Abstract
Environmental pollution is becoming a serious threat to the human society, and photocatalysis is recognized as an environmental benign technology to remediate organic pollutants from aqueous environment. Mainstream research related to pollutant remediation and energy production is based on heterogeneous photocatalysis, a modified advanced oxidation process. Being a green technology, it can have further applications if the vast and inexpensive solar light can be utilized in place of harmful ultraviolet rays.
This chapter focuses on some important nano-semiconductor photocatalysts like TiO2, ZnO and graphitic carbon nitride (g-C3N4 or CN) and various strategies adopted for improving their photocatalytic activity under sunlight. Different methods for improving visible light active photocatalysts including metal/non-metal doping, the addition of photosensitive materials, incorporation of other nanoparticles, composite formation with other semiconductors and formation of heterojunctions and nanohybrids are discussed. These fundamental information can serve as knowledge base in constructing next-generation photocatalysts with better properties.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Nano-semiconductor materials
- Solar/visible light
- Photocatalysts
- Pollutant remediation
- Advanced oxidation process
- TiO2
- ZnO graphitic carbon nitride
- Titanates
- Nano-metal sulphides
8.1 Introduction
Solar energy is the most abundant, clean and renewable source of energy available on earth. It was proposed that the amount of sunlight that strikes on earth’s surface in an hour is enough to power the world economy for an entire year (Izumi 2013). Sunlight can be effectively employed for energy production and pollutant remediation , two major issues the world is most concerned with. Solar energy utilization techniques have attracted great attention because of low cost, but the developments of materials which can efficiently harvest sunlight require extensive research.
In the development of solar energy utilization technologies, efficient solar energy conversion systems in which solar energy is efficiently used to generate sustainable electrons and holes triggering the reduction and oxidation (redox) reactions are vitally prerequisite. Nowadays advanced oxidation processes (AOPs) offer a feasible strategy to address energy production and pollutant remediation . AOP involves the generation of hydroxyl radicals with very high oxidizing power in an aqueous medium which endorses energy production and degradation of pollutants. UV-mediated ozonation, oxidation using Fenton’s reagent (a mixture of H2O2 with Fe2+) and electrocoagulation are some common techniques involving AOP. All these techniques produce hydroxyl radicals, which destroy various POPs, present in polluted water via series of chemical reactions. Complete degradation of pollutants, zero waste production and rapid reaction rates are achieved by AOP, but the main drawbacks are inflated cost and reduced efficiency due to the presence of turbidity or UV-absorbing species, i.e. the efficiency of AOP is determined by the wastewater composition and treatment conditions.
Nowadays photocatalysis , a technique based on AOP, has emerged as a fascinating process towards the degradation of various POPs in the environment and also for fuel production, as it is a cost-effective, renewable, non-toxic and versatile technique. According to the IUPAC gold book, photocatalysis is defined as the change in the rate of a chemical reaction or its initiation under the action of light in the presence of a substance called photocatalyst (Glossary of terms used in photochemistry 2006). In 1972, Fujishima and Honda discovered that water can be split into hydrogen and oxygen using TiO2 electrode under UV light (Fujishima and Honda 1972). This discovery inspired many researchers to work in the area of heterogeneous photocatalysis. Photocatalytic activity is the ability of a material to create an electron-hole pair as a result of exposure to suitable light irradiation. Design and development of novel photocatalytic systems with high efficiencies have attracted worldwide scientific interests. Semiconductor nanomaterial-based photocatalysis is recognized as a promising alternative to the conventional methods for pollutant removal because it opens green pathways for the complete mineralization of environmental pollutants in the presence of light (Li et al. 2017a).
Photocatalysis is extensively employed in various fields like water and air purification, self-cleaning surfaces, selective and green synthesis of organic compounds, hydrogen generation, etc. Heterogeneous photocatalysts (where photocatalyst and reactants are in different phases) involving nano semiconductors are extensively employed for the remediation of POPs attributed to low cost, non-toxicity, reusability and high stability. TiO2, ZnO, graphitic carbon nitride (g-C3N4 or g-CN) , Fe2O3, CdS, ZnS, WO3, SrTiO3, CdTe, MoS2, ZrO2, titanates, etc. are the commonly used semiconductors for these purposes (Bhethanabotla et al. 2017; Qin et al. 2017; Liu et al. 2014a, 2017a, b; Shi et al. 2012; Gupta et al. 2017; Shahrokhi 2016; Gao et al. 2013; Hanifehpour et al. 2016; Li et al. 2016a; Basahel et al. 2015).
In this chapter, we discuss some important nano-photocatalysts with a special emphasis on various strategies for improving their photocatalytic activity under visible light in order to exploit sunlight for the photocatalytic degradation of pollutants and also for energy production.
8.2 Mechanism of Semiconductor-Mediated Photocatalysis
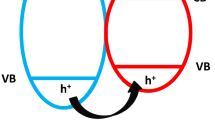
Semiconductors are characterized by filled valance band and empty conduction bands. They can act as sensitizers of light-induced redox reactions due to their electronic structure (Hoffmann et al. 1995). Semiconductor photocatalysis is initiated by electron-hole pairs after bandgap excitation. When photocatalyst is irradiated with light whose energy is equal to or greater than bandgap energy of photocatalyst, the valence band electrons (e−) can be excited to the conduction band, leaving a positive hole (h+) in the valence band (Fig. 8.1). These excited charge carriers (e− and h+) can recombine, releasing the input energy as heat, with no chemical effect. If photoinduced charge carriers travel to the surface of photocatalyst without recombination, they can take part in various redox reactions with surface-absorbed species like oxygen, water and other organic and inorganic species. The valence band holes are powerful oxidants (+1.0 to +3.5 V vs NHE depending on the semiconductor and pH), while the conduction band electrons are good reductants (+0.5 to −1.5 V vs NHE) (Hoffmann et al. 1995). Most organic photodegradation reactions utilize the oxidizing power of the holes either directly or indirectly. These redox reactions are the basic mechanism of photocatalytic energy production, pollutant remediation , water purification, etc.
The bandgap energies and band edge positions of various semiconductors are listed in Fig. 8.2. Wide-bandgap semiconductors like ZnS (3.6 eV), SrTiO3 (3.2 eV), TiO2 (anatase phase- 3.2 eV; rutile phase-3.0 eV), ZnO (3.2 eV) and ZrO2 (5.0 eV) require high-energy UV light for their photocatalytic reactions. However, CdTe (1.4 eV), CdSe (1.7 eV), MoS2 (1.75 eV), CdS (2.4 eV), Fe2O3 (2.3 eV), CN (2.7 eV) and WO3 (2.8 eV) are narrow-bandgap semiconductors, and they utilize low-energy visible light for photocatalysis. Both wide- and narrow-bandgap semiconductors suffer from disadvantages like rapid recombination and low generation of photoinduced charge carriers. These factors make their photocatalytic efficiency very low. The requirement of harmful UV light for the photocatalytic reactions for wide-bandgap semiconductors (i.e. low solar energy utilization efficiency) and ultrafast recombination of photoinduced charge carriers are the bottleneck of photocatalysts to satisfy the requirements of applications in a practical way. Use of UV light practically rules out the application of sunlight as an energy source in photocatalysis. Nowadays, research is intensively focused on creating visible/solar light active semiconductor photocatalysts attributed to the fact that sunlight consists of 45% visible light and only 3–5% UV light (rest is other type of electromagnetic radiations). Herein, we present an overview of current research activities that centre on nano-photocatalysts like TiO2, ZnO and graphitic carbon nitride (g-C3N4 or CN).
Bandgap energies (eV) and band edge positions of different semiconductors . The wide-bandgap semiconductors uses UV light, and narrow-bandgap semiconductors uses visible light for photocatalysis . (Reprinted from Wu et al. 2015 with permission)
8.2.1 Nano-TiO2 as Photocatalysts
Among the different photocatalysts mentioned above, TiO2 is a widely used photocatalyst for pollutant degradation and energy production as it is cheap, nontoxic and inert. TiO2 exist mainly in three crystalline phases: anatase, brookite and rutile (Fig. 8.3). Anatase and brookite (kinetic products) will transform to the thermodynamically stable rutile upon calcination at a temperature beyond 600 °C (Hu et al. 2003). Rutile phase has more chemical stability and high refractive index over anatase phase, but despite these advantages, rutile TiO2 has attracted fewer attention in producing photocatalysts attributed to the lower electrochemical performance than the anatase phase, primarily due to the difference in their electronic structure. Anatase phase TiO2 is a good photocatalyst, but owing to the wide bandgap of 3.2 eV, it requires UV light for photocatalysis which inhibits its applications in visible light or solar light-mediated photocatalysis. The low photo-generated charge transfer rate is another downside of TiO2 photocatalysts (Taheri et al. 2017). Reduction of bandgap by introducing energy levels between the conduction band and valence band, using various techniques, permits TiO2 to be active under the solar light which will be discussed in the following session (Bingham and Daoud 2011).
Schematic representations of anatase, brookite and rutile phases of TiO2. Rutile phase TiO2 has more chemical stability and high refractive index over anatase phase, but anatase phase TiO2 is a good photocatalyst (bandgap 3.2 eV). (Reprinted from Haggerty et al. 2017 with permission)
8.2.2 Nano-ZnO as Photocatalysts
ZnO, a wide-bandgap (3.2 eV) semiconductor, has also emerged as a promising candidate in green environmental management system because of its unique characteristics, such as direct bandgap in the near-UV spectral region, strong oxidation ability and good photocatalytic property (Lee et al. 2016; Tripathy et al. 2016). Moreover, it is relatively cheaper compared to TiO2. As an important semiconductor material, ZnO has been applied in catalysis, paint industries, ceramic bodies, cosmetics, etc. ZnO received much attention in the degradation and complete mineralization of environmental pollutants (Muthulingam et al. 2015; Zyoud et al. 2016; Sun et al. 2016). Since ZnO has almost same bandgap energy as TiO2, its photocatalytic capability is anticipated to be similar to that of TiO2. The light absorption of ZnO also is limited in the visible light region due to the wideband energy. Major drawbacks of ZnO are the wide-bandgap energy, photocorrosion and fast recombination of charge carriers which result in the low photocatalytic efficiency (Sun et al. 2016).
ZnO crystallizes in three phases – thermodynamically stable wurtzite, the cubic zinc blende and rock salt (NaCl) structures (Fig. 8.4.). The cubic structures are rarely reported and the rock salt structure is obtained only at relatively high pressure; thus, wurtzite structure is predominant and stable [under ambient conditions, the thermodynamically stable phase is that of wurtzite symmetry]. Wurtzite ZnO belongs to the space group of P63mc [lattice parameters a = 0.3296 and c = 0.52065 nm] and has a hexagonal close-packed lattice. Each Zn2+ sublattice contains four Zn2+ ions surrounded by four O2− ions and vice versa, coordinated at the edges of a tetrahedron, stacked alternately along the c-axis (Jagadish and Pearton 2006; Morkoç and Özg€ur n.d.).
(a) Cubic rock salt, (b) cubic zinc blende and (c) hexagonal wurtzite structure of ZnO crystal (Stick and ball representation). The shaded grey and black spheres denote zinc and oxygen atoms. (Reprinted from (Morkoç and Özg€ur n.d. with permission)
8.2.3 Graphitic Carbon Nitride as Photocatalysts
In the year 2009, graphitic carbon nitride (g-C3N4), the most stable allotrope among various carbon nitrides, was introduced into the field of heterogeneous catalysis. In the 1830s, Berzelius and Liebig invented “melon”, the sprouting form of g-C3N4, which is a linear polymer consisting of interconnected tri-s-triazines via secondary nitrogen (Wang et al. 2012). g-C3N4 in the form of two-dimensional sheets consists of tri-s-triazines interconnected via tertiary amines (Fig. 8.5) (Zheng et al. 2012a; Thomas et al. 2008).
Various functionalities of graphitic carbon nitride. (Reprinted from Thomas et al. 2008 with permission)
Graphitic carbon nitride, the metal-free semiconductor photocatalysts, attracted the attention of scientists attributed to their narrow bandgap and aromatic π-conjugated structure. Unlike TiO2, which is active only under UV region, g-C3N4 possesses a bandgap of 2.7 eV (corresponding to visible light of 460 nm) and exhibits promising potential in solar photocatalysis . Hence, g-C3N4, an inexpensive and environmentally benign material, is developing as an ideal solar photocatalytic material to replace TiO2-based materials. Nevertheless, the photocatalytic efficiency of the pristine g-C3N4 is still slow under sunlight irradiation because of the high recombination rate of photo-generated electron-hole pairs, low-specific surface area and low visible light utilization efficiency (Thomas et al. 2018). Scientists are now focused on creating novel g-C3N4 photocatalysts with enhanced physicochemical properties and high photocatalytic activities under sunlight.
8.2.4 Titanates as Photocatalysts
Titanates are another group of widely used photocatalysts (Rutar et al. 2015). They have the general formula, A2TinO2n+1, where A represents ions of metal like Ba, Ca, Sr, Mn, Fe, Co, Ni, etc. In titanates, metal ions occupy the interlayer space resulting in the mobile nature of metal ions. On treatment with acids, they get easily replaced by protons, resulting in the formation of hydrogen titanate. Hence metal titanate can also be used for the synthesis of TiO2 with different morphologies (Rutar et al. 2015). Titanate perovskites are a class of titanates which have been widely studied for photocatalytic applications for a long time. Perovskites are the class of compounds with general formula ABO3 in which A site is occupied by the larger cation, while the B site is occupied by the smaller cation. The perovskite crystal structure has corner connected BO6 octahedra and 12 oxygen coordinated A cations, located in between the eight BO6 octahedra (Fig. 8.6). MTiO3 (M = Sr, Ba, Ca, Mn, Co, Fe, Pb, Cd, Ni) titanate systems shown excellent photocatalytic properties towards photooxidation reactions and pollutant degradation (Kanhere and Chen 2014).
Crystal structure of simple perovskite, BaTiO3 (red, oxygen; green, A site cation; grey, BO6 octahedra). (Reprinted from Kanhere and Chen 2014 with permission)
8.2.5 Nano-metal Sulphides as Photocatalysts
Nanosized metal sulphides like CdS, ZnS and MoS2 are widely employed for photocatalysis , manufacturing short wavelength light-emitting devices (LED), sensors, etc. CdS is a transition metal sulphide with a bandgap of 2.4 eV with high optical absorption coefficient (Kanhere and Chen 2014). ZnS with a direct bandgap of 3.68 eV makes it suitable for a number of optical applications. MoS2 with a layered 2D structure similar to carbon nitride (Fig. 8.7) has gained a lot of interest in the area of catalysis and energy production attributed to the van der Waals forces existing between S-Mo-S sandwiches (Shahrokhi 2016; Li et al. 2016a).
Top (a) and side views (b) of the graphene-like 2D-ZnS. Big grey and small yellow balls in geometrical models represent zinc and sulphide atoms, respectively. (Reprinted from Shahrokhi 2016 with permission)
8.3 Strategies for Making Solar/Visible Light Active Photocatalysts
As mentioned above, the two major disadvantages in semiconductor -mediated photocatalysis are (i) the ultrafast recombination of photoinduced charge carriers like holes and electrons, which are generated in the valance band and conduction band of photocatalyst after light irradiation, and (ii) requirement of UV light for photocatalysis which accounts for only 3–5% of solar spectrum (Jesty et al. 2011). This charge recombination process results in the reduction of quantum efficiency resulting in the diminution of photocatalytic performance. Extension of the photocatalytically active region of the semiconductor to the visible region will result in widespread real-life applications (Jesty and Yoon 2012). Hence reduction of the recombination process and the improvement of visible light harvest for photocatalytic activity are essential in developing solar light active photocatalyst systems.
Different methods adopted for developing visible light active photocatalysts include metal/non-metal doping, the addition of photosensitive materials, incorporation of other nanoparticles, composite formation with other semiconductors and formation of heterojunctions and nanohybrids (Kumar et al. 2016; Shi et al. 2016; Yao et al. 2015; Moussawi and Patra 2016; Zhu et al. 2015, 2017; Sridharan et al. 2015; Sharma et al. 2016; Bai et al. 2015; Li et al. 2016b, c; Zalfani et al. 2016; Tobajas et al. 2017; Ghasemi et al. 2017) which are discussed below.
8.3.1 Metal/Non-metal Doping
Incorporation of nanoparticles of metals/non-metals is a well-received technique for efficient separation of photoinduced charge carriers and absorption of visible light in semiconductor photocatalysts. Metal-/non-metal-doped semiconductors are more efficient than undoped semiconductor resulting in the improved performance for photocatalysis . Numerous defects and distortions are introduced into semiconductor system when a dopant is added which result in the changes of electronic properties. The diameters of heteroatoms of dopant must be equal to or smaller than semiconductor atoms (Liu et al. 2016a).
Nanoparticles show properties (both physical and chemical) different from their bulk materials. When nanoparticles of metals/non-metals are incorporated into the photocatalyst, it will exhibit altered properties attributed to the presence of nanoparticles. The strong absorption of visible light caused by the surface plasmon resonance (SPR) of metal nanoparticles has gained considerable attention. When metal nanoparticles are incorporated into the semiconductor, they act as electron trapping sites and diminish the recombination of charge carriers, resulting in the enhanced photocatalytic activity. They also exhibit resonant photon scattering, which in turn increases the interaction time with semiconductor and more electron-hole pairs are generated. Depending upon the nature of neighbouring material or size of metal nanoparticles, changes in photocatalytic performance are expected. Nanoparticles of Au, Ag, Pt, etc. (noble metals ) have the ability to absorb visible light owing to localized surface plasmonic resonance. They can redshift the absorption edge of wide-bandgap semiconductors to the visible region when these nanoparticles are doped into semiconductors . For example, Au clusters (formed by the assembly of Au nanospheres and nanorods) doped TiO2 showed enhanced photocatalytic performance towards the degradation of methylene blue (MB) dye and a hazardous fungicide, carbendazim. Au nanoclusters play dual roles: as a co-catalyst under ultraviolet radiation and as a light harvester in visible light. Compared to bare TiO2, Au-TiO2 exhibited enhanced performance attributed to the proficient photo-generated charge separation on the Au-TiO2 surface served by Au nanostructures (Jesty and Yoon 2012; Thomas and Chitra 2014). Photocatalytic pollutant degradation mechanism by Au-TiO2 nanoassemblies under UV light irradiation and visible light irradiation is schematized in Fig. 8.8.
Schematic representation of photocatalytic pollutant degradation mechanism by Au-TiO2 nanoassemblies under (a) UV light irradiation and (b) visible light irradiation. (Reprinted from Sharma et al. 2016 with permission)
Nano-Au-doped ZnO nanoparticles also showed enhanced photocatalytic activity. Glutathione-protected Au nanoparticles with different diameters (1.1, 1.6 and 2.8 nm) were doped into nano-ZnO. The assessment of the photocatalytic performance by monitoring the degradation of dyes like thionine and rhodamine 6G revealed that ZnO-Au nanocomposites showed an increase in photocatalytic activity with an increase in nanogold size (Lee et al. 2011).
Similarly improved photocatalytic activity under visible light was observed in Au nanoparticles (AuNPs) loaded on g-C3N4 nanosheets also. The photocatalyst was prepared by ultrasonication-assisted liquid exfoliation of bulk g-C3N4 and photoreduction of Au(III) under visible light irradiation. This system showed superior photocatalytic activity under visible light irradiation towards the decomposition of methyl orange compared to bulk g-C3N4/g-C3N4 nanosheets (Cheng et al. n.d.).
Analogous to nanogold, silver nanoparticles also have been used for improving the visible light activity of the photocatalysts (Jesty et al. 2011). Visible light active nano-Ag/TiO2 nanocomposites were prepared (Cao et al. 2014) via the combination of a sol-gel process of a titanium ethoxide in inverse mini emulsions and in situ reductions of Ag ions in the AgBF4/TiO2 by hydrazine. The in situ reduction of Ag ions on the surface of AgBF4/TiO2 proved that Ag salt content does not influence the particle morphology and colloidal stability of the reaction systems. Visible light-induced photocatalytic degradation of rhodamine B (RhB) was studied with respect to different phases of TiO2 (Ag/rutile or amorphous or anatase TiO2) and various Ag contents. Nano-Ag/anatase TiO2 showed enhanced degradation of RhB.
Using a combination of solvothermal technique with photoreduction method, a photocatalyst, Ag-AgBr/TiO2 heterostructured nanofibres were fabricated on electrospun TiO2 nanofibres (Sui et al. 2015). Ag-AgBr/TiO2 heterostructured nanofibres shown enhanced photocatalytic activity towards the degradation of methylene blue dye, attributed to the surface plasmon resonance effect of Ag nanoparticles and the synergetic effect between Ag, AgBr and TiO2.
It was observed that Ag-doped graphitic carbon nitride films (Ag/g-C3N4) synthesized on ITO substrates by a liquid-based reaction process exhibited high photoelectrocatalytic activity for the degradation of methylene blue. Enhancement of photoelectrocatalysis was due to the more visible light harvesting and also due to the easy electron transfer between photo-generated electron and interfacial electron in Ag/g-C3N4 film (Qi 2016). Visible light-induced photocatalytic properties of a composite system consisting of silver quantum clusters [Ag9(H2MSA)7] (H2MSA= mercaptosuccinic acid) embedded on graphitic carbon nitride nanosheets (AgQCs-CN) showed extended visible light absorption through multiple single-electron transitions in Ag quantum clusters and an effective electronic structure for hydroxyl radical generation, which enabled increased activity in the photocatalytic degradation of methylene blue and methyl orange dye molecules compared with pristine graphitic carbon nitride (CN) and silver nanoparticle grafted CN (AgNPs-CN). The rate of hydrogen generated using AgQCs-CN was slightly less than that of AgNPs-CN because of surface hydroxyl radical formation (Sridharan et al. 2015).
Effect of different transition metals on the photocatalytic activity of TiO2 was studied. For this, a series of metal-doped TiO2 [M/TiO2 (M = Cu, Ni, Co, Fe, Mn, Cr)] photocatalysts were prepared via modified precipitation method using corresponding metal nitrates and titanium (IV) butoxide (Kuyumcu et al. 2015). These different metal-doped TiO2 photocatalysts were used to access the degradation of commercial dyes like methyl orange (MO, azo dye) and methylene blue (MB, thiazine dye group) under visible light. Among the different metals doped TiO2, Cu/TiO2 sample exhibited the highest photocatalytic activity under visible light attributed to the low bandgap energy and delayed electron-hole recombination. Cu was found to be a good dopant for CN also. Cu-doped mesoporous graphitic carbon nitride (Cu/mpg-C3N4) photocatalysts were prepared using cupric chloride and melamine as precursors. The photocatalytic degradation rate of MO reached 90.2% in 120 min onto Cu2+-doped mpg-C3N4. The rate constant for Cu2+−doped mpg-C3N4 was two times as high as that of pure g-C3N4 due to an increase in the electron/hole separation rate (Le et al. 2016).
Co, Cu and Ni cations were used for ZnO doping and stabilized in aqueous colloidal solutions using different surface capping agents. High PL emission, smallest particle size and most stable colloid were obtained for Co-ZnO showing that Co is the best dopant among three metals. Another photocatalyst, Sn loaded Au-ZnO, was prepared by precipitation-decomposition method (Senthilraja et al. 2016). Sn-Au-ZnO at optimum pH of 11 and catalyst loading of 2 g/L is found to be more efficient than bare ZnO and other commercial catalysts for the mineralization of AR 18 dye under UV-A light (Fig. 8.9). The photocatalytic degradation of Naphthol Blue Black (NBB) dye in aqueous solution under solar light irradiation was achieved using Ce co-doped Ag-ZnO (Ce-Ag-ZnO) prepared via solvothermal method (Subash et al. 2012). Co-doping of Ce and Ag resulted in the red-shifted absorption edge of ZnO and inhibition of photo-generated charge recombination.
The mechanism for degradation of AR 18 by Sn-Au-ZnO under UV-A light. Catalyst loading of 2 g/L is found to be more efficient than bare ZnO and other commercial catalysts for the mineralization of AR 18 dye. (Reprinted from Senthilraja et al. 2016 with permission)
It was found that doping of two or more metals into semiconductors yields enhanced photocatalytic performance; however, their photocatalytic mechanism will be different. Kumar et al. reported a bimetallic-TiO2 photocatalyst using Cu and Ag metals (Kumar et al. 2016). The bimetallic system shifted the optical response of the TiO2-based catalyst from UV to the visible region with a shift of bandgap from 3.22 to 2.82 eV. Improved electron-hole separation was also observed as Ag, Cu and/or Ag-Cu metal sites act as electron traps and functioned as co-catalysts . The promotional effect of Ag-Cu particles is induced by plasmon activation under visible light and is followed by consecutive electron transfer from Ag-Cu to the valence band of TiO2 (Fig. 8.10). The SPR induced local electric field of activated Ag-Cu particles to drive their electrons to combine with the holes on the valance band of TiO2. Ag-Cu/TiO2 catalyst was used to study H2 production (5683 μmoles g−1h−1) from H2O splitting, and the enhanced H2 production was attributed to additional active sites created by synergetic effect between Ag and Cu nanoparticles.
Schematic representation of electron transfer mechanism by surface plasmon resonance in Ag-Cu/TiO2 and is followed by consecutive electron transfer from Ag-Cu to the valence band of TiO2for H2 production under solar light irradiation. (Reprinted from Kumar et al. 2016 with permission)
Rare earth metal oxides are an excellent choice for metal doping attributed to the good thermal stability provided by their 4f multielectron configuration. Lanthanide ions act as electron scavenger and prevent the electron-hole recombination by reaction with superoxide ions which improves the photocatalytic efficiency. The augmentation in photoluminescence of ZnO co-doped with Li+ and trivalent rare earth metal ions (RE3+) was studied (Bachir et al. 1997). A series of rare earth metal (La, Ce, Pr, Nd, Sm, Eu, Dy, Gd)-doped TiO2 were prepared via an ecologically friendly single-step, one-pot, no post-calcination synthesis route for the production of low-cost photocatalytic pigments (Stengl et al. 2009). Among the different rare earth dopants, Nd3+ doped TiO2 showed enhanced performance towards the degradation of orange II dye aqueous solution. Polymer pyrolysis method was employed to synthesize Ln (La3+, Nd3+ or Sm3+)-doped ZnO nanoparticles (Khatamian et al. 2012) and was used to study the photocatalytic degradation of 4-nitrophenol. The photocatalytic performance of ZnO was enhanced by Ln doping, and 4 wt% Nd-doped ZnO was the most active sample showing high photocatalytic activity for the degradation of 4-nitrophenol. He et al. reported Gd-La co-doped TiO2 microspheres showing excellent photocatalytic activity towards the degradation of methyl orange dye (Lin et al. 2013). Abundant oxygen vacancies and surface defects for electron trapping and dye adsorption enhanced the performance of Gd-La co-doped TiO2.
Eu3+-doped graphitic carbon nitride photocatalyst (0.38 wt.% Eu3+) also showed enhanced activity, and near-complete degradation of methylene blue was observed. Eu3+ ions act as an electron acceptor and diminish the photoinduced charge recombination ensuing the improved photocatalytic performance. Sm3+−doped g-C3N4 nanosheets also showed efficient solar photocatalytic activity due to the decreases in the bandgap and increases in visible light absorption. Here hydroxyl radicals and holes play a significant role in the degradation of dye effluent and salicylaldehyde. Optimization of weight percentage of Sm3+ revealed that the degradation of the photocatalytic activities of different samples under sunlight is in the order CN-0.02 wt% Sm3+ > CN-0.03 wt% Sm3+ > CN-0.05 wt% Sm3+ > CN-0.01 wt% Sm3+ > g-C3N4 (Rutar et al. 2015). It has been found that addition of “Ce” to graphitic carbon nitride also enhanced visible light activity due to the crystal growth inhibition, bandgap reduction and efficient separation of charge carriers (Jin et al. 2015).
Molten metal salts are unique and novel high-temperature ionic liquids that can be used for the development of visible light active photocatalysts. A heterojunction-type g-C3N4-based photocatalysts were synthesized using molten salt KCl/LiCl mixtures and melamine in microwave irradiation medium (Liu et al. 2017b). Modified g-C3N4 heterojunction showed a visible light-driven photocatalytic hydrogen evolution rate of 1480 μmol g−1h−1 and has an apparent quantum yield of 10.7%. This heterojunction provides novel electronic band structures for efficient separation of photoinduced charge carriers, resulting in a large enhancement of hydrogen evolution. Here the synergistic effects of microwave heating and molten salt liquid polycondensation are well employed.
Instead of metals, non-metals are also used for doping in photocatalysts. Atomic orbitals of non-metals have potential energy greater than that of O2p orbital of TiO2, ZnO, etc.; hence, non-metal doping has attracted much attraction in making visible light active photocatalysts. Non-metals like B, C, O, Si, P, S, halogens, etc. are commonly used for doping in TiO2, ZnO and graphitic carbon nitride (Shen et al. 2016a; Yu et al. 2016, 2017; Sagara et al. 2016; Zhang et al. 2013; Wang et al. 2017; Suryawanshi et al. 2012; Bu and Chen 2014; Liu et al. 2016b; Qiu et al. 2017; Su et al. 2008; Kwon et al. 2017; Chen et al. 2017; Ansari et al. n.d.; Chaudhuri and Paria 2014; Fan et al. 2017; Samsudin et al. 2016; Carroll et al. 2017; Cai et al. 2016; Han et al. 2015; Bakar and Ribeiro 2016). Previous studies demonstrated that g-C3N4 materials showed improved light-harvesting and pronounced changes in the energy band structure when doped with heteroatoms such as sulphur, iodine, boron, phosphorus, oxygen halogens, etc. These dopants may trap electrons and reduce the chances of electron-hole recombination that deactivates the photocatalytic system.
For example, photocatalytic degradation of methylene blue dye was studied by visible light active S-doped TiO2 nanorods which were synthesized using oxidant peroxide method (Bakar and Ribeiro 2016). Mechanism of MB photocatalytic degradation over S-doped TiO2 nanorods is outlined in Fig. 8.11. S-doping decreased the crystal size of TiO2 attributed to the substitution of Ti4+ by S6+ in the S-doped sample as S6+ (0.29 A) has smaller atomic radius compared to Ti4+ (0.64 A). S-doping increased the number of adsorbed active groups at the catalyst surface, and the formation of Ti-O-S bond favours the partial transfer of electrons from S to O atoms which helped the electron-deficient S atoms to hold/capture electrons, reducing the electron-hole recombination.
Schematic representation of MB photocatalytic degradation over S-doped TiO2 nanorods. The transfer of electrons from S to O atoms helped the electron-deficient S atoms to hold/capture electrons thus reducing the electron-hole recombination. (Reprinted from Bakar and Ribeiro 2016 with permission)
S-doped g-C3N4 also showed enhanced photocatalytic activity. Nanoporous sulphur-doped g-C3N4 microrods were prepared by direct thermal condensation of a melamine-trithiocyanuric acid supramolecular cocrystal. It acts as a highly active photocatalyst for H2 evolution under visible light irradiation (Feng et al. 2014). Mesoporous sulphur-doped graphitic carbon nitride (MCNS) was synthesized using thiourea and SiO2 gel solution as a template through a simple thermal condensation method. Among bulk graphitic carbon nitride (g-C3N4), undoped g-C3N4, sulphur-doped g-C3N4 without template and TiO2 (Degussa P25) samples, the optimized MCNS-4 (sample synthesized at the reaction temperature of 500 °C for 240 min with the SiO2/thiourea weight ratio of 0.3) illustrated the highest photocatalytic activity towards the removal of MO under visible light irradiation. The enhanced performance was originated from the synergistic effects of high surface area, mesoporous texture, sulphur doping and high visible light absorption, which were helpful for the separation and transportation of the photo-generated electron-hole pairs (Jourshabani et al. 2017). S and O co-doped g-C3N4 nanosheets with mesoporous structures synthesized by the polymerization of melamine and H2O2-bonded trithiocyanuric acid (TCA) also gave an enhanced photocatalytic performance for RhB degradation. The S-O co-doped g-C3N4 gave sixfold increase in the activity, by enhancing visible light adsorption and decreasing its bandgap compared to pristine g-C3N4 nanosheets (You et al. 2017).
It was demonstrated that the incorporation of carbon-based materials into semiconductors results in the enhanced photocatalytic performance. Carbon is a good electron acceptor and absorbs over a wide range of visible light. Nanosized carbon materials have higher porosity and various morphologies based on synthesis routes. A series of carbon xerogels-TiO2 samples were prepared using sol-gel synthesis (García et al. 2017). These samples have homogeneous and three-dimensional mesoporous structure, and the interesting feature is that, even though synthesis route involves the calcination of carbon xerogels-TiO2 at 900 °C, anatase phase of TiO2 was stable owing to the good dispersion of TiO2 in the carbon matrix. During sintering, carbon matrix favoured titania reduction, keeping anatase phase stable. Also, carbon favours electron transfer from TiO2 nanoparticles, reducing the charge recombination, leading to higher photocatalytic activity. Carbon-doped ZnO at different temperatures was synthesized using commercially available melamine and zinc nitrate hexahydrate as the starting materials. The photocatalytic performance of the as-prepared photocatalyst towards the degradation of a model pollutant, rhodamine B, under visible light irradiation showed that C-doped ZnO exhibited a significantly higher degradation response than bare ZnO due to the narrowing of the bandgap and improved electron/hole separation efficiency (Ansari et al. 2017).
8.3.2 Addition of Photosensitive Materials
Introduction of sensitizers is another popular method because sensitizers can directly absorb visible light and get excited. The excited sensitizers can release electrons to the conduction band of semiconductors , thus improving the photocatalytic efficiency (Ansari et al. 2017). Photosensitive materials like porphyrins , phthalocyanines, fluorescein, organic dyes, etc. are widely used for enhancing the photocatalytic performance of semiconductors (Radhika and Thomas 2017; Thomasa et al. 2017).
Semiconductivity and visible light photosensitivity of porphyrins/metalloporphyrins make them an important group of photosensitizers. They can boost the separating efficiency of the photoinduced charge carriers by their electron transportability. Their molecules are arranged regularly through the π-π stacking, with which they can form heterojunction structure with semiconductors. Porphyrin compounds (H- or J-type) have good chromophore activities over the solar spectrum and good electron donating properties due to their large π-electron systems. When incorporated into semiconductors , porphyrin aggregates act as light-harvesting assemblies to gather and transfer energy to the assembled devices, resulting in the higher incident photon-to-photocurrent generation efficiency (Li et al. 2010). This was illustrated by the photocatalytic degradation employing copper(II)porphyrin-sensitized TiO2/ZnO photocatalyst (Li et al. 2010; Yu et al. 2015). Cu (II) porphyrins absorb visible light and act as a small-bandgap semiconductor. When incorporated in TiO2, it effectively separates the charge carriers and enhances the photocatalytic activity. A porphyrin heteroaggregate ((ZnO/TAPPI-CoTPPS), where TAPPI = tetrakis (4-trimethylaminophenyl) porphyrin and CoTPPS = tetrakis (4-sulfonatophenyl) porphyrin cobalt(II)), has been anchored on the surface of ZnO which forms a strong interaction with the semiconductor. The excited porphyrin heteroaggregate (Por*) injects its excited electrons to the conduction band of ZnO, thus enhancing the photocatalytic performance (Li et al. 2010).
Curcumin, another photosensitive molecule, also was selected as an aspirant to improve the visible light activity of semiconductor photocatalysts. Curcumin-conjugated ZnO nanostructures synthesized by a simple impregnation method showed enhanced photocatalytic performance with a bandgap of 1.2 eV. The enhanced photocatalytic degradation by curcumin-conjugated nano-ZnO compared to bare ZnO was observed depending on the extent of curcumin conjugation. This work has shed some insight into the large-scale utilization of heterogeneous cost-effective photocatalysis via visible light to tackle water contamination and environmental pollution.
Dyes extracted from plants also have been used to sensitize semiconductors. Curcumin (from turmeric), beta-carotene (from root vegetables like carrot, beetroot, etc.), lawsone (from henna leaves), etc. are some useful compounds for this purpose. Ahed Zyoud et al. reported curcumin-sensitized anatase TiO2 nanoparticles for the photodegradation of organic pollutant (phenazopyridine) under visible light. Curcumin-sensitized anatase TiO2 was able to completely degrade the pollutant and is a promising alternative for hazardous sensitizing dyes (Zyoud and Hilal 2014).
Phthalocyanines are a group of chromophores having characteristic absorption bands in both UV/blue (Soret band) and the red/near-IR spectral regions (Q band, 650−800 nm). They have excellent photochemical/thermal stability and redox potential, which makes them an attractive candidate for the dye sensitization of wide-bandgap semiconductors . Zinc phthalocyanine-sensitized polymeric graphitic carbon nitride (Zn-tri-PcNc/g-C3N4) photocatalyst was synthesized by impregnation method (Zhang et al. 2014). Zinc phthalocyanine extended spectral response region of g-C3N4 from 450 nm to >800 nm. Addition of chenodeoxycholic acid as co-adsorbent to Zn-tri-PcNc/g-C3N4 significantly improved photoactivity for H2 production (125.2 μmol h−1) attributed to the enhancement in electron injection efficiency and impediment of the charge recombination. Photocatalytic degradation of plasticizer dimethyl phthalate (DMP) under xenon-lamp irradiation was achieved by magnetic hybrid photocatalysts formed by layer by layer deposition of Fe2O3, SiO2, TiO2 and copper phthalocyanine (CuPc) (Chang and Man 2014). The presence of CuPc enhanced the effective inhibition of exciton annihilation and promotion of electron injection into the semiconductors.
Polyoxometalates (POMs) are complex transition metal-oxygen clusters having excellent photoelectrochemical activities which can act as electron pools attributed to their distinctive structures (Sivakumar et al. 2012). These POMs can be incorporated with semiconductors for enhanced photocatalytic performance due to the efficient photo-generated charge separation facilitated by transfer of photo-generated electrons to the d-orbital of POMs, during photocatalysis . Among the different POMs, heteropoly phosphotungstic acid (HPA or HPW, H3PW12O40) is widely used for POM/semiconductor nanocomposites . Visible light active H3PW12O40/TiO2 (PW12/TiO2) nano-photocatalyst was synthesized through a modified sol-gel hydrothermal method and the degradation of fuchsin acid, malachite green and p-nitrophenol under simulated sunlight (320 nm< λ <780 nm) irradiation gave first-order rate constants(Zhao et al. 2013). High photocatalytic degradation efficiency was ascribed to the synergistic effect of H3PW12O40 and TiO2, which resulted in enhanced quantum efficiency and high light-harvesting efficiency. A one-step hydrothermal method was employed for synthesizing PMo12@g-C3N4 and PW12@g-C3N4 (He et al. 2015). Under UV-vis light irradiations, PMo12@g-C3N4-6% showed better photocatalytic performance towards the degradation of methylene blue (MB) and phenol, attributed to the higher surface area and pore volume (He et al. 2015). Phosphotungstic acid (H3PW12O40, HPW) immobilized on mesoporous graphitic carbon nitride (mpg-C3N4) contributed a good photocatalyst with high catalytic activity for the oxidative desulfurization process of dibenzothiophene (Zhu et al. 2015). After 15 recycles, catalyst retained its activity towards oxidative desulfurization process, and the Keggin structure of HPW active species was kept after being immobilized on the mpg-C3N4 surface.
Organic dye nanoparticles such as perylene nanoparticles (PeNPs) exhibit distinctive optoelectronic properties superior to their bulk counterparts, and they are expected to have higher photosensitization of semiconductors. Stable PeNPs synthesized from perylene-3,4,9,10-tetracarboxylic dianhydride using D-glucosamine hydrochloride are incorporated into g-C3N4. The resulting g-C3N4-based nanocomposites show greatly enhanced photocatalytic activity under illumination of simulated solar light as compared to those of free g-C3N4 and TiO2 (Degussa P25). When PeNPs – the g-C3N4 system (PeNPs-CN) – was irradiated by sunlight, electrons are excited from valance band to conduction band of g-C3N4 and generate photoexcited electrons and holes. Perylene nanoparticles get excited (perylene*) under solar light irradiation and rapid e− transfer occur between excited perylene (Perylene*) and g-C3N4. The adsorbed perylene* nanoparticles can inject its e− into the conduction band of g-C3N4. The photo-generated holes (h+), ·O2 − and OH. radicals formed are mainly responsible for the degradation of pollutants through its successive attacks via the formation of several intermediate products. The mechanism is outlined in Fig. 8.12. (Thomasa et al. 2017).
Mechanism of pollutant degradation using PeNPs-CN under sunlight. The photo-generated holes (h+), ·O2 − and OH. radicals formed are mainly responsible for the degradation of pollutants in this system. (Reprinted from Thomasa et al. 2017 with permission)
TiO2 co-sensitized with copper phthalocyanine (CuPc) and perylene diimide (TiO2/PTCDI)-CuPc) prepared by hydrothermal method showed higher photocatalytic activity (Zhao et al. n.d.). N,N′-di(octadecyl)perylene-3,4,9,10-tetracarboxylic bisimide (DPBI) is highly fluorescent and exhibited strong absorption and emission in the visible spectral region. Loading of DPBI in TiO2 results in increased absorbance from 400 to 650 nm, and DPBI-loaded TiO2 showed higher efficiency in Reactive Orange 4 degradation than pure TiO2 in UV and solar light (Senthilrajaa et al. 2014).
Xanthene dyes are also good sensitizers and tend to be fluorescent, yellow to pink to bluish red depending upon the functional groups on the xanthene moiety (McNaught and Wilkinson 1997). Large absorption, luminescence, excellent light resistance, low toxicity and relatively high solubility in water are the characteristic features of xanthene dyes which makes them excellent choices for optical materials and dye-sensitized solar cells. Fluorescein treatment enhanced the photovoltaic efficiency and inhibited fluorescence quenching in reactant solution. Xanthene dyes like fluorescein, dibromofluorescein, eosin Y and erythrosine B were used to sensitize carbon nitride (Zhang et al. 2015). Picosecond time-resolved fluorescence measurements were employed to derive the electron transfer rate from the LUMO of each photoexcited xanthene dye to the conduction band of carbon nitride. Similar results were obtained from cyclic voltammetry measurements. These results suggest that electron present in LUMO level of these xanthene dyes can inject its electron to the conduction band of C3N4 in case of dye-sensitized C3N4 composites. On EY sensitization, g-C3N4 prepared at 600 °C from urea exhibited the highest sensitization activity and produced 18.8% hydrogen evolution under optimum conditions (Xu et al. 2013). Factors like pure composition, the higher dye adsorption amount and the lowest defect concentration of the above-mentioned g-C3N4 resulted in its highest activity.
8.3.3 Construction of Heterojunctions/Composites
Construction of heterojunction is an effective method for improving the photocatalytic activity of a photocatalyst if the energy band of hetero material is well matched with that of the semiconductor . Heterojunctions can be formed between semiconductors of different bandgap energies. In heterojunctions, photo-generated electrons and holes move into opposite directions by the electric field created in situ, thus overcoming the charge transfer barrier. Cu2O-TiO2 nanocomposites were synthesized using a wet impregnation method with TiO2 nanorods and CuSO4 and showed enhanced hydrogen production (50,339 mmolh−1g−1 cat) from biomass-derived glycerol under solar irradiation. TiO2 nanorods with nanocavities provide a suitable environment for reactions (Kumar et al. 2015). Cu2O incorporation increased the absorption of UV-visible light from the natural solar spectrum, inhibited the recombination of electron-hole pairs, while TiO2 nanorods with nanocavities provide multiple internal reflections of light within nanocavities, which improved the surface-interface reactions. Mechanism of photocatalysis of the catalysts is given in Fig. 8.13. Mixed oxide dye-sensitized solar cells (DSSCs) based on CuO-ZnO nanocomposite films as working electrodes with and without titania blocking layer (TBL) were investigated (Habibi et al. 2013) and found that charge recombination in the working electrode was slow when titania blocking layer was present in DSSC.
Proposed reaction mechanism of Cu2O-TiO2 nanocomposites for photocatalytic H2 production with aqueous glycerol-water mixture under solar irradiation. (Reprinted from Kumar et al. 2015 with permission)
Visible light-induced dendritic BiVO4/TiO2 photocatalysts synthesized using hydrothermal and sol-gel method gave a good result for the photocatalytic degradation of methylene blue (MB) under visible light irradiation. The photocatalyst with a ratio of BiVO4/TiO2 (mass ratio) equal to 60% prepared at 400 °C exhibited the best photocatalytic activity. In this system BiVO4 acts as a photocatalyst, which can be excited under visible light irradiation, with the generation of holes and electrons. The degradation processes in BiVO4/TiO2 mainly depended on the synergistic effect of photocatalysis and photosensitization (Li et al. 2016d).
Conjugated polymers are another group of organic semiconductors having wide applications in energy production and pollutant degradation. Based on varying or modifying polymeric monomer, their semiconducting property and light absorption ability can be easily tuned. Poly(benzothiadiazole)-TiO2-based composite (BBT/TiO2) is conveniently fabricated through an in situ polycondensation procedure of 4,7-dibromo benzo [1,2,5]thiadiazole and 1,3,5-triethynylbenzene in presence of TiO2 (Degussa P25) (Hou et al. 2017). BBT/TiO2 exhibited enhanced visible light photocatalytic activities towards H2 evolution and ciprofloxacin degradation compared to BBT alone. The enhancement of photocatalytic performance is ascribed to the formation of heterojunction and reduction of the rate of photoinduced charge carriers.
Pure Ag3PO4 is a narrow-bandgap (2.33 eV) photocatalyst with inadequate long-term stability due to decomposition in the absence of a sacrificial agent . But when it is incorporated with TiO2, a highly visible light-responsive Ag3PO4/TiO2 photocatalyst was formed with a molar ratio 75:25(Taheri et al. 2017). Ag3PO4/TiO2 completely degraded bisphenol A pollutant within 4 min of solar irradiation. The synergetic effect between Ag3PO4 and TiO2 which helps in the interfacial charge transfer and suppression of electron-hole recombination resulted in the superior photocatalytic performance of Ag3PO4/TiO2. The visible light-responsive Ag3PO4@g-C3N4 core shell composites were also synthesized using ultrasonication/chemisorption method (Li et al. 2016b). Photocatalytic degradation of methylene blue (MB) and bisphenol A (BPA) in the presence of these composites showed that Ag3PO4@g-C3N4 (7.0 wt.%) was able to degrade MB and BPA after visible light irradiation within 30 min. The photocurrent and EIS measurements confirmed the efficient photo-generated charge separation originated from a strong interaction in the intimately contacted interface (Li et al. 2016b).
Graphene having carbon atoms arranged in a honeycomb structure is an excellent electrical conductor. Graphene oxide (GO) with introduced hydroxyl and carboxyl groups also possesses similar properties of graphene. The presence of special surface functional groups makes GO as a good choice for supporting metal or metal oxide particles. High electron mobility and flexible sheet nature of reduced graphene oxide (rGO) make it as a good candidate for photocatalytic applications. They act both as electron-acceptor and as electron-transport material facilitating the migration of photoinduced charge carriers and hinder the charge recombination to enhance the photocatalytic performance during the photocatalysis experiments. Reduced graphene oxide (rGO) incorporated with semiconductor nanoparticles also showed improved photocatalytic performance (Li et al. 2017b). ZnO microspheres-rGO nanocomposites synthesized via a simple solution method were performed as efficient photocatalysts in comparison with ZnO microspheres and TiO2 because rGO sheets could reduce the photoinduced charge recombination processes (Li et al. 2017b).
Large Ag cores decorated with small clusters of CuO nanoparticles on the surface of TiO2 (DegussaP25) (Ag@CuO/TiO2 nanocomposite), synthesized via radiolytic reduction technique, showed enhanced activity towards phenol degradation and acetic acid oxidation under visible light. Introduction of nanoparticles of CuO decreases the bandgap, and Ag introduces localized surface plasmon resonance which improved the photocatalytic activity of TiO2 (Medrano et al. 2016). In2O3 and WO3 are well-known semiconductors having bandgaps of 2.8 and 2.7 eV, respectively, with excellent conductivity and stability in aqueous systems. Modified photoelectrodes based on TiO2/In2O3 and TiO2/WO3 gave enhanced hydrogen production under visible light conditions . Hydrogen production rates of 11.8 and 13.8 lh−1m−2were obtained using TiO2/In2O3 and TiO2/WO3, respectively, by these photoelectrodes (Upadhyay et al. 2013). Enhancement in hydrogen production with modified TiO2 photoelectrodes was due to the improved spectral response resulting from the decrease of energy bandgap. In2O3-modified ZnO photocatalyst also showed enhanced activity and stability due to the reduction in both charge recombination and size of ZnO after the loading of In2O3, which contributed to the improved hydrogen production from methanol (Martha et al. 2014).
8.3.4 Construction of Nanohybrid Materials
Hybrid materials are composed of two or more components of organic and inorganic origin, which are well dispersed in the molecular level. Hybrid materials of suitable composition are proved as good candidates for visible light active photocatalysts. Lanthanide ion doping and incorporation of photosensitive materials have attracted a lot of interest in the area of solar/visible light photocatalysis . Indeed few reports are available on lanthanide ion doped semiconductor incorporated with photosensitive molecules as hybrid materials . Exploiting the advantages of bandgap reduction by lanthanide ion doping and the electron-hole recombination prevention by incorporation of photosensitive materials, some efficient photocatalytic systems were synthesized (Thomas et al. 2016). Novel photocatalysts, neodymium (Nd3+)-doped TiO2 nanoparticles incorporated with heteropoly phosphotungstic acid (HPA) (Nd3+-TiO2-HPA) were applied for the degradation of pollutants like methylene blue and organochlorine toxic chemical, 4-chlorophenol in water. Complete mineralization of both the pollutants upon sunlight illumination indicates that Nd3+-TiO2-HPA nanocomposites would be very useful for cleaning polluted surface water by sunlight through the advanced oxidation process . The enhanced photocatalytic activity of Nd3+-TiO2-HPA nanocomposites is mainly due to two factors: (i) doping of Nd3+ions decreased the bandgap of TiO2 (from 3.22 to 2.87 eV) and (ii) HPA addition prevented the recombination of electrons and holes.
Nano-ZnO-based dye-sensitized solar cells were synthesized using zinc porphyrin (an electron donor) and fullerene (C60 acid, an electron acceptor) via spin coating method (Hayashi et al. 2009). These dye-sensitized bulk heterojunction solar cells have both features of dye sensitization and bulk heterojunction devices. The porphyrin-fullerene-ZnO nanorod devices exhibited efficient photocurrent generation compared to that of the reference systems. Fullerene can inject its electrons to the conduction band of ZnO and suppresses the recombination of charges. The porphyrin-fullerene composite layer on ZnO surface also efficiently separates the photoinduced charges.
Metal-doped semiconductor incorporated with another semiconductor of low bandgap energy is another way to enhance the visible light-induced photocatalytic performance of a UV light active semiconductor. Such a hybrid nanocomposite, CdS-loaded Ag-ZnO catalyst was obtained using a simple precipitation-thermal decomposition method (Subash et al. 2016). The novel photocatalysts can absorb visible light and has reduced recombination of the photo-generated electron-hole pairs, attributed to the presence of Ag and CdS. Studies showed that loss of Zn during the photocatalytic reaction, one of the main problems associated with nano-ZnO-based photocatalysts, was prevented ascribed to the strong interaction between ZnO with Ag and CdS.
Photosensitizer-metal sulphide-semiconductor nanohybrids show enhanced photocatalytic performance (Yuan et al. 2015). A system for visible light-driven hydrogen evolution was constructed by using Zn(II)-5,10,15,20-tetrakis(4-carboxyphenyl)porphyrin dye-sensitized MoS2/ZnO as a photocatalyst. It was used for the photocatalytic H2 production from water in presence of triethanolamine as the sacrificial electron donor under visible light irradiation. MoS2, having 2D structure, is similar to graphene and is widely used as co-catalyst for photochemical H2 evolution reaction because of its high edge plane exposure.
It was observed that the incorporation of graphene and nano-Au into TiO2 significantly enhanced the electron transport and therefore impeded the charge recombination of excited TiO2 which resulted in the decrease of the crystalline size, an increase of surface area and redshift to the visible region (Ghasemi et al. 2017). Exploiting the surface plasmon resonance of Au nanoparticles and superior electron transport property of carbon nanotubes (CNT) , g-C3N4-based hybrid (g-C3N4/CNTs/Au) was synthesized using ultrasonication method, for water purification and water splitting (Pawar et al. 2015). The large-specific surface area, reduction in the charge recombination and the high visible light absorption due to the SPR of Au nanoparticles were observed in ternary g-C3N4/CNTs/Au hybrid. All these properties resulted in the enhancement of photocatalytic activity and photocurrent response of the ternary g-C3N4/CNTs/Au hybrid. Photo-generation and electron transport mechanism are shown in Fig. 8.14.
Electron transport mechanism under visible light for the degradation of organic compounds with the ternary g-C3N4/CNTs/Au hybrid which showed enhanced photocatalytic activity and photocurrent response. (Reprinted from Pawar et al. 2015 with permission)
The size-dependent role of metal nanoparticles in hybrid systems for photocatalysis is well explained (Bai et al. 2016). Au nanoparticles having two different sizes (100–150 nm and 10–20 nm) were added to synthesize Au/g-C3N4/BiOBr system (where BiOBr = Bismuth oxybromide). Au/g-C3N4/BiOBr system with big Au nanoparticles showed higher photocatalytic activity under UV light (380 nm monochromatic light irradiation) attributed to the Z-scheme bridge roles displayed by big Au nanoparticles. However, Au/g-C3N4/BiOBr system with small Au nanoparticles exhibited surface plasmon resonance and showed enhanced performance under visible light (550 nm monochromatic light irradiation). The size-dependent role of Au nanoparticles for the photocatalytic system was due to the SPR effect of small Au nanoparticles and electron transfer centre of big Au nanoparticles.
Synthesis of nanocomposite consisting of metal-organic framework (MOF) and g-C3N4 is a new way to improve the drawbacks of the g-C3N4 photocatalyst. g-C3N4 /Ti-benzenedicarboxylate hybrid nanocomposite {represented as CMTi} was synthesized by employing ultrasonication followed by solvothermal treatment (Wang et al. 2015). The near-complete photocatalytic degradation of RhB was obtained after 60 min of experimental conditions by the hybrid photocatalyst. The enhanced performance of this hybrid nanocomposite was attributed to intervalence electron transfer between Ti3+ and Ti4+ and synergetic effect between MOF and g-C3N4.
Immobilization of photocatalysts on supports like clay, zeolites, MCM-41, silica, etc. not only enhances the photocatalytic performance but also improves the stability of photocatalyst (Belver et al. 2017; Tongon et al. 2014; Vadivel et al. 2016a; Hagura et al. 2011; Suligoj et al. 2016; Wee et al. 2016). Properties like high surface area, layered or fibrous structure and high adsorption capacity have made clay minerals as one of the extensively used supports in heterogeneous catalysis. Semiconductor-clay composites with superior photocatalytic performance have been reported by many research groups, combining the adsorption capacity of the clay and the photocatalytic ability of semiconductor. The one-step sol-gel route is employed to synthesize solar light active Zr-doped TiO2 nanoparticles immobilized on delaminated clay materials (Belver et al. 2017). High degradation rates of antipyrine (an analgesic drug) by Zr-doped TiO2/clay catalyst under solar irradiation show the potential application of this catalyst in environmental remediation. The incorporation of Zr reduced the charge recombination, while the addition of delaminated clay resulted in the high surface area and a disordered mesoporous structure. Construction of novel photocatalysts based on semiconductor-heterojunctions on a delaminated clay surface can enhance their photocatalytic activity, by reducing the probability of electron-hole recombination and promoting the charge carrier migration. Photocatalytic degradation of two widely used pharmaceuticals, acetaminophen and antipyrine, was achieved using TiO2-ZnO/cloisite photocatalysts based on the heterojunction between semiconductors (TiO2-ZnO) and delaminated-layered clay (cloisite) (Tobajas et al. 2017). Silica (SiO2) also is a widely used inorganic supporting material, possessing high chemical stability except towards strong acid or base. SiO2 has a low refractive index, good light transmission and adsorption of contaminants which result in the enhancement of photoactivity of SiO2-semiconductor composites. TiO2-SiO2 (SBA-15) films free of organic contents were prepared from metatitanic acid as a precursor for TiO2 (Suligoj et al. 2016). TiO2-SiO2 films were immobilized on glass carriers by brush deposition and showed 100 and 91% decompositions of toluene and formaldehyde, respectively, at room temperature under UV-A irradiation. Six times higher turnover frequency was shown by TiO2-SiO2 films in comparison to pure TiO2. COK-12 (mesoporous silica nanoplatelets having a thickness of 200–300 nm, prepared via spontaneous precipitation of sodium silicate and citrate/citric acid buffered P123 triblock copolymer solutions) support was used to enhance the photocatalytic activity of TiO2 nanoparticles (Wee et al. 2016). TiO2-COK-12 photocatalyst has shown excellent photocatalytic activity than Degussa P25. ZnO/silica gel nanocomposite (ZnO/SG) showing the excellent antibacterial property was synthesized via environmentally friendly method by heating a mixture of ZnCl2 and silica gel at 450 °C without the addition of any reducing agent (Lotfiman and Ghorbanpour 2017). The antibacterial test against Escherichia coli and Staphylococcus aureus revealed that the ZnO/SG prepared in longer deposition times showed improved antibacterial activity due to the enhancement in the contents of ZnO nanoparticles on the silica surface.
Carbon nitride-silica-based hybrid photocatalyst was developed by loading carbon nitride into titanium-incorporated SBA-15 (Ti-SBA15-CN). Photocatalytic reduction of Cr (VI) in aqueous solution and synergistic oxidation of phenol was investigated using Ti-SBA15-CN under visible light irradiation. By introducing Ti into the SBA-15 framework structure, visible light-driven photocatalytic activity of Ti-SBA15-CN can be improved compared to SBA15-CN, and the photocatalytic activity exhibited a rise with the increase of Ti contents. It was because the presence of Ti moiety could promote the separation of the photo-generated charge carriers in carbon nitride, leading to the enhancement of the photocatalytic activity. The addition of phenol further enhanced the photocatalytic reduction of Cr (VI). Similarly, the presence of Cr (VI) promoted the degradation of phenol. The synergistic effect of the reduction of Cr (VI) and the degradation of phenol provided a salutary method for purification of the complex wastewater and environmental restoration (Liu et al. 2017a).
Visible light-responsive Ag/TiO2/MCM-41 nanocomposite films were synthesized by an inexpensive and convenient microwave-assisted sol-gel technique (Tongon et al. 2014). Ag/TiO2/MCM-41 films were used to study the degradation of methylene blue dye and showed degradation rates of 80% and 30% under UV and visible light, respectively. Ag reduced the bandgap energy and prevented recombination of photoinduced charges, whereas adsorption capacity of the nanocomposite films was enhanced by MCM-41. MCM-41 also supplies active hydroxyl radicals attributed to the presence of silanol groups (Si-OH). Graphitic carbon nitride (g-C3N4) also showed superior photocatalytic hydrogen evolution when loaded onto the MCM-41 mesoporous silica. 1.2 times higher H2 evolution was obtained on using MCM-41/g-C3N4 compared with pure g-C3N4 and is ascribed to the high dispersion of g-C3N4 on MCM-41 which in turn lead to the improved separation efficiency of photo-generated charge carriers of g-C3N4 (Shen et al. 2016b). Introduction of Ti into the MCM-41 framework structure created (Ti4+-O2−) active centres, resulting in the utilization of visible light.
Semiconductors modified with zeolites, another class of hybrid catalysts, also show enhanced photocatalytic performance attributed to the presence of broad-specific area, high amount of hydrophobic and hydrophilic active sites and adequate crystallinity (Zheng et al. 2012b). The presence of molecular dimensional pores and channels, high surface area, excellent ion exchange properties, etc. nominate zeolite as an excellent candidate in the field of catalysis. Strong acidic functional groups can be introduced in zeolites to incorporate various metals and metal oxides . Functional form of TiO2-zeolite nanocomposite synthesized via two-step sol-gel method showed a high apparent pseudo-first-order reaction rate constant of 0.0419 min−1 at lower dye concentration, following a more adsorption-oriented photocatalytic degradation of water pollutants, which is useful for removing trace and untreated dye compounds in the advanced industrial dye wastewater treatment stage (Chong et al. 2015). Modification of ZnO using different zeolites has potential applications in heterogeneous photocatalysis (Batistela et al. 2017). ZnO photocatalyst modified by supporting on three different zeolites, zeolite Y (NaY), zeolite A (NaA) and zeolite Y ultrastable (USY) showed improved activity. The crystallization of ZnO occurred on the external and internal surfaces of zeolite NaA and zeolites NaY and USY. Zero point charge analysis (ZPC) showed that ZnO modification using NaA caused a significant decrease in ZPC due to synergic effect of ZnO and NaA. ZPC increased in ZnO-NaY and ZnO-USY catalysts indicating that ZnO acts as an integral part of the structure of the catalysts, due to its incorporation into the pores.
Bismuth oxyfluoride (BiOF), a UV-sensitive semiconductor (bandgap of 3.5 eV) which attracted a lot of interest in the area of environmental remediation, was incorporated into other semiconductors to form hybrid materials. Ag modified BiOF/CN organic-inorganic hybrid photocatalysts exhibited advanced photocatalytic activity towards the degradation of MB under visible light (Vadivel et al. 2016b). The photoinduced electrons can be easily transferred from CN to BiOF ascribed to the fact that conduction band edge potential of CN is more negative than that of BiOF. Ag particles acted as an electron-conduction bridge between CN and BiOF.
CaFe2O4 belongs to the family of spinel ferrites and is an excellent material showing interesting catalytic properties. Vadivel et al. reported CN-CaFe2O4 nanocomposites prepared by ultrasonication method (Vadivel et al. 2016a). Among the different synthesized samples, CaFe2O4 (30%)-CN showed superior photocatalytic activity towards the degradation of methylene blue (MB) under visible light. Addition of CaFe2O4 leads to strong visible light absorption and efficient charge separation which resulted in the superior photocatalytic activity of CN-CaFe2O4 nanocomposites.
ZnIn2S4, a well-known ternary chalcogenide semiconductor having 2D layered structure and the narrow bandgap of 2.6 eV, has been widely studied in photocatalysis . “Sheet-on-sheet” hierarchical nanocomposite consisting of CN and ZnIn2S4 exhibit remarkably enhanced photocatalytic H2 production (Zhang et al. 2016). Efficient interfacial photoinduced charge carrier transfer from CN to ZnIn2S4 resulted in reduced charge recombination in CN and enhanced a number of electrons and holes in ZnIn2S4.
The photocatalytic degradation of RhB was studied using Fe2O3/CN, Co3O4/CN and NiO/CN synthesized via one-step pyrolysis of urea and corresponding metal nitrates (Sridharan et al. 2014). During pyrolysis, urea acts as reducing agent and simultaneously gets converted to CN. The resultant photocatalysts have wide absorption in visible spectrum making them suitable choice as optical limiters, which protects eyes from the harmful effects of lasers or other radiations.
8.3.5 Surface Modification
Surface properties of nano-semiconductor photocatalysts have significant effects on their photochemical applications. Especially, in the photocatalytic degradation of organic pollutants, the surface charge of photocatalysts plays a significant role. The point of zero charge (zpc) is a point when electrical charge density on a surface is zero. The semiconductor surface is positively charged below its zpc value and is negatively charged when exceeded its zpc. The zpc values of TiO2, ZnO and g-C3N4 are 6.2 (Wen et al. 2015), 9.5 (Radzimska and Jesionowski 2014) and 5.6, respectively (Fronczak et al. n.d.).
Bird’s nest-like anatase TiO2 microstructure with the exposed highly active surface has been synthesized using a facile one-step solvothermal method (Zhang et al. 2017). Photocatalytic activity of the bird’s nest-like sample was more effective than P25 under the simulated solar light for the degradation of MB and was due to the unique mesoporous structure, high specific surface area and highly active crystal facet exposure.
Anatase TiO2 nanoparticles with shape controlled bipyramidal morphology (TiO2-A-bipy) were also reported recently as good photocatalyst (Rémy et al. 2017). The sol-gel method was utilized for the preparation of TiO2-A-bipy nanoparticles and then used for the photodegradation of three model pollutants – rhodamine B, phenol and formic acid under UV-A radiation exposure. TiO2-A-bipy showed better photocatalytic activity towards RhB degradation than the commercial TiO2-P25 attributed to the presence of more acidic sites on the TiO2-A-bipy surface. However, in the case of phenol and formic acid degradation, commercial TiO2-P25 showed better activity, may be due to better generation and separation of photo-generated charges. This work proved that dye degradation tests alone cannot confirm the photocatalytic performance of photocatalysts.
The stability of semiconductor nanoparticles can be enhanced by the use of capping agents like polyethylene glycol (PEG), cetyltrimethylammonium bromide (CTAB), etc. However, the photocatalytic activity of capped semiconductor nanoparticles will be different. PEG capped ZnO nanoparticles gave reduced photocatalytic activity than bare ZnO towards the photodegradation of rhodamine B dye (Sudha et al. 2013). But CTAB-modified TiO2 photocatalysts show enhanced photocatalytic activity than bare TiO2 attributed to the high separation of photoinduced charge carriers in CTAB-modified TiO2 (Zhong et al. 2013). ZnO with doughnut-like morphology was synthesized via calcination of ZnO-starch biocomposites (Carp et al. 2015). Here starch acted as a template and stabilizing/capping agent. It forces the nucleation and growth of ZnO along polysaccharides regions of high Zn2+ concentrations. Complete mineralization and degradation of phenol were obtained using this doughnut-like ZnO photocatalyst under visible light irradiation attributed to the large specific surface area, high crystallinity and narrow bandgap . Mesoporous TiO2 with the large surface area was synthesized from titanium isopropoxide by sol-gel method using Triton-X and oleic acid as surfactants and diethanolamine as hydrolysis rate controller (Athanasiou et al. 2014). Photocatalytic activity of mesoporous TiO2 was evaluated by accessing the photocatalytic degradation of methylene blue (MB) solution and NO oxidation.
In the case of graphitic carbon nitride photocatalysts, a proficient approach is exfoliation of bulk g-C3N4 (Chang et al. 2014). Exfoliation will create corresponding nanosheets by destroying the multilayer structure of g-C3N4. This technique will produce several thin segments having enlarged surface area. Efficient separation of charge carriers along with increased surface area provides a large number of active sites on photocatalysts surface which in turn results in the improved visible light absorption and better photocatalytic activity.
8.4 Conclusion
Growing concern over solar energy utilization and pollutant degradation has generated a strong interest in the development of solar light active photocatalysts, which would provide an environmentally sustainable photocatalytic treatment process for the degradation of organic pollutants and also for energy production using sunlight in place of artificial light. In this chapter we discussed some important photocatalysts, nanosized TiO2, ZnO and graphitic carbon nitride, and various strategies adopted for improving their photocatalytic efficiency under sunlight. Fundamental information indicated in this chapter about semiconductor photocatalysts would be useful in constructing next-generation photocatalysts with higher spectral response in the visible region for efficient solar energy utilization.
References
Ansari SA, Ansari SG, Foaud H, Cho MH (2017) New J Chem 41:9314–9320. https://doi.org/10.1039/C6NJ04070E
Ansari SA, Ansari MO, Cho MH. Sci Rep, 6:27713. https://doi.org/10.1038/srep27713.
Athanasiou A, Mitsionis A, Vaimakis T, Pomonis P, Petrakis D, Loukatzikou L, Todorova N, Trapalis C, Ladas S (2014) Appl Surf Sci 319:143–150. https://doi.org/10.1016/j.apsusc.2014.06.086
Bachir S, Azuma K, Kossanyi J, Valat P, Haret JCR (1997) J Lumin 75:35–49. https://doi.org/10.1016/S0022-2313(97)00093-8
Bai Z, Yan X, Kang Z, Hu Y, Zhang X, Zhang Y (2015) Nano Energy 14:392–400. https://doi.org/10.1016/j.nanoen.2014.09.005
Bai Y, Chen T, Wang P, Wang L, Ye L, Shi X, Bai W (2016) Sol Energy Mater Sol Cells 157:406–414. https://doi.org/10.1016/j.solmat.2016.07.001
Bakar SA, Ribeiro C (2016) J Mol Catal A Chem 412:78–92. https://doi.org/10.1016/j.molcata.2015.12.002
Basahel SN, Ali TT, Mokhtar M, Narasimharao K (2015) Nanoscale Res Lett 10:73–86. https://doi.org/10.1186/s11671-015-0780-z
Batistela VR, Fogac LZ, Fávaro SL, Caetano W, Machado NRCF, Hioka N (2017) Colloids Surf A Physicochem Eng Asp 513:20–27. https://doi.org/10.1016/j.colsurfa.2016.11.023
Belver C, Bedia J, Rodriguez JJ (2017) J Hazard Mater 322 (233–242. https://doi.org/10.1016/j.jhazmat.2016.02.028
Bhethanabotla VC, Russell DR, Kuhn JN (2017) Appl Catal B Environ 202:156–164. https://doi.org/10.1016/j.apcatb.2016.09.008
Bingham S, Daoud WA (2011) J Mater Chem 21:2041–2050. https://doi.org/10.1039/C0JM02271C
Bu Y, Chen Z (2014) Electrochim Acta 144:42–49. https://doi.org/10.1016/j.electacta.2014.08.095
Cai A, Du L, Wang Q, Chang Y, Wang X, Guo X (2016) Mater Sci Semicond Process 43:25–33. https://doi.org/10.1016/j.mssp.2015.11.017
Cao Z, Zhu S, Qu H, Qi D, Ziener U, Yang L, Yan Y, Yang H (2014) J Colloid Interface Sci 435:51–58. https://doi.org/10.1016/j.jcis.2014.08.021
Carp, Tirsoaga A, Jurca B, Ene R, Somacescu S, Ianculescu A (2015) Carbohydr Polym 115:285–293. https://doi.org/10.1016/j.carbpol.2014.08.061
Carroll JP, Myles A, Quilty B, McCormack DE, Fagan R, Hinder SJ, Dionysiou DD, Pillai SC (2017) J Hazard Mater 324(A):39–47. https://doi.org/10.1016/j.jhazmat.2015.12.038
Chang CF, Man CY (2014) Colloids Surf A Physicochem Eng Asp 441:255–261. https://doi.org/10.1016/j.colsurfa.2013.09.009
Chang F, Zhang J, Xie Y, Chen J, Li C, Wang J, Luo J, Deng B, Hu X (2014) Appl Surf Sci 311:574–581. https://doi.org/10.1016/j.apsusc.2014.05.111
Chaudhuri RG, Paria S (2014) Dalton Trans 43:5526–5534. https://doi.org/10.1039/C3DT53311E
Chen Z, Ge Ma Z, Chen Y, Zhang Z, Zhang J, Gao Q, Meng M, Yuan X, Wang J, Liu GZ (2017) Appl Surf Sci 396:609–615. https://doi.org/10.1016/j.apsusc.2016.10.203
Cheng N, Tian J, Liu Q, Ge C, Qusti A, Asiri A, Al-Youbi A, Sun X. ACS Appl Mater Interfaces 5, 15, 6815–6819. https://doi.org/10.1021/am401802r
Chong MN, Tneu ZY, Poh PE, Jin B, Aryal R (2015) J Taiwan Inst Chem Eng 50:288–296. https://doi.org/10.1016/j.jtice.2014.12.013
Fan Q, Liu J, Yu Y, Zuo S, Li B (2017) Appl Surf Sci 391(B):360–368. https://doi.org/10.1016/j.apsusc.2016.04.055
Feng LL, Zou Y, Li C, Gao S, Zhou LJ, Sun Q, Fan M, Wang H, Wang D, Li G-D, Zou X (2014) Int J Hydrog Energy 39(28):15373–15379. https://doi.org/10.1016/j.ijhydene.2014.07.160
Fronczak M, Krajewska M, Demby K, Bystrzejewski M (n.d.) J Phys Chem C 121(29):15756–15766. https://doi.org/10.1021/acs.jpcc.7b03674
Fujishima, Honda K (1972) Nature 238:37. https://doi.org/10.1038/238037a0
Gao X, Su X, Yang C, Xiao F, Wang J, Cao X, Wang S, Lu Z (2013) Sensors Actuators B 181:537–543. https://doi.org/10.1016/j.snb.2013.02.031
García EB, Elmouwahidi A, Álvarez MA, Marín FC, Cadenas AFP, Hódar FJM (2017) Appl Catal B Environ 201:29–40. https://doi.org/10.1016/j.apcatb.2016.08.015
Ghasemi S, Hashemian SJ, Alamolhoda AA, Gocheva I, Setayesh SR (2017) Mater Res Bull 87:40–47. https://doi.org/10.1016/j.materresbull.2016.11.020
Glossary of terms used in photochemistry (2006) IUPAC recommendations. 3rd edn., p 384. https://doi.org/10.1351/pac200779030293
Gupta R, Eswar NKR, Modak JM, Madras G (2017) Chem Eng J 307:966–980. https://doi.org/10.1016/j.cej.2016.08.142.
Habibi MH, Karimi B, Zendehdel M, Habibi M (2013) Spectrochim Acta A Mol Biomol Spectrosc 116:374–380. https://doi.org/10.1016/j.saa.2013.07.046
Haggerty JES, Schelhas LT, Kitchaev DA, Mangum JS, Garten LM, Sun W, Stone KH, Perkins JD, Toney MF, Ceder G, Ginley DS, Gorman BP, Tate J (2017) Sci Rep 7:15232. https://doi.org/10.1038/s41598-017-15364-y
Hagura N, Ogi T, Shirahama T, Iskandar F, Okuyama K (2011) J Lumin 131:921–925. https://doi.org/10.1016/j.jlumin.2010.12.024
Han Q, Hu C, Zhao F, Zhang Z, Chen N, Qu L (2015) J Mater Chem A 3:4612–4619. https://doi.org/10.1039/C4TA06093H
Hanifehpour Y, Hamnabard N, Khomami B, Joo SW, Min BK, Jung JH (2016) J Rare Earths 34(1):45–54. https://doi.org/10.1016/j.molliq.2016.06.076.
Hayashi H, Kira A, Umeyama T, Matano Y, Charoensirithavorn P, Sagawa T, Yoshikawa S, Tkachenko NV, Lemmetyinen H, Imahori H (2009) J Phys Chem C 113:10819–10828. https://doi.org/10.1021/jp902623g
He J, Sun H, Indrawirawan S, Duan X, Tade MO, Wang S (2015) J Colloid Interface Sci 456:15–21. https://doi.org/10.1016/j.jcis.2015.06.003
Hoffmann MR, Martin ST, Choi W, Bahnemannt DW (1995) Chem Rev 95:69–96. https://doi.org/10.1021/cr00033a004
Hou HJ, Zhang XH, Huang DK, Ding X, Wang SY, Yang XL, Li SQ, Xiang YG, Chen H (2017) Appl Catal B Environ 203:563–571. https://doi.org/10.1016/j.apcatb.2016.10.059
Hu Y, Tsai HL, Huangk CL (2003) J Eur Ceram Soc 23:691–696. https://doi.org/10.1016/S0955-2219(02)00194-2.
Izumi Y (2013) Coord Chem Rev 257:171–186. https://doi.org/10.1016/j.ccr.2012.04.018
Jagadish C, Pearton S (2006) Zinc oxide – Bulk, thin films and nanostructures, 1st edn. Elsevier Science, eBook, Amsterdam. ISBN:9780080464039
Jesty T, Yoon M (2012) Appl Catal B Environ 111–112:502–508. https://doi.org/10.1016/j.apcatb.2011.10.039
Jesty T, Kumar KP, Suresh M (2011) Sci Adv Mater 3:59–65. https://doi.org/10.1166/sam.2011.1131
Jin R, Hu S, Gui J, Liu D (2015) Bull Kor Chem Soc 36(1):17–23. https://doi.org/10.1002/bkcs.10001
Jourshabani M, Shariatinia Z, Badie A (2017) Langmuir 33(28):7062–7078. https://doi.org/10.1021/acs.langmuir.7b01767
Kanhere P, Chen Z (2014) Molecules 19:19995–20022. https://doi.org/10.3390/molecules191219995
Khatamian M, Khandar AA, Divband B, Haghighi M, Ebrahimiasl S (2012) J Mol Catal A Chem 365:120–127. https://doi.org/10.1016/j.molcata.2012.08.018
Kumar DP, Reddy NL, Kumari MM, Srinivas B, Kumari VD, Sreedhar B, Roddatis V, Bondarchuk O, Karthik M, Neppolian B, Shankar MV (2015) Sol Energy Mater Sol Cells 136:157–166. https://doi.org/10.1016/j.solmat.2015.01.009
Kumar MK, Bhavani K, Naresh G, Srinivas B, Venugopal A (2016) Appl Catal B Environ 199:282–291. https://doi.org/10.1016/j.apcatb.2016.06.050
Kuyumcu ÖK, Kibar E, Glu KD, Gedik F, Akın AN, Glu ŞÖA (2015) J Photochem Photobiol A Chem 311:176–185. https://doi.org/10.1016/j.jphotochem.2015.05.037
Kwon JH, Park J, Park GS, Zuo JM, Kang SY, Oh JY, Kim M (2017) Curr Appl Phys 17(2):152–156. https://doi.org/10.1016/j.cap.2016.11.013
Le S, Jiang T, Zhao Q, Liu XF, Li Y, Fang B, Gong M (2016) RSC Adv 6:38811–38819. https://doi.org/10.1039/C6RA03982K
Lee J, Shim HS, Lee M, Song JK, Lee D (2011) J Phys Chem Lett 2:2840–2845. https://doi.org/10.1021/jz2013352
Lee KM, Lai CW, Ngai KS, Juan JC (2016) Water Res 88:428–448. https://doi.org/10.1016/j.watres.2015.09.045
Li X, Cheng Y, Kang S, Mu J (2010) Appl Surf Sci 256:6705–6709. https://doi.org/10.1016/j.apsusc.2010.04.074
Li M, Zhang L, Fan X, Wu M, Du Y, Wang M, Kong Q, Zhang L, Shi J (2016a) Appl Catal B Environ 190:36–43. https://doi.org/10.1016/j.apcatb.2016.02.060
Li L, Qi Y, Lu J, Lin S, An W, Liang Y, Cui W (2016b) Appl Catal B Environ 183:133–141. https://doi.org/10.1016/j.apcatb.2015.10.035
Li Z, Wu Y, Lu G (2016c) Appl Catal B Environ 188:56–64. https://doi.org/10.1016/j.apcatb.2016.01.057
Li W, Ze W, Kong D, D D, Zhou M, Y D, Yan T, You J, Kong D (2016d) J Alloys Compd 688:703–711. https://doi.org/10.1016/j.jallcom.2016.07.249
Li J, Xu X, Liu X, Qin W, Wang M, Pan L (2017a) J Alloys Compd 690:640–646. https://doi.org/10.1016/j.jallcom.2016.08.176
Li X, Shen R, Mab S, Chen X, Xie J (2017b) Appl Surf Sci 430:53–107. https://doi.org/10.1016/j.apsusc.2017.08.194
Lin Y, Chai Y, Yang X, Wang DH, Tang Q, Ghoshroy S (2013) Int J Hydrog Energy 38:2634–2640. https://doi.org/10.1016/j.ijhydene.2012.11.100
Liu Y, Wang Z, Wang W, An X, Mi S, Tang J, Huang W (2014a) Appl Surf Sci 315:314–322. https://doi.org/10.1016/j.apsusc.2014.07.143
Liu Y, Wang Z, Wang W, Huang W (2014b) J Catal 310:16–23. https://doi.org/10.1016/j.jcat.2013.03.024
Liu S, Li D, Sun H, Ang HM, Tadé MO, Wang S (2016a) J Colloid Interface Sci 468:176–182. https://doi.org/10.1016/j.jcis.2016.01.051
Liu T, Li X, Yuan X, Wang Y, Li F (2016b) J Mol Catal A Chem 414:122–129. https://doi.org/10.1016/j.molcata.2016.01.011
Liu F, Yu J, Tu G, Qu L, Xiao J, Liu Y, Wang L, Lei J, Zhang J (2017a) Appl Catal B Environ 201:1–11. https://doi.org/10.1016/j.apcatb.2016.08.001
Liu H, Chen D, Wang Z, Jing H, Zhang R (2017b) Appl Catal B Environ 203:300–313. https://doi.org/10.1016/j.apcatb.2016.10.014
Lotfiman S, Ghorbanpour M (2017) Surf Coat Technol 310:129–133. https://doi.org/10.1016/j.surfcoat.2016.12.032
Martha S, Reddy KH, Parida KM (2014) J Mater Chem A 2:3621–3631. https://doi.org/10.1039/C3TA14285J
McNaught AD, Wilkinson A (1997) IUPAC- compendium of chemical terminology, 2nd edn. Blackwell Scientific Publications, Oxford. https://doi.org/10.1351/goldbook.X06695
Medrano MGM, Kowalska E, Lehoux A, Herissan A, Ohtani B, Bahena D, Briois V, Justin CC, López JLR, Remita H (2016) J Phys Chem C 120(9):5143–5154. https://doi.org/10.1021/acs.jpcc.5b10703
Morkoç H, Özg€ur Ü (n.d.) Zinc oxide: fundamentals, materials and device technology. ISBN:978-3-527-40813-9
Moussawi RN, Patra D (2016) Sci Rep 6:24565. https://doi.org/10.1038/srep24565
Muthulingam S, Lee IH, Uthirakumar P (2015) J Colloid Interface Sci 455:101–109. https://doi.org/10.1016/j.jcis.2015.05.046
Pawar RC, Kang S, Ahn SH, Lee CS (2015) RSC Adv 5:24281–24292. https://doi.org/10.1039/C4RA15560B
Qi F, Li Y, Wang Y, Wang Y, Liu S, Zhao X (2016) RSC Adv 6:81378–81385. https://doi.org/10.1039/C6RA17613E.
Qin J, Zhang X, Yang C, Cao M, Ma M, Liu R (2017) Appl Surf Sci 392:196–203. https://doi.org/10.1016/j.apsusc.2016.09.043
Qiu P, Xu C, Chen H, Jiang F, Wang X, Lu R, Zhang X (2017) Appl Catal B Environ 206:319–327. https://doi.org/10.1016/j.apcatb.2017.01.058
Radhika S, Thomas J (2017) J Environ Chem Eng 5:4239–4250. https://doi.org/10.1016/j.jece.2017.08.013
Radzimska AK, Jesionowski T (2014) Materials 7:2833–2881. https://doi.org/10.3390/ma7042833
Rémy SP, Dufour F, Herissan A, Ruaux V, Maugé F, Hazime R, Foronato C, Guillard C, Chaneac C, Durupthy O, Justin CC, Cassaignon S (2017) Appl Catal B Environ 203:324–334. https://doi.org/10.1016/j.apcatb.2016.10.031
Rutar M, Rozman N, Pregelj M, Bittencourt C, Korošec RC, Škapin AS, Mrzel A, Škapin SD, Umek P (2015) Beilstein J Nanotechnol 6:831–844. https://doi.org/10.3762/bjnano.6.86.
Sagara N, Kamimura S, Tsubota T, Ohno T (2016) Appl Catal B Environ 192:193–198. https://doi.org/10.1016/j.apcatb.2016.03.055
Samsudin EM, Hamid SBA, Juan JC, Basirun WJ, Centi G (2016) Appl Surf Sci 370:380–393. https://doi.org/10.1016/j.apsusc.2016.02.172
Senthilraja, Krishnakumar B, Subash B, Sobral AJFN, Swaminathan M, Shanthi M (2016) J Ind Eng Chem 33:51–58. https://doi.org/10.1016/j.jiec.2015.09.010
Senthilrajaa A, Krishnakumara B, Swaminathana M, Nagarajanb S (2014) New J Chem 38:1573–1580. https://doi.org/10.1039/C3NJ01356A
Shahrokhi M (2016) Appl Surf Sci 390:377–384. https://doi.org/10.1016/j.apsusc.2016.08.055.
Sharma V, Kumar S, Krishnan V (2016) ChemistrySelect 1:2963–2970. https://doi.org/10.1002/slct.201600671
Shen SJ, Yang TS, Wong MS (2016a) Surf Coat Technol 303:184–190. https://doi.org/10.1016/j.surfcoat.2016.03.042
Shen S, Zhao D, Chen J, Guo L, Mao SS (2016b) Appl Catal A Gen 521:111–117. https://doi.org/10.1016/j.apcata.2015.11.004
Shi Y, Li H, Wang L, Shen W, Chen H (2012) ACS Appl Mater Interfaces 4:4800–4806. https://doi.org/10.1021/am3011516
Shi L, Wang F, Zhang J, Sun J (2016) Ceram Int 42:18116–18123. https://doi.org/10.1016/j.ceramint.2016.08.124
Sivakumar R, Jesty T, Yoon M (2012) J Photochem Photobiol C: Photochem Rev 13:277–298. https://doi.org/10.1016/j.jphotochemrev.2012.08.001
Sridharan K, Kuriakose T, Philip R, Park TJ (2014) Appl Surf Sci 308:139–147. https://doi.org/10.1016/j.apsusc.2014.04.121.
Sridharan K, Jang E, Park JH, Kim JH, Lee JH, Park TJ (2015) Chem Eur J 21:1–8. https://doi.org/10.1002/chem.201500163
Stengl V, Bakardjieva S, Murafa N (2009) Mater Chem Phys 114:217–226. https://doi.org/10.1016/j.matchemphys.2008.09.025
Su Y, Chen S, Quan X, Zhao H, Zhang Y (2008) Appl Surf Sci 255(5,1):2167–2172. https://doi.org/10.1016/j.apsusc.2008.07.053
Subash B, Krishnakumar R, Velmurugan, Swaminathan M, Shanthi M (2012) Catal Sci Technol 2:2319–2326. https://doi.org/10.1039/C2CY20254A
Subash B, Krishnakumar B, Sobral AJFN, Surya C, John NAA, Senthilraja A, Swaminathan M, Shanthi M (2016) Sep Purif Technol 164:170–181. https://doi.org/10.1016/j.seppur.2016.03.029
Sudha M, Senthilkumar S, Hariharan R, Suganthi A, Rajarajan M (2013) J Sol-Gel Sci Technol 65(3):301–310. https://doi.org/10.1007/s10971-012-2936-y
Sui Y, Su C, Yang X, Hu J, Lin X (2015) J Mol Catal A Chem 410:226–234. https://doi.org/10.1016/j.molcata.2015.09.018
Suligoj A, Stangar UL, Ristic A, Mazaj M, Verhovsek D, Tusara NN (2016) Appl Catal B Environ 184:119–131. https://doi.org/10.1016/j.apcatb.2015.11.007
Sun Y, Chen L, Bao Y, Zhang Y, Wang J, Fu M, Wu J, Ye D (2016) Catalysts 6:188. https://doi.org/10.3390/catal6120188
Suryawanshi A, Dhanasekaran P, Mhamane D, Kelkar S, Patil S, Gupta N, Ogal S (2012) Int J Hydrog Energy 37:9584–9589. https://doi.org/10.1016/j.ijhydene.2012.03.123
Taheri E, Petala A, Mantzavinos D, Kondarides DI (2017) Catal Today 280:99–107. https://doi.org/10.1016/j.cattod.2016.05.047.
Thomas J, Chitra KR (2014) Adv Mater Res 938:292–296. https://doi.org/10.4028/www.scientific.net/AMR.938.292
Thomas AF, Goettmann F, Antonietti M, Müller JO, Carlsson JM (2008) J Mater Chem 18:4893–4908. https://doi.org/10.1039/B800274F
Thomas J, Radhika S, Yoon M (2016) J Mol Catal A Chem 411:146–156. https://doi.org/10.1016/j.molcata.2015.10.021
Thomas J, Ambili KS, Radhika S (2018) Catal Today 310:11–18. https://doi.org/10.1016/j.cattod.2017.06.029
Thomasa J, Radhika S, Yoon M (2017) Mol Catal 433:274–281. https://doi.org/10.1016/j.mcat.2016.12.016
Tobajas M, Belver C, Rodriguez JJ (2017) Chem Eng J 309:596–606. https://doi.org/10.1016/j.cej.2016.10.002
Tongon W, Chawengkijwanich C, Chiarakorn S (2014) Superlatices Microstruct 69:108–121. https://doi.org/10.1016/j.spmi.2014.02.003
Tripathy N, Ahmad R, Kuk H, Lee DH, Hahn YB, Khang G, Photochem J (2016) Photobiol B: Bio 161:312–317. https://doi.org/10.1016/j.jphotobiol.2016.06.003
Upadhyay R, Tripathi M, Pandey A (2013) High Energy Chem 47(6):308–314. https://doi.org/10.1134/S0018143913060106
Vadivel S, Maruthamani D, Habibi-Yangjeh A, Paul B, Dhar SS, Selvam K (2016a) J Colloid Interface Sci 480:126–136. https://doi.org/10.1016/j.jcis.2016.07.012
Vadivel S, Kamalakannan VP, Kavitha NP, Priya TS, Balasubramanian N (2016b) Mater Sci Semicond Process 41:59–66. https://doi.org/10.1016/j.mssp.2015.07.023
Wang Y, Wang X, Antonietti M (2012) Angew Chem Int Ed 51:68–89. https://doi.org/10.1002/anie.201101182
Wang H, Yuan X, Wu Y, Zeng G, Chen X, Leng L, Li H (2015) Appl Catal B Environ 174–175:445–454. https://doi.org/10.1016/j.apcatb.2015.03.037
Wang S, Zhang X, Li S, Fang Y, Pan L, Zou JJ (2017) J Hazard Mater 331:235–245. https://doi.org/10.1016/j.jhazmat.2017.02.049
Wee LH, Meledina M, Turner S, Custers K, Kerkhofs S, Sree SP, Gobechiya E, Kirschhock CEA, Van Tendeloo G, Martens JA (2016) RSC Adv 6:46678–46685. https://doi.org/10.1039/C6RA06141A
Wen J, Li X, Liu W, Fang Y, Xie J, Xu Y (2015) Chin J Catal 36:2049–2070. https://doi.org/10.1016/S1872-2067(15)60999-8
Wu W, Jiang C, Roy VAL (2015) Nanoscale 7:38–58. https://doi.org/10.1039/C4NR04244A
Xu J, Li Y, Peng S, Lu G, Li S (2013) Phys Chem Chem Phys 15:7657–7665. https://doi.org/10.1039/C3CP44687E
Yao B, Peng C, Zhang W, Zhang Q, Niu J, Zhao J (2015) Appl Catal B Environ 174–175:77–84. https://doi.org/10.1016/j.apcatb.2015.02.030
You R, Dou H, Chen L, Zheng S, Zhang Y (2017) RSC Adv 7:15842. https://doi.org/10.1039/C7RA01036B
Yu MM, Wang C, Li J, Yuan L, Sun WJ (2015) Appl Surf Sci 342:47–54. https://doi.org/10.1016/j.apsusc.2015.03.020
Yu W, Zhang J, Peng T (2016) Appl Catal B Environ 181:220–227. https://doi.org/10.1016/j.apcatb.2015.07.031
Yu Q, Jiang L, Gao S, Zhang S, Ai T, Feng X, Wang W (2017) Ceram Int 43(2):2864–2866. https://doi.org/10.1016/j.ceramint.2016.10.190
Yuan YJ, Tu JR, Ye ZJ, Lu HW, Ji ZG, Hu B, Li YH, Cao DP, Yu ZT, Zou ZG (2015) Dyes Pigments 123:285–292. https://doi.org/10.1016/j.dyepig.2015.08.014
Zalfani M, van der Schueren B, Mahdouani M, Bourguig R, Yu WB, Wu M, Deparis O, Li Y, Su BL (2016) Appl Catal B Environ 199:187–198. https://doi.org/10.1016/j.apcatb.2016.06.016.
Zhang Z, Zhou Y, Zhang Y, Sheng X, Zhou S, Xiang S (2013) Appl Surf Sci 286:344–350. https://doi.org/10.1016/j.apsusc.2013.09.084
Zhang X, L Y, Zhuang C, Peng T, Li R, Li X (2014) ACS Catal 4:162–170. https://doi.org/10.1021/cs400863c
Zhang H, Li S, Lu R, Yu A (2015) ACS Appl Mater Interfaces 7:21868–21874. https://doi.org/10.1021/acsami.5b06309
Zhang Z, Liu K, Feng Z, Bao Y, Dong B (2016) Sci Rep 6:19221–19232. https://doi.org/10.1038/srep19221.
G. Zhang, S. Zhang, L. Wang, R. Liu, Y. Zeng, X. Xia, Y. Liu, S. Luo, Appl Surf Sci 391 (2017) 228–235, https://doi.org/10.1016/j.apsusc.2016.04.095.
Zhao K, Lu Y, Lu N, Zhao Y, Yuan X, Zhang H, Teng L, Li F (2013) Appl Surf Sci 285:616–624. https://doi.org/10.1016/j.apsusc.2013.08.101
Zhao F, Shang J, Liu J (n.d.) Beijing Daxue Xuebao Ziran Kexue Ban/Acta Scientiarum Naturalium Universitatis Pekinensis 46(2):293–297
Zheng Y, Liu J, Liang J, Jaroniec M, Qiao SZ (2012a) Energy Environ Sci 5:6717–6731. https://doi.org/10.1039/C2EE03479D
Zheng Y, Li X, Dutta PK (2012b) Sensors 12:5170–5194. https://doi.org/10.3390/s120405170
Zhong J, Li J, Feng F, Huang S, Zeng J (2013) Mater Lett 100:195–197. https://doi.org/10.1016/j.matlet.2013.03.030
Zhu Y, Zhu M, Kang L, Yu F, Dai B (2015) Ind Eng Chem Res 54:2040–2047. https://doi.org/10.1021/ie504372p
Zhu Y, Shah MW, Wang C (2017) Appl Catal B Environ 203:526–532. https://doi.org/10.1016/j.apcatb.2016.10.056
Zyoud AH, Hilal HS (2014) Phyto Chem Bio Sub J 8(3):127–137. https://doi.org/10.163.pcbsj/2014.8.3.127
Zyoud, Dwikat M, Al-Shakhshir S, Ateeq S, Shteiwi J, Zu’bi A, Helal MHS, Campet G, Park DH, Kwon H, Kim TW, Kharoof M, Shawahna R, Hilal HS (2016) J Photochem Photobiol A Chem 328:207–216. https://doi.org/10.1016/j.jphotochem.2016.05.020
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Thomas, J., Ambili, K.S. (2019). Solar Light Active Nano-photocatalysts. In: Inamuddin, Ahamed, M., Asiri, A., Lichtfouse, E. (eds) Nanophotocatalysis and Environmental Applications . Environmental Chemistry for a Sustainable World, vol 31. Springer, Cham. https://doi.org/10.1007/978-3-030-04949-2_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-04949-2_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-04948-5
Online ISBN: 978-3-030-04949-2
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)