Abstract
In recent decades, water pollution with organic and inorganic contaminants is a chief environmental concern. Enormously increasing industrial sectors such as textile dyes, pigments, ink, polymers, plastics, medicine and cosmetics effluent discharges are the main contributors toward the depleting water reservoir qualities. Which potentially have toxic effect on the organism depending on it that also includes humans too. Many of them are destruction resistant by conventional degradation methods. Thus, optimization of photocatalytic degradation methods has proven a new era in global water pollution remediation and cleaning related fields. Photocatalysts are considered as a great potential, economical, eco-friendly, sustainable and show promising role in water remediation of wastewater without generating secondary waste. However, the development of such an advanced system in large scale is still in the optimization phase. The design and development of efficiency, photocatalyst with optimum operational parameters, configuration and integration of photocatalysis is still need to be provoked generously. This chapter will be emphasized on the mechanism and diversifying factors influencing on photocatalysis degradation that should be taken into consideration during optimization and development of the photocatalysis system depending upon the load of the organic and inorganic contaminants. Various electromagnetic spectrum-based excitable nanoparticles or nanomaterials have been studied as photocatalyst that potentially degrade the contaminants from water. TiO2, Fe2O3, CuO, ZnO, CdS, SnO2, ZnS, etc. were extensively studied for the efficiency controlled optimizing factors in the degradation of pollutants. Optimization of Influencing factors and their effect on photocatalytic degradation activity of the photocatalyst on organic and inorganic water pollutants will be the point of convergence of this chapter. Within this frame of reference surface area, morphology of photocatalyst, higher light intensity, presence of oxidant and doping agents were perceived to be effective toward photocatalytic activity. Moreover, the general mechanism of photocatalysis and recent development in nanoparticles/nanomaterials and composite material will be discussed here.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
8.1 Introduction
Population explosion and the rapidly increasing industrial development sectors nearly hit the highly complex and difficult to manageable water pollution that ultimately threatened the massive effect on the climate (Lai et al. 2014) changes in many regions of the world. Also draining the increase in water consumption throughout the globe. Thus, it becomes more challenging to figure out an eco-friendly and more sustainable approaches to remediate the water pollution. Treated waste water becomes a general necessity of the human needs these days. Although the sewer systems, collection of waste water systems and treatment were not much accommodated as per the generated ratio. In addition to this, the present infrastructure facing the increasing demands to produce safer quality of water utilizing less amount of energy inputs (Soutsas et al. 2010). According to the existing planning it is practically difficult to design and develop such a huge system in the rapidly developing regions of the world. These challenges acquired attention of the more advanced but effective system toward the sustainable applications.
In the current scenario of the safe water available for the consumption is continuously deteriorated due to the direct or indirect discharge of the effluents from the industries such as pharmaceutical, food processing, textile, dyes, ink, rubber, plastic, polymers, chemical batteries and domesticated sewers as well as agricultural fertilizers or pesticide. All these industrial sectors contribute the contaminants are containing organic (industrial or textile dyes, herbicides or pesticides, halogenated hydrocarbons, pharmaceuticals by-products, some aromatic compounds such as polycyclic aromatic hydrocarbons) and inorganic (heavy metal ions, free radicals, carcinogens) pollutant molecules which are usually difficult to degrade by traditional methods and can cause the nuisance to the natural water reservoirs (Cacho et al. 1999; Gupta et al. 2006). These pollutants can cause the severe health damages to vital organs like kidneys, reproductive system, nervous system, lungs, lever even at very minute quantities (at ppm and ppb levels).
Several methods (in Fig. 8.1) already been applied to the waste water treatment before discharging out of the mainstreams. Each of processes from the method manifests with advantages and disadvantages.
Meanwhile, photocatalytic degradation treatment of pollutants is one of the best sustainable methods and proven a promising approach to clean the water sustainably using renewable sources and nanohybrid semiconductor materials. For efficient optimum photocatalytic degradation of waste water pollutants, influencing operational factors of the reaction should be taken into consideration. This chapter reviews the most important properties of the nanoparticles as photocatalysts and also focuses on the rigorous introduction of photocatalysis mechanism and factors that influencing the rate of photocatalytic degradation reaction of pollutants present in wastewater (Dahiya and Patel 2021) (Fig. 8.2).
In the context, Nanotechnology offers better opportunities that can develop hyphened techniques relay for the next generation water supply systems. This chapter reviews the promising role of the nanoparticles/nanomaterials and nanocomposites in the water treatment processes to transform or degrade the possible organic and inorganic pollutants from the wastewater (Dave et al. 2021a). The tremendous versatility of the nanoparticles harnesses the great abilities such as high surface area, photosensitivity, catalytic activity, electromagnetic properties, anti-microbial effects and the regulatory pore size capacities etc. are the aspects in the many applications. This application may include quality monitoring sensors, catalytic sites, disinfection agents and distinctive performing matrix/membrane (Gupta and Mondal 2021) s. The development of such a nanotechnological-based application in the waste water treatment processes must be conjointly soothe with the environmental health and safety research values that hand out the sustainable development. Highly expected prosecution of the nanotechnology in the water treatment may require the consideration of the expensive nanomaterials that might play vital role in obtaining reusable water to relieve the risks of the public and environmental health by potential minimizing the nanoparticles in the water and lift the safer ways of purification/decontaminations (Prajapati and Mondal 2021; Mallick et al. 2021).
8.2 Significance of Photocatalysis in Water Treatment
Photocatalysis is mediated through the light irradiations on photocatalyst particles have vital significance that leads to degradation of toxic pollutants from waste water. Photons are absorbed to obtain the charge which takes place in the redox reaction of toxic pollutant oxidation. Surface area plays a vital role in the mechanism. Succession of the reaction ultimately gives rise to form a hydroxyl free radical and acts as a potent oxidant to degrade the toxicants (Sarangapany and Mohanty 2021; Díez et al. 2021; Merouani and Hamdaoui 2021) (Fig. 8.3).
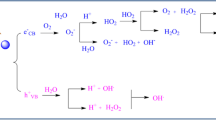
8.3 Mechanism of Nanoparticles (Photocatalyst) Involved in Photocatalysis of Wastewater Pollutants
A general mechanism of photocatalysis is driven by the photochemical transformation organic and inorganic pollutants from the waste water by absorbing the light radiation through the surface chemistry of the photocatalyst that leads to redox reaction. Nanocatalyst semiconductor material captures the energy released by the charge carriers which transfer the charge to the redox reaction of pollutants (Kumar et al. 2021). Apparently, photocatalyst can absorb the light radiation only on an appropriate illumination causing the excitation of charge carriers. Photochemical reaction mechanism produced light induced by the charge carriers and separated as Holes (H+) and Electrons (e−) which are responsible for oxidation of pollutants and degradation of pollutants from the waste water respectively (as shown in Fig. 8.4).
8.4 Factors Influencing on the Photocatalysis Mechanism
-
(A)
Ultrastructure of Photocatalyst:
-
a.
Effect of Size and Surface area: Size and surface area of the photocatalyst are most crucial factor to affect the photocatalytic degradation as the adsorption is directly involved. As the smaller size corresponds to higher surface area available for direct toward number of active sites, adsorption of contaminants and absorption of photons in efficient mechanism that ultimately lead to oxidation and degradation of pollutants. Thus, Nanoparticles play a vital role in the photocatalytic degradation as it exhibits better optical activity and also possesses the electrical photochemical properties (Zulkifili et al. 2018; Leroy et al. 2020; Isac et al. 2019).
-
b.
Effect of Morphology(shape): Morphology is definitely controlling the efficiency of photocatalysis of pollutants as the active sites and photon capture are based on it. It has been tested that ZnO nanorods, ZnO spindles and ZnO Nanoflowers can degrade the several chemical dyes. In which, the ZnO Nanorods found to be the most effective against the photocatalytic degradation. This is due to the higher number of reactive species and active site interaction on the ZnO Nanorods (Haruna et al. 2020).
-
a.
-
(B)
Photocatalyst Doping Element
Some Impurities are artificially added to the photocatalyst during synthesis that can potentially increase the photochemical activities. Such impurities were called as Doping agent or dopants. It increases the photochemical activity by means of elevated energy levels of dopants, better trapping of electrons, efficiently generating the oxygen deficient sites, producing more active sites for the adsorption of pollutant molecules and most importantly altering the light capturing bandgaps for more reactivity. Doping photocatalyst can be broadly classified as follows (Song et al. 2021; Ojha et al. 2020; Wang et al. 2019; Nada et al. 2020; Li et al. 2020; Teh et al. 2020; Zhang et al. 2019):
-
a.
Noble Metal Doping
-
b.
Metal Doping
-
c.
Rare Earth Metal Doping
-
d.
Non-Metallic Doping
-
e.
Co-Doping
-
f.
Self-Doping
-
a.
-
(C)
Reactant Accessibility
-
a.
Amount of Catalyst: Photocatalysis rate of reaction is directly proportional to the hydroxyl ion free radical and the positive holes under the radiation. These two primarily take a part in the photocatalytic reaction of the pollutants present in the water. Photocatalysis may increase with the amount of catalyst but exceeding to certain limit it inhibits the rate of reaction. With the higher concentration of photocatalyst turbidity of the solution increases and prohibited the light entry in the water, also causes the scattering of UV–Vis radiation. Agglomeration is also a main cause of high concentration of nanocatalyst which ultimately blocks the active site and leads to lowering down the photocatalysis (Laurenti et al. 2020; Anju Chanu et al. 2019; Deveci and Mercimek 2019; Tichapondwa et al. 2020).
-
b.
Concentration of Pollutants: Surface area is associated with the amount of pollutant where the photocatalysis depends upon pollutants adsorbs. This adsorption is also depending upon the initial concentration pollutants and gradually increases in the water. However, higher than a certain limit, here is significant decrease in the photocatalysis observed due to the higher initial concentration of pollutants adsorbed (Hu et al. 2016). As the photocatalyst's surface is totally covered with the adoption of pollutants, thus light photons were absorbed by the pollutant molecules instead of photocatalyst. Ultimately this reduces the production of hydroxyl ion free radicals and the positive hole. The occupied active sites of photocatalyst by pollutants show reduction in photochemical process (Zelekew et al. 2021).
-
a.
-
(D)
Heterogeneity of Oxidants
Effect of H2O2, KBrO3, (NH4)2S2O8/K2S2O8, HNO3 was seen while photocatlytic dye degradation (Sadik et al. 2004; Sadik et al. 2004; Ovhal et al. 2021). The study on the effect of oxidants such as (NH4)2S2O8, KBrO3 and H2O2 on the photooxidation of AR18 reveals that the addition of (NH4)2S2O8 and KBrO3 increases the dye removal whereas the addition of H2O2 decreases the photocatalytic degradation. The unusual decrease by the addition of H2O2 is due to its low adsorption on the ZnO surface (Sobana and Swaminathan 2007)
-
(E)
Miscellany Factors
-
a.
Effect of pH: One of the preliminary factors in the photocatalysis is pH. At higher pH (alkaline) free radicals are the predominantly active, while at lower pH (acidic) oxidation of pollutants and positives holes with high oxidation potential are the key factors for photocatalysis of contaminants in the wastewater (Yeganeh et al. 2020; Vijay et al. 2019; Deshmukh et al. 2020; Laishram et al. 2018). As the pH value increases in the solution hydroxyl ion was generated in between the reaction of positive holes and hydroxyl free radicals which cause increase in the degradation rate. If the pH reaches too high (pH > 11/12) the excessively formed hydroxyl ions it starts competing with the pollutants and get absorbed on the photocatalyst, as a result the active site for acidic pollutants gets blocked. Vice-versa, when pH lowers, surface of the photocatalyst gets protonated causing the reduction in the cationic pollutants adsorption which end up with significant decrease in the photodegradation (Suthar et al. 2021; Dave et al. 2021b; Purohit et al. 2021).
-
b.
Effect of Temperature: Photocatalysis can even occur at room temperature. However, degradation and recombination of electrons and positive hole pairs, releases energy, thus the temperature of the reaction medium gradually increases. Artificially increase in the temperature can gradually increase the rate of photocatalytic degradation reaction. But higher than 80 °C temperature can reduce the lifespan of the charge carriers through recombination with each other. Also, in temperature lower than 20 °C the reaction medium causes increase in the apparent activation of energy. Therefore, 20–80 °C is considered optimum temperature range for the photocatalysis which shows minimum apparent activation energy and low dependency of the rate of degradation (Dahiya and Patel 2021; Gupta and Mondal 2021; Prajapati and Mondal 2021; Mallick et al. 2021; Dave et al. 2021c; Satapathy et al. 2021).
-
c.
Effect of Light Intensity available: The photocatalytic dye degradation in presence of sunlight when conducted in between 10 a.m. to 4 p.m. has resulted in the inference that when the maximum quantity of sunlight is available with high intensity and energy then the catalytic activity is more. The reports demonstrate that sunlight has capability to degrade the dye in presence of these photo catalyst which clearly indicates its energy economy approach thus making it more economic and green method for the industries (Mehta et al. 2016; Ali and Ameta 2013; Borhade et al. 2020; Adhikari et al. 2020).
-
d.
Effect of Inorganic Ions: Waste water significantly consists of various inorganic, anionic and cationic pollutants. Presence of such dissolved inorganic ions are large enough to affect the photocatalysis efficiency. These inorganic ions may compete with the pollutant molecules and adsorbed on the surface of the photocatalyst ultimately reduced the active site for photochemical reaction. Inorganic ions affect the photocatalysis particularly by precipitation on the surface, blocking active sites, reacting with catalyst and scavenging radical and positive holes. Also, the Cl−, SO42−, HCO3− and PO43− inorganic anions were revealed as holes and radical scavengers and reduce the rate of photocatalysis, along with inorganic cations such as Mg2+, Zn2+, Fe3+, Cu2+ etc., in wastewater can also significantly affect the photodegradation activity.
-
a.
8.5 Conclusion
As a future perspective there will be emphasis on the mechanism and diversify factors influencing on photocatalytic degradation that should be taken into consideration during optimization and development of the photocatalysis system depends upon the load of the organic and inorganic contaminants. Various electromagnetic spectrum-based excitable nanoparticles or nanomaterials are been studied as photocatalyst that potentially degrade the contaminants from water.
References
Adhikari C, Kaur M, Ravichandran (2020) Sunlight assisted degradation of methylene blue as a model dye using bismuth oxychloride nanoparticles: ecofriendly and industry efficient photocatalysis for waste chemical treatment. Asian J Chem 32(1)
Ali Y, Ameta A (2013) Degradation and decolouration of amaranth dye by photo-fenton and fenton reagents: a comparative study. Int J Chem Sci 11(3
Anju Chanu L, Joychandra Singh W, Jugeshwar Singh K, Nomita Devi K (2019) Effect of operational parameters on the photocatalytic degradation of Methylene blue dye solution using manganese doped ZnO nanoparticles. Results Phys 12
Borhade A, Tope D, Kushare S (2020) Mercenaria shell powder as a cost-effective and eco-friendly photocatalyst for the degradation of Eriochrome Black T Dye. Iran J Sci Technol Trans A Sci 44(1)
Cacho J, Fierro I, Deban L, Vega M, Pardo R (1999) Monitoring of the photochemical degradation of metamitron and imidacloprid by micellar electrokinetic chromatography and differential-pulse polarography. Pestic Sci
Dahiya A, Patel BK (2021) Photocatalytic degradation of organic dyes using heterogeneous catalysts. In: Photocatalytic degradation of dyes
Dave S, Jagtap P, Verma S, Nehra R, Dave S, Mohanty P et al (2021a) Mathematical modeling and surface response curves for green synthesized nanomaterials and their application in dye degradation. In: Photocatalytic degradation of dyes
Dave S, Khan AM, Purohit SD, Suthar DL (2021b) Application of green synthesized metal nanoparticles in the photocatalytic degradation of dyes and its mathematical modelling using the caputo-fabrizio fractional derivative without the singular Kernel. J Math 2021b
Dave S, Dave S, Das J (2021c) Photocatalytic degradation of dyes in textile effluent: a green approach to eradicate environmental pollution. In: The future of effluent treatment plants
Deshmukh SP, Kale DP, Kar S, Shirsath SR, Bhanvase BA, Saharan VK et al (2020) Ultrasound assisted preparation of rGO/TiO2 nanocomposite for effective photocatalytic degradation of methylene blue under sunlight. Nano-Struct Nano-Objects 21
Deveci İ, Mercimek B (2019) Performance of SiO2/Ag Core/Shell particles in sonocatalalytic degradation of Rhodamine B. Ultrason Sonochem 51
Díez AM, Pazos M, Sanromán MA (2021) Hybrid systems to improve photo-based processes and their importance in the dye degradation. In: Photocatalytic degradation of dyes
Gupta GK, Mondal MK (2021) Fundamentals and mechanistic pathways of dye degradation using photocatalysts. In: Photocatalytic degradation of dyes
Gupta AK, Pal A, Sahoo C (2006) Photocatalytic degradation of a mixture of crystal violet (basic violet 3) and methyl red dye in aqueous suspensions using Ag+ doped TiO2. Dye Pigment
Haruna A, Abdulkadir I, Idris SO (2020) Photocatalytic activity and doping effects of BiFeO3 nanoparticles in model organic dyes. Heliyon 6
Hu E, Wu X, Shang S, Tao XM, Jiang SX, Gan L (2016) Catalytic ozonation of simulated textile dyeing wastewater using mesoporous carbon aerogel supported copper oxide catalyst. J Clean Prod 112
Isac L, Cazan C, Enesca A, Andronic L (2019) Copper sulfide based heterojunctions as photocatalysts for dyes photodegradation. Front Chem 7
Kumar M, Swain G, Sonwani RK, Singh RS, Verma A, Rai B (2021) Effect of operating parameters on photocatalytic degradation of dyes by using graphitic carbon nitride. In: Photocatalytic degradation of dyes
Lai CW, Juan JC, Ko WB, Bee Abd Hamid S (2014) An overview: recent development of titanium oxide nanotubes as photocatalyst for dye degradation. Int J Photoenergy
Laishram D, Shejale KP, Gupta R, Sharma RK (2018) Heterostructured HfO2/TiO2 spherical nanoparticles for visible photocatalytic water remediation. Mater Lett 231
Laurenti M, Garino N, Garino N, Canavese G, Hernandéz S, Cauda V (2020) Piezo- and photocatalytic activity of ferroelectric ZnO:Sb thin films for the efficient degradation of rhodamine-β dye pollutant. ACS Appl Mater Interfaces 12(23)
Leroy S, Blach JF, Huvé M, Léger B, Kania N, Henninot JF et al (2020) Photocatalytic and sonophotocatalytic degradation of rhodamine B by nano-sized La2Ti2O7 oxides synthesized with sol-gel method. J Photochem Photobiol A Chem 401
Li Z, Chen Q, Lin Q, Chen Y, Liao X, Yu H et al (2020) Three-dimensional P-doped porous g-C3N4 nanosheets as an efficient metal-free photocatalyst for visible-light photocatalytic degradation of Rhodamine B model pollutant. J Taiwan Inst Chem Eng 114
Mallick A, Patil PD, Tiwari MS, Kane P, Khonde D (2021) Green and sustainable methods for dye degradation employing photocatalytic materials. In: Photocatalytic degradation of dyes
Mehta A, Mishra A, Sharma M, Singh S, Basu S (2016) Effect of silica/titania ratio on enhanced photooxidation of industrial hazardous materials by microwave treated mesoporous SBA-15/TiO2 nanocomposites. J Nanoparticle Res 18(7)
Merouani S, Hamdaoui O (2021) Sonophotocatalytic degradation of refractory textile dyes. In: Photocatalytic degradation of dyes
Nada AA, El Rouby WMA, Bekheet MF, Antuch M, Weber M, Miele P et al (2020) Highly textured boron/nitrogen co-doped TiO2 with honeycomb structure showing enhanced visible-light photoelectrocatalytic activity. Appl Surf Sci 505
Ojha N, Bajpai A, Kumar S (2020) Enhanced and selective photocatalytic reduction of CO2 by H2O over strategically doped Fe and Cr into porous boron carbon nitride. Cataly Sci Technol 10(8)
Ovhal SD, Rodrigues CSD, Madeira LM (2021) Photocatalytic wet peroxide assisted degradation of Orange II dye by reduced graphene oxide and zeolites. J Chem Technol Biotechnol 96(2)
Prajapati AK, Mondal MK. (2021) Emerging nanocomposites as highly efficient materials for photocatalysis of dyes: synthesis routes, characterization, and reaction mechanism. In: Photocatalytic degradation of dyes
Purohit SD, Khan AM, Suthar DL, Dave S (2021) The impact on raise of environmental pollution and occurrence in biological populations pertaining to incomplete H-function. Natl Acad Sci Lett 44(3)
Sadik WA, Sadek OM, El-Demerdash AM (2004) The use of heterogenous advanced oxidation processes to degrade neutral red dye in aqueous solution. Polym Plast Technol Eng 43(6)
Sadik WA, El-Demerdash AM, Nashed AW (2004) UV-induced decolorization of indophenol by heterogenous advanced oxidation processes. Polym Plast Technol Eng 43(6)
Sarangapany S, Mohanty K (2021) A facile biogenic-mediated synthesis of Ag nanoparticles over anchored ZnO for enhanced photocatalytic degradation of organic dyes. In: Photocatalytic degradation of dyes
Satapathy S, Acharya D, Dixit PK, Mishra G, Das J, Dave S (2021) Mechanistic aspects and rate-limiting steps in green synthesis of metal and metal oxide nanoparticles and their potential in photocatalytic degradation of textile dye. In: Photocatalytic degradation of dyes
Sobana N, Swaminathan M (2007) The effect of operational parameters on the photocatalytic degradation of acid red 18 by ZnO. Sep Purif Technol 56(1)
Song H, Liu L, Wang H, Feng B, Xiao M, Tang Y et al (2021) Adjustment of the band gap of co-doped KCl/NH4Cl/g-C3N4 for enhanced photocatalytic performance under visible light. Mater Sci Semicond Process 128
Soutsas K, Karayannis V, Poulios I, Riga A, Ntampegliotis K, Spiliotis X et al (2010) Decolorization and degradation of reactive azo dyes via heterogeneous photocatalytic processes. Desalination
Suthar DL, Purohit SD, Khan AM, Dave S (2021) Impacts of environmental pollution on the growth and conception of biological populations involving incomplete I-function. In: Lecture notes on data engineering and communications technologies
Teh YW, Chee MKT, Kong XY, Yong ST, Chai SP (2020) An insight into perovskite-based photocatalysts for artificial photosynthesis. Sustain Energy Fuels 4(3)
Tichapondwa SM, Newman JP, Kubheka O (2020) Effect of TiO2 phase on the photocatalytic degradation of methylene blue dye. Phys Chem Earth 118–119
Vijay S, Balakrishnan RM, Rene ER, Priyanka U (2019) Photocatalytic degradation of Irgalite violet dye using nickel ferrite nanoparticles. J Water Supply Res Technol AQUA 68(8)
Wang K, Fu J, Zheng Y (2019) Insights into photocatalytic CO2 reduction on C3N4: Strategy of simultaneous B, K co-doping and enhancement by N vacancies. Appl Catal B Environ 254
Yeganeh FE, Yousefi M, Hekmati M, Bikhof M (2020) Photocatalytic degradation of coomassie blue G-250 by magnetic NiFe2O4/ZnO nanocomposite. Comptes Rendus Chim 23(6–7)
Zelekew OA, Fufa PA, Sabir FK, Duma AD (2021) Water hyacinth plant extract mediated green synthesis of Cr2O3/ZnO composite photocatalyst for the degradation of organic dye. Heliyon 7(7)
Zhang H, Han X, Yu H, Zou Y, Dong X (2019) Enhanced photocatalytic performance of boron and phosphorous co-doped graphitic carbon nitride nanosheets for removal of organic pollutants. Sep Purif Technol 226
Zulkifili AN, Fujiki A, Kimijima S (2018) Flower-like BiVO4 microspheres and their visible light-driven photocatalytic activity. Appl Sci 8(2)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Dave, S., Jagtap, P. (2022). Optimizing Nanocatalyst’s and Technological Factors Influencing on Photocatalytic Degradation of Organic and Inorganic Pollutants. In: Dave, S., Das, J. (eds) Trends and Contemporary Technologies for Photocatalytic Degradation of Dyes. Environmental Science and Engineering. Springer, Cham. https://doi.org/10.1007/978-3-031-08991-6_8
Download citation
DOI: https://doi.org/10.1007/978-3-031-08991-6_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-08990-9
Online ISBN: 978-3-031-08991-6
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)