Abstract
Tyrosine kinases inhibitors (TKIs) are small molecules that have been developed as targeted therapies for various medical conditions. They act specifically on tyrosine kinases, a family of membrane-bound or intracellular molecules that regulate a variety of important cellular functions. TKIs have emerged as treatment for hematologic malignancies and autoimmune diseases. Each class of TKI has unique features and often acts on a specific receptor, minimizing the risk of adverse effects and infectious sequelae. Despite this targeted therapy, however, infectious complications have emerged as a major obstacle for many of these agents. Depending on the specific class of TKI, there is often an increased risk of neutropenia, or a higher risk for pneumonia and other respiratory infections. Reactivation of latent infections and opportunistic infections may also occur. Screening for latent infections prior to initiation of therapy and prophylaxis should be considered depending on the TKI agents used.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Tyrosine kinases are a family of membrane-bound or intracellular molecules that regulate a variety of important cellular functions. They are involved in transferring phosphate groups to tyrosine residues in substrate proteins transducing intracellular signals engaged in many cellular functions, including the modulation of growth factors related to carcinogenesis. Many tyrosine kinase inhibitors (TKIs) play a key role in cell cycle regulation and carry significant potential for oncogenesis if mutated [1]. As such, protein kinases have become one of the most intensively investigated target classes for therapeutic intervention with consensus guidelines for clinicians who care for immune compromised hosts [2]. So far, more than ten classes of small molecular weight protein kinase inhibitors have been approved for cancer treatment, and over 100 kinase inhibitors are currently in clinical development [3].
The first TKI, imatinib, was approved in 2001 as an inhibitor of breakpoint cluster region (BCR)-v-abl Abelson murine leukemia viral oncogene homolog (ABL) tyrosine kinase fusion protein (BCR-ABL). It represents the first-in-class agent targeting this specific mutation and subsequently spawned a new class of targeted therapies [1]. There are now many other approved TKIs for patients with hematologic or other malignancies. In this chapter, we review this family of small molecules with special attention to BCR-ABL TKI inhibitors. We also highlight the unique features of TKIs and focus on their specific indications, the risks of infection and recommendations for prophylaxis.
The Tyrosine Kinase Inhibitor Family
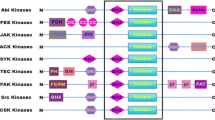
Tyrosine kinases are involved in intracellular signaling cascades and play a crucial role in initiating or perpetuating a signaling cascade within the cell, leading to cell growth, transformation, activation, or apoptosis. Inhibitors of these enzymes, known as TKIs, are small molecules that have been considered as “targeted therapies” because of their specific mechanisms of action [4] as seen in (Fig. 15.1). The tyrosine kinases are often selectively overexpressed in malignancies due to point mutations or chromosomal rearrangements. Hence, TKIs have mostly been developed for the treatment of malignancies. TKIs have good oral bioavailability and may be prone to cytochrome P450 drug-drug interactions [5, 6]. TKIs, because of their unique mechanism of action, have the potential to increase the risk of several infectious complications, which are detailed in Table 15.1.
BCR-ABL Inhibitors
There are currently five agents in this class—imatinib, dasatinib, nilotinib, bosutinib, and ponatinib—which are all used to treat primary hematologic malignancies that arise as a result of the BCR-ABL gene and fusion protein. BCR-ABL inhibitors also target other receptor tyrosine kinases and a wide range of non-receptor kinases [7]. They all share a common mechanism of binding to the ATP-binding site of the mutant BCR-ABL fusion protein with high affinity.
Mechanism of Action (MOA) and Indications
Imatinib was the first TKI approved for chronic myelogenous leukemia (CML). It competitively inhibits the ATP-binding site of the BCR-ABL tyrosine kinase, inhibiting the phosphorylation of tyrosine proteins involved in BCR-ABL signal transduction. It also specifically acts on the receptor for platelet-derived-growth factor (PDGFR) and c-KIT tyrosine kinases. As a result, it is also clinically useful for certain diseases such as gastrointestinal stromal tumors (GIST) and systemic mastocytosis, as well as diseases such as hypereosinophilic syndromes (HES) and chronic myelomonocytic leukemia (CMML) [1].
Dasatinib is a second-generation inhibitor of BCR-ABL kinases approved for the treatment of Ph + CML or acute lymphocytic leukemia (ALL) [8,9,10]. It was developed as a dual SRC/ABL inhibitor, but it also affects a wider array of kinases, including TEC family kinases and Bruton tyrosine kinase (BTK) [11]. This increase in off-target activity may be responsible for in vitro data that hint at a strongest immunosuppressive effect for this TKI [12].
Nilotinib is a second-generation BCR-ABL inhibitor that is also approved for use in the treatment of Ph + CML [13, 14]. It is an analogue of imatinib with more potent BCR-ABL kinase inhibition.
Bosutinib is a small, orally bioavailable molecule, inhibiting both SRC and BCR-ABL with activity against imatinib-resistant CML cell lines [15]. It is approved for use in various phases of CML [16, 17]. Bosutinib has demonstrated activity and manageable tolerability in a phase I/II study of patients with chronic phase CML following resistance/intolerance to imatinib only, or imatinib plus dasatinib and/or nilotinib [15, 18,19,20].
Ponatinib is a TKI active against disease resistant to other BCR-ABL TKIs [21]. It is approved for use in patients with CML or PH+ ALL resistant to other therapies, or with the T315I mutation.
Risk of Infection
Imatinib is known to induce neutropenia, inhibit T-cell proliferation and activation, reduce specific CD8+ responses to CMV and EBV, and cause dysfunction of dendritic cells [22,23,24]. The phase 3 IRIS study in 2003 [25] using imatinib as initial treatment of newly diagnosed chronic phase CML among 553 patients showed 60.8% all-grade neutropenia with imatinib, of which only 14.3% were grades 3 (i.e., neutrophil count between 500–1000/mm3 and 4 (neutrophil count <500/mm3), based on the Common Toxicity Criteria of the National Cancer Institute. Mild respiratory infections were commonly low grade and viral in origin. Long-term data showed that nearly all infection complications occurred within the first year. Only 1 patient each was reported as having treatment-related neutropenia, febrile neutropenia, and an anorectal infection in the first year of treatment, whereas only 1 patient each developed appendicitis and cellulitis in years 6 and 11, respectively [26].
Patients receiving imatinib for GIST did not have significant infection risk, although grade 3 or higher neutropenia occurred in 7% [27, 28]. There were also no significant infectious complications in a phase II study investigating its use in other malignancies [29].
However, long-term experience with imatinib has demonstrated increased risk of HBV or VZV reactivation, occurring in 2% of patients in a single retrospective study [30]. In another, 13–16% of patients developed a variety of infections, most commonly VZV and pneumonia [31]. Sporadic cases of tuberculosis [9, 32, 33], leishmaniasis [34], cryptococcosis, and other fungal infections have also been reported [35, 36].
Dasatinib appears to be associated with a greater risk of infection compared to other BCR-ABL inhibitors. In a clinical trial [37], infections of any grade occurred in 27 (11%) of dasatinib-treated patients compared to 18 (7%) imatinib-treated patients. Five patients versus 1 patient died due to infection, in the dasatinib and imatinib arm, respectively; however, the investigators deemed these infections unrelated to the drug. Interestingly, the majority of infections did not occur during the period of neutropenia.
In a safety analysis of two major clinical trials [38] inclusive of 1150 patients, serious infections were rare and only one grade 3–4 opportunistic infection was observed for dasatinib. However, a comparative analysis of dasatinib with nilotinib demonstrated that there was a higher proportion of infection-related healthcare resource utilization costs among those receiving dasatinib than in those receiving nilotinib, largely attributable to a larger proportion of infection-related inpatient-days [39].
The risk of CMV reactivation appears to be increased following hematopoietic stem cell transplantation (HSCT); in one study of 109 patients, dasatinib was associated with an increased incidence of CMV reactivation in the first year after transplantation (adjusted hazard ratio = 7.65, 95% CI, 1.84–31.7) [40].
Nilotinib was not associated with a greater risk of infection and caused less neutropenia compared to imatinib among patients with newly diagnosed CML. This was corroborated by subsequent cohorts [41,42,43]. Phase II studies examining its use in treated patients with chronic phase CML also failed to demonstrate evidence of significant infections, although neutropenia more commonly occurred [44, 45].
In a review of 169 patients with CML receiving first-line nilotinib therapy or therapy after imatinib failure, 9 (10%) patients among the frontline therapy group developed any infection, whereas 29 of 79 (37%) patients treated with nilotinib after imatinib failure developed any infection [46].
Phase I/II studies of bosutinib monotherapy in patients with imatinib-resistant chronic phase CML found no evidence of infectious complications but reported grade 3 or higher neutropenia in 18% of patients [15]. On long-term follow-up of this cohort [47], serious adverse events (SAEs) occurred in 59% (99/167) of patients with the most frequently occurring individual SAEs (>5% of patients overall) being pneumonia (10%), pyrexia (7%), febrile neutropenia (6%), thrombocytopenia (6%), disease progression (5%), headache (5%), and pleural effusion (5%). The only newly occurring individual SAE reported in more than two patients within year 2, 3, or 4 was pneumonia (three patients with events in year 2).
Ponatinib is relatively new, and post-marketing surveillance is limited. However, in phase II studies investigating its use in previously treated patients with CML or ALL did not demonstrate increased risk for infectious complications, although grade 3 or 4 neutropenia occurred in 12–26% [48], or in 14% of patients, respectively [49].
Prevention of Infection
Given the potentially increased risk of HBV reactivation infection among patients treated with TKIs, all patients should be screened for HBV prior to starting treatment [7] (Table 15.2). Those with evidence of HBV infection should initiate antiviral therapy or prophylaxis with entecavir or tenofovir, which should continue up to 6–12 months after cessation of immune suppressive therapy [50]. Susceptible patients should be vaccinated according to society guidelines [51], although the response may be impaired because of the underlying condition or because of the TKI.
Janus-Associated Kinases (JAK) Inhibitors
JAKs are a family of four non-receptor protein tyrosine kinases—JAK1, JAK2, JAK3, and tyrosine-kinase 2—which mediate signaling of cytokine receptors [52]. The pathways are involved in the growth, development, and differentiation of various cells but are crucial to the function of immune and hematopoietic cells [53]. There are three currently available JAK inhibitors—tofacitinib, baricitinib, and ruxolitinib. Their mechanisms of action, indications, and infection risk are summarized in Table 15.1.
MOA and Indication
Tofacitinib is a reversible competitive inhibitor of JAK1, JAK2, and JAK3 that inhibits lymphocyte proliferation and cytokine production, affecting the maturation of monocyte-derived dendritic cells and capacity to stimulate T cells [54, 55]. It was approved by the US Food and Drug Administration (FDA) for the treatment of rheumatoid arthritis (RA) and psoriatic arthritis (PsA) [56, 57].
Baricitinib, the second JAK inhibitor approved by the FDA for use in the treatment of RA [58, 59], is a selective and reversible JAK1 and 2 inhibitor. It suppresses the differentiation of plasmablasts, Th1, and Th17 cells [60]. Ruxolitinib is an inhibitor of JAK 1 and 2, approved for treatment of myelofibrosis and polycythemia vera (PCV) [61, 62]. Upadacitinib, a selective JAK-1 inhibitor, was recently approved in 2019 for the treatment of moderate to severe RA nonresponsive to methotrexate.
Risk of Infection
All JAK inhibitors may reduce T-cell number and function, inhibit T-cell proliferation, and impair NK cell maturation, which may be responsible for the increased risk of infectious complications [63]. In general, JAK inhibitors are associated with an increased risk of mild infections, such as respiratory tract infections, a small but increased risk of serious infections (3 per 100 patient years), and a consistent signal for a heightened risk for herpes zoster and tuberculosis [64].
In a review of pooled data from phase 2, phase 3 (P2P3), and long-term extension (LTE) studies of tofacitinib, among 4789 patients with RA, the overall rate of serious infection was 3.1 events/100 patient-years [65]. Age, corticosteroid dose, diabetes, and tofacitinib dose were independently linked to the risk of serious infection. Lymphocyte counts of <0.5 × 103/mm3 were rare but were associated with an increased risk of treated and/or serious infection. The most frequent infection was pneumonia, but skin and soft tissue infections were also commonly reported. Nonserious or serious herpes zoster virus infections were reported in 346 patients in the P2P3LTE population (incidence rate 4.27 events per 100 patient-years [95% CI 3.85–4.75]), while only 25 patients experienced an opportunistic infection (0.30 events per 100 patient-years [95% CI 0.20–0.44]). TB was reported in 16 patients (6 cases in the pooled P3 population [all receiving higher-dose tofacitinib at 10 mg twice daily], and 10 in the LTE group [5 receiving tofacitinib at a dosage of 5 mg twice daily and 5 receiving tofacitinib at a dosage of 10 mg twice daily]). The risk of serious infection did not increase over time with an incidence rate of 3.09 events/100 patient-years at 8.5 years follow-up.
Twenty-one studies were included in a recent meta-analysis [66] specifically evaluating the risk of serious infection and herpes zoster among RA patients receiving JAK inhibitors—11 tofacitinib (5888 patients), 6 baricitinib (3520 patients), and 4 upadacitinib studies (1736 patients). For serious infections, the incidence rates were relatively low at 1.97 (95% CI: 1.41, 2.68), 3.16 (95% CI: 2.07, 4.63), and 3.02 (95% CI: 0.98, 7.04), respectively. For herpes zoster, the incidence rates were 2.51 (95% CI: 1.87, 3.30), 3.16 (95% CI: 2.07, 4.63), and 2.41 (95% CI: 0.66, 6.18), respectively. The risk of herpes zoster appears to be higher overall than the general population, and it was numerically greatest with baricitinib.
Prevention of Infection
Screening for and treatment of latent tuberculosis infection and hepatitis B infection are advised in all patients before commencing treatment (Table 15.2). Given the higher risk of varicella zoster virus (VZV), vaccination against VZV with recombinant vaccine should also be considered for patients with positive serology or prior history of illness 2–3 weeks prior to starting therapy [67] (Table 15.2).
Bruton’s Tyrosine Kinase (BTK) Inhibitors
BTK is a non-receptor protein kinase expressed in B cells, myeloid cells, mast cells, and platelets. B-cell receptor (BCR) signaling via BTK is imperative for B-cell activation, proliferation, and survival (945). There are two currently approved agents in this class – ibrutinib and acalabrutinib (Table 15.1).
MOA and Indication
Ibrutinib is a selective and reversible inhibitor of the BTK protein, approved for the treatment of CLL, small lymphocytic lymphoma (SLL), and mantle cell lymphoma (MCL), Waldenstrom’s macroglobulinemia (WM), marginal zone lymphoma (MZL), and chronic graft versus host disease (cGVHD) [68, 69].
Acalabrutinib is an irreversible BTK inhibitor for the treatment of MCL. The highly selective and potent BTK inhibition provided by acalabrutinib is thought to translate into an improved safety profile compared with other targeted therapies [70, 71].
Risk of Infection
Infections in patients treated with ibrutinib relate primarily to B-cell dysfunction [72]. However, invasive fungal infection has also frequently been reported in association with BTK inhibitors, possibly as a result of its off-target effect on other kinases [72, 73].
In one study [74], 70% of 195 patients with relapsed or refractory CLL/SLL treated with ibrutinib developed an infection. Neutropenia occurred in 42 patients (22%), and pneumonia and urinary tract infections occurred in 14–17%.
The use of ibrutinib as first-line therapy for CLL/SLL was not clearly associated with an increased risk of infection in clinical trials, although severe pneumonia did occur [75]. In another study of 64 patients given ibrutinib for relapse or refractory MZL, 19% of patients experienced grade 3 or higher infections, most commonly pneumonia (8%) and cellulitis (5%) [76].
In a meta-analysis of prospective studies evaluating the use of ibrutinib among patients with a lymphoid malignancy [77], infectious complications were reported in almost all (44/48, 92%) trials. Infectious adverse events of any grade occurred in 56% of patients (approximately N = 900) treated with single-agent ibrutinib and in 52% of patients (approximately N = 250) receiving combination therapy. Grade 3–4 infectious adverse events occurred in 26% of patients on a single agent, and 20% of patients receiving combination therapy. The rate of grade 5 infectious adverse events was 2% in both cohorts. Eighteen of 22 single-agent trials and 15 of 28 combination therapy trials noted grade 3–4 pneumonia. The patient-affected rates for grade 3–4 pneumonia were 13% in single-agent studies and 8% in the combination setting. Grade 5 pneumonia occurred in 2% of all patients. These fatal infectious events included opportunistic pathogens such as Pneumocystis jirovecii, Histoplasma capsulatum, Cryptococcus neoformans, Nocardia species, and Aspergillus species.
In a more recent systematic review of phase III randomized controlled trials only [78], (7 studies, n = 2167 patients), ibrutinib was associated with a significantly increased risk of infection (any grade and grade 3–5) in patients with B-cell malignancies [pooled risk ratio (RR) = 1.34, 95% confidence interval [CI], 1.06–1.69, P = 0.015; and RR = 1.35, 95% CI, 1.05–1.74, P = 0.018, respectively]. In patients with CLL, a significantly increased risk of grade 3–5 infection was noted in the ibrutinib group [pooled RR = 1.24, 95% CI, 1.02–1.50, P = 0.028]. Pneumonia and URTI were the two most commonly reported infections in the studies included in this analysis.
A recent study looked at 124 patients treated with acalabrutinib in the phase II ACE-LY-004 trial [70], who were adjusted to match average baseline characteristics of populations from studies using alternative targeted treatment regimens for relapsed/refractory MCL for either monotherapy or combination therapies. The overall safety profile of acalabrutinib was similar to or better than that of the monotherapies; however, there was an increased risk of grade 3/4 infections versus the combination bendamustine + rituximab, and an increased risk of anemia compared with lenalidomide + rituximab and ibrutinib + rituximab.
Prevention of Infection
Recommendations for antifungal prophylaxis are currently not well defined. Further systemic and large-scale evaluation of the risk for pneumocystis pneumonia and other fungal infections are required before formal guidance can be issued. In general, pneumocystis prophylaxis may be given to those who have received prior chemoimmunotherapy, have refractory disease, or other risk factors such as concomitant high-dose steroid use [79, 80]. All patients should be screened for serological evidence of hepatitis B virus prior to commencement of ibrutinib, with prophylaxis provided to those who are hepatitis B surface antigen positive [81]. Vaccination against influenza and pneumococcus is recommended prior to initiation of therapy, but immune response is typically poor because of the underlying condition [82,83,84] (Table 15.2).
Phosphatidylinositol 3-Kinase (PI3K) Inhibitors
Phosphatidylinositol 3-kinase (PI3K) is a signaling pathway activated downstream of BCR. Along with protein kinase B (AKT) and mammalian target of rapamycin (mTOR), it is responsible for B-cell proliferation, cell survival, and angiogenesis [85]. There are two currently available agents in this class—idelalisib and copanlisib.
MOA and Indication
Idelalisib is a reversible inhibitor of PI3K-δ and is approved for use in the treatment of CLL, SLL, and follicular lymphoma (FL) [86,87,88]. Inhibition of PI3K-δ impairs both B- and T-cell-mediated function [81, 89, 90].
Copanlisib is a second-generation, intravenous PI3K inhibitor with predominant activity in PI3K-α and δ isoforms [91] approved for relapsed FL.
Risk of Infection
Pneumonia is one of the most common infectious complications associated with idelalisib use, with an incidence of about 20%; majority are grade 3 or higher [92]. Atypical infections, such as pneumocystis pneumonia, invasive fungal infection, and noninfectious (autoimmune) pneumonitis also occur [93].
In a retrospective analysis among 2198 patients receiving idelalisib [94], the overall incidence of pneumocystis pneumonia infection was 2.5% in patients receiving idelalisib, with or without rituximab, with or without bendamustine, compared to only 0.2% in patients receiving only rituximab with or without bendamustine. In this cohort, pneumocystis pneumonia prophylaxis reduced the incidence from 3.4% to 1.3%.
Fatal and serious infections have been reported in 21–48% of patients receiving idelalisib [86], and a warning was issued related to this risk. The increased risk of infection related death from either sepsis or pneumonia frequently occurred within the first 180 days of starting treatment [95]. Given this substantial risk, the FDA recommended that it is not indicated for first-line treatment of malignancy or in combination with bendamustine and/or rituximab for patients with FL [86].
Two phase II studies have evaluated the infection profile of copanlisib; in the smaller study [96] (n = 33), neutropenia occurred in 34.5% with grade 3 or higher neutropenia in majority (29.8%); infections were reported in 64.3% of patients, with grade 3 or higher infections including pneumonia in 14.3%, febrile neutropenia in 3.6%, and urinary tract and skin and soft tissue infections in 2.4%. In the larger study (n = 142) [97], pneumonia occurred in 21% of patients overall, and 15% experience grade 3 or higher pneumonia. In both cohorts, infection from unusual organisms such as P. jirovecii, C. neoformans, and Aspergillus species also occurred.
Prevention of Infection
Universal prophylaxis against P. jirovecii is recommended for all patients during treatment and for 2–6 months after cessation of idelalisib therapy [81] (Table 15.2). Monthly preemptive monitoring for CMV among those with positive IgG serology is also recommended upon initiation of idelalisib treatment [86,87,88]. Monitoring of absolute neutrophil count should be monitored at least every 2 weeks for the first 6 months of treatment, and the drug should not be started in patients with an ongoing or active infection [86,87,88]. Screening for latent TB infection and HBV are also recommended although there is insufficient data regarding the risk of reactivation infection. Both pneumococcal and influenza vaccination are also recommended [98]. Until further data are available, the recommendations for idelalisib should also be applied to copanlisib (Table 15.2).
Anaplastic Lymphoma Kinase (ALK) Inhibitors
The ALK gene codes for the ALK receptor tyrosine kinase whose exact function is unknown but may be related to neuronal cell proliferation [64]. Activation of this gene usually occurs via chromosomal rearrangement, and ALK rearrangements are seen in 3–5% of non-small cell lung cancers (NSCLC), most commonly in adenocarcinomas.
MOA and Indication
Crizotinib was the first ALK inhibitor approved for the treatment of advanced non-small cell lung carcinoma (NSCLC) with an ALK or ROS1 gene rearrangement [99,100,101]. The other ALK inhibitors currently approved for use are summarized in Table 15.1.
Risk of Infection
Data from two randomized controlled trials [102, 103] did not show evidence of increased risk of infection with crizotinib, although neutropenia occurred in 11–13% of patients. Upper respiratory tract infections also occurred at a higher rate but were not associated with significant morbidity or mortality [102]. A unique feature of crizotinib is the propensity to develop complex renal cysts, which may be secondarily infected [104]. Interstitial pneumonitis has also been reported with all ALK inhibitors [103, 105,106,107,108].
Prevention of Infection
At this time, there are no specific recommendations regarding prevention of infectious complications from the use of ALK inhibitors.
Spleen (Syk) TKI
Spleen tyrosine kinase (Syk) is an intracellular cytoplasmic tyrosine kinase that is widely expressed in hematopoietic stem cells, particularly B cells [109]. It also plays a pivotal role in signaling and activating Fc receptors [110], regulating both T-cell and B-cell expansion and proliferation, and mediating signaling in inflammatory cells [111]. In vitro, Syk inhibition leads to diminished proliferation of antigen-specific CD4+ T cells and reduced production of inflammatory cytokines such as IFN λ and IL-17 [112].
MOA and Indication
Fostamatinib is the only currently approved agent in this class for use in patients with chronic immune thrombocytopenia (ITP) [113]; it is used off label for RA [111]. Its active metabolite, R406, inhibits signal transduction by Fcγ receptors involved in the antibody-mediated destruction of platelets by immune cells in chronic ITP [110], which results in increased platelet counts in this population.
Risk of Infection
Given the role of Syk in the immune response, one would expect its inhibition to have the potential to make the patient critically ill, but this has not been the case thus far [64]. Early phase II studies in patients receiving fostamatinib reported dose-related neutropenia in 6 [109] to 15% [114] of patients, higher than those receiving placebo.
In a small phase II study of 16 patients with chronic, refractory ITP [115], the use of various doses of fostamatinib led to a small but statistically significant decrease in total WBC, without increasing the infection risk. Two paired phase III studies compared fostamatinib with placebo using different doses [116], and rates of moderate to severe infections were similar compared to placebo at 8% vs. 6%. The risk of mild respiratory infections was slightly higher for patients on fostamatinib at 11% compared to 6% of those on placebo.
Prevention of Infection
There are currently no specific recommendations about preventive measures for patients taking fostamatinib. However, monthly monitoring of the absolute neutrophil count is recommended, with dose reduction or temporary cessation of the drug if it falls to <1 × 109/liter [113].
Conclusion
Several TKI are now available for use as primary therapy for hematologic malignancies and autoimmune diseases and has made the treatment of these diseases easier and more successful. However despite this targeted approach, the risk of infection remains, and is higher for those with refractory disease and those with history of prior immune suppression. The types of infection differ depending on the TKI class, although infection risk with TKIs appears to be related to the degree of neutropenia, and include viral or fungal pneumonia and other respiratory tract infections. Reactivation infections, such as tuberculosis and hepatitis B, can also occur, and a thorough evaluation and screening of these patients prior to initiation therapy must be performed.
References
Kin A, Schiffer CA. Infectious complications of tyrosine kinase inhibitors in hematological malignancies. Infect Dis Clin N Am. 2020;34(2):245–56.
Reinwald M, Silva JT, Mueller NJ, Fortún J, Garzoni C, de Fijter JW, et al. ESCMID study Group for Infections in compromised hosts (ESGICH) consensus document on the safety of targeted and biological therapies: an infectious diseases perspective (intracellular signaling pathways: tyrosine kinase and mTOR inhibitors). Clin Microbiol Infect. 2018;24(Suppl 2):S53–s70.
Zsila F, Fitos I, Bencze G, Kéri G, Orfi L. Determination of human serum alpha1-acid glycoprotein and albumin binding of various marketed and preclinical kinase inhibitors. Curr Med Chem. 2009;16(16):1964–77.
Hartmann JT, Haap M, Kopp HG, Lipp HP. Tyrosine kinase inhibitors—a review on pharmacology, metabolism and side effects. Curr Drug Metab. 2009;10(5):470–81.
Pajares B, Torres E, Trigo JM, Sáez MI, Ribelles N, Jiménez B, et al. Tyrosine kinase inhibitors and drug interactions: a review with practical recommendations. Clin Transl Oncol. 2012;14(2):94–101.
Drenberg CD, Baker SD, Sparreboom A. Integrating clinical pharmacology concepts in individualized therapy with tyrosine kinase inhibitors. Clin Pharmacol Ther. 2013;93(3):215–9.
Knoll BM, Seiter K. Infections in patients on BCR-ABL tyrosine kinase inhibitor therapy: cases and review of the literature. Infection. 2018;46(3):409–18.
Sprycel (dasatinib) 2018. Prescribing information. Reference ID 4179887. Food and Drug Administration, Silver Spring, MD. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021986s020lbl.pdf. Accessed October 2020.
Therapeutic Goods Administration. Australian product information—Sprycel (dasatinib) 2018. Therapeutic Goods Administration, Woden, Australia. https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id%03CP-2010-PI-02657-3. Accessed October 2020.
European Medicines Agency. Sprycel, INN—dasatinib. Product information. European Medicines Agency, Amsterdam, The Netherlands. https://www.ema.europa.eu/documents/product-information/sprycel-epar-product-information_en.pdf. Accessed October 2020 [Internet]. 2018 [cited October 2020].
Hantschel O, Rix U, Superti-Furga G. Target spectrum of the BCR-ABL inhibitors imatinib, nilotinib and dasatinib. Leuk Lymphoma. 2008;49(4):615–9.
Hayashi Y, Nakamae H, Katayama T, Nakane T, Koh H, Nakamae M, et al. Different immunoprofiles in patients with chronic myeloid leukemia treated with imatinib, nilotinib or dasatinib. Leuk Lymphoma. 2012;53(6):1084–9.
Therapeutic Goods Administration. 2018. Australian product information— Tasigna (nilotinib). Therapeutic goods Administration, Woden, Australia. https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id%03CP-2009-PI-00292-3. Accessed October 2020.
Anonymous. 2018. Tasigna (nilotinib). Prescribing information. Reference ID 4309495. Food and Drug Administration, Silver Spring, MD.https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/022068s029lbl.pdf. Accessed October 2020.
Cortes JE, Kantarjian HM, Brümmendorf TH, Kim DW, Turkina AG, Shen ZX, et al. Safety and efficacy of bosutinib (SKI-606) in chronic phase Philadelphia chromosome-positive chronic myeloid leukemia patients with resistance or intolerance to imatinib. Blood. 2011;118(17):4567–76.
European Medicines Agency. 2018. Bosulif, INN—bosutinib. Product information. European Medicines Agency, Amsterdam, The Netherlands. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_Product_Information/human/002373/WC500141721.pdf. Accessed October 2020.
Anonymous 2018. Bosulif (bosutinib). Prescribing information. Reference ID 4197387. Food and Drug Administration, Silver Spring, MD. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/ 203341s009lbl.pdf. Accessed October 2020.
Gambacorti-Passerini C, Brümmendorf TH, Kim DW, Turkina AG, Masszi T, Assouline S, et al. Bosutinib efficacy and safety in chronic phase chronic myeloid leukemia after imatinib resistance or intolerance: minimum 24-month follow-up. Am J Hematol. 2014;89(7):732–42.
Kantarjian HM, Cortes JE, Kim DW, Khoury HJ, Brümmendorf TH, Porkka K, et al. Bosutinib safety and management of toxicity in leukemia patients with resistance or intolerance to imatinib and other tyrosine kinase inhibitors. Blood. 2014;123(9):1309–18.
Khoury HJ, Cortes JE, Kantarjian HM, Gambacorti-Passerini C, Baccarani M, Kim DW, et al. Bosutinib is active in chronic phase chronic myeloid leukemia after imatinib and dasatinib and/or nilotinib therapy failure. Blood. 2012;119(15):3403–12.
Cortes JE, Kantarjian H, Shah NP, Bixby D, Mauro MJ, Flinn I, et al. Ponatinib in refractory Philadelphia chromosome-positive leukemias. N Engl J Med. 2012;367(22):2075–88.
Appel S, Boehmler AM, Grünebach F, Müller MR, Rupf A, Weck MM, et al. Imatinib mesylate affects the development and function of dendritic cells generated from CD34+ peripheral blood progenitor cells. Blood. 2004;103(2):538–44.
Dietz AB, Souan L, Knutson GJ, Bulur PA, Litzow MR, Vuk-Pavlovic S. Imatinib mesylate inhibits T-cell proliferation in vitro and delayed-type hypersensitivity in vivo. Blood. 2004;104(4):1094–9.
Seggewiss R, Loré K, Greiner E, Magnusson MK, Price DA, Douek DC, et al. Imatinib inhibits T-cell receptor-mediated T-cell proliferation and activation in a dose-dependent manner. Blood. 2005;105(6):2473–9.
O'Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348(11):994–1004.
Hochhaus A, Larson RA, Guilhot F, Radich JP, Branford S, Hughes TP, et al. Long-term outcomes of imatinib treatment for chronic myeloid leukemia. N Engl J Med. 2017;376(10):917–27.
Blanke CD, Rankin C, Demetri GD, Ryan CW, von Mehren M, Benjamin RS, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008;26(4):626–32.
Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347(7):472–80.
Heinrich MC, Joensuu H, Demetri GD, Corless CL, Apperley J, Fletcher JA, et al. Phase II, open-label study evaluating the activity of imatinib in treating life-threatening malignancies known to be associated with imatinib-sensitive tyrosine kinases. Clin Cancer Res. 2008;14(9):2717–25.
Mattiuzzi GN, Cortes JE, Talpaz M, Reuben J, Rios MB, Shan J, et al. Development of varicella-zoster virus infection in patients with chronic myelogenous leukemia treated with imatinib mesylate. Clin Cancer Res. 2003;9(3):976–80.
Breccia M, Girmenia C, Latagliata R, Loglisci G, Santopietro M, Federico V, et al. Low incidence rate of opportunistic and viral infections during imatinib treatment in chronic myeloid leukemia patients in early and late chronic phase. Mediterr J Hematol Infect Dis. 2011;3(1):e2011021.
Daniels JM, Vonk-Noordegraaf A, Janssen JJ, Postmus PE, van Altena R. Tuberculosis complicating imatinib treatment for chronic myeloid leukaemia. Eur Respir J. 2009;33(3):670–2.
Senn L, Kovacsovics T, Tarr PE, Meylan P. Peritoneal tuberculosis after imatinib therapy. Arch Intern Med. 2009;169(3):312–3.
Quixada A, Filho PA, Filho TP, Duarte FB, Moreira-Nunes CA, Lemes RP. Pancytopenia during tyrosine kinase inhibitor treatment—coexistence of chronic myeloid leukemia and visceral leishmaniasis: a case report. J Med Case Rep. 2016;10:207.
Crisan AM, Ghiaur A, Stancioaca MC, Bardas A, Ghita C, Manea CM, et al. Mucormycosis during imatinib treatment: case report. J Med Life. 2015;8(3):365–70.
Speletas M, Vyzantiadis TA, Kalala F, Plastiras D, Kokoviadou K, Antoniadis A, et al. Pneumonia caused by Candida krusei and Candida glabrata in a patient with chronic myeloid leukemia receiving imatinib mesylate treatment. Med Mycol. 2008;46(3):259–63.
Kantarjian HM, Shah NP, Cortes JE, Baccarani M, Agarwal MB, Undurraga MS, et al. Dasatinib or imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: 2-year follow-up from a randomized phase 3 trial (DASISION). Blood. 2012;119(5):1123–9.
Al-Ameri A, Kantarijan H., Burton E. Low risk of infectious events in patients (pts) with chronic myeloid leukemia (CML) in chronic phase (CP) treated with dasatinib. Abstract 3291 ASH Annual Meeting 2009.
Seiter K, Latremouille-Viau D, Guerin A, Ndife B, Habucky K, Tang DH, et al. Burden of infections among chronic myeloid leukemia patients receiving Dasatinib or nilotinib: a real-world retrospective healthcare claims study in the United States. Adv Ther. 2018;35(10):1671–85.
Prestes DP, Arbona E, Nevett-Fernandez A, Woolley AE, Ho VT, Koo S, et al. Dasatinib use and risk of cytomegalovirus reactivation after allogeneic hematopoietic-cell transplantation. Clin Infect Dis. 2017;65(3):510–3.
Hochhaus A, Rosti G, Cross NC, Steegmann JL, le Coutre P, Ossenkoppele G, et al. Frontline nilotinib in patients with chronic myeloid leukemia in chronic phase: results from the European ENEST1st study. Leukemia. 2016;30(1):57–64.
Hochhaus A, Saglio G, Hughes TP, Larson RA, Kim DW, Issaragrisil S, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016;30(5):1044–54.
Larson RA, Hochhaus A, Hughes TP, Clark RE, Etienne G, Kim DW, et al. Nilotinib vs imatinib in patients with newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: ENESTnd 3-year follow-up. Leukemia. 2012;26(10):2197–203.
le Coutre PD, Giles FJ, Hochhaus A, Apperley JF, Ossenkoppele GJ, Blakesley R, et al. Nilotinib in patients with Ph+ chronic myeloid leukemia in accelerated phase following imatinib resistance or intolerance: 24-month follow-up results. Leukemia. 2012;26(6):1189–94.
Nicolini FE, Turkina A, Shen ZX, Gallagher N, Jootar S, Powell BL, et al. Expanding nilotinib access in clinical trials (ENACT): an open-label, multicenter study of oral nilotinib in adult patients with imatinib-resistant or imatinib-intolerant Philadelphia chromosome-positive chronic myeloid leukemia in the chronic phase. Cancer. 2012;118(1):118–26.
Al-Ameri AM, Cortes JE, Kantarjian H, Burton E, Quintas-Cardama A, Jabbour E, et al. Infectious events in patients with chronic myeloid leukemia treated with nilotinib as a front line therapy and after imatinib failure. Blood. 2010;116:1233.
Gambacorti-Passerini C, Kantarjian HM, Kim DW, Khoury HJ, Turkina AG, Brümmendorf TH, et al. Long-term efficacy and safety of bosutinib in patients with advanced leukemia following resistance/intolerance to imatinib and other tyrosine kinase inhibitors. Am J Hematol. 2015;90(9):755–68.
Cortes JE, Kim DW, Pinilla-Ibarz J, le Coutre P, Paquette R, Chuah C, et al. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med. 2013;369(19):1783–96.
Jain P, Kantarjian H, Jabbour E, Gonzalez GN, Borthakur G, Pemmaraju N, et al. Ponatinib as first-line treatment for patients with chronic myeloid leukaemia in chronic phase: a phase 2 study. Lancet Haematol. 2015;2(9):e376–83.
Reddy KR, Beavers KL, Hammond SP, Lim JK, Falck-Ytter YT. American Gastroenterological Association Institute guideline on the prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2015;148(1):215–9; quiz e16–7
Rubin LG, Levin MJ, Ljungman P, Davies EG, Avery R, Tomblyn M, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58(3):309–18.
Cai B, Cai JP, Luo YL, Chen C, Zhang S. The specific roles of JAK/STAT signaling pathway in sepsis. Inflammation. 2015;38(4):1599–608.
Hodge JA, Kawabata TT, Krishnaswami S, Clark JD, Telliez JB, Dowty ME, et al. The mechanism of action of tofacitinib - an oral Janus kinase inhibitor for the treatment of rheumatoid arthritis. Clin Exp Rheumatol. 2016;34(2):318–28.
Kubo S, Yamaoka K, Kondo M, Yamagata K, Zhao J, Iwata S, et al. The JAK inhibitor, tofacitinib, reduces the T cell stimulatory capacity of human monocyte-derived dendritic cells. Ann Rheum Dis. 2014;73(12):2192–8.
Maeshima K, Yamaoka K, Kubo S, Nakano K, Iwata S, Saito K, et al. The JAK inhibitor tofacitinib regulates synovitis through inhibition of interferon-γ and interleukin-17 production by human CD4+ T cells. Arthritis Rheum. 2012;64(6):1790–8.
Anonymous. 2018. Xeljanz (tofacitinib). Prescribing information. Reference ID 4308396. Food and Drug Administration, Silver Spring, MD. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/203214s021,208246s007lbl.pdf. Accessed October 2020.
European Medicines Agency. 2018. Xeljanz, INN—tofacitinib citrate. Product information. European Medicines Agency, Amsterdam, The Netherlands. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/004214/WC500224911.pdf. Accessed October 2020.
European Medicines Agency. 2018. Olumiant, INN—baricitinib. Product information. European Medicines Agency, Amsterdam, The Netherlands. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/004085/WC500223723.pdf. Accessed October 2020.
Anonymous. 2018. Olumiant (baricitinib). Prescribing information. Reference ID 4271150. Food and Drug Administration, Silver Spring, MD. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/207924Orig1s000lbl.pdf. Accessed October 2020.
Kubo S, Nakayamada S, Sakata K, Kitanaga Y, Ma X, Lee S, et al. Janus kinase inhibitor Baricitinib modulates human innate and adaptive immune system. Front Immunol. 2018;9:1510.
Therapeutic Goods Administration. 2018. Australian product information—Jakavi (ruxolitinib). Therapeutic Goods Administration, Woden, Australia. https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/ pdf?OpenAgent&id_CP-2013-PI-01918-1&d_201809111016933. October 2020.
Anonymous. 2018. Jakafi (ruxolitinib). Prescribing information. Reference ID 4191667. Food and Drug Administration, Silver Spring, MD. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/202192s015lbl.pdf. Accessed October 2020.
McLornan DP, Khan AA, Harrison CN. Immunological consequences of JAK inhibition: friend or foe? Curr Hematol Malig Rep. 2015;10(4):370–9.
Davis JS, Ferreira D, Paige E, Gedye C, Boyle M. Infectious complications of biological and small molecule targeted immunomodulatory therapies. Clin Microbiol Rev. 2020;33(3)
Cohen S, Radominski SC, Gomez-Reino JJ, Wang L, Krishnaswami S, Wood SP, et al. Analysis of infections and all-cause mortality in phase II, phase III, and long-term extension studies of tofacitinib in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66(11):2924–37.
Bechman K, Subesinghe S, Norton S, Atzeni F, Galli M, Cope AP, et al. A systematic review and meta-analysis of infection risk with small molecule JAK inhibitors in rheumatoid arthritis. Rheumatology (Oxford). 2019;58(10):1755–66.
Winthrop KL, Park SH, Gul A, Cardiel MH, Gomez-Reino JJ, Tanaka Y, et al. Tuberculosis and other opportunistic infections in tofacitinib-treated patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75(6):1133–8.
Anonymous. 2018. Imbruvica (ibrutinib). Prescribing information. Reference ID 4222705. Food and Drug Administration, Silver Spring, MD. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210563s000lbl.pdf. Accessed October 2020.
Therapeutic Goods Administration. 2018. Australian product information—Imbruvica (ibrutinib). Therapeutic goods Administration, Woden, Australia. https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id%03CP-2015-PI-01676-1&d%03201808251016933. Accessed October 2020.
Telford C, Kabadi SM, Abhyankar S, Song J, Signorovitch J, Zhao J, et al. Matching-adjusted indirect comparisons of the efficacy and safety of acalabrutinib versus other targeted therapies in relapsed/refractory mantle cell lymphoma. Clin Ther. 2019;41(11):2357–79.e1.
Wu J, Zhang M, Liu D. Acalabrutinib (ACP-196): a selective second-generation BTK inhibitor. J Hematol Oncol. 2016;9:21.
Hilal T, Gea-Banacloche JC, Leis JF. Chronic lymphocytic leukemia and infection risk in the era of targeted therapies: linking mechanisms with infections. Blood Rev. 2018;32(5):387–99.
Varughese T, Taur Y, Cohen N, Palomba ML, Seo SK, Hohl TM, et al. Serious infections in patients receiving ibrutinib for treatment of lymphoid cancer. Clin Infect Dis. 2018;67(5):687–92.
Byrd JC, Brown JR, O'Brien S, Barrientos JC, Kay NE, Reddy NM, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371(3):213–23.
Burger JA, Tedeschi A, Barr PM, Robak T, Owen C, Ghia P, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25):2425–37.
Noy A, de Vos S, Thieblemont C, Martin P, Flowers CR, Morschhauser F, et al. Targeting Bruton tyrosine kinase with ibrutinib in relapsed/refractory marginal zone lymphoma. Blood. 2017;129(16):2224–32.
Tillman BF, Pauff JM, Satyanarayana G, Talbott M, Warner JL. Systematic review of infectious events with the Bruton tyrosine kinase inhibitor ibrutinib in the treatment of hematologic malignancies. Eur J Haematol. 2018;100(4):325–34.
Ball S, Das A, Vutthikraivit W, Edwards PJ, Hardwicke F, Short NJ, et al. Risk of infection associated with ibrutinib in patients with B-cell malignancies: a systematic review and meta-analysis of randomized controlled trials. Clin Lymphoma Myeloma Leuk. 2020;20(2):87–97.e5.
Gribben JG, Bosch F, Cymbalista F, Geisler CH, Ghia P, Hillmen P, et al. Optimising outcomes for patients with chronic lymphocytic leukaemia on ibrutinib therapy: European recommendations for clinical practice. Br J Haematol. 2018;180(5):666–79.
Reinwald M, Boch T, Hofmann WK, Buchheidt D. Risk of infectious complications in Hemato-oncological patients treated with kinase inhibitors. Biomark Insights. 2015;10(Suppl 3):55–68.
Teh BW, Tam CS, Handunnetti S, Worth LJ, Slavin MA. Infections in patients with chronic lymphocytic leukaemia: mitigating risk in the era of targeted therapies. Blood Rev. 2018;32(6):499–507.
Andrick B, Alwhaibi A, DeRemer DL, Quershi S, Khan R, Bryan LJ, et al. Lack of adequate pneumococcal vaccination response in chronic lymphocytic leukaemia patients receiving ibrutinib. Br J Haematol. 2018;182(5):712–4.
Douglas AP, Trubiano JA, Barr I, Leung V, Slavin MA, Tam CS. Ibrutinib may impair serological responses to influenza vaccination. Haematologica. 2017;102(10):e397–e9.
Sun C, Gao J, Couzens L, Tian X, Farooqui MZ, Eichelberger MC, et al. Seasonal influenza vaccination in patients with chronic lymphocytic leukemia treated with ibrutinib. JAMA Oncol. 2016;2(12):1656–7.
Greenwell IB, Ip A, Cohen JB. PI3K inhibitors: understanding toxicity mechanisms and management. Oncology (Williston Park). 2017;31(11):821–8.
Anonymous. 2018. Zydelig (idelalisib). Prescribing information. Reference ID 4213201. Food and Drug Administration, Silver Spring, MD. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/205858s009lbl.pdf. Accessed October 2020.
European Medicines Agency. 2018. Zydelig, INN—idelalisib. Product information. European Medicines Agency, Amsterdam, The Netherlands. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003843/WC500175377.pdf. Accessed October 2020.
Therapeutic Goods Administration. 2018. Product information—Zydelig (idelalisib). Therapeutic goods Administration, Woden, Australia https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id%03CP-2015-PI-01225-1&d%03201809151016933. Accessed October 2020.
Martinelli S, Maffei R, Fiorcari S, Quadrelli C, Zucchini P, Benatti S, et al. Idelalisib impairs T-cell-mediated immunity in chronic lymphocytic leukemia. Haematologica. 2018;103(12):e598–601.
Mensah FA, Blaize JP, Bryan LJ. Spotlight on copanlisib and its potential in the treatment of relapsed/refractory follicular lymphoma: evidence to date. Onco Targets Ther. 2018;11:4817–27.
Liu N, Rowley BR, Bull CO, Schneider C, Haegebarth A, Schatz CA, et al. BAY 80-6946 is a highly selective intravenous PI3K inhibitor with potent p110α and p110δ activities in tumor cell lines and xenograft models. Mol Cancer Ther. 2013;12(11):2319–30.
de Weerdt I, Koopmans SM, Kater AP, van Gelder M. Incidence and management of toxicity associated with ibrutinib and idelalisib: a practical approach. Haematologica. 2017;102(10):1629–39.
Coutré SE, Barrientos JC, Brown JR, de Vos S, Furman RR, Keating MJ, et al. Management of adverse events associated with idelalisib treatment: expert panel opinion. Leuk Lymphoma. 2015;56(10):2779–86.
Sehn LH, Hallek M, Jurczak W, Brown JR, Barr PM, Catalano J, et al. A retrospective analysis of Pneumocystis jirovecii pneumonia infection in patients receiving idelalisib in clinical trials. Blood. 2016;128:3705.
Australian Government Department of Health. 2017. Idelalisib (zydelig): safety advisory—change to indications and addition of warnings. https://www.tga.gov.au/alert/idelalisib-zydelig. Accessed October 2020.
Dreyling M, Morschhauser F, Bouabdallah K, Bron D, Cunningham D, Assouline SE, et al. Phase II study of copanlisib, a PI3K inhibitor, in relapsed or refractory, indolent or aggressive lymphoma. Ann Oncol. 2017;28(9):2169–78.
Dreyling M, Santoro A, Mollica L, Leppä S, Follows GA, Lenz G, et al. Phosphatidylinositol 3-kinase inhibition by Copanlisib in relapsed or refractory indolent lymphoma. J Clin Oncol. 2017;35(35):3898–905.
Cuneo A, Barosi G, Danesi R, Fagiuoli S, Ghia P, Marzano A, et al. Management of adverse events associated with idelalisib treatment in chronic lymphocytic leukemia and follicular lymphoma: a multidisciplinary position paper. Hematol Oncol. 2019;37(1):3–14.
European Medicines Agency. 2018. Xalkori, INN—crizotinib. Product Information. European Medicines Agency, Amsterdam, The Netherlands. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002489/WC500134759.pdf. Accessed October 2020.
Anonymous. 2018. Xalkori (crizotinib). Prescribing information. Reference ID 4216933. Food and Drug Administration, Silver Spring, MD. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/202570s023lbl.pdf. Accessed October 2020.
Therapeutic Goods Administration. 2018. Australian product information—Xalkori (crizotinib). Therapeutic goods Administration, Woden, Australia. https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id%03CP-2013-PI-02261-1. Accessed October 2020.
Shaw AT, Kim DW, Nakagawa K, Seto T, Crinó L, Ahn MJ, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368(25):2385–94.
Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371(23):2167–77.
Yoneshima Y, Okamoto I, Arimura-Omori M, Kimura S, Hidaka-Fujimoto N, Iwama E, et al. Infected complex renal cysts during crizotinib therapy in a patient with non-small cell lung cancer positive for ALK rearrangement. Investig New Drugs. 2015;33(2):510–2.
Faderl S, Talpaz M, Estrov Z, O'Brien S, Kurzrock R, Kantarjian HM. The biology of chronic myeloid leukemia. N Engl J Med. 1999;341(3):164–72.
Ikeda S, Yoshioka H, Arita M, Sakai T, Sone N, Nishiyama A, et al. Interstitial lung disease induced by alectinib (CH5424802/RO5424802). Jpn J Clin Oncol. 2015;45(2):221–4.
Kim DW, Mehra R, Tan DSW, Felip E, Chow LQM, Camidge DR, et al. Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): updated results from the multicentre, open-label, phase 1 trial. Lancet Oncol. 2016;17(4):452–63.
Yamamoto Y, Okamoto I, Otsubo K, Iwama E, Hamada N, Harada T, et al. Severe acute interstitial lung disease in a patient with anaplastic lymphoma kinase rearrangement-positive non-small cell lung cancer treated with alectinib. Investig New Drugs. 2015;33(5):1148–50.
Weinblatt ME, Kavanaugh A, Genovese MC, Musser TK, Grossbard EB, Magilavy DB. An oral spleen tyrosine kinase (Syk) inhibitor for rheumatoid arthritis. N Engl J Med. 2010;363(14):1303–12.
Braselmann S, Taylor V, Zhao H, Wang S, Sylvain C, Baluom M, et al. R406, an orally available spleen tyrosine kinase inhibitor blocks fc receptor signaling and reduces immune complex-mediated inflammation. J Pharmacol Exp Ther. 2006;319(3):998–1008.
Kunwar S, Devkota AR, Ghimire DK. Fostamatinib, an oral spleen tyrosine kinase inhibitor, in the treatment of rheumatoid arthritis: a meta-analysis of randomized controlled trials. Rheumatol Int. 2016;36(8):1077–87.
Platt AM, Benson RA, McQueenie R, Butcher JP, Braddock M, Brewer JM, et al. The active metabolite of spleen tyrosine kinase inhibitor fostamatinib abrogates the CD4+ T cell-priming capacity of dendritic cells. Rheumatology (Oxford). 2015;54(1):169–77.
Anonymous. 2018. Tavalisse (fostamatinib disodium hexahydrate).Reference ID 4249943. Prescribing information. Food and Drug Administration, Silver Spring, MD. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/209299lbl.pdf. Accessed October 2020.
Weinblatt ME, Kavanaugh A, Burgos-Vargas R, Dikranian AH, Medrano-Ramirez G, Morales-Torres JL, et al. Treatment of rheumatoid arthritis with a Syk kinase inhibitor: a twelve-week, randomized, placebo-controlled trial. Arthritis Rheum. 2008;58(11):3309–18.
Podolanczuk A, Lazarus AH, Crow AR, Grossbard E, Bussel JB. Of mice and men: an open-label pilot study for treatment of immune thrombocytopenic purpura by an inhibitor of Syk. Blood. 2009;113(14):3154–60.
Bussel J, Arnold DM, Grossbard E, Mayer J, Treliński J, Homenda W, et al. Fostamatinib for the treatment of adult persistent and chronic immune thrombocytopenia: results of two phase 3, randomized, placebo-controlled trials. Am J Hematol. 2018;93(7):921–30.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Abad, C.L.R., Razonable, R.R. (2022). Tyrosine-Kinase Inhibitors. In: Cervera, C., Aguado, J.M. (eds) Infectious Complications in Biologic and Targeted Therapies. Springer, Cham. https://doi.org/10.1007/978-3-031-11363-5_15
Download citation
DOI: https://doi.org/10.1007/978-3-031-11363-5_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-11362-8
Online ISBN: 978-3-031-11363-5
eBook Packages: MedicineMedicine (R0)