling pathways is therefore critical to the successful formation of an intact kidney and urinary tract. While a large number of transcription factors and ligand-receptor signalling pathways have been identified that regulate kidney development [28, 30], we will focus on the function of a few selected molecules that have been implicated in human congenital renal malformations: Gdnf-Ret, EYA1, Six1, Sall1, Pax2, HNF1b, Shh, and components of the renin-angiotensin-system (RAS).
The central ligand-receptor signalling pathway that leads to the outgrowth of the ureteric bud from the mesonephric duct is the GDNF-GFRα1-RET signalling pathway. Glial cell derived neurotrophic factor (GDNF) is a ligand expressed by the metanephric blastema that interacts with the tyrosine kinase receptor, RET, and its co-receptor GFRα1, both expressed on the surface of the mesonephric duct, to initiate outgrowth of the ureteric bud. Mutational inactivation of Gdnf, Gfra1, or Ret in mice causes bilateral renal agenesis due to failure of ureteric bud outgrowth, demonstrating the importance of this pathway [31,32,33,34]. Similarly, when GDNF-soaked beads are positioned adjacent to cultured murine mesonephric ducts, multiple ectopic ureteric buds emerge, demonstrating the potency of this signalling pathway [35]. The expression domain of GDNF is therefore tightly regulated in the nephrogenic mesenchyme and the metanephric mesenchyme.
A network of transcription factors promotes Gdnf expression: Eya1, Six1, Sall1, and Pax2, while Foxc1 restricts Gdnf expression [36]. Another ligand-receptor complex that limits the domain of Gdnf expression is the secreted factor SLIT2 and its receptor ROBO2 [37]. Slit2 is expressed in the mesonephric duct, while Robo2 is expressed in the nephrogenic mesenchyme Bone morphogenetic protein 4 also negatively regulates the expression domain of Gdnf such that the ureteric bud emerges in the correct location [38].
EYA1 is expressed in metanephric mesenchymal cells in the same spatial and temporal pattern as GDNF. Mice with EYA1 deficiency demonstrate renal agenesis and failure of GDNF expression [39]. EYA1 functions in a molecular complex that includes SIX1 and together they translocate to the nucleus to regulate GDNF expression. Therefore, mutational inactivation of Six1 in mice also results in renal agenesis or severe dysgenesis [40]. Like GDNF, SIX1 and EYA1, SALL1 is expressed in the metanephric mesenchyme prior to and during ureteric bud invasion. Mutational inactivation of Sall1 in mice causes renal agenesis or severe dysgenesis and a marked decrease in GDNF expression [41]. Thus, EYA1, SIX1 and SALL1 function upstream of GDNF to positively regulate its expression, thereby controlling ureteric bud outgrowth.
PAX2 is another transcription factor that is expressed in the mesonephric duct, the ureteric bud and in metanephric blastema cells induced by ureteric bud branch tips [42]. Mice with a Pax2 mutation identical in type to that found in humans with renal coloboma syndrome (RCS) exhibit decreased ureteric bud branching and renal hypoplasia. Investigation of the mechanisms controlling abnormal ureteric bud branching in a murine model of RCS (Pax21Neu) revealed that increased ureteric bud cell apoptosis decreases the number of ureteric bud branches and glomeruli formed. Remarkably, rescue of ureteric bud cell apoptosis normalizes the mutant phenotype [43]. Pax2 appears to function upstream of Gdnf since in Pax2 null mice no Gdnf expression is detected and the PAX2 protein can activate the Gdnf promoter [44].
PAX2 and HNF1β, another transcription factor, are co-expressed in the mesonephric duct and the ureteric bud lineage. Constitutive inactivation of HNF1β is embryonic-lethal in the mouse at gastrulation prior to the formation of the kidneys, but by using tetraploid and diploid embryo complementation, homozygous mutant embryos were able to proceed past gastrulation. The latter study demonstrated that HNF1β is critical for mesonephric duct integrity, ureteric bud branching morphogenesis, and early nephron formation [45]. Another group conditionally inactivated HNF1β in the proximal tubule, loop of Henle and collecting ducts and noted that null mice had cystic kidneys with cysts arising predominantly from collecting duct and loop of Henle segments [46]. The renal phenotype was severe, leading to death from renal failure in the newborn period. Importantly, cystic kidneys from null animals demonstrated downregulation of uromodulin, Pkd2, and Pkhd1, suggesting that HNF1β may regulate genes associated with cyst formation [46]. Compound heterozygous mice bearing null alleles for Pax2 and Hnf1β show severe CAKUT phenotypes, including hypoplasia of the kidneys, caudal ectopic aborted ureteric buds, duplex kidneys, megaureters and hydronephrosis [47]. These phenotypes were much more severe than Pax2 heterozygous null or Hnf1β heterozygous null mice, strongly suggesting that Pax2 and Hnf1β genetically interact in a common kidney developmental pathway.
Sonic hedgehog (SHH) is a secreted protein that controls a variety of critical processes during embryogenesis. In mammals, SHH acts to control gene transcription via three members of the GLI family of transcription factors, GLI1, GLI2 and GLI3. A pathogenic role for truncated Gli3 was demonstrated in mice engineered such that the normal GLI3 allele was replaced with the truncated isoform. These mice are characterized by renal agenesis or dysplasia similar to humans with Pallister Hall Syndrome (PHS) [48]. Subsequent analysis of renal embryogenesis in mice deficient in SHH suggests that the truncated form of GLI3 represses genes like Pax2 and Sall1 that are required for the initiation of renal development [49]. Loss of Hedgehog signalling has also been implicated in nonobstructive hydronephrosis and urinary pacemaker dysfunction in mice [50]. Shh is expressed in the epithelium of the ureter during embryonic development and signals to the developing ureteric mesenchyme. Shh deficiency results in lack of smooth muscle cell differentiation in the ureter and nonobstructive hydronephrosis [51]. Hedgehog signaling is also active in the embryonic metanephric mesenchyme; genetic deficiency of Hedgehog signaling in this spatial domain also results in nonobstructive hydronephrosis but by a different mechanism. In mice so affected, there is loss of pacemaker cell activity in the renal pelvis and ureter [50] The observation that constitutive expression of GLI3 repressor also causes nonobstructive hydronephrosis and that genetic deficiency of Gli3 in mice with decreased Hedgehog rescues hydronephrosis demonstrates the critical role of GLI3 repressor downstream of Hedgehog signaling. Interestingly, some patients with Pallister Hall Syndrome manifest hydronephrosis, although the underlying mechanisms in these patients are unclear.
Analysis of mice with constitutive activation of Hedgehog signaling and human ureter tissues, implicates Hedgehog signaling in the pathogenesis of UPJO. Mice with deficiency of Patched1, a cell surface receptor that inhibits Hedgehog signaling, in renal progenitors that give rise to both nephrogenic and stromal cells are characterized by UPJO due to formation of an ectopic cluster of stromal cells that block the UPJ. Analysis of obstructing ureteric tissue in infants and children with congenital UPJO demonstrated upregulation of hedgehog signaling effectors and stromal genes, suggesting that increased hedgehog signaling may contribute to the pathogenesis of human UPJO, as well [52].
In postnatal renal physiology, the RAS plays a critical role in fluid and electrolyte homeostasis and in the control of blood pressure. Renin cleaves angiotensinogen (AGT) to generate angiotensin (Ang) I which is cleaved by angiotensin-converting enzyme (ACE) to yield Ang II. Ang II is the main effector peptide growth factor of the RAS and acts on two major receptors: AT1R and AT2R. The role of the RAS during kidney development appears to differ somewhat in humans versus rodents, but the metanephric kidney expresses all components of the pathway in both species. Ang is expressed in the ureteric bud lineage and the stromal mesenchyme, while renin is expressed by mesenchymal cells destined to form vascular precursors in the kidney. ACE is expressed slightly later during kidney development in differentiated mesenchymal structures including glomeruli, proximal tubules and collecting ducts. The receptors AT1R and AT2R are expressed in the ureteric bud lineage and in metanephric mesenchymal cells [53]. Mutations of AGT, renin, ACE, or AT1R all result in CAKUT phenotypes in the mouse that are characterized by renal malformations with hypoplasia of the medulla and the papillae and hydronephrosis [54]. Mice with mutations in ATR2 also exhibit CAKUT, but a wider range of phenotypes is observed that includes renal hypo/dysplasia, duplicated collecting systems, VUR, and hydronephrosis [55]. Importantly, genetic inactivation of the RAS pathway in mice does not result in renal tubular dysgenesis (RTD) as observed in humans with similar mutations. It is postulated this may be due to differences between the species: in humans, RAS activity (renin and ANG II levels) peaks during fetal life while nephrogenesis is occurring, while in rodents, RAS activity peaks postnatally from weeks 2–6, when nephrogenesis has ceased. These temporal differences likely explain the lack of concordance between genetic mouse models and affected humans [56].
Ureteric bud branching and modelling of the lower urinary tract with its insertion into the bladder is also controlled by vitamin A and its signaling effectors [57, 58]. Expression of RET, the receptor for GDNF, is controlled by members of the retinoic acid receptor family of transcription factors that function in the vitamin A signaling pathway. These members, including RAR alpha and RAR beta2, are expressed in stromal cells surrounding Ret-expressing ureteric bud branch tips [58, 59]. Mice deficient in these receptors exhibit fewer ureteric bud branches and diminished expression of Ret. These observations are consistent with the finding that vitamin A deficiency during pregnancy causes renal hypoplasia in the rat fetus [60]. A similar observation has been noted in a human study where maternal vitamin A deficiency was associated with congenital renal malformation [61].
In summary, genetic and nutritional factors like vitamin A and folic acid [61,62,63] interact to control ureteric bud outgrowth, ureteric bud branching, nephrogenesis, and ureter formation. The number of nephrons is likely determined by a complex combination of factors including genetic variants, environmental events and stochastic factors. This could explain the variable number of nephrons in humans, ranging from approximately 230,000 to 1,800,000 [64]. Loss-of-function mutations in developmental genes can impair nephron formation in utero and depending on the magnitude of this effect, renal insufficiency may present at birth, childhood, adolescence or adulthood. Despite evidence in animals that depletion of protein, total calories or micronutrients causes renal hypoplasia, their contribution to human CAKUT remains unclear and an important area of future investigation.
Human Renal Malformations with a Defined Genetic Etiology
In humans, congenital renal malformations are more frequently sporadic than familial in occurrence. This may be due to the fact that infants with severe renal malformations have only recently survived; prior to the late 1970s, chronic dialysis was not offered as a therapy for children, and this continues to be the case in much of the developing world because of a lack of resources. Therefore, it is only in the past 30 years that children with congenital renal malformations have survived and been able to reproduce and potentially transmit deleterious gene mutations. Therefore, congenital renal malformations appear as sporadic events over time. Genetic haploinsufficiency for many of the aforementioned transcription factors (EYA1, SIX1, PAX2, etc.) can result in a severe renal developmental phenotype, therefore de novo heterozygous mutations continue to arise. However, as reported by others, incomplete penetrance with variable expressivity is frequently observed in genetic studies of CAKUT, especially in relation to many of the transcription factors described previously [65]. Congenital renal malformations can occur in isolation, as part of CAKUT, or as part of a syndrome with organ malformations. Importantly, familial cases and extra-renal symptoms are sometimes unrecognized if carefully phenotyping is not performed. A careful evaluation of family history reveals a clustering of isolated or syndromic urinary tract and renal malformations in more than 10% of the cases [66]. Knowledge of the most frequent syndromes, a careful clinical examination and appropriately selected investigations are critical to the clinical approach to these disorders.
Mutations in more than 30 genes have been identified in children with renal development anomalies, generally as part of a multiorgan syndrome (Table 8.2). Some of these syndromes and their associated genes are described here or in some recent reviews [16, 17]. The most frequent syndromes in which renal malformations are encountered are listed in Tables 8.1 and 8.2. For a complete list of syndromes featuring renal malformations, the reader is referred to McKusick’s Online Mendelian Inheritance in Man.Footnote 1
For most children with renal malformations, neither a syndrome nor a Mendelian pattern of inheritance is obvious. However, genetic studies incorporating chromosomal microarrays, targeted gene panels or whole exome sequencing (WES) have identified a genetic cause for the renal malformation in anywhere from 5% to 30% of cases [16]. One of the first such studies of 100 patients with RHD and renal insufficiency demonstrated that 15% had mutations in two transcription factors [65]: TCF2 (HNF1β) (especially in the subset with kidney cysts) and PAX2. EYA1 and SALL1 mutations were found in single cases. Some of the mutations that were identified in these genes were de novo mutations explaining the sporadic appearance of RHD. Careful analysis of patients with TCF2 and PAX2 mutations revealed the presence of extrarenal symptoms in only half, supporting previous reports that TCF2 and PAX2 mutations can be responsible for isolated renal tract anomalies or at least CAKUT malformations with minimal extrarenal features [67, 68]. This study demonstrates that subtle extrarenal symptoms in syndromal RHD can easily be missed. Genetic testing in children with RHD should be preceded by a thorough clinical evaluation for extrarenal symptoms, including eye, ear, and metabolic anomalies. The presence of nonrenal anomalies increases the likelihood of detecting a specific genetic abnormality (Table 8.5). In addition, mutations in genes that are usually associated with syndromes can occur in patients with isolated RHD.
The GDNF/RET Signaling Pathway
The proto-oncogene RET, a tyrosine kinase receptor, and its ligand, GDNF, play a pivotal role during early nephrogenesis and enteric nervous system development. Activating RET mutations cause multiple endocrine neoplasia, whereas inactivating mutations lead to Hirschsprung disease. A number of human studies have demonstrated that patients with CAKUT have mutations in the RET/GDNF signaling pathway [69,70,71,72]. A study of 122 patients with CAKUT identified heterozygous deleterious sequence variants in GDNF or RET in 6/122 patients, 5%, while another group screened 749 families from all over the world and identified 3 families with heterozygous mutations in RET [69, 73]. Similar findings have been reported in studies of fetuses with bilateral or unilateral renal agenesis [70, 71].
Branchio-Oto-Renal Syndrome
The association of branchial (B), otic (O) and renal (R) anomalies was first described by Fraser and Melnick [74, 75]. Major diagnostic criteria consist of hearing loss (95%), branchial defects (49–69%), ear pits (83%) and renal anomalies (38–67%) [76, 77]. The association of these three major features defines the classical BOR syndrome (OMIM # 113650). Yet, many patients have only one or two of these major features in association with other minor features such as external ear anomalies, preauricular tags or other facial abnormalities (Table 8.3). Hearing loss can be conductive, sensorineural, or mixed.
The frequency of BOR syndrome has been estimated to be 1 in 40,000 births [78]. The transmission is autosomal dominant with incomplete penetrance and variable expressivity. Renal malformations include unilateral or bilateral renal agenesis, hypodysplasia as well as malformation of the lower urinary tract including VUR, pyeloureteral obstruction, and ureteral duplication. Different renal malformations can be observed in the same family; moreover, some individuals have normal kidneys (BO syndrome, OMIM 120502). Other infrequent abnormalities have been described in patients with the BOR syndrome. These include aplasia of the lacrimal ducts, congenital cataracts and anterior segment anomalies [74, 75]. Characteristic temporal bone findings include cochlear hypoplasia (4/5 of normal size with only 2 turns), dilation of the vestibular aqueduct, bulbous internal auditory canals, deep posterior fossae, and acutely angled promontories [77].
Approximately 40% of patients with BOR syndrome have a mutation in EYA1 [76]. Mutations in in SIX1, SIX5, and SALL1 have also been identified in patients with BOR syndrome, but at lower frequencies [79,80,81]. Both EYA1 and SIX1 are co-expressed in the developing otic, branchial and renal tissue, where they function in a transcriptional complex that regulates cell proliferation and cell survival [82, 83]. EYA1 and SIX1 control the expression of PAX2 and GDNF in the metanephric mesenchyme [84]. The EYA1 protein contains a highly conserved region called the eyes absent homologous region encoded within exons 9–16, which is the site of most mutations identified to date.
A reasonable approach is to perform genetic analysis in families in which at least one member fulfils the criteria for classical BOR syndrome (Table 8.3). Investigations should include a family history, and examination of relatives to look for preauricular pits, lacrimal duct stenosis, and branchial fistulae and/or cysts. Hearing studies and renal ultrasound should be done in all first-degree relatives.
Molecular testing can confirm the diagnosis and provide genetic recurrence risk information to families. However, variability of the phenotype even with the same mutation does not permit accurate prediction of the disease severity. Within the same family, a given mutation may be associated with renal malformation in some individuals, but not in others. This discrepancy might be explained by stochastic factors that impact the formation of the kidneys or by other unlinked genetic events that may act in synergy with the EYA1 protein during nephrogenesis.
Townes-Brocks Syndrome and VATER/VACTERL Associations
Townes-Brocks syndrome (TBS) is an autosomal dominant malformation syndrome usually defined by a triad of anomalies including imperforate anus, dysplastic ears, and thumb malformations [85]. A wide spectrum of additional features includes renal malformations, congenital heart defects, hand and foot malformations, hearing loss, and eye anomalies [86, 87]. Intelligence is usually normal. REAR Syndrome (renal-ear-anal-radial) has also been used to describe this condition [88]. Its incidence is reported to be 1:250,000 live births [89]. The presentation of TBS is highly variable within and between affected families. Importantly, SALL1, is the only gene implicated in TBS and it encodes a C2H2 zinc finger transcription factor that is required for the normal development of the limbs, nervous system, ears, anus, heart and kidneys [90, 91].
The detection rate of SALL1 mutations in patients with TBS appears to be higher when malformations of the hands, the ears, and the anus are present [92]. However genetic testing is further complicated by the fact that the phenotypic features of TBS can resemble other disorders like VACTERL association, Goldenhar syndrome, Oculo-Auriculo-Vertebral spectrum, Pallister-Hall syndrome and even BOR syndrome. TBS features overlap those seen in the VACTERL association (anal, radial and renal malformations). In contrast to VACTERL association, TBS is associated with ear anomalies and deafness and it is not characterized by tracheo-oesophageal fistula or vertebral anomalies.
VACTERL association is defined by the presence of at least three of the following congenital malformations: vertebral anomalies, anal atresia, cardiac defects, tracheo-esophageal defects, renal malformations, and limb anomalies [93]. It is reported to occur in 1:10,000–40,000 of all live births. Renal anomalies are reported in 50–80% of patients and include unilateral or bilateral renal agenesis, horseshoe kidney, cystic kidneys, and dysplastic kidneys; they can be accompanied by urinary tract and genital defects [93, 94]. Ninety percent of VACTERL cases appear to be sporadic with little evidence of heritability [93]. In a subset of patients there is evidence of heritability [93, 95, 96], and genes that interact with the Sonic Hedgehog pathway have been implicated [97, 98]. The presence of a single umbilical artery on ultrasound has been associated with a variety of congenital birth defects, including VACTERL syndrome [99]. It has been hypothesized that the single umbilical artery is a risk factor for a placental defect that may affect nutrient supply for multiple organs simultaneously during development [100].
An important diagnosis to consider in patients suspected to have VACTERL syndrome is Fanconi’s anemia. Patients with Fanconi’s anemia can phenocopy VACTERL syndrome, but also exhibit bone marrow failure manifest as pancytopenia. They can also develop malignancies like acute myelogenous leukemia secondary to their propensity for chromosomal instability manifest as spontaneous cytogenetic aberrations. Patients with Fanconi’s anemia also frequently demonstrate skin pigmentation (café au lait spots), microcephaly, growth retardation and microphthalmia. There are at least nine different gene mutations implicated in Fanconi’s anemia and they are inherited as X-linked or recessive disorders. It has been reported that approximately 5% of patients with confirmed Fanconi’s anemia have features consistent with VACTERL syndrome [101]. Therefore, the diagnosis of Fanconi’s anemia needs to be carefully considered in all patients with VACTERL syndrome and confirmed if needed by performing chromosomal breakage studies [101].
Renal-Coloboma Syndrome
Renal Coloboma Syndrome (RCS) (also named papillo-renal syndrome) is an autosomal dominant disorder characterized by the association of renal hypoplasia, VUR and optic nerve coloboma from a mutation in PAX2 [102]. The prevalence of the syndrome is unknown, but approximately 100 families have been reported [103]. A wide range of renal malformations are observed in RCS. Oligomeganephronic hypoplasia, renal dysplasia and VUR are the most frequent malformations, but multicystic dysplasia [104] and UPJO have also been described [104]. Similarly, the ocular phenotype is extremely variable. The most common finding is an optic disc pit associated with vascular abnormalities and cilio-retinal arteries, with mild visual impairment limited to blind spot enlargement, the “morning glory” anomaly [105]. In other cases, the only ocular anomaly is optic nerve dysplasia with an abnormal vessel pattern and no functional consequence (Fig. 8.2). In contrast, a large coloboma of the optic nerve or of the choroid and retina and the morning glory anomaly can be responsible for a severe visual impairment [106]. Coloboma and the related anomalies are probably the consequence of an incomplete closure of the embryonic fissure of the optic cup. Other extrarenal manifestations can include sensorineural hearing loss, joint laxity, Arnold-Chiari malformation and seizures of unknown cause [107, 108]. In addition to its expression in the developing kidney and in the optic fissure, PAX2 is also expressed in the hindbrain during its development. However, neurological symptoms are not usually present in RCS.
Optic disc appearance in two patients with Renal Coloboma Syndrome and PAX2 mutations: (a) Characteristic features of optic disk coloboma with a deep temporal excavation (arrowheads). (b) The optic disk is dysplastic with thickening (arrow) and emergence of abnormal vessels (“morning glory anomaly”)
PAX2 is a transcription factor of the paired-box family of homeotic genes that is expressed in the mesonephros and in the metanephros during renal development. In 1995, Sanyanusin et al reported heterozygous mutations in two RCS families [109]. Since then, more than 30 mutations have been reported, most of them lying in exons 2–4 that encode the paired domain that binds to DNA or in exons 7–9 that encode the transactivation domain [103]. Other gene(s) are probably also responsible for this syndrome since PAX2 mutations are not found in approximately 50% of RCS patients. Importantly the RCS phenotype is highly variable, even in patients harboring the same PAX2 mutation, suggesting that modifier genes might be implicated.
Optic nerve coloboma occurs frequently as an isolated anomaly or as a feature of many other multiorgan syndromes such as the CHARGE association, the COACH syndrome and the acro-renal-ocular syndrome. As optic nerve coloboma and the related disorders can be easily misdiagnosed, it is likely that the prevalence of RCS is underestimated. It is wise to examine the fundus in every patient with RHD, and conversely to perform renal ultrasound and serum creatinine in every patient with optic nerve coloboma.
Even in the absence of optic nerve colobomas, mutations in PAX2 are one of the more common genetic causes of RHD [65] and they also appear to be associated with low nephron number. Barua et al. described families diagnosed with FSGS anywhere from 7 to 68 years of age due to dominantly inherited mutations in PAX2. One patient had a kidney biopsy sample that exhibited glomerulomegaly, which could be secondary to low nephron endowment at birth [110]. Some of the affected individuals had imaging studies that revealed other CAKUT phenotypes including small kidneys and hydronephrosis. Vivante et al. identified heterozygous mutations in PAX2 in three families and one child with steroid-resistant nephrotic syndrome [111]. The patients developed their steroid-resistant nephrotic syndrome or FSGS either during infancy or in adolescence. Here again, the FSGS lesion could be secondary to low nephron endowment at birth.
Renal Cyst and Diabetes Syndrome
Mutations in the TCF2 gene encoding the transcription factor HNF1β were initially found in patients with maturity onset diabetes of the young, type 5 (MODY5), an autosomal dominant disorder [112, 113]. Diabetes mellitus is present in approximately 60% of all the cases reported, usually occurs before 25 years of age, and is often associated with pancreatic atrophy [114,115,116]. In some patients, a subclinical deficiency of pancreatic exocrine functions has been demonstrated. Additional features have been described, including a wide spectrum of renal phenotypes (Table 8.4). The presence of cysts is the most consistent feature of the renal phenotype, leading to the name, “Renal Cysts and Diabetes (RCAD) Syndrome”. The cysts are usually cortical, bilateral, and small [68]. Mutations in the TCF2 gene have also been found in association with a variety of isolated renal development disorders such as RHD, MCDKs, renal agenesis, horseshoe kidneys, UPJO as well as clubbing and tiny diverticula of the calyces [117,118,119]. The most specific finding when histology is available is the presence of cortical glomerular cysts with dilatation of the Bowman spaces (glomerulocystic dysplasia) [120]. Other nonspecific lesions such as cystic renal dysplasia, interstitial fibrosis or oligomeganephronia have also been reported. Antenatal presentations with enlarged hyperechoic kidneys or macroscopic cysts can occur [118, 121].
Various genital tract malformations have been reported mostly in females. These include vaginal aplasia, rudimentary uterus, bicornuate uterus, uterus didelphys and double vagina. In males, hypospadias, epididymal cysts, and agenesis of the vas deferens have been reported [114]. These genital anomalies have been described in approximately 10–15% of patients with TCF2 mutations, but these malformations might be underestimated especially in paediatric reports. Reduced fractional excretion of uric acid (<15%) and moderate hyperuricemia is observed in some cases and is usually asymptomatic. The hyperuricemia is thought to reflect altered urate transport by the kidney and impaired glomerular filtration [114]. Serum hypomagnesemia has also been reported and this may be due to the fact that HNF1b regulates FXYD2 that is needed for distal tubule reabsorption of magnesium [122]. A similar mechanism may explain the altered urate transport observed in these patients since HNF1b can activate the promoter of URAT1 that regulates urate transport in the proximal tubule [123]. Moderate elevation of liver enzymes is a common finding, but severe hepatopathy has not been reported.
HNF1β is a homeobox-containing basic helix-turn-helix transcription factor, which is involved in the development of the pancreas, the kidneys, the liver and intestine. More than 50 mutations have been reported, most of which are located in the first four exons that encode the DNA-binding domain. In more than one-third of the cases, the gene is entirely deleted [68, 115, 123]. Such alterations are not detected by conventional amplification and screening methods. Importantly, deletions are infrequently transmitted by the parents but appear de novo in the proband. Analysis of TCF2 can thus be recommended not only in patients with a family history of RCAD syndrome but also in cases with renal cysts when polycystic disease or nephronophthisis are unlikely. The presence of cortical bilateral cysts is probably the most typical finding. Reduced uric acid fractional excretion, elevation of liver enzymes, hypomagnesemia, glucose intolerance and abnormalities of the genital tract should be systemically sought and TCF2 analyzed if one of these symptoms is present. As observed in other syndromes, phenotypic variability can be observed between families and also in family members with the same mutation, suggesting a role for environmental and genetic factors.
Longitudinal follow-up of genetically proven HNF1β nephropathy has been reported in a group of 62 children and adolescents, of whom 87% were diagnosed with bilateral renal dysplasia. Among these patients, 74% and 16% had visible bilateral or unilateral cysts, respectively, at the end of an average of 4 years of follow-up [124]. During this period, 28% of patients had an increase in cyst number, which was associated with a greater decline in GFR compared to patients without an increase in cyst number. Eight percent of patients developed ESKD at a median age of 15 months. Hypomagnesemia was present in 19 of 52 patients evaluated by a median age of 1 year, while recurrent hyperglycemia was observed in 4 of 50 evaluated patients. Increased uric acid was detected in 37% of patients. HNF1β mutations were varied in type, were familial or de novo, and were not correlated with phenotype. The issue of which patients should be screened for a mutation in HNF1β has been investigated in a cohort of 433 pediatric patients with known HNF1β status and CAKUT using a 17-item weighted score inclusive of abnormalities in renal, pancreatic, genital, electrolyte, and liver function as well as family history. A score of >8 was reported to have a sensitivity of 98.2%, specificity of 41.1%, positive predictive value of 19.8% and a negative predictive value 99.4% [125]. The possible utility of this scoring system, particularly in predicting absence of a HNF1β mutation, needs to be validated in other cohorts with HNF1β-associated disease.
Kallmann Syndrome
Kallmann syndrome (KS) is defined by the presence of hypogonadotropic hypogonadism and deficiency of the sense of smell (anosmia or hyposmia) [126]. Some affected individuals exhibit unilateral renal agenesis, cleft lip and/or palate, selective tooth agenesis, bimanual synkinesis and hearing impairment [127]. Other CAKUT phenotypes including duplex systems, hydronephrosis, and VUR have been rarely reported. Anosmia/hyposmia is related to the absence or hypoplasia of the olfactory bulbs and tracts. Hypogonadism is due to a deficiency in gonadotropin-releasing hormone (GnRH). The GnRH-synthesizing neurons migrate during development from the olfactory epithelium to the forebrain along the olfactory nerve pathway [128]. KS is genetically heterogeneous with at least 8 genes reported including KAL1, an X-chromosome encoded gene that gives rise to the extracellular matrix protein anosmin-1 [129], FGF8 (Fibroblast growth factor 8) [130], FGFR1 (Fibroblast Growth Factor Receptor 1) [131], PROK2 (prokinectin-2) and PROKR2 (prokinectin-2 receptor) [132], CHD7, NELF, and HS6ST1 [133]. Chromodomain helicase DNA-binding protein 7 (CHD7) is a transcriptional regulator that binds to enhancer elements in the nucleus. It is implicated in CHARGE syndrome, that is characterized by choanal atresia, malformations of the heart, the inner ear, and the retina, and in Kallmann syndrome. In the largest study to date of 219 patients with Kallmann syndrome, mutations were most commonly observed in the FGF pathway (either FGF8 or FGFR1), in KAL1, in the PROK2/PROKR2 pathway and in CHD7 [133]. Importantly in this study, unilateral renal agenesis was only observed in patients with KAL1 mutations (reported in 18%, 3/17), or in patients with no mutation in the above-mentioned 8 genes, where the frequency was similar at 17% (4/23). Patients with KAL1 mutations are typically male since the disorder is X-linked and they demonstrate a much more severe reproductive phenotype compared to patients with other mutations with small testes, absent puberty, and micropenis. Females with KAL1 mutations typically present with partial pubertal development manifesting as spontaneous breast development in the absence of hormonal treatment. KAL1 is expressed in the developing human metanephric kidney at 11 weeks of gestation [134].
Renal Tubular Dysgenesis and Mutations of RAS System Elements
The differential diagnosis of oligohydramnios with neonatal renal failure includes a spectrum of diagnoses including bilateral renal dysplasia, posterior urethral valves, and PKD. All of these diagnoses are detectable and distinguishable on antenatal ultrasound imaging of the kidneys and the urinary tracts. The presence of normal kidneys on antenatal ultrasound in combination with oligo- or anhydramnios should strongly suggest the diagnosis of RTD [135]. RTD is a severe perinatal disorder characterized by absence or paucity of differentiated proximal tubules, early severe oligohydramnios, and perinatal death. The latter is usually due to pulmonary hypoplasia and skull ossification defects [136]. This condition has also been described in clinical conditions associated with renal ischemia, including the twin–twin transfusion syndrome, major cardiac malformations, severe liver diseases, fetal or infantile renal artery stenosis [137] and in fetuses that are exposed in utero to ACE inhibitors, Ang II receptor antagonists [138] or non-steroidal anti-inflammatory medications [135]. All of these environmental insults are postulated to lead to chronic hypoperfusion of the fetal kidneys with upregulation of the RAS. The absence or paucity of proximal tubules is believed to be secondary to chronic renal hypoperfusion [56]. Mutations in the genes which encode components of the RAS have been identified in some families [139]. Mutations in the ACE gene are seen in 65.5 % of cases, while mutations in the renin (REN) are observed in 20 % of cases. Mutations in AGT and in the AGT type I receptor (ATR1) occur much less frequently [56]. It has been suggested that if there is no expression of the renin protein on immunohistochemistry of the kidneys, then the renin gene should first be assessed. Similarly, the plasma renin activity should be measured in the newborn with suspected genetic RTD and if elevated, this should prompt an analysis of genes downstream of the REN gene [140].
CHD1L, CHD7 and CHARGE Syndrome
Chromodomain helicase DNA binding protein 1-like protein, CHD1L, belongs to the Snf2 family of helicase-related ATP-hydrolyzing proteins and contains a helicase-like region that is similar to other family members, such as CHD7 which is the major gene that causes CHARGE syndrome. CHARGE syndrome is characterized by Colobomas, Heart defects, choanal Atresia, Retarded growth and development, Genital hypoplasia, and Ear anomalies with deafness. CHARGE syndrome is associated with CAKUT phenotypes including horseshoe kidneys, renal agenesis, VUR and renal cysts [141]. Chromatin-remodelling and -modifying enzymes like CHD1L and CHD7 are predicted to play key roles in differentiation, development and tumour pathogenesis via effects on chromatin structure and accessibility. Brockeschmidt et al. screened 85 patients with CAKUT and identified 3 patients with heterozygous missense variants in CHD1L [142]. The same paper reported that CHD1L was expressed in early ureteric bud and comma- and S-shaped structures during human kidney development. In the postnatal human kidney, CHD1L was expressed in the cytoplasm of tubular cells in all nephron segments. Similarly, Hwang et al. reported that 5 out of 650 families had heterozygous mutations in CHD1L: the affected individuals had a spectrum of CAKUT phenotypes including renal dysplasia, posterior urethral valves, UVJ obstruction and horseshoe kidneys [73]. It is not yet known if these patients will also be at greater risk for malignancies given that CHD1L is known to be an oncogene in hepatocellular carcinoma [143].
DSTYK and CAKUT
DSTYK is a dual serine-threonine and tyrosine protein kinase that is co-expressed with fibroblast growth factor receptors in the developing mouse and human kidney in both metanephric mesenchyme and ureteric bud cells. Sanna-Cherchi et al. discovered that 7/311 patients with CAKUT had heterozygous mutations in this gene [144]. The CAKUT phenotypes observed in these patients included UPJO, VUR, and RHD.
Copy Number Variants, CAKUT and Neuropsychiatric Disorders
Copy number variants are stretches of DNA that are larger than 1 kb in length with the potential to contribute to functional variation and disease. Rare CNVs have been implicated in neuropsychiatric and craniofacial syndromes, and in syndromes with CAKUT [145, 146]. Sanna-Cherchi et al. examined the burden of rare CNVs in individuals with congenital renal malformations and identified disease-causing CNVs and potentially pathogenic CNVs in 10% and 6%, respectively, of the 522 affected individuals analyzed. This burden of CNVs in CAKUT was compared to 0.2% in population controls [146]. A subsequent analysis of 2824 individuals with CAKUT highlighted an increased prevalence of large, rare, exonic CNVs compared to population-based controls [147]. In this study, genomic abnormalities were identified in 4% of patients with the majority within six pathogenic loci including chromosome 17q12 (RCAD Syndrome), 22q11.2 (DiGeorge Syndrome), 16p11.2, 1q21.1, 4p_ (Wolf-Hirschhorn Syndrome), and 16p13.11. In addition, 90% of the CNVs associated with congenital renal malformations were previously reported to predispose to developmental delay or neuropsychiatric disease, suggesting that there are shared pathways implicated in renal and central nervous system development. Similarly, Handrigan et al. demonstrated that copy number variants at chromosome 16q24.2 are associated with autism spectrum disorder, intellectual disability, and congenital renal malformations [145].
Environmental Factors and Renal Malformations
As mentioned earlier in this chapter, genetic causes of CAKUT can be identified in at most 30% of all cases, which suggests that environmental factors or epigenetic factors explain the remaining cases (Table 8.5). Epigenetics refers to changes in gene expression rather than changes in the gene sequence itself and usually arises from DNA methylation and histone modifications that can silence or enhance gene expression. Maternal obesity and diabetes are major risk factors for CAKUT. From questionnaire data of 562 parents of children with CAKUT, maternal obesity was more highly associated with duplex kidneys and VUR, while maternal diabetes was particularly associated with posterior urethral valves [148]. Dart et al. demonstrated that pregestational diabetes was significantly associated with CAKUT (odds ratio, 1.67; 95% confidence interval, 1.14–2.46), which implies a 67% increased chance of CAKUT in the children of mothers with pregestational diabetes compared to the general population (8.3 vs. 5.0 per 1000 births, respectively) [149]. These findings in humans are strongly supported by animal models in which the pregnant dam has diabetes. Animal models have also shown that maternal undernutrition and uteroplacental insufficiency are risk factors for impaired nephrogenesis and CAKUT. Maternal use of medications, alcohol and illicit drugs like cocaine are also risk factors for impaired nephrogenesis and CAKUT. Maternal use of RAS inhibitors such as ACE inhibitors, Ang II receptor blockers and direct renin inhibitors during pregnancy have been linked to an increased risk of fetopathy in humans. The majority of children manifest hyperechogenic and enlarged kidneys with proximal tubular dysplasia, thickening of arterial walls and multiple small cysts [150, 151]. Maternal supplementation with folate may also decrease the risk of CAKUT. CAKUT phenotypes have been observed in infants exposed to folic acid antagonists in utero, such as carbamazepine, phenytoin, primidone, phenobarbital or valproic acid, suggesting that folate is important for kidney and urinary tract development [63, 152]. Fertility treatment with in vitro fertilization or intrauterine semination are also risk factors for CAKUT, possibly through epigenetic effects on the developing zygote. Identifying the environmental risk factors that predispose to CAKUT needs to be addressed in future research.
Clinical Approach to Renal Malformation
The majority of renal malformations are now diagnosed antenatally, largely because of the widespread use and sensitivity of fetal ultrasound. The sensitivity of prenatal ultrasound screening for renal malformations is about 82% and the mean time at which these malformations are detected is 23 weeks gestation [12]. In general, urinary tract malformations detected antenatally are isolated and present as mild hydronephrosis with no therapeutic consequences. Parents should be reassured. In contrast, bilateral forms of renal agenesis, severe dysgenesis, bilateral ureteric obstruction, or obstruction of the bladder outlet or the urethra can cause severe oligohydramnios as early as 18 weeks. Because amniotic fluid is critical to lung development, oligohydramnios as early as the second trimester can result in lung hypoplasia, a potentially fatal disorder. The oligohydramnios sequence, termed Potter’s syndrome, in its most severe form consists of a typical facial appearance characterized by epicanthal folds, recessed chin, posteriorly rotated, flattened ears and flattened nose, as well as decreased fetal movement, musculoskeletal features including clubfoot and clubhand, hip dislocation, joint contractures and pulmonary hypoplasia. The renal prognosis can be evaluated antenatally. Poor outcome can be predicted when there is severe oligohydramnios, and small and hyperechogenic kidneys. Normative data on kidney dimensions including kidney length from antenatal ultrasound imaging is available from the 15th week of gestation and can be used to determine if a kidney is small, suggesting some type of renal dysplasia, or increased in size, as observed in autosomal recessive PKD (ARPKD) or autosomal dominant PKD (ADPKD) [153]. Indeed, fetal renal hyperechogenicity with renal cysts suggests the fetus may have ARPKD, ADPKD or a mutation in HNF1B. If there is concurrent severe oligohydramnios, then ARPKD is the most likely diagnosis. Amniotic fluid analysis may be of help in some cases if the fetus is suspected to have a trisomy. Trisomy 21, 18, and 13 are all associated with CAKUT [154,155,156]. Antenatal diagnosis and assessment of the renal prognosis are important for consideration of early termination in cases of fatal (or eventually severe renal disease) and to prepare parents and medical staff for the likelihood of neonatal renal insufficiency. Other organ malformations should be sought carefully and, if detected, a karyotype should be done. Some authors have suggested that fetal urine analysis may be helpful to determine fetal renal prognosis and to decide on in utero therapy if congenital lower urinary tract obstruction is noted. Morris et al. performed a systematic review of the literature on fetal urine analysis and concluded that none of the analytes examined had sufficient accuracy to predict poor postnatal renal function [157].
Oligo/anhydramnios from CAKUT is associated with a high incidence of fetal death in utero, severe pulmonary hypoplasia, umbilical cord compression and perinatal asphyxia. The earlier that renal oligohydramnios (ROH) is identified in the pregnancy, the more severe the pulmonary hypoplasia [158, 159]. Pulmonary hypoplasia is defined as deficiency in the number of lung cells, airways and alveoli, leading to a reduced surface area for gas exchange. Postnatally, pulmonary hypoplasia is suspected in cases of ROH when there is respiratory failure with the need for high ventilatory support and the chest x-ray reveals a bell-shaped chest, an elevated diaphragm, and/or pneumomediastinum or pneumothorax. Ultrasound and MRI have been used to assess lung volumes antenatally, but there is no consensus on whether they are reliable predictors of pulmonary hypoplasia postnatally. Therefore, it remains difficult to predict pulmonary prognosis postnatally.
When isolated CAKUT or CAKUT with other organ defects is diagnosed in the fetus, genetic counseling and referral to a multidisciplinary team should be offered. An individualized approach that is in accordance with the parental wishes for more information is advised [160]. A screen for gross chromosomal abnormalities can be performed using a chromosomal microarray from the amniotic fluid (if less than 25 weeks) or from fetal blood (greater than 25 weeks or severe oligohydramnios). If the chromosomal microarray is normal, then a targeted gene panel or WES on the parents and the fetus could be considered. Given the incomplete penetrance and variable expressivity of many mutations implicated in CAKUT, it remains to be demonstrated whether targeted gene panels or WES are beneficial for antenatal decision-making.
The clinical presentation of renal malformation in the postnatal period is dependent on the amount of functioning renal mass, the presence of bilateral urinary tract obstruction and the occurrence of urinary tract infection. Bilateral renal agenesis or severe dysplasia is likely to present soon after birth with decreased renal function. This may be accompanied by oliguria. Alternatively, patients may present with a flank mass or an asymptomatic abnormality detected by renal imaging.
A detailed history and careful physical examination should be carried out on all infants with an antenatally detected renal malformation. An early (within 24 h of life) renal ultrasound is recommended for newborns with a history of oligohydramnios, progressive antenatal hydronephrosis, distended bladder on antenatal sonograms, and bilateral severe hydroureteronephrosis. In male infants, a distended bladder and bilateral hydroureteronephrosis may be secondary to posterior urethral valves, a condition which requires immediate renal imaging and clinical intervention. In general, unilateral anomalies do not require urgent investigation after birth. Renal ultrasound for unilateral hydronephrosis is not recommended within the first 72 h of life because urine output gradually increases over the first 24–48 h of life as renal plasma flow and glomerular filtration rate increase [161]. Thus, the degree of urinary tract dilatation can be underestimated during this period of transition.
A careful examination of the genitalia and the position of the anus are part of the initial assessment since CAKUT can occur in the context of cloacal malformations and with genital tract defects in females and males. The mesonephric duct gives rise to the developing kidneys, urinary tracts and the male genital tracts; therefore, a careful examination of the testes, the epididymis, and the ductus deferens is important. Congenital epididymal cysts are the most frequent anomaly noted in association with mesonephric duct anomalies and are usually asymptomatic. Other male genital duct anomalies that may occur in the context of CAKUT include an absent, ectopic or duplicated ductus deferens. Seminal vesicle cysts may also arise and typically present after puberty as pelvic pain or with urinary symptoms like dysuria, polyuria, or urinary retention [162]. Adjacent to the mesonephric ducts are the paired Müllerian or paramesonephric ducts that give rise to the fallopian tubes, the uterus, the cervix, and the upper two thirds of the vagina. Because Müllerian duct development is tightly linked to the growth and elongation of the mesonephric ducts, CAKUT is also observed with concurrent female Müllerian duct anomalies. Indeed, the Mayer-Rokitansky-Kuster-Hauser syndrome describes women with normal female external genitalia, but Müllerian duct anomalies that include aplasia of the uterus, the cervix, and the upper vagina. In a large cohort of 284 women with this syndrome, roughly 30% of them had associated CAKUT anomalies including renal agenesis, horseshoe kidney, ectopic kidney, and urinary tract defects including duplications [163]. Females with Müllerian ducts anomalies are typically discovered because of primary amenorrhea, dyspareunia, infertility, and/or obstetric complications [164]. In females with CAKUT and a suspected Müllerian duct anomaly, MRI imaging may be indicated to define the anatomical defect with better precision.
Clinical Approach to Specific Malformations
Unilateral Renal Agenesis
A diagnosis of unilateral renal agenesis depends on the certainty that a second kidney does not exist in the pelvis or some other ectopic location. Since absence of one kidney induces compensatory hypertrophy in the existing kidney, the presence of a large kidney on one side suggests the possibility of unilateral renal agenesis. Interestingly, compensatory hypertrophy has been observed to begin as early as 20 weeks of gestation: van Vuuren et al. examined 67 fetuses with a diagnosis of MCDK or unilateral renal agenesis and noted that 87% of the cases of MCDK and 100% of the cases of unilateral renal agenesis exhibited compensatory hypertrophy of the contralateral kidney with kidney length greater than the 95th percentile for gestational age [165]. Since unilateral agenesis is associated with contralateral urinary tract abnormalities including UPJO and VUR in 20–40% of the cases [166, 167], imaging of the contralateral side is suggested. Management of affected patients involves determining the functional status of the contralateral kidney. If the contralateral kidney is normal, the long-term renal functional outcome is usually excellent, although a recent study suggests that some patients may in fact have a poor long-term outcome and require dialysis [25]. It is therefore reasonable to propose that individuals with a single functioning kidney should have their blood pressure measured, urine tested for protein, and renal function measured periodically throughout life. While some have suggested that children with single kidneys should avoid contact/collision sports, at least one study suggests that kidney injuries occur much less frequently than other organ injuries, and thus sports restriction may not be indicated solely on the basis of having a single kidney [168].
Renal Hypoplasia
Unless associated with other malformations, renal hypoplasia can be asymptomatic. Unilateral hypoplasia is often discovered as an incidental finding during an abdominal sonogram or other imaging study. In contrast, patients with bilateral renal hypoplasia are at risk for decreased renal function and CKD.
Renal Dysplasia
The dysplastic kidney is generally smaller than normal. However, cystic elements can contribute to large kidney size, the most extreme example being the MCDK (see below). During the antenatal period, unilateral disease is likely to be discovered as an incidental finding. This may also be the case for bilateral renal dysplasia unless it is associated with oligohydramnios. After birth, bilateral renal dysplasia may limit GFR, causing renal failure that is usually progressive. Postnatal ultrasonography of the dysplastic kidney is characterized by small size, increased echogenicity, loss of corticomedullary differentiation and cortical cysts. Renal dysplasia is strongly associated with dilatation of the upper and lower urinary tract from VUR, posterior urethral valves, and/or other urinary tract obstruction [169]. Accordingly, imaging of the lower urinary tract should be performed to determine whether these abnormalities are present.
Multicystic Dysplastic Kidney
The MCDK presents by ultrasonography as a large cystic non-reniform mass in the renal fossa and by palpation as a flank mass. The MCDK is nonfunctional, a condition that can be demonstrated by imaging with MAG3 or DTPA radionuclide scanning. The MCDK is usually unilateral. If bilateral, it is fatal. Complications of MCDK include hypertension (0.01–0.1%). Wilms tumour and renal cell carcinoma have also been described in MCDK, but the incidence of malignant complications is not significantly different from the general population [170]. In 25% of cases, the contralateral urinary tract is abnormal. Contralateral abnormalities can include rotational or positional anomalies, renal hypoplasia, VUR and UPJO [18]. Contralateral UPJO occurs in 5–10% of cases.
Gradual reduction in renal size and eventual resolution of the mass of the MCDK is common. At two years, an involution in size by ultrasound has been noted in up to 60% of the affected kidneys. Complete disappearance of the MCDK can occur in a minority of patients (3–4%) by the time of birth, and in 20–25% by two years. Increase in the size of MCDK can be seen in some cases. Several reports suggest that if the kidney length of the MCDK is less than 6.2 cm on the initial postnatal US, then complete resolution is likely to occur [171, 172]. The contralateral kidney usually shows compensatory hypertrophy by ultrasound evaluation. If the contralateral kidney does not show hypertrophy, it could be hypoplastic.
Management of patients with MCDK has shifted from routine nephrectomy in the past, to observation and medical therapy. Because of the risk of associated anomalies in the contralateral kidney, the possibility of VUR should be evaluated and blood pressure should be measured. For children with isolated MCDK and a contralateral kidney that is structurally normal with compensatory hypertrophy, the prognosis is excellent. While there exist no evidence-based guidelines for long-term follow-up of these children, a review of published evidence and expert opinion supports serial investigation by ultrasound and urinalysis within the first two years of life to monitor MCDK involution and contralateral renal growth, and then very intermittent examination of renal growth, blood pressure and urine protein excretion through the end of puberty [173]. For the small number of patients with unilateral MCDK who develop hypertension, estimated to be 5.4 out of 1000 children [170], medical therapy is usually effective. Nephrectomy may be curative in resistant cases.
Renal Ectopia
Normally, the kidneys lie on either side of the spine in the lumbar region and are located in the retroperitoneal renal fossae. Rapid caudal growth during embryogenesis results in migration of the developing kidney from the pelvis to the retroperitoneal renal fossa. With ascension, comes a 90° rotation from a horizontal to a vertical position with the renal hilum finally directed medially. Migration and rotation are complete by 8 weeks of gestation.
Simple congenital ectopy refers to a low-lying kidney that failed to ascend normally. It most commonly lies over the pelvic brim or in the pelvis and is termed a pelvic kidney. Less commonly, the kidney may lie on the contralateral side of the body, a state that is termed crossed ectopy without fusion. Clinical presentation can be asymptomatic or symptomatic. Asymptomatic presentation is when the ectopic kidney has been diagnosed coincidentally such as might occur during routine antenatal sonography. Symptomatic presentation occurs with urinary tract infections. Symptoms such as abdominal pain or fever may occur. On examination, an abdominal mass may be palpable. Other presenting features include hematuria, incontinence, renal insufficiency and hypertension [21]. A high incidence of urological abnormalities has been associated with renal ectopia. VUR is the most common, occurring in 20% of crossed renal ectopia and 30% of simple renal ectopia. In bilateral simple renal ectopia, there is a higher incidence of VUR, occurring in 70% of cases. Other associated urological abnormalities include contralateral renal dysplasia (4%), cryptorchidism (5%) and hypospadias (5%) [21]. Reduced renal function is commonly observed by radionuclide scan in the ectopic kidney. Female genital anomalies such as agenesis of the uterus and vagina [174] or unicornuate uterus [175] have also been associated with ectopic kidneys. Other anomalies described include adrenal, cardiac and skeletal anomalies. Clinical assessment should therefore include a careful physical examination for other anomalies. Renal ultrasonography will help with diagnosis and defining the underlying anatomy. A VCUG should be undertaken, particularly if there is hydronephrosis, given the risk of VUR and obstruction. A DMSA scan is also recommended to assess for differential renal function.
Renal Fusion
Renal fusion is defined as the fusion of two kidneys. The most common fusion anomaly is the horseshoe kidney, in which fusion occurs at one pole of each kidney, usually the lower pole. The fused kidney may lie in the midline (symmetric horseshoe kidney) or the fused part may lie lateral to the midline (asymmetric horseshoe kidney). In a crossed fused ectopic kidney, the kidney from one side has crossed the midline to fuse with the kidney on the other side. Fusion is thought to occur before the kidneys ascend from the pelvis to their normal dorsolumbar position. This is usually between the fourth to ninth week of gestation. As a result, fusion anomalies seldom assume the high position of normal kidneys. The blood supply may therefore come from vessels such as the iliac arteries. Abnormal rotation is also associated with early fusion of the developing kidneys. The pelvis of each kidney lies anteriorly and the ureter, therefore, traverses over the isthmus of a horseshoe kidney or the anterior surface of the fused kidney. Ureteric compression may occur due to external compression by a traversing aberrant artery. The majority of patients are asymptomatic. Some, however, develop obstruction which presents with loin pain, hematuria and may be associated with urinary tract infections due to urinary stasis or VUR. Renal calculi may occur in up to 20% of cases [176]. Other associated urological anomalies include ureteral duplication, ectopic ureter and retrocaval ureter. Genital anomalies such as bicornuate and/or septate uterus, hypospadias, and undescended testis have also been described. Associated nonrenal anomalies involve the gastrointestinal tract (anorectal malformations such as imperforate anus, malrotation, and Meckel diverticulum) the central nervous system (neural tube defects), and the skeleton (rib defects, clubfoot, or congenital hip dislocation). Investigations should include static imaging (renal ultrasound) and functional imaging (DMSA scan) and a VCUG.
Notes
References
Annual report 2011. Available from: https://naprtcs.org/registries/annual-report.
Ardissino G, Dacco V, Testa S, Bonaudo R, Claris-Appiani A, Taioli E, et al. Epidemiology of chronic renal failure in children: data from the ItalKid project. Pediatrics. 2003;111(4 Pt 1):e382–7.
Harambat J, van Stralen KJ, Kim JJ, Tizard EJ. Epidemiology of chronic kidney disease in children. Pediatr Nephrol. 2012;27(3):363–73.
Potter EL. Normal and abnormal development of the kidney. Chicago: Year Book Medical Publishers; 1972.
Dinkel E, Ertel M, Dittrich M, Peters H, Berres M, Schulte-Wissermann H. Kidney size in childhood. Sonographical growth charts for kidney length and volume. Pediatr Radiol. 1985;15(1):38–43.
Han BK, Babcock DS. Sonographic measurements and appearance of normal kidneys in children. AJR Am J Roentgenol. 1985;145(3):611–6.
Montini G, Busutti M, Yalcinkaya F, Woolf AS, Weber S, European Society for Paediatric Nephrology Working Group on Congenital Anomalies of the Kidney, et al. A questionnaire survey of radiological diagnosis and management of renal dysplasia in children. J Nephrol. 2018;31(1):95–102.
Crump C, Sundquist J, Winkleby MA, Sundquist K. Preterm birth and risk of chronic kidney disease from childhood into mid-adulthood: national cohort study. BMJ. 2019;365:l1346.
Eriksson JG, Salonen MK, Kajantie E, Osmond C. Prenatal growth and CKD in older adults: longitudinal findings from the Helsinki Birth Cohort Study, 1924-1944. Am J Kidney Dis. 2018;71(1):20–6.
Keller G, Zimmer G, Mall G, Ritz E, Amann K. Nephron number in patients with primary hypertension. N Engl J Med. 2003;348(2):101–8.
Melo BF, Aguiar MB, Bouzada MC, Aguiar RL, Pereira AK, Paixao GM, et al. Early risk factors for neonatal mortality in CAKUT: analysis of 524 affected newborns. Pediatr Nephrol. 2012;27(6):965–72.
Wiesel A, Queisser-Luft A, Clementi M, Bianca S, Stoll C, Group ES. Prenatal detection of congenital renal malformations by fetal ultrasonographic examination: an analysis of 709,030 births in 12 European countries. Eur J Med Genet. 2005;48(2):131–44.
Jain S, Chen F. Developmental pathology of congenital kidney and urinary tract anomalies. Clin Kidney J. 2019;12(3):382–99.
Piscione TD, Rosenblum ND. The malformed kidney: disruption of glomerular and tubular development. Clin Genet. 1999;56(5):341–56.
Queisser-Luft A, Stolz G, Wiesel A, Schlaefer K, Spranger J. Malformations in newborn: results based on 30,940 infants and fetuses from the Mainz congenital birth defect monitoring system (1990-1998). Arch Gynecol Obstet. 2002;266(3):163–7.
Sanna-Cherchi S, Westland R, Ghiggeri GM, Gharavi AG. Genetic basis of human congenital anomalies of the kidney and urinary tract. J Clin Invest. 2018;128(1):4–15.
van der Ven AT, Vivante A, Hildebrandt F. Novel insights into the pathogenesis of monogenic congenital anomalies of the kidney and urinary tract. J Am Soc Nephrol. 2018;29(1):36–50.
Winyard P, Chitty L. Dysplastic and polycystic kidneys: diagnosis, associations and management. Prenat Diagn. 2001;21(11):924–35.
Harris J, Robert E, Kallen B. Epidemiologic characteristics of kidney malformations. Eur J Epidemiol. 2000;16(11):985–92.
Roodhooft AM, Birnholz JC, Holmes LB. Familial nature of congenital absence and severe dysgenesis of both kidneys. N Engl J Med. 1984;310(21):1341–5.
Guarino N, Tadini B, Camardi P, Silvestro L, Lace R, Bianchi M. The incidence of associated urological abnormalities in children with renal ectopia. J Urol. 2004;172(4 Pt 2):1757–9; discussion 9.
Weizer AZ, Silverstein AD, Auge BK, Delvecchio FC, Raj G, Albala DM, et al. Determining the incidence of horseshoe kidney from radiographic data at a single institution. J Urol. 2003;170(5):1722–6.
Quirino IG, Diniz JS, Bouzada MC, Pereira AK, Lopes TJ, Paixao GM, et al. Clinical course of 822 children with prenatally detected nephrouropathies. Clin J Am Soc Nephrol. 2012;7(3):444–51.
Gonzalez Celedon C, Bitsori M, Tullus K. Progression of chronic renal failure in children with dysplastic kidneys. Pediatr Nephrol. 2007;22(7):1014–20.
Sanna-Cherchi S, Ravani P, Corbani V, Parodi S, Haupt R, Piaggio G, et al. Renal outcome in patients with congenital anomalies of the kidney and urinary tract. Kidney Int. 2009;76(5):528–33.
Wuhl E, van Stralen KJ, Verrina E, Bjerre A, Wanner C, Heaf JG, et al. Timing and outcome of renal replacement therapy in patients with congenital malformations of the kidney and urinary tract. Clin J Am Soc Nephrol. 2013;8(1):67–74.
Costantini F. Genetic controls and cellular behaviors in branching morphogenesis of the renal collecting system. Wiley Interdiscip Rev Dev Biol. 2012;1(5):693–713.
Chai OH, Song CH, Park SK, Kim W, Cho ES. Molecular regulation of kidney development. Anat Cell Biol. 2013;46(1):19–31.
Schwarz RD, Stephens FD, Cussen LJ. The pathogenesis of renal dysplasia. II. The significance of lateral and medial ectopy of the ureteric orifice. Invest Urol. 1981;19(2):97–100.
Hu MC, Rosenblum ND. Genetic regulation of branching morphogenesis: lessons learned from loss-of-function phenotypes. Pediatr Res. 2003;54(4):433–8.
Enomoto H, Araki T, Jackman A, Heuckeroth RO, Snider WD, Johnson EM Jr, et al. GFR alpha1-deficient mice have deficits in the enteric nervous system and kidneys. Neuron. 1998;21(2):317–24.
Pichel JG, Shen L, Sheng HZ, Granholm AC, Drago J, Grinberg A, et al. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature. 1996;382(6586):73–6.
Schuchardt A, D’Agati V, Larsson-Blomberg L, Costantini F, Pachnis V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature. 1994;367(6461):380–3.
Schuchardt A, D’Agati V, Pachnis V, Costantini F. Renal agenesis and hypodysplasia in ret-k-mutant mice result from defects in ureteric bud development. Development. 1996;122(6):1919–29.
Maeshima A, Sakurai H, Choi Y, Kitamura S, Vaughn DA, Tee JB, et al. Glial cell-derived neurotrophic factor independent ureteric bud outgrowth from the Wolffian duct. J Am Soc Nephrol. 2007;18(12):3147–55.
Kume T, Deng K, Hogan BL. Murine forkhead/winged helix genes Foxc1 (Mf1) and Foxc2 (Mfh1) are required for the early organogenesis of the kidney and urinary tract. Development. 2000;127(7):1387–95.
Grieshammer U, Le M, Plump AS, Wang F, Tessier-Lavigne M, Martin GR. SLIT2-mediated ROBO2 signaling restricts kidney induction to a single site. Dev Cell. 2004;6(5):709–17.
Miyazaki Y, Oshima K, Fogo A, Hogan BL, Ichikawa I. Bone morphogenetic protein 4 regulates the budding site and elongation of the mouse ureter. J Clin Invest. 2000;105(7):863–73.
Xu PX, Adams J, Peters H, Brown MC, Heaney S, Maas R. Eya1-deficient mice lack ears and kidneys and show abnormal apoptosis of organ primordia. Nat Genet. 1999;23(1):113–7.
Xu PX, Zheng W, Huang L, Maire P, Laclef C, Silvius D. Six1 is required for the early organogenesis of mammalian kidney. Development. 2003;130(14):3085–94.
Nishinakamura R, Matsumoto Y, Nakao K, Nakamura K, Sato A, Copeland NG, et al. Murine homolog of SALL1 is essential for ureteric bud invasion in kidney development. Development. 2001;128(16):3105–15.
Dressler GR, Deutsch U, Chowdhury K, Nornes HO, Gruss P. Pax2, a new murine paired-box-containing gene and its expression in the developing excretory system. Development. 1990;109(4):787–95.
Dziarmaga A, Eccles M, Goodyer P. Suppression of ureteric bud apoptosis rescues nephron endowment and adult renal function in Pax2 mutant mice. J Am Soc Nephrol. 2006;17(6):1568–75.
Brophy PD, Ostrom L, Lang KM, Dressler GR. Regulation of ureteric bud outgrowth by Pax2-dependent activation of the glial derived neurotrophic factor gene. Development. 2001;128(23):4747–56.
Lokmane L, Heliot C, Garcia-Villalba P, Fabre M, Cereghini S. vHNF1 functions in distinct regulatory circuits to control ureteric bud branching and early nephrogenesis. Development. 2010;137(2):347–57.
Gresh L, Fischer E, Reimann A, Tanguy M, Garbay S, Shao X, et al. A transcriptional network in polycystic kidney disease. EMBO J. 2004;23(7):1657–68.
Paces-Fessy M, Fabre M, Lesaulnier C, Cereghini S. Hnf1b and Pax2 cooperate to control different pathways in kidney and ureter morphogenesis. Hum Mol Genet. 2012;21(14):3143–55.
Bose J, Grotewold L, Ruther U. Pallister-Hall syndrome phenotype in mice mutant for Gli3. Hum Mol Genet. 2002;11(9):1129–35.
Hu MC, Mo R, Bhella S, Wilson CW, Chuang PT, Hui CC, et al. GLI3-dependent transcriptional repression of Gli1, Gli2 and kidney patterning genes disrupts renal morphogenesis. Development. 2006;133(3):569–78.
Cain JE, Islam E, Haxho F, Blake J, Rosenblum ND. GLI3 repressor controls functional development of the mouse ureter. J Clin Invest. 2011;121(3):1199–206.
Yu J, Carroll TJ, McMahon AP. Sonic hedgehog regulates proliferation and differentiation of mesenchymal cells in the mouse metanephric kidney. Development. 2002;129(22):5301–12.
Sheybani-Deloui S, Chi L, Staite MV, Cain JE, Nieman BJ, Henkelman RM, et al. Activated hedgehog-GLI signaling causes congenital ureteropelvic junction obstruction. J Am Soc Nephrol. 2018;29(2):532–44.
Yosypiv IV. Renin-angiotensin system in ureteric bud branching morphogenesis: implications for kidney disease. Pediatr Nephrol. 2014;29(4):609–20.
Yosypiv IV. Renin-angiotensin system in ureteric bud branching morphogenesis: insights into the mechanisms. Pediatr Nephrol. 2011;26(9):1499–512.
Nishimura H, Yerkes E, Hohenfellner K, Miyazaki Y, Ma J, Hunley TE, et al. Role of the angiotensin type 2 receptor gene in congenital anomalies of the kidney and urinary tract, CAKUT, of mice and men. Mol Cell. 1999;3(1):1–10.
Gubler MC, Antignac C. Renin-angiotensin system in kidney development: renal tubular dysgenesis. Kidney Int. 2010;77(5):400–6.
Batourina E, Choi C, Paragas N, Bello N, Hensle T, Costantini FD, et al. Distal ureter morphogenesis depends on epithelial cell remodeling mediated by vitamin A and Ret. Nat Genet. 2002;32(1):109–15.
Batourina E, Gim S, Bello N, Shy M, Clagett-Dame M, Srinivas S, et al. Vitamin A controls epithelial/mesenchymal interactions through Ret expression. Nat Genet. 2001;27(1):74–8.
Mendelsohn C, Batourina E, Fung S, Gilbert T, Dodd J. Stromal cells mediate retinoid-dependent functions essential for renal development. Development. 1999;126(6):1139–48.
Lelievre-Pegorier M, Vilar J, Ferrier ML, Moreau E, Freund N, Gilbert T, et al. Mild vitamin A deficiency leads to inborn nephron deficit in the rat. Kidney Int. 1998;54(5):1455–62.
Goodyer P, Kurpad A, Rekha S, Muthayya S, Dwarkanath P, Iyengar A, et al. Effects of maternal vitamin A status on kidney development: a pilot study. Pediatr Nephrol. 2007;22(2):209–14.
Czeizel AE, Dobo M, Vargha P. Hungarian cohort-controlled trial of periconceptional multivitamin supplementation shows a reduction in certain congenital abnormalities. Birth Defects Res A Clin Mol Teratol. 2004;70(11):853–61.
Hernandez-Diaz S, Werler MM, Walker AM, Mitchell AA. Folic acid antagonists during pregnancy and the risk of birth defects. N Engl J Med. 2000;343(22):1608–14.
Nyengaard JR, Bendtsen TF. Glomerular number and size in relation to age, kidney weight, and body surface in normal man. Anat Rec. 1992;232(2):194–201.
Weber S, Moriniere V, Knuppel T, Charbit M, Dusek J, Ghiggeri GM, et al. Prevalence of mutations in renal developmental genes in children with renal hypodysplasia: results of the ESCAPE study. J Am Soc Nephrol. 2006;17(10):2864–70.
Schwaderer AL, Bates CM, McHugh KM, McBride KL. Renal anomalies in family members of infants with bilateral renal agenesis/adysplasia. Pediatr Nephrol. 2007;22(1):52–6.
Salomon R, Tellier AL, Attie-Bitach T, Amiel J, Vekemans M, Lyonnet S, et al. PAX2 mutations in oligomeganephronia. Kidney Int. 2001;59(2):457–62.
Ulinski T, Lescure S, Beaufils S, Guigonis V, Decramer S, Morin D, et al. Renal phenotypes related to hepatocyte nuclear factor-1beta (TCF2) mutations in a pediatric cohort. J Am Soc Nephrol. 2006;17(2):497–503.
Chatterjee R, Ramos E, Hoffman M, VanWinkle J, Martin DR, Davis TK, et al. Traditional and targeted exome sequencing reveals common, rare and novel functional deleterious variants in RET-signaling complex in a cohort of living US patients with urinary tract malformations. Hum Genet. 2012;131(11):1725–38.
Jeanpierre C, Mace G, Parisot M, Moriniere V, Pawtowsky A, Benabou M, et al. RET and GDNF mutations are rare in fetuses with renal agenesis or other severe kidney development defects. J Med Genet. 2011;48(7):497–504.
Skinner MA, Safford SD, Reeves JG, Jackson ME, Freemerman AJ. Renal aplasia in humans is associated with RET mutations. Am J Hum Genet. 2008;82(2):344–51.
Yang Y, Houle AM, Letendre J, Richter A. RET Gly691Ser mutation is associated with primary vesicoureteral reflux in the French-Canadian population from Quebec. Hum Mutat. 2008;29(5):695–702.
Hwang DY, Dworschak GC, Kohl S, Saisawat P, Vivante A, Hilger AC, et al. Mutations in 12 known dominant disease-causing genes clarify many congenital anomalies of the kidney and urinary tract. Kidney Int. 2014;85(6):1429–33.
Fraser FC, Ling D, Clogg D, Nogrady B. Genetic aspects of the BOR syndrome—branchial fistulas, ear pits, hearing loss, and renal anomalies. Am J Med Genet. 1978;2(3):241–52.
Melnick M, Bixler D, Silk K, Yune H, Nance WE. Autosomal dominant branchiootorenal dysplasia. Birth Defects Orig Artic Ser. 1975;11(5):121–8.
Chang EH, Menezes M, Meyer NC, Cucci RA, Vervoort VS, Schwartz CE, et al. Branchio-oto-renal syndrome: the mutation spectrum in EYA1 and its phenotypic consequences. Hum Mutat. 2004;23(6):582–9.
Chen A, Francis M, Ni L, Cremers CW, Kimberling WJ, Sato Y, et al. Phenotypic manifestations of branchio-oto-renal syndrome. Am J Med Genet. 1995;58(4):365–70.
Fraser FC, Sproule JR, Halal F. Frequency of the branchio-oto-renal (BOR) syndrome in children with profound hearing loss. Am J Med Genet. 1980;7(3):341–9.
Hoskins BE, Cramer CH, Silvius D, Zou D, Raymond RM, Orten DJ, et al. Transcription factor SIX5 is mutated in patients with branchio-oto-renal syndrome. Am J Hum Genet. 2007;80(4):800–4.
Kochhar A, Orten DJ, Sorensen JL, Fischer SM, Cremers CW, Kimberling WJ, et al. SIX1 mutation screening in 247 branchio-oto-renal syndrome families: a recurrent missense mutation associated with BOR. Hum Mutat. 2008;29(4):565.
Krug P, Moriniere V, Marlin S, Koubi V, Gabriel HD, Colin E, et al. Mutation screening of the EYA1, SIX1, and SIX5 genes in a large cohort of patients harboring branchio-oto-renal syndrome calls into question the pathogenic role of SIX5 mutations. Hum Mutat. 2011;32(2):183–90.
Li X, Oghi KA, Zhang J, Krones A, Bush KT, Glass CK, et al. Eya protein phosphatase activity regulates Six1-Dach-Eya transcriptional effects in mammalian organogenesis. Nature. 2003;426(6964):247–54.
Ruf RG, Xu PX, Silvius D, Otto EA, Beekmann F, Muerb UT, et al. SIX1 mutations cause branchio-oto-renal syndrome by disruption of EYA1-SIX1-DNA complexes. Proc Natl Acad Sci U S A. 2004;101(21):8090–5.
Sajithlal G, Zou D, Silvius D, Xu PX. Eya 1 acts as a critical regulator for specifying the metanephric mesenchyme. Dev Biol. 2005;284(2):323–36.
Miller EM, Hopkin R, Bao L, Ware SM. Implications for genotype-phenotype predictions in Townes-Brocks syndrome: case report of a novel SALL1 deletion and review of the literature. Am J Med Genet A. 2012;158A(3):533–40.
O’Callaghan M, Young ID. The Townes-Brocks syndrome. J Med Genet. 1990;27(7):457–61.
Townes PL, Brocks ER. Hereditary syndrome of imperforate anus with hand, foot, and ear anomalies. J Pediatr. 1972;81(2):321–6.
Kurnit DM, Steele MW, Pinsky L, Dibbins A. Autosomal dominant transmission of a syndrome of anal, ear, renal, and radial congenital malformations. J Pediatr. 1978;93(2):270–3.
Martinez-Frias ML, Bermejo Sanchez E, Arroyo Carrera I, Perez Fernandez JL, Pardo Romero M, Buron Martinez E, et al. [The Townes-Brocks syndrome in Spain: the epidemiological aspects in a consecutive series of cases]. An Esp Pediatr. 1999;50(1):57–60.
Kohlhase J. SALL1 mutations in Townes-Brocks syndrome and related disorders. Hum Mutat. 2000;16(6):460–6.
Kohlhase J, Wischermann A, Reichenbach H, Froster U, Engel W. Mutations in the SALL1 putative transcription factor gene cause Townes-Brocks syndrome. Nat Genet. 1998;18(1):81–3.
Marlin S, Blanchard S, Slim R, Lacombe D, Denoyelle F, Alessandri JL, et al. Townes-Brocks syndrome: detection of a SALL1 mutation hot spot and evidence for a position effect in one patient. Hum Mutat. 1999;14(5):377–86.
Solomon BD, Pineda-Alvarez DE, Raam MS, Bous SM, Keaton AA, Velez JI, et al. Analysis of component findings in 79 patients diagnosed with VACTERL association. Am J Med Genet A. 2010;152A(9):2236–44.
Rittler M, Paz JE, Castilla EE. VACTERL association, epidemiologic definition and delineation. Am J Med Genet. 1996;63(4):529–36.
Brown AK, Roddam AW, Spitz L, Ward SJ. Oesophageal atresia, related malformations, and medical problems: a family study. Am J Med Genet. 1999;85(1):31–7.
van Rooij IA, Wijers CH, Rieu PN, Hendriks HS, Brouwers MM, Knoers NV, et al. Maternal and paternal risk factors for anorectal malformations: a Dutch case-control study. Birth Defects Res A Clin Mol Teratol. 2010;88(3):152–8.
Garcia-Barcelo MM, Wong KK, Lui VC, Yuan ZW, So MT, Ngan ES, et al. Identification of a HOXD13 mutation in a VACTERL patient. Am J Med Genet A. 2008;146A(24):3181–5.
Stankiewicz P, Sen P, Bhatt SS, Storer M, Xia Z, Bejjani BA, et al. Genomic and genic deletions of the FOX gene cluster on 16q24.1 and inactivating mutations of FOXF1 cause alveolar capillary dysplasia and other malformations. Am J Hum Genet. 2009;84(6):780–91.
Tongsong T, Wanapirak C, Piyamongkol W, Sudasana J. Prenatal sonographic diagnosis of VATER association. J Clin Ultrasound. 1999;27(7):378–84.
Murphy-Kaulbeck L, Dodds L, Joseph KS, Van den Hof M. Single umbilical artery risk factors and pregnancy outcomes. Obstet Gynecol. 2010;116(4):843–50.
Faivre L, Portnoi MF, Pals G, Stoppa-Lyonnet D, Le Merrer M, Thauvin-Robinet C, et al. Should chromosome breakage studies be performed in patients with VACTERL association? Am J Med Genet A. 2005;137(1):55–8.
Weaver RG, Cashwell LF, Lorentz W, Whiteman D, Geisinger KR, Ball M. Optic nerve coloboma associated with renal disease. Am J Med Genet. 1988;29(3):597–605.
Bower M, Salomon R, Allanson J, Antignac C, Benedicenti F, Benetti E, et al. Update of PAX2 mutations in renal coloboma syndrome and establishment of a locus-specific database. Hum Mutat. 2012;33(3):457–66.
Fletcher J, Hu M, Berman Y, Collins F, Grigg J, McIver M, et al. Multicystic dysplastic kidney and variable phenotype in a family with a novel deletion mutation of PAX2. J Am Soc Nephrol. 2005;16(9):2754–61.
Dureau P, Attie-Bitach T, Salomon R, Bettembourg O, Amiel J, Uteza Y, et al. Renal coloboma syndrome. Ophthalmology. 2001;108(10):1912–6.
Parsa CF, Silva ED, Sundin OH, Goldberg MF, De Jong MR, Sunness JS, et al. Redefining papillorenal syndrome: an underdiagnosed cause of ocular and renal morbidity. Ophthalmology. 2001;108(4):738–49.
Eccles MR, Schimmenti LA. Renal-coloboma syndrome: a multi-system developmental disorder caused by PAX2 mutations. Clin Genet. 1999;56(1):1–9.
Schimmenti LA, Cunliffe HE, McNoe LA, Ward TA, French MC, Shim HH, et al. Further delineation of renal-coloboma syndrome in patients with extreme variability of phenotype and identical PAX2 mutations. Am J Hum Genet. 1997;60(4):869–78.
Sanyanusin P, Schimmenti LA, McNoe LA, Ward TA, Pierpont ME, Sullivan MJ, et al. Mutation of the PAX2 gene in a family with optic nerve colobomas, renal anomalies and vesicoureteral reflux. Nat Genet. 1995;9(4):358–64.
Barua M, Stellacci E, Stella L, Weins A, Genovese G, Muto V, et al. Mutations in PAX2 associate with adult-onset FSGS. J Am Soc Nephrol. 2014;25(9):1942–53.
Vivante A, Chacham OS, Shril S, Schreiber R, Mane SM, Pode-Shakked B, et al. Dominant PAX2 mutations may cause steroid-resistant nephrotic syndrome and FSGS in children. Pediatr Nephrol. 2019;34(9):1607–13.
Coffinier C, Thepot D, Babinet C, Yaniv M, Barra J. Essential role for the homeoprotein vHNF1/HNF1beta in visceral endoderm differentiation. Development. 1999;126(21):4785–94.
Kolatsi-Joannou M, Bingham C, Ellard S, Bulman MP, Allen LIS, Hattersley AT, et al. Hepatocyte nuclear factor-1beta: a new kindred with renal cysts and diabetes and gene expression in normal human development. J Am Soc Nephrol. 2001;12(10):2175–80.
Bellanne-Chantelot C, Chauveau D, Gautier JF, Dubois-Laforgue D, Clauin S, Beaufils S, et al. Clinical spectrum associated with hepatocyte nuclear factor-1beta mutations. Ann Intern Med. 2004;140(7):510–7.
Bellanne-Chantelot C, Clauin S, Chauveau D, Collin P, Daumont M, Douillard C, et al. Large genomic rearrangements in the hepatocyte nuclear factor-1beta (TCF2) gene are the most frequent cause of maturity-onset diabetes of the young type 5. Diabetes. 2005;54(11):3126–32.
Edghill EL, Bingham C, Ellard S, Hattersley AT. Mutations in hepatocyte nuclear factor-1beta and their related phenotypes. J Med Genet. 2006;43(1):84–90.
Bingham C, Bulman MP, Ellard S, Allen LI, Lipkin GW, Hoff WG, et al. Mutations in the hepatocyte nuclear factor-1beta gene are associated with familial hypoplastic glomerulocystic kidney disease. Am J Hum Genet. 2001;68(1):219–24.
Bingham C, Ellard S, Allen L, Bulman M, Shepherd M, Frayling T, et al. Abnormal nephron development associated with a frameshift mutation in the transcription factor hepatocyte nuclear factor-1 beta. Kidney Int. 2000;57(3):898–907.
Lindner TH, Njolstad PR, Horikawa Y, Bostad L, Bell GI, Sovik O. A novel syndrome of diabetes mellitus, renal dysfunction and genital malformation associated with a partial deletion of the pseudo-POU domain of hepatocyte nuclear factor-1beta. Hum Mol Genet. 1999;8(11):2001–8.
Rizzoni G, Loirat C, Levy M, Milanesi C, Zachello G, Mathieu H. Familial hypoplastic glomerulocystic kidney. A new entity? Clin Nephrol. 1982;18(5):263–8.
Decramer S, Parant O, Beaufils S, Clauin S, Guillou C, Kessler S, et al. Anomalies of the TCF2 gene are the main cause of fetal bilateral hyperechogenic kidneys. J Am Soc Nephrol. 2007;18(3):923–33.
Adalat S, Woolf AS, Johnstone KA, Wirsing A, Harries LW, Long DA, et al. HNF1B mutations associate with hypomagnesemia and renal magnesium wasting. J Am Soc Nephrol. 2009;20(5):1123–31.
Kikuchi R, Kusuhara H, Hattori N, Kim I, Shiota K, Gonzalez FJ, et al. Regulation of tissue-specific expression of the human and mouse urate transporter 1 gene by hepatocyte nuclear factor 1 alpha/beta and DNA methylation. Mol Pharmacol. 2007;72(6):1619–25.
Okorn C, Goertz A, Vester U, Beck BB, Bergmann C, Habbig S, et al. HNF1B nephropathy has a slow-progressive phenotype in childhood-with the exception of very early onset cases: results of the German Multicenter HNF1B Childhood Registry. Pediatr Nephrol. 2019;34(6):1065–75.
Faguer S, Chassaing N, Bandin F, Prouheze C, Garnier A, Casemayou A, et al. The HNF1B score is a simple tool to select patients for HNF1B gene analysis. Kidney Int. 2014;86(5):1007–15.
Karstensen HG, Tommerup N. Isolated and syndromic forms of congenital anosmia. Clin Genet. 2012;81(3):210–5.
Tsai PS, Gill JC. Mechanisms of disease: insights into X-linked and autosomal-dominant Kallmann syndrome. Nat Clin Pract Endocrinol Metab. 2006;2(3):160–71.
Schwanzel-Fukuda M, Bick D, Pfaff DW. Luteinizing hormone-releasing hormone (LHRH)-expressing cells do not migrate normally in an inherited hypogonadal (Kallmann) syndrome. Brain Res Mol Brain Res. 1989;6(4):311–26.
Cariboni A, Andre V, Chauvet S, Cassatella D, Davidson K, Caramello A, et al. Dysfunctional SEMA3E signaling underlies gonadotropin-releasing hormone neuron deficiency in Kallmann syndrome. J Clin Invest. 2015;125(6):2413–28.
Falardeau J, Chung WC, Beenken A, Raivio T, Plummer L, Sidis Y, et al. Decreased FGF8 signaling causes deficiency of gonadotropin-releasing hormone in humans and mice. J Clin Invest. 2008;118(8):2822–31.
Dode C, Levilliers J, Dupont JM, De Paepe A, Le Du N, Soussi-Yanicostas N, et al. Loss-of-function mutations in FGFR1 cause autosomal dominant Kallmann syndrome. Nat Genet. 2003;33(4):463–5.
Dode C, Teixeira L, Levilliers J, Fouveaut C, Bouchard P, Kottler ML, et al. Kallmann syndrome: mutations in the genes encoding prokineticin-2 and prokineticin receptor-2. PLoS Genet. 2006;2(10):e175.
Costa-Barbosa FA, Balasubramanian R, Keefe KW, Shaw ND, Al-Tassan N, Plummer L, et al. Prioritizing genetic testing in patients with Kallmann syndrome using clinical phenotypes. J Clin Endocrinol Metab. 2013;98(5):E943–53.
Duke VM, Winyard PJ, Thorogood P, Soothill P, Bouloux PM, Woolf AS. KAL, a gene mutated in Kallmann’s syndrome, is expressed in the first trimester of human development. Mol Cell Endocrinol. 1995;110(1–2):73–9.
John U, Benz K, Hubler A, Patzer L, Zenker M, Amann K. Oligohydramnios associated with sonographically normal kidneys. Urology. 2012;79(5):1155–7.
Kumar D, Moss G, Primhak R, Coombs R. Congenital renal tubular dysplasia and skull ossification defects similar to teratogenic effects of angiotensin converting enzyme (ACE) inhibitors. J Med Genet. 1997;34(7):541–5.
Mahieu-Caputo D, Dommergues M, Delezoide AL, Lacoste M, Cai Y, Narcy F, et al. Twin-to-twin transfusion syndrome. Role of the fetal renin-angiotensin system. Am J Pathol. 2000;156(2):629–36.
Barr M Jr, Cohen MM Jr. ACE inhibitor fetopathy and hypocalvaria: the kidney-skull connection. Teratology. 1991;44(5):485–95.
Gribouval O, Gonzales M, Neuhaus T, Aziza J, Bieth E, Laurent N, et al. Mutations in genes in the renin-angiotensin system are associated with autosomal recessive renal tubular dysgenesis. Nat Genet. 2005;37(9):964–8.
Uematsu M, Sakamoto O, Ohura T, Shimizu N, Satomura K, Tsuchiya S. A further case of renal tubular dysgenesis surviving the neonatal period. Eur J Pediatr. 2009;168(2):207–9.
Jongmans MC, Admiraal RJ, van der Donk KP, Vissers LE, Baas AF, Kapusta L, et al. CHARGE syndrome: the phenotypic spectrum of mutations in the CHD7 gene. J Med Genet. 2006;43(4):306–14.
Brockschmidt A, Chung B, Weber S, Fischer DC, Kolatsi-Joannou M, Christ L, et al. CHD1L: a new candidate gene for congenital anomalies of the kidneys and urinary tract (CAKUT). Nephrol Dial Transplant. 2012;27(6):2355–64.
Ma NF, Hu L, Fung JM, Xie D, Zheng BJ, Chen L, et al. Isolation and characterization of a novel oncogene, amplified in liver cancer 1, within a commonly amplified region at 1q21 in hepatocellular carcinoma. Hepatology. 2008;47(2):503–10.
Sanna-Cherchi S, Sampogna RV, Papeta N, Burgess KE, Nees SN, Perry BJ, et al. Mutations in DSTYK and dominant urinary tract malformations. N Engl J Med. 2013;369(7):621–9.
Handrigan GR, Chitayat D, Lionel AC, Pinsk M, Vaags AK, Marshall CR, et al. Deletions in 16q24.2 are associated with autism spectrum disorder, intellectual disability and congenital renal malformation. J Med Genet. 2013;50(3):163–73.
Sanna-Cherchi S, Kiryluk K, Burgess KE, Bodria M, Sampson MG, Hadley D, et al. Copy-number disorders are a common cause of congenital kidney malformations. Am J Hum Genet. 2012;91(6):987–97.
Verbitsky M, Westland R, Perez A, Kiryluk K, Liu Q, Krithivasan P, et al. The copy number variation landscape of congenital anomalies of the kidney and urinary tract. Nat Genet. 2019;51(1):117–27.
Groen In’t Woud S, Renkema KY, Schreuder MF, Wijers CH, van der Zanden LF, Knoers NV, et al. Maternal risk factors involved in specific congenital anomalies of the kidney and urinary tract: a case-control study. Birth Defects Res A Clin Mol Teratol. 2016;106(7):596–603.
Dart AB, Ruth CA, Sellers EA, Au W, Dean HJ. Maternal diabetes mellitus and congenital anomalies of the kidney and urinary tract (CAKUT) in the child. Am J Kidney Dis. 2015;65(5):684–91.
Cooper WO, Hernandez-Diaz S, Arbogast PG, Dudley JA, Dyer S, Gideon PS, et al. Major congenital malformations after first-trimester exposure to ACE inhibitors. N Engl J Med. 2006;354(23):2443–51.
Martinovic J, Benachi A, Laurent N, Daikha-Dahmane F, Gubler MC. Fetal toxic effects and angiotensin-II-receptor antagonists. Lancet. 2001;358(9277):241–2.
Huot C, Gauthier M, Lebel M, Larbrisseau A. Congenital malformations associated with maternal use of valproic acid. Can J Neurol Sci. 1987;14(3):290–3.
van Vuuren SH, Damen-Elias HA, Stigter RH, van der Doef R, Goldschmeding R, de Jong TP, et al. Size and volume charts of fetal kidney, renal pelvis and adrenal gland. Ultrasound Obstet Gynecol. 2012;40(6):659–64.
Cereda A, Carey JC. The trisomy 18 syndrome. Orphanet J Rare Dis. 2012;7:81.
Iliopoulos D, Sekerli E, Vassiliou G, Sidiropoulou V, Topalidis A, Dimopoulou D, et al. Patau syndrome with a long survival (146 months): a clinical report and review of literature. Am J Med Genet A. 2006;140(1):92–3.
Kupferman JC, Druschel CM, Kupchik GS. Increased prevalence of renal and urinary tract anomalies in children with Down syndrome. Pediatrics. 2009;124(4):e615–21.
Morris RK, Quinlan-Jones E, Kilby MD, Khan KS. Systematic review of accuracy of fetal urine analysis to predict poor postnatal renal function in cases of congenital urinary tract obstruction. Prenat Diagn. 2007;27(10):900–11.
Klaassen I, Neuhaus TJ, Mueller-Wiefel DE, Kemper MJ. Antenatal oligohydramnios of renal origin: long-term outcome. Nephrol Dial Transplant. 2007;22(2):432–9.
Mehler K, Beck BB, Kaul I, Rahimi G, Hoppe B, Kribs A. Respiratory and general outcome in neonates with renal oligohydramnios—a single-centre experience. Nephrol Dial Transplant. 2011;26(11):3514–22.
Talati AN, Webster CM, Vora NL. Prenatal genetic considerations of congenital anomalies of the kidney and urinary tract (CAKUT). Prenat Diagn. 2019;39(9):679–92.
Bueva A, Guignard JP. Renal function in preterm neonates. Pediatr Res. 1994;36(5):572–7.
Mure PY, Sabatier-Laval E, Dodat H. [Malformations of male internal genitalia originating from the Wolffian duct]. Arch Pediatr. 1997;4(2):163–9.
Oppelt PG, Lermann J, Strick R, Dittrich R, Strissel P, Rettig I, et al. Malformations in a cohort of 284 women with Mayer-Rokitansky-Kuster-Hauser syndrome (MRKH). Reprod Biol Endocrinol. 2012;10:57.
Behr SC, Courtier JL, Qayyum A. Imaging of mullerian duct anomalies. Radiographics. 2012;32(6):E233–50.
van Vuuren SH, van der Doef R, Cohen-Overbeek TE, Goldschmeding R, Pistorius LR, de Jong TP. Compensatory enlargement of a solitary functioning kidney during fetal development. Ultrasound Obstet Gynecol. 2012;40(6):665–8.
Cascio S, Paran S, Puri P. Associated urological anomalies in children with unilateral renal agenesis. J Urol. 1999;162(3 Pt 2):1081–3.
Kaneyama K, Yamataka A, Satake S, Yanai T, Lane GJ, Kaneko K, et al. Associated urologic anomalies in children with solitary kidney. J Pediatr Surg. 2004;39(1):85–7.
Grinsell MM, Butz K, Gurka MJ, Gurka KK, Norwood V. Sport-related kidney injury among high school athletes. Pediatrics. 2012;130(1):e40–5.
Shibata S, Nagata M. Pathogenesis of human renal dysplasia: an alternative scenario to the major theories. Pediatr Int. 2003;45(5):605–9.
Narchi H. Risk of hypertension with multicystic kidney disease: a systematic review. Arch Dis Child. 2005;90(9):921–4.
Hains DS, Bates CM, Ingraham S, Schwaderer AL. Management and etiology of the unilateral multicystic dysplastic kidney: a review. Pediatr Nephrol. 2009;24(2):233–41.
Rabelo EA, Oliveira EA, Silva GS, Pezzuti IL, Tatsuo ES. Predictive factors of ultrasonographic involution of prenatally detected multicystic dysplastic kidney. BJU Int. 2005;95(6):868–71.
Jawa NA, Rosenblum ND, Radhakrishnan S, Pearl RJ, Levin L, Matsuda-Abedini M. Choosing wisely: improving the quality of care by reducing unnecessary testing in children with isolated unilateral multicystic dysplastic kidney or solitary kidney. Pediatrics. 2021;148(2):e2020035550.
D’Alberton A, Reschini E, Ferrari N, Candiani P. Prevalence of urinary tract abnormalities in a large series of patients with uterovaginal atresia. J Urol. 1981;126(5):623–4.
Fedele L, Bianchi S, Agnoli B, Tozzi L, Vignali M. Urinary tract anomalies associated with unicornuate uterus. J Urol. 1996;155(3):847–8.
Raj GV, Auge BK, Assimos D, Preminger GM. Metabolic abnormalities associated with renal calculi in patients with horseshoe kidneys. J Endourol. 2004;18(2):157–61.
Oda T, Elkahloun AG, Pike BL, Okajima K, Krantz ID, Genin A, et al. Mutations in the human Jagged1 gene are responsible for Alagille syndrome. Nat Genet. 1997;16(3):235–42.
Kamath BM, Podkameni G, Hutchinson AL, Leonard LD, Gerfen J, Krantz ID, et al. Renal anomalies in Alagille syndrome: a disease-defining feature. Am J Med Genet A. 2012;158A(1):85–9.
McDaniell R, Warthen DM, Sanchez-Lara PA, Pai A, Krantz ID, Piccoli DA, et al. NOTCH2 mutations cause Alagille syndrome, a heterogeneous disorder of the notch signaling pathway. Am J Hum Genet. 2006;79(1):169–73.
Wilkie AO, Slaney SF, Oldridge M, Poole MD, Ashworth GJ, Hockley AD, et al. Apert syndrome results from localized mutations of FGFR2 and is allelic with Crouzon syndrome. Nat Genet. 1995;9(2):165–72.
Seyedzadeh A, Kompani F, Esmailie E, Samadzadeh S, Farshchi B. High-grade vesicoureteral reflux in Pfeiffer syndrome. Urol J. 2008;5(3):200–2.
Hatada I, Ohashi H, Fukushima Y, Kaneko Y, Inoue M, Komoto Y, et al. An imprinted gene p57KIP2 is mutated in Beckwith-Wiedemann syndrome. Nat Genet. 1996;14(2):171–3.
Goldman M, Smith A, Shuman C, Caluseriu O, Wei C, Steele L, et al. Renal abnormalities in beckwith-wiedemann syndrome are associated with 11p15.5 uniparental disomy. J Am Soc Nephrol. 2002;13(8):2077–84.
Abdelhak S, Kalatzis V, Heilig R, Compain S, Samson D, Vincent C, et al. Clustering of mutations responsible for branchio-oto-renal (BOR) syndrome in the eyes absent homologous region (eyaHR) of EYA1. Hum Mol Genet. 1997;6(13):2247–55.
Houston CS, Opitz JM, Spranger JW, Macpherson RI, Reed MH, Gilbert EF, et al. The campomelic syndrome: review, report of 17 cases, and follow-up on the currently 17-year-old boy first reported by Maroteaux et al in 1971. Am J Med Genet. 1983;15(1):3–28.
Wagner T, Wirth J, Meyer J, Zabel B, Held M, Zimmer J, et al. Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell. 1994;79(6):1111–20.
Sakaki-Yumoto M, Kobayashi C, Sato A, Fujimura S, Matsumoto Y, Takasato M, et al. The murine homolog of SALL4, a causative gene in Okihiro syndrome, is essential for embryonic stem cell proliferation, and cooperates with Sall1 in anorectal, heart, brain and kidney development. Development. 2006;133(15):3005–13.
McGregor L, Makela V, Darling SM, Vrontou S, Chalepakis G, Roberts C, et al. Fraser syndrome and mouse blebbed phenotype caused by mutations in FRAS1/Fras1 encoding a putative extracellular matrix protein. Nat Genet. 2003;34(2):203–8.
Alazami AM, Shaheen R, Alzahrani F, Snape K, Saggar A, Brinkmann B, et al. FREM1 mutations cause bifid nose, renal agenesis, and anorectal malformations syndrome. Am J Hum Genet. 2009;85(3):414–8.
Jadeja S, Smyth I, Pitera JE, Taylor MS, van Haelst M, Bentley E, et al. Identification of a new gene mutated in Fraser syndrome and mouse myelencephalic blebs. Nat Genet. 2005;37(5):520–5.
Vogel MJ, van Zon P, Brueton L, Gijzen M, van Tuil MC, Cox P, et al. Mutations in GRIP1 cause Fraser syndrome. J Med Genet. 2012;49(5):303–6.
Peco-Antic A, Bogdanovic R, Paripovic D, Paripovic A, Kocev N, Golubovic E, et al. Epidemiology of chronic kidney disease in children in Serbia. Nephrol Dial Transplant. 2012;27(5):1978–84.
Chenouard A, Isidor B, Allain-Launay E, Moreau A, Le Bideau M, Roussey G. Renal phenotypic variability in HDR syndrome: glomerular nephropathy as a novel finding. Eur J Pediatr. 2013;172(1):107–10.
Franco B, Guioli S, Pragliola A, Incerti B, Bardoni B, Tonlorenzi R, et al. A gene deleted in Kallmann’s syndrome shares homology with neural cell adhesion and axonal path-finding molecules. Nature. 1991;353(6344):529–36.
Bamshad M, Lin RC, Law DJ, Watkins WC, Krakowiak PA, Moore ME, et al. Mutations in human TBX3 alter limb, apocrine and genital development in ulnar-mammary syndrome. Nat Genet. 1997;16(3):311–5.
Kang S, Graham JM Jr, Olney AH, Biesecker LG. GLI3 frameshift mutations cause autosomal dominant Pallister-Hall syndrome. Nat Genet. 1997;15(3):266–8.
Bohn S, Thomas H, Turan G, Ellard S, Bingham C, Hattersley AT, et al. Distinct molecular and morphogenetic properties of mutations in the human HNF1beta gene that lead to defective kidney development. J Am Soc Nephrol. 2003;14(8):2033–41.
Pilia G, Hughes-Benzie RM, MacKenzie A, Baybayan P, Chen EY, Huber R, et al. Mutations in GPC3, a glypican gene, cause the Simpson-Golabi-Behmel overgrowth syndrome. Nat Genet. 1996;12(3):241–7.
Tint GS, Irons M, Elias ER, Batta AK, Frieden R, Chen TS, et al. Defective cholesterol biosynthesis associated with the Smith-Lemli-Opitz syndrome. N Engl J Med. 1994;330(2):107–13.
Preuss N, Brosius U, Biermanns M, Muntau AC, Conzelmann E, Gartner J. PEX1 mutations in complementation group 1 of Zellweger spectrum patients correlate with severity of disease. Pediatr Res. 2002;51(6):706–14.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Rosenblum, N.D., Gupta, I.R. (2023). Disorders of Kidney Formation. In: Schaefer, F., Greenbaum, L.A. (eds) Pediatric Kidney Disease. Springer, Cham. https://doi.org/10.1007/978-3-031-11665-0_8
Download citation
DOI: https://doi.org/10.1007/978-3-031-11665-0_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-11664-3
Online ISBN: 978-3-031-11665-0
eBook Packages: MedicineMedicine (R0)