Abstract
The Janus kinase-signal transducer and activator of transcription (JAK/STAT) pathway is a principal signaling pathway for the signal transduction of many pivotal cytokines involved in sepsis. Binding of cytokines to corresponding receptors can activate associated JAK kinases, which selectively phosphorylate STATs. Activated STATs then translocate to the nucleus and play a critical role in the transcription of target genes. During the past several years, significant progress has been made in the understanding of the roles of JAK/STAT pathway in sepsis. The aims of this review are to describe the present knowledge about JAK/STAT signaling pathway, describe the specific roles of JAK/STAT pathway in sepsis, and put forward the prospect for future studies of JAK/STAT signaling pathway in sepsis. A PubMed database search was performed for studies of JAKs and STATs in sepsis. It has been shown that a variety of cytokines can exert their biological effects via the JAK/STAT signaling pathway. JAK/STAT pathway has been shown related to the release of various cytokines and inflammatory mediators and involved in the regulation of immune response in sepsis. Moreover, JAK/STAT pathway has been shown involved in organ damage and other dysfunctions in sepsis models.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Sepsis is a syndrome with multiple organ or tissue damage caused by systematic inflammatory reactions resulting from infection and trauma. It was estimated that sepsis occurred in 750,000 patients in the USA annually [1], and the annual total costs have been estimated to be approximately $16.7 billion nationally [2]. Although the total inhospital mortality rate fell from 27.8 % during the period from 1979 to 1984 to 17.9 % during the period from 1995 to 2000, yet the total number of deaths continued to increase [3]. The Centers for Disease Control and Prevention has estimated that sepsis is the 10th leading cause of death overall in the USA [4] and the second leading cause of death among patients in noncoronary intensive care units (ICUs) [5]. In mainland China, a latest data from the China Critical Care Clinical Trials Group (CCCCTG) showed that 37.3 per 100 ICU admissions were diagnosed with severe sepsis or septic shock. The most frequent sites of infection were the lung and abdomen. And the overall ICU and hospital mortality rates were 28.7 and 33.5 % (n = 162), respectively [6].
Cytokines refer to a functional class of small protein mediators with low molecular weights; their half-life is very short, only a few minutes to a few hours, but play an important role in the inflammatory process. Varied kinds of cells including macrophages, fibroblasts, endothelial cells, lymphocytes, and smooth muscle cells can release a wide range of cytokines, including pro-inflammatory cytokines, such as interleukin (IL)-1, IL-6, and tumor necrosis factor (TNF)-α, as well as anti-inflammatory cytokines, such as IL-10, transforming growth factor (TGF)-β, and IL-4. Plasma levels of cytokines are significantly elevated in response to the initial host-microbial interaction and believed to play a crucial role in the pathogenesis of sepsis. Once released, cytokines lead to an ensuing activation of the innate or the adaptive immune response, characterized by the further production of immunoregulatory or effector cytokines [7].
Cytokines utilize complex signaling cascades to exert their biological effects. The Janus kinase-signal transducer and activator of transcription (JAK/STAT) pathway is one of the major signaling pathways believed to be involved in sepsis. The JAK/STAT pathway is an essential pathway for the signal transduction of many pivotal cytokines in the pathogenesis of sepsis, such as IL-4, IL-6, IL-10, IL-12, and interferon (IFN)-γ [8–12]. JAKs represent a family of four non-receptor tyrosine kinases: Jak1, Jak2, Jak3, and Tyk2. STATs comprise a family of seven structurally and functionally related proteins: Stat1, Stat2, Stat3, Stat4, Stat5a, Stat5b, and Stat6. JAK kinases can selectively phosphorylate STATs, leading to their activation. Once activated, STATs can transfer to the nucleus and play a critical role in the transcription of target genes, regulating innate and acquired host immune responses. To date, knowledge of JAK/STAT pathway in sepsis is limited. Here, we reviewed all relevant articles and tried to describe the specific roles of JAK/STAT signaling pathway in sepsis.
JAK FAMILY AND COMPONENTS
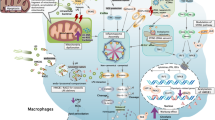
In mammals, JAK family includes four members, Jak1, Jak2, Jak3, and Tyk2. They are over 1000 amino acids in length, ranging in molecular weight from 120 to 140 kDa. Three members of the family (Jak1, Jak2, and Tyk2) were expressed ubiquitously, whereas the fourth member, Jak3, was found to be expressed predominantly in hematopoietic cells [13, 14]. Each JAK protein contains four main domains (Fig. 1). The N-terminal four-point-one, ezrin, radixin, moesin (FERM) domain, consisting of three sub-domains, is able to mediate protein-protein interactions, including adaptor and scaffolding interactions with cytokine receptors and membrane-bound proteins [15]. The Src-homology 2 (SH2) domain, a ~100-residue motif that binds to phosphotyrosine residues, provides a mechanism for reading the code [16]. The “pseudokinase” domain, with no catalytic function, plays a regulatory role [17]. And the classical protein tyrosine kinase (PTK) domain, including an ATP-binding site and a catalytic region, is able to phosphorylate tyrosine residues, thereby writing the code [18].
STAT FAMILY AND COMPONENTS
STAT family consists of seven members, Stat1, Stat2, Stat3, Stat4, Stat5a, Stat5b, and Stat6. The seven STAT proteins range in size from 750 to 850 amino acids. STATs possess a common structural organization with highly conserved regions, including the N-terminal domain, the coiled-coiled domain (CCD), the DNA binding domain (DBD), the linker domain, and the SH2/tyrosine activation domain. On the contrary, the carboxy-terminal transcriptional activation domain (TAD) is quite divergent [17] (Fig. 1). The N-terminal domain is associated with the combination of STATs with tandem gamma-activated site (GAS) elements [19, 20], the interaction of STATs with receptor domains [21], and the nuclear translocation [22]. The CCD is involved with specific interactions with other helical proteins [23, 24]; in addition, it is related to tyrosine phosphorylation and nuclear export [25, 26]. The DBD is important in effective transcriptional activity. The linker domain is also related to transcriptional regulation [27]. The SH2 domain plays an important role in recruitment to the cytokine receptor and recognition of specific receptor phosphotyrosine motifs; in addition, it is associated with the JAK activation and STAT dimerization [28, 29]. The C-terminal TAD mediates transactivation [30].
THE CANONICAL JAK/STAT SIGNALING PATHWAY
Signaling through the JAK/STAT pathway is initiated when a peptide ligand (e.g., a cytokine) binds to its corresponding homodimeric or heterodimeric receptors; then, receptor-associated JAKs are brought into close proximity through receptor oligomerization. This leads to conformational changes in the cytoplasmic portion of the receptor, initiating activation of receptor-associated members of the JAK family of kinases. The activated JAKs subsequently phosphorylate cytokine receptors, allowing STATs to bind via SH2-phosphotyrosine interactions. STATs also become phosphorylated when recruited to the receptor. Activated STATs dissociate from the receptor, dimerize by reciprocal phosphotyrosine and SH2 domain, and translocate to the nucleus by a mechanism that is dependent on importin α-5 and the Ran nuclear import pathway. In the nucleus, dimerized STATs bind specific regulatory sequences to activate or repress transcription of target genes (Fig. 2) [31, 32].
Using gene-deficient mice and cell lines, many cytokine-specific JAKs and STATs have been determined. For example, Jak2-deficient mice were embryonic lethal due to the absence of definitive erythropoiesis, and Jak2-deficient cells failed to respond to IL-3, granulocyte/macrophage colony-stimulating factor (GM-CSF) and IFN-γ [33, 34]. For another example, Stat1-deficient mice displayed a complete lack of responsiveness to either IFN-α or IFN-γ and were highly sensitive to infection by microbial pathogens and viruses [35]. Table 1 summarizes some cytokine-specific JAKs and STATs.
NEGATIVE PATHWAY REGULATORS
The JAK/STAT pathway has some negative regulators that modulate the signaling, such as protein tyrosine phosphatases (PTPs), suppressors of cytokine signaling (SOCS), protein inhibitors of activated stats (PIAS). SH2-containing protein tyrosine phosphatase (SHP-1) is one of the most characterized PTPs. SHP-1 contains two SH2 domains, a catalytic PTP domain, and a divergent C-terminal region. The SH2 domains can bind to either phosphorylated JAKs or phosphorylated receptors to facilitate dephosphorylation of these activated signaling molecules. The divergent C-terminal region contains several tyrosine residues that serve as docking sites for other signaling proteins when phosphorylated. Other PTPs, such as CD45, appear to have a role in regulating JAK/STAT signaling through a subset of receptors. SOCS family contains eight proteins: CIS, SOCS1, SOCS2, SOCS3, SOCS4, SOCS5, SOCS6, and SOCS7. Each SOCS consists of a N-terminal region with variable length and sequence, a central SH2 domain responsible for binding to cytokine receptors, JAKs and other substrates, and a C-terminal SOCS box domain that facilitates protein degradation of the cytokine receptor signaling molecules. The SOCS completes a negative feedback loop in the JAK/STAT pathway: activated STATs stimulate transcription of the SOCS genes; on the contrary, SOCS proteins bind phosphorylated JAKs and their receptors to turn off the pathway. SOCSs can bind phosphotyrosines on the receptors to block the recruitment of STATs to the receptor. SOCSs can also bind directly to JAKs or to the receptors to specifically inhibit JAK kinase activity. In addition, SOCSs can facilitate the ubiquitination of JAKs and the receptors. Ubiquitination of these targets decreases their stability by targeting them for proteasomal degradation [36, 37]. Using computer-aided studies, Paracha et al. [38] found intervention in the downregulation of pro-inflammatory cytokines by SOCS-1 was desirable to boost the immune responses; and they suggested that this intervention may produce useful results in the case of immunocompromised septic patients. PIAS proteins include PIAS1, PIAS3, PIASx, and PIASy. PIAS protein contains a SAF-A/B, Acinus and PIAS (SAP) domain, a Zn-binding RING-finger domain, and a less well-conserved C-terminal serine/threonine rich region. The C-terminal domain is involved in target protein binding. PIAS proteins can bind to activated STAT dimers and prevent them from binding DNA [32].
JAK/STAT PATHWAY IN SEPSIS
Jak2 and Stat3 in CLP-Induced Sepsis
Hui et al. [39] investigated the potential roles of JAK/STAT pathway in regulation of systemic inflammatory response induced by septic challenge in vivo. They found Jak2 could be quickly activated in septic rats. Activation of Jak2 peaked at 2 h in pulmonary tissues and 6 h in hepatic tissues. In addition, nuclear translocation of Stat3 was markedly increased in liver and lungs of cecal ligation and puncture (CLP) rats. It was detectable 2 h after CLP, maintaining at high values up to 48 h. They demonstrated that JAK/STAT pathway could modulate multiple organ damage in CLP-induced septic rats. Inhibition of Jak2 or Stat3 attenuated the multiple organ damage and lethality resulted from CLP-induced severe sepsis. In addition, a study about anti-inflammation effects of LXM-10 (2, 4-dimethyl-9-b-phenylethyl-3-oxo-6, 9-diazaspiro [5. 5] undecane chloride) in both acute and chronic inflammation models also demonstrated that inhibition of Jak2/Stat3 signaling pathway could reduce the production of pro-inflammatory cytokines of TNF-α and IL-6. Both IL-6 and TNF-α played critical roles in the inflammatory response and were capable of causing end-organ dysfunction that occurred in severe sepsis [40].
Another study also strongly suggested that Jak2 contributed to the innate immune responses induced during sepsis. Pena et al. [41] found Jak2 inhibition with AG490, a well-established Jak2 inhibitor, could rescue animals from polymicrobial sepsis in a clinically relevant time frame. Moreover, AG490 could prevent nuclear factor-κB (NF-κB) activation, modulate macrophage activation, and restrain the production of inflammatory cytokines. They found Jak2 inhibition restrained serum levels of TNF by modulating TNF production in the lung and the spleen and protected mice from lethal endotoxemia in a concentration-dependent manner. In addition, they demonstrated that Jak2 inhibition specifically prevented lipopolysaccharide (LPS)-induced Stat3 tyrosine phosphorylation. The researchers further investigated the immunological implications of tyrosine-unphosphorylated Stat3 in infectious diseases [42]. They found Stat3 mediated the anti-inflammation pathway of the α7 nicotinic receptor (α7nAChR) to control innate immune responses to infection. Inhibition of Stat3 tyrosine phosphorylation restrained the inflammatory responses, inhibiting NF-κB and cytokine production in macrophages. In vivo inhibition of Stat3 tyrosine phosphorylation by stattic prevented systemic inflammation and improved survival in experimental sepsis in mice. Moreover, they found inhibition of Stat3 protein expression enhanced cytokine production and abrogated α7nAChR signaling. In addition, Claire et al. [43] found genetic reduction of Stat3 activity alleviated LPS hypersensitivity and reduced LPS-induced IL-6 production in mice. They suggested that IL-6 trans-signaling via Stat3 was a critical modulator of LPS-driven pro-inflammatory responses.
Jak1, Stat3, and Stat1 in IL-10-Mediated Inflammatory Responses
IL-10 is an important cytokine in immune responses. One of the most unique roles of IL-10 is to inhibit the production of pro-inflammatory cytokines, such as TNF-α, IL-1, and IL-12, which are synthesized by macrophages in response to bacterial products, such as LPS. This activity results in decreased IFN-γ production and inhibition of cell-mediated immune responses, accompanied by enhancement of humoral immunity [44]. It has been reported that the characteristic ability of IL-10 to inhibit TNF-α production in LPS-stimulated macrophages displayed an obligate dependence on Jak1 [45]. Rodig et al. found cells from Jak1-deficient mice failed to develop biologic responses to ligands of class II cytokine receptors (i.e., the receptors for IFN-α/β, IFN-γ, and IL-10).
In addition, Riley et al. [44] demonstrated that macrophages derived from mice engineered to express a genetic Stat3 deficiency in the myeloid cell compartment failed to respond to IL-10 and secrete high levels of TNF-α upon stimulation with IL-10 plus LPS. This result thus confirmed the requirement of Stat3 for the anti-inflammation functions of IL-10 in macrophages. Takeda et al. [46] also demonstrated that Stat3 activation in macrophages was indispensable for IL-10-mediated anti-inflammatory responses in a murine model of endotoxin shock. They found dramatically increased levels of TNF-α, IL-1β, IL-12, and IFN-γ in Stat3-deficient mice. They further identified the functional role of Stat3 in innate immune cells during sepsis. Using a murine model of sepsis induced by CLP, they found that mice with Stat3 deficiency in macrophages and neutrophils were highly susceptible to sepsis induced by CLP. And the increased lethality resulted from aberrant inflammation, characterized by excessive systemic inflammatory responses with serious multiple organ failure (MOF) [47].
IL-10 has also shown to exhibit stimulatory functions including CD14 upregulation on human monocytic cells. CD14 is the primary LPS receptor. The interaction of LPS with CD14 on monocytic cells plays a critical role in the activation of these cells during inflammation and sepsis [48]. Rahimi et al. [49] found that endogenously produced IL-10 played an important role in LPS-induced CD14 expression in monocytic cells, and IL-10-induced CD14 expression involved the activation of Stat1. IL-10 failed to stimulate CD14 expression in cells transfected with Stat1 siRNA plasmid compared with the cells transfected with the control plasmid. They suggested that IL-10-induced CD14 upregulation in human monocytic cells may be mediated by Stat1 activation.
Tyk2, STAT1, and STAT4 in LPS-Induced Endotoxin Shock
Kamezaki et al. [50] found mice lacking Tyk2, Stat1, or Stat4 were resistant to LPS-induced endotoxin shock. They injected a high dose of LPS into wild type (WT) and Tyk2-, Stat1-, or Stat-4 deficient mice, causing the activation of macrophages and the resulting secretion of a variety of cytokines and mediators. Results of survival showed Tyk2-deficient mice were extremely resistant to LPS shock, and Stat1- and Stat4-deficient mice were moderately resistant to LPS shock when compared with WT mice. Analyses of serum concentrations of several inflammatory cytokines after LPS challenge were shown in WT mice; serum concentrations of TNF-α and IL-12 were dramatically increased 2 h after LPS challenge. While in Tyk2-, Stat1-, or Stat-4-deficient mice, the elevation of TNF-α and IL-12 after LPS challenge was moderately decreased, measuring about 50~70 % of the levels observed in WT mice. IFN-γ was elevated by LPS challenge in WT mice. However, the level of serum IFN-γ remained low after LPS injection in Tyk2- and Stat4-deficient mice. In addition, IFN-β mRNA was induced by LPS stimulation in macrophages from wild-type mice. On the contrary, very little IFN-β mRNA induction was observed in macrophages from Tyk2- or Stat1-deficient mice. They suggested that the signaling pathway via Tyk2 played a substantial role in sensitivity to LPS, and this signal was transduced by the activation of both Stat1 and Stat4.
Based on the above results, Herzig et al. [51] further studied the responses of Stat1- and Tyk2-deficient mice to septic shock caused by CLP. Results showed that the survival rate was significantly higher in Stat1-deficient mice compared to wild-type controls (80 vs. 10 %). The improved survival of Stat1-deficient mice was associated with less hypothermia, metabolic acidosis, hypoglycemia, and hepatocellular injury. In addition, plasma IL-6, macrophage inflammatory protein (MIP)-2, C-X-C motif chemokine 10 (CXCL10), and IFN-α concentrations were significantly lower in Stat1-deficient mice than that in WT mice. In the absence of antibiotic treatment, bacteria colony-forming units (CFU) in blood and lungs were significantly lower in Stat1-deficient mice than in WT mice. Interestingly, they found survival was not significantly different between Tyk2-deficient and WT mice after CLP. However, plasma IL-6 and CXCL10 concentrations were significantly lower in Tyk2-deficient mice than that in WT mice. They suggested that Stat1 activation was an important factor in the pathogenesis of CLP-induced septic shock and was associated with the development of systemic inflammation and organ injury. Tyk2 activation also appeared to contribute to CLP-induced inflammation but to a lesser extent than Stat1.
Jak2 and Stat1 in LPS-Induced iNOS Expression
A previous report found that pretreatment with AG490 inhibited IFN-γ-induced Jak2, Stat1 tyrosine phosphorylation in astroglial cells. Moreover, pretreatment with AG490 inhibited IFN-γ-induced iNOS expression. They suggested Jak2 and Stat1 tyrosine phosphorylation was an early event involved in the expression of iNOS in astroglial cells [52]. This suggestion was consistent with another study, which found that silencing of Stat1 prevented the induction of iNOS and production of NO in response to stimulation of LPS/IFN-γ or TNF/IFN-γ in intestinal epithelial cells [53]. It has been reported that LPS stimulation could induce iNOS expression in macrophages. After stimulation of macrophages with LPS, significant NO accumulation in the culture medium was observed [54]. Large amount of NO production due to iNOS induction was one of the important characteristics in sepsis. Tsoyi et al. [55] tested the role of Jak2/Stat1 pathway on the LPS-mediated iNOS production in macrophages. They found pretreatment with AG490, or fludarabine (a Stat1 inhibitor), could significantly attenuate iNOS expression and resulted in a significant reduction of NO in macrophages. Moreover, transfection with siStat1 (siRNA against mouse STAT1 p84/p91) decreased the iNOS induction in LPS-treated macrophages. Thus, they suggested that Jak2/Stat1 pathway was involved in the regulation of iNOS in activated macrophages [56].
Jak2 and Stat5 in LPS-Induced IL-6 Production
IL-6 is a pro-inflammatory mediator. IL-6 production during experimental sepsis has been demonstrated to be harmful in rodents [57]. It has also been shown that blockade of IL-6 with antibodies at the start of CLP could greatly improve survival in mice in a dose-dependent manner [58]. Kimura et al. [59] investigated the role of Jak2 in LPS signaling and found LPS-induced IL-6 production was significantly impaired by AG490 at the protein level as well as at the mRNA level. In addition, they found Stat5 has a similar effect as that of Jak2 on LPS-induced IL-6 production. LPS-induced IL-6 production was reduced by the dominant negative form of Stat5 at the protein and mRNA levels in macrophage cells. These findings indicated that Jak2 and Stat5 participated in LPS signaling and had important roles in the LPS-induced IL-6 production pathway. In addition, they suggested that it was through regulation of Jak2 and Stat5 that SOCS-1, one of the negative JAK/STAT pathway regulators, selectively inhibited LPS-induced IL-6 production.
Stat4 and Stat6 in Endotoxin-Induced Organ Damage
Matsukawa et al. [60] explored the role of Stat4 and Stat6 in innate immunity during septic peritonitis. They found that Stat4-deficient mice were resistant to the lethality initiated by CLP. However, the bacterial load in the peritoneum in Stat4-deficient mice was similar to WT mice, and there were no differences in the numbers of infiltrating neutrophils and macrophages between WT and Stat4-deficient mice. In addition, the serum level of AST, ALT, BUN, and creatinine increased in WT mice after CLP, indicating that CLP caused liver and kidney damage in WT mice. However, the levels of AST, ALT, BUN, and creatinine in Stat4-deficient mice after CLP were remarkably reduced compared with WT mice, and the levels were comparable to those in untreated mice, suggesting that organ injury was evaded in Stat4-deficient mice. Therefore, they suggested that in Stat4-deficient mice, organ damage during sepsis was significantly ameliorated without affecting the bacterial load in the peritoneum. In addition, they measured organ-associated cytokine levels in Stat4-deficient mice. They found hepatic levels of IL-10 and IL-13 increased, while levels of MIP-2 and keratinocyte chemoattractant (KC) decreased in liver. Sepsis-induced renal injury was also abrogated in Stat4-deficient mice, which was accompanied by decreased renal levels of MIP-2 and KC without altering IL-10 and IL-13 levels. These results indicated that the cytokine profile in the organs during sepsis was altered in Stat4-deficient mice which may contribute to the improvement of organ injury and resistant to lethal sepsis.
The above study also found Stat6-deficient mice were resistant to the lethality initiated by CLP. It demonstrated that the mean peritoneal CFU in Stat6-deficient mice was 10-fold lower than that in WT mice. Likewise, the bacterial load recovered from peripheral blood in Stat6-deficient mice was lower than that in WT mice. In addition, the number of neutrophils after CLP in Stat6-deficient mice was significantly increased compared with WT mice. These results suggested that the bacterial clearance and neutrophil influx were augmented in Stat6-deficient mice. The researchers also found in Stat6-deficient mice, the peritoneal levels of IL-12, TNF-α, macrophage-derived chemokine (MDC), and C10 were augmented. Thus, they suggested Stat6-deficient mice resisted to septic peritonitis by an altered cytokine profile in the peritoneum, which was in favor of bacterial clearance [60].
However, several studies found contrasting observations. Lentsch et al. [61] demonstrated that mice deficient for Stat4 and Stat6 were far more susceptible to lethal endotoxemia than WT mice. They found after administration of endotoxin, 20 % of WT mice died within 24 h, but none died thereafter; in Stat4-deficient mice, 40 % of them died within 2 days, 60 % died after 3 days, and 80 % died after 4 days; while in Stat6-deficient mice, 36 % died after 24 h, 91 % died after 2 days, and 100 % died by 3 days. In addition, they found in Stat6-deficient mice that dysregulated activation of the transcription factor NF-κB led to augmented production of pro-inflammatory cytokines and chemokines in the lung and liver, including MIP-1α, MIP-2, interferon-inducible protein (IP)-10, and monocyte chemotactic protein (MCP)-1. Moreover, Stat6-deficient mice displayed increased accumulation of neutrophils and leukocytes in the liver and lung following endotoxin, which may contribute to organ damage. They suggested Stat6 appeared to be required for the regulation of cytokine and chemokine production during endotoxemia.
Jak3 and Stat3 in Myocardial Vascular Permeability
On the contrary to other JAK family members, Jak3 is preferentially expressed in the lymphoid or myeloid cell lineages [62]. However, recent studies demonstrated that vascular endothelial cells and non-lymphoid and non-myeloid cells could also express and induce significant levels of Jak3 via treatment with IL-1β, TNF-α, IFN-γ, and LPS [63]. Sepsis is characterized by a blunting of the endothelial response and an increase in vascular permeability [64]. Sepsis-induced blunting of the endothelial response results in the dysregulation of arteriolar tone and contributes to myocardial dysfunction. Lee et al. [65] investigated the effect of Jak3 inhibition on TNF-α-induced expression of cell adhesion molecules in vascular endothelial cells and evaluated the therapeutic potential of Jak3 for myocardial vascular permeability in endotoxemic mice. Results showed that TNF-α significantly increased the levels of intercellular adhesion molecule (ICAM)-1, vascular cell adhesion molecule (VCAM)-1, and fractalkine in their endotoxemic model. However, pretreatment of JANEX-1 (a Jak3 inhibitor) blocked the effect of TNF-α on the expression of ICAM-1, VCAM-1, and fractalkine. In addition, they found the injection of LPS into mice increased the levels of phosphorylated Stat3 and ICAM-1 in the heart tissue, and JANEX-1 could improve myocardial vascular leakage by decreasing Stat3 phosphorylation and ICAM-1 expression in endotoxemic mice. These observations suggested that the regulation of Jak3 and Stat3 may ameliorate the myocardial vascular leakage caused by endotoxemia and protect myocardial function in sepsis.
Jak2, Stat1, and Stat3 in HMGB1 Expression
It was reported that JAK/STAT pathway involved in the secretion of high mobility group β protein-1 (HMGB1), a late pro-inflammatory mediator which contributed to the progression of sepsis [66]. HMGB1 was a 30-kD heparin-binding protein involved in process extension and migration of cells [67]. High levels of HMGB1 were released to serum during septic shock, significantly stimulating the release of TNF, IL-1α, IL-1β, IL-1RA, IL-6, IL-8, and MIP-1α in human monocyte cultures [68]. Studies showed LPS-induced HMGB1 release was associated with TLR4, TRIF, IFN-β, and JAK/STAT signaling. Inhibition of any of these signals could dramatically reduce the HMGB1 release [69]. This result was consistent with another study which examined the role of JAK/STAT pathway in the regulation of expression of HMGB1. They found stimulation of LPS in vitro significantly increased HMGB1 expression and prompted activation of JAK/STAT pathway. However, administration of AG490 (a specific inhibitor for Jak2), fludarabine (a specific inhibitor for Stat1), or rapamycin (a specific inhibitor for Stat3) markedly suppressed HMGB1 expression. In addition, when macrophages were stimulated with HMGB1 in vitro, significant increases in TNF-α expression were demonstrated. On the contrary, mRNA expression of TNF-α could be suppressed by JAK/STAT pathway inhibitors [70]. Lu et al. [71] demonstrated that Jak/Stat1 pathway played a critical role in mediating HMGB1 cytoplasmic accumulation for subsequent release. They found pharmacological inhibition of Jak/Stat1 inhibited LPS-induced HMGB1 nuclear translocation. Conversely, activation of Jak/Stat1 by IFN-β stimulation induced HMGB1 translocation from the nucleus to cytoplasm. In addition, LPS stimulation induced lysine acetylation within both HMGB1 nuclear localization sequence (NLS) sites and genetic deletion of Stat1 abrogated LPS- and IFN-β-induced HMGB1 acetylation within the NLS sites, indicting that Jak/Stat1 was required for LPS- or IFN-β-induced HMGB1 hyperacetylation within NLS sites and nucleus to cytoplasm translocation of HMGB1.
CONCLUSION AND OUTLOOK
Cytokines play an important role in sepsis, affecting the anti-inflammatory and pro-inflammatory responses and the outcome of the disease. The JAK/STAT pathway is one of the principal signaling pathways for a wide range of cytokines. With the components and functions of the JAKs and STATs family were discovered, the canonical JAK/STAT signaling pathway and its negative regulators, as well as various cytokine-specific JAKs and STATs have been demonstrated. During the past several years, significant progress has been made in the roles of JAK/STAT pathway on sepsis. JAK/STAT pathway was related to the release of various cytokines and inflammatory mediators such as IL-6, IL-10, iNOS, and HMGB1 and involved in the regulation of immune response in sepsis. Moreover, the JAK/STAT pathway was involved in endotoxin shock, organ damage, and myocardial vascular permeability in sepsis models. Therefore, we suggest that JAK/STAT pathway can serve as a potential target for therapeutic intervention in sepsis. And more in-depth analyses about the regulatory function of JAK/STAT pathway in sepsis are the focus of future researches. In addition, we should turn more attention to the roles of negative regulators of JAK/STAT pathway on the treatment of sepsis.
References
Angus, D.C., W.T. Linde-Zwirble, J. Lidicker, G. Clermont, J. Carcillo, and M.R. Pinsky. 2001. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Critical Care Medicine 29(7): 1303–1310.
Chalupka, A.N., and D. Talmor. 2012. The economics of sepsis. Critical Care Clinics 28(1): 57–76. doi:10.1016/j.ccc.2011.09.003. 1.
Martin, G.S., D.M. Mannino, S. Eaton, and M. Moss. 2003. The epidemiology of sepsis in the United States from 1979 through 2000. New England Journal of Medicine 348(16): 1546–1554. doi:10.1056/Nejmoa022139.
Hoyert, D.L., E. Arias, B.L. Smith, S.L. Murphy, and K.D. Kochanek. 2001. Deaths: Final data for 1999. National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System 49(8): 1–113.
Parrillo, J.E., M.M. Parker, C. Natanson, A.F. Suffredini, R.L. Danner, R.E. Cunnion, et al. 1990. Septic shock in humans. Advances in the understanding of pathogenesis, cardiovascular dysfunction, and therapy. Annals of Internal Medicine 113(3): 227–242.
Zhou, J., C. Qian, M. Zhao, X. Yu, Y. Kang, X. Ma, et al. 2014. Epidemiology and outcome of severe sepsis and septic shock in intensive care units in mainland China. PloS One 9(9): e107181. doi:10.1371/journal.pone.0107181.
Cavaillon, J.M., C. Munoz, C. Fitting, B. Misset, and J. Carlet. 1992. Circulating cytokines: the tip of the iceberg? Circulatory Shock 38(2): 145–152.
Wu, H.P., C.L. Wu, C.K. Chen, K. Chung, J.C. Tseng, Y.C. Liu, et al. 2008. The interleukin-4 expression in patients with severe sepsis. Journal of Critical Care 23(4): 519–524. doi:10.1016/j.jcrc.2007.11.008.
Steinhauser, M.L., C.M. Hogaboam, N.W. Lukacs, R.M. Strieter, and S.L. Kunkel. 1999. Multiple roles for IL-12 in a model of acute septic peritonitis. Journal of Immunology 162(9): 5437–5443.
Song, G.Y., C.S. Chung, I.H. Chaudry, and A. Ayala. 1999. What is the role of interleukin 10 in polymicrobial sepsis: Anti-inflammatory agent or immunosuppressant? Surgery 126(2): 378–383.
Nijsten, M.W., C.E. Hack, M. Helle, H.J. ten Duis, H.J. Klasen, and L.A. Aarden. 1991. Interleukin-6 and its relation to the humoral immune response and clinical parameters in burned patients. Surgery 109(6): 761–767.
Heinzel, F.P. 1990. The role of IFN-gamma in the pathology of experimental endotoxemia. Journal of Immunology 145(9): 2920–2924.
Jaime-Figueroa, S., J. De Vicente, J. Hermann, A. Jahangir, S. Jin, A. Kuglstatter, et al. 2013. Discovery of a series of novel 5H-pyrrolo[2,3-b]pyrazine-2-phenyl ethers, as potent JAK3 kinase inhibitors. Bioorganic & Medicinal Chemistry Letters 23(9): 2522–2526. doi:10.1016/j.bmcl.2013.03.015.
O’Shea, J.J., M. Pesu, D.C. Borie, and P.S. Changelian. 2004. A new modality for immunosuppression: Targeting the JAK/STAT pathway. Nature Reviews Drug Discovery 3(7): 555–564. doi:10.1038/nrd1441.
Tepass, U. 2009. FERM proteins in animal morphogenesis. Current Opinion in Genetics & Development 19(4): 357–367. doi:10.1016/j.gde.2009.05.006.
Olsen, J.V., B. Blagoev, F. Gnad, B. Macek, C. Kumar, P. Mortensen, et al. 2006. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell 127(3): 635–648. doi:10.1016/j.cell.2006.09.026.
Kisseleva, T., S. Bhattacharya, J. Braunstein, and C.W. Schindler. 2002. Signaling through the JAK/STAT pathway, recent advances and future challenges. Gene 285(1–2): 1–24.
Lim, W.A., and T. Pawson. 2010. Phosphotyrosine signaling: Evolving a new cellular communication system. Cell 142(5): 661–667. doi:10.1016/j.cell.2010.08.023.
Vinkemeier, U., I. Moarefi, J.E. Darnell Jr., and J. Kuriyan. 1998. Structure of the amino-terminal protein interaction domain of STAT-4. Science 279(5353): 1048–1052.
Vinkemeier, U., S.L. Cohen, I. Moarefi, B.T. Chait, J. Kuriyan, and J.E. Darnell Jr. 1996. DNA binding of in vitro activated Stat1 alpha, Stat1 beta and truncated Stat1: Interaction between NH2-terminal domains stabilizes binding of two dimers to tandem DNA sites. The EMBO Journal 15(20): 5616–5626.
Murphy, T.L., E.D. Geissal, J.D. Farrar, and K.M. Murphy. 2000. Role of the Stat4 N domain in receptor proximal tyrosine phosphorylation. Molecular and Cellular Biology 20(19): 7121–7131.
Strehlow, I., and C. Schindler. 1998. Amino-terminal signal transducer and activator of transcription (STAT) domains regulate nuclear translocation and STAT deactivation. The Journal of Biological Chemistry 273(43): 28049–28056.
Collum, R.G., S. Brutsaert, G. Lee, and C. Schindler. 2000. A Stat3-interacting protein (StIP1) regulates cytokine signal transduction. Proceedings of the National Academy of Sciences of the United States of America 97(18): 10120–10125. doi:10.1073/pnas.170192197.
Zhu, M., S. John, M. Berg, and W.J. Leonard. 1999. Functional association of Nmi with Stat5 and Stat1 in IL-2- and IFN gamma-mediated signaling. Cell 96(1): 121–130.
Zhang, T., W.H. Kee, K.T. Seow, W. Fung, and X. Cao. 2000. The coiled-coil domain of Stat3 is essential for its SH2 domain-mediated receptor binding and subsequent activation induced by epidermal growth factor and interleukin-6. Molecular and Cellular Biology 20(19): 7132–7139.
Begitt, A., T. Meyer, M. van Rossum, and U. Vinkemeier. 2000. Nucleocytoplasmic translocation of Stat1 is regulated by a leucine-rich export signal in the coiled-coil domain. Proceedings of the National Academy of Sciences of the United States of America 97(19): 10418–10423. doi:10.1073/pnas.190318397.
Yang, E., Z. Wen, R.L. Haspel, J.J. Zhang, and J.E. Darnell Jr. 1999. The linker domain of Stat1 is required for gamma interferon-driven transcription. Molecular and Cellular Biology 19(7): 5106–5112.
Barahmand-Pour, F., A. Meinke, B. Groner, and T. Decker. 1998. Jak2-Stat5 interactions analyzed in yeast. The Journal of Biological Chemistry 273(20): 12567–12575.
Gupta, S., H. Yan, L.H. Wong, S. Ralph, J. Krolewski, and C. Schindler. 1996. The SH2 domains of Stat1 and Stat2 mediate multiple interactions in the transduction of IFN-alpha signals. The EMBO Journal 15(5): 1075–1084.
Park, C., M.J. Lecomte, and C. Schindler. 1999. Murine Stat2 is uncharacteristically divergent. Nucleic Acids Research 27(21): 4191–4199.
Matsukawa, A. 2007. STAT proteins in innate immunity during sepsis: Lessons from gene knockout mice. Acta Medica Okayama 61(5): 239–245.
Rawlings, J.S., K.M. Rosler, and D.A. Harrison. 2004. The JAK/STAT signaling pathway. Journal of Cell Science 117(Pt 8): 1281–1283. doi:10.1242/jcs.00963.
Parganas, E., D. Wang, D. Stravopodis, D.J. Topham, J.C. Marine, S. Teglund, et al. 1998. Jak2 is essential for signaling through a variety of cytokine receptors. Cell 93(3): 385–395.
Neubauer, H., A. Cumano, M. Muller, H. Wu, U. Huffstadt, and K. Pfeffer. 1998. Jak2 deficiency defines an essential developmental checkpoint in definitive hematopoiesis. Cell 93(3): 397–409.
Meraz, M.A., J.M. White, K.C. Sheehan, E.A. Bach, S.J. Rodig, A.S. Dighe, et al. 1996. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell 84(3): 431–442.
Yoshimura, A., H. Nishinakamura, Y. Matsumura, and T. Hanada. 2005. Negative regulation of cytokine signaling and immune responses by SOCS proteins. Arthritis Research & Therapy 7(3): 100–110. doi:10.1186/ar1741.
Liongue, C., and A.C. Ward. 2013. Evolution of the JAK-STAT pathway. Jak-Stat 2(1): e22756. doi:10.4161/jkst.22756.
Paracha, R.Z., J. Ahmad, A. Ali, R. Hussain, U. Niazi, S.H. Tareen, et al. 2014. Formal modelling of toll like receptor 4 and JAK/STAT signalling pathways: Insight into the roles of SOCS-1, interferon-beta and proinflammatory cytokines in sepsis. PloS One 9(9): e108466. doi:10.1371/journal.pone.0108466.
Hui, L., Y. Yao, S. Wang, Y. Yu, N. Dong, H. Li, et al. 2009. Inhibition of Janus kinase 2 and signal transduction and activator of transcription 3 protect against cecal ligation and puncture-induced multiple organ damage and mortality. The Journal of Trauma 66(3): 859–865. doi:10.1097/TA.0b013e318164d05f.
Zhang, W., Q. Sun, X. Gao, Y. Jiang, R. Li, and J. Ye. 2013. Anti-inflammation of spirocyclopiperazinium salt compound LXM-10 targeting alpha7 nAChR and M4 mAChR and inhibiting JAK2/STAT3 pathway in rats. PloS One 8(6): e66895. doi:10.1371/journal.pone.0066895.
Pena, G., B. Cai, E.A. Deitch, and L. Ulloa. 2010. JAK2 inhibition prevents innate immune responses and rescues animals from sepsis. Journal of Molecular Medicine 88(8): 851–859. doi:10.1007/s00109-010-0628-z.
Pena, G., B. Cai, J. Liu, E.P. van der Zanden, E.A. Deitch, W.J. de Jonge, et al. 2010. Unphosphorylated STAT3 modulates alpha 7 nicotinic receptor signaling and cytokine production in sepsis. European Journal of Immunology 40(9): 2580–2589. doi:10.1002/eji.201040540.
Greenhill, C.J., S. Rose-John, R. Lissilaa, W. Ferlin, M. Ernst, P.J. Hertzog, et al. 2011. IL-6 trans-signaling modulates TLR4-dependent inflammatory responses via STAT3. Journal of Immunology 186(2): 1199–1208. doi:10.4049/jimmunol.1002971.
Riley, J.K., K. Takeda, S. Akira, and R.D. Schreiber. 1999. Interleukin-10 receptor signaling through the JAK-STAT pathway. Requirement for two distinct receptor-derived signals for anti-inflammatory action. The Journal of Biological Chemistry 274(23): 16513–16521.
Rodig, S.J., M.A. Meraz, J.M. White, P.A. Lampe, J.K. Riley, C.D. Arthur, et al. 1998. Disruption of the Jak1 gene demonstrates obligatory and nonredundant roles of the Jaks in cytokine-induced biologic responses. Cell 93(3): 373–383.
Takeda, K., B.E. Clausen, T. Kaisho, T. Tsujimura, N. Terada, I. Forster, et al. 1999. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity 10(1): 39–49.
Matsukawa, A., K. Takeda, S. Kudo, T. Maeda, M. Kagayama, and S. Akira. 2003. Aberrant inflammation and lethality to septic peritonitis in mice lacking STAT3 in macrophages and neutrophils. Journal of Immunology 171(11): 6198–6205.
Triantafilou, M., and K. Triantafilou. 2002. Lipopolysaccharide recognition: CD14, TLRs and the LPS-activation cluster. Trends in Immunology 23(6): 301–304.
Rahimi, A.A., K. Gee, S. Mishra, W. Lim, and A. Kumar. 2005. STAT-1 mediates the stimulatory effect of IL-10 on CD14 expression in human monocytic cells. Journal of Immunology 174(12): 7823–7832.
Kamezaki, K., K. Shimoda, A. Numata, T. Matsuda, K. Nakayama, and M. Harada. 2004. The role of Tyk2, Stat1 and Stat4 in LPS-induced endotoxin signals. International Immunology 16(8): 1173–1179. doi:10.1093/intimm/dxh118.
Herzig, D., G. Fang, T.E. Toliver-Kinsky, Y. Guo, J. Bohannon, and E.R. Sherwood. 2012. STAT1-deficient mice are resistant to cecal ligation and puncture-induced septic shock. Shock 38(4): 395–402. doi:10.1097/SHK.0b013e318265a2ab.
Dell’Albani, P., R. Santangelo, L. Torrisi, V.G. Nicoletti, J. de Vellis, and A.M. Giuffrida Stella. 2001. JAK/STAT signaling pathway mediates cytokine-induced iNOS expression in primary astroglial cell cultures. Journal of Neuroscience Research 65(5): 417–424.
Stempelj, M., M. Kedinger, L. Augenlicht, and L. Klampfer. 2007. Essential role of the JAK/STAT1 signaling pathway in the expression of inducible nitric-oxide synthase in intestinal epithelial cells and its regulation by butyrate. The Journal of Biological Chemistry 282(13): 9797–9804. doi:10.1074/jbc.M609426200.
Hong, J., S. Sang, H.J. Park, S.J. Kwon, N. Suh, M.T. Huang, et al. 2006. Modulation of arachidonic acid metabolism and nitric oxide synthesis by garcinol and its derivatives. Carcinogenesis 27(2): 278–286. doi:10.1093/carcin/bgi208.
Tsoyi, K., H.J. Kim, J.S. Shin, D.H. Kim, H.J. Cho, S.S. Lee, et al. 2008. HO-1 and JAK-2/STAT-1 signals are involved in preferential inhibition of iNOS over COX-2 gene expression by newly synthesized tetrahydroisoquinoline alkaloid, CKD712, in cells activated with lipopolysacchride. Cellular Signalling 20(10): 1839–1847. doi:10.1016/j.cellsig.2008.06.012.
Tsoyi, K., I.T. Nizamutdinova, H.J. Jang, L. Mun, H.J. Kim, H.G. Seo, et al. 2010. Carbon monoxide from CORM-2 reduces HMGB1 release through regulation of IFN-beta/JAK2/STAT-1/INOS/NO signaling but not COX-2 in TLR-activated macrophages. Shock 34(6): 608–614. doi:10.1097/SHK.0b013e3181e46f15.
Riedemann, N.C., T.A. Neff, R.F. Guo, K.D. Bernacki, I.J. Laudes, J.V. Sarma, et al. 2003. Protective effects of IL-6 blockade in sepsis are linked to reduced C5a receptor expression. Journal of Immunology 170(1): 503–507.
Riedemann, N.C., R.F. Guo, T.J. Hollmann, H. Gao, T.A. Neff, J.S. Reuben, et al. 2004. Regulatory role of C5a in LPS-induced IL-6 production by neutrophils during sepsis. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology 18(2): 370–372. doi:10.1096/fj.03-0708fje.
Kimura, A., T. Naka, T. Muta, O. Takeuchi, S. Akira, I. Kawase, et al. 2005. Suppressor of cytokine signaling-1 selectively inhibits LPS-induced IL-6 production by regulating JAK-STAT. Proceedings of the National Academy of Sciences of the United States of America 102(47): 17089–17094. doi:10.1073/pnas.0508517102.
Matsukawa, A., M.H. Kaplan, C.M. Hogaboam, N.W. Lukacs, and S.L. Kunkel. 2001. Pivotal role of signal transducer and activator of transcription (Stat)4 and Stat6 in the innate immune response during sepsis. The Journal of Experimental Medicine 193(6): 679–688.
Lentsch, A.B.., A. Kato, B. Davis, W. Wang, C. Chao, and M.J. Edwards. 2001. STAT4 and STAT6 regulate systemic inflammation and protect against lethal endotoxemia. The Journal of Clinical Investigation 108(10): 1475–1482. doi:10.1172/JCI13763.
Gurniak, C.B., and L.J. Berg. 1996. Murine JAK3 is preferentially expressed in hematopoietic tissues and lymphocyte precursor cells. Blood 87(8): 3151–3160.
Verbsky, J.W., E.A. Bach, Y.F. Fang, L. Yang, D.A. Randolph, and L.E. Fields. 1996. Expression of Janus kinase 3 in human endothelial and other non-lymphoid and non-myeloid cells. The Journal of Biological Chemistry 271(24): 13976–13980.
Aird, W.C. 2003. The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood 101(10): 3765–3777. doi:10.1182/blood-2002-06-1887.
Lee, J.E., A.S. Lee, D.H. Kim, Y.J. Jung, S. Lee, B.H. Park, et al. 2012. Janex-1, a JAK3 inhibitor, ameliorates tumor necrosis factor-alpha-induced expression of cell adhesion molecules and improves myocardial vascular permeability in endotoxemic mice. International Journal of Molecular Medicine 29(5): 864–870. doi:10.3892/ijmm.2012.920.
Wang, H., O. Bloom, M. Zhang, J.M. Vishnubhakat, M. Ombrellino, J. Che, et al. 1999. HMG-1 as a late mediator of endotoxin lethality in mice. Science 285(5425): 248–251.
Rouhiainen, A., J. Kuja-Panula, E. Wilkman, J. Pakkanen, J. Stenfors, R.K. Tuominen, et al. 2004. Regulation of monocyte migration by amphoterin (HMGB1). Blood 104(4): 1174–1182. doi:10.1182/blood-2003-10-3536.
Andersson, U., H. Wang, K. Palmblad, A.C. Aveberger, O. Bloom, H. Erlandsson-Harris, et al. 2000. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. The Journal of Experimental Medicine 192(4): 565–570.
Kim, J.H., S.J. Kim, I.S. Lee, M.S. Lee, S. Uematsu, S. Akira, et al. 2009. Bacterial endotoxin induces the release of high mobility group box 1 via the IFN-beta signaling pathway. Journal of Immunology 182(4): 2458–2466. doi:10.4049/jimmunol.0801364.
Liu, H., Y.M. Yao, Y. Yu, N. Dong, H.N. Yin, and Z.Y. Sheng. 2007. Role of Janus kinase/signal transducer and activator of transcription pathway in regulation of expression and inflammation-promoting activity of high mobility group box protein 1 in rat peritoneal macrophages. Shock 27(1): 55–60. doi:10.1097/01.shk.0000233197.40989.31.
Lu, B., D.J. Antoine, K. Kwan, P. Lundback, H. Wahamaa, H. Schierbeck, et al. 2014. JAK/STAT1 signaling promotes HMGB1 hyperacetylation and nuclear translocation. Proceedings of the National Academy of Sciences of the United States of America 111(8): 3068–3073. doi:10.1073/pnas.1316925111.
Acknowledgments
This article is supported by the National Natural Science Foundation of China (grant no. 81160235).
Conflicts of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.