Abstract
Parkinson's disease (PD) is caused by a lack of dopamine, causing an imbalance in the cognitive and motor regions of the brain. Therefore, the search for new inhibitors is important to increase the therapeutic possibilities. Thus, medicinal plants are more studied because they have many substances with varied biological activities. With Medicinal Chemistry, through in silico studies, it is possible to design molecules with defined activity, then confirm this activity in in vitro and in vivo tests. In this study, the animal model used was Danio rerio (zebrafish), due to the similarities of its central nervous system with that of humans. Thus, the study aimed to in silico drug design and evaluate the in vivo acute toxicity of molecules with the prediction of MAO-B activity for the development of drugs that are candidates for the treatment of PD. According to the results obtained in silico, it was possible to identify Amburoside A as a promising bioligand from which the analogs PMC1, PMC2, PMC3 were obtained, the last being tested in zebrafish for acute toxicity test with a negative result, indicating that it is a promising molecule to treat Parkinson's disease.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The diagnosis of neurodegenerative diseases is gradually increasing in the world population, due to the growing number of people diagnosed. The most diagnosed neurodegenerative disease is Alzheimer's Disease, and the second most common is Parkinson's Disease (PD), which affects people aged 55–60 years [1]. And in Brazil, there was an increase in the frequency of neurodegenerative diseases diagnosed in individuals over the age of 60, including Parkinson's disease. PD is a neurodegenerative disease caused by the death of dopaminergic neurons which are responsible for the synthesis of the neurotransmitter dopamine (AD), affecting the cognitive and motor regions of the brain [2]. The cause of neuron degradation has not yet been discovered, but some hypotheses have emerged to explain the pathophysiology of PD, one of which is the oxidative stress caused by monoamine oxidase B (MAO-B) by degrading dopamine, releasing free radicals, in addition to decreasing the amount of the neurotransmitter in the synaptic cleft [3, 4].
Given the relationship between MAO-B and the pathophysiology of PD, the focus of many studies is on inhibiting this enzyme to decrease its activity and increase AD in the synaptic cleft, in addition to reducing the production of free radicals. However, this class of medication is not widely used in treatment due to its side effects, and this causes patients to fail to adhere to treatment [5, 6]. Thus, there is a growing number of studies with medicinal plants, considering their variety of natural substances, in which they can assist in the development of drugs with better activities, in addition to always seeking to reduce side effects, and there is also greater adherence to the treatment by the Brazilian population when the medication is of natural origin. Among the medicinal plants found in the Brazilian flora, there is Passiflora incarnata and Amburama cearensis with a potent antioxidant and neuroprotective effect, from which natural substances can be used to plan antiparkinsonian drugs [7, 8].
Since the 18th century, the discovery of new drugs was made at random based on traditional knowledge, empirical practice of using natural products to treat diseases. Although they have not been analyzed correctly, it was possible to identify many molecules with important pharmacological activity. With the advancement of computer technology, there was, consequently, an increase in structural databases making the in silico study through molecular modeling, bioinformatics, and artificial intelligence, more accessible and interesting for research, due to its quick access and result with lower cost [9]. A widely used in silico approach is the virtual screening process, based on in vitro and/or in vivo tests, several molecules are subjected to computational tools to simulate various tests, such as pharmacokinetic and toxicological properties, activity prediction and synthetic viability, among others, this approach to drug discovery is called Medicinal Chemistry [10].
The choice of the animal model in the in vivo test depends on the objective of the study and the pathology to be studied. In the case of PD, since the 1980s, the fish Danio rario, popularly known as Zebrafish, started to use, as this fish has many important characteristics for carrying out various studies, such as rapid growth facilitating studies of reproductive toxicity, and lower financial cost for both purchasing and maintaining the environment [11]. In addition, Zebrafish fish have anatomical and functional characteristics similar to those of mammals, such as the brain where dopaminergic neurons, Purkinje cells of the cerebellum and motor neurons are found. Some studies have suggested that the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) is metabolized in Zebrafish embryos and larvae by a monoamine oxidase similar to mammalian MAO-B [12].
According to data obtained in the scientific literature, this study aimed to plan MAO-B inhibitor drug candidates from the prototype of natural origin, through Medicinal Chemistry, using in silico planning, synthesis of analogs and evaluation in vivo toxicity.
2 Results and Discussion
2.1 A Bibliographic Search of Natural Substances
In Fig. 1, the medication, used as a standard, and the natural substances described in the literature and used in this study are observed, respectively: Selegiline (1), Amburoside A (2), Harman (3), Harmaline (4), Harmalol (5), which were designed in the ChemSketch 12.0 software [3, 4, 7, 12,13,14].
Selegiline was the first MAO-B inhibitor to be discovered. The molecule was synthesized by Zoltán Ecseri at Chinoin Pharmaceuticals (Budapest, Hungary) in 1962, and its selective inhibitory effect of selegiline on one of the MAO isoforms was identified by Knoll and Magyar (1972). Since then, this drug has been used for the treatment of PD, as it is an irreversible inhibitor that in high doses has antidepressant activity, it also has a neuroprotective action against MPTP (1-methyl-4-phenyl-1,2,3, 6-tetrahydropyridine) and its sympathomimetic effects are related to methamphetamine metabolites. However, due to its adverse effects such as insomnia, dizziness, headache, bradykinesia, among others, patients abandon treatment [4, 6, 15].
Of the various plants reported in the literature, two plants belonging to the Brazilian flora Amburana cearensis, which is native to the Brazilian northeastern region and Passiflora incarnata native to the Amazon, were found to enhance the plants of the national flora, in which experimental studies with activity were reported neuroprotective effects in induced PD models [8, 13, 16].
A. cearensis (sin. Torresea cearensis) is a plant belonging to the Leguminosea family, popularly known by several designations, such as imburana of smell, cherry and coumarou [16]. In a review study of Brazilian plants with inflammatory activity, the natural substances isokaempferide (12.5, 25 and 50 mg/kg) and amburoside A (25 and 50 mg/kg), isolated from the shells of A. cearensis, with considerable anti-inflammatory activity, as they inhibited the migration of neutrophils and leukocytes and the production of TNF-a and prostaglandins E2 [17]. In another study, the phenolic glycosides found in A. cearensis, Amburosideo A and B had their biological activity evaluated in cell cultures exposed to the neurotoxin 6-hydroxydopamine (6-OHDA), a hydroxylated derivative of dopamine that is possibly formed endogenously in patients with PD, however, only amburoside A showed neuroprotection [7, 13].
P. incarnata popularly known as passion fruit, a word of indigenous origin (tupi), is a herbaceous, climbing plant from northern South America. The main constituents of P. incarnata leaves are flavonoids (0.25%), such as vitexin, isovitexin, orientin, isoorientin, apigenin and kampferol. Alkaloids based on the ß-carboline ring system, harman, harmin, harmaline and harmalol are said to be effective anti-Parkinson compounds [15]. Flavonoids with potential antioxidant activity such as antiparkinsonian and for improving memory in the treatment of Alzheimer's disease were also evaluated in which the results showed a significant decrease in free radicals [8]. Other studies with P. incarnata, with the aqueous extract of dry leaves, evaluated the effects of the extract in albino mice that were experimentally induced by neuroleptics to develop DP catalepsy, as this is an animal model used to screen for drugs and obtained positive results in terms of activity [18].
2.2 Derivation of the Pharmacophoric Pattern
Pharmacophore is the set of electronic and steric characteristics that identifies one or more functional groups or structural subunits, necessary for better molecular recognition by the receptor and, therefore, for the desired pharmacological effect, these are classified by a score [20]. Selegiline and the 4 substances of natural origin were submitted to the webserver PharmaGist obtaining the pharmacophore. Figure 2 shows the result of the pharmacophore with Selegiline as the pivot molecule, however, in the grouping there was only the natural product Harmalina with the score of 4.825 showing the spatial characteristics: a hydrogen bond acceptor group (yellow sphere), a hydrophobic group (sphere green) and an aromatic region (purple sphere). On the basis of these results similar essential regions were identified in the other natural substances.
Hagenow and collaborators [21] identified, for the MAO-B inhibitor, the pharmacophoric pattern with a hydrophobic region, two acceptor regions and an aromatic region. While Mathew et al. [22], find a model with a hydrogen acceptor, a hydrophobic and two aromatic regions. In the present study, the aromatic, hydrogen-accepting and hydrophobic regions were similar to previous studies.
2.3 Molecular Docking Study
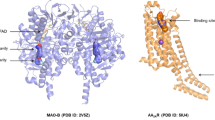
For the study of docking simulation, the standard drug Selegiline (1) and the natural substances Amburoside A (2), Harman (3), Harmaline (4), Harmalol (5) were considered as a ligand and, as an enzyme, MAO-B. For that, first, the selection was made from the Protein Data Bank (PDB) database of the crystallographic structure of MAO-B deposited in the form of a complex under the code 4A79, with the resolution of 1.89 Å. The software used was GOLD 5.4, which uses the genetic algorithm for the purpose of flexible ligand docking experiments within protein binding sites [23]. This was used to identify the MAO-B binding site so that the interaction between the ligands and the enzyme can be simulated.
The validation of the docking simulation software consists of the replication of the experimental result of the positions of the atoms of the ligand in relation to the active site of the enzyme, acquired from the crystallographic complex, and through the GOLD 5.4 software, several coupling tests were performed in an attempt to obtain the same positions of the atoms of the experimental result. The most used method to analyze if the software managed to reproduce the experimental result is the calculation of the Mean Quadratic Deviation (RMSD) in which previous studies showed that results smaller than 2 Å have a high success rate for replicating the experimental result [24]. Through the Discovery Studio Visualizer software [14] it was possible to visualize the RMSD value of 1.381 Å compared to the position of the ligand from the experimental result of the 4A79 complex, shown in Fig. 3.
Hydrophobic interactions occur between nonpolar regions such as aromatic rings and methyls. Conventional hydrogen bonds are characterized when a proton acceptor region interacts with a proton donor region due to the difference in electronegativity. This type of coupling occurs between electronegative atoms such as oxygen and nitrogen [25]. Hydrogen bonds with a distance below or equal to 3 Å are relevant to affirm that there is interaction, while hydrophobic bonds have a distance below or equal to 5 Å so that there is an interaction between amino acids and the natural binding product [26].
Figure 4 shows the possible interactions between the amino acid residues of the active site that were selected as described in the literature: TYR398, TYR435, PHE343, LEU171, TYR326, ILE316, PRO104, PRO104, ILE199, LEU171, CYS172, TRP119 [27] and the standard medicine, Selegiline, along with the studied natural substances. The analysis showed that the possible connections were mostly hydrophobic interactions and had two amino acids in common LEU171 and CYS172, and all the studied molecules had interaction with more than 3 amino acids among the 12 of the active site.
Table 1 shows the results of the docking simulation with the possible interactions for the standard molecule Selegiline (1), and the substances of natural origin Amburoside A (2), Harman (3), Harmaline (4) and Harmalol (5).
The docking simulation between Selegiline and the MAO-B amino acid residues showed 8 interactions in 6 different amino acid residues from the active site: CYS172, TYR326, PRO104, ILE316, ILE199 and LEU171. All interactions were hydrophobic, with the exception of the interaction with the amino acid residue CYS172 in which a van der Waals interaction was obtained between the sulfur atom of the amino acid CYS172 and the resonance of the aromatic ring of the molecule with a distance of 4.73 Å. With the amino acid residue TYR326, two hydrophobic interactions were observed, one between the resonance of the aromatic ring of the amino acid and the carbon of the methyl radical of the molecule, and the other between the aromatic rings of both the amino acid and the molecule, with distances 5.28 Å and 5.46 Å, respectively. With the amino acid residue PRO104, a hydrophobic interaction was obtained between an alkyl group of the amino acid and the hydrogen atom of the molecule with a distance of 3.99 Å. With the amino acid residue ILE316, two hydrophobic interactions were observed, between an alkyl group of the amino acid and the carbon of the methyl radical, and another interaction between the alkyl group of the amino acid residue and the tertiary carbon of Selegiline, with distances of 4.84 Å and 4.64 Å, respectively. With the amino acid residue ILE199, a hydrophobic interaction was obtained between an alkyl group of the amino acid and the carbon of the methyl radical of the molecule with a distance of 4.22 Å. With the amino acid residue LEU171, a hydrophobic bond was obtained between an alkyl group of the amino acid and the aromatic ring of the molecule with a distance of 4.24 Å, with a score of 64.54.
The simulation between Amburoside A and the MAO-B amino acid residues showed 7 bonds in 5 different amino acids: LEU171, CYS172, TYR398, TYR435 and ILE199. Most of the interactions are of the hydrophobic type (4), and there have also been three interactions of hydrogen between the hydrogen (H) and oxygen (O) atoms. With the amino acid residue LEU171, a hydrophobic interaction was obtained between the alkyl group of the amino acid residue and the aromatic ring of the natural substance with a distance of 3.97 Å. The amino acid residue CYS172 had two interactions, one of the hydrogen interaction type between the H of the amino acid and O of the natural substance with a distance of 2.65 Å, and the other of the hydrophobic type between an alkyl group of the amino acid residue and the ring. aromatic of the natural substance, with a distance of 5.06 Å. With the amino acid residue TYR398, a hydrogen interaction was obtained between the O atoms of the amino acid residue and the H atoms of Amburoside A with a distance of 2.11 Å. The amino acid residue TYR435 showed a hydrogen interaction between the hydroxyl O of the amino acid and the H of Amburoside A with a distance of 2.51 Å. The amino acid residue ILE199 showed two hydrophobic interactions, between alkyl groups of the amino acid residue and the aromatic ring of Amburoside A, with distances of 3.83 and 4.89 Å, with a score of 105.20.
In the simulation between Harman and the MAO-B amino acid residues, 7 interactions were observed in 4 different amino acids: TYR435, TYR398, LEU171 and CYS172. Most interactions are of the hydrophobic type and only one of the hydrogen interaction type. With the amino acid residue TYR435, two interactions were observed, one of the hydrogen interaction type between the O of the amino acid residue and the H of Harman, and another of the hydrophobic type between the aromatic ring of the amino acid residue and the alkyl group, with distances of 2.09 Å and 3.84 Å, respectively. With the amino acid residue TYR398, three hydrophobic interactions were observed, one between the aromatic rings of the amino acid residue and the Harman, another between the aromatic ring of the amino acid residue and the pyrrolidine ring of the Harman, and one between the aromatic ring of the amino acid residue and the alkyl group of the Harman with distances 4.51 Å, 5.36 Å, 4.06 Å, respectively. The amino acid residue LEU171 showed a hydrophobic interaction between the alkyl group of the amino acid residue and the aromatic ring of Harman with a distance of 4.52 Å. The amino acid CYS172 showed a hydrophobic interaction between the alkyl group of the amino acid residue and the aromatic ring of the Harman at a distance of 4.83 Å, with a score of 64.01.
In the simulation between Harmaline and the MAO-B amino acid residues, 8 interactions were observed in 5 different amino acids: ILE199, CYS172, TYR326, ILE316 and LEU171. Most of the bonds were of the hydrophobic type, with only one of the hydrogen interaction type. With the amino acid residue ILE199, a hydrogen interaction was obtained between the O atoms of the amino acid residue and H of Harmaline, and three hydrophobic interactions, one between the alkyl group of the amino acid residue, and the three aromatic rings of the natural product, with distances of 3.08 Å, 4.79 Å, 4.86 Å and 4.22 Å, respectively. With the amino acid residue CYS172, an interaction was obtained between the sulfur atom (S) of the amino acid and the aromatic ring of Harmaline with a distance of 4.76 Å. With the amino acid residue TYR326, a hydrophobic interaction was obtained between the aromatic rings of the amino acid residue and the alkyl group of Harmaline with a distance of 3.36 Å. The amino acid residue ILE316 presented a hydrophobic interaction between alkyl groups of the amino acid residue and the pyridine of Harmaline with a distance of 4.87 Å. The amino acid residue LEU171 presented two hydrophobic interactions, one between the alkyl group of the amino acid and the aromatic ring of Harmaline with a distance of 4.21 Å, with a score of 57.32.
In the simulation between Harmalol and the MAO-B amino acid residues, 7 interactions in 5 different amino acids were observed. All interactions are hydrophobic. With the amino acid CYS172, an interaction was obtained between the sulfur (S) of the amino acid residue and the aromatic ring of Harmalol with a distance of 4.88 Å. With the amino acid residue TYR326, two interactions were observed, one between the aromatic rings of the amino acid residue, with the alkyl group of Harmalol, with distances of 5.34 Å and 4.92 Å, respectively. With the amino acid residue LEU171, an interaction was obtained between the alkyl group of the amino acid residue and the aromatic ring of Harmalol with a distance of 4.19 Å. The amino acid residue ILE199 showed an interaction between the alkyl groups of the amino acid and the pyridine group of Harmalol with a distance of 4.20 Å. The amino acid residue ILE316 showed two interactions between the alkyl groups of the amino acid residue and the aromatic ring of Harmalol with distances of 5.28 and 4.72 Å, with a score of 57.50.
However, the standard molecule Selegiline an irreversible MAO-B inhibitor drug, this irreversibility characteristic is associated with the adverse effects of the inhibitors due to inactivating, totally or partially, the enzyme for a long period of time, in some cases causing the destruction of some functional groups of the active site, in which it is related to the type of bonds covalent, and in this case, it is assumed that the covalent bond is between the sulfur atom of the CYS172 residue and the molecule, in addition to being the only one that presented weak interaction with the amino acid residue PRO104 but important for conformation and stabilization of the interaction, with it is assumed that these two interactions may be the cause of the side effects of this drug. For the docking results, Selegiline showed 8 interactions in six different amino acids, being CYS172, TYR326, PRO104, ILE316, ILE199, LEU171, and the closest result to the standard molecule was the natural product Harmaline for presenting 8 interactions with the same amino acids the which Selegiline also interacted, with the exception of PRO104. Therefore, it can be deduced that Harmaline is more likely among the four substances of natural origin to present inhibitory activity of the MAO-B enzyme, in the same way as Selegiline, indicating irreversible inhibition. However, the natural substance Amburoside A showed 7 possible interactions in which 3 were with amino acids equal to which Selegiline interacted, and 2 different amino acids belonging to the active site, and its score was higher (105.20) in relation to the other molecules, indicating that probably the inhibitory action of Amburoside A is different from Selegiline and may be reversible, a characteristic that is desired in the planning of drugs with inhibitory action on MAO-B.
The study by Dhiman et al. [28]. presented the docking for the piperine, alkali of Piper nigrum in which they demonstrated possible links with TYR326, TYR398, PHE168, TRP119, PHE103, ILE199, CYS172, PHE343 and TYR188. Emphasizing that the natural substances in the present study also showed interactions with these amino acid residues, with Amburoside A being the highlight, out of the five amino acid residues that interacted, three are present in the study by Dhiman et al. [29].
Hagenow and collaborators [21] docked two compounds for MAO-A and MAO-B. With that, it was observed that the compounds had interaction with the amino acid residues LEU164, PHE168, PRO102, GLN206 and CYS172, the latter also interacting with the studied natural products.
2.4 Prediction of Pharmacokinetics (ADME) and Toxicological Properties
2.4.1 Prediction of Pharmacokinetic Properties
The in silico models of ADME properties, in comparison with traditional experimental tests, have greater applicability to meet the huge demand generated in the large-scale screening of new molecules. In addition, in vitro and in vivo tests have disadvantages that limit their use on a large scale: they are complex and expensive in terms of materials, infrastructure and qualified personal. Therefore, the in silico study is used as a complementary tool in research, being more used in the screening stage [29, 30].
For these reasons, there is great interest in the industry in the generation of ADME models in silico that can quickly assist in the selection of promising molecules and guide the elimination of compounds with an inappropriate pharmacokinetic profile. On the other hand, the integration of ADME models (in silico, in vitro and in vivo) seems to be an essential path to be followed in all stages of the drug discovery process. For the pharmacokinetic properties, the QikProp program was used, in which it predicts important parameters to be evaluated, mainly, in the absorption and distribution of a certain molecule in comparison with 95% of other molecules already known in the database. In addition, the program performs screening of molecules with the potential to be orally administered drugs through the Lipinski rule [31]. In this study, the parameters included for evaluation were: Human Oral Absorption (HOA), Caco-2 cells, Madin-Darby canine kidney cells (MDCK), blood–brain barrier (BBB), LogP, hydrogen donor, hydrogen acceptor. The results of the ADME prediction of this study are shown in Table 2.
To estimate Human Oral Absorption (HOA), the QikProp software predicts through the number of violations of Lipinski's Rule of Five (RO5) and the percentage of HOA. The analysis of RO5 is made by the number of violations of this rule, being allowed only one violation for the following parameters to be considered a molecule with good bioavailability by oral route: less than 5 hydrogen donors, less than 10 hydrogen acceptors, molecular weight less than 500 daltons and LogP less than 5 [32]. All the molecules under study, including the pivot, showed zero violations in relation to RO5, with the exception of Amburoside A, which presented only one violation, 6 hydrogen bonding donor groups, however, it continues to have a satisfactory result for oral absorption. While for the percentage result, where >80% high absorption, 25–80% medium and <25% low absorption, natural substances showed between medium and high absorption. Therefore, both Selegiline (standard molecule) and substances of natural origin present satisfactory results of physicochemical properties to be considered good drugs for oral administration.
Caco-2 cells are useful for measuring parameters of cell permeability in vitro, an important test to assess the intestinal absorption of drugs. In addition, in vitro cell permeability is often also determined with Madin-Darby canine kidney cells (MDCK), which have a shorter growth time than Caco-2 cells. Values above 500 nm/s for both properties are considered ideal. For the permeability parameter in Caco-2 cells, the studied molecules showed results above 500 nm/s, with the exception of Amburoside A and Harmalol that showed values below 500 nm/s, while the molecules that showed results above 500 nm/s were Harman (3) and Harmaline (4), indicating that most will probably be well absorbed in the body. For MDCK cells, most of the studied molecules also showed satisfactory results with greater evidence for Harman (3), Harmaline (4) and Harmalol (5) (Table 2).
The blood–brain barrier (BBB) is a complex structure consisting of cellular elements and an extracellular matrix. It plays a fundamental role in determining the types of molecules that can selectively act in the CNS, therefore, this is one of the most important parameters for this study, considering that the studied molecules and the proposed analogs must have an action in the CNS, that is, present good permeability in the blood–brain barrier. The range indicating the possibility of crossing this barrier is −3.0 and 1.2. And for this parameter, the studied molecules showed good permeability, emphasizing that the natural substances Harman (3) and Harmalol (5) presented better results of LogBB; (Table 2).
Dhiman et al. [28] performed the ADMET in the QiqProp software of compounds derived from the alkaline piperine prediction of natural substances derived from piperine that have potential inhibitory activity for MAO. In this study, natural substances showed between medium and good results for the analyzed parameters, and none showed more than one violation of Lipinski's RO5. Jiang et al. [33] produced a pharmacokinetic study of Harman and Harmaline in vitro and in vivo using the rat animal model, with intravenous administration, natural substances were detected in the striatum 300 min after administration, indicated that they have good blood–brain barrier permeability, confirming the in silico result obtained in the present study.
According to the two studies mentioned above, natural substances with MAO-B inhibitory activity have satisfactory results to be considered good in the absorption and distribution properties, for further research. Although the natural substance Amburoside A has presented results that are not so satisfactory, it can be modified based on the pharmacophore and molecular docking, highlighting the result obtained for this last parameter, to improve its pharmacokinetic characteristics, as in vitro results were quite satisfactory in the study by Ribeiro et al. [18] and Almeida [7], indicating that it has potential activity.
2.4.2 Prediction of Toxicological Properties
In the planning and development of new drugs, the study of toxicological properties is very important, since the relationship between damage and effectiveness is the factor in choosing a drug for a given treatment [34].
The study of the mutagenic and carcinogenic properties of molecules is carried out in animals to obtain results that they would probably have in humans. However, the cost of researching toxicological properties is quite high when the study involves several molecules, with which new tools and methodologies for the prediction of these properties appear [35].
As an example, the DEREK software, which contains a subset of about 50 rules that describe toxicophoric substructures responsible, for example, for skin sensitization [36] and makes this prediction considering the presence of alkyl halides, aldehydes, α, β compounds unsaturated, aromatic amines, phenols, hydroquinones, isothiazolinones, alkyl sulfonates and aromatic nitro groups in the test molecules [37]. Improvements are constantly being made to alerts and predictions are included, such as alerts for 1,2-diketones and isothiazolinones improving the definition of the toxicophoric group [38].
In the prediction of toxicological properties, only Amburoside A (2) presented a toxic action of altering the chromosome responsible for human reasoning, indicating the catechol group as the toxicophoric group, this group did not present interaction in the molecular docking and is also not part of the pharmacophore, therefore, it can be modified to not present more toxicity. And no natural substance showed warnings about Genotoxicity, Mutagenicity and Carcinogenicity, irritation, reproductive effects and neurotoxicity (Table 3).
Mathew et al. [22] carried out in vitro toxicity studies with chalcones that had potential MAO inhibitory activity. The results showed that the majority of chalcones did not show toxicity to liver cells at 1 μM. Upon increasing the concentration to 5 μM, some compounds exhibited moderate toxicity.
Is et al. [39] presented a study with MAO inhibitors in which the toxicological properties were evaluated in silico, in which the two molecules were subjected to 26 different QSAR models of toxicity, such as mutagenicity, carcinogenicity, cytotoxicity, anemia, genotoxicity, hepatotoxicity, liver necrosis and neurotoxicity, presented low probability and/or negative results for the QSAR models, indicating that they are probably not toxic.
With the results of in silico toxicity of natural substances, it can be deduced that the probability of these substances being toxic is minimal, mainly because they have in silico studies of MAO-B inhibitors in which most of the molecules showed no or moderate toxicity, confirming the study conducted in that research.
2.5 Structural Modifications, Activity Prediction and Synthetic Viability
2.5.1 Structural Modifications
First, the bioligand Amburoside A was selected because it presented experimental results for in vitro MAO-B inhibitory activity described in the literature and in the present in silico study of molecular docking it presented quantities of interactions similar to Selegiline (standard molecule) in some different amino acids, indicating that it probably has an inhibitory action differently from Selegiline, and may be reversible inhibition since it did not present covalent binding. With that, the modifications had the objective to improve the pharmacokinetic and toxicological properties and to specify the biological activity. In general, based on the probable pharmacophore, glucose was removed and the radicals of the aromatic ring of the catechol group were modified, in which it was identified as a probable toxicological region, the ester group was removed to increase liposolubility to improve the passage in the blood–brain barrier and thus, facilitating the synthetic process (Fig. 5).
The molecules obtained are from the chalcone family, in which they have an open chain containing a phenyl ring attached to the carbonyl group and the other benzene ring linked by a three-carbon enone fragment, that is, they are α, β- ketones unsaturated, in which one aromatic ring is directly linked to carbonyl and the other to carbon β of olefinic function, this family is widely used due to its various biological activities such as cancer, inflammation and diabetes, with this, many drugs based on chalcones have been approved for clinical use [40].
In the study by Tran et al. [41], chalcone analogs were synthesized for antibacterial activity of methicillin-resistant Staphylococcus aureus. One of these synthesized chalcones has the same structure as PMC1 and has low antibacterial activity. Another study carried out with the synthesis of chalcones as potential antidiabetic agents, also used the structure of PMC1, but it did not obtain results for antidiabetic activity [42].
Salama et al. [43] carried out a study on the synthesis of new tetrazole derivatives, in which one of these synthesized molecules has the same structure as PMC2. However, no biological activity tests were performed, the study aimed to show the possibility of synthesizing through the reaction of dienones with the reagent tetrachlorosilane-sodium azide and its structural contribution of NMR.
In the study by Bai et al. [44], molecules were synthesized to assess antitumor activity. Among these molecules, one has a chemical structure similar to PMC3, however, it did not show antitumor activity in vitro.
In general, there are few in vivo studies of neuroprotective and antioxidant biological activity, mainly in PD, with chalcones. In a study by Chen et al. [45], they obtained a positive result for the cytoprotective activity of a chalcone, in which the cells were subjected to a toxin that decreases the Parkin, Parkin1 and DJ-1 proteins that are associated with PD, and after the treatment with the synthesized chalcone was observed a significant increase of these proteins. Therefore, using the chalcone family to treat neurodegenerative diseases is very promising.
2.5.2 Activity Prediction
The activity prediction performed by the PASS web server is based on the structural formula of the substance, with great precision for more than 3500 pharmacotherapeutic effects, being the parameter of the probability of being active (Pa) and the probability of being inactive (Pi) and it is only considered a molecule probably active when Pa > Pi. If Pa > 0.7, the substance is very likely to exhibit activity in the experiments, but the possibility that the substance is the analog of a known pharmaceutical agent is also high. If 0.5 < Pa < 0.7, the substance is likely to exhibit activity in the experiments, but the probability is less, and it is different from the substance in which the pharmaceutical action is known. If Pa < 0.5, they are unlikely to exhibit activity in the substance test. However, if the presence of this activity is confirmed in the experiment, the substance may be a new chemical entity [46, 47].
However, for the prediction of MAO-B inhibitory activity, analog 1 (PMC1) presented the value of Pa 0.238, analog 2 (PMC2) presented the value of Pa 0.198 and analog 3 (PMC3) presented the value of Pa 0.282. These results demonstrate great progress for the development of these molecules planned as potential MAO inhibitors, specifically MAO-B, as they were compared with a variety of molecules that already have activity, mostly, proven to inhibit MAO-B, indicating that the planning is following the objective of the study (Table 4).
The SEA web server performs the prediction of biological activity indicating the biological target based on chemical similarity, and can assume that a particular molecule may have an affinity with a certain target. As in the study by McCarrol and collaborators, who used SEA for a virtual screening in order to identify whether some molecules would have action on targets other than the Zebrafish GABA receptor and compare with the in vivo assay [48].
SEA mainly uses two values to perform the activity prediction by chemical similarity. The E-value is the value of the expectation that the similarity is not random between the sets, in which the values closer to zero or less than 1 × 10−10 consider that the activity prediction is not likely to be occurring by chance. While the Tanimoto Coefficient (Tc) is the expected chemical similarity of value 1 [49].
For the PMC1 molecule, DP-related activities were obtained, represented by the values of E-value 4.426e−35 and Tc of 0.86 for human enzyme MAO-B, and the values of E-value 2.509e−11 and Tc of 0.69 for human MAO-A enzyme, indicating that PMC1 has a greater affinity for the MAO-B enzyme and greater probability of not being a random alignment. For PMC2, results were obtained for E-value 2.342e−24 and Tc 0.58 for human MAO-B enzyme, E-value 9.906e−08 and Tc 0.29 for MAO-B mouse enzyme, and E- value 1.408e−07 and Tc 0.57 for human MAO-A enzyme, indicating that PMC2 has similar characteristics to interact with both the human enzyme MAO-A and MAO-B. For PMC3, E-value 7.283e−14 and Tc 1 were obtained for human MAO-A enzyme, E-value 2.505e−28 and Tc 0.73 for human MAO-B enzyme, and E-value 2.683e−08 and Tc 0.35 for COMT rat enzyme, indicating that PMC3 has a non-significant tendency towards MAO-A instead of MAO-B and still has a probable interaction with COMT thus deducing that its action may be multi-target (Table 5).
2.5.3 Synthetic Viability
To predict the synthetic viability of the compounds, the SYLVIA software was used, which verifies through calculations the levels of difficulty of synthesis scoring from 1 to 3 for compounds that are easy to synthesize with the background of the image colored green, 3–6 for medium-sized ones with a yellow background and 6–10 for compounds that are very difficult to synthesize with red background [50]. Analog 1 (PMC1) showed a value of 2.52 indicating it is easy to synthesize, analog 2 (PMC2) showed a value of 3.74, that is, medium ease in synthesis, and analog 3 (PMC3) was 2.77, easily synthesized. The proposals are promising because they present biological activity for MAO-B inhibitors and mainly because they are possible to synthesize.
2.6 Synthesis of Planned Molecules by Claisen-Schmidt Reaction
The Claisen-Schmidt reaction is a classic reaction (Fig. 6), described by R. L. Claisen and J. G. Schmidt, widely used in the synthesis of chalcones through the aldolic condensation of an acetophenone with an aromatic aldehyde [51]. This reaction can occur in both acidic and basic environments, generating an enol or enolate from the ketone, respectively, followed by the aldol addition and dehydration, resulting in the aldol adduct. Despite being simple, the Claisen-Schmidt reaction has some disadvantages, such as slow reaction, production of artifacts and most have low yield, ranging from <10% to 100> conversion. To increase the likelihood of better yield, aluminum chloride (AlCl3) is also used as a Lewis acid for the synthesis of chalcone [40].
Since it is a basic reaction for the synthesis of chalcones, with easily accessible reagents and relatively simple protocol, it was decided to synthesize the analogs proposed in this work to have their toxicities tested in Zebrafish. Molecules 6, 7 and 8, where synthesized with 15%, 46% and 5% yield, respectively. All molecules were synthesized more than once to obtain the minimum amount of 1 g, to be used in the biological experiments.
The structures of the synthesized products were confirmed using the techniques of nucler magnetic resonance (NMR), infrared spectroscopy (IR), gas chromatography-mass spectrometry (GC-MS) and melting point (m.p.). The 1H and 13C NMR spectra of molecules 6 and 8 showed the characteristic signals expected for these compounds, with emphasis on the 1H–1H coupling constant of 15,6 Hz, characteristic of double bonds in the trans geometry with values close of to the expected value of 16 Hz. For molecule 7 it was not possible to obtain the coupling constant for double bonds because of the formation of multiplets, but the chemical shifts of 1H and 13C confirmed the structure of the product. The analysis by mass spectrometry showed the m/z for molecules 6, 7 and 8 equals to 224, 248 and 238 Da, consistent with the calculated values for their molecular ions.
2.7 Pharmacological Activity in a Zebrafish Model
2.7.1 Subjects and Creation Procedure
The animals were kept on the Zebrafish Platform of the Drug Research Laboratory, Biological Sciences and Health Department of the Federal University of Amapá (UNIFAP), Brazil. At a temperature of 26 ± 2 °C with a light cycle of 10 h light/14 h dark. Standardized water (ISSO 1996) was used for the maintenance of adult fish. For the production of standardized water, deionized water was used and the following salts were added: CaCl2 × 2H2O (117 mg/L), MgSO4 × 7 H2O (49.3 mg/L), NaHCO3 (25.9 mg/L) and potassium chloride (2.3 mg/L) (Sigma Aldrich).
The adult fish were fed with commercial feed and Artemia saline. These were treated according to the guide for the care and use of experimental animals. The behavior of the fish was assessed by a human observer and filmed, after a week under normal conditions and treatment. This study was submitted and approved by the Committee on Ethics in Animal Use—CEAU of UNIFAP, receiving protocol number 013/2018.
2.7.2 Toxicity Test of the Studied Substance
The acute in vivo toxicity test in an animal model Danio rerio, known as Zebrafish, has a lower cost and time, and can be performed at the beginning of preclinical development, and this justifies the acceptance of this methodology by the drug regulatory bodies [53].
Of the three synthesized molecules, PMC1, PMC2 and PMC3, PMC3 was chosen for the toxicity test because it does not have scientific studies of activity in the central nervous system, and in the prediction test of biological activity in silico it showed possible inhibition in two enzymes that are related to the pathophysiology of PD. For acute oral toxicity, the administration of a single dose of PMC3 is considered in this study and observation after 24 h to identify whether there was death in the groups, administering concentrations of 350, 750, 1500 and 2500 mg/kg, according to the recommendations of the OECD protocol 236 [54] after the highest level of the administered dose (2000 mg/kg) it is suggested that there is no mortality in the tested population. Based on this methodology, the acute toxicity test was also performed by the intraperitoneal route, to confirm the possible absence of toxicity of the PMC3 analog by both routes, in which for both routes there was no death or behavior change of the 30 fish tested in total. The results are shown in Table 6.
Due to the chalcones not having good solubility in water, it was necessary to add DMSO and Tween 80 to the distilled water to obtain a mother concentration of 200 mg/mL, so it was possible to dilute according to the bodyweight of each fish. Soon after administration, it was noted that there was an increase in the swimming activity of the fish in both routes, with the same behavior for the control group. The fish were evaluated for 7 days and showed no significant differences in behavior and had a mortality rate of 0%.
3 Conclusion
This study was divided into three parts: molecular modeling, synthetic route and acute toxicity in vivo, to obtain a drug candidate with MAO-B inhibitory activity for the treatment of Parkinson's Disease. Thus, the natural substances (Amburoside A, Harman, Harmaline and Harmalol) selected for the in silico study of the pharmacophore, molecular docking, pharmacokinetic and toxicological properties demonstrated satisfactory results indicating a greater probability of good absorption and distribution, in addition to MAO inhibitory activity. −B due to interaction with the active enzyme site provided by docking.
Based on these results, Amburosídeo A was indicated as a bioligant to propose the modifications, and thus 3 molecules of the chalcone family named PMC1, PMC2 and PMC3 were obtained, although they already exist in the virtual database, these molecules do not have studies for the treatment of disease of the central nervous system, and the majority being for antimicrobial activity in which they did not show good results. Therefore, predictions of activity and synthetic viability were also performed, in which the three molecules showed activity to inhibit MAO. The PMC3 prediction for COMT inhibitory activity is also noteworthy, indicating that this molecule probably has a multi-target action, in addition to presenting easy synthesis. In the synthesis stage, it was possible to synthesize the three molecules with an average yield of 40%, confirming the prediction of synthetic viability. Subsequently, PMC3 was selected to perform the acute toxicity test on Zebrafish, due to its likely multi-target action, and even with the highest dose (2000 mg/kg) administered, the mortality rate in the study population was 0%, indicating the lack of toxicity in this parameter. Thus, this study demonstrates the importance of Molecular Modeling in decreasing the time and cost of research, in addition to proposing three new potential drug candidates for the treatment of Parkinson's Disease.
4 Experimental Session
4.1 Search for the Structures of Natural Substances
To propose new drug candidates with antiparkinsonian biological activity with the inhibition of the monoamine oxidase B (MAO-B) enzyme for the treatment of PD, first, an online database search was performed with the descriptors “medicinal plants”, “Antiparkinsonian activity” and “natural compounds”, to find natural substances that are described in the literature with antiparkinsonian, antioxidant or neuroprotective activity in vitro or in vivo experiments on animal models with induced PD.
4.2 Derivation of the Pharmacophoric Pattern
The pharmacophore represents the set of functional domains of the studied bioligands through which the possible types of interaction that the ligands in common can make with the receptor site are defined [55]. The pharmacophoric pattern was determined from the online server PharmaGist (http://bioinfo3d.cs.tau.ac.il/PharmaGist/) [56] and observed its image on the ZINCPharmer web server (http://zincpharmer.csb.pitt.edu/pharmer.html).
4.3 Molecular Docking Study
Molecular docking is a computational method to identify the mode of interaction of ligands at the enzyme or receptor binding site through specific key interactions and to predict the binding affinity between protein-ligand complexes [57]. The GOLD 5.4 software (Genetic Optimization for Ligand Docking) was used to simulate the interaction between the ligands and the MAO-B enzyme through calculations that employs the genetic algorithm for flexible ligand docking experiments within protein binding sites [17].
4.4 Prediction of Pharmacokinetics (ADME) and Toxicological Properties
4.4.1 Prediction of Pharmacokinetic Properties (ADME)
For the pharmacokinetic properties, the parameters of human oral absorption (AOH), cell permeability in Caco-2 and MDCK cells and the penetration of the blood–brain barrier (pBHE) were used, as well as the properties related to the rule of five (logP, molecular mass, hydrogen donors, and hydrogen acceptors) for natural substances, using the QikProp module of the Schrödinger software [58].
4.4.2 Prediction of Toxicological Properties
The natural substance toxicological properties were predicted using the DEREK software (Deductive Estimate of Risk from Existing Knowedge) [34]. DEREK has a system that makes predictions of toxicity, such as mutagenicity, carcinogenicity, skin sensitization, irritation, reproductive effects, neurotoxicity, among others, through the correlation rules implemented in the software [12, 28]
4.5 Structural Modifications, Activity Prediction and Synthetic Viability
The changes in the structure of the studied molecules were carried out based on the study of docking, on the properties of significant pharmacokinetics and toxicology. In addition, it was also possible to predict the activity of the proposed new compounds using the PASS webserver (http://www.akosgmbh.de/pass/index.html), which predicts with high accuracy (70–80%) up to 2000 biological activities for chemical compounds. And SEA (http://sea.bkslab.org/) uses the protein-related set of molecules similarity based on the chemical similarity established between its ligands, by searching large compounds databases and creating cross-destination similarity maps.
The prediction of synthetic viability was performed using the SYLVIA software (http://www.molecular-networks.com/online_demos/sylvia/), in which it indicates whether the molecule has characteristics of easy (green), medium (yellow), or difficult (red) synthesis.
4.6 Synthesis of Planned Molecules by Claisen-Schmidt Reaction
The search for organic compounds that show activity as MAO-B inhibitors for the treatment of PD was based on chalcone nuclei. The chalcones were synthesized by a Claisen-Schmidt reaction that describes a process in which a benzaldehyde and a methyl ketone are condensed in the presence of catalysts, this reaction is considered one of the most classic reactions in organic chemistry, with the catalysts being strong or acid bases [52].
Typical procedure for PMC1 (6): In a 250 mL flask, 30 mL of ethanol, 4-hydroxybenzaldehyde (5.1 mmol) and acetophenone (5.0 mmol) were stirring for 10 min, then added 6 M NaOH (5 mL) dropwise and remaining in magnetic stirring for 24 h at room temperature. Small pieces of aluminum foil were added to reaction in an attempt to increase the yield. After this period, the mixture was transferred to a Beaker (500 mL) containing approximately 100 g of crushed ice and 10% HCl (15 mL) in manual stirring with a glass stick until the formation of the precipitate. Vacuum filtration was performed to obtain the precipitated solid 6.
Typical procedure for PMC2 (7): In a 250 mL flask, 30 mL of ethanol, cinnemaldehyde (5.1 mmol) and 4-methylacetophenone (5.0 mmol) were stirring for 10 min, then added 6 M NaOH (5 mL) dropwise and remaining in magnetic stirring for 24 h at room temperature. Small pieces of aluminum foil were added to reaction in an attempt to increase the yield. After this period, the mixture was transferred to a Beaker (500 mL) containing approximately 100 g of crushed ice and 10% HCl (15 mL) in manual stirring with a glass stick until the formation of the precipitate. Vacuum filtration was performed to obtain the precipitated solid 7.
Typical procedure for PMC3 (8): In a 250 mL flask, 30 mL of ethanol, 4-hydroxybenzaldehyde (5.1 mmol) and 4-methylacetophenone (5.0 mmol) were stirring for 10 min, then added 6 M NaOH (5 mL) dropwise and remaining in magnetic stirring for 24 h at room temperature. Small pieces of aluminum foil were added to reaction in an attempt to increase the yield. After this period, the mixture was transferred to a Beaker (500 mL) containing approximately 100 g of crushed ice and 10% HCl (15 mL) in manual stirring with a glass stick until the formation of the precipitate. Vacuum filtration was performed to obtain the precipitated solid 8.
All substances were characterized by 1H and 13C NMR, infrared spectroscopy, gas chromatography–mass spectrometry and melting point (supplementary material).
4.7 Acute Toxicity in a Zebrafish Model
4.7.1 Subjects and Creation Procedure
The adult Zebrafish (approximately 0.3 g in weight) were created on the ZebraFish Platform of the Pharmaceutical Research Laboratory, from the Federal University of Amapá, following standard fish care and maintenance protocols [53]. The environmental variance was maintained, at a minimum, for all behavioral experiments. The adult Zebrafish were kept in deionized water containing 200 mg/L of salt. All animal care procedures were submitted to the Animal Use Ethics Committee of the Federal University of Amapá.
4.7.2 Acute Toxicity Test
This test consists of administering the studied molecule to the fish in a certain dose and, if the fish survives at least 7 days, it indicates a high probability of the substance not being toxic 60. To perform this test, two routes of administration were used, both orally, due to the planned and synthesized substance being indicated as a drug candidate by the oral route, and the intraperitoneal route because it is the route of administration of the neurotoxin used in other studies. For the test, 12 adult fish were used, divided into four groups, containing three fish in each group, and the substance PMC3 fasted once in four different concentrations, 350, 750, 1500 and 2500 mg/kg, with 0.250 g of PMC3 diluted in 900 μg/mL of water, 90 μg/mL of DMSO and 10 μg/mL of Tween 80.
5 Ethical considerations
The Ethics Committee on the Use of Animals of the Federal University of Amapá—CEUA-UNIFAP is a deliberative and advisory body of the Higher Administration of the University in normative and consultative matters, in questions about the use of animals for teaching and research. This work was submitted and approved by protocol 013/2018 by this Animal Use Ethics Committee (CEUA).
References
Savica, R., Grossardt, B.R., Rocca, W.A., Bower, J.H.: Mov Disord. 33(4), 537–543 (2018)
Wang, D., Hong, R.-Y., Guo, M., Liu, Y., Chen, N., Li, X., Kong D.-X.: Molecules, 4003, 24 (2019)
Campos, H.C., Rocha, M.D., Viegas, F.P.D., Nicastro, P.C., Fossaluzza, P.C., Fraga, C.A.M., Barreiro, E.J., Viegas, C., Jr.: CNS Neurol. Disord. Drug Targets, 10(2), 239–250 (2011)
Follmer, C., Netto, H.J.C.B.: Quim. Nova 36(2), 306–313 (2013)
Finberg, J.P.M., Rabey, J.M.: Front. Pharmacol. 340, 7 (2016)
Marino, B.L.B., Souza, L.R., Sousa, K.P.A., Ferreira, J.V., Padilha, E.C., Silva, C.H.T.P., Taft, C.A., Hage-Melim, L.I.S.: Mini. Rev. Med. Chem. 20(9), 754–767 (2020)
Almeida, J.R.G.S.: Sci. Plena, 6, 11 (2010)
Ingale, S.P., S.B.: Life, 36(4), 200–206 (2017). https://www.ncbi.nlm.nih.gov/pubmed/?term=Kasture%20SB%5BAuthor%5D&cauthor=true&cauthor_uid=29269972"Kasture. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5726187/. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5726187/"Sci.
Pinzi, L., Rastelli, G.: Int. J. Mol. Sci. 20, 4331 (2019)
Maia, E.H.B., Assis, L.C., Oliveira, T.A., Silva, A.M., Taranto, A.G.: Front. Chem. 343, 8 (2020)
Espino-Saldaña, A.E., Ortiz, R.R., Jaramillo, E.P., Torres, A.M.: Curr. Neuropharmacol. 18, 136–152 (2020)
Cariello, N.F., Wilson, J.D., Britt, B.H., Wedd, D.J., Burlinson, B., Gombar, V.: Mutagenesis 17, 321–329 (2002)
Leal, L.K., Júnior, H.V.N., Cunha, G.M.A., Moraes, M.O., Pessoa, C., Oliveira, R.A., Silveira, E.R., Canuto, K.M., Viana, G.S.B.: Neurosci. Lett. 2, 86–90 (2005)
Accelrys discovery studio. v. 4.0, T.C., San Diego, CA 92121 USA (2007)
Ingale, S.P., Sanjay, B.K.: Orient. Pharm. Exp. Med. (2014)
Canuto, K.M., Silveira, E.R.: Quim. Nova, 29(6), 1241–1243 (2006)
Tábi, T., Vécsei, L., Youdim, M.B., Riederer, P., Szökő, É.: J. Neural Transm. 127, 831–842 (2019)
Ribeiro, V.P., Arruda, C., El-Salam, M.A., Bastos, J.K.: Pharm. Biol. 56(1), 253–268 (2018)
Dhawan, K., Kumar, S.: A, Sharma. J. Ethnopharmacol. 78, 165–170 (2001)
Vyas, V., Jain, A., Jain, A., Gupta, A.: Virtual screening: a fast tool for drug design. Sci. Pharm. 333–360 (2008)
Hagenow, J., Hagenow, S., Grau, K., Khanfar, M., Hefke, L., Proschak, E., Stark, H.: Drug Des. Dev. Therapy, 14, 371–393 (2020)
Mathew, B., Adeniyi, A.A., Dev, S., Yurtsever, M., Joy, G., Ucar, G.E., Mathew, Singh-Pillay, A., Soliman, M.E.: J. Phys. Chem. B. 121(6), 1186–1203 (2017). https://www.ncbi.nlm.nih.gov/pubmed/?term=Matew+2017+pharmacophore+monoamine+oxidase
Verdonk, M.L., Cole, J.C., Hartshom, M.J., Murray, C.W., Taylor, R.D.: Proteins 52(4), 609–623 (2003)
Cole, J.C., Murray, C.W., Nissink, J.W.M., Taylor, R.D., Taylor, R.: Proteins 60, 325–332 (2005)
Picanço, L.C.S., Castro, L.L., Pinheiro, A.A., Silva, K.R., Souza, L.R., Braga, F.S., Silva, C.H.T.P., Santos, C.B.R., Hage-Melim, L.I.S.: B. J. Pharm. Res. 7, 152–175 (2015)
Rodrigues, J.A.R.: Química Nova, 6, 23 (2000)
Binda, C., Aldeco, M., Geldenhuys, W.J., Tortorici, M., Mattevi, A., Edmondson, D.E.: Med. Chem. Lett. 3, 39–42 (2012)
Dhiman, P., Malik, N., Khatkar, A.: BMC Chem. 14,12 (2020)
Yamashita, S., Furubayashi, T., Kataoka, M., Sakane, T., Sezaki, H., Tokuda, H.: Eur. J. Pharm. 10, 195 (2000)
Silva, N.S.R., Santos, C.F., Gonçalves, L.K.S., Braga, F.S., Almeida, J.R., Lima, C.S., Brasil, D.S.B., Silva, C.H.T.P., Hage-Melim, L.I.S., Santos, C.B.R.: B. J. Pharm. Res. 7, 247–263 (2015)
Fu, C., Shi, H., Chen, H., Zhang, K., Wang, M., Qiu, F.: ACS Omega 6, 889–899 (2021)
Lipinski, C.A., Lombardo, F., Dominy, B.W., Feeney, P.J.: Adv. Drug Deliv. Rev. 46(3), 3–26 (2001)
Jiang, B., Meng, L., Zou, N., Wang, H., Li, S., Cheng, X., Wang, Z., Chen, W., Wang, C.: Phytomedicine, 62, 152–967 (2019)
Ridings, J.E., Barrattb, M.D., Gary, R., Earnshawd, C.G., Eggingtond, C.E., Ellis, M.K., Judsonf, P.N., Langowskig, J.J., Marchantg, C.A., Watson, W.P., Yih, T.D.: Toxicology, 106, 267–279 (1996)
Muster, W., Breidenbach, A., Fischer, H.: Drug Discov Today. 13, 303–310 (2008)
Barratt, M.D., Langowski, J.J.: J. Chem. Inf. Comput. Sci. 39(2), 294–298 (1999)
Estrada, E., Patlewicz, G., Gutierrez, Y.: J. Chem. Inf. Comput. Sci. 44(2), 688–698 (2004)
Langton, K., Patlewicz, G., Long, A., Marchant, C., Basketter, D.: Contact Dermatitis 55, 342–347 (2006)
Is, Y.S., Durdagi, S., Aksoydan, B., Yurtsever, M.: ACS Chem. Neurosci. 9, 1768–1782 (2018)
Zhuang, C., Zhang, W., Sheng, C., Zhang, W., Xing, C., Miao, Z.: Chem. Rev. 117(12), 7762–7810 (2017)
Tran, T.-D., Do, T.-H., Tran, N.-C., Ngo, T.-D., Huynh, T.-N.-P., Tran, C.-D., Thai, K.-M.: Bioorg. Med. Chem. Lett. 4555–4560 (2012)
Hsieh, C.-T., Hsieh, T.-J., El-Shazly, M., Chuang, D.-W., Tsai, Y.-H., Yen, C.-T., Wua, S.-F., Wua, Y.-C., Chang, F.-R.: Bioorg. Med. Chem. 3912–3915 (2012)
Salama, T.A., El-Ahl, A.-A.S., Khalil, A.-G.M., Girges, M.M., Lackner, B., Steindl, C., Elmorsy, S.S.: Monatshefte fur Chemie, 134, 1241–1252 (2003)
Bai, X., Shi, W.Q., Chen, H.F., Zhang, P., Li, Y., Yin, S.F.: Chem. Nat. Compounds, 48, 1 (2012)
Chen, Y.-F., Wu, S.-N., Gao, J.-M., Liao, Z.-Y., Tseng, Y.-T., Fülöp, F., Chang, F.-R., Lo, Y.-C.: Molecules, 25, 2907 (2020)
Filimonov, D., Poroikov, V., Borodina, Y., Gloriozova, T.: J. Chem. Inf. Comput. Sci. 39, 666–670 (1999)
Ferreira, J.V., Chaves, G.A., Marino, B.L.B., Sousa, K.P.A., Souza, L.R., Brito, M.F.B., Teixeira, H.R.C., Da Silva, C.H.T.P., Santos, C.B., Hage-Melim, L.I.S.: ChemMedChem 12(16), 408–1416 (2017). https://www.ncbi.nlm.nih.gov/pubmed/?term=Santos%20CBR%5BAuthor%5D&cauthor=true&cauthor_uid=28417566. https://www.ncbi.nlm.nih.gov/pubmed/?term=Santos%20CBR%5BAuthor%5D&cauthor=true&cauthor_uid=28417566"R
McCarroll, M.N., Gendelev, L., Kinser, R., Taylor, J., Bruni, G., Myers-Turnbull, D., Helsell, C., Carbajal, A., Rinaldi, C., Kang, H.J., Gong, J.H., Sello, J.K., Tomita, S., Peterson, R.T., Keiser, M.J., Kokel, D.: Nat. Commun. 10, 4078 (2019)
Keiser, M.J., Roth, B.L., Armbruster, B.N., Ernsberger, P., Irwin, J.J., Shoichet, B.K.: Nat Biotech 25, 197–206 (2007)
Boda, K., Gasteiger, J., Seidel, T.: J. Comput. Aided Mol. Des. 21(6), 311–325 (2007)
Rammohan, A., Reddy, J.S., Sravya, G., et al.: Chalcone synthesis, properties and medicinal applications: a review. Environ. Chem. Lett. 18, 433–458 (2020)
Se, W.D.: Bioorg. Med. Chem. Lett. 15(24), 5514–5516 (2005)
Hyacientha, B.M.S., Picanco, K.R.T., Sanchez-Ortiza, B.L., Silvad, L.B., Pereira, A.C.M., Goes, L.D.M., Borgesa, R.S., Ataide, R.C., Santos, C.B.R., Carvalho, H.O., Anduaga, G.M.G., Navarretec, A., Carvalho, J.C.T.: Toxicol. Rep. 217–232 (2020)
Organização Para A Cooperação E Desenvolvimento Económico—OECD. Test no. 236: fish embryo acute toxicity test (FET), 22 p. OECD, Paris (2013)
Inbar, Y., Schneidman-Duhovny, D., Dror, O., Nussinov, R., Wolfson, H.J.: Research in Computational Molecular Biology: 11th Annual International Conference, RECOMB, Oakland, CA, USA (2007)
Schneidman-Duhovny, D., Dror, O., Inbar, Y., Nussinov, R., Wolfson, H.J.: Nucl. Acids. Res. 36, W223–W228 (2008)
Gupta, S., Mohan, C.G.: Biomed. Res. Int. (2014)
Schrödinger Release 2018-1: QikProp, Schrödinger, LLC, New York (2018)
Bretaud, S., Lee, S., Guo, S.: Neurotoxicol. Teratol. 26, 857–864 (2004)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
1 Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Marino, B.L.B. et al. (2022). In Silico Drug Design and in Vivo Acute Toxicity Assay of Chalcone Analogs with Biological Antiparkinsonian Activity. In: Taft, C.A., de Lazaro, S.R. (eds) Research Topics in Bioactivity, Environment and Energy. Engineering Materials. Springer, Cham. https://doi.org/10.1007/978-3-031-07622-0_14
Download citation
DOI: https://doi.org/10.1007/978-3-031-07622-0_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-07621-3
Online ISBN: 978-3-031-07622-0
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)